Abstract
Objectives:
We consider whether it is the healthiest dementia caregivers who experience a mortality benefit and whether a protective association is consistent for leading causes of mortality.
Methods:
Using the Health and Retirement study (2000–2012), Cox survival models predict time to death for dementia caregivers, including an interaction between dementia caregiver status and self-rated health. The nationally representative sample consisted of 10,650 married adults aged 51 or older (917 dementia caregivers).
Results:
A significant interaction between dementia caregiver status and self-rated health suggested that relative to non-caregivers, dementia caregivers had reduced mortality, with this effect particularly strong at lower levels of self-rated health. The protective effect of dementia caregiver status was consistent across death by heart disease, cancer, and cerebrovascular disease.
Discussion:
These findings add to a growing body of literature suggesting that caregiving may provide a mortality benefit and a reason to maintain health.
Keywords: mortality, dementia caregiving, self-rated health
Despite favorable trends in dementia risk (Langa, 2015), the rapid growth in the elderly population in the United States (US) means that the number of persons living with dementia (PLWD) is rising dramatically. Caregiving for PLWD is intensive, and the caregiving costs exceed that of other common chronic conditions (Hurd, Martorell, Delavande, Mullen, & Langa, 2013). Caregiving for a PLWD can be rewarding, yet the negative impact on caregivers’ physical and mental health has been well-documented (Cuijpers, 2005; Pinquart & Sörensen, 2006). Aspects of the caregiving role may be stressful, from role overload to helping with challenging care tasks. Schulz and Beach’s (1999) seminal study reported that caregivers experiencing strain had a 63% higher mortality risk than same-aged peers who were not caregivers. Since this foundational work, however, a number of studies have found that caregiving is associated with lower mortality risk (Brown et al., 2009; Caputo, Pavalko, & Hardy, 2016; Fredman, Lyons, Cauley, Hochberg, & Applebaum, 2015; Maguire, Rosato, & O’Reilly, 2016; O’Reilly, Connolly, Rosato, & Patterson, 2008; O’Reilly, Rosato, Maguire, & Wright, 2015; Ramsay, Grundy, & Reilly, 2013; Roth, Fredman, & Haley, 2015). Given the expected future increase in the number of PLWD, it will be increasingly important to expand our understanding of the risks and potential benefits to individuals and society of caring for PLWD. This study is the first to investigate mortality risks, all-cause and leading causes of death, for caregivers of PLWD in a national sample of older US adults.
Caregiving and mortality.
Stress is commonly reported by caregivers of PLWD (e.g., 59% report high levels of emotional distress and thus serves as a posited mediator whereby caregiving may impact mortality (Alzheimer’s Association, 2017). Studies examining caregiver stress in association with differential mortality outcomes offer mixed results, with some suggesting that it is the effects of stress broadly as opposed to caregiving specifically that impacts mortality (Fredman et al., 2010; Pinquart & Sorensen, 2007; Schulz & Beach, 1999). Yet, an increasing number of studies are finding lower mortality risk among caregivers in spite of increased stress and depression (Brown et al., 2009; Caputo et al., 2016; Fredman et al., 2015; Maguire et al., 2016; O’Reilly et al., 2008; O’Reilly et al., 2015; Ramsay et al., 2013; Roth, Brown, Rhodes, & Haley, 2018; Roth et al., 2015). Studies have assessed the intensity of the caregiving situation as associated with mortality, with even the most intensive care situations (50+ hours) showing reduced mortality (Brown et al., 2009; Maguire et al., 2016; O’Reilly et al., 2015). This benefit has been found over short and long-term follow-ups and relative to multiple comparison groups- even relative to those residing with a PLWD just not providing care (Maguire et al., 2016). A benefit may be due to a healthy survivor effect; whereby less healthy older adults are lost to follow-up. However an additional explanation may be the Healthy Caregiver Hypothesis, which suggests that healthy individuals enter the caregiving role and that caregiving may subsequently maintain health (Bertrand et al., 2011; Fredman et al., 2010).
Yet prior research, has found a caregiving survival advantage even among those with the poorest initial health status. For example, Ramsay and colleagues found that caregivers who had worse health initially had lower mortality over time than non-caregivers (Ramsay et al., 2013). While some of these studies have controlled for health of the caregiver as a correlate of caregiver mortality, no study has investigated the intersection of health status and dementia care on mortality, particularly in a nationally representative population of US older adults. Thus, we sought to consider the role of the Healthy Caregiver Hypothesis whereby varying levels of caregiver health may be maintained by care activity, or vary in terms of how caregiving takes its toll. This is important because the activity of caregiving may promote health maintenance even among those in the poorest health to enable them to continue in their caregiver role.
Leading causes of death and mortality.
Another limitation of prior studies is a focus on all-cause mortality which may lead to contrasting findings due to the great variability in cause of death. For example, among leading causes of death, caregivers may not be at increased risk of death by cancer or kidney disease, whereas they may be at greater risk of death from heart disease for which stress is a key risk factor. While a range of studies have examined caregiving as associated with increased risk for many of the most common chronic conditions (Fredman et al., 2010; Haley, Roth, Howard, & Safford, 2010; Lee, Colditz, Berkman, & Kawachi, 2003; Roth et al., 2013; Von Känel et al., 2008), few have investigated death from those diseases associate with caregiving (O’Reilly et al., 2015) and none in the context of dementia.
Mortality is an advantageous measure to assess the health effects of caregiving as it does not carry self-report bias and is of clear public health concern. Caregivers must maintain their own health to continue care provision. Further, just as the beneficial association of caregiving on mortality has been shown across levels of care intensity, we sought a more nuanced understanding of how health status may intersect with caregiving and mortality, namely, is the protective effect of caregiving present for all levels of health? We consider spousal caregivers for a PLWD, specifically. Dementia is a topic of growing concern and importance as it is a chronic condition resulting in high care need and stress for caregivers relative to other conditions. Moreover, dementia caregivers may be simultaneously dealing with their own health concerns (Alzheimer’s Association, 2017; Caregiving & Institute, 2015; Oldenkamp et al., 2016; Polenick et al., 2017). Underscoring the importance of considering spouses as a risk group, a meta-analysis found that spouse caregivers tend to report more depressive symptoms than adult child caregivers (Pinquart & Sörensen, 2011). The current study used data from a nationally representative sample of older adults to examine two research hypotheses. First, building on the Healthy Caregiver Hypothesis, we posit that the strength of association between caregiving for PLWD and mortality varies by level of self-rated health (Hypothesis 1). We also hypothesize that caregiving will have less benefit for leading causes of mortality related to the stress process (e.g., cardiovascular disease) (Hypothesis 2).
Methods
Sample
Our sample consisted of 10,650 married adults drawn from the Health and Retirement Study (HRS), a biennial longitudinal survey of a nationally representative US sample over the age of 50. In this study, we define three categories related to caregiving: non-caregivers, non-dementia caregivers, and dementia caregivers. Non-caregivers are respondents whose spouse did not report receiving help with activities of daily living (ADLs) (getting across a room, dressing, bathing, eating, getting in/out of bed, using the toilet) at any wave between 2000 and 2012. Non-dementia caregivers are those whose spouse reported that they were aided by the caregiver (their spouse) with an ADL in any wave between 2000 and 2012, and whose spouse was not classified as having dementia across any wave under study. Dementia caregivers are those whose spouse reported that they were aided by the caregiver (their spouse) with an ADL in any given wave under study, and whose spouse was classified as having dementia at the same wave in which they (the dementia caregiver) provided ADL support. If the interview is done by proxy, the proxy respondent would indicate this information.
Dementia status was identified following the Langa-Weir classification cut-points of normal, cognitively impaired but not demented, and demented based on tests of immediate and delayed recall, working memory, speed of mental processing (Crimmins, Kim, Langa, & Weir, 2011; Langa, Weir, Kabeto, & Sonnega, 2018).
Both HRS public release and RAND HRS data were utilized (Bugliari et al., 2018). The HRS features a multi-stage area probability sampling design with clustering and geographic stratification with oversampling of Black and Hispanic households. HRS is funded by the National Institute on Aging (NIA U01AG0097) and housed at the University of Michigan Institute for Social Research. All participants provided written informed consent, and the study was approved by the UM Institutional Review Board.
Measures
Outcome.
HRS determines mortality status through a linkage with the National Death Index (NDI), a national database which includes information on cause and date of death. Time-to-death in months was calculated from 2000 (or first wave of reported caregiving for a spouse with dementia) to the NDI reported date of death for all-cause mortality (Hypothesis 1) and for three specific causes of death: heart disease, cancer, and cerebrovascular disease (Hypothesis 2).
Effect modifier.
Self-reported health status was a ranking of five health categories: excellent, very good, good, fair or poor. For Hypothesis 1, we evaluated the intersection of self-rated health status and dementia caregiving status by creating a 10 category variable with the following categories: non-caregiver in excellent health, non-caregiver in very good health, non-caregivers in good health, non-caregivers in fair health, non-caregiver in poor health, dementia caregiver in excellent health, dementia caregiver in very good health, dementia caregiver in good health, dementia caregiver in fair health, and dementia caregiver in poor health. The referent category was non-caregivers with excellent self-reported health. For Hypothesis 2, dementia caregiver status was the exposure of interest.
Demographic covariates.
We included controls for age, gender, race (White/Black/Other), and widowed, namely, whether the respondent was widowed over the course of the follow-up period.
Health covariates.
Other health factors known to be associated with mortality were controlled including: a history of smoking, depressive symptoms, and activities of daily living (ADLs). Depressive symptoms were measured with an 8-item version of the Center for Epidemiologic Studies Depression (CESD) scale (Steffick, 2000). Participants reported the extent in the past week to which they felt: depressed, could not get going, everything was an effort, lonely, enjoyed life, sleep was restless, sad, and happy. RAND dichotomizes these because of response category differences across waves. Positive items were recoded, and a sum of depressive symptoms was created ranging from 0 to 8 (Zivin et al., 2010). The RAND measure of participant’s ADLs (from RAND) includes a dichotomized (some or greater versus no difficulty) count of five activities: bathing, dressing, eating, getting in and out of bed, and walking across a room (Wallace & Herzog, 1995). All covariates were taken from the participant’s initial wave of data in the case of non-caregivers, and from the first reported wave of dementia caregiving or non-dementia caregiving.
Data Analysis
Sample characteristics were estimated for the full sample, non-caregivers, non-dementia caregivers, and dementia caregivers. Next, Cox proportional hazards models were run in STATA with the outcome time-to-death. Time-to-death or censoring was measured starting from 2000 (or first wave of reported caregiving for a spouse with dementia) to the final available NDI data (2012). In Model 1, we estimated hazard ratios (HR) to evaluate the main effects of caregiving by comparing dementia caregivers and non-dementia caregivers relative to non-caregivers. Participants who were alive in 2012 and those attrited from the study were censored at their last available wave. We addressed Hypothesis 1 in two ways: First, we tested the overall interaction and report the omnibus test for that interaction in the text. In Model 2, we further report hazard ratios for each of 10 possible combinations of caregiving status and self-rated health controlling for demographics, smoking status, depressive symptoms, and ADLs. Note that non-dementia caregivers were considered for comparison in our Model 1 considering the main effect of dementia caregiving on mortality, but our main hypothesis (evaluated in Model 2) tested an interaction between dementia caregivers relative to non-caregivers.
Addressing Hypothesis 2, a second set of models considered dementia caregiver status as a predictor of leading causes of mortality (i.e., cardiovascular disease, cancer, cerebrovascular disease) in a competing risks framework. Competing risks analysis is a generalization of standard survival analysis in instances where there may be multiple causes of failure (in this case, causes of death) (Fine & Gray, 1999). When analyzing cause-specific mortality, death due to an alternative cause than the cause of interest precludes death which might otherwise have occurred due to the cause of interest. If the competing causes are correlated or dependent, it could distort cause-specific mortality estimates. We estimated cause-specific hazards for each of the potential causes, which is the instantaneous risk of death from the specific cause given that death from any cause has not yet happened.
Analytical sample weights were used to account for the complex sample design and non-response, except in the latter set of models since the SVY module in STATA is not available for competing risk models.
Results
In this large population-based sample of older adults, 8.6% (n=917) reported having served as a dementia caregiver. Relative to non-dementia caregivers and non-caregivers, dementia caregivers were older, a larger proportion were female and White, had more depressive symptoms, and a smaller proportion had a history of smoking. Further, 53% were widowed over follow-up relative to 15.2% being widowed among the non-caregiver group. Over the 12 years of follow-up, 26% of dementia caregivers died compared to 28% of non-caregivers. The primary causes of dementia caregiver death included heart disease (31.6%), cancer (21.3%), and other causes (15.8%). Complete sample characteristics can be found in Table 1.
Table 1.
Sample Characteristics
| Total Sample | Non - Caregiver | Non-dementia Caregiver | Dementia Caregiver | |
|---|---|---|---|---|
| N=10,650 | N=7841 | N=1892 | N=917 | |
| Sample Characteristics | N(%) | N(%) | N(%) | N(%) |
| Age | ||||
| 51–64 | 5310 (49.86) | 4176 (53.26) | 850 (44.93) | 284 (30.97) |
| 65–74 | 3453 (32.42) | 2437 (31.08) | 652 (34.46) | 364 (39.69) |
| 75–84 | 1604 (15.06) | 1024 (13.06 | 346 (18.29) | 234 (25.52) |
| 85+ | 283 (2.66) | 204 (2.60) | 44 (2.33) | 35 (3.82) |
| Female | 7401 (69.49) | 5341 (68.12) | 1345 (71.09) | 715 (77.97) |
| Race | ||||
| Caucasian American | 9258 (86.93) | 6786 (86.55) | 1644 (86.89) | 828 (90.29) |
| African American | 1125 (10.56) | 849 (10.83) | 203 (10.73) | 73 (7.96) |
| Other | 267 (2.51) | 206 (2.63) | 45 (2.38) | 16 (1.74) |
| History of smoking | 6303 (59.23) | 4744 (60.56) | 1098 (58.06) | 461 (50.27) |
| Depressive symptoms (0–8) | 1.42 (1.80) | 1.20 (1.68) | 1.85 (1.90) | 2.36 (2.07) |
| Activities of Daily Living limitations (0–5) | 0.23 (0.74) | 0.23 (0.76) | 0.25 (0.71) | 0.34 (0.83) |
| Self-rated health (1–5) | ||||
| Excellent | 1400 (13.15) | 1192 (15.20) | 146 (7.72) | 62 (6.76) |
| Very good | 3539 (33.23) | 2580 (32.91) | 625 (33.03) | 334 (36.42) |
| Good | 3385 (31.79) | 2396 (30.56) | 672 (35.52) | 317 (34.57) |
| Fair | 1629 (15.30) | 1183 (15.09) | 319 (16.86) | 127 (13.85) |
| Poor | 696 (6.54) | 489 (6.24) | 130 (6.87) | 77 (8.40) |
| Widowed | 2280 (21.41) | 1193 (15.21) | 601 (31.77) | 486 (53.00) |
| Percent died during 12 years of follow-up | 2916 (27.4) | 2218 (28.29) | 457 (24.15) | 241 (26.28) |
| Median survival (yrs)1 | 10.26 | 10.18 | 10.34 | 10.34 |
Notes. n=10,650 ;
Note that survival time is bounded by 12 year follow-up with the majority of participants surviving over this period.
Age, smoking history, and functional disability were associated with greater risk of death whereas having been widowed over follow-up and serving as a dementia caregiver or non-dementia caregiver was associated with lower risk of mortality (dementia caregiver HR=0.71, 95% CI=0.63–0.80; non-dementia caregiver HR=0.74, 95% CI=0.62–0.89; see Table 2, Model 1). Considering Hypothesis 1, the overall interaction between dementia care and self-rated health was statistically significant as indicated by the omnibus test (F(4,52)=38.61, p<.01) suggesting that dementia caregiver status statistically reduced risk for mortality, particularly at lower levels of self-rated health. Given the presence of a significant interaction, we stratified models according to the effect modifier, self-rated health status. Figure 1 depicts the comparison in hazard ratios of mortality between dementia caregivers and non-caregivers stratified by self-rated health. Model 2 in Table 2 shows the intersection of dementia caregiver status and level of self-rated health. Relative to non-caregivers with excellent health (referent category), non-caregivers had significantly higher hazards of death, each decreasing level of self-rated health associated with an increased hazard of death, respectively (Hazards ranging from 1.35 to 4.78). On the other hand, only dementia caregivers who reported good and fair health had a significantly higher hazard of death relative to the referent category, non-caregivers with excellent health (dementia caregiver’s hazards ranging from 0.72–2.18). Relative to the referent group, non-caregivers and dementia caregiver’s hazards were in sharp contrast particularly at lower levels of self-rated health (e.g. compared to the referent of non-caregivers in excellent health, the hazard of death for non-caregivers in poor health was 4.78 whereas for dementia caregivers in poor health it was 1.51).
Table 2.
Hazard Ratios for the Interaction between Provision of Dementia Care and Levels of Self-Rated Health Predicting All-Cause Mortality
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Age | ||||
| 51–64 (ref.) | -- | -- | -- | -- |
| 65–74 | 3.13 | (2.48 – 3.95) | 3.18 | (2.52 – 4.02) |
| 75–84 | 8.44 | (7.45 – 9.56) | 8.51 | (7.38 – 9.81) |
| 85+ | 19.27 | (17.31 – 21.44) | 18.97 | (16.42 – 21.92) |
| Female | 1.18 | (1.02 – 1.37) | 1.10 | (0.96 – 1.26) |
| Black | 0.90 | (0.73 – 1.11) | 0.88 | (0.71 – 1.10) |
| Other | 0.88 | (0.60 – 1.29) | 0.79 | (0.53 – 1.18) |
| Smoking History | 1.50 | (1.38 – 1.63) | 1.51 | (1.35 – 1.70) |
| Depressive Symptoms | 1.03 | (0.99 – 1.06) | 1.03 | (1.00 – 1.07) |
| ADL limitations | 1.19 | (1.13 – 1.26) | 1.15 | (1.05 – 1.25) |
| Widowed | 0.42 | (0.39 – 0.45) | 0.40 | (0.36 – 0.43) |
| Self-rated health | ||||
| Excellent (ref.) | -- | -- | -- | -- |
| Very good | 1.16 | (0.83 – 1.63) | -- | -- |
| Good | 1.70 | (1.37 – 2.12) | -- | -- |
| Fair | 2.71 | (2.04 – 3.61) | -- | -- |
| Poor | 3.74 | (2.52 – 5.57) | -- | -- |
| Caregiving status | ||||
| Non caregiver | -- | -- | -- | -- |
| Dementia caregiver | 0.71 | (0.63 – 0.80) | -- | -- |
| Non-dementia caregiver | 0.74 | (0.62 – 0.89) | -- | -- |
| Self-rated health by caregiver status | ||||
| Non-caregiver excellent health | -- | -- | -- | -- |
| Non-caregiver very good health | -- | -- | 1.35 | (1.04 – 1.75) |
| Non-caregiver good health | -- | -- | 1.69 | (1.26 – 2.27) |
| Non-caregiver fair health | -- | -- | 3.00 | (1.96 – 4.59) |
| Non-caregiver poor health | -- | -- | 4.78 | (2.79 – 8.19) |
| Dementia caregiver excellent health | -- | -- | 1.23 | (0.79 – 1.94) |
| Dementia caregiver very good health | -- | -- | 0.72 | (0.47 – 1.12) |
| Dementia caregiver good health | -- | -- | 1.98 | (1.40 – 2.81) |
| Dementia caregiver fair health | -- | -- | 2.18 | (1.65 – 2.88) |
| Dementia caregiver poor health | -- | -- | 1.51 | (0.85 – 2.70) |
Notes. Model 1 n=10,650, Model 2 excluding other caregivers n=8,758; Results are adjusted for all other covariates in the model.
Figure 1.
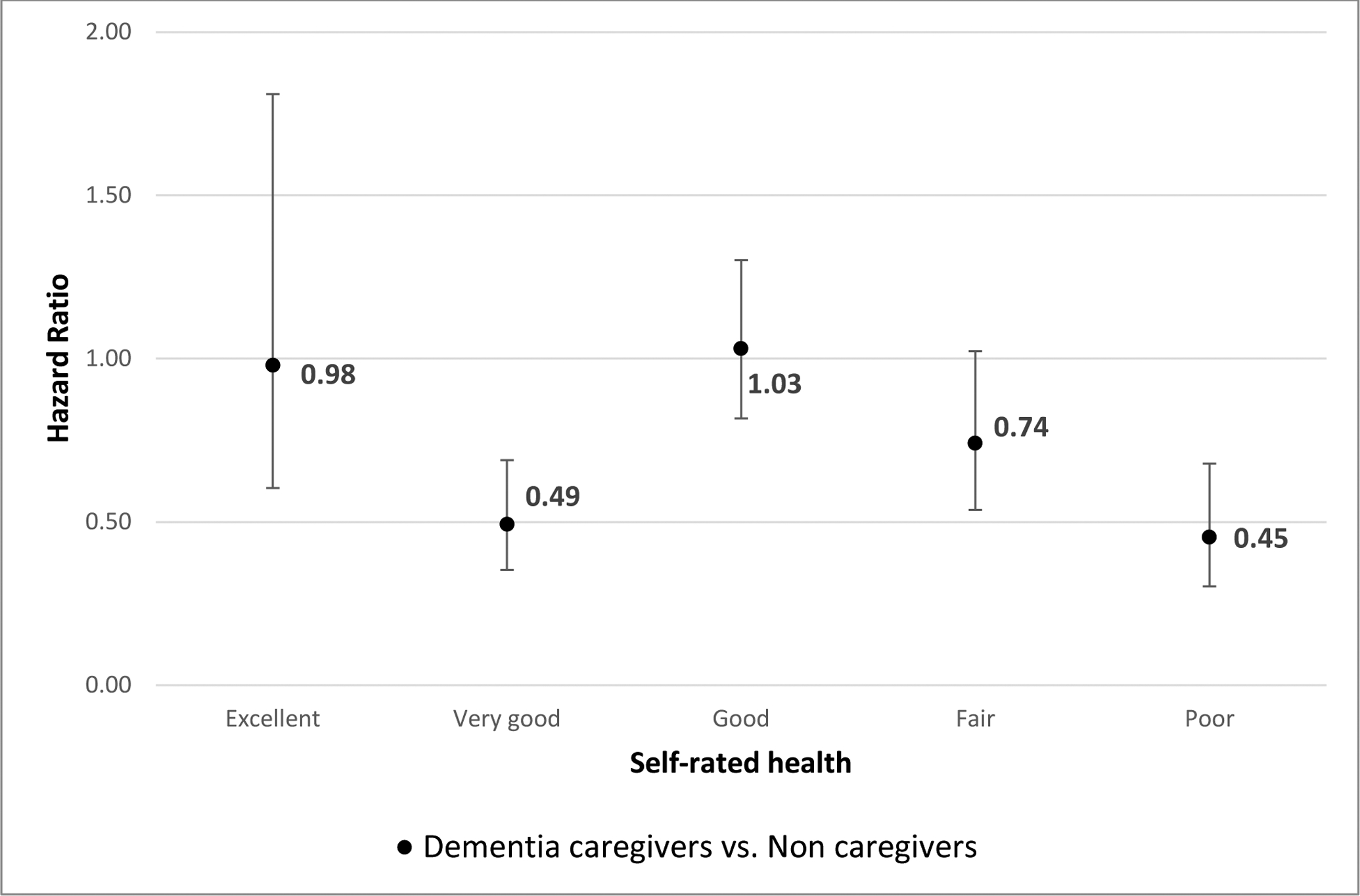
Model estimated hazard ratio of death comparing dementia caregivers and non-caregivers, stratified by self-rated health
Note: Reference category is non-caregivers with the same level of self-reported health
In addition, results of the competing risks analyses (Hypothesis 2) showed that, controlling for demographic and health characteristics, the protective association of dementia care was consistent for heart disease (HR: 0.77, CI: 0.59–0.99), cancer (HR: 0.79, CI: 0.59–1.05), and cerebrovascular disease (HR: 0.69; CI: 0.36–1.30) mortality.
Discussion
Considering married adults from the nationally representative HRS, we found that dementia caregiving was associated overall with a lower hazard of death, and this association was moderated by self-rated health. Specifically, we found support for Hypothesis 1 with a significant dementia caregiver by self-rated health interaction. The difference in hazard of death between dementia caregivers and non-caregivers was greatest among individuals reporting poorer self-reported health. Contrary to Hypothesis 2, this beneficial mortality association for dementia caregiving was also seen across three leading causes of death, so it does not seem to be specific to a certain pathology or disease.
Overall, our findings of a mortality benefit for dementia caregiving is in contrast with a wealth of social science research that describes the increased burden, strain, and depression faced by many caregivers for persons living with dementia, which would seemingly translate into a greater risk of death (Cuijpers, 2005; Pinquart & Sörensen, 2006). Prior work has shown that caregivers have increased risk for a number of chronic health conditions associated with mortality such as cardiovascular disease (O’Reilly et al., 2015). And while research has shown the advantages to health of marriage in late adulthood, spousal caregivers commonly report the greatest stress (Kaplan & Kronick, 2006). Thus, comparing spousal caregivers against spousal non-caregivers may be comparing the most stressed versus the healthiest, which makes a beneficial mortality association for dementia caregivers particularly striking (Fredman et al., 2010). Yet our findings do align with other studies which suggest caregivers have a reduced mortality risk even in those with poor baseline health (O’Reilly et al., 2008; O’Reilly et al., 2015; Ramsay et al., 2013).
Our findings may make sense in the context of research showing a number of salutogenic effects of caregiving that may increase resilience in spite of caregiver stress (Cohen, Colantonio, & Vernich, 2002; Lopez, Lopez-Arrieta, & Crespo, 2005). Caregiving may give spouses a reason to maintain their health to be able to provide the intensive care that dementia requires (Kaplan & Kronick, 2006). However, for those already in good health, caregiving may have less room to further improve health (relating to the statistical concept of regression to the mean), and thus the biggest impact can be seen among those with the greatest margin for health improvement. As a similar example of health promotion, the Experience Corps intervention, which places older adult volunteers in schools, found improved mental and physical well-being in its volunteers. The physical activity improvements were particularly seen in volunteers with low activity at baseline (Tan, Xue, Li, Carlson, & Fried, 2006). It was hypothesized that the activity and life purpose the new volunteer role brings may make positive improvements in health (Hong & Morrow-Howell, 2010). It may be that spousal caregiving plays a similar role whereby those with current good health have little to gain in the way of improvement (i.e., regression to the mean), yet those with poor health stand to gain greatly from increased life purpose, commitment and physical activity that the care role entails. In spite of caregiving stress, for caregivers in poor health, gains may outweigh stresses in regards to lifespan. In support of this, prior work on dementia caregivers found that caregivers report benefits and rewards in the caregiving role (Fisher Gwenith et al., 2011).
Other benefits of caregiving may help compensate for the risks and associated stress. Caregivers may have a greater sense of life purpose. For example, reduced mortality has been found for older adults who report that they feel useful (Gruenewald, Karlamangla, Greendale, Singer, & Seeman, 2007). Erik Erikson’s developmental stages theory is also relevant as the middle-adulthood to early late-life state, which most caregivers are positioned within, is stage 7 or “generativity versus stagnation” whereby Erikson posits that it is critical for adults to give back to continue developing successfully. Helping behaviors may be a form of resilience against stressors for older adults. Brown, Brown, and Preston (2012) postulate a “caregiver system model” that contains emotions, cognition, and neurophysiology as factors which motivate helping behaviors and promote health (Poulin, Brown, Dillard, & Smith, 2013). Other studies of caregiver mortality have also posited such positive models including prosocial helping behaviors and the stress buffering hypothesis (Brown & Brown, 2015; Roth et al., 2018).
Furthermore, accounting for competing risks, a similar protective association of dementia caregiving was found for three leading causes of mortality: cancer, heart disease, and cerebrovascular disease. While dementia caregiving did not significantly protect against cancer and cerebrovascular disease deaths, (the smaller sample sizes of these models may increase the confidence intervals) the hazards still suggest a protective effect. This suggests that the caregiving effect is not solely associated with stress-related causes of death but applies more broadly.
Limitations.
While the current study uses national, longitudinal data of older adults and includes the gold standard of mortality reports (the NDI), several limitations should be noted. First, the temporality of caregiving is variable in the current study. Though we start caregivers’ time from the initial wave in which they report serving as a caregiver, we do not have data on the start and stop date of caregiving and thus cannot account for length of the caregiving role or the amount of time between exiting the caregiving role and mortality. Future work should attend to selection effects and reverse causation to consider whether healthier individuals enter and stay in a caregiving role because those with poor health have already attrited from the population.
Further, while self-reported health is an imperfect proxy for actual health, there was good variability in the sample using this item and our controlling for ADLs accounts for functional ability. As chronic medical conditions are collinear with mortality (e.g., cardiovascular disease is perfectly related to death by cardiovascular disease), we felt that self-rated health was the best proxy for an individual’s overall health status. Sample restriction limited exploration of other, lower frequency causes of death. Finally, HRS has a breadth of data on psychosocial and health information of middle-aged and older adults, but does not have a particular focus on caregiving. Specific aspects which may be posited to impact mortality (such as perceived burden of caregiving) are not included in the data and may be important to consider in future studies.
Finally, in contrast with prior work which generally has found an increased risk of mortality following spousal bereavement, we find a significant protective association of being widowed over follow-up (King, Lodwick, Jones, Whitaker, & Petersen, 2017; Prior et al., 2017). This finding may be an artifact of confounding by caregiver status (53% of dementia caregivers were widowed relative to 15% of non-caregivers) or study design. For example, widows may have less follow-up time relative to married participants, potentially resulting in an artificially lower risk of mortality. Future work should consider this effect in greater detail and over varying lengths of follow-up.
In conclusion, these findings add to a growing body of literature suggesting that caregiving may provide a mortality benefit. We uniquely find that this protective association spans causes of death and is strongest amongst those reporting poor health. Clinicians and researchers should keep in mind the potential health and mental health risks of caregiving for PLWD, but also emphasize the benefits and growth that may result from a care role in later life. Maintaining activity and well-being in spite of declining health may be life-extending for both care partners.
References
- Alzheimer’s Association. (2017). Alzheimer’s Disease Facts and Figures. Alzheimer’s and Dementia, 13, 325–373. [DOI] [PubMed] [Google Scholar]
- Bertrand RM, Saczynski JS, Mezzacappa C, Hulse M, Ensrud K, & Fredman L (2011). Caregiving and Cognitive Function in Older Women: Evidence for the Healthy Caregiver Hypothesis. Journal of Aging and Health, 24(1), 48–66. doi: 10.1177/0898264311421367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SL, & Brown RM (2015). Connecting prosocial behavior to improved physical health: Contributions from the neurobiology of parenting. Neuroscience & Biobehavioral Reviews, 55, 1–17. doi: 10.1016/j.neubiorev.2015.04.004 [DOI] [PubMed] [Google Scholar]
- Brown SL, Brown RM, & Preston S (2012). The human caregiving system: A neuroscience model of compassionate motivation and behavior In Brown S, Brown R, & Penner L (Eds.), Moving beyond self-interest: Perspectives from evolutionary biology, neuroscience, and the social sciences (pp. 75–88). New York: Oxford University Press. [Google Scholar]
- Brown SL, Smith DM, Schulz R, Kabeto MU, Ubel PA, Poulin M, … Langa KM (2009). Caregiving Behavior Is Associated With Decreased Mortality Risk. Psychological Science, 20(4), 488–494. doi: 10.1111/j.1467-9280.2009.02323.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugliari D, Campbell N, Chan C, Hayden O, Hayes J, & Hurd M (2018). RAND HRS Longitudinal File 2014 (V2) Documentation. Retrieved from Santa Monica, CA: [Google Scholar]
- Caputo J, Pavalko EK, & Hardy MA (2016). The Long-Term Effects of Caregiving on Women’s Health and Mortality. Journal of Marriage and Family, 78(5), 1382–1398. doi: 10.1111/jomf.12332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caregiving, N. A. f., & Institute, A. P. P. (2015). Caregiving in the U.S Retrieved from https://www.aarp.org/content/…/caregiving-in-the-united-states-2015-report-revised.pdf
- Cohen CA, Colantonio A, & Vernich L (2002). Positive aspects of caregiving: rounding out the caregiver experience. International Journal of Geriatric Psychiatry, 17(2), 184–188. doi: 10.1002/gps.561 [DOI] [PubMed] [Google Scholar]
- Crimmins EM, Kim JK, Langa KM, & Weir DR (2011). Assessment of Cognition Using Surveys and Neuropsychological Assessment: The Health and Retirement Study and the Aging, Demographics, and Memory Study. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 66B(suppl 1), i162–i171. doi: 10.1093/geronb/gbr048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuijpers P (2005). Depressive disorders in caregivers of dementia patients: A systematic review. Aging & Mental Health, 9(4), 325–330. doi: 10.1080/13607860500090078 [DOI] [PubMed] [Google Scholar]
- Elwert F, & Christakis NA (2008). The Effect of Widowhood on Mortality by the Causes of Death of Both Spouses. American Journal of Public Health, 98(11), 2092–2098. doi: 10.2105/AJPH.2007.114348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine JP, & Gray RJ (1999). A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association, 94(446), 496–509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- Fisher G, Franks M, Plassman BL, Brown SL, Potter G, Llewellyn D, … Langa KM (2011). Caring for Individuals with Dementia and Cognitive Impairment, Not Dementia: Findings from the Aging, Demographics, and Memory Study. Journal of the American Geriatrics Society, 59(3), 488–494. doi: 10.1111/j.1532-5415.2010.03304.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredman L, Cauley JA, Hochberg M, Ensrud KE, Doros G, & for the Study of Osteoporotic, F. (2010). Mortality Associated with Caregiving, General Stress, and Caregiving-Related Stress in Elderly Women: Results of Caregiver-Study of Osteoporotic Fractures. Journal of the American Geriatrics Society, 58(5), 937–943. doi: 10.1111/j.1532-5415.2010.02808.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredman L, Lyons JG, Cauley JA, Hochberg M, & Applebaum KM (2015). The Relationship Between Caregiving and Mortality After Accounting for Time-Varying Caregiver Status and Addressing the Healthy Caregiver Hypothesis. The Journals of Gerontology: Series A, 70(9), 1163–1168. doi: 10.1093/gerona/glv009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenewald TL, Karlamangla AS, Greendale GA, Singer BH, & Seeman TE (2007). Feelings of Usefulness to Others, Disability, and Mortality in Older Adults: The MacArthur Study of Successful Aging. The Journals of Gerontology: Series B, 62(1), P28–P37. doi: 10.1093/geronb/62.1.P28 [DOI] [PubMed] [Google Scholar]
- Haley WE, Roth DL, Howard G, & Safford MM (2010). Caregiving Strain and Estimated Risk for Stroke and Coronary Heart Disease Among Spouse Caregivers. Differential Effects by Race and Sex, 41(2), 331–336. doi: 10.1161/strokeaha.109.568279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SI, & Morrow-Howell N (2010). Health outcomes of Experience Corps®: A high-commitment volunteer program. Social Science & Medicine, 71(2), 414–420. doi: 10.1016/j.socscimed.2010.04.009 [DOI] [PubMed] [Google Scholar]
- Hurd MD, Martorell P, Delavande A, Mullen KJ, & Langa KM (2013). Monetary costs of dementia in the United States. New England Journal of Medicine, 368(14), 1326–1334. doi: 10.1056/NEJMsa1204629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RM, & Kronick RG (2006). Marital status and longevity in the United States population. Journal of Epidemiology and Community Health, 60(9), 760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M, Lodwick R, Jones R, Whitaker H, & Petersen I (2017). Death following partner bereavement: A self-controlled case series analysis. PLoS One, 12(3), e0173870. doi: 10.1371/journal.pone.0173870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langa KM (2015). Is the risk of Alzheimer’s disease and dementia declining? Alzheimer’s Research and Therapy, 7(1), 34. doi: 10.1186/s13195-015-0118-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langa KM, Weir DR, Kabeto M, & Sonnega A (2018). Langa-Weir Classification of Cognitive Function (1995 onward). Retrieved from Institute for Social Research: [Google Scholar]
- Lee S, Colditz GA, Berkman LF, & Kawachi I (2003). Caregiving and risk of coronary heart disease in U.S. women. American Journal of Preventive Medicine, 24(2), 113–119. doi: 10.1016/S0749-3797(02)00582-2 [DOI] [PubMed] [Google Scholar]
- Lopez J, Lopez-Arrieta J, & Crespo M (2005). Factors associated with the positive impact of caring for elderly and dependent relatives. Arch Gerontol Geriatr, 41(1), 81–94. doi: 10.1016/j.archger.2004.12.001 [DOI] [PubMed] [Google Scholar]
- Maguire A, Rosato M, & O’Reilly D (2016). Mental health and morbidity of caregivers and co-residents of individuals with dementia: a quasi-experimental design. International Journal of Geriatric Psychiatry, n/a–n/a. doi: 10.1002/gps.4573 [DOI] [PubMed] [Google Scholar]
- O’Reilly D, Connolly S, Rosato M, & Patterson C (2008). Is caring associated with an increased risk of mortality? A longitudinal study. Social Science & Medicine, 67(8), 1282–1290. doi: 10.1016/j.socscimed.2008.06.025 [DOI] [PubMed] [Google Scholar]
- O’Reilly D, Rosato M, Maguire A, & Wright D (2015). Caregiving reduces mortality risk for most caregivers: a census-based record linkage study. International Journal of Epidemiology, 44(6), 1959–1969. doi: 10.1093/ije/dyv172 [DOI] [PubMed] [Google Scholar]
- Oldenkamp M, Hagedoorn M, Slaets J, Stolk R, Wittek R, & Smidt N (2016). Subjective burden among spousal and adult-child informal caregivers of older adults: results from a longitudinal cohort study. BMC Geriatrics, 16(1), 208. doi: 10.1186/s12877-016-0387-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinquart M, & Sorensen S (2007). Correlates of physical health of informal caregivers: a meta-analysis. J Gerontol B Psychol Sci Soc Sci, 62(2), P126–137. [DOI] [PubMed] [Google Scholar]
- Pinquart M, & Sörensen S (2006). Gender Differences in Caregiver Stressors, Social Resources, and Health: An Updated Meta-Analysis. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 61(1), P33–P45. [DOI] [PubMed] [Google Scholar]
- Pinquart M, & Sörensen S (2011). Spouses, adult children, and children-in-law as caregivers of older adults: A meta-analytic comparison. Psychology and Aging, 26(1), 1–14. doi: 10.1037/a0021863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polenick CA, Leggett AN, Webster NJ, Han BH, Zarit SH, & Piette JD (2017). Multiple Chronic Conditions in Spousal Caregivers of Older Adults With Functional Disability: Associations With Caregiving Difficulties and Gains. The Journals of Gerontology: Series B, gbx118–gbx118. doi: 10.1093/geronb/gbx118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin MJ, Brown SL, Dillard AJ, & Smith DM (2013). Giving to Others and the Association Between Stress and Mortality. American Journal of Public Health, 103(9), 1649–1655. doi: 10.2105/AJPH.2012.300876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior A, Fenger-Grøn M, Davydow DS, Olsen J, Li J, Guldin MB, & Vestergaard M (2017). Bereavement, multimorbidity and mortality: a population-based study using bereavement as an indicator of mental stress. Psychological Medicine, 48(9), 1437–1443. doi: 10.1017/S0033291717002380 [DOI] [PubMed] [Google Scholar]
- Ramsay S, Grundy E, & Reilly D (2013). The relationship between informal caregiving and mortality: an analysis using the ONS Longitudinal Study of England and Wales. Journal of Epidemiology and Community Health, 67(8), 655. [DOI] [PubMed] [Google Scholar]
- Roth DL, Brown SL, Rhodes JD, & Haley WE (2018). Reduced mortality rates among caregivers: Does family caregiving provide a stress-buffering effect? Psychol Aging, 33(4), 619–629. doi: 10.1037/pag0000224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth DL, Fredman L, & Haley WE (2015). Informal Caregiving and Its Impact on Health: A Reappraisal From Population-Based Studies. The Gerontologist, 55(2), 309–319. doi: 10.1093/geront/gnu177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth DL, Haley WE, Hovater M, Perkins M, Wadley VG, & Judd S (2013). Family Caregiving and All-Cause Mortality: Findings from a Population-based Propensity-matched Analysis. American Journal of Epidemiology, 178(10), 1571–1578. doi: 10.1093/aje/kwt225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, & Beach SR (1999). Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA, 282(23), 2215–2219. [DOI] [PubMed] [Google Scholar]
- Shah SM, Carey IM, Harris T, DeWilde S, Victor CR, & Cook DG (2016). The mental health and mortality impact of death of a partner with dementia. International Journal of Geriatric Psychiatry, 31(8), 929–937. doi: 10.1002/gps.4411 [DOI] [PubMed] [Google Scholar]
- Steffick DE (2000). Documentation of Affective Functioning Measures in the Health and Retirement Study. Retrieved from Ann Arbor, MI: http://hrsonline.isr.umich.edu/docs/userg/dr-005.pdf [Google Scholar]
- Tan EJ, Xue Q-L, Li T, Carlson MC, & Fried LP (2006). Volunteering: A Physical Activity Intervention for Older Adults—The Experience Corps® Program in Baltimore. Journal of Urban Health, 83(5), 954–969. doi: 10.1007/s11524-006-9060-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Känel R, Mausbach BT, Patterson TL, Dimsdale JE, Aschbacher K, Mills PJ, … Grant I (2008). Increased Framingham Coronary Heart Disease Risk Score in dementia caregivers relative to non-caregiving controls. Gerontology, 54(3), 131–137. [DOI] [PubMed] [Google Scholar]
- Wallace RB, & Herzog AR (1995). Overview of the Health Measures in the Health and Retirement Study. Journal of Human Resources, 30 Supplement, S84–S107. [Google Scholar]
- Zivin K, Llewellyn DJ, Lang IA, Vijan S, Kabeto MU, Miller EM, & Langa KM (2010). Depression Among Older Adults in the United States and England. American Journal of Geriatric Psych, 18(11), 1036–1044 1010.1097/JGP.1030b1013e3181dba1036d1032. [DOI] [PMC free article] [PubMed] [Google Scholar]


