Clostridioides difficile spores can survive in the environment in either mono- or mixed-species biofilms. However, no previous studies have investigated chemical disinfection of C. difficile spores embedded in biofilms. Thus, the purpose of this study was to assess the in vitro effectiveness of hospital disinfectants against C. difficile spores embedded within biofilms.
KEYWORDS: in vitro study, anaerobic infections, biofilm, environmental decontamination, ribotype 027
ABSTRACT
Clostridioides difficile spores can survive in the environment in either mono- or mixed-species biofilms. However, no previous studies have investigated chemical disinfection of C. difficile spores embedded in biofilms. Thus, the purpose of this study was to assess the in vitro effectiveness of hospital disinfectants against C. difficile spores embedded within biofilms. Five unique C. difficile strains embedded in three different biofilm types grown for 72 or 120 h were exposed to seven different hospital disinfectants. C. difficile abundance [as log(number of CFU/milliliter)] was calculated after manufacturer-determined contact times along with biofilm biomass and microscopy. The primary analysis compared differences between C. difficile vegetative cell and spore counts as well as amounts of biomass after exposure to disinfectants. C. difficile vegetative cells and spores were recovered from biofilms regardless of the type of biofilm growth or biofilm growth time. No disinfectant was able to completely eliminate C. difficile from the biofilms. Overall, Clorox, ortho-phthalaldehyde (OPA), and Virex were most effective at killing C. difficile spores regardless of biofilm age, ribotype, or wash conditions (whether biofilms are washed or unwashed) (P = 0.001, each). Clorox and OPA were also effective at killing total vegetative cell growth (P = 0.001, each), but Virex was found to be ineffective against vegetative cell growth in biofilms (P = 0.77). Clorox and Virex were most effective in reducing biomass, followed by Nixall, OPA, and Vital Oxide. No disinfectant was able to completely eliminate C. difficile embedded within biofilms although differences among disinfectants were noted. Future research will be required to determine methods to eradicate this persister reservoir.
INTRODUCTION
Clostridioides difficile is a Gram-positive, obligate, anaerobic, spore-forming bacterium and the most common health care-acquired infection in the United States (1). Spores can be transmitted via symptomatic and asymptomatic carriers to the environment or via health care personnel (2, 3). To break the transmission cycle, hospitals and health care institutions commonly use chemical sporicidal agents for environmental surface cleaning (4, 5). Current guidelines of the United Kingdom Department of Health recommend the use of chlorine-based disinfectants to reduce C. difficile spore levels in clinical settings (5, 6). As per the Infectious Diseases Society of America (IDSA), daily cleaning with a sporicidal agent in conjunction with other modalities is recommended in the case of an outbreak, hyperendemic settings, or a high rate of repeated infection (7). However, clinical studies investigating chlorine-based disinfectants found that no disinfectant tested achieved adequate disinfection within the labeled determined contact time in either a clean or dirty environment (6). The reasons for this lack of efficacy are unclear but may relate to the presence of biofilms.
In vitro studies that evaluate the efficacy of sporicidal disinfectants generally test the compound against planktonic bacteria or spores (8). However, it is likely that C. difficile survives in the abiotic environment in either mono- or mixed-species biofilms (9). Biofilm formation by C. difficile was first reported in 2012 (10, 11). Although biofilm production can vary between strains, C. difficile biofilms have been shown to form a complex multilayered protein, containing DNA and polysaccharide, in the gut as well as on abiotic surfaces (9, 12, 13). The exopolysaccharide (EPS) matrix provides an anaerobic scaffold that supports both vegetative cell growth and spores (10). Multiple variables influence biofilm formation, including virulence-associated proteins (cwp84, flagella, transcription factors, and SpoA) and the quorum sensing regulator LuxS (9, 11). Using mixed-species biofilms, a coinfection model of Finegoldia magna and C. difficile enhanced biofilm formation of both bacteria (11), and another polymicrobial biofilm model described C. difficile spores embedded in the biofilm (14).
Most sporicidal disinfectants are not as effective against biofilm embedded spores (15–18). Microorganisms in biofilm may be up to 1,000-fold more resistant to disinfectants than their planktonic counterparts. For example, spores of Bacillus cereus embedded in a biofilm are highly resistant to cleaning procedures (19). After 6 days of exposure to chemical disinfection, Bacillus spores could still be observed within a biofilm using microslicing techniques (20). Penicillium brevicompactum spores were also resistant to chlorine disinfection when embedded within a biofilm but were killed as free spores (21). Biofilm properties that affect the efficacy of chemical disinfectants include the age of the biofilm and cell density (22, 23). Despite this known association on spores and biofilms, few studies have investigated chemical disinfection of C. difficile spores embedded in biofilms. Thus, the purpose of this study was to assess the in vitro effectiveness of hospital-based disinfectants against C. difficile spores within a mono- or mixed-species biofilm at various stages of biofilm development.
(This research was conducted by T. Rashid in partial fulfillment of the requirements for a Ph.D. from the University of Texas School of Public Health, Houston, TX, 2019.)
RESULTS
C. difficile biofilm.
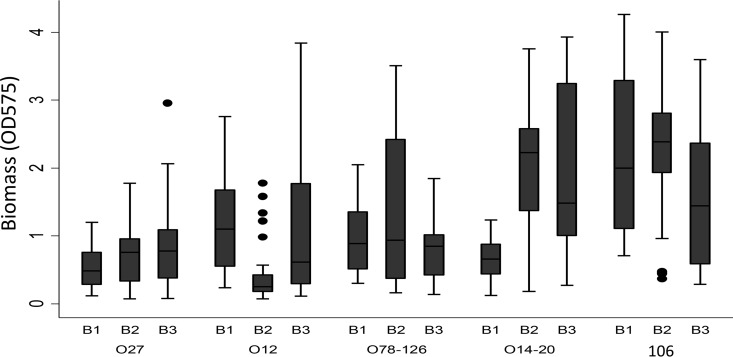
C. difficile vegetative cells and spores were grown from all ribotypes regardless of the type of biofilm growth (single versus mixed species or anaerobic versus aerobic) or biofilm growth time. Biofilms were visualized starting on day 2 of growth attached to the base of 24-well plates. No statistically significant difference was observed among the three modes of biofilm formation (P = 0.243). Overall, all ribotypes were able to form biofilms although amounts of biomass differed among them (Fig. 1). The mean log CFU counts of vegetative cells and spores for 72-h biofilm between ribotypes were 2.77 ± 0.07 and 1.77 ± 0.06 CFU/ml, respectively. Average log CFU counts of vegetative cells and spores were 3.11 ± 1.62 CFU/ml and 1.99 ± 1.46 CFU/ml, respectively, for C. difficile grown in 72-h biofilms, 2.84 ± 1.43 CFU/ml and 1.78 ± 1.14 CFU/ml, respectively, for C. difficile grown in monospecies anaerobic biofilms, and 2.35 ± 1.36 and 1.54 ± 0.97 CFU/ml, respectively, for multispecies anaerobic biofilms. Average log CFU counts of vegetative cells and spore counts for 72-h biofilm by precleaning status were 3.06 ± 1.40 CFU/ml and 2.04 ± 1.22 CFU/ml, respectively, for washed biofilm and 2.47 ± 1.54 CFU/ml and 1.51 ± 1.17 CFU/ml, respectively, for unwashed biofilms. Similar results were seen in 120-h biofilms with the exception that the vegetative cell counts were lower than those observed in biofilms grown for 72 h (P < 0.01).
FIG 1.
Biofilm mass for different C. difficile ribotypes. B1, C. difficile monospecies biofilm; B2, mixed-species biofilm grown anaerobically; B3, mixed-species biofilm grown aerobically.
C. difficile spore and vegetative cells embedded within biofilms after disinfectant exposure.
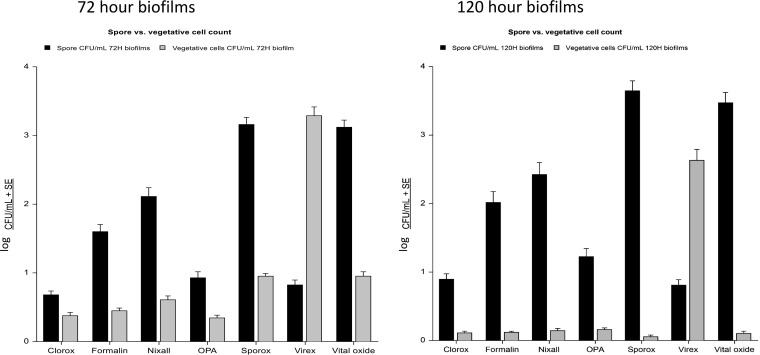
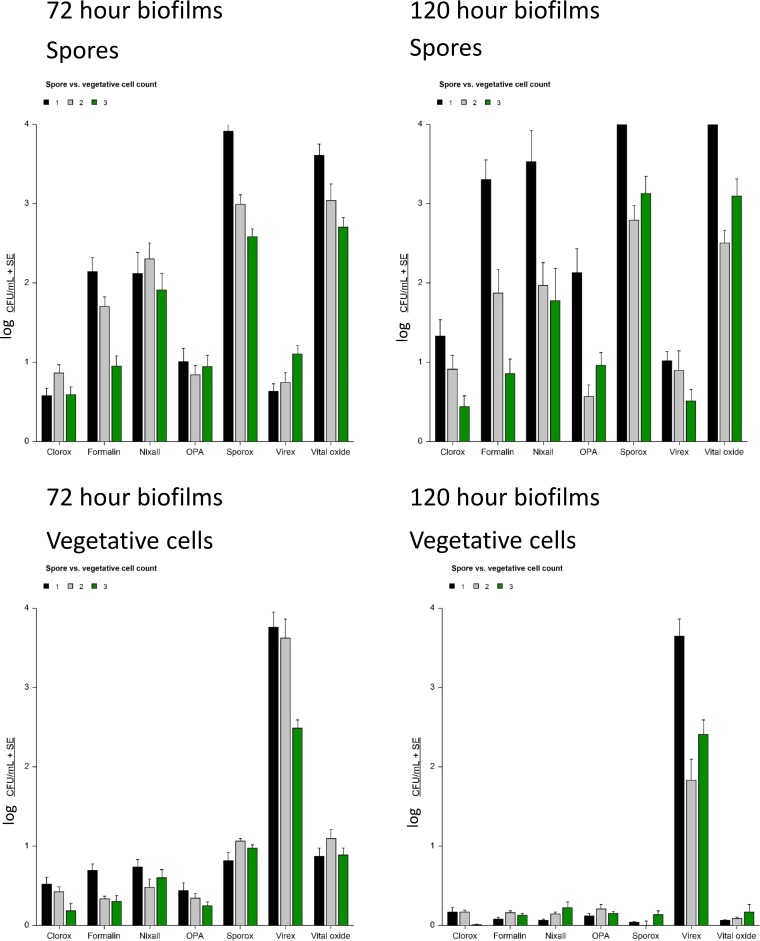
The efficacy of seven hospital disinfectants to kill C. difficile embedded within biofilms grown for 72 h and 120 h is shown in Fig. 2. No disinfectant was able to completely eliminate C. difficile from the biofilms. Overall, Clorox, ortho-phthalaldehyde (OPA), and Virex were most effective at killing C. difficile spores regardless of biofilm age, ribotype, or wash conditions (i.e., washed or unwashed biofilm) (P = 0.001, each). Clorox and OPA were also effective at killing vegetative cell growth (P = 0.001, each) but Virex was found to be ineffective against the vegetative cell growth (P = 0.77). This same effect was noted in biofilms grown for 72 and 120 h. Formalin and Nixall were not as effective as Clorox, OPA, or Virex but were more effective than Sporox, which did not have a sporicidal effect. Similar results were observed regardless of biofilm preparation method (C. difficile single species, anaerobic mixed species, or aerobic mixed species) (Fig. 3).
FIG 2.
Biofilm embedded C. difficile spore and vegetative cell counts (log CFU counts/milliliter) after exposure to disinfectants grown in biofilms for 72 or 120 h, as indicated.
FIG 3.
Killing effect of disinfectant based on biofilm type.
Efficacy of disinfectants against C. difficile in a biofilm.
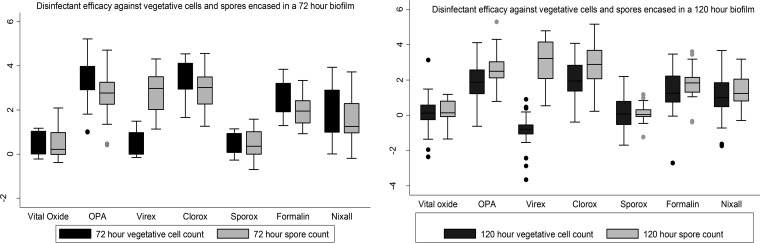
Figure 4 shows the log reduction in CFU count for vegetative cells and spores in 72- and 120-h biofilms. The mean log reduction of CFU count for all disinfectants was 1.74 ± 0.07 and 1.83 ± 0.06 CFU/ml, respectively, for vegetative cell counts and spores in a 72-h biofilm. Similar results were observed for 120-h biofilm. Most of the disinfectants were ineffective in reducing the vegetative cell and spore counts by more than 2 logs. A regression analysis was done to look at the effect of disinfectants on total and spore counts of 3- and 5-day-old biofilms adjusting for ribotypes, duration of exposure, type of biofilm, and presence of organic matter. As Vital Oxide was found to be ineffective against planktonic C. difficile and all three stages of C. difficile biofilm, the efficacy of other disinfectants was compared to that of Vital Oxide. There was mostly a statistically significant (P < 0.001, all) reduction in total and spore counts for both 3- and 5-day-old biofilms except for Sporox and Virex. Sporox increased the total and spore counts in all cases, and Virex increased the total count but reduced the spore count.
FIG 4.
C. difficile vegetative and spore cell counts based on disinfectant and biofilm growth time.
Biomass of biofilms after exposure to disinfectants.
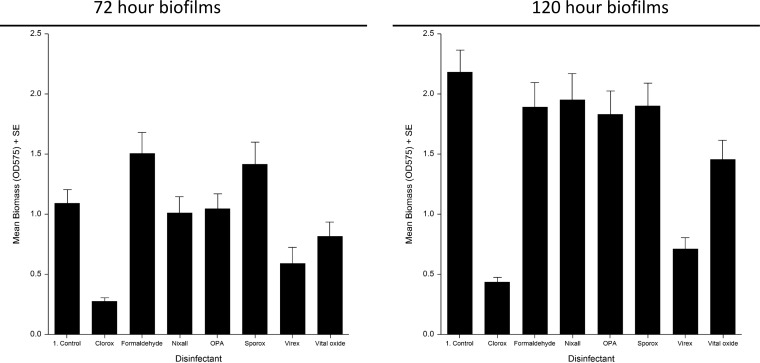
The effect of disinfectants on biomass of biofilms is shown in Fig. 5. Overall, Clorox and Virex were most effective in reducing biomass, followed by Nixall, OPA and Vital Oxide. There was no reduction of biomass postexposure to Sporox and formalin. The same effect was noted in biofilms grown for 72 h and 120 h. An F test revealed a strong association between disinfectant efficacy in reducing vegetative cell count and biofilm mass (P = 0.0002), which remained significant even after adjusting for the type of disinfectant, ribotype, and stage of biofilm growth (P < 0.01). Similar associations were observed for disinfectant efficacy in reducing spore count.
FIG 5.
Effect of disinfectants on biomass of biofilms grown for 72 and 120 h.
Biofilm visualization and viability testing.
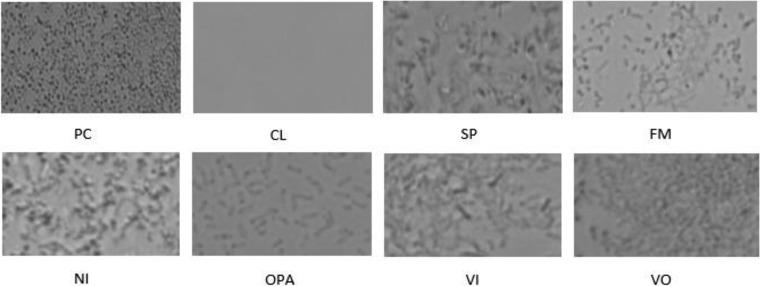
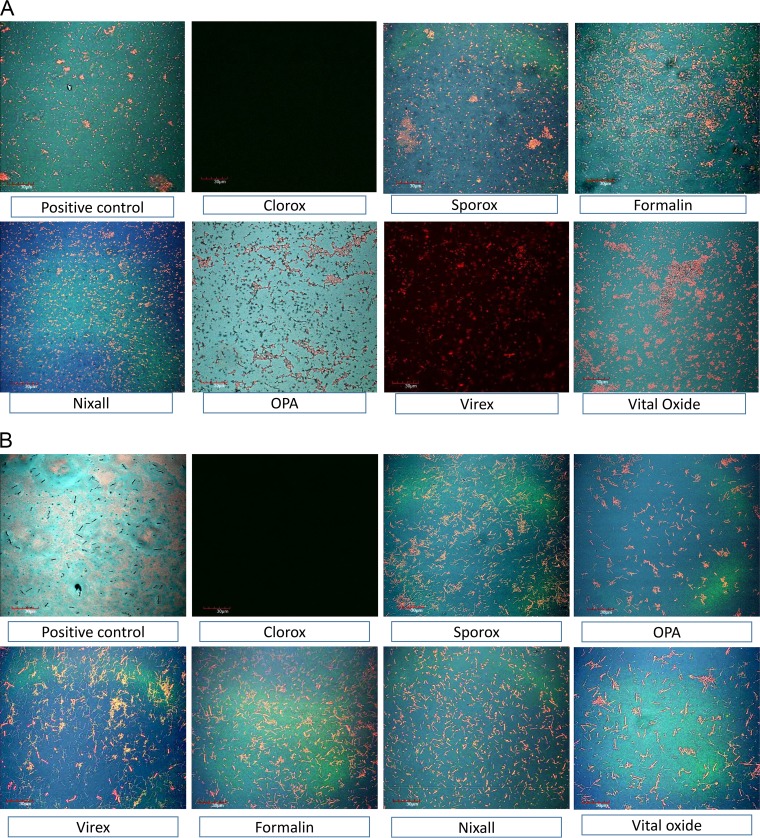
Using inverted microscopy, biofilms were not evident after treatment with Clorox and were markedly reduced after treatment with OPA or formalin (Fig. 6). Visualization and viability of cells were also assessed using live/dead biofilm viability staining by confocal microscopy (Fig. 7a and b) for both monospecies anaerobic and multispecies aerobic biofilms. Visually, no difference was found in the viability of cells between the two types of biofilm. More live cells were observed in 3-day-old biofilm than in 5-day-old biofilm for both ribotypes.
FIG 6.
Visualization of killing effect of disinfectants on monocellular C. difficile biofilm on a surface using light microscopy. PC, positive control; CL, Clorox; SP, Sporox; FM, formalin; NI, Nixall; OPA, ortho-phthalaldehyde; VI, Virex; VO, Vital Oxide.
FIG 7.
(A) Visualization of killing effect of disinfectants on a 72-h anaerobic C. difficile biofilm on a surface using confocal microscopy. (B) Visualization of killing effect of disinfectants on a 72-h aerobic multispecies C. difficile biofilm on a surface using confocal microscopy.
DISCUSSION
The purpose of this study was to assess the in vitro effectiveness of hospital-based disinfectants against C. difficile spores within a mono- or mixed-species biofilm at various stages of biofilm development. Three disinfectants (Clorox, OPA, and Virex) were most effective at killing C. difficile spores regardless of experimental conditions. Clorox and OPA were also effective at killing vegetative C. difficile within biofilms although Virex was found to be ineffective against vegetative cell growth. Biomass studies were consistent with time-kill results, and light and confocal microscopy demonstrated a significant reduction in biofilm grown on slides after exposure to Clorox. Multivariate analysis demonstrated differences in disinfectant killing effects based on experimental conditions of the studies (ribotype, biofilm age, biofilm type, and washed versus unwashed). To the best of our knowledge, this is the first comprehensive study to evaluate the effect of hospital disinfectants on C. difficile embedded in biofilms. Strengths of the study include a large number of experiments under different growth conditions using a large number of clinically significant C. difficile ribotypes.
No previous study has investigated the killing effects of disinfectants on C. difficile embedded in a biofilm. However, previous studies have investigated the impact of disinfectants against other organisms embedded in biofilms (24). For example, 11% of multidrug-resistant Staphylococcus aureus (MRSA) and 80% of Pseudomonas cells survived in a biofilm after disinfectant exposure (25). Not only was Escherichia coli embedded in a biofilm resistant to sodium hypochlorite disinfectant but also the biofilm was able to reform on disinfectant-treated surfaces after initial disruption (26). Efficacy of sodium hypochlorite disinfectant was also dependent on the composition of the biofilm, with disinfectants having decreased killing effect in multispecies biofilms, results that are consistent with our study (27). Hypochlorite and quaternary ammonium disinfectants were ineffective against B. cereus embedded in biofilms even at high concentrations (24, 28). Studies using OPA have demonstrated decreased killing effects for Pseudomonas fluorescence (13) but not for Klebsiella pneumonia (29) when the organism was embedded in a biofilm. Studies using H2O2-based disinfectants were effective against P. aeruginosa and S. aureus embedded in biofilms, which differs from results of our study in which H2O2 was ineffective against C. difficile embedded in a biofilm (12). A hydrogen peroxide disinfectant (Sporox) displayed a reduced killing effect against P. aeruginosa embedded in a biofilm. This was postulated to be due to genetic products released by cells in a biofilm that may reduce their susceptibility to oxidative disinfectants such as H2O2 (30). Hospital and nursing home environments are known to harbor C. difficile spores and may be important sources for health care-associated infection. Biofilm removal methods may be required to properly clean these environments (31). An interesting observation from our study was that ammonium-based disinfectants (Virex) consistently reduced the spore count in a biofilm but had no effect on vegetative cells. It is possible that quaternary ammonium compounds may interfere with the formation of negatively charged biofilm matrix due to their cationic nature and may also affect spore germination (32). Sodium hypochlorite-based disinfectants denatured proteins in a biofilm and inhibited major enzymatic function of bacterial cells (32).
There are certain limitations to this study. This study used in vitro techniques to compare killing effects of hospital-based disinfectants against C. difficile embedded in biofilms. Future clinical studies to confirm these results are required. Accurate quantification of the organism within biofilms by sonication or scraping is sometimes limited by the ability to remove all of the organism from the biofilm (25). To overcome this issue, we used manual scraping in addition to sonication. Although our study looked at the killing effects of disinfectant using label-determined contact times and concentrations, it did not investigate any off-label use or effect of other environmental factors. This study was done on a polystyrene surface and, hence, may not necessarily represent how cells grow on other surfaces (25). For example, the rate of biofilm formation was enhanced on stainless steel surfaces compared to that on other metals or plastic surfaces, possibly due to the hydrophilic nature of the material or to surface irregularities leading to increased surface area (25). The viable cells after disinfectant exposure may have the ability to resurrect the biofilm and act as a reservoir for spread and preservation of recalcitrant infection or simply as a source of environmental contaminant (33). Thus, there is a need to combine disinfectant use with other modalities of treatment to eliminate the biofilm structure as well as persister cells.
Conclusion.
In this in vitro study to assess the effect of hospital-based disinfectant to kill C. difficile embedded in biofilms, no disinfectant was able to completely eliminate C. difficile. Overall, Clorox, OPA, and Virex were most effective at killing C. difficile spores regardless of biofilm age, ribotype, or wash conditions. Future research will be required to determine methods to eradicate this persister reservoir.
MATERIALS AND METHODS
Bacterial strains.
Two laboratory strains of C. difficile (R20291, ribotype 027; CD630, ribotype 012) and three clinical strains of different ribotypes (ribotypes 014-20, 078-126, and 106) were used for all experiments.
Chemical disinfectants.
Seven hospital disinfectants were used in this study, six with sporicidal properties and one nonsporicidal disinfectant as an active control (Table 1). Hospital disinfectants were defined as chemicals used for general-purpose disinfection of hospital buildings, patient rooms, or wards and did not include instrument-grade disinfectant, antibacterial clothes preparations, sanitary fluid, or hand sanitizers. For each disinfectant, contact time experiments were conducted based on manufacturer-determined contact times or a standardized contact time.
TABLE 1.
Commercial hospital disinfectants used in this study
| Active ingredient(s) (concn)a | Disinfectant name | Biocide typeb | Manufacturer | Minimum contact time (min)c | Sporicidal |
|---|---|---|---|---|---|
| Sodium hypochlorite (10%) | Clorox | CRA | Clorox Company | 1.5 | Yes |
| Formaldehyde (4%) | Formalin | Formaldehyde | Pure Health | 60 | Yes |
| Hypochlorous acid (0.046%) | Nixall | CRA | Nixall Company | 10 | Yes |
| OPA solution (0.575%, wt/vol) | Cidex OPA | OPA | McKesson | 10 | Yes |
| Hydrogen peroxide solution (7.5%) | Sporox | H2O2 | Sultan Healthcare | 30 | Yes |
| QAC (5–10%) | Virex | QAC | Diversey | 10 | Yes |
| Chlorine dioxide (0.2%) + QAC (0.125% + 0.125%) | Vital Oxide | CRA + QAC | Vital Oxide Company | 10 | No |
OPA, ortho-phthalaldehyde; QAC, quaternary ammonium compounds.
CRA, chlorine-releasing agents.
Per the manufacturer’s label.
Growth of biofilms containing C. difficile vegetative and spore cells on 24-well polystyrene plates.
Biofilms containing C. difficile vegetative and spore cells were grown under three different conditions, namely, strictly anaerobic, mixed aerobic and anaerobic, and strictly aerobic. To form a C. difficile single-species biofilm grown anaerobically (monospecies anaerobic biofilm), overnight cultures of C. difficile were diluted 1:100 in fresh brain heart infusion-supplemented (BHIS) broth containing 0.1 M glucose. One milliliter of broth was then pipetted into each well of a 24-well polystyrene plate and incubated anaerobically at 37°C for 3 and 5 days. To form a mixed multispecies biofilm grown anaerobically (multispecies anaerobic biofilm), 1 ml of overnight cultures of Enterococcus faecium and Staphylococcus aureus diluted 1:100 in BHIS was added to a 2-day-old C. difficile biofilm grown anaerobically. The mixture was then incubated aerobically at 37°C for an additional 24 or 72 h to maintain a consistent biofilm at an age of 3 or 5 days. Finally, to grow a multispecies biofilm in an aerobic environment (multispecies aerobic biofilm), an overnight culture of C. difficile grown anaerobically along with both E. faecium and S. aureus cultures was diluted as described above and pipetted simultaneously into a 24-well polystyrene plate. The plates were then incubated aerobically for 3 and 5 days for biofilm formation.
Quantification of biofilm biomass.
Three- or 5-day biofilms were washed twice with sterile phosphate-buffered saline (PBS) and allowed to air dry for 10 min. Following drying, 1 ml of filter-sterilized 0.2% crystal violet was added to each well and incubated for 30 min. The strictly anaerobic biofilm was incubated anaerobically, and the other two biofilms were incubated aerobically. After this time, the crystal violet was removed by washing the wells twice with sterile phosphate-buffered saline. Crystal violet dye was reeluted from the biofilm by the addition of 1 ml of methanol and incubation at room temperature for 30 min. Methanol-extracted dye was then diluted 1:1 and 1:10, and the optical density was measured on a plate reader at 570 nm (OD570).
Biofilm imaging and viability assay.
Biofilm was imaged using both an inverted light microscope (Evos Cell Imaging System; Thermo Fisher) and confocal laser scanning microscope. Light microscopy was done to visualize structure and formation differences of 3- and 5-day-old biofilms pre- and postexposure to disinfectants. Cells in the biofilms were also visualized using a confocal laser scanning microscope according to the protocol of Jurcisek et al. (34). Briefly, bacterial biofilms from all stages were formed on a four-well chamber slide. Following incubation, treated or untreated biofilms were washed, stained with BacLight live/dead stain, and fixed with neutral buffered formalin (8, 10, 34). A live/dead viability kit contained Syto9, and propidium iodide stain was added to visualize in vitro killing effects. Slides were fixed and washed with PBS, and plastic wells were removed from the slide. Saline was added to plastic wells and covered with coverslips. The edges of the coverslip were sealed with mounting medium and air dried for 1 h before microscopy. Samples were imaged under oil immersion using a laser confocal microscope (34). The excitation and emission wavelengths for Syto9 were 488 nm and 505 to 550 nm, respectively, and 543 and >650 nm, respectively, for propidium iodide (34). All assays were performed in duplicate.
Experimental procedures.
Immediately prior to experiments, the liquid supernatant of each well was gently removed with a pipette without disturbing the biofilm. Half of the biofilms were gently washed twice with sterile PBS, and the rest were left unwashed. This was done to look at the effect of organic substrate on disinfectant efficacy. For each experiment, 500 μl of disinfectant at the original concentration was added in duplicate to both the washed and unwashed biofilms based on label-determined contact times (Table 1). PBS (500 μl) was added to the wells as a positive control. Following exposure for the appropriate time, the disinfectants were removed, and wells were washed with 1 ml of sterile PBS. Biofilms were then detached from the bottom of the wells using a sonicator at 42 Hz for 10 min, followed by manual scraping for exactly 1 min, and pipetted into an Eppendorf tube. Removal of the biofilms was confirmed by light microscopy. For total counts of viable cells or spores, the cells in the Eppendorf tube were serially diluted, and 100 μl was plated on blood agar plates and incubated anaerobically at 37°C for 48 h. For spore counts, the detached cells were heated at 65°C for 30 min to kill the vegetative cells. Vegetative cell and spore C. difficile CFU counts were measured using the dilution and plating method (35). In the case of multispecies biofilms, the morphology of the colonies for the three types of organism used were distinct, allowing for accurate C. difficile counts. All experiments were performed at least in duplicate using the appropriate positive and negative controls.
Statistical analysis.
The primary analysis was to compare C. difficile vegetative cell and spore counts as well as biomass measured by a crystal violet assay, pre- and postexposure to disinfectants. Secondary aims were to compare the log reduction of C. difficile vegetative cell and spore counts based on the type of biofilm (monospecies, mixed, or mixed aerobic), duration of biofilm formation (3 or 5 days), ribotype, and presence of organic matter (washed versus unwashed biofilm). The third aim was to visually compare the overall efficacies of disinfectants against the three different types of biofilm for different ages of biofilm. The vegetative cell and spore mean log10 reduction in CFU counts ± standard error (SE) was calculated for each disinfectant by biofilm type, age, ribotype, and experimental procedure. Linear regression analysis was done comparing the efficacy of sporicidal disinfectants against the efficacy of the nonsporicidal disinfectant Vital Oxide for biofilms of different ages. SAS, version 9.3 (SAS Institute, Cary NC), or STATA/IC, version 12.1 (STATACorp LLC, College Station, TX), was used for all analyses. A P value of <0.05 was considered significant.
ACKNOWLEDGMENT
This study was funded, in part, from the NIH NIAID (U01AI124290).
REFERENCES
- 1.Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, Farley MM, Holzbauer SM, Meek JI, Phipps EC, Wilson LE, Winston LG, Cohen JA, Limbago BM, Fridkin SK, Gerding DN, McDonald LC. 2015. Burden of Clostridium difficile infection in the United States. N Engl J Med 372:825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rashid T, Vonville H, Hasan I, Garey K. 2017. Mechanisms for floor surfaces or environmental ground contamination to cause human infection: a systematic review. Epidemiol Infect 145:347–357. doi: 10.1017/S0950268816002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kenters N, Huijskens E, de Wit S, Sanders I, van Rosmalen J, Kuijper E, Voss A. 2017. Effectiveness of various cleaning and disinfectant products on Clostridium difficile spores of PCR ribotypes 010, 014 and 027. Antimicrob Resist Infect Control 6:54. doi: 10.1186/s13756-017-0210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uwamahoro MC, Massicotte R, Hurtubise Y, Gagné-Bourque F, Mafu AA, Yahia L. 2018. Evaluating the sporicidal activity of disinfectants against Clostridium difficile and Bacillus amyloliquefaciens spores by using the improved methods based on ASTM E2197-11. Front Public Health 6:18. doi: 10.3389/fpubh.2018.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rutala WA, Gergen MF, Weber DJ. 2012. Efficacy of different cleaning and disinfection methods against Clostridium difficile spores: importance of physical removal versus sporicidal inactivation. Infect Control Hosp Epidemiol 33:1255–1258. doi: 10.1086/668434. [DOI] [PubMed] [Google Scholar]
- 6.Speight S, Moy A, Macken S, Chitnis R, Hoffman PN, Davies A, Bennett A, Walker JT. 2011. Evaluation of the sporicidal activity of different chemical disinfectants used in hospitals against Clostridium difficile. J Hosp Infect 79:18–22. doi: 10.1016/j.jhin.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 7.McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, Dubberke ER, Garey KW, Gould CV, Kelly C, Loo V, Sammons JS, Sandora TJ, Wilcox MH. 2018. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 66:e1–e8. doi: 10.1093/cid/cix1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Semenyuk EG, Laning ML, Foley J, Johnston PF, Knight KL, Gerding DN, Driks A. 2014. Spore formation and toxin production in Clostridium difficile biofilms. PLoS One 9:e87757. doi: 10.1371/journal.pone.0087757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ðapa T, Dapa T, Leuzzi R, Ng YK, Baban ST, Adamo R, Kuehne SA, Scarselli M, Minton NP, Serruto D, Unnikrishnan M. 2013. Multiple factors modulate biofilm formation by the anaerobic pathogen Clostridium difficile. J Bacteriol 195:545–555. doi: 10.1128/JB.01980-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawson LF, Valiente E, Faulds-Pain A, Donahue EH, Wren BW. 2012. Characterisation of Clostridium difficile biofilm formation, a role for Spo0A. PLoS One 7:e50527. doi: 10.1371/journal.pone.0050527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dapa T, Unnikrishnan M. 2013. Biofilm formation by Clostridium difficile. Gut Microbes 4:397–402. doi: 10.4161/gmic.25862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeQueiroz GA, Day DF. 2007. Antimicrobial activity and effectiveness of a combination of sodium hypochlorite and hydrogen peroxide in killing and removing Pseudomonas aeruginosa biofilms from surfaces. J Appl Microbiol 103:794–802. doi: 10.1111/j.1365-2672.2007.03299.x. [DOI] [PubMed] [Google Scholar]
- 13.Simões M, Carvalho H, Pereira MO, Vieira MJ. 2003. Studies on the behaviour of pseudomonas fluorescens biofilms after ortho-phthalaldehyde treatment. Biofouling 19:151–157. doi: 10.1080/0892701031000072127. [DOI] [PubMed] [Google Scholar]
- 14.Crowther GS, Chilton CH, Todhunter SL, Nicholson S, Freeman J, Baines SD, Wilcox MH. 2014. Development and validation of a chemostat gut model to study both planktonic and biofilm modes of growth of Clostridium difficile and human microbiota. PLoS One 9:e88396. doi: 10.1371/journal.pone.0088396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campanac C, Pineau L, Payard A, Baziard-Mouysset G, Roques C. 2002. Interactions between biocide cationic agents and bacterial biofilms. Antimicrob Agents Chemother 46:1469–1474. doi: 10.1128/aac.46.5.1469-1474.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grobe KJ, Zahller J, Stewart PS. 2002. Role of dose concentration in biocide efficacy against Pseudomonas aeruginosa biofilms. J Ind Microbiol Biotechnol 29:10–15. doi: 10.1038/sj.jim.7000256. [DOI] [PubMed] [Google Scholar]
- 17.Belessi C-E, Gounadaki AS, Psomas AN, Skandamis PN. 2011. Efficiency of different sanitation methods on Listeria monocytogenes biofilms formed under various environmental conditions. Int J Food Microbiol 145:S46–S52. doi: 10.1016/j.ijfoodmicro.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 18.Bonez PC, dos Santos Alves CF, Dalmolin TV, Agertt VA, Mizdal CR, da Costa Flores V, Marques JB, Santos RCV, de Campos M. 2013. Chlorhexidine activity against bacterial biofilms. Am J Infect Control 41:e119–e122. doi: 10.1016/j.ajic.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Majed R, Faille C, Kallassy M, Gohar M. 2016. Bacillus cereus biofilms—same, only different. Front Microbiol 7:1054. doi: 10.3389/fmicb.2016.01054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosni AA, Szabo JG, Bishop PL. 2011. Efficacy of chlorine dioxide as a disinfectant for Bacillus spores in drinking-water biofilms. J Environ Eng 137:569–574. doi: 10.1061/(ASCE)EE.1943-7870.0000355. [DOI] [Google Scholar]
- 21.de Siqueira VM, Lima N. 2010. Efficacy of free chlorine against water biofilms and spores of Penicillium brevicompactum, p 157–165. In Borchers U, Gray J, Thompson KC (ed), Water contamination emergencies: monitoring, understanding and acting. RSC Publishing, Cambridge, UK. [Google Scholar]
- 22.Stewart PS. 2015. Antimicrobial tolerance in biofilms. Microbiol Spectr 3:MB-0010-2014. doi: 10.1128/microbiolspec.MB-0010-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beauchamp CS, Dourou D, Geornaras I, Yoon Y, Scanga J, Belk K, Smith G, Nychas G, Sofos J. 2012. Sanitizer efficacy against Escherichia coli O157: H7 biofilms on inadequately cleaned meat-contact surface materials. Food Prot Trends 32:173–182. [Google Scholar]
- 24.Otter JA, Vickery K, Walker JT, deLancey Pulcini E, Stoodley P, Goldenberg SD, Salkeld JA, Chewins J, Yezli S, Edgeworth JD. 2015. Surface-attached cells, biofilms and biocide susceptibility: implications for hospital cleaning and disinfection. J Hosp Infect 89:16–27. doi: 10.1016/j.jhin.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Smith K, Hunter IS. 2008. Efficacy of common hospital biocides with biofilms of multi-drug resistant clinical isolates. J Med Microbiol 57:966–973. doi: 10.1099/jmm.0.47668-0. [DOI] [PubMed] [Google Scholar]
- 26.Leung CY, Chan YC, Samaranayake LP, Seneviratne CJ. 2012. Biocide resistance of Candida and Escherichia coli biofilms is associated with higher antioxidative capacities. J Hosp Infect 81:79–86. doi: 10.1016/j.jhin.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 27.Behnke S, Parker AE, Woodall D, Camper AK. 2011. Comparing the chlorine disinfection of detached biofilm clusters with those of sessile biofilms and planktonic cells in single- and dual-species cultures. Appl Environ Microbiol 77:7176–7184. doi: 10.1128/AEM.05514-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng JS, Tsai WC, Chou CC. 2002. Inactivation and removal of Bacillus cereus by sanitizer and detergent. Int J Food Microbiol 77:11–18. doi: 10.1016/s0168-1605(02)00060-0. [DOI] [PubMed] [Google Scholar]
- 29.Yu FP, Pyle BH, McFeters GA. 1993. A direct viable count method for the enumeration of attached bacteria and assessment of biofilm disinfection. J Microbiol Methods 17:167–180. doi: 10.1016/0167-7012(93)90044-I. [DOI] [PubMed] [Google Scholar]
- 30.Cochran WL, McFeters GA, Stewart PS. 2000. Reduced susceptibility of thin Pseudomonas aeruginosa biofilms to hydrogen peroxide and monochloramine. J Appl Microbiol 88:22–30. doi: 10.1046/j.1365-2672.2000.00825.x. [DOI] [PubMed] [Google Scholar]
- 31.Stiefel P, Mauerhofer S, Schneider J, Maniura-Weber K, Rosenberg U, Ren Q. 2016. Enzymes enhance biofilm removal efficiency of cleaners. Antimicrob Agents Chemother 60:3647–3652. doi: 10.1128/AAC.00400-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lineback CB, Nkemngong CA, Wu ST, Li X, Teska PJ, Oliver HF. 2018. Hydrogen peroxide and sodium hypochlorite disinfectants are more effective against Staphylococcus aureus and Pseudomonas aeruginosa biofilms than quaternary ammonium compounds. Antimicrob Resist Infect Control 7:154. doi: 10.1186/s13756-018-0447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis K. 2001. Riddle of biofilm resistance. Antimicrob Agents Chemother 45:999–1007. doi: 10.1128/AAC.45.4.999-1007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jurcisek JA, Dickson AC, Bruggeman ME, Bakaletz LO. 2011. In vitro biofilm formation in an 8-well chamber slide. J Vis Exp 47:2481. doi: 10.3791/2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sieuwerts S, De Bok FA, Mols E, De Vos WM, van Hylckama Vlieg J. 2008. A simple and fast method for determining colony forming units. Lett Appl Microbiol 47:275–278. doi: 10.1111/j.1472-765X.2008.02417.x. [DOI] [PubMed] [Google Scholar]