Cefiderocol is a siderophore-cephalosporin conjugate with greater in vitro potency under iron-depleted conditions. During infection, iron is scarce in host tissue; however, it is not known whether iron overload in the host, such as in cases of hereditary hemochromatosis, alters the efficacy of cefiderocol. We compared cefiderocol efficacy between iron-overloaded and standard murine thigh infection models.
KEYWORDS: Gram-negative bacteria, animal models, cephalosporin, iron regulation, siderophores, soft-tissue infection
ABSTRACT
Cefiderocol is a siderophore-cephalosporin conjugate with greater in vitro potency under iron-depleted conditions. During infection, iron is scarce in host tissue; however, it is not known whether iron overload in the host, such as in cases of hereditary hemochromatosis, alters the efficacy of cefiderocol. We compared cefiderocol efficacy between iron-overloaded and standard murine thigh infection models. Female CD-1 mice rendered neutropenic received 2 weeks of iron dextran at 100 mg/kg of body weight/day intraperitoneally (iron-overloaded model) or no injections (standard model). Mice were inoculated (107 CFU/ml) with Enterobacterales, Acinetobacter baumannii, and Pseudomonas aeruginosa with previously determined cefiderocol MICs from 0.25 to 64 mg/liter. Human-simulated regimens of cefiderocol or meropenem (2 g every 8 h [q8h], 3-h infusion) were administered for 24 h (31 strains) or 72 h (15 strains; cefiderocol only). Procedures were simultaneously performed in standard and iron-overloaded models. Mean bacterial burdens (log10 CFU/thigh) at baseline were 5.75 ± 0.47 versus 5.81 ± 0.51 in standard versus iron-overloaded models, respectively. At 24 h, mean burdens in standard versus iron-overloaded models decreased by −0.8 ± 1.9 versus −1.2 ± 2.0 (P = 0.25) in meropenem-treated mice and by −1.5 ± 1.4 versus −1.6 ± 1.5 (P = 0.54) in cefiderocol-treated mice. At 72 h, mean burdens in cefiderocol-treated mice decreased by −2.5 ± 1.5 versus −2.5 ± 1.4. No overall differences in efficacy between the models were observed for meropenem or cefiderocol. Human-simulated exposure of cefiderocol is equally efficacious in iron-overloaded and normal hosts. The potential clinical use of cefiderocol to treat Gram-negative infections in patients with iron overload is supported.
INTRODUCTION
Cefiderocol is a siderophore-conjugated cephalosporin that is recognized by outer membrane transporters of iron in Gram-negative bacteria, facilitating uptake into the periplasmic space, its site of activity. The expression of these transporters, along with native bacterial siderophores, is upregulated when environmental iron is low (1). Evidence supporting this Trojan horse mechanism is found in measurements of the in vitro MIC of cefiderocol for Gram-negative bacteria in broth with various iron concentrations (2). When excess iron is added to the broth, cefiderocol MIC increases up to that of ceftazidime, which contains the same cephalosporin core structure and side chain but lacks a siderophore moiety. When iron is removed, the in vitro MIC of cefiderocol decreases below that observed in standard cation-adjusted Mueller-Hinton Broth, indicating increased potency of the drug when iron is not available.
Free (non-protein-bound) iron is sparse in living tissue because of its well-known propensity to catalyze the production of damaging oxygen free radicals. It is further restricted via the hypoferremic response to infection in mammalian hosts, where iron is sequestered intracellularly to deprive infectious organisms of this essential nutrient (3). Therefore, the low availability of iron in vivo during infection is similar to that of iron-depleted broth in vitro and may contribute to the efficacy of cefiderocol. However, the hypoferremic response is mediated through hepcidin, a protein with expression under the control of the inflammatory cascade as well as overall iron status of the host (4); therefore, patients with iron overload may have a diminished hypoferremic response. Furthermore, while iron sequestration is accomplished by multiple mechanisms (5), iron overload may exceed the capacity of these pathways to restrict iron. This potential for increased bacterial iron availability, coupled with in vitro observations of increased MIC with excess iron, raises a concern that the efficacy of siderophore-antibiotic conjugates like cefiderocol could be impacted in the setting of host iron overload.
Several diseases can lead to chronic iron overload, including hereditary hemochromatosis, a family of disorders involving mutations to the hepcidin gene, as well as thalassemia, which necessitates chronic blood transfusions (4). Iron overload can also be caused acutely in the case of massive transfusion or pharmacological iron overdose. Recently, we developed a murine model of thigh infection with comorbid iron overload that replicates chronic deposition of iron in the liver and spleen, important organs of iron storage, as well as elevated plasma iron from acute iron overload (6). We compared the efficacy of 24 h of human-simulated plasma exposure of cefiderocol 2 g q8h (3-h infusion) in this translational iron-overloaded thigh infection model to the standard murine thigh infection model against a collection of Gram-negative bacteria, including Enterobacterales, Pseudomonas aeruginosa, and Acinetobacter baumannii, which have been studied previously in the standard model. For a subset of bacterial isolates, we extended the treatment duration in separate experimental runs up to 72 h to determine if adaptive resistance developed in the presence of iron overload.
RESULTS
Bacterial density studies.
The efficacy of human-simulated exposure of cefiderocol was compared between the standard neutropenic murine thigh infection model and the iron-overloaded murine thigh infection model. To control for any effect of the iron-overloading treatment on pathogen fitness, the efficacy of a non-siderophore-conjugated antibiotic, meropenem, was also compared between models for a subset of bacterial strains. Bacterial densities (means ± standard deviations [SD]) averaged across all isolates are presented in Table 1. In the absence of antimicrobial treatment, all isolates grew adequately in both standard and iron-overloaded models.
TABLE 1.
Bacterial burdens (means ± SD) in standard and iron-overloaded murine thigh infection models averaged across all strains studied
| Treatment and parametera | Values (log10 CFU/thigh) for each model |
P value | |
|---|---|---|---|
| Standard model | Iron overloaded | ||
| Meropenem 24-h efficacy (12 strains) | |||
| Baseline | 5.77 ± 0.45 | 5.92 ± 0.47 | 0.064 |
| Saline (Δ) | +2.94 ± 0.99 | +2.90 ± 0.77 | 0.833 |
| Meropenem HSR (Δ) | −0.81 ± 1.94 | −1.19 ± 2.04 | 0.247 |
| Cefiderocol 24-h efficacy (31 strains) | |||
| Baseline | 5.75 ± 0.47 | 5.81 ± 0.51 | 0.227 |
| Saline (Δ) | +3.17 ± 1.07 | +3.43 ± 0.77 | 0.008 |
| Cefiderocol HSR (Δ) | −1.46 ± 1.40 | −1.55 ± 1.48 | 0.536 |
| Cefiderocol 72-h efficacy (15 strains) | |||
| Baseline | 5.52 ± 0.44 | 5.54 ± 0.38 | 0.697 |
| Saline 24 h (Δ) | +3.54 ± 0.77 | +3.53 ± 0.71 | 0.905 |
| Cefiderocol HSR 24h (Δ) | −1.77 ± 1.12 | −1.95 ± 0.77 | 0.187 |
| Saline 48 h (Δ) | +3.13 ± 1.15 | +3.88 ± 0.47 | <0.001 |
| Cefiderocol HSR 48 h (Δ) | −2.05 ± 1.82 | −2.35 ± 1.12 | 0.179 |
| Saline 72 h (Δ) | +2.02 ± 1.83 | +3.73 ± 0.16 | 0.037 |
| Cefiderocol HSR 72 h (Δ) | −2.48 ± 1.48 | −2.53 ± 1.42 | 0.807 |
Burdens in saline-dosed controls and treatment groups are presented as changes from baseline. Δ, change in bacterial density relative to baseline. HSR, human-simulated regimen.
Human-simulated meropenem exposure produced no significant difference in overall efficacy between the standard and iron-overloaded murine thigh infection models when averaged over 12 strains (Table 1). Efficacy by strain is presented in Fig. 1. Seven strains treated with the meropenem human-simulated regimen had significant differences in efficacy between the models, yet only one of these differences resulted in a categorical difference: ACB 127, which had growth in the standard model and stasis in the iron-overloaded model. No trends were observed favoring the efficacy of meropenem in either model.
FIG 1.
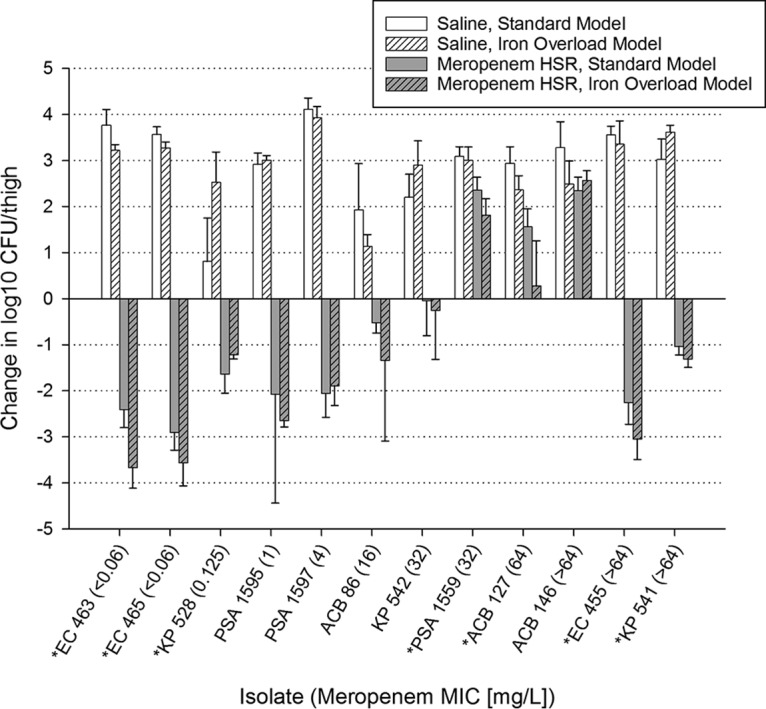
Efficacy of 24 h of a meropenem human-simulated regimen (HSR) (2 g q8h, 3-h infusion) against selected Gram-negative strains in the standard and iron-overloaded murine thigh infection models. Isolates for which meropenem had a significantly different change in log10 CFU/thigh between standard and iron-overloaded models are denoted with an asterisk.
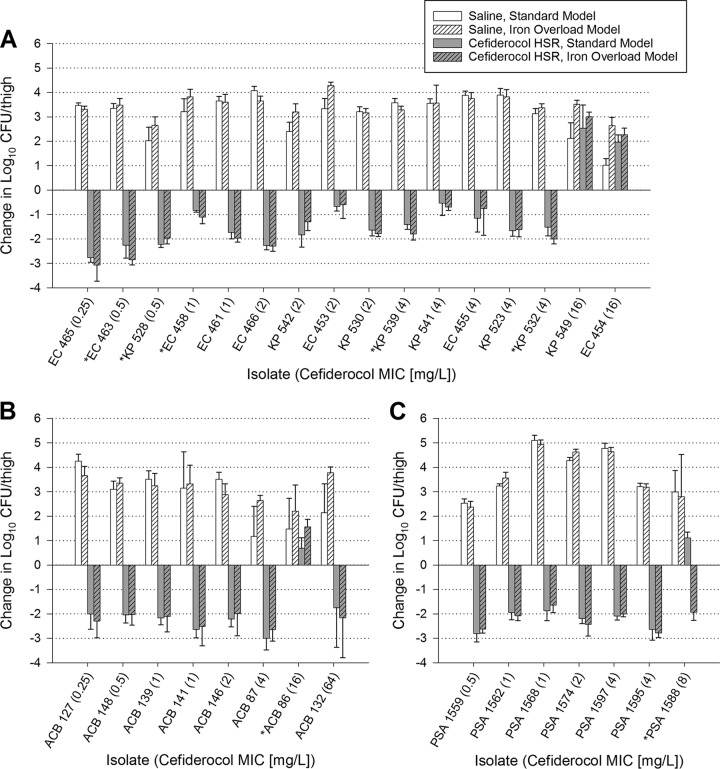
In the 31 strains studied with 24 h of the cefiderocol human-simulated regimen, no significant difference in overall efficacy was observed between the models (Table 1). While placebo-dosed control mice had significantly greater overall bacterial growth at 24 h in the iron-overloaded model, both models exceeded 3 log10 CFU/thigh overall growth, supporting the fitness of these strains in both models. For individual isolates (Fig. 2), significant differences in efficacy between models were observed in 5/16 Enterobacterales, 1/8 A. baumannii, and 1/7 P. aeruginosa strains. Three of these isolates had categorical differences in efficacy between models: EC 458, with stasis in the standard model and killing in the iron-overloaded model; ACB 86, with stasis in the standard model and growth in the iron-overloaded model; and PSA 1588, with growth in the standard model and killing in the iron-overloaded model. No trends favoring cefiderocol efficacy in either model were observed.
FIG 2.
Efficacy of 24 h of a cefiderocol human-simulated regimen (HSR) (2 g q8h, 3-h infusion) against Enterobacterales (A), A. baumannii (B), and P. aeruginosa (C) in standard and iron-overloaded murine thigh infection models. Isolates for which cefiderocol had a significantly different change in log10 CFU/thigh between standard and iron-overloaded models are denoted with an asterisk.
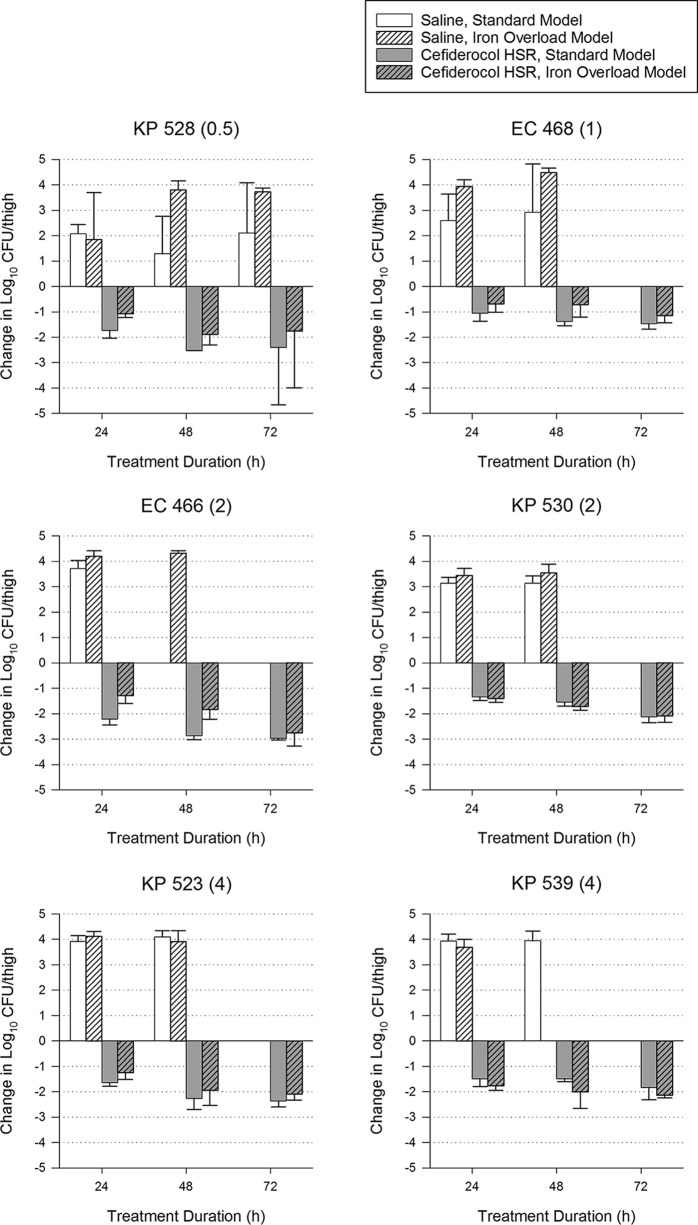
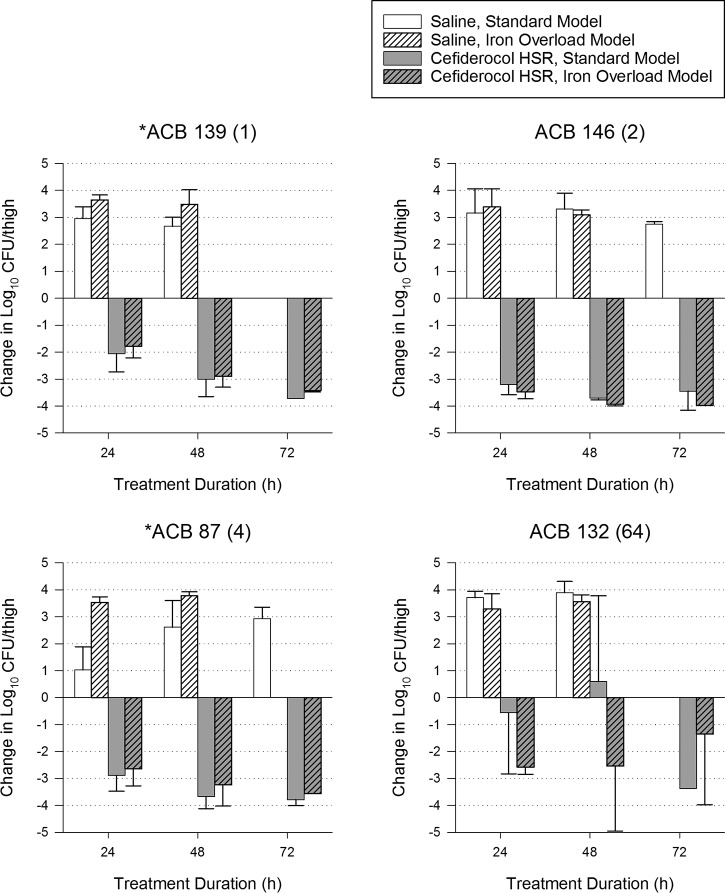
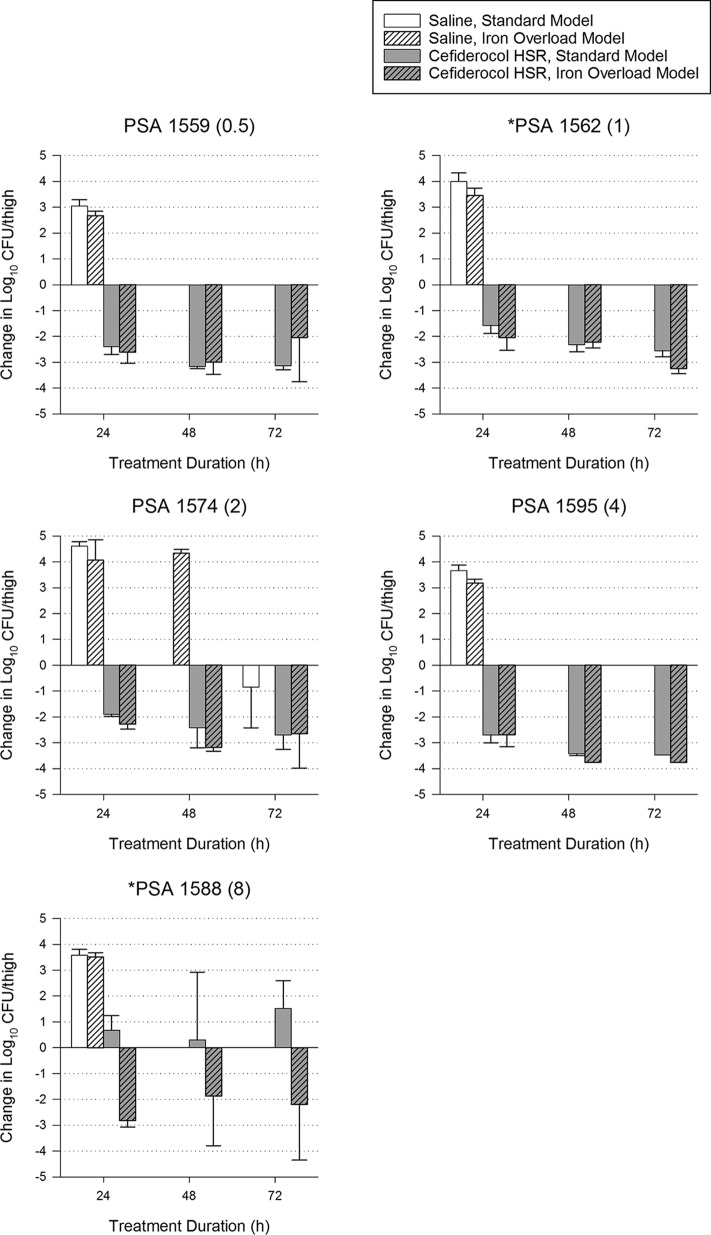
For 15 bacterial strains, additional groups of mice were treated for up to 72 h (with matching controls) to assess the development of adaptive resistance, which has been observed with other siderophore-antibiotic conjugates. Overall efficacy of the cefiderocol regimen was not significantly different among models at 24, 48, and 72 h despite higher growth of the strains in the iron overload model at 48 and 72 h (Table 1). When analyzed individually (Fig. 3–5), 4 isolates had statistically significant differences in efficacy at 72 h. For 2 of these isolates (ACB 139 and ACB 87), most thigh samples in both models were sterilized (<100 CFU/thigh, the lower limit of detection), indicating that maximum efficacy was achieved in both models and the differences are reflective of different baseline bacterial burdens. The only isolate with a categorical difference in efficacy between models was PSA 1588, which had growth in the standard model and killing in the iron-overloaded model, similar to observations made in the 24-h studies. No isolates in either model showed evidence of adaptive resistance, since efficacy at 72 h did not decline relative to 24-h efficacy.
FIG 3.
Efficacy of 72 h of a cefiderocol human-simulated regimen (HSR) (2 g q8h, 3-h infusion) against 6 Enterobacterales isolates in standard and iron-overloaded murine thigh infection models. Cefiderocol MIC (mg/liter) is shown in parentheses after the isolate name. Isolates for which cefiderocol had a significantly different change in log10 CFU/thigh at 72 h between standard and iron overload models are denoted with an asterisk.
FIG 4.
Efficacy of 72 h of a cefiderocol human-simulated regimen (HSR) (2 g q8h, 3-h infusion) against 4 A. baumannii isolates in standard and iron-overloaded murine thigh infection models. Cefiderocol MIC (mg/liter) is shown in parentheses after the isolate name. Isolates for which cefiderocol had a significantly different change in log10 CFU/thigh at 72 h between standard and iron overload models are denoted with an asterisk.
FIG 5.
Efficacy of 72 h of a cefiderocol human-simulated regimen (HSR) (2 g q8h, 3-h infusion) against 5 P. aeruginosa isolates in standard and iron-overloaded murine thigh infection models. Cefiderocol MIC (mg/liter) is shown in parentheses after the isolate name. Isolates for which cefiderocol had a significantly different change in log10 CFU/thigh at 72 h between standard and iron overload models are denoted with an asterisk.
DISCUSSION
This is, to our knowledge, the first study comparing the in vivo efficacy of a siderophore-antibiotic conjugate in a host with iron overload to a host with normal iron status. Iron overload could alter the host immune ability to restrict iron, potentially causing in vivo conditions similar to those of in vitro iron excess, which has been shown to increase the MIC of cefiderocol (2). In murine thigh and lung infection models, cefiderocol MIC as measured in iron-depleted broth was best predictive of cefiderocol efficacy, and this methodology is recommended by the Clinical and Laboratory Standards Institute (7, 8). Given the predictive value of iron-depleted MIC on in vivo efficacy, this preclinical investigation is necessary to determine if in vivo iron overload could compromise the drug’s efficacy before clinical studies of cefiderocol in patients with iron overload could be performed.
In order to make a comparison between the standard and iron-overloaded models, it was important to establish that the iron overload model did not impact the fitness of the bacteria relative to the standard model and that the bactericidal activity of a non-siderophore beta-lactam was unaffected by iron overload. Iron is a regulator of several virulence factors in Escherichia coli (9), Klebsiella pneumoniae (10), Acinetobacter baumannii (11), and Pseudomonas aeruginosa (12); therefore, the iron-overloading treatments could have altered the fitness of these organisms in vivo and produced variation in response to antibiotics. Bacterial fitness, as measured by growth in the absence of treatment, was equivalent in both models for all species studied. In addition, meropenem was equally efficacious in both models. Therefore, any differences in cefiderocol efficacy between the models could be attributed to the siderophore-conjugated antibiotic itself rather than differences between the models or general responsiveness to beta-lactams.
Cefiderocol efficacy was not diminished by clinically relevant host iron overload. Liver iron deposition in the iron overload model is within the range seen in patients with hereditary hemochromatosis, while the plasma iron concentrations exceed thresholds of acute iron toxicity (13, 14). Furthermore, the plasma iron content in this model exceeds the total iron binding capacity in mice (15), suggesting that bacteria could have been exposed to excess free iron, similar to conditions which increased cefiderocol MIC in vitro. Under these conditions representing extremes of iron overload, most strains had no significant difference in response to cefiderocol, and among those that did, only one exceeded 0.5 log10 CFU/thigh, which is generally regarded as the magnitude of difference considered clinically meaningful in the murine thigh infection model. In a further assessment of possible clinical significance, only 3 strains had a categorical difference in efficacy between models at 24 h, of which 2 had greater efficacy in the setting of iron overload. The duration of treatment did not impact these conclusions: with 72 h of human-simulated cefiderocol exposure, there was no evidence of diverging efficacy between the models.
Our work builds upon prior studies in the standard neutropenic murine thigh infection model using the same isolates. Monogue and colleagues studied the same human-simulated regimen of cefiderocol over 24 h in a larger set of Enterobacterales, P. aeruginosa, and A. baumannii isolates, finding consistent activity of approximately 1 to 2 log10 killing against isolates with cefiderocol MIC of ≤4 mg/liter (16). The present results from the 24-h standard thigh infection model reproduce these observations with remarkable agreement on the magnitude of killing for individual isolates between both studies. Additional investigation on a subset of these isolates using 72 h of human-simulated cefiderocol exposure was performed by Stainton and colleagues, finding that during an extended treatment period, efficacy was sustained, and only one isolate had a significant (two 2-fold dilutions) increase in postexposure cefiderocol MIC, which the authors interpreted as evidence against adaptive resistance (17). Three isolates (KP 539, ACB 87, and PSA 1595) from this study were included in the present experiments, where similar magnitudes of killing at 72 h were seen in both standard and iron-overloaded models.
Only one isolate studied showed a major difference in response to cefiderocol exposure between the models: PSA 1588 was killed by 2 log10 CFU/thigh in the iron-overloaded model but grew approximately 1 log10 CFU/thigh in the standard model. After observing this unexpected response in the 24-h experiments, the isolate was included in the 72-h experiments, where the findings were replicated and sustained over the 72-h treatment period. When previously studied by Monogue and colleagues, this isolate grew in the standard murine thigh infection model as observed presently. The reason for the enhanced efficacy of cefiderocol against this isolate in the iron-overloaded model is unknown and warrants further investigation, given the complex role of iron in the regulation of P. aeruginosa virulence (9, 18).
In summary, human-simulated cefiderocol exposure was similarly efficacious against a large collection of Gram-negative bacteria with a wide range of cefiderocol MICs regardless of the presence of host iron overload. No evidence was found to support the hypothesis that diminished in vitro activity of cefiderocol in the presence of excess iron could be translated to the iron-overloaded host. Cefiderocol efficacy in both models against particular isolates closely matched previous studies at both the 24-h and 72-h endpoints. This fact, along with the similar responses in both models to a non-siderophore beta-lactam, improves the validity of these findings. Clinical study of cefiderocol in patients with disorders of iron overload is supported.
MATERIALS AND METHODS
Antimicrobial test agents.
Cefiderocol (S-649266) 500-mg vials (lot 12M01) were provided by Shionogi (Osaka, Japan). Commercially available meropenem 500-mg vials (lot 4B18F05; Fresenius Kabi USA, Lake Zurich, IL) were obtained from Cardinal Health (Dublin, OH). Vials were reconstituted with 0.9% sodium chloride (normal saline; NS) per manufacturer instructions and then further diluted with NS to the final dosing concentrations.
Bacterial isolates.
Thirty-one clinical Gram-negative isolates, including 16 Enterobacterales, 7 P. aeruginosa, and 8 A. baumannii isolates, were studied. MICs to cefiderocol in iron-depleted cation-adjusted Mueller-Hinton broth (CAMHB) and meropenem in standard CAMHB for each isolate were previously determined in triplicate (17); cefiderocol MICs ranged from 0.25 to 16 mg/liter in Enterobacterales, 0.5 to 8 mg/liter in P. aeruginosa, and 0.25 to 64 mg/liter in A. baumannii. All isolates were previously frozen in skim milk at –80°C. Bacterial suspensions were prepared from the second of two transfers on Trypticase soy agar plates with 5% sheep blood (TSA II; Becton, Dickinson, & Co., Sparks, MD, USA), which were incubated for 18 to 24 h at 37°C.
Animals.
Animal usage was approved by the Hartford Hospital Institutional Animal Care and Use Committee (assurance number A3185-01). Specific-pathogen-free outbred female mice (CD-1; Charles River Laboratories, Wilmington, MA) were allowed to acclimate for 48 h after arrival at the facility before study procedures began. Groups of 3 mice were housed together in HEPA-filtered cages (Innovive, San Diego, CA) with paper nesting material for enrichment; food and water were available ad libitum. Controlled room temperature and a 12-h light/dark cycle were employed. During study procedures, mice were monitored at least 3 times daily for signs of morbidity and were euthanized via CO2 asphyxiation followed by cervical dislocation if moribund.
Neutropenic thigh infection models.
The standard murine thigh infection model was utilized as previously described (16, 17). In brief, neutropenia was produced with 150 mg/kg cyclophosphamide at day 4 and 100 mg/kg at day 1 prior to inoculation, and 5 mg/kg uranyl nitrate was used at day 3 prior to inoculation to decrease renal elimination of the study compounds. In addition, an iron-overloaded murine thigh infection model was used in which mice received the treatments described above plus 14 days of iron dextran (100 mg/kg/day intraperitoneally). This model has been shown to produce supranormal iron concentrations in plasma, liver, and spleen (6). In both models, mice were intramuscularly inoculated in both thighs with 0.05 ml of bacterial suspensions of approximately 107 CFU/ml.
Bacterial density studies.
All experiments were performed in the standard murine thigh infection model and the iron-overloaded murine thigh infection model simultaneously. After inoculation, mice were randomized into baseline (0 h), growth control, or treatment groups with 3 mice (6 thighs) each. Two hours after inoculation (0 h), mice in baseline groups were harvested, while mice in treatment or growth control groups began receiving subcutaneous doses of antimicrobial test agents or NS placebo injections, respectively.
A cefiderocol dosing regimen in mice that simulates the human free cefiderocol plasma profile after clinical doses of 2 g q8h (3-h infusion) was studied (19). The human-simulated regimen has been shown to produce similar exposures in standard and iron-overloaded murine thigh infection models (6) and consisted of cefiderocol doses of 15 mg/kg, 20 mg/kg, 25 mg/kg, 10 mg/kg, and 5 mg/kg given at 0, 1, 2, 4, and 6 h, respectively. In addition, a meropenem regimen shown to simulate free plasma exposure in both standard and iron-overloaded models after clinical doses of 2 g q8h (3-h infusion) was utilized, consisting of doses of 65 mg/kg, 65 mg/kg, 45 mg/kg, and 45 mg/kg given at 0, 1.25, 3.5, and 6 h, respectively (6, 16). Both human-simulated regimens were given every 8 h over the treatment period.
At the scheduled time of harvest, mice were euthanized via CO2 asphyxiation followed by cervical dislocation, after which both thighs were aseptically harvested, homogenized in NS, and plated to determine bacterial CFU/thigh. Mice which succumbed to infection prior to the scheduled time of harvest were included in the next group due for harvest.
Data analysis.
Efficacy was defined as the change in log10 CFU/thigh at the end of the treatment period compared with 0-h controls in the same model and from the same experimental run. The overall efficacy of a treatment was defined as the mean efficacy for all isolates receiving that treatment and was calculated using data from individual thighs. Comparisons of overall efficacy and efficacy by isolate were made between the standard and iron-overloaded models using Student's t test, with two-tailed P values of ≤0.05 deemed significant. For individual isolates with a statistically significant difference in efficacy between the models, comparisons were also made based on categorical efficacy, with killing defined as a decrease of ≥1 log10 CFU/thigh, growth defined as an increase of the same magnitude, and stasis defined as a change of <1 log10 CFU/thigh in either direction.
ACKNOWLEDGMENT
This study was funded by a grant from Shionogi & Co. Ltd., Osaka, Japan.
Footnotes
For a companion article on this topic, see https://doi.org/10.1128/AAC.01961-19.
REFERENCES
- 1.Chu BC, Garcia-Herrero A, Johanson TH, Krewulak KD, Lau CK, Peacock RS, Slavinskaya Z, Vogel HJ. 2010. Siderophore uptake in bacteria and the battle for iron with the host; a bird’s eye view. Biometals 23:601–611. doi: 10.1007/s10534-010-9361-x. [DOI] [PubMed] [Google Scholar]
- 2.Ito A, Nishikawa T, Matsumoto S, Yoshizawa H, Sato T, Nakamura R, Tsuji M, Yamano Y. 2016. Siderophore cephalosporin cefiderocol utilizes ferric iron transporter systems for antibacterial activity against Pseudomonas aeruginosa. Antimicrob Agents Chemother 60:7396–7401. doi: 10.1128/AAC.01405-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cassat JE, Skaar EP. 2013. Iron in infection and immunity. Cell Host Microbe 13:509–519. doi: 10.1016/j.chom.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleming RE, Ponka P. 2012. Iron overload in human disease. N Engl J Med 366:348–359. doi: 10.1056/NEJMra1004967. [DOI] [PubMed] [Google Scholar]
- 5.Ganz T. 2009. Iron in innate immunity: starve the invaders. Curr Opin Immunol 29:63–67. doi: 10.1016/j.coi.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kidd JM, Abdelraouf K, Nicolau DP. 2020. Development of neutropenic murine models of iron overload and depletion to study the efficacy of siderophore-antibiotic conjugates. Antimicrob Agents Chemother 64:e01961-19. doi: 10.1128/AAC.01961-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakamura R, Ito-Horiyama T, Takemura M, Toba S, Matsumoto S, Ikehara T, Tsuji M, Sato T, Yamano Y. 2019. In vivo pharmacodynamic study of cefiderocol, a novel parenteral siderophore cephalosporin, in murine thigh and lung infection models. Antimicrob Agents Chemother 63:e02031-18. doi: 10.1128/AAC.02031-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. 2019. Performance standards for antimicrobial susceptibility testing, 29th ed, CLSI supplement M100 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 9.Carpenter BM, Whitmire JM, Merrell DS. 2009. This is not your mother’s repressor: the complex role of Fur in pathogenesis. Infect Immun 77:2590–2601. doi: 10.1128/IAI.00116-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsieh P, Lin T, Lee C, Tsai S, Wang J. 2008. Serum-induced iron-acquisition systems and TonB contribute to virulence in Klebsiella pneumoniae causing primary pyogenic liver abscess. J Infect Dis 197:1717–1727. doi: 10.1086/588383. [DOI] [PubMed] [Google Scholar]
- 11.Runci F, Gentile V, Frangipani E, Rampioni G, Leoni L, Lucidi M, Visaggio D, Harris G, Chen W, Stahl J, Averhoff B, Visca P. 2019. Contribution of active iron uptake to Acinetobacter baumannii pathogenicity. Infect Immun 87:e00755-18. doi: 10.1128/IAI.00755-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minandri F, Imperi F, Frangipani E, Bonchi C, Visaggio D, Facchini M, Pasquali P, Bragonzi A, Visca P. 2016. Role of iron uptake systems in Pseudomonas aeruginosa virulence. Infect Immun 84:2324–2335. doi: 10.1128/IAI.00098-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brittenham GM, Farrell DE, Harris JW, Feldman ES, Danish EH, Muir WA, Tripp JH, Bellon EM. 1982. Magnetic-susceptibility measurement of human iron stores. N Engl J Med 307:1671–1675. doi: 10.1056/NEJM198212303072703. [DOI] [PubMed] [Google Scholar]
- 14.Chyka PA, Butler AY. 1993. Assessment of acute iron poisoning by laboratory and clinical observations. Am J Emerg Med 11:99–103. doi: 10.1016/0735-6757(93)90099-w. [DOI] [PubMed] [Google Scholar]
- 15.Ward PP, Mendoza-Meneses M, Cunningham GA, Conneely OM. 2003. Iron status in mice carrying a targeted disruption of lactoferrin. Mol Cell Biol 23:178–185. doi: 10.1128/mcb.23.1.178-185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monogue ML, Tsuji M, Yamano Y, Echols R, Nicolau DP. 2017. Efficacy of humanized exposures of cefiderocol (S-649266) against a diverse population of Gram-negative bacteria in the murine thigh infection model. Antimicrob Agents Chemother 61:e01022-17. doi: 10.1128/AAC.01022-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stainton SM, Monogue ML, Tsuji M, Yamano Y, Echols R, Nicolau DP. 2018. Efficacy of humanized cefiderocol exposures over 72 hours against a diverse group of Gram-negative isolates in the neutropenic murine thigh infection model. Antimicrob Agents Chemother 63:e01040-18. doi: 10.1128/AAC.01040-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nadal Jimenez P, Koch G, Thompson JA, Xavier KB, Cool RH, Quax WJ. 2012. The multiple signaling systems regulating virulence in Pseudomonas aeruginosa. Microbiol Mol Biol Rev 76:46–65. doi: 10.1128/MMBR.05007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghazi IM, Monogue ML, Tsuji M, Nicolau DP. 2018. Humanized exposures of cefiderocol, a siderophore cephalosporin, display sustained in vivo activity against siderophore-resistant Pseudomonas aeruginosa. Pharmacology 101:278–275. doi: 10.1159/000487441. [DOI] [PMC free article] [PubMed] [Google Scholar]