We documented the adjunctive bacteriophage therapy to treat a chronic relapsing periprosthetic joint infection of the knee and chronic osteomyelitis of the femur caused by multidrug-resistant Pseudomonas aeruginosa. The combined antibiotic-phage treatment eradicated the infection, and no side effects to phages were observed.
KEYWORDS: periprosthetic joint infection, biofilm infections, MDR Pseudomonas aeruginosa, bacteriophage therapy
ABSTRACT
We documented the adjunctive bacteriophage therapy to treat a chronic relapsing periprosthetic joint infection of the knee and chronic osteomyelitis of the femur caused by multidrug-resistant Pseudomonas aeruginosa. The combined antibiotic-phage treatment eradicated the infection, and no side effects to phages were observed.
INTRODUCTION
Periprosthetic joint infection (PJI) caused by microorganisms that grow in biofilms is challenging to treat and requires both surgery and long-term antimicrobials (1). Gram-negative bacilli are reported in 10% to 23% of cases and are often isolated as part of polymicrobial PJI. Multidrug resistance (MDR) is increasingly observed in Gram-negative bacilli, accounting up to 44%, including Pseudomonas aeruginosa (2). Due to limited antimicrobial options, alternative therapeutic approaches are needed, such as the use of bacteriophages (3, 4). The application of strictly lytic bacteriophages selectively infecting only host bacterial cells without affecting the normal microflora can potentially serve as an alternative or as a complement to local or systemic antibiotic therapy (5–8). Phage therapy has been used mainly in the Eastern European countries for the treatment of human bacterial infections (9). However, with the emergence of multidrug-resistant bacteria, phage therapy has been rediscovered by Western medicine. Although regulatory authorities have not approved it yet, successful applications of bacteriophages have been reported (10). In addition, new data suggest that phage therapy may also be applied in the treatment of nonbacterial infections (viruses and fungi) to interact with human immune system cells, leading to clinically useful immunomodulatory and anti-inflammatory effects (11).
CASE PRESENTATION
An 80-year-old woman with metabolic syndrome (diabetes mellitus type 2, obesity with a body mass index of 33 kg/m2, essential hypertension) and chronic kidney failure with an estimated glomerular filtration rate (eGFR) of <30 ml/min presented with a diagnosis of relapsing right knee PJI and chronic osteomyelitis of the femur after a gunshot injury. One year earlier, the knee prosthesis was explanted, the distal femur resected, and an antibiotic-loaded bone spacer implanted. At the time of explantation, multidrug-resistant (MDR) Klebsiella pneumoniae and Providencia stuartii were cultured and treated in the prosthesis-free interval with cefepime and trimethoprim-sulfamethoxazole. Eight weeks later, the prosthesis was reimplanted and treated with ceftazidime-avibactam for 3 weeks, followed by suppression with trimethoprim-sulfamethoxazole with renal dose adjustments. Three months after reimplantation, the right knee was painful and swollen, C-reactive protein was increased (51 mg/liter), and no loosening of the prosthesis was seen on X ray. Persistent infection was suspected, the antibiotic was discontinued, and diagnostic joint aspiration was performed 2 weeks later.
Two morphologically distinct MDR Pseudomonas aeruginosa isolates grew from the aspirated synovial fluid: one was resistant to all antibiotics except colistin, the other was susceptible to colistin and ceftazidime only (Table 1). The knee prosthesis was explanted, and the surgical site was debrided and extensively rinsed with a 2% to 3% sodium bicarbonate solution; no antiseptic solution was applied. An antibiotic-loaded cement spacer was inserted containing 1 g gentamicin and 1 g clindamycin per 40 g poly(methyl methacrylate). During surgery, four drainage tubes were placed: one into the femoral and one into tibial canal via drill holes, and additional two tubes were placed into the former prosthesis area.
TABLE 1.
Antimicrobial susceptibility of clinical isolates
| Antibiotica | Susceptibility (MIC [μg/ml])b |
|||||
|---|---|---|---|---|---|---|
| P. aeruginosa | P. aeruginosa | K. pneumoniae | P. stuartii | S. epidermidis | S. haemolyticus | |
| OXA | NA | NA | NA | NA | R (≥4) | S (≤0.25) |
| PIP | R (≥128) | R (≥128) | R | R | R | S |
| PIP-TAZ | R (≥128) | R (≥128) | R (≥128) | R (≥128) | R | S |
| CAZ | S (4) | R (≥64) | R (≥16) | R (≥16) | R | R |
| CAF-AVI | S (2) | R (≥256) | S (1) | S (0.5) | NA | NA |
| IMI | R (≥16) | R (≥16) | R (≥16) | R (≥16) | R | S |
| AZT | R (≥64) | R (≥64) | NA | NA | NA | NA |
| CEF | R (≥64) | R (≥64) | R (32) | S (≤1) | R | S |
| MER | R (≥16) | R (≥16) | R (≥16) | I (4) | R | S |
| CIP | R (≥2) | R (≥2) | R (≥4) | R | R | S |
| LEV | R | R | R | R | R (≥8) | S (≤0.12) |
| GEN | R (≥16) | R (≥16) | S (≤16) | R (≤1) | R (8) | S (≤0.5) |
| TOB | R (≥16) | R (≥16) | R (8) | R (2) | R | S |
| AMI | R (16) | R (≥64) | I (≤2) | I (≤16) | NA | NA |
| FOS | R (≥256) | R (≥256) | NA | NA | S (≤8) | R (≥128) |
| COL | S (0.5) | S (1) | NA | NA | NA | NA |
| DOX | NA | NA | NA | NA | S (≤1) | S (≤1) |
| RIF | NA | NA | NA | NA | S (≤0.03) | S (≤0.03) |
| VAN | NA | NA | NA | NA | S (2) | S (≤0.5) |
| DAP | NA | NA | NA | NA | S (0.25) | S (≤0.12) |
| TMP-SMX | NA | NA | S (≤20) | R (≥320) | S (20) | S (≤10) |
OXA, oxacillin; PIP, piperacillin; PIP-TAZ, piperacillin-tazobactam; CAZ, ceftazidime; CAZ-AVI, ceftazidime-avibactam; IMI, imipenem; AZT, aztreonam; CEF, cefepime; MER, meropenem; CIP, ciprofloxacin; LEV, levofloxacin; GEN, gentamicin; TOB, tobramycin; AMI, amikacin; FOS, fosfomycin; COL, colistin; DOX, doxycycline; RIF, rifampin; VAN, vancomycin; DAP, daptomycin; TMP-SMX, trimethoprim-sulfamethoxazole.
R, resistant; S, susceptible; NA, not available. Interpretations according to CLSI guidelines.
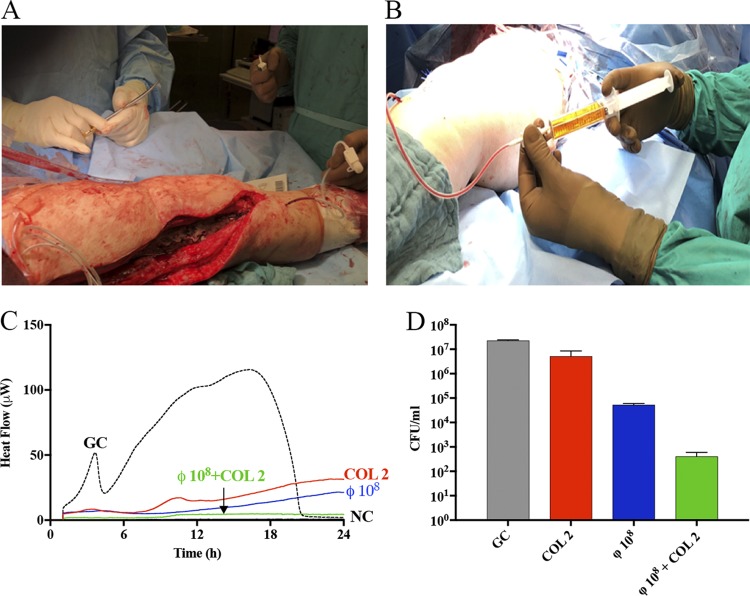
According to the principals of the Declaration of Helsinki (article 37), adjunctive local bacteriophage therapy against MDR P. aeruginosa was applied. Before phage therapy, the patient gave informed consent. A single 100-ml loading dose of purified bacteriophage was applied locally during surgery, followed by administration of 5 ml of bacteriophage solution containing 108 PFU/ml every 8 h through each of the four drains as a local delivery system for 5 days (Fig. 1A and B). After surgery, intravenous treatment with colistin (150 mg every 24 h), meropenem (1 g every 12 h), and ceftazidime (2 g every 12 h) was started (adapted to the eGFR of 20 ml/min). The explanted prosthesis was sent for sonication. The sonication fluid and four intraoperatively collected periprosthetic tissue samples grew MDR P. aeruginosa with same resistance profile as the isolate cultured from the preoperatively aspirated synovial fluid. In addition, one tissue culture grew an oxacillin-susceptible and rifampin-susceptible Staphylococcus haemolyticus (Table 1). The drainage fluid was collected for culture before bacteriophage instillation on days three, four, and five of phage treatment. No P. aeruginosa was isolated, but methicillin-resistant Staphylococcus epidermidis (MRSE) grew in all sampled drainage fluid samples.
FIG 1.
(A) Intraoperatively placed drains as local delivery system. (B) Local application of P. aeruginosa bacteriophages. (C) Evaluation of MDR P. aeruginosa biofilm susceptibility to antimicrobials alone and to staggered exposure of phages (8 h prior) followed by colistin. Each curve shows the heat produced by viable bacteria present in the biofilm after the treatment. Numbers above and near curves represent antibiotic concentrations (in μg/ml) and titers of phage (in PFU/ml). GC, growth control (dashed line); NC, negative control. (D) Effect of P. aeruginosa phage on viability of biofilm-embedded P. aeruginosa. Biofilm formed on porous glass beads was exposed to phages for 24 h. Data are reported as mean CFU/ml ± standard error of the mean from at least three independent experiments. GC, growth control.
Two weeks after explantation of the prosthesis, purulent discharge occurred. Debridement and exchange of the spacer was performed. Sonication fluid of the explanted spacer grew MRSE with the same resistance profile as earlier, but no P. aeruginosa was isolated. Meropenem was replaced by daptomycin (700 mg every 48 h), which was added to intravenous colistin and ceftazidime. Four weeks later, reimplantation of the prosthesis was performed. All intraoperatively collected periprosthetic tissue samples remained negative in culture. Four weeks after reimplantation, colistin and ceftazidime were discontinued due to nephrotoxicity (eGFR, 12 ml/min) and neurotoxicity (confusion, disorientation, and agitation), respectively, and the patient was discharged with oral rifampin 600 mg once daily and doxycycline 100 mg twice daily for 6 weeks. For MDR P. aeruginosa, no oral antibiotic treatment was available. At a follow-up visit 10 months after reimplantation, the patient reported no pain in the right knee; the soft tissue at the surgical site was unremarkable and the mobility satisfactory. The serum C-reactive protein was normal. Conventional X ray showed good position of the knee prosthesis, without signs of implant loosening.
CHALLENGE QUESTION
What is the rationale for using bacteriophages in the treatment of periprosthetic joint infection caused by a multidrug-resistant bacterium?
-
A.
Bacteriophages can infect and replicate within the target bacterium, resulting in viral replication that generates high concentrations of bacteriophages, in turn, infecting surrounding bacteria.
-
B.
Some bacteriophages can enzymatically degrade biofilm matrix.
-
C.
Addition of bacteriophages to antibiotics can improve the antimicrobial efficacy against bacterial biofilm.
-
D.
Bacterial persister cells can be infected and killed by bacteriophages.
-
E.
All of the above.
TREATMENT AND OUTCOME
The treatment outcome of chronic PJI largely depends on adequate surgical debridement after removal of all foreign material (prosthetic components, necrotic tissue, and remaining bone cement), along with biofilm-active antibiotics. Limited clinical experience exists for the management of PJI caused by MDR P. aeruginosa. The risk of treatment failure increases when the prosthesis is retained and the pathogens are resistant to fluoroquinolones—the only biofilm-active antibiotic for Gram-negative PJI (12). In MDR P. aeruginosa PJI, where colistin is the only active antibiotic, the prolonged intravenous use is limited by its side effects and toxicity.
By nature, bacteriophages are good candidates for antibacterial and biofilm-active therapy. They are able to degrade biofilm matrix, thereby enhancing the efficacy of antibiotics and reducing the concentration of antibiotics required to eradicate infection (13, 14). Recent studies have demonstrated that phages can eradicate biofilms by their synergistic action with antibiotics (15, 16).
Both P. aeruginosa isolates obtained from the patient were screened against existing bacteriophages from the collection at the George Eliava Institute of Bacteriophages, Microbiology and Virology (Tbilisi, Georgia). The most active phage was purified and supplied to our hospital at the titer of 109 PFU/ml. Customizing phage approach is time consuming comparing to that for “off-the-shelf” cocktails but can provide the strain specificity required for favorable clinical outcomes (17). However, a phage cocktail is more effective in reducing bacterial mutation frequency and has more therapeutic potential for infections caused by MDR pathogens (18).
Adoption of phage therapy into human medicine requires phage susceptibility testing to select a phage or phage combinations for individual patients’ isolates and to assess potential emergence of resistance to particular phages (19). The susceptibility of two MDR P. aeruginosa strains to bacteriophage was tested by spot test and enumeration of bacteriophage was assessed by a double-layer plaque assay test (20). In addition, an interaction between the selected phage, antibiotics, and biofilm P. aeruginosa was observed in vitro in real time by isothermal microcalorimetry, as previously described (21). Isothermal microcalorimetry is a highly sensitive laboratory tool that provides precise real-time measurements of the bacterial metabolic status and replication activity related to heat production. We evaluated the interaction of simultaneous versus staggered treatment with phage and antibiotics to investigate a potential synergistic effect on the biofilm. Pretreatment of a P. aeruginosa biofilm with phages 8 h prior to colistin exposure demonstrated the strongest reduction of biofilm biomass evaluated by isothermal microcalorimetry (Fig. 1C) and a sonication/colony counting test (Fig. 1D).
In the presented case, a two-stage surgical revision was performed due to the difficulty in the treatment of MDR P. aeruginosa using local and systemic antibiotics. Combined phage-antibiotic therapy was well tolerated, resulting in microbiological eradication, whereas repeated previous courses of antibiotics and surgical treatment failed. Advantages of the adjunctive phage approach included rapid bactericidal action of bacteriophages and high synergistic activity with antibiotics.
Bacteriophages are self-replicating microorganisms, and a single administration should theoretically be sufficient to treat a bacterial infection. However, several studies have shown that multiple doses of phages may be better than a single dose (22, 23). In our report, no dosing recommendations for bacteriophages were available. Empirically, a multiple-dose treatment regimen for a maximum of 5 days was chosen to minimize the risk for secondary drain-associated infection.
In conclusion, we described a successful local application of personalized bacteriophage treatment in combination with surgery and conventional antimicrobial therapy of MDR P. aeruginosa PJI. This case indicates a potential adjunctive role of phages for eradication of MDR biofilms with limited therapeutic options. Further preclinical and clinical studies need to evaluate the phage dose, application form, treatment duration, and combination with local and systemic antimicrobials to optimize the treatment success.
ACKNOWLEDGMENTS
We thank Mzia Kutateladze, director and head of the scientific council of G. Eliava Institute of Bacteriophages, Microbiology and Virology, for help.
This work was supported by PRO-IMPLANT Foundation (www.pro-implant-foundation.org) in Berlin, Germany.
We declare no conflicts of interest.
This Journal section presents a real, challenging case involving a multidrug-resistant organism. The case authors present the rationale for their therapeutic strategy and discuss the impact of mechanisms of resistance on clinical outcome. Two expert clinicians then provide a commentary on the case.
Footnotes
For the commentary, see https://doi.org/10.1128/AAC.01987-19.
REFERENCES
- 1.Zimmerli W, Trampuz A, Ochsner PE. 2004. Prosthetic-joint infections. N Engl J Med 351:1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
- 2.Ribera A, Benavent E, Lora-Tamayo J, Tubau F, Pedrero S, Cabo X, Ariza J, Murillo O. 2015. Osteoarticular infection caused by MDR Pseudomonas aeruginosa: the benefits of combination therapy with colistin plus β-lactams. J Antimicrob Chemother 70:3357–3365. doi: 10.1093/jac/dkv281. [DOI] [PubMed] [Google Scholar]
- 3.Akanda ZZ, Taha M, Abdelbary H. 2018. Current review-the rise of bacteriophage as a unique therapeutic platform in treating peri-prosthetic joint infections. J Orthop Res 36:1051–1060. doi: 10.1002/jor.23755. [DOI] [PubMed] [Google Scholar]
- 4.Kortright KE, Chan BK, Koff JL, Turner PE. 2019. Phage therapy: a renewed approach to combat antibiotic-resistant bacteria. Cell Host Microbe 25:219–232. doi: 10.1016/j.chom.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Jennes S, Merabishvili M, Soentjens P, Pang KW, Rose T, Keersebilck E, Soete O, François P-M, Teodorescu S, Verween G, Verbeken G, De Vos D, Pirnay J-P. 2017. Use of bacteriophages in the treatment of colistin-only-sensitive Pseudomonas aeruginosa septicaemia in a patient with acute kidney injury–a case report. Crit Care 21:129. doi: 10.1186/s13054-017-1709-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright A, Hawkins CH, Anggard EE, Harper DR. 2009. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clin Otolaryngol 34:349–357. doi: 10.1111/j.1749-4486.2009.01973.x. [DOI] [PubMed] [Google Scholar]
- 7.Fish R, Kutter E, Wheat G, Blasdel B, Kutateladze M, Kuhl S. 2018. Compassionate use of bacteriophage therapy for foot ulcer treatment as an effective step for moving toward clinical trials. Methods Mol Biol 1693:159–170. doi: 10.1007/978-1-4939-7395-8_14. [DOI] [PubMed] [Google Scholar]
- 8.Vogt D, Sperling S, Tkhilaishvili T, Trampuz A, Pirnay JP, Willy C. 2017. Beyond antibiotic therapy - future antiinfective strategies - update 2017. Unfallchirurg 120:573–584. doi: 10.1007/s00113-017-0374-6 (In German.) [DOI] [PubMed] [Google Scholar]
- 9.Kutateladze M, Adamia R. 2010. Bacteriophages as potential new therapeutics to replace or supplement antibiotics. Trends Biotechnol 28:591–595. doi: 10.1016/j.tibtech.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Furfaro LL, Payne MS, Chang BJ. 2018. Bacteriophage therapy: clinical trials and regulatory hurdles. Front Cell Infect Microbiol 8:376–376. doi: 10.3389/fcimb.2018.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Górski A, Bollyky PL, Przybylski M, Borysowski J, Międzybrodzki R, Jończyk-Matysiak E, Weber-Dąbrowska B. 2019. Perspectives of phage therapy in non-bacterial infections. Front Microbiol 9:3306. doi: 10.3389/fmicb.2018.03306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodríguez-Pardo D, Pigrau C, Lora-Tamayo J, Soriano A, del Toro MD, Cobo J, Palomino J, Euba G, Riera M, Sánchez-Somolinos M, Benito N, Fernández-Sampedro M, Sorli L, Guio L, Iribarren JA, Baraia-Etxaburu JM, Ramos A, Bahamonde A, Flores-Sánchez X, Corona PS, Ariza J, REIPI Group for the Study of Prosthetic Infection. 2014. Gram-negative prosthetic joint infection: outcome of a debridement, antibiotics and implant retention approach. A large multicentre study. Clin Microbiol Infect 20:O911–O919. doi: 10.1111/1469-0691.12649. [DOI] [PubMed] [Google Scholar]
- 13.Tkhilaishvili T, Lombardi L, Klatt AB, Trampuz A, Di Luca M. 2018. Bacteriophage Sb-1 enhances antibiotic activity against biofilm, degrades exopolysaccharide matrix and targets persisters of Staphylococcus aureus. Int J Antimicrob Agents 52:842–853. doi: 10.1016/j.ijantimicag.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Dickey J, Perrot V. 2019. Adjunct phage treatment enhances the effectiveness of low antibiotic concentration against Staphylococcus aureus biofilms in vitro. PLoS One 14:e0209390. doi: 10.1371/journal.pone.0209390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumaran D, Taha M, Yi Q, Ramirez-Arcos S, Diallo J-S, Carli A, Abdelbary H. 2018. Does treatment order matter? Investigating the ability of bacteriophage to augment antibiotic activity against Staphylococcus aureus biofilms. Front Microbiol 9:127–127. doi: 10.3389/fmicb.2018.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaudhry WN, Concepcion-Acevedo J, Park T, Andleeb S, Bull JJ, Levin BR. 2017. Synergy and order effects of antibiotics and phages in killing Pseudomonas aeruginosa biofilms. PLoS One 12:e0168615. doi: 10.1371/journal.pone.0168615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schooley RT, Biswas B, Gill JJ, Hernandez-Morales A, Lancaster J, Lessor L, Barr JJ, Reed SL, Rohwer F, Benler S, Segall AM, Taplitz R, Smith DM, Kerr K, Kumaraswamy M, Nizet V, Lin L, McCauley MD, Strathdee SA, Benson CA, Pope RK, Leroux BM, Picel AC, Mateczun AJ, Cilwa KE, Regeimbal JM, Estrella LA, Wolfe DM, Henry MS, Quinones J, Salka S, Bishop-Lilly KA, Young R, Hamilton T. 2017. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob Agents Chemother 61:e00954-17. doi: 10.1128/AAC.00954-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu J, Liu X, Li Y, Han W, Lei L, Yang Y, Zhao H, Gao Y, Song J, Lu R, Sun C, Feng X. 2012. A method for generation phage cocktail with great therapeutic potential. PLoS One 7:e31698. doi: 10.1371/journal.pone.0031698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caflisch KM, Patel R. 2019. Implications of bacteriophage- and bacteriophage component-based therapies for the clinical microbiology laboratory. J Clin Microbiol 57:e00229-19. doi: 10.1128/JCM.00229-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan Mirzaei M, Nilsson AS. 2015. Isolation of phages for phage therapy: a comparison of spot tests and efficiency of plating analyses for determination of host range and efficacy. PLoS One 10:e0118557. doi: 10.1371/journal.pone.0118557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tkhilaishvili T, Di Luca M, Abbandonato G, Maiolo EM, Klatt AB, Reuter M, Moncke-Buchner E, Trampuz A. 2018. Real-time assessment of bacteriophage T3-derived antimicrobial activity against planktonic and biofilm-embedded Escherichia coli by isothermal microcalorimetry. Res Microbiol 169:515–521. doi: 10.1016/j.resmic.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Patey O, McCallin S, Mazure H, Liddle M, Smithyman A, Dublanchet A. 2018. Clinical indications and compassionate use of phage therapy: personal experience and literature review with a focus on osteoarticular infections. Viruses 11:18. doi: 10.3390/v11010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ly-Chatain MH. 2014. The factors affecting effectiveness of treatment in phages therapy. Front Microbiol 5:51. doi: 10.3389/fmicb.2014.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]