The use of the Sensititre YeastOne YO10 alamarBlue assay for the in vitro susceptibility testing of Madurella mycetomatis was evaluated in M. mycetomatis isolates with and without pyomelanin secretion. Pyomelanin secretion did not influence visual endpoint reading; however, it caused a shift in peak absorbance from 570 nm to 620 nm when read spectrophotometrically. Therefore, when choosing the method for endpoint reading, the presence of pyomelanin should be considered.
KEYWORDS: Madurella mycetomatis, azole, melanin, mycetoma, pigment, pyomelanin, susceptibility testing
ABSTRACT
The use of the Sensititre YeastOne YO10 alamarBlue assay for the in vitro susceptibility testing of Madurella mycetomatis was evaluated in M. mycetomatis isolates with and without pyomelanin secretion. Pyomelanin secretion did not influence visual endpoint reading; however, it caused a shift in peak absorbance from 570 nm to 620 nm when read spectrophotometrically. Therefore, when choosing the method for endpoint reading, the presence of pyomelanin should be considered.
INTRODUCTION
Madurella mycetomatis is the major causative agent of eumycetoma, a poverty-associated neglected tropical disease (1). It is characterized by a painless subcutaneous lesion, an inflammation reaction, multiple draining sinuses, and the formation of grains (2, 3). Although eumycetoma can be treated with a combination of antifungal therapy and surgery, the success rates are relatively low (25.9%) and recurrences are common (4).
The relatively low success rate frustrates both patients and physicians, and it is difficult to predict which patient will respond to treatment and which patient will not. Currently, antifungal susceptibility assays for M. mycetomatis are only used for research purposes and not used in the clinical management of patients (4, 5). However, determining MICs and correlating them to therapeutic outcome could improve the clinical management of mycetoma (6). For this, an easy-to-use in vitro susceptibility assay is needed. The currently used method is based on the use of hyphal suspensions as a starting material, which requires a viability dye, such as 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT), for endpoint-visualization (7–11). This dye is expensive, which limits its use for diagnostic purposes, especially in regions of endemicity. An alternative assay is the commercially available Sensititre YeastOne system (Thermo Fisher Scientific). This system has been shown to be reproducible and in agreement with the CLSI (12) and, more importantly, can be kept at room temperature-friendly conditions, which makes it useful in settings of endemicity. The endpoints can be read out visually or by using the Thermo Fisher Scientific Sensititre Vizion system or spectrophotometrically. However, the agreement between the three readouts (visual, Sensititre Vizion, and spectrophotometry) in isolates of M. mycetomatis has not been evaluated. A high agreement between the methods will enable the application of visual readings in resource-limited settings of endemicity, where a microplate reader or Sensititre automated SWIN software platform (Trek Diagnostic Systems) may not be available.
M. mycetomatis has been shown to secrete pyomelanin, and in a previous study, we demonstrated that this pyomelanin influences the color change from blue to pink in the Sensititre YeastOne system (YO02) (7). Visually, this color change does not influence the readout system; whether this pyomelanin influences the endpoint determination with the Sensititre Vizion or the spectrophotometric readout system is not known. Therefore, in this study, we aim to determine the MICs by the colorimetric YO10 Sensititre YeastOne alamarBlue assay (Thermo Fisher Scientific) for eight pyomelanin-producing (P1, MM25, MM30, MM44, MM45, MM49, MM55, MM68) and eight non-pyomelanin-producing (MM14, MM22, MM50, MM52, MM54, MM58, MM64, MM83) M. mycetomatis isolates. All isolates were previously identified to the species level using M. mycetomatis-specific PCR and by sequencing of the internal transcribed spacer (ITS) regions (13). MICs were obtained independently in triplicate as described before (10). For visual endpoint reading, MIC endpoints were determined as the first blue/purple well for each antifungal agent. For the automatic plate reader, readings were done using the Thermo Fisher Scientific Sensititre Vizion system, and results were interpreted by SWIN software according to the manufacturer’s instructions. For spectrophotometric readings, the plates were centrifuged (Centr GP8R, IEC; Centra, Germany) for 1 minute at 3,400 rpm, and 50 μl of the supernatant was transferred to flat-bottom 96-well plates (Greiner Bio-One GmbH, Germany). Absorbance was measured at 570 nm and 620 nm using a microplate reader (Epoch 2; BioTek Instruments, Inc., Winooski, VT, USA). The relative percentage reduction of alamarBlue for 3 independent experiments was calculated with the formula described elsewhere ([(AC – AT)/AC] × 100), where AC is the absorbance of the negative control and AT is the absorbance of the test (14–16). The MIC was defined as the lowest drug concentration resulting in a ≤20% reduction of alamarBlue. Furthermore, the growth control and the negative control were used to perform an absorbance curve to determine peak absorbance for pyomelanin-secreting and non-pyomelanin-secreting isolates.
Regardless of the readout system, M. mycetomatis isolates were most susceptible for the azoles and amphotericin B, except fluconazole. This was in accordance with previous studies (17–19). The echinocandins and 5-flucytosine did not inhibit growth (Table 1), which was again in agreement with previous studies (10, 18–20). The lowest MICs were obtained when the endpoints were determined with the Vizion system. The highest MICs were obtained after spectrophotometric endpoint reading at 570 nm.
TABLE 1.
MIC distribution of 16 M. mycetomatis isolates against 9 antifungal agents
| Antifungal agent | MIC90 (μg/ml) for: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All isolates |
Pyomelanin |
Non-pyomelanin |
||||||||
| Visual | Vizion | Spectrophotometric |
Visual | Spectrophotometric |
Visual | Spectrophotometric |
||||
| 570 nm | 620 nm | 570 nm | 620 nm | 570 nm | 620 nm | |||||
| Itraconazole | 0.12 | 0.06 | 0.5 | 0.12 | 0.12 | 0.06 | 0.12 | 0.12 | 0.12 | 0.12 |
| Voriconazole | 0.12 | 0.12 | 0.5 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.25 |
| Posaconazole | 0.12 | 0.12 | 0.25 | 0.25 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 |
| Fluconazole | 16 | 8 | 64 | 16 | 16 | 16 | 16 | 8 | 32 | 8 |
| Amphotericin B | 1 | 1 | 2 | 1 | 1 | 0.5 | 0.5 | 1 | 0.5 | 1 |
| 5-Flucytosine | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| Caspofungin | >8 | >8 | >8 | >8 | >8 | >8 | >8 | >8 | >8 | >8 |
| Micafungin | >8 | >8 | >8 | >8 | >8 | >8 | >8 | >8 | >8 | >8 |
| Anidalufungin | >8 | >8 | >8 | >8 | >8 | >8 | >8 | >8 | >8 | >8 |
Similar to Aspergillus fumigatus and Sporothrix schenckii, M. mycetomatis forms pyomelanin through homogentisic acid polymerization and 1,8-dihydroxynaphthalene (DHN)-melanin by polyketide synthase (21, 22). As demonstrated in Table 1, no statistically significantly different MICs were obtained in pyomelanin-secreting isolates versus non-pyomelanin-secreting isolates (Mann-Whitney, P > 0.5 for all antifungals). This observation was also found for Sporothrix schenckii and Sporothrix brasiliensis when pyomelanin biosynthesis was induced in these Sporothrix spp., and similar MICs were obtained for itraconazole, ketoconazole, terbinafine, and amphotericin B (22). However, inhibition of pyomelanin production in one S. brasiliensis isolate significantly reduced the minimal fungicidal concentration of terbinafine but not itraconazole (22). In contrast, when DHN melanin isolated from M. mycetomatis was added to the Sensititre YeastOne YO02 plates, an MIC rise of 16- and 32-fold for itraconazole and ketoconazole, respectively, was obtained. (7). This suggests that the different melanins from M. mycetomatis have different effects on susceptibility to antifungals.
Although pyomelanin secretion did not influence the MIC, it could potentially influence the readout system. To evaluate similarities between the three readout systems, we calculated the interexperimental agreements between the various readout systems for each antifungal agent at 570 nm (Table 2). The agreement between visual and Vizion was generally better than the agreement between visual reading or Vizion reading and spectrophotometric readings. The agreement between the visual endpoint reading and spectrophotometric endpoint reading was poor when measured at 570 nm. The percentage agreement obtained at 570 nm was 56.3 to 100%, with the highest agreement observed for noninhibiting antifungals and the lowest, for fluconazole.
TABLE 2.
Effects of pyomelanin secretion on the readout systema
| Antifungal agent | Agreement (%) at 570 nm for: |
Agreement (%) at 620 nm for: |
|||
|---|---|---|---|---|---|
| Visual vs Vizion | Visual vs spectrophotometric | Vizion vs spectrophotometric | Visual vs spectrophotometric | Vizion vs spectrophotometric | |
| MICs within 1 dilution | MICs within 1 dilution | MICs within 1 dilution | MICs identical | MICs within 1 dilution | |
| Itraconazole | 100 | 81.5 | 80 | 92.3 | 80 |
| Voriconacozole | 100 | 81.5 | 70 | 92.3 | 70 |
| Posaconazole | 100 | 81.5 | 80 | 92.3 | 80 |
| Fluconazole | 70 | 56.25 | 50 | 92.3 | 60 |
| Amphotericin B | 100 | 87.5 | 80 | 100 | 80 |
| 5-Flucytosine | 100 | 93.75 | 90 | 100 | 90 |
| Caspofungin | 100 | 93.75 | 90 | 100 | 90 |
| Micafungin | 100 | 93.75 | 90 | 100 | 90 |
| Anidalufungin | 100 | 100 | 100 | 100 | 100 |
Percentage agreement between visual, SWIN, and spectrophotometric readouts at 570 nm and 620 nm for the Sensititre YeastOne assay for isolates of 16 M. mycetomatis strains. The agreement is presented as MIC90 within a one-step dilution difference.
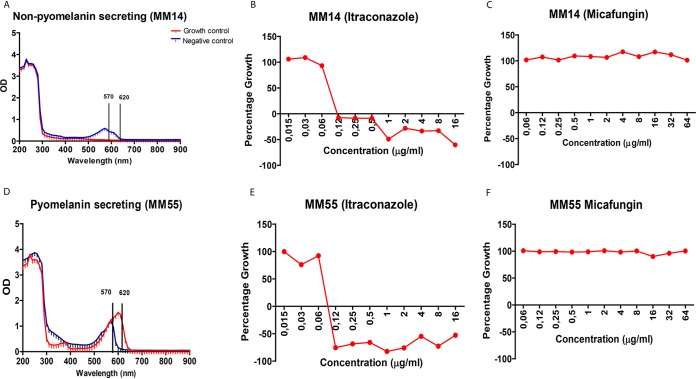
To determine if the low agreement between the visual and spectrophotometric readout systems was due to pyomelanin secretion, we compared the absorbance curves of pyomelanin-secreting isolates and non-pyomelanin-secreting isolates. A pink color was observed when the non-pyomelanin-secreting isolates grew, while for pyomelanin-secreting isolates, a brown color was observed. In pyomelanin-secreting isolates, a shift in wavelength (peak absorbance) from 570 nm to 620 nm was noted in the negative control, and a shift from 450 nm to 570 nm was noted in the growth controls (Fig. 1A). Resazurin assays are based on the reduction of the oxidized blue resazurin, with a peak absorbance of 600 nm to a pink fluorescent resorufin product with a peak absorbance of 570 nm. Upon prolonged incubation, resorufin can be further reduced to the colorless hydroresorufin (23). In our experiments, a prolonged incubation was used, which can explain why in the growth control of the non-pyomelanin-secreting isolates, no peak was observed at 570 nm. A similar situation was observed in HepG2 cells, with no peak noted at 570 nm, indicating that no resorufin was produced or converted to colorless hydroresorufin (23). In contrast, in the pyomelanin-secreting isolates, the resazurin peak at 620 nm was still observed. This absorbance was due to resazurin, as pyomelanin on its own did not give an absorbance peak at this wavelength (data not shown).
FIG 1.
Pyomelanin secretion causes a shift in wavelength absorbance. (A) Absorbance curve for non-pyomelanin-secreting isolate with growth control displaying no peak absorbance (red) and negative-control (blue) peak absorbance at 570 nm. (B and C) The percentage growth curve (red curve) for an inhibiting antifungal (itraconazole) (B) and noninhibiting antifungal (C) (micafungin) at 570 nm. (D) The absorbance curve for a pyomelanin-secreting isolate with peak absorbance for the growth control at 620 nm (red) and peak absorbance for the negative control at 570 nm (blue). (E and F) The percentage growth curve for itraconazole and micafungin, respectively, at 620 nm.
Melanin is known to be redox-active, and in a reduced state it can quench free radicals by donating electrons, or in the oxidized state by accepting electrons (24). Indeed, strong oxidizing and reducing radicals have been found to react with different types of melanin (25). Therefore, the pyomelanin could have influenced the reduction rate of resorufin to hydroresorufin, explaining why in the presence of pyomelanin, the resazurin peak was observed. Another possibility is that pyomelanin did form a complex with resazurin, thereby preventing its reduction to resorufin. However, the color of the well was brown instead of pink, indicating that upon forming a complex, the blue color is lost.
Since we noted a shift in absorbance with pyomelanin-secreting isolates, we evaluated the influence of pyomelanin on the agreement between visual reading and spectrophotometric readings at 570 nm and 620 nm for pyomelanin- and non-pyomelanin-secreting isolates. For the non-pyomelanin isolates, a good agreement was observed at 570 nm, with the percentage agreement ranging from 75 to 100% (Table 3). For the pyomelanin-secreting isolates, the percentage agreement was lower at 570 nm, ranging from 62.5 to 100% (Table 3).
TABLE 3.
Percentage agreement between 8 pyomelanin-secreting and 8 non-pyomelanin-secreting isolates for visual versus spectrophotometric endpoint reading at 570 nm and 620 nma
| Antifungal agent | Agreement at 570 nm (%) for: |
Agreement at 620 nm (%) for: |
||
|---|---|---|---|---|
| Pyomelanin secreting | Non-pyomelanin secreting | Pyomelanin secreting | Non-pyomelanin secreting | |
| MICs within 1 dilution | MICs within 1 dilution | Identical MICs | MICs within 1 dilution | |
| Itraconazole | 62.5 | 100 | 100 | 85.7 |
| Voriconacozole | 75 | 87.5 | 100 | 85.7 |
| Posaconazole | 75 | 87.5 | 100 | 85.7 |
| Fluconazole | 37.5 | 75 | 100 | 85.7 |
| Amphotericin B | 87.5 | 87.5 | 100 | 100 |
| 5-Flucytosine | 87.5 | 100 | 100 | 100 |
| Caspofungin | 87.5 | 100 | 100 | 100 |
| Micafungin | 87.5 | 100 | 100 | 100 |
| Anidalufungin | 100 | 100 | 100 | 100 |
Agreements are based on MIC90s within 1 dilution step difference.
The percentage agreement for pyomelanin-secreting isolates improved remarkably when spectrophotometric endpoint readings were taken at 620 nm, with 100% agreement for all tested antifungals. The percentage agreement for non-pyomelanin-secreting isolates also improved, ranging from 85.7 to 100% (Table 3). We therefore recommend that when the Sensititre YeastOne system is used for M. mycetomatis, but also for other Madurella species producing a similar pigment, the results should be read visually or spectrophotometrically at 620 nm.
REFERENCES
- 1.Hay RJ, Fahal AH. 2015. Mycetoma: an old and still neglected tropical disease. Trans R Soc Trop Med Hyg 109:169–170. doi: 10.1093/trstmh/trv003. [DOI] [PubMed] [Google Scholar]
- 2.van de Sande WW. 2013. Global burden of human mycetoma: a systematic review and meta-analysis. PLoS Negl Trop Dis 7:e2550. doi: 10.1371/journal.pntd.0002550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zijlstra EE, van de Sande WWJ, Welsh O, Mahgoub ES, Goodfellow M, Fahal AH. 2016. Mycetoma: a unique neglected tropical disease. Lancet Infect Dis 16:100–112. doi: 10.1016/S1473-3099(15)00359-X. [DOI] [PubMed] [Google Scholar]
- 4.van de Sande W, Fahal A, Ahmed SA, Serrano JA, Bonifaz A, Zijlstra E, Eumycetoma Working Group. 2018. Closing the mycetoma knowledge gap. Med Mycol 56:153–164. doi: 10.1093/mmy/myx061. [DOI] [PubMed] [Google Scholar]
- 5.Welsh O, Al-Abdely HM, Salinas-Carmona MC, Fahal AH. 2014. Mycetoma medical therapy. PLoS Negl Trop Dis 8:e3218. doi: 10.1371/journal.pntd.0003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reis CMS, Reis-Filho E. 2018. Mycetomas: an epidemiological, etiological, clinical, laboratory and therapeutic review. An Bras Dermatol 93:8–18. doi: 10.1590/abd1806-4841.20187075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van de Sande WW, de Kat J, Coppens J, Ahmed AO, Fahal A, Verbrugh H, van Belkum A. 2007. Melanin biosynthesis in Madurella mycetomatis and its effect on susceptibility to itraconazole and ketoconazole. Microbes Infect 9:1114–1123. doi: 10.1016/j.micinf.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins SG, Schuetz AN. 2012. Current concepts in laboratory testing to guide antimicrobial therapy. Mayo Clin Proc 87:290–308. doi: 10.1016/j.mayocp.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed SA, Kloezen W, Duncanson F, Zijlstra EE, de Hoog GS, Fahal AH, van de Sande WW. 2014. Madurella mycetomatis is highly susceptible to ravuconazole. PLoS Negl Trop Dis 8:e2942. doi: 10.1371/journal.pntd.0002942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van de Sande WW, Luijendijk A, Ahmed AO, Bakker-Woudenberg IA, van Belkum A. 2005. Testing of the in vitro susceptibilities of Madurella mycetomatis to six antifungal agents by using the Sensititre system in comparison with a viability-based 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5- [(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) assay and a modified NCCLS method. Antimicrob Agents Chemother 49:1364–1368. doi: 10.1128/AAC.49.4.1364-1368.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kloezen W, Meis JF, Curfs-Breuker I, Fahal AH, van de Sande WW. 2012. In vitro antifungal activity of isavuconazole against Madurella mycetomatis. Antimicrob Agents Chemother 56:6054–6056. doi: 10.1128/AAC.01170-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espinel-Ingroff A, Barchiesi F, Cuenca-Estrella M, Fothergill A, Pfaller MA, Rinaldi M, Rodriguez-Tudela JL, Verweij PE. 2005. Comparison of visual 24-hour and spectrophotometric 48-hour MICs to CLSI reference microdilution MICs of fluconazole, itraconazole, posaconazole, and voriconazole for Candida spp.: a collaborative study. J Clin Microbiol 43:4535–4540. doi: 10.1128/JCM.43.9.4535-4540.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed SA, van de Sande WW, Stevens DA, Fahal A, van Diepeningen AD, Menken SB, de Hoog GS. 2014. Revision of agents of black-grain eumycetoma in the order Pleosporales. Persoonia 33:141–154. doi: 10.3767/003158514X684744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vega B, Liberti D, Harmon PF, Dewdney MM. 2012. A rapid resazurin-based microtiter assay to evaluate QoI sensitivity for Alternaria alternata isolates and their molecular characterization. Plant Dis 96:1262–1270. doi: 10.1094/PDIS-12-11-1037-RE. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Liu H, Zhao J, Gao H, Zhou L, Liu Z, Chen Y, Sui P. 2010. Antimicrobial and antioxidant activities of the root bark essential oil of Periploca sepium and its main component 2-hydroxy-4-methoxybenzaldehyde. Molecules 15:5807–5817. doi: 10.3390/molecules15085807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Repp KK, Menor SA, Pettit RK. 2007. Microplate Alamar blue assay for susceptibility testing of Candida albicans biofilms. Med Mycol 45:603–607. doi: 10.1080/13693780701581458. [DOI] [PubMed] [Google Scholar]
- 17.van de Sande WW, Fahal AH, Riley TV, Verbrugh H, van Belkum A. 2007. In vitro susceptibility of Madurella mycetomatis, prime agent of Madura foot, to tea tree oil and artemisinin. J Antimicrob Chemother 59:553–555. doi: 10.1093/jac/dkl526. [DOI] [PubMed] [Google Scholar]
- 18.van Belkum A, Fahal AH, van de Sande WW. 2011. In vitro susceptibility of Madurella mycetomatis to posaconazole and terbinafine. Antimicrob Agents Chemother 55:1771–1773. doi: 10.1128/AAC.01045-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed AO, van de Sande WW, van Vianen W, van Belkum A, Fahal AH, Verbrugh HA, Bakker-Woudenberg IA. 2004. In vitro susceptibilities of Madurella mycetomatis to itraconazole and amphotericin B assessed by a modified NCCLS method and a viability-based 2,3-bis(2-methoxy-4-nitro-5- sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) assay. Antimicrob Agents Chemother 48:2742–2746. doi: 10.1128/AAC.48.7.2742-2746.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van de Sande WW, Fahal AH, Bakker-Woudenberg IA, van Belkum A. 2010. Madurella mycetomatis is not susceptible to the echinocandin class of antifungal agents. Antimicrob Agents Chemother 54:2738–2740. doi: 10.1128/AAC.01546-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heinekamp T, Thywißen A, Macheleidt J, Keller S, Valiante V, Brakhage AA. 2012. Aspergillus fumigatus melanins: interference with the host endocytosis pathway and impact on virulence. Front Microbiol 3:440. doi: 10.3389/fmicb.2012.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almeida-Paes R, Figueiredo-Carvalho MH, Brito-Santos F, Almeida-Silva F, Oliveira MM, Zancopé-Oliveira RM. 2016. Melanins protect Sporothrix brasiliensis and Sporothrix schenckii from the antifungal effects of terbinafine. PLoS One 11:e0152796. doi: 10.1371/journal.pone.0152796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Brien J, Wilson I, Orton T, Pognan F. 2000. Investigation of the Alamar blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem 267:5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- 24.Kim E, Kang M, Tschirhart T, Malo M, Dadachova E, Cao G, Yin JJ, Bentley WE, Wang Z, Payne GF. 2017. Spectroelectrochemical reverse engineering demonstrates that melanin’s redox and radical scavenging activities are linked. Biomacromolecules 18:4084–4098. doi: 10.1021/acs.biomac.7b01166. [DOI] [PubMed] [Google Scholar]
- 25.Rózanowska M, Sarna T, Land EJ, Truscott TG. 1999. Free radical scavenging properties of melanin interaction of eu- and pheo-melanin models with reducing and oxidising radicals. Free Radic Biol Med 26:518–525. doi: 10.1016/s0891-5849(98)00234-2. [DOI] [PubMed] [Google Scholar]