Miltefosine is an alkylphosphocholine compound that is used primarily for treatment of leishmaniasis and demonstrates in vitro and in vivo antiamebic activity against Acanthamoeba species. Recommendations for treatment of amebic encephalitis generally include miltefosine therapy. Data indicate that treatment with an amebicidal concentration of at least 16 μg/ml of miltefosine is required for most Acanthamoeba species.
Keywords: ameba, HIV, AIDS, miltefosine, cerebrospinal fluid
ABSTRACT
Miltefosine is an alkylphosphocholine compound that is used primarily for treatment of leishmaniasis and demonstrates in vitro and in vivo antiamebic activity against Acanthamoeba species. Recommendations for treatment of amebic encephalitis generally include miltefosine therapy. Data indicate that treatment with an amebicidal concentration of at least 16 μg/ml of miltefosine is required for most Acanthamoeba species. Although there is a high level of mortality associated with amebic encephalitis, a paucity of data regarding miltefosine levels in plasma and cerebrospinal fluid in vivo exists in the literature. We found that despite aggressive dosing (oral miltefosine 50 mg every 6 h) and therapeutic plasma levels, the miltefosine concentration in cerebrospinal fluid was negligible in a patient with AIDS and Acanthamoeba encephalitis.
INTRODUCTION
Acanthamoeba is a free-living ameba existing in fresh and salt water. Transmission is usually via nasal passages to the lower respiratory tract or through cutaneous lesions, with the risk for hematogenous spread to the central nervous system (CNS) resulting in granulomatous amebic encephalitis (1). Typically opportunistic in nature, Acanthamoeba infections are more commonly seen in immunocompromised hosts, such as individuals with AIDS or those who have undergone organ transplantation. There is no standard treatment for amebic encephalitis, which carries a high rate of mortality (1). However, a combination of therapeutic agents, including miltefosine, is recommended by the Centers for Disease Control and Prevention (CDC). Nonetheless, data regarding the plasma and cerebrospinal fluid (CSF) concentrations after administration of miltefosine for the treatment of amebic encephalitis in vivo are lacking.
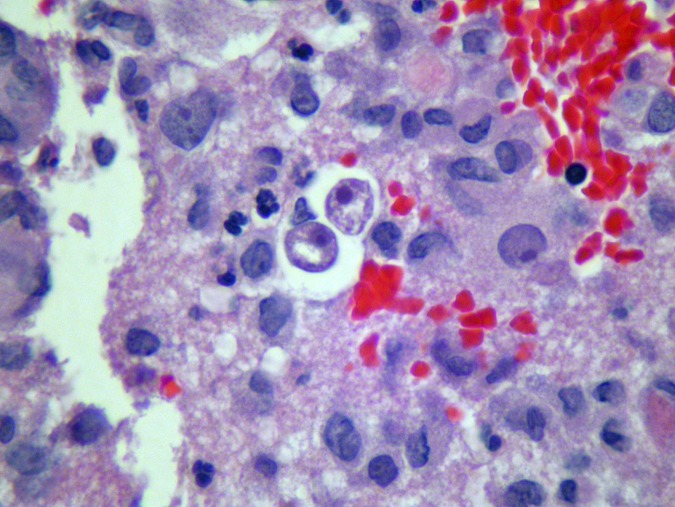
Acanthamoeba encephalitis was diagnosed via surgical biopsy specimen, with pathology revealing encapsulated cysts and trophozoites consistent with free-living ameba isolates (Fig. 1) in a 35-year-old man found to be positive for HIV (CD4 lymphocyte count of 30 cells/μl and HIV RNA by PCR of 95,900 copies/ml). Magnetic resonance imaging of the brain with gadolinium revealed multiple ring-enhancing lesions with hemorrhage and leptomeningeal enhancement along the basal cisterns. Before identification of the ameba species and after a review of the scant available literature for treatment of amebic encephalitis, the patient was started empirically on oral miltefosine 50 mg every 6 h, fluconazole 800 mg daily, and albendazole 400 mg daily and intravenous (i.v.) trimethoprim-sulfamethoxazole 320 mg every 6 h, rifampin 600 mg daily, and azithromycin 500 mg daily (1).
FIG 1.
Brain biopsy specimen demonstrating amebic trophozoites (hematoxylin and eosin stain, ×50 magnification).
Brain tissue from a surgical biopsy was sent to the CDC and further identified by immunohistochemistry and PCR as being infected with Acanthamoeba species a week after the initial empirical regimen was prescribed. Therefore, the treatment regimen was consolidated further to oral miltefosine 50 mg every 8 h, sulfadiazine 1.5 g every 6 h, flucytosine 37.5 mg/kg every 6 h, and fluconazole 12 mg/kg daily and i.v. pentamidine 4 mg/kg daily per CDC recommendations.
Plasma and CSF miltefosine concentrations were collected on day 7 of treatment after the patient had received higher-than-recommended doses of the medication (200-mg-total/day versus the recommended 150-mg/day regimen). A total of 25 doses (50 mg over 6 h) were administered before pharmacokinetic sampling. Quantification of miltefosine was performed using a liquid chromatography-tandem mass spectrometry method adapted from a previously published method (2). The method was validated on a Waters Xevo TQ-S triple quadrupole mass spectrometer coupled with a Waters Acquity UPLC I-class system. Because the purpose of this study was to quantify the levels of miltefosine in patient plasma and CSF, only the lower limit of quantification (LOQ), which is generally well above the detection limit, was determined during assay development. In this study, the LOQ of miltefosine in sample was 1 nM or, due to the 20-fold dilution during the extraction, 0.05 nM in the extract. The 0.007-μg/ml miltefosine concentration was equivalent to 0.02 nM miltefosine, assuming a molecular weight of 407.6 g/mol. The plasma concentration of miltefosine taken 8 h after the last dose on day 7 of therapy was 16.2 μg/ml, whereas the CSF concentration of miltefosine taken at the same time was only 0.007 μg/ml.
Despite surgical intervention and continued anti-infective therapies, the patient never regained neurological function and ultimately succumbed to his infection more than a month after initial presentation.
The pathogens Acanthamoeba and Balamuthia mandrillaris are free-living ameba isolates existing in fresh water. Infection can occur via inhalation of the organism through nasal passages with spread to the lower respiratory tract, direct inoculation of the eye causing keratitis, or skin contact resulting in cutaneous lesions, all of which can secondarily result in hematogenous spread to the CNS, leading to often-fatal granulomatous amebic encephalitis from Acanthamoeba or Balamuthia isolates (3). These are typically opportunistic pathogens affecting immunocompromised hosts, such as individuals with AIDS, as in the current case (4). There is no standard of treatment for amebic encephalitis, particularly that caused by Acanthamoeba infection, which has an extremely low rate of survival, especially in patients with concomitant HIV infection. In the literature, there are only three reported cases of patients with HIV/AIDS who survived Acanthamoeba infections, one of which involved use of miltefosine (3–6).
Miltefosine, an alkylphosphocholine compound, initially failed as an anticancer agent because of an intolerable side-effect profile. However, the agent’s life span was extended with the discovery of its antiprotozoal activity. Miltefosine is currently approved by the FDA for treatment of cutaneous, visceral, and mucosal leishmaniasis (7). Today, miltefosine is still used primarily for treatment of leishmaniasis, but further research has identified in vitro and in vivo antiamebic activity against Acanthamoeba species, making miltefosine a drug of interest for the treatment of amebic encephalitis (8, 9).
In vivo amebastatic activity has been observed with miltefosine exposures of 16.3 μg/ml (40 μM), with variation in sensitivity between strains (8, 10). Notably, drug concentrations of ≥16.3 μg/ml at the site of infection may be required because of Acanthamoeba spp. recovery at this concentration, which was not noted with a concentration of 32.6 μg/ml (80 μM) (10). Miltefosine concentrations of ≥16.3 μg/ml have been noted to be amebicidal (10). Together, these data support a minimum amebicidal concentration (MAC) of at least 16 μg/ml for most isolates (8, 10).
Standard dosing of miltefosine for the treatment of leishmaniasis in patients weighing >44 kg is 1 50-mg oral capsule 3 times daily. Notably, there are limited data on the effectiveness of this dose for the treatment of amebic encephalitis. Miltefosine is highly protein bound (95%) with poor CNS penetration (9). The CSF concentration of miltefosine in an 11-year-old child with amebic encephalitis was 0.4 μg/ml after 5 days of treatment (9), which was substantially lower than 16-μg/ml MAC (8, 10). Given the questionable blood-brain barrier penetration, unknown brain parenchyma accumulation, and >90% mortality of amebic encephalitis in this particular case, a miltefosine dose of 50 mg 4 times daily was administered for the first week of amebic encephalitis treatment, followed by the standard 50 mg 3 times daily for the remainder of treatment.
This is the first report to describe plasma and CSF concentrations of miltefosine after administration of a higher-than-recommended dose (200 mg/day) for the treatment of amebic encephalitis. The administration of high-dose miltefosine resulted in trough plasma concentrations (i.e., 8 h postdose) at or below the suggested MAC for Acanthamoeba spp. Furthermore, the miltefosine blood-brain penetration was dismal, with a plasma-to-CSF ratio of 2,310:1. Miltefosine human brain parenchyma concentrations (both total and unbound) have yet to be described in the literature, which warrants further investigation because CSF may not always be an accurate surrogate for unbound drug concentrations in the brain. In rats, the highest miltefosine concentrations were found in the kidney, adrenal glands, and spleen; after 18 days of miltefosine administration, the miltefosine brain concentration was 61 nmol/g, which was much lower than those in the kidney (1,390 nmol/g) and serum (160 nmol/g) (11).
High-dose miltefosine was initially investigated in several phase 1 and 2 studies for the treatment of solid tumors. Dose-limiting gastrointestinal adverse events resulted in discontinuation of the drug for these indications (12). During the course of high-dose therapy in the current case, gastrointestinal adverse events were not observed. Moreover, the patient’s creatinine level remained stable during the high-dose therapy. Mild elevations in liver enzymes were observed on admission, albeit the levels never reached more than 3 times the upper limit of normal during high-dose miltefosine treatment.
Miltefosine dosing recommendations for the treatment of amebic encephalitis currently follow leishmaniasis treatment recommendations. This report reveals that standard and higher-than-recommended doses of miltefosine are likely suboptimal for the treatment of amebic encephalitis. Further research is needed to assess miltefosine brain parenchyma penetration to evaluate the role of miltefosine in the treatment of amebic encephalitis caused by Acanthamoeba spp.
ACKNOWLEDGMENTS
We appreciate David E. Greenberg for assistance with storage and transport of the plasma and CSF samples and William N. Tharpe for assistance with miltefosine acquisition.
We have no conflicts of interest or external funding sources to report.
REFERENCES
- 1.Zamora A, Henderson H, Swiatlo E. 2014. Acanthamoeba encephalitis: a case report and review of therapy. Surg Neurol Int 5:68. doi: 10.4103/2152-7806.132239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dorlo TPC, Hillebrand MJX, Rosing H, Eggelte TA, de Vries PJ, Beijnen JH. 2008. Development and validation of a quantitative assay for the measurement of miltefosine in human plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 865:55–62. doi: 10.1016/j.jchromb.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Visvesvara G, Moura H, Schuster FL. 2007. Pathogenic and opportunistic free-living amoeba: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol 50:1–26. doi: 10.1111/j.1574-695X.2007.00232.x. [DOI] [PubMed] [Google Scholar]
- 4.Sahly H, Udayamurthy M, Parkerson G, Hasbun R. 2017. Survival of an AIDS patient after infection with Acanthamoeba sp. of the central nervous system. Infection 45:715–718. doi: 10.1007/s15010-017-1037-9. [DOI] [PubMed] [Google Scholar]
- 5.Carter WW, Gompf SG, Toney JF, Greene JN, Cutolo EP. 2004. Disseminated Acanthamoeba sinusitis in a patient with AIDS: a possible role for early antiretroviral therapy. AIDS Read 14:41–49. [PubMed] [Google Scholar]
- 6.Seijo Martinez M, Gonalez-Mediero G, Santiago P, Rodrigez de Lope A, Diz J, Conde C, Visvesvara GS. 2000. Granulomatous amebic encephalitis in a patient with AIDS: isolation of Acanthamoeba sp. group II from brain tissue and successful treatment with sulfadiazine and fluconazole. J Clin Microbiol 38:3892–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castro MM, Gomez MA, Kip AE, Cossio A, Ortiz E, Navas A, Dorlo TPC, Saravia NG. 2017. Pharmacokinetics of miltefosine in children and adults with cutaneous leishmaniasis. Antimicrob Agents Chemother 61:e02198-16. doi: 10.1128/AAC.02198-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walochnik J, Duchêne M, Seifert K, Obwaller A, Hottkowitz T, Wiedermann G, Eibl H, Aspöck H. 2002. Cytotoxic activities of alkylphosphocholines against clinical isolates of Acanthamoeba spp. Antimicrob Agents Chemother 46:695–701. doi: 10.1128/aac.46.3.695-701.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roy SL, Atkins JT, Gennuso R, Kofos D, Sriram RR, Dorlo TP, Hayes T, Qvarnstrom Y, Kucerova Z, Guglielmo BJ, Visvesvara GS. 2015. Assessment of blood-brain barrier penetration of miltefosine used to treat a fatal case of granulomatous amebic encephalitis possibly caused by an unusual Balamuthia mandrillaris strain. Parasitol Res 114:4431–4439. doi: 10.1007/s00436-015-4684-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuster FL, Guglielmo BJ, Visvesvara GS. 2006. In-vitro activity of miltefosine and voriconazole on clinical isolates of free-living amebas: Balamuthia mandrillaris, Acanthamoeba spp., and Naegleria fowleri. J Eukaryot Microbiol 53:121–126. doi: 10.1111/j.1550-7408.2005.00082.x. [DOI] [PubMed] [Google Scholar]
- 11.Marschner N, Kötting J, Eibl H, Unger C. 1992. Distribution of hexadecylphosphocholine and octadecyl-methyl-glycero-3-phosphocholine in rat tissues during steady-state treatment. Cancer Chemother Pharmacol 31:18–22. doi: 10.1007/bf00695989. [DOI] [PubMed] [Google Scholar]
- 12.Planting AST, Stoter G, Verweij J. 1993. Phase II study of daily oral miltefosine (hexadecylphosphocholine) in advanced colorectal cancer. Eur J Cancer 29:518–519. doi: 10.1016/S0959-8049(05)80142-X. [DOI] [PubMed] [Google Scholar]