We report a case of a 58-year-old renal transplant patient who developed a recurrent urinary tract infection with an extended-spectrum β-lactamase (ESBL)-positive Klebsiella pneumoniae strain in the first month posttransplant. Even though it tested susceptible to carbapenems and despite repeated meropenem treatment, his infection recurred. The infection eventually evolved into epididymitis that was successfully treated with meropenem and bacteriophages.
KEYWORDS: bacteriophages, urinary tract infection, Klebsiella pneumoniae, extended spectrum β-lactamase, renal transplant
ABSTRACT
We report a case of a 58-year-old renal transplant patient who developed a recurrent urinary tract infection with an extended-spectrum β-lactamase (ESBL)-positive Klebsiella pneumoniae strain in the first month posttransplant. Even though it tested susceptible to carbapenems and despite repeated meropenem treatment, his infection recurred. The infection eventually evolved into epididymitis that was successfully treated with meropenem and bacteriophages. This case demonstrates the difficulty of treating relapsing ESBL-positive Gram-negative infections in renal transplant patients.
INTRODUCTION
Urinary tract infections (UTIs) with extended-spectrum β-lactamase (ESBL)-positive Enterobacterales (ESBL-E) are a common infectious complication of renal transplant recipients, with 10% of patients suffering from UTIs with ESBL-E within the first year posttransplant. Moreover, recurrence rates of UTI caused by ESBL-E are almost three times higher than those by cephalosporin-susceptible Enterobacterales (1), demonstrating the decreased efficacy of antibiotics in the treatment of these UTIs.
Due to increasing antibiotic resistance worldwide, in particular multidrug-resistance in Gram-negative bacteria, a renewed interest in treatment with bacteriophages has awakened in the medical field (2).
The very existence of bacteriophages was first discovered in 1915. However, even after promising studies, further elucidation of their capacities and potential use as antibacterial agents was abandoned in the United States and in most of Europe when penicillin was discovered and implemented as a antibacterial agent in the 1940s. In Georgia, Poland, and Russia, bacteriophages and phage therapy continued to be studied after 1940, but despite longstanding experience, there is scant evidence for the therapeutic application of bacteriophages in chronic relapsing UTIs. In this era of increasing prevalence of infections with multidrug-resistant bacterial strains, both physicians and patients are turning to phage therapy to try to improve treatment outcomes (3–5).
CASE PRESENTATION
The patient is a 58-year-old Dutch male who was referred to our hospital in 2015 for biphasic kidney removal for polycystic kidney disease. Renal transplant followed in late 2017. One week posttransplant, our patient developed a UTI with leukocytosis due to ESBL-producing Klebsiella pneumoniae that tested meropenem and amikacin susceptible but resistant to other β-lactams, fluoroquinolones, and co-trimoxazole (Table 1). His urinary catheter and double-J catheter were removed before the patient went home with oral fosfomycin trometamol prophylaxis (8 g every third day; fosfomycin MIC, 32 μg/ml). The patient returned with urosepsis and was treated for 2 weeks with meropenem, 1,000 mg intravenously thrice daily.
TABLE 1.
Susceptibility profile of Klebsiella pneumoniae from our patient as tested by Phoenix M50 system (BD Diagnostic Systems, Sparks, MD, USA) using EUCAST breakpointsa
| Antibiotic | MIC (μg/ml) | S/I/R according to EUCASTb |
|---|---|---|
| Amoxicillin-clavulanic acid | >32 | R |
| Piperacillin-tazobactam | 32 | R |
| Meropenem | ≤0.125 | S |
| Ceftriaxone | >4 | R |
| Ceftazidime | >16 | R |
| Gentamicin | >4 | R |
| Tobramycin | >4 | R |
| Amikacin | ≤4 | S |
| Trimethoprim-sulfamethoxazole | >4 | R |
| Ciprofloxacin | >1 | R |
| Levofloxacin | 1 | I |
| Tigecycline | 2 | S |
| Tetracycline | 256 | R |
| Fosfomycin | 32 | No EUCAST breakpoint |
ESBL was phenotypically confirmed with the double-disc method of cefotaxime and ceftazidime with and without clavulanic acid with zone differences of 15 and 14 mm, respectively.
S, susceptible; I, intermediately susceptible; R, resistant; EUCAST, European Committee on Antimicrobial Susceptibility Testing.
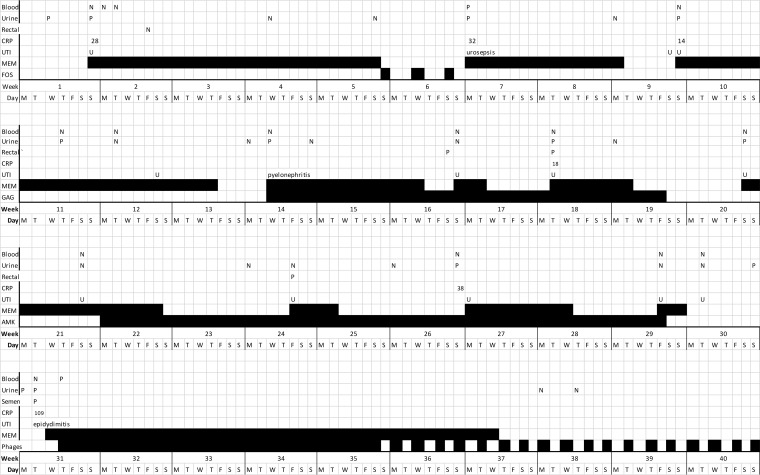
More episodes of UTI followed, and intravesical glycosaminoglycan (6) and intravesical amikacin (250 mg) adjunctive therapies were given by intermittent catheterization besides intravenous meropenem but failed (Fig. 1). Prostatitis was dismissed as a diagnosis due to a lack of symptoms, negative physical examinations, and two low prostate-specific antigen (PSA) values over 3 months (0.20 and 0.21 μg/liter). Also, fluorodeoxyglucose positron emission tomography (FDG-PET) imaging showed no signs of infection in the prostate, kidneys, or hepatic cysts. Interestingly, a bladder ultrasound showed inflammatory thickening of the wall near the urethra, corresponding with recurring urethral symptoms. These symptoms did not respond to meropenem treatment.
FIG 1.
Duration of treatment with meropenem (MEM; first course given 2 weeks posttransplant), prophylaxis with fosfomycin (FOS), chondroitin sulfate (GAG), and phages (bacteriophages) and blood cultures (blood), urine cultures (urine), rectal swabs, and symptoms of UTI present. Intermittent phage treatment continued for 8 weeks and is not completely shown.
Meanwhile, our patient had turned to the Eliava Institute in Tbilisi, Georgia, for bacteriophage therapy. After sending his urine to Tbilisi, he received vials with anti-Klebsiella pneumoniae bacteriophages. The instructions from the Eliava Institute for use were twice daily oral intake of one vial content and twice daily bladder irrigation with a vial for 2 weeks, followed by 2 weeks of one vial of bacteriophages taken orally as well as bladder irrigation once daily for 2 weeks, and finally one vial orally and one intravesically every second day during 8 weeks. All bacteriophage bladder installations were conducted by the patient through intermittent catheterization, allowing maximum dwell time before the next urination. No adverse events occurred from oral or intravesical administration.
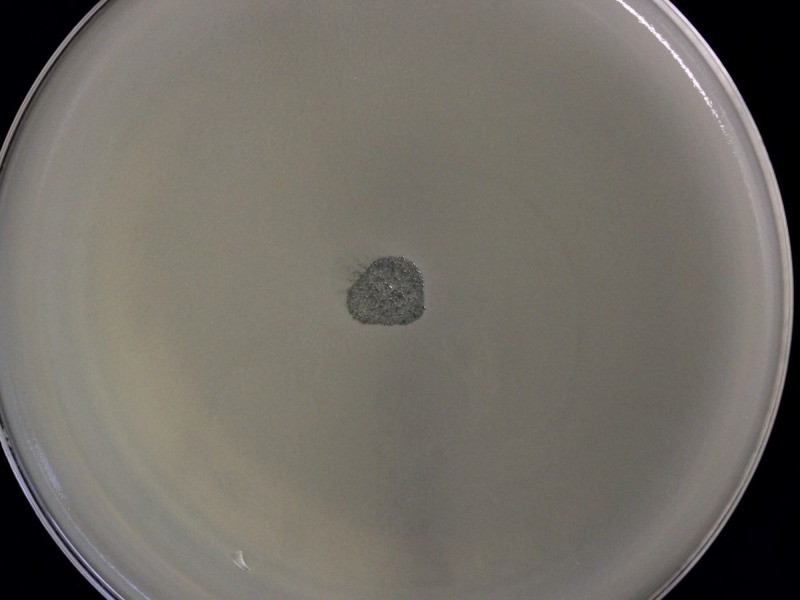
In our laboratory, we tested if the bacteriophage suspension our patient had received from Tbilisi was active against his causative ESBL-producing Klebsiella pneumoniae strain. To this end, we performed the bacteriophage spot test using the plaque assay for identification and enumeration of bacteriophages by the double-layer agar method (6). The spot test, which was performed in duplicates, showed lytic activity of the bacteriophage solution (Fig. 2). As a control, three other Klebsiella pneumoniae strains were tested. There was lytic activity of the bacteriophages toward the Klebsiella pneumoniae ATCC 700603 strain, but two different urinary strains from patients from a general practitioner were nonsusceptible to the bacteriophages. We could not obtain exact details from the Eliava Institute about the solution regarding the dose, endotoxin concentration, or whether it was a mixture of multiple active bacteriophages.
FIG 2.
Lytic activity of anti-Klebsiella pneumoniae bacteriophages on the double-layer agar.
CHALLENGE QUESTION
What is the rationale for combining an antibiotic with bacteriophages in the treatment of persisting urinary tract infections caused by ESBL-producing Klebsiella pneumoniae?
-
A.
Bacteriophage heads will easily attach to lipopolysaccharide (LPS) of Gram-negative bacteria, making them more susceptible to antibiotic treatment.
-
B.
Bacteriophages can only attach to a bacterial capsule, thereby enabling antibiotics to penetrate and lyse the bacteria, eradicating infection.
-
C.
A recent randomized controlled trial provides clinical evidence of combining antibiotics with bacteriophages for treating UTIs.
-
D.
Together with antibiotics, bacteriophages will resolve the urinary tract infection by acting as an adjuvant for the immune system.
-
E.
Bacteriophages will lyse susceptible bacteria, and the bacteriophage-resistant bacteria may be eradicated by antibiotics to which they tested susceptible.
TREATMENT AND OUTCOME
Urinary tract infections (UTIs) with ESBL-producing Enterobacterales in renal transplant recipients pose a considerable treatment challenge for physicians. Additionally, these UTIs relapse more often (1), and repeated carbapenem treatment may select for carbapenemase-producing strains, especially in high-endemic settings.
What are the treatment options when a renal transplant patient is failing first-line therapy for UTIs and other treatment possibilities are limited? The Klebsiella pneumoniae isolates of our patient were multiple-drug resistant, and at first, the MIC for fosfomycin seemed susceptible, but prophylaxis with oral fosfomycin every third day failed. Carbapenems remained the best treatment option.
It is known that bladder irrigation with glycosaminoglycans can lead to fewer UTI recurrences and longer time to UTI recurrence (6). However, intravesical glycosaminoglycans did not result in prevention of UTI in our patient nor did intravesical installation with amikacin during 8 weeks. Bacteriophages as a therapeutic option in UTIs have not yet been researched in a randomized clinical trial, although studies are under way (7). Bacteriophages lyse susceptible bacteria, as can be demonstrated in vitro, and the antibiotic will eradicate those bacteria susceptible to the antibiotic.
Intestinal carriage of an ESBL-producing Klebsiella pneumoniae strain was confirmed in our patient by three rectal swabs taken over 3 months. Hypothetically, oral intake of lytic bacteriophages may eliminate intestinal carriage of UTI-causing pathogens.
In our case, we were left with only intravenous options for treatment of a persistent infection of the urinary tract. As there is increasing public awareness in the Netherlands about bacteriophage therapy in refractory infections due to documentaries on television, our patient sought additional help at the Eliava Institute in Tbilisi, Georgia, to which he sent a urine sample. When our patient received vials of anti-Klebsiella pneumoniae bacteriophages, we were able to test the content for lytic activity against his ESBL-producing K. pneumoniae strain. The oral and intravesical bacteriophage treatment coincided with the 6-week meropenem treatment for epididymitis. After initiating bacteriophage therapy, our patient’s urethral symptoms subsided completely within one day and have not recurred since. In the 14 months following treatment, urine cultures have remained negative.
In this case, persistently recurrent UTI by an ESBL-positive Klebsiella pneumoniae strain was successfully treated with a combination of meropenem and bacteriophages after meropenem therapy of 10 days up to 4 weeks had failed seven times. Upon initiation of bacteriophages, the urethritis symptoms disappeared rapidly. Together, these suggest that the bacteriophages contributed to the treatment success. Ideally, treatment with bacteriophages should be given using a set of multiple bacteriophages with proven activity against the bacterial strain causing the infection (5). More insight must be gained in the pharmacokinetics and pharmacodynamics of the bacteriophage (cocktails) when used orally and intravesically. Also, quality control of bacteriophage solutions regarding endotoxin, bacterial, and viral contamination should be conducted (5).
Compassionate usage of bacteriophages combined with antibiotics should be possible for those patients with UTI who fail to respond to conventional treatment, while all legal issues regarding prescribing of bacteriophages for compassionate use must be solved in the near future. This case also illustrates that patients can easily access medical treatment through other countries. In this case, it would have been good to have had more insight in the work of the Eliava Institute and more knowledge about the contents of the bacteriophage solution. In the meantime, we are looking forward to the results of trials of bacteriophages for urinary tract infections that are currently being performed (4, 7).
ACKNOWLEDGMENTS
We thank M. Kutateladze and staff from the Eliava Institute for providing personalized anti-Klebsiella pneumoniae bacteriophages to our patient.
This Journal section presents a real, challenging case involving a multidrug-resistant organism. The case authors present the rationale for their therapeutic strategy and discuss the impact of mechanisms of resistance on clinical outcome. Two expert clinicians then provide a commentary on the case.
Footnotes
For the commentary, see https://doi.org/10.1128/AAC.01987-19.
REFERENCES
- 1.Alevizakos M, Nasioudis D, Mylonakis E. 2017. Urinary tract infections caused by ESBL-producing Enterobacteriaceae in renal transplant recipients: a systematic review and meta-analysis. Transpl Infect Dis 19:e12759. doi: 10.1111/tid.12759. [DOI] [PubMed] [Google Scholar]
- 2.Kortright KE, Chan BK, Koff JL, Turner PE. 2019. Phage therapy: a renewed approach to combat antibiotic-resistant bacteria. Cell Host Microbe 25:219–232. doi: 10.1016/j.chom.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 3.Sybesma W, Zbinden R, Chanishvili N, Kutateladze M, Chkhotua A, Ujmajuridze A, Mehnert U, Kessler TM. 2016. Bacteriophages as potential treatment for urinary tract infections. Front Microbiol 7:465. doi: 10.3389/fmicb.2016.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leitner L, Sybesma W, Chanishvili N, Goderdzishvili M, Chkhotua A, Ujmajuridze A, Schneider MP, Sartori A, Mehnert U, Bachmann LM, Kessler TM. 2017. Bacteriophages for treating urinary tract infections in patients undergoing transurethral resection of the prostate: a randomized, placebo-controlled, double-blind clinical trial. BMC Urol 17:90. doi: 10.1186/s12894-017-0283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schooley RT, Biswas B, Gill JJ, Hernandez-Morales A, Lancaster J, Lessor L, Barr JJ, Reed SL, Rohwer F, Benler S, Segall AM, Taplitz R, Smith DM, Kumaraswamy M, Nizet V, Lin L, McCauley MD, Strathdee SA, Benson CA, Pope RK, Leroux BM, Picel AC, Mateczun AJ, Cilwa KE, Regeimbal JM, Estrella LA, Wolfe DM, Henry MS, Quinones J, Salka S, Bishop-Lilly KA, Young R, Hamilton T. 2017. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob Agents Chemother 61:e00954-17. doi: 10.1128/AAC.00954-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lazzeri M, Hurle R, Casale P, Buffi N, Lughezzani G, Fiorini G, Peschechera R, Pasini L, Zandegiacomo S, Benetti A, Taverna G, Guazzoni G, Barbagli G. 2016. Managing chronic bladder diseases with the administration of exogenous glycosaminoglycans: an update on the evidence. Ther Adv Urol 8:91–99. doi: 10.1177/1756287215621234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ujmajuridze A, Chanishvili N, Goderdzishvili M, Leitner L, Mehnert U, Chkhotua A, Kessler TM, Sybesma W. 2018. Adapted bacteriophages for treating urinary tract infections. Front Microbiol 9:1832. doi: 10.3389/fmicb.2018.01832. [DOI] [PMC free article] [PubMed] [Google Scholar]