A novel, plasmid-mediated, high-level tigecycline resistance tet(X) gene variant, tet(X5), was detected in a clinical Acinetobacter baumannii isolate from China in 2017. Tet(X5) shows 84.5% and 90.5% amino acid identity to Tet(X3) and Tet(X4), respectively, with similar binding sites and a comparable affinity for tetracyclines. The tet(X5)-containing plasmid could only be transferred to A. baumannii via electrotransformation.
KEYWORDS: tigecycline, tet(X5), Acinetobacter baumannii
ABSTRACT
A novel, plasmid-mediated, high-level tigecycline resistance tet(X) gene variant, tet(X5), was detected in a clinical Acinetobacter baumannii isolate from China in 2017. Tet(X5) shows 84.5% and 90.5% amino acid identity to Tet(X3) and Tet(X4), respectively, with similar binding sites and a comparable affinity for tetracyclines. The tet(X5)-containing plasmid could only be transferred to A. baumannii via electrotransformation. This report follows the recent study describing the identification of tet(X3) and tet(X4).
INTRODUCTION
Tigecycline, the original member of the glycylcyclines class of antibiotics, is commonly used to treat infections due to extensively drug-resistant Gram-negative bacteria, against which it shows satisfactory antimicrobial efficacy (1). In particular, treatment regimens for infections caused by carbapenem-resistant Acinetobacter species are invariably reliant on colistin and tigecycline, either individually or in combination with carbapenems (2). Nevertheless, two novel, plasmid-mediated, high-level tigecycline resistance genes, tet(X3) and tet(X4), classed as tet(X) tetracycline-inactivating variants, have recently been detected in Acinetobacter and Enterobacteriaceae isolates from animals, animal-derived foods, and humans in China (3). The emergence and dissemination of these mobile genes will render tigecycline ineffective, further limiting treatment options. Hence, these genes are a significant global threat to public health. In the current study, we screened clinical tigecycline-resistant Acinetobacter baumannii isolates from China for the presence of tet(X) variants and identified a novel, plasmid-mediated variant, designated tet(X5).
A total of 74 clinically isolated tigecycline-resistant (MIC > 2 mg/liter; tigecycline breakpoint for A. baumannii has been set by the European Committee on Antimicrobial Susceptibility Testing [EUCAST] at >2 mg/liter) (4) A. baumannii isolates were identified from among 671 nonduplicate clinical A. baumannii isolates collected from patients in 19 hospitals between June 2017 and June 2018 as part of the China Antimicrobial Resistance Surveillance Trial (CARST). Only one isolate, AB17H194, recovered from a wound sample in Hebei Province in 2017, was PCR-positive for tet(X) (0.1%, 1/671) using the universal primers tet(X)-F (5ʹ-CCGTTGGACTGACTATGGC-3ʹ) and tet(X)-R (5ʹ-TCAACTTGCGTGTCGGTAA-3ʹ). AB17H194 exhibited resistance to all tested tetracyclines (Table 1), particularly tigecycline, eravacycline, and omadacycline (MICs were determined using the broth microdilution method according to the 2018 recommendation of CLSI) (5). To confirm the sequence of tet(X) from AB17H194, whole-genome sequencing was performed using the Illumina HiSeq 2500 system, with contigs de novo assembled and annotated using SPAdes (v3.9.0) and RAST. Interestingly, we obtained a 1,167-bp gene sequence encoding a 338-amino acid (aa) protein that exhibited 90.0%, 84.5%, and 90.5% identity to Tet(X2), Tet(X3), and Tet(X4), respectively (see Fig. S2 in the supplemental material). Therefore, this novel tet(X) variant was designated tet(X5). Genomic mining revealed that no identical protein sequence was found in the NCBI GenBank database, yet similar proteins were found from Flavobacterium marinum from an unknown source (GenBank accession number WP_091095665.1) with 96.7% aa identity to Tet(X5), followed by two Riemerella anatipestifer isolates from China (GenBank accession number WP_064970078.1, 95.4% aa identity) and Germany (GenBank accession number WP_107046667.1, 95.1% aa identity) (see Fig. S1 in the supplemental material). To verify the role of the novel variant, a single intact copy of tet(X5) together with the upstream sequence was amplified and ligated into the shuttle vector, pAM401, using primers Ptet(X5)-F, 5′-TTATTAATCAGATAAAATATTTCTAGACACCAAGGGAATGG-AACAAT-3′, and Ptet(X5)-R, 5′-ACGATGCGTCCGGCTAGAGGATCCTGCTCCCGCAATC-AAGAG-3’, which contained the predicted promoters (6). The recombinant vector was then transferred to Escherichia coli DH5α by electroporation as previously described (3). A 4- to 32-fold increase in the MICs of all tested tetracyclines was observed for the E. coli transformant, designated DH5α/pAM401-tet(X5) (Table 1), indicating that Tet(X5) mediated slightly lower levels of resistance than Tet(X3) and Tet(X4) (3). The double agar dilution method (except for polymyxins, for which the broth microdilution method was used) revealed that A. baumannii isolate AB17H194 also exhibited resistance to β-lactams, aminoglycosides, fluoroquinolones, macrolides, and tetracyclines and was only susceptible to polymyxins and carbapenems (MICs were determined using the broth microdilution method according to the 2018 recommendation of CLSI) (5) (see Table S1 in the supplemental material).
TABLE 1.
Antimicrobial susceptibility profiles of bacterial hosts carrying tet(X5) or tet(X5)-containing plasmids
| Strain | Description | MIC (mg/liter) of tetracycline antimicrobialsa
|
|||||
|---|---|---|---|---|---|---|---|
| TET | DOX | MIN | TIG | ERA | OMA | ||
| A. baumannii AB17H194 | Donor of tet(X5)-carrying plasmid pAB17H194 | 256 | 32 | 8 | 32 | 32 | 32 |
| E. coli DH5α/pAM401 | Recipient for tet(X5) gene | 1 | 2 | 2 | 0.5 | 0.5 | 1 |
| E. coli DH5α/pAM401-tet(X5) | Transformants | 64 | 16 | 8 | 16 | 8 | 64 |
| A. baumannii 5AB | Recipient for pAB17H194, Human | 16 | 1 | 2 | 2 | 4 | 4 |
| A. baumannii 5AB/pAB17H194 | Transformants | 64 | 8 | 8 | 16 | 32 | 32 |
TET, tetracycline; DOX, doxycycline; MIN, minocycline; TIG, tigecycline; ERA, eravacycline; OMA, omadacycline.
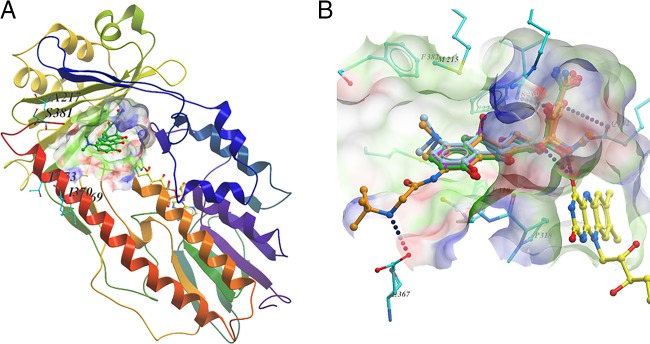
To understand the hydroxylation of tetracyclines by Tet(X5), the structure of Tet(X) complexed with minocycline (PDB accession number 4A99) was used as the template for homology modeling (7). Superpositioning of this model onto the Tet(X2) X-ray structure (PDB accession number 4A99) showed that the tetracycline-binding site of Tet(X5) was essentially identical to that of Tet(X2), with similar results observed also for Tet(X3) and Tet(X4) (3). Similarities in docking models are a likely result of the high sequence identity among this group of proteins (Fig. 1A). Subsequently, four tetracyclines were docked into the substrate-binding site using LeDock (Zurich, Switzerland). The tetracycline core showed similar binding modes, preoriented for regioselective hydroxylation to 11a-hydroxytetracyclines (Fig. 1B; see also Fig. S3 in the supplemental material). Briefly, the two hydroxyl groups at C-12 and C-12a of the tetracycline core act like tweezers, pinching the isoalloxazine of flavin adenine dinucleotide (FAD) by forming two hydrogen bonds. The C-3 enolate is hydrogen bonded to the side chain of Gln192, while the carbonyl oxygen at C-1 interacts with the side chain of Arg213 (Fig. 1B; see also Fig. S3). Notably, tigecycline had the highest binding affinity (Fig. 1B; see also Fig. S3 and Table S2 in the supplemental material), resulting in salt bridge formation between its amide moiety and Glu367, as described previously by Volkers et al. (7, 8).
FIG 1.
Homology modeling and molecular docking of Tet(X5). (A) Cartoon representation of the modelled Tet(X5) structure in complex with ligand (carbon in green) and FAD (carbon in yellow). Nonconserved residues within 10 Å of the ligand are shown as sticks. A surface representation of the substrate-binding site is illustrated. (B) Predicted binding conformation of four tetracyclines at the substrate-binding site of the modelled Tet(X5) structure. The binding conformations suggest that the tetracycline core has essentially the same interactions with Tet(X5). Hydrogen bonds are depicted by blue dotted lines.
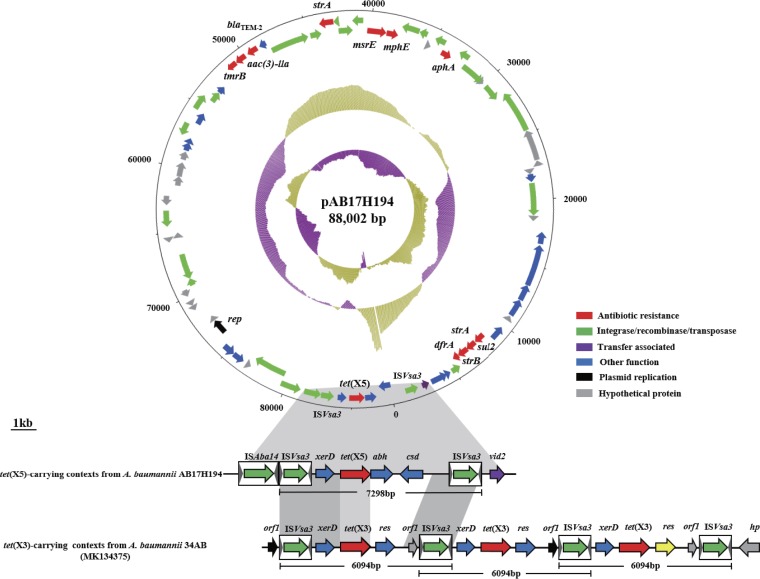
To characterize the genetic environment of tet(X5), single-molecule real-time (SMRT) sequencing was performed to generate the complete sequence of AB17H194, as described previously (3). Results revealed that AB17H194 harbored two plasmids. The 88,002-bp plasmid, designated pAB17H194, carried tet(X5) (Fig. 2). pAB17H194 had an average GC content of 45.0% and contained 89 predicted open reading frames (ORFs), including 12 additional resistance genes (Fig. 2). Notably, tet(X5) was located within a 7,299-bp region with the gene arrangement ISVsa3-xerD-tet(X5)-abh-csd-ISVsa3 (the ISVsa3 corresponds to ISCR2). Similar genetic environments have been observed for tet(X3) and tet(X4), with two intact copies of ISVsa3 in the same orientation flanking the tet(X5) central region (Fig. 2) (3). Therefore, a potentially mobile 6,324-bp circular intermediate consisting of the tet(X5)-carrying central region and one ISVsa3 element was created by inverse PCR (Fig. 2). Conjugation experiments failed to transfer pAB17H194 into either A. baumannii or E. coli as previously described (3), likely because of the lack of conjugative elements in the plasmid. Only one A. baumannii strain was successfully transformed via electrotransformation, indicating that the plasmid can only replicate in Acinetobacter species. The pAB17H194-positive transformant showed resistance to all tested tetracycline antibiotics (Table 1).
FIG 2.
Structures of tet(X5)-carrying plasmids and comparison of the genetic environments of tet(X5)-carrying regions. The arrows indicate the direction of transcription of the genes. Genes are differentiated by colors. Regions of >99% homology are marked by dark gray shading. Regions of >80% homology are marked by light gray shading. Black arrowheads indicate the positions of primers used for amplification of the ISVsa3-xerD-tet(X5)-abh-csd minicircles.
In recent years, the clinical use of tigecycline has resulted in the global emergence of resistance. Although three tet(X) variants, tet(X), tet(X1), and tet(X2), have been reported in Bacteroides, Enterobacteriaceae, and Acinetobacter, no experimental verification showed that these tet(X) genes could be horizontally transferred and mediate high-level resistance to tigecycline (9–12) until a report describing two novel plasmid-borne high-level tigecycline resistance genes, tet(X3) and tet(X4), in A. baumannii and E. coli (3, 13). The acquisition of tet(X3) or tet(X4) by pathogenic species in a clinical setting may render tigecycline ineffective, severely limiting treatment options. Thus, we screened clinical A. baumannii isolates collected as part of the CARST project for the presence of other tet(X) variants, subsequently identifying the novel resistance gene, tet(X5). The high similarity (95.1 to 96.7%) of Tet(X) variants observed in Flavobacterium marinum, Riemerella anatipestifer, Bacteroides vulgatus, and uncultured bacteria from various animals and public reservoirs suggests that the tet(X5) gene is disseminated among clinical and nonclinical pathogens.
Tigecycline is only used in clinical settings and has never been licensed for animal husbandry. Paradoxically, these data and previous studies reveal that tet(X) variants in human pathogens appear to be rare and that detection rates are significantly lower than those from isolates originating from animals or retail meat products (3). This may be because other tetracycline antibiotics, including tetracycline, oxytetracycline, chlortetracycline, and doxycycline, are widely available and heavily utilized in animal production. This selective pressure may enrich tet(X) variants in bacteria from animal and animal-derived foods. Tet(X5) showed nearly 90.0% amino acid identity to Tet(X2) and, thus, like Tet(X3) and Tet(X4), contains a similar binding site and affinity for tetracyclines (3). We, therefore, hypothesize that amino acid substitutions in the Tet(X) variants are restricted to defined limits in order to maintain efficacy against tetracyclines.
Although our results showed that the tet(X5)-harboring plasmid could not be horizontally transferred via conjugation, the resistance gene is flanked by insertion sequence ISVsa3 in an arrangement similar to that observed in tet(X3)- or tet(X4)-harboring plasmids (3). Additionally, circular intermediates containing ISVsa3 and tet(X5) were detected by inverse PCR assays. A previous study showed that the circular intermediate of tet(X4) could be transferred via transposition in E. coli (3), suggesting that ISVsa3 might play an important role in the dissemination of all detected tet(X) variants.
In summary, we report the discovery of a unique plasmid-mediated tigecycline resistance gene, tet(X5), in A. baumannii isolated from a clinical secretion sample. Tet(X5) has a similar function and structure to other Tet(X) variants and is likely to utilize similar transfer elements for dissemination. Therefore, more detailed studies should be conducted to further compare all known tet(X) variants and tet(X) variant-positive strains.
Accession number(s).
The complete nucleotide sequence of AB17H194 has been deposited in GenBank under accession numbers CP040911, CP040912, and CP040913.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported in part by grants from the National Key Research and Development Program of China (2018YFD0500300), the National Natural Science Foundation of China (grants 81572033, 31930110 and 81661138002), and Medical Research Council grants DETER-XDRE-CHINA (MR/P007295/1) and DETER-XDRE-CHINA-HUB (MRC MR/S013768/1).
We thank our colleagues from the Institute of Clinical Pharmacology of Peking University First Hospital; Beijing Advanced Innovation Center for Food Nutrition and Human Health, College of Veterinary Medicine of China Agricultural University; and the Department of Medical Microbiology and Infectious Disease, Institute of Infection and Immunity.
We declare that we have no conflicts of interest.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Tasina E, Haidich AB, Kokkali S, Arvanitidou M. 2011. Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. Lancet Infect Dis 11:834–844. doi: 10.1016/S1473-3099(11)70177-3. [DOI] [PubMed] [Google Scholar]
- 2.Hsu LY, Apisarnthanarak A, Khan E, Suwantarat N, Ghafur A, Tambyah PA. 2017. Carbapenem-resistant Acinetobacter baumannii and Enterobacteriaceae in South and Southeast Asia. Clin Microbiol Rev 30:1–22. doi: 10.1128/CMR.00042-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tao H, Ran W, Dejun L, Walsh TR, Zhang R, Lv Y, Ke Y, Ji Q, Wei R, Liu Z, Shen Y, Wang G, Sun L, Lei L, Lv Z, Li Y, Pang M, Wang L, Sun Q, Fu Y, Song H, Hao Y, Shen Z, Wang S, Chen G, Wu C, Shen J, Wang Y. 2019. Emergence of plasmid-mediated high-level tigecycline resistance genes in animals and humans. Nat Microbiol 4:1450–1456. doi: 10.1038/s41564-019-0445-2. [DOI] [PubMed] [Google Scholar]
- 4.European Committee on Antimicrobial Susceptibility Testing. 2019. Breakpoint tables for interpretation of MICs and zone diameters. Version 9.0, 2019. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_9.0_Breakpoint_Tables.pdf.
- 5.Clinical and Laboratory Standards Institute. 2019. Performance standards for antimicrobial susceptibility testing. CLSI M100-S29 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 6.Wirth R, An FY, Clewell DB. 1986. Highly efficient protoplast transformation system for Streptococcus faecalis and a new Escherichia coli-S. faecalis shuttle vector. J Bacteriol 165:831–836. doi: 10.1128/jb.165.3.831-836.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volkers G, Damas JM, Palm GJ, Panjikar S, Soares CM, Hinrichs W. 2013. Putative dioxygen-binding sites and recognition of tigecycline and minocycline in the tetracycline-degrading monooxygenase TetX. Acta Crystallogr D Biol Crystallogr 69:1758–1767. doi: 10.1107/S0907444913013802. [DOI] [PubMed] [Google Scholar]
- 8.Volkers G, Palm GJ, Weiss MS, Wright GD, Hinrichs W. 2011. Structural basis for a new tetracycline resistance mechanism relying on the TetX monooxygenase. FEBS Lett 585:1061–1066. doi: 10.1016/j.febslet.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Bartha NA, Sóki J, Urbán E, Nagy E. 2011. Investigation of the prevalence of tetQ, tetX and tetX1 genes in Bacteroides strains with elevated tigecycline minimum inhibitory concentrations. Int J Antimicrob Agents 38:522–525. doi: 10.1016/j.ijantimicag.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Deng M, Zhu MH, Li JJ, Bi S, Sheng ZK, Hu FS, Zhang JJ, Chen W, Xue XW, Sheng JF, Li LJ. 2014. Molecular epidemiology and mechanisms of tigecycline resistance in clinical isolates of Acinetobacter baumannii from a Chinese university hospital. Antimicrob Agents Chemother 58:297–303. doi: 10.1128/AAC.01727-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leski TA, Bangura U, Jimmy DH, Ansumana R, Lizewski SE, Stenger DA, Taitt CR, Vora GJ. 2013. Multidrug-resistant tet(X)-containing hospital isolates in Sierra Leone. Int J Antimicrob Agents 42:83–86. doi: 10.1016/j.ijantimicag.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh S, Sadowsky MJ, Roberts MC, Gralnick JA, LaPara TM. 2009. Sphingobacterium sp. strain PM2-P1-29 harbours a functional tet(X) gene encoding for the degradation of tetracycline. J Appl Microbiol 106:1336–1342. doi: 10.1111/j.1365-2672.2008.04101.x. [DOI] [PubMed] [Google Scholar]
- 13.Bai L, Du P, Du Y, Sun H, Zhang P, Wan Y, Lin Q, Fanning S, Cui S, Wu Y. 2019. Detection of plasmid-mediated tigecycline-resistant gene tet(X4) in Escherichia coli from pork, Sichuan and Shandong provinces, China, February 2019. Euro Surveil 24(25):pii=1900340 10.2807/1560-7917.ES.2019.24.25.1900340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.