Abstract
Objective
To examine the association of baseline elevated brain amyloid and neurodegeneration with changes in activities of daily living in participants without dementia (ND; i.e., cognitively unimpaired and participants with mild cognitive impairment) at baseline in the population‐based Mayo Clinic Study of Aging.
Methods
We included 1747 ND participants with 11C‐PiB PET and MR imaging in the study, with data on activities of daily living (as assessed by the Functional Activities Questionnaire (FAQ) and the Clinical Dementia Rating scale Sum of Boxes for functional domains (CDR‐SOB (functional)), with a median (range) of 4.3 (0.0–12.7) years of follow‐up. Abnormal (elevated; A+) 11C‐PiB‐PET retention ratio was defined as standardized uptake value ratio ≥ 1.48, and abnormal (reduced) AD signature cortical thickness as ≤ 2.68 mm (neurodegeneration; N+). Associations were examined with mixed effects models, adjusting for age, sex, education, apolipoprotein E ε4 allele carrier status, and global cognitive z‐score.
Results
Mean age (SD) was 71.4 years (10.1), 46.7% were females, 195 (11.2%) had A+N‐, 442 (25.3%) had A‐N+, and 339 (19.4%) had A+N+ biomarkers. The A+N+ group had the largest annualized change in the FAQ score from baseline (difference in annual change A+N+ vs. A‐N‐; ß (SE): 0.80 (0.07)); associations were substantially attenuated when a time‐varying global cognitive composite was included in the model (A+N+ vs. A‐N‐; ß (SE): 0.31 (0.05)). CDR‐SOB (functional) findings partly agreed with FAQ score findings.
Interpretation
The longitudinal increase in functional limitations is greater for individuals with abnormal neuroimaging biomarkers, especially for those with both elevated brain amyloid and neurodegeneration.
Introduction
In dementia, functional impairment is manifested by the inability to perform instrumental activities of daily living (IADL; e.g., managing finances, preparing a balanced meal, traveling out of the neighborhood) and more basic ADL (e.g., feeding, dressing, toileting). IADL are complex, involving multiple higher cognitive functions, are important for autonomous living and decline earlier than more basic ADL. Although cognitive outcomes in association with brain amyloid accumulation have been described more thoroughly, functional trajectories of activities of daily living have been studied in less detail. 1
In our previous research, 2 we found that higher scores on the Functional Activities Questionnaire (FAQ; indicating greater IADL limitations) were cross‐sectionally associated with abnormal (elevated) 11C‐PiB‐PET standardized uptake value ratio (SUVR; A+) and abnormal (reduced) Alzheimer’s disease (AD) signature cortical thickness (N+) in individuals without dementia (ND). In addition, previous research suggests that elevated brain amyloid is associated with lower ADL and IADL abilities, 3 , 4 , 5 , 6 and that there is an association between IADL disability and brain hypometabolism, 7 , 8 , 9 and MRI neurodegeneration biomarkers. 10 , 11 , 12 Studies also suggested that baseline MRI biomarkers predict future IADL worsening across the AD spectrum, 10 and that ND individuals might have different trajectories in IADL performance depending on brain amyloid status. 1
The objective of this study was to examine the association of baseline elevated brain amyloid and reduced AD signature cortical thickness with change in activities of daily living among ND participants (i.e., cognitively unimpaired (CU) and participants with mild cognitive impairment (MCI)) in the population‐based Mayo Clinic Study of Aging (MCSA).
Methods
Study population
The MCSA study design has been published in detail. 13 , 14 In summary, MCSA is a population‐based study initially established in 2004 in Olmsted County, MN, to identify MCI and dementia risk factors. Using the Rochester Epidemiology Project (REP) resources, 15 the Olmsted County residents, aged 70–89 years in October 2004, were enumerated and an age‐ and sex‐stratified random sample was invited to participate in the MCSA. Exclusion criteria included terminal illness, hospice admission, or dementia. In 2012, recruitment of residents 50–69 years began, and ongoing recruitment has maintained the study sample. Magnetic resonance imaging (MRI) was initiated in 2005 and 11C‐PiB positron emission tomography (PET) scans in 2008. The institutional review boards of the Mayo Clinic and the Olmsted Medical Center approved all study protocols. All participants provided written informed consent prior to participation in the study. Participants in the current study were all MCSA participants with a baseline PET scan by the end of 2017. All participants were followed up through 2018. The present study includes 1747 participants with amyloid PET, MRI, Functional Activities Questionnaire 16 (FAQ), and Clinical Dementia Rating scale 17 (CDR) data available, with a mean (SD) of 4.4 (2.6) and a median (range) of 4.3 (0.0–12.7) years of follow‐up [i.e., (mean (SD) of 4.6 (2.5) and median (range) of 4.7 ( 0–12.7) years of follow‐up for CU participants, and mean (SD) of 3.1 (2.6) and median (range) of 2.7 (0–11.5) years of follow‐up for participants with MCI)].
Evaluation of participants and diagnostic assessment
At baseline and each follow‐up visit approximately every 15 months, a nurse or study coordinator collected sociodemographic factors, asked questions on memory, and administered the Beck Depression Inventory (BDI‐II) 18 to participants and the CDR and the FAQ to an informant (i.e., someone who knew the participant well). A physician reviewed the participant’s medical history, administered the Short Test of Mental Status 19 and performed a neurological examination. Nine neuropsychological tests, administered by a psychometrist, were used to assess cognitive performance in four domains: (1) memory, (2) attention/executive function, (3) language, and (4) visuospatial skills. 13 , 14 An expert consensus panel of evaluators,(i.e., the nurse or study coordinator, the physician, and a neuropsychologist) review all the information for each participant and adjudicate a final diagnosis (CU, MCI, dementia) by consensus based on published criteria. 20 , 21 , 22 Individuals who performed in the normative range and did not meet criteria for MCI 20 or dementia 21 , 22 were classified as CU.
Imaging
All MCSA participants are invited to undergo neuroimaging studies at baseline and every 30 months (if cognitively stable) or sooner if a participant changes cognitive status (e.g., if one progresses from CU to MCI). Exclusions include any participant with a partial brain resection and routine MRI safety restrictions.
11C‐PiB PET methods
Details are presented in previous reports. 23 Amyloid PET imaging was performed with Pittsburg Compound B. 24 An amyloid PET SUVR was calculated from the voxel number weighted average of the median uptake in the parietal, temporal, prefrontal, orbitofrontal, anterior and posterior cingulate, and precuneus regions of interest, referenced to the cerebellar gray crus region. The cut point for amyloid PET signifying normal or abnormal was the SUVR value of 1.48 (centiloid 22 25 ), 23 , 26 beyond that rates of amyloid PET reliably increased. Amyloid positivity (A+) was defined as SUVR ≥ 1.48. The first 11C‐PiB‐PET was used as the baseline in the current analysis.
MRI measures methods
All MRI images were acquired on 3T GE MRI (GE Medical Systems, Milwaukee, WI). The MRI measure of neurodegeneration was a FreeSurfer (version 5.3)‐derived cortical thickness meta‐Region of interest (ROI) (entorhinal cortex, fusiform inferior temporal, and middle temporal gyri). 23 The cut point value for cortical thickness was 2.68 mm, which most accurately discriminated between cognitively impaired individuals with abnormal amyloid PET and cognitively unimpaired young (aged 30–49 years) individuals with normal amyloid PET (i.e., neurodegeneration (N+; reduced AD signature cortical thickness) was defined as ≤ 2.68mm). 23 , 26
Assessment of activities of daily living
Functional performance was assessed by the FAQ 16 and the CDR 17 over the previous year. We computed the CDR‐sum of boxes (SOB) by summing the scores for each of the domain boxes only for the three functional domains (community affairs, home and hobbies, and personal care), 27 which we call in the present study “CDR‐SOB (functional),” resulting in a score range from 0 to 9; higher scores indicate greater impairment. 28 The FAQ is a 10‐item questionnaire that assesses IADL (e.g., writing checks, assembling tax records, shopping alone for groceries, working on a hobby, turning off the stove after use, traveling out of the neighborhood, preparing a balanced diet). The informant rates the participant’s abilities (0 = normal, 1 = has difficulty but does by self, 2 = requires assistance, 3 = dependent; 8 = not applicable (e.g., never did) was not scored)), resulting in a score range from 0 to 30; higher scores indicate greater impairment. The FAQ was considered complete if 70% of the questions were answered. 28 Both FAQ and CDR were administered to an informant at baseline and each follow‐up. For 246 participants, the CDR and FAQ information was reported by informants or participants interchangeably during follow‐up, and 88 participants self‐reported the CDR and FAQ during their entire follow‐up.
Covariates
The chronic disease burden at the study baseline was assessed from a weighted Charlson Comorbidity Index (CCI) 29 score based on electronic diagnosis codes (HICDA, ICD‐9, ICD‐10) using the REP resources. Apolipoprotein E (APOE) ε4 status (carrier vs. noncarrier) was determined from a blood draw at MCSA baseline assessment.
Statistical analysis
Participant characteristics were compared using Kruskal–Wallis tests or χ2 tests as appropriate. For analytical purposes, the raw scores for tests in each cognitive domain were z‐scored and averaged to create domain‐specific cognitive z‐scores. In addition, a global z‐score for overall cognitive performance was also created by averaging the four domain z‐scores. We examined the longitudinal associations of baseline neuroimaging biomarkers (exposure: A+, N+ and their combinations) and change in functional status (outcome) in ND participants (i.e., CU and participants with MCI at baseline) during follow‐up. Mixed effects models allowing fixed effects for age, sex, and education (model 1), as well as individual random intercepts and slopes to account for within‐person correlation, were used to examine the association between baseline neuroimaging biomarkers and change in FAQ and CDR‐SOB (functional). We adjusted additionally for APOE ε4 allele status (model 2) and for time‐varying global cognitive z‐score (model 3). An additional model was also run including baseline depressive symptoms (BDI‐II ≥ 13) and CCI. Estimates did not change appreciably, thus findings are not presented in tables. We also tested whether AN biomarker status predicted rates of change compared to a model without using a 3‐df likelihood ratio test and since this was significant for both FAQ as well as CDR‐SOB outcomes, we followed up with pairwise comparisons between the AN biomarker groups. Potential effect modification by age, sex, APOE ɛ4 carrier status, depressive symptoms, and cognitive status (i.e., CU, MCI) at baseline was examined using interaction terms in the models. We created figures (using mean covariate values for age, sex, and education) only for the predicted FAQ scores, in the case of statistically significant interaction terms. Sensitivity analyses were performed (1) excluding those that ever self‐reported the FAQ and the CDR, (2) excluding participants who were younger than 60 years of age, as very few had elevated brain amyloid (n = 8) and (3) excluding 15 participants with FAQ> 9 at study baseline. Sensitivity analyses’ findings were not appreciably different than findings from the main analysis that included all participants. Estimates are presented as beta coefficients with standard errors (SE). We utilized a standard 0.05 alpha level to determine statistical significance. Analyses were performed using the SAS statistical software version 9.4 (SAS Institute, Cary, North Carolina).
Results
Participants’ characteristics
There were overall 1,747 participants included in the present study (mean age (standard deviation (SD)): 71.4 (10.1) years; 816 (46.7%) females), 492 (28.4%) were APOE ε 4 positive and 203 (11.6%) had MCI at baseline (Table 1). There were 771 (44.1%) individuals with A‐N‐, 195 (11.2%) with A+N‐, 442 (25.3%) with A‐N+, and 339 (19.4%) with A+N+ biomarkers. Participants with A+N+ were older, had lower mean global cognitive z‐score, higher mean CCI, higher mean CDR‐SOB, and FAQ score.
Table 1.
Characteristics of participants without dementia (including CU and MCI) at baseline by AN biomarker status.
| A‐N‐ (N = 771) | A+N‐ (N = 195) | A‐N+ (N = 442) | A+N+ (N = 339) | P‐value* | |
|---|---|---|---|---|---|
| Age, mean (SD) | 65.3 (8.7) | 73.0 (7.7) | 74.8 (8.9) | 80.0 (6.4) | <0.001 |
| Sex, female | 379 (49.2) | 111 (56.9) | 173 (39.1) | 153 (45.1) | <0.001 |
| Education (yrs), mean (SD) | 15.0 (2.5) | 14.6 (2.6) | 14.3 (2.8) | 14.4 (2.8) | <0.001 |
| APOE ε24/ε34/ε4 1 | 181 (23.9) | 94 (48.5) | 76 (17.3) | 141 (41.6) | <0.001 |
| CCI, mean (SD) | 1.91 (2.26) | 2.77 (2.46) | 3.69 (3.35) | 4.18 (3.23) | <0.001 |
| Depressive symptoms 2 | 38 (4.9) | 13 (6.7) | 39 (8.8) | 32 (9.5) | 0.015 |
| Global z‐score, 3 mean (SD) | 0.48 (0.94) | 0.003 (0.95) | −0.17 (1.06) | −0.79 (1.18) | <0.001 |
| Mild cognitive impairment | 41 (5.3) | 18 (9.2) | 50 (11.3) | 94 (27.7) | <0.001 |
| FAQ score, mean (SD) | 0.08 (0.43) | 0.35 (1.36) | 0.52 (1.76) | 1.25 (2.71) | <0.001 |
| median (range) | 0 (0–6) | 0 (0–11) | 0 (0–19) | 0 (0–20) | |
| FAQ score >0 | 36 (4.7) | 23 (11.8) | 69 (15.6) | 103 (30.4) | <0.001 |
| No. of FAQ tests, mean (SD) | 4.1 (1.7) | 4.5 (2.1) | 4.4 (2.07) | 4.0 (2.1) | 0.005 |
| Have baseline FAQ data only | 58 (7.5) | 14 (7.2) | 35 (7.9) | 45 (13.3) | 0.011 |
| CDR score, mean (SD) | 0.02 (0.11) | 0.05 (0.15) | 0.06 (0.16) | 0.15 (0.24) | <0.001 |
| median (range) | 0 (0–0.5) | 0 (0–0.5) | 0 (0–1) | 0 (0–1) | |
| CDR score> 0 | 36 (4.7) | 18 (9.2) | 49 (11.1) | 98 (28.9) | <0.001 |
| CDR SOB, mean (SD) | 0.04 (0.20) | 0.12 (0.48) | 0.14 (0.47) | 0.45 (0.92) | <0.001 |
| median (range) | 0 (0–2.5) | 0 (0–4) | 0 (0–4.5) | 0 (0–6.5) | |
| CDR SOB> 0 | 40 (5.2) | 20 (10.3) | 57 (12.9) | 105 (31.0) | <0.001 |
| CDR SOB (func.), mean (SD) | 0.003 (0.04) | 0.02 (0.14) | 0.04 (0.22) | 0.13 (0.39) | <0.001 |
| median (range) | 0 (0–1) | 0 (0–1.5) | 0 (0–3) | 0 (0–3.5) | |
| CDR SOB (func.) >0 | 3 (0.4) | 5 (2.6) | 19 (4.3) | 46 (13.6) | <0.001 |
| No. of CDR tests, mean (SD) | 4.2 (1.8) | 4.5 (2.1) | 4.5 (2.1) | 4.0 (2.1) | 0.004 |
| Have Baseline CDR data only | 58 (7.5) | 14 (7.2) | 30 (6.8) | 41 (12.1) | 0.033 |
| Years since baseline, mean (SD) | 4.3 (2.3) | 4.7 (2.7) | 4.8 (2.7) | 4.1 (2.8) | 0.001 |
| median (range) | 4.2 (0–10.5) | 4.8 (0–11.5) | 5.0 (0–12.7) | 4.0 (0–11.7) |
APOE, apolipoprotein E; CCI, Charlson Comorbidity Index; BDI II, Beck Depression Inventory; A+, Elevated amyloid (A+), is defined as 11C‐Pittsburgh compound B standardized uptake value ratio ≥ 1.48; N+, Abnormal (reduced) AD signature cortical thickness (N+) was defined as ≤ 2.68mm; CDR SOB, Clinical Dementia Rating scale sum of boxes (0–18); CDR (func.), CDR‐SOB only for the three functional (community affairs, home and hobbies and personal care) domains (0–9); FAQ score, Functional activities questionnaire total score (0–30, completed if 70% of questions answered).
N (%) unless otherwise stated.
17 missing.
BDI‐II ≥ 13, 8 missing.
Cognitive z‐scores computed after scaling raw cognitive test scores (mean = 0, SD = 1) using data for cognitively normal subjects at baseline. Domain‐specific z‐scores are summed and scaled to obtain the global z‐score; 87 missing.
Kruskal–Wallis or Chi‐Square test.
During the follow‐up period (mean (SD) 4.4 years (2.6)), overall 177 CU individuals at baseline progressed to MCI (30 A‐N‐, 26 A+N‐, 54 A‐N+, 67 A+N+ at baseline) with 32 progressing further to dementia (2 A‐N‐, 5 A+N‐, 7 A‐N+, 18 A+N+ at baseline). Forty individuals with MCI at baseline progressed to dementia (1 A‐N‐, 2 A+N‐, 13 A‐N+, 24 A+N+). In participants aged 50–69 (N = 750) at baseline, only 36 (4.8%) progressed (increased) in FAQ score over their follow‐up time, while in those 70+ years old at baseline, there were 227 of 997 (22.8%) who progressed in FAQ over their follow‐up time. Participants’ characteristics by amyloid and neurodegeneration status are presented in Table 2.
Table 2.
Participants' baseline characteristics by Amyloid and Neurodegeneration status.
| Characteristics | Elevated Amyloid (A+) | Neurodegeneration (N+) | ||||
|---|---|---|---|---|---|---|
| No, N = 1213 | Yes, N = 534 | P‐value* | No, N = 966 | Yes, N = 781 | P‐value* | |
| Age, mean (SD) | 68.8 (9.9) | 77.4 (7.7) | <0.001 | 66.9 (9.1) | 77.1 (8.3) | <0.001 |
| Sex, female | 552 (45.5) | 264 (49.4) | 0.13 | 476 (49.3) | 455 (58.3) | <0.001 |
| Education (yrs), mean (SD) | 14.8 (2.6) | 14.4 (2.7) | 0.008 | 14.9 (2.5) | 14.4 (2.8) | <0.001 |
| APOE ε24/ε34/ε4 1 | 257 (21.5) | 235 (44.1) | <0.001 | 275 (28.9) | 217 (27.9) | 0.63 |
| CCI | 2.6 (2.8) | 3.7 (3.1) | <0.001 | 2.1 (2.3) | 3.9 (3.3) | <0.001 |
| Depressive symptoms 2 | 77 (6.4) | 45 (8.5) | 0.11 | 51 (5.3) | 71 (9.1) | 0.002 |
| Global cognitive z‐score 3 , * | 0.2 (1.0) | −0.5 (1.2) | <0.001 | 0.4 (1.0) | −0.4 (1.2) | <0.001 |
| Mild cognitive impairment | 91 (7.5) | 112 (21.0) | <0.001 | 59 (6.1) | 144 (18.4) | <0.001 |
| FAQ score, mean (SD) | 0.2 (1.1) | 0.9 (2.3) | <0.001 | 0.1 (0.7) | 0.8 (2.2) | <0.001 |
| median (range) | 0 (0–19) | 0 (0–20) | 0 (0–11) | 0 (0–20) | ||
| FAQ score> 0 | 105 (8.7) | 126 (23.6) | <0.001 | 59 (6.1) | 172 (22.0) | <0.001 |
| CDR score, mean (SD) | 0.04 (0.13) | 0.11 (0.21) | <0.001 | 0.03 (0.1) | 0.1 (0.2) | <0.001 |
| median (range) | 0 (0–1) | 0 (0–1) | 0 (0–0.5) | 0 (0–1) | ||
| CDRf score> 0 | 85 (7.0) | 116 (21.7) | <0.001 | 54 (5.6) | 147 (18.8) | <0.001 |
| CDR SOB, mean (SD) | 0.1 (0.3) | 0.3 (0.8) | <0.001 | 0.06 (0.3) | 0.3 (0.7) | <0.001 |
| median (range) | 0 (0–4.5) | 0 (0–6.5) | 0 (0–4) | 0 (0–6.5) | ||
| CDR SOB> 0 | 97 (8.0) | 125 (23.4) | <0.001 | 60 (6.2) | 162 (20.7) | <0.001 |
| CDR SOB (func.), mean (SD) | 0.02 (0.1) | 0.09 (0.3) | <0.001 | 0.01 (0.1) | 0.1 (0.3) | <0.001 |
| median (range) | 0 (0–3) | 0 (0–3.5) | 0 (0–1.5) | 0 (0–3.5) | ||
| CDR SOB (func.) >0 | 22 (1.8) | 51 (9.6) | <0.001 | 8 (0.8) | 65 (8.3) | <0.001 |
APOE, apolipoprotein E; CCI, Charlson Comorbidity Index; BDI II, Beck Depression Inventory; A+, Elevated amyloid (A+), is defined as 11C‐Pittsburgh compound B standardized uptake value ratio ≥ 1.48; N+, Abnormal (reduced) AD signature cortical thickness (N+) was defined as ≤ 2.68mm; CDR SOB, Clinical Dementia rating Scale sum of boxes (0–18); CDR (func.), CDR‐SOB only for the three functional (community affairs, home and hobbies and personal care) domains (0–9); FAQ score = Functional activities questionnaire score (0–30, completed if 70% of questions answered).
N (%) unless otherwise stated.
17 missing
BDI‐II ≥ 13, 8 missing
Cognitive z‐scores computed after scaling raw cognitive test scores (mean = 0, SD = 1) using data for cognitively normal subjects at baseline. Domain‐specific z‐scores are summed and scaled to obtain global z‐scores; 87 missing.
Kruskal–Wallis or Chi‐Square test.
Association of neuroimaging biomarkers and change in FAQ score and CDR‐SOB (functional)
When we consider both amyloid and neurodegeneration status together, the A+N+ group had the highest annualized change in FAQ score from baseline than the other three biomarker groups (Table 3 and Fig. 1). When a time‐varying global cognitive composite was included in the model, associations between the biomarkers and functional measures of daily living were substantially attenuated (Tables 3 and 4), indicating that cognitive and activities of daily living measures share a substantial amount of variance. Interaction terms were statistically significant for age, APOE ɛ4 status, and cognitive status at baseline (Figs. 2, 3). As only 4.8% of participants aged 50–69 at baseline progressed in FAQ overtime, a sensitivity analysis was performed only in those 70+ years old at baseline and findings agree with the overall main analysis (Table 5).
Table 3.
Regression coefficients from linear mixed‐effects models for FAQ and CDR‐SOB (functional) by AN biomarker status.
| FAQ score | CDR‐SOB (functional) | |||||
|---|---|---|---|---|---|---|
| Model 11 | Model 22 | Model 33 | Model 11 | Model 22 | Model 33 | |
| ß (Standard Error) | ||||||
| Intercept | 0.16 (0.07) | 0.09 (0.08) | 0.26 (0.07) | 0.002 (0.01) | −0.005 (0.01) | 0.02 (0.01) |
| Baseline age, in years | 0.02 (0.005) | 0.02 (0.005) | 0.00 (0.005) | 0.002 (0.001) | 0.002 (0.001) | 0.00 (0.001) |
| Male | 0.05 (0.08) | 0.05 (0.08) | −0.05 (0.07) | 0.02 (0.01) | 0.02 (0.01) | 0.003 (0.01) |
| Education, in years | −0.04 (0.01) | −0.04 (0.01) | 0.04 (0.01) | 0.00 (0.002) | 0.001 (0.002) | 0.01 (0.002) |
| APOE ε4 | 0.31 (0.09) | 0.22 (0.08) | 0.03 (0.01) | 0.02 (0.01) | ||
| Global cognitive z‐score | −0.49 (0.03) | ‐0.06 (0.005) | ||||
| A+N‐ | 0.06 (0.13) | −0.03 (0.14) | 0.01 (0.12) | −0.02 (0.02) | −0.03 (0.02) | −0.02 (0.02) |
| A‐N+ | 0.15 (0.11) | 0.16 (0.11) | 0.08 (0.10) | 0.01 (0.02) | 0.01 (0.02) | 0.008 (0.01) |
| A+N+ | 0.49 (0.13) | 0.40 (0.13) | 0.32 (0.12) | 0.06 (0.02) | 0.05 (0.02) | 0.05 (0.02) |
| Time | 0.03 (0.04) | 0.03 (0.04) | 0.03 (0.02) | 0.005 (0.01) | 0.004 (0.01) | 0.004 (0.003) |
| A+N‐ x Time | 0.24 (0.08)** | 0.24 (0.08)** | 0.12 (0.05)* | 0.03 (0.01)* | 0.03 (0.01)* | 0.01 (0.01)* |
| A‐N+ x Time | 0.23 (0.06)*** | 0.23 (0.06)*** | 0.09 (0.04)* | 0.04 (0.01)*** | 0.04 (0.01)*** | 0.01 (0.005) |
| A+N+ x Time | 0.80 (0.07)*** | 0.80 (0.07)*** | 0.31 (0.05)*** | 0.09 (0.01)*** | 0.09 (0.01)*** | 0.02 (0.01)*** |
| Annual Rate of Change | ||||||
| A+N‐ slope | 0.28 (0.07) | 0.28 (0.07) | 0.15 (0.05) | 0.04 (0.01) | 0.04 (0.01) | 0.02 (0.01) |
| A‐N+ slope | 0.26 (0.05) | 0.26 (0.05) | 0.13 (0.03) | 0.04 (0.01) | 0.04 (0.01) | 0.01 (0.004) |
| A+N+ slope | 0.83 (0.06) | 0.83 (0.06) | 0.34 (0.04) | 0.10 (0.01) | 0.10 (0.01) | 0.02 (0.005) |
| Difference in annual rate of change | ||||||
| A+N‐ vs A‐N+ | 0.02 (0.08) | 0.01 (0.08) | 0.02 (0.06) | −0.01 (0.01) | −0.01 (0.01) | 0.01 (0.01) |
| A+N‐ vs A+N+ | −0.56 (0.09)*** | −0.56 (0.09)*** | −0.20 (0.06)** | −0.06 (0.02)*** | −0.06 (0.02)*** | −0.01 (0.01) |
| A‐N+ vs A+N+ | −0.57 (0.07)*** | −0.57 (0.07)*** | −0.22 (0.05)*** | −0.06 (0.01)*** | −0.06 (0.01)*** | −0.01 (0.01)* |
A+, Elevated amyloid (A+), defined as 11C‐Pittsburgh compound B standardized uptake value ratio ≥ 1.48; N+, Abnormal (reduced) AD signature cortical thickness (N+), defined as ≤ 2.68mm; CDR SOB (functional), Clinical dementia rating scale sum of boxes only for the 3 functional domains (community affairs, home and hobbies, and personal care); FAQ score, Functional activities questionnaire score. Fixed model results from a linear mixed effects model allowing for random individual intercepts and slopes; 1model 1 allows fixed effects for age, sex, education; 2model 2 allows fixed effects for age, sex, education, apolipoprotein E ε4 carrier status; 3model 3 allows fixed effects for age, sex, education, APOE ε4, global cognitive z‐score. Intercept refers to the mean score at baseline for a 70‐year‐old female with 14 years of education, no ApoE ε4 allele (models 2 and 3) and a z‐global score of 0 (model 3) and with A‐N‐ biomarker status. Each AN biomarker status refers to the difference in mean score at baseline compared to A‐N‐ status. Time value refers to the annual rate of change in score for those with A‐N‐ status. Interaction effects refer to the difference in annual change in score for each AN biomarker status compared to A‐N‐. In the longitudinal models, individuals contribute from 1 to 11 observations to the analysis. Model 1 includes data from 1747 participants, Model 2 includes data from 1730 participants (17 participants missing information for ApoE) and Model 3 includes data from 1644 participants (86 participants missing information additionally for z‐global).
0.01 ≤ P‐value ≤ 0.05
0.001 ≤ P‐value < 0.01
P‐value < 0.001
Figure 1.
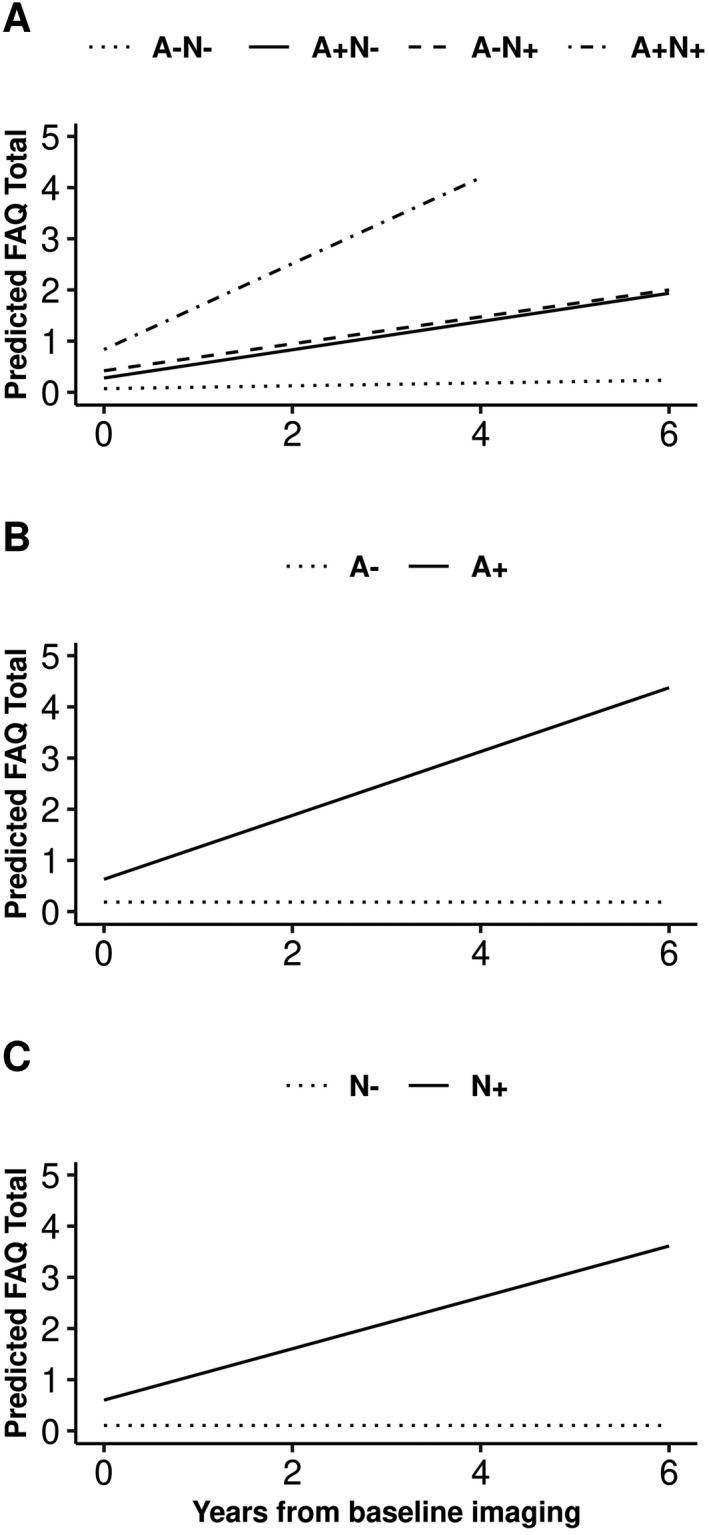
Predicted FAQ score from baseline in individuals without dementia by AN biomarker groups (panel A), elevated brain amyloid (A+/A‐; panel B) and neurodegeneration (N+/N‐; panel C) status. Derived from mixed‐effects models using mean covariate values for age, sex, and education, allowing for random subject‐specific intercepts and slopes.
Table 4.
Regression coefficients from linear mixed‐effects models for FAQ and CDR‐SOB (functional) by amyloid and neurodegeneration status.
| FAQ score | CDR‐SOB (functional) | |||||
|---|---|---|---|---|---|---|
| Model 11 | Model 22 | Model 33 | Model 11 | Model 22 | Model 33 | |
| ß (Standard Error) | ||||||
| Amyloid status | ||||||
| Intercept | 0.21 (0.06) | 0.15 (0.07) | 0.29 (0.06) | 0.004 (0.01) | −0.003 (0.01) | 0.02 (0.01) |
| Baseline age, in years | 0.02 (0.004) | 0.03 (0.004) | 0.002 (0.004) | 0.002 (0.001) | 0.003 (0.001) | 0.00 (0.001) |
| Male | 0.07 (0.08) | 0.07 (0.08) | −0.04 (0.07) | 0.02 (0.01) | 0.02 (0.01) | 0.005 (0.01) |
| Education, in years | −0.04 (0.01) | −0.04 (0.01) | 0.04 (0.01) | 0.00 (0.002) | 0.00 (0.002) | 0.01 (0.002) |
| APOE ε4 | 0.30 (0.09) | 0.22 (0.08) | 0.03 (0.01) | 0.02 (0.01) | ||
| Global cognition z‐score | −0.51 (0.03) | −0.06 (0.005) | ||||
| A+ | 0.24 (0.09) | 0.15 (0.10) | 0.15 (0.09) | 0.02 (0.01) | 0.01 (0.01) | 0.02 (0.01) |
| Time | 0.12 (0.03) | 0.12 (0.03) | 0.07 (0.02) | 0.02 (0.005) | 0.02 (0.01) | 0.01 (0.002) |
| A+ x Time | 0.50 (0.05)*** | 0.50 (0.05)*** | 0.19 (0.04)*** | 0.06 (0.01)*** | 0.06 (0.01)*** | 0.01 (0.004)** |
| Annual Rate of Change | ||||||
| A+ slope | 0.62 (0.04) | 0.62 (0.04) | 0.26 (0.03) | 0.08 (0.01) | 0.08 (0.01) | 0.02 (0.004) |
| Neurodegeneration | ||||||
| Intercept | 0.18 (0.07) | 0.09 (0.07) | 0.27 (0.06) | −0.001 (0.01) | −0.01 (0.01) | 0.01 (0.01) |
| Baseline age, in years | 0.02 (0.005) | 0.02 (0.005) | 0.001 (0.004) | 0.002 (0.001) | 0.002 (0.001) | −0.001 (0.001) |
| Male | 0.04 (0.08) | 0.04 (0.08) | −0.06 (0.07) | 0.02 (0.01) | 0.02 (0.01) | 0.001 (0.01) |
| Education, in years | −0.04 (0.01) | −0.03 (0.01) | 0.04 (0.01) | 0.001 (0.002) | 0.001 (0.002) | 0.01 (0.002) |
| APOE ε4 | 0.33 (0.09) | 0.24 (0.08) | 0.04 (0.01) | 0.03 (0.01) | ||
| Global cognition z‐score | −0.51 (0.03) | −0.06 (0.005) | ||||
| N+ | 0.26 (0.09) | 0.26 (0.09) | 0.16 (0.08) | 0.03 (0.01) | 0.03 (0.01) | 0.03 (0.01) |
| Time | 0.08 (0.03) | 0.08 (0.03) | 0.05 (0.02) | 0.01 (0.01) | 0.01 (0.01) | 0.007 (0.003) |
| N+ x Time | 0.41 (0.05)*** | 0.41 (0.05)*** | 0.15 (0.03)*** | 0.05 (0.01)*** | 0.05 (0.01)*** | 0.01 (0.004)* |
| Annual Rate of Change | ||||||
| N+ slope | 0.50 (0.04) | 0.50 (0.04) | 0.21 (0.03) | 0.07 (0.01) | 0.07 (0.01) | 0.02 (0.003) |
A+ =Elevated amyloid (A+), defined as 11C‐Pittsburgh compound B standardized uptake value ratio ≥ 1.48; N+ = Abnormal (reduced) AD signature cortical thickness (N+), defined as ≤ 2.68mm; CDR SOB (functional) = Clinical dementia rating scale sum of boxes only for the three functional domains (community affairs, home and hobbies, and personal care); FAQ score = Functional activities questionnaire score. Fixed model results from a linear mixed effects model allowing for random individual intercepts and slopes; 1model 1 allows fixed effects for age, sex, education; 2model 2 allows fixed effects for age, sex, education, apolipoprotein E ε4 carrier status; 3model 3 allows fixed effects for age, sex, education, APOE ε4, global cognitive z‐score. Intercept refers to the mean score at baseline for a 70–year‐old female with 14 years of education, no ApoE ε4 allele (models 2 and 3) and a z‐global score of 0 (model 3) and with A‐ or N‐ biomarker status. A+ or N+ biomarker status refers to the difference in mean score at baseline compared to A‐ or N‐ status. Time value refers to annual change in score for those with A‐ or N‐ status. Interaction effects refer to the difference in annual change in score for A+ or N+ biomarker status compared to A‐ or N‐. In the longitudinal models, individuals contribute from 1 to 11 observations to the analysis. Model 1 includes data from 1747 participants, Model 2 includes data from 1730 participants (17 participants missing information for ApoE) and Model 3 includes data from 1644 participants (86 participants missing information additionally for z‐global).
0.01 ≤ P‐value ≤ 0.05
0.001 ≤ P‐value < 0.01
P‐value < 0.001
Figure 2.
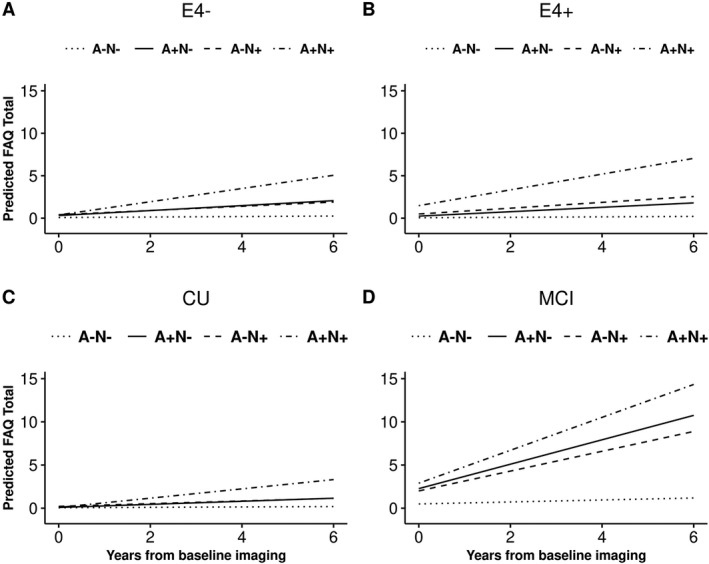
Predicted FAQ score from baseline in individuals without dementia by AN biomarker groups, and APOE ε4 status (E4; panels A and B; APOE ε4 p for interaction: <0.001) and cognitive status (cognitively unimpaired (CU)/mild cognitive impairment (MCI) panels C and D; cognitive status at baseline p for interaction: <0.001). Derived from mixed‐effects models using mean covariate values for age, sex, and education, allowing for random subject‐specific intercepts and slopes.
Figure 3.
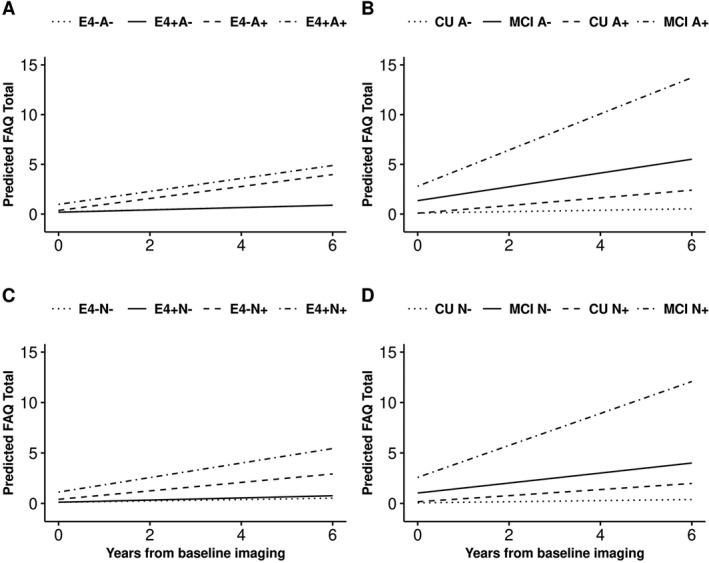
Predicted FAQ score from baseline in individuals without dementia by: (i) elevated brain amyloid status (A+/A‐), APOE ε4 status (E4, panel A; APOE ε4 p for interaction: 0.01) and cognitive status (cognitively unimpaired (CU)/mild cognitive impairment (MCI), panel B; cognitive status at baseline p for interaction: <0.001); and (ii) by neurodegeneration (N+/N‐), APOE ε4 status (E4, panel C; APOE ε4 p for interaction: <0.001) and cognitive status (CU/MCI), panel D; cognitive status at baseline p for interaction: <0.001). Derived from mixed‐effects models using mean covariate values for age, sex, and education, allowing for random subject‐specific intercepts and slopes.
Table 5.
Regression coefficients from linear mixed effects models for FAQ and CDR‐SOB (functional) by AN biomarker status in participants 70 + years old.
| FAQ score | CDR‐SOB (functional) | |||||
|---|---|---|---|---|---|---|
| Model 1 1 | Model 22 | Model 33 | Model 11 | Model 22 | Model 33 | |
| ß (Standard Error) | ||||||
| Intercept | −0.16 (0.16) | −0.30 (0.17) | −0.10 (0.15) | −0.03 (0.02) | −0.05 (0.03) | −0.001 (0.02) |
| Baseline age, in years | 0.04 (0.01) | 0.05 (0.01) | 0.03 (0.01) | 0.004 (0.002) | 0.01 (0.002) | 0.001 (0.001) |
| Male | 0.08 (0.13) | 0.08 (0.13) | −0.04 (0.12) | 0.03 (0.02) | 0.03 (0.02) | 0.01 (0.01) |
| Education, in years | −0.05 (0.02) | −0.05 (0.02) | 0.04 (0.02) | 0.001 (0.003) | 0.001 (0.003) | 0.01 (0.003) |
| APOE ε4 | 0.57 (0.15) | 0.45 (0.14) | 0.06 (0.02) | 0.03 (0.02) | ||
| Global cognition z‐score | −0.61 (0.05) | −0.05 (0.01) | ||||
| A+N‐ | 0.17 (0.22) | 0.01 (0.22) | 0.06 (0.20) | −0.02 (0.03) | −0.04 (0.03) | −0.02 (0.02) |
| A‐N+ | 0.23 (0.18) | 0.25 (0.18) | 0.15 (0.16) | 0.01 (0.03) | 0.01 (0.03) | 0.01 (0.02) |
| A+N+ | 0.52 (0.19) | 0.36 (0.19) | 0.20 (0.17) | 0.07 (0.03) | 0.05 (0.03) | 0.06 (0.02) |
| Time, in years | 0.06 (0.08) | 0.06 (0.08) | 0.02 (0.05) | 0.01 (0.01) | 0.01 (0.01) | 0.005 (0.01) |
| A+N‐ x Time | 0.33 (0.13) | 0.32 (0.13)* | 0.16 (0.09) | 0.04 (0.02) | 0.04 (0.03) | 0.02 (0.02) |
| A‐N+ x Time | 0.28 (0.11) | 0.28 (0.11)** | 0.12 (0.07) | 0.05 (0.02)* | 0.05 (0.02)* | 0.02 (0.01) |
| A+N+ x Time | 0.88 (0.11)** | 0.88 (0.11)*** | 0.35 (0.08)*** | 0.10 (0.02)*** | 0.10 (0.02)*** | 0.03 (0.01)** |
| Annual Rate of Change | ||||||
| A+N‐ slope | 0.39 (0.11) | 0.38 (0.11) | 0.18 (0.06) | 0.05 (0.02) | 0.05 (0.02) | 0.03 (0.01) |
| A‐N+ slope | 0.34 (0.07) | 0.34 (0.07) | 0.14 (0.05) | 0.06 (0.01) | 0.06 (0.01) | 0.02 (0.01) |
| A+N+ slope | 0.94 (0.08) | 0.94 (0.08) | 0.36 (0.06) | 0.11 (0.01) | 0.11 (0.01) | 0.04 (0.01) |
| Difference in annual rate of change | ||||||
| A+N‐ vs A‐N+ | 0.05 (0.13) | 0.04 (0.13) | 0.04 (0.09) | −0.01 (0.02) | −0.01 (0.02) | 0.003 (0.01) |
| A+N‐ vs A+N+ | −0.55 (0.13)*** | −0.56 (0.13)*** | −0.19 (0.09)* | −0.06 (0.02)* | −0.05 (0.02)* | −0.01 (0.02) |
| A‐N+ vs A+N+ | −0.60 (0.10)*** | −0.60 (0.10)*** | −0.22 (0.07)** | −0.06 (0.02)** | −0.05 (0.02)* | −0.01 (0.01) |
A+, Elevated amyloid (A+), defined as 11C‐Pittsburgh compound B standardized uptake value ratio ≥ 1.48; N+, Abnormal (reduced) AD signature cortical thickness (N+), defined as ≤ 2.68mm; CDR SOB (functional), Clinical dementia rating scale sum of boxes only for the three functional domains (community affairs, home and hobbies, and personal care); FAQ score = Functional activities questionnaire score. Fixed model results from a linear mixed effects model allowing for random individual intercepts and slopes; 1model 1 allows fixed effects for age, sex, education; 2model 2 allows fixed effects for age, sex, education, apolipoprotein E ε4 carrier status; 3model 3 allows fixed effects for age, sex, education, APOE ε4, global cognitive z‐score. Intercept refers to the mean score at baseline for a 70‐year‐old female with 14 years of education, no ApoE ε4 allele (models 2 and 3) and a z‐global score of 0 (model 3) and with A‐N‐ biomarker status. Each AN biomarker status refers to the difference in mean score at baseline compared to A‐N‐ status. Time value refers to annual change in score for those with A‐N‐ status. Interaction effects refer to the difference in annual change in score for each AN biomarker status compared to A‐N‐. In the longitudinal models, individuals contribute from 1 to 11 observations to the analysis. Model 1 includes data from 1747 participants, Model 2 includes data from 1730 participants (17 participants missing information for ApoE) and Model 3 includes data from 1644 participants (86 participants missing information additionally for z‐global).
0.01 ≤ P‐value ≤ 0.05
0.001 ≤ P‐value < 0.01
P‐value < 0.001
Findings related to CDR‐SOB (functional) partly agreed with FAQ score findings, with the A+N+ group having the highest annualized change in activities of daily living from baseline, but this relationship was not statistically significant for all comparisons when adjusting also for the time‐varying cognitive performance (Table 3; model 3). However, we need to acknowledge that there were very few participants (4.2%) who had a CDR‐SOB (functional)>0 resulting in lower power to detect differences.
Discussion
Elevated brain amyloid and reduced cortical thickness in an AD signature region were associated with faster IADL decline in participants without dementia at baseline. Participants who were A+N+ had the greatest decline in IADL performance (vs. A‐N‐; especially when assessed by the FAQ). This association was considerably attenuated, as expected, after adjusting additionally for time‐varying cognitive performance and remained statistically significant (i.e., for annualized change in the FAQ score). The association between biomarkers and change in activities of daily living was modified by age, APOE ε4 status, and cognitive status at baseline.
Current findings are in line with a recent study suggesting that amyloid‐positive and ‐negative individuals might have different IADL trajectories, 1 with previous studies suggesting that baseline MRI biomarkers could predict IADL worsening over time regardless of cognitive status 10 and that higher disability in ADL ability is associated with greater pathological burden (e.g., neuritic plaque and neurofibrillary tangle counts) in patients with dementia due to AD. 30 A review 31 showed that neuroanatomical changes (e.g., hippocampal atrophy, white matter changes) were associated with IADL, independent of cognition, but to a lesser extent; cognitive function seemed to be a stronger IADL predictor than brain morphological changes. Baseline temporal and parietal atrophy has been associated with IADL worsening overtime in all cognitive status groups, 10 but most studies have focused on white matter and not gray matter changes. 31 In addition, in individuals with AD, medial frontal and temporo‐parietal regional gray matter atrophy has been associated with decreased cognition, physical functioning, and independence. 12
Cognitive decline is a determinant of functional decline. 31 , 32 Thus, we considered useful to examine the associations using multiple models, with or without adjustment for time‐varying cognitive performance during the follow‐up. The association between AD biomarkers and IADL score was largely attenuated when models were adjusted for time‐varying cognitive performance, keeping however, its statistical significance. Whether this remaining association signifies unmeasured (until present) parameters in the preclinical stage (e.g., very early subtle cognitive and/or activities of daily living functional impairments not captured well with current assessments), remains to be examined.
Cognitive decline begins many years before dementia diagnosis and test scores could remain in the expected, for age, range, concealing the decline. 33 In a similar manner, subtle changes in activities of daily living could occur for some time in the preclinical period until changes of clinical significance (e.g., limiting or threatening independence) are detected. However, most instruments available to detect IADL or ADL decline might be geared toward the later stages of dementia and fewer toward early (prodromal to mild) AD and MCI, 34 even though this period, where daily living abilities have more subtle limitations and independence is not disabled, could be the opportune time for interventions that support daily living abilities as cognition declines 35 and help promote continued independence, 36 or to provide disease‐modifying therapies once they become available. In present study, there were very few participants with a CDR‐SOB (functional)>0 at baseline, thus it is hard to delineate why the A+N+ group had very similar CDR‐SOB (functional) difference from A‐N‐, as the other two groups (A+N‐, A‐N+; model 3), especially when adjusting for time‐varying cognitive performance; it could be due to lack of statistical power or could mean that the FAQ provided a better (more comprehensive) assessment of IADL performance than the functional component of the CDR‐SOB. Findings highlight the necessity to develop sensitive measures that capture both daily living functional and cognitive changes for the earliest stages of preclinical AD. 37 , 38 , 39
The group with A+N+ biomarkers had the steepest decline in IADL performance. This is a significant finding, as we also know that the A+N+ group might have accelerated cognitive decline (compared to A‐N‐), 40 and could be a group prioritized for close follow‐up and preventive interventions when available. These results complement MCSA work 41 showing that those with both abnormal amyloid and neurodegeneration had much higher MCI/dementia progression risk; while, as in the current study, having only one biomarker positive also increased the progression risk (although less so). Although not everyone that is A+N+ will develop dementia in their lifetime, IADL limitations are a major concern in all older adults given their quality of life implications and importance for the management of chronic conditions. 36 We need to note also that this study population includes participants in midlife (31.5% of the study population), allowing for time to accumulate further amyloid or neurodegeneration. On the other hand, individuals in late life who have accumulated non‐amyloid related neurodegeneration might be more vulnerable for cognitive decline in lower than usually set amyloid levels, 40 we could hypothesize that the same could be true for activities of daily living functional decline, as well.
We can assume that IADL decline in the A+N‐ group (or A‐N+ group) is driven by the progression of neurodegeneration. Not all neurodegeneration will be captured by our cortical thickness measure. As a consequence of the multiplicity of neurodegenerative processes, and the fact that the field lacks biomarkers for non‐AD tauopathy or TDP43 proteinopathy, we can only speculate on which of these processes or others is linked to IADL loss. We 42 have previously shown that the prevalence of AN biomarker abnormality is higher in older age groups, but demographics or other clinical features do not substantially aid in establishing etiologies.
The relationship between cognitive and activities of daily living functional changes may differ by diagnostic status (e.g., CU aging vs. MCI vs. dementia), 36 , 43 which is in agreement with our findings that the functional trajectories are different with steepest IADL changes in those with MCI. In addition, in A‐ or N‐ participants, the annualized change in the predicted FAQ score was similar regardless of APOE ε4 status and this is consistent with previous research 44 showing no significant difference in memory performance between APOE ε4‐negative and ‐positive groups in the absence of elevated amyloid.
The study has potential limitations. The study is lacking data on other important biomarkers (e.g., tau, vascular biomarkers and includes dichotomized (abnormal or not) AD biomarkers, but it is probable (as mentioned earlier) that lower – than the cutoff values – burden of biomarker abnormality is associated with IADL abilities decline. We “dichotomize” (as positive/negative) biomarkers that represent an underlying continuous process and classification errors (in particular close to cut points) could occur. We tried to include a measure of the burden of chronic conditions in the analysis (i.e., CCI), but other physical or visual limitations could limit IADL performance. 45 Although we considered in analysis cognitive performance, cognition involves multiple discrete domains (e.g., memory, language, attention) and dysfunction in different cognitive domains might have a differential effect on IADL decline (independent or not of AD biomarkers). 33 Nevertheless, exploring adjustment by multiple different cognitive domains was not one of the current study’s aims, and awaits additional research.
This study also has several strengths. The study has a large number of participants without dementia with well‐defined AD biomarkers, utilizing multimodal, state‐of‐the‐art imaging. We were able to adjust for a composite measure of time‐varying cognitive performance beyond considering only baseline cognitive performance. The study was also able to do multiple sensitivity analyses, generally supporting the similar findings throughout. In addition, the study used informants’ reports, that research has suggested provide more precise IADL information as individuals with MCI might underestimate their limitations. 46 A better understanding of the long‐term trajectories of IADL functional decline would be essential in earlier identification of individuals at risk for dementia and eligible for outcome modifying interventions. 47 Further studies are needed to understand if and when subtle changes in IADL performance are present early in the preclinical AD stages.
Author Contributions
M.V., R.O.R., and R.C.P. contributed to study concept and design; all authors contributed to data acquisition, analysis, and interpretation; M.V., J.A.A. contributed to drafting the text and preparing the figures and tables. All authors critically revised the manuscript for important intellectual content.
Conflicts of Interest
The aim of this study was supported by a research grant by F. Hoffman‐La Roche. M.V. was the primary investigator of this grant.
Acknowledgment
The study was supported by National Institutes of Health (NIH) Grants U01 AG006786, P50 AG016574, R37 AG011378, R01 AG041851, R01 NS097495; F. Hoffman‐La Roche; the GHR Foundation; the Elsie and Marvin Dekelboum Family Foundation; the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic; the Mayo Foundation for Medical Education and Research; the Liston Award; and the Schuler Foundation and was made possible by the Rochester Epidemiology Project (R01 AG034676).
Funding Information
The study was supported by National Institutes of Health (NIH) Grants U01 AG006786, P50 AG016574, R37 AG011378, R01 AG041851, R01 NS097495; F. Hoffman‐La Roche; the GHR Foundation; the Elsie and Marvin Dekelboum Family Foundation; the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic; the Mayo Foundation for Medical Education and Research; the Liston Award; and the Schuler Foundation and was made possible by the Rochester Epidemiology Project (R01 AG034676).
Funding Statement
This work was funded by National Institutes of Health grants P50 AG016574, R01 AG034676, R01 AG041851, R01 NS097495, R37 AG011378, and U01 AG006786; Elsie and Marvin Dekelboum Family Foundation grant ; F. Hoffmann‐La Roche grant ; Alexander Family Alzheimer's Disease Research Professorship of the Mayo Clinic grant ; GHR Foundation grant ; Liston Award grant ; Schuler Foundation grant ; Mayo Foundation for Medical Education and Research grant .
References
- 1. Lilamand M, Cesari M, Cantet C, et al. Relationship Between Brain Amyloid Deposition and Instrumental Activities of Daily Living in Older Adults: A Longitudinal Study from the Multidomain Alzheimer Prevention Trial. J Am Geriatr Soc. 2018;66:1940–1947. [DOI] [PubMed] [Google Scholar]
- 2. Vassilaki M, Aakre JA, Kremers WK, et al. Association Between Functional Performance and Alzheimer's Disease Biomarkers in Individuals Without Dementia. J Am Geriatr Soc. 2018;66:2274–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lilamand M, Cesari M, del Campo N, et al. Amyloid deposition is associated with lower instrumental activities of daily living abilities in older adults. Results From the MAPT Study. J Gerontol a‐Biol 2016;71:391–397. [DOI] [PubMed] [Google Scholar]
- 4. Grimmer T, Henriksen G, Wester HJ, et al. Clinical severity of Alzheimer's disease is associated with PIB uptake in PET. Neurobiol Aging 2009;30:1902–1909. [DOI] [PubMed] [Google Scholar]
- 5. Rowe CC, Ng S, Ackermann U, et al. Imaging beta‐amyloid burden in aging and dementia. Neurology 2007;68:1718–1725. [DOI] [PubMed] [Google Scholar]
- 6. Marshall GA, Olson LE, Frey MT, et al. Instrumental activities of daily living impairment is associated with increased amyloid burden. Dement Geriatr Cogn Disord 2011;31:443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Landau SM, Harvey D, Madison CM, et al. Associations between cognitive, functional, and FDG‐PET measures of decline in AD and MCI. Neurobiol Aging 2011;32:1207–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roy K, Pepin LC, Philiossaint M, et al. Regional fluorodeoxyglucose metabolism and instrumental activities of daily living across the Alzheimer's disease spectrum. J Alzheimers Dis 2014;42:291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Salmon E, Lespagnard S, Marique P, et al. Cerebral metabolic correlates of four dementia scales in Alzheimer's disease. J Neurol 2005;252:283–290. [DOI] [PubMed] [Google Scholar]
- 10. Marshall GA, Lorius N, Locascio JJ, et al. Regional cortical thinning and cerebrospinal biomarkers predict worsening daily functioning across the Alzheimer's disease spectrum. J Alzheimers Dis 2014;41:719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cahn‐Weiner DA, Farias ST, Julian L, et al. Cognitive and neuroimaging predictors of instrumental activities of daily living. J Int Neuropsychol Soc 2007;13:747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vidoni ED, Honea RA, Burns JM. Neural correlates of impaired functional independence in early Alzheimer's disease. J Alzheimers Dis 2010;19:517–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 2008;30:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men: the Mayo Clinic Study of Aging. Neurology 2010;75:889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. St Sauver JL, Grossardt BR, Yawn BP, et al. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol 2011;173:1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pfeffer RI, Kurosaki TT, Harrah CH Jr, et al. Measurement of functional activities in older adults in the community. J Gerontol 1982;37:323–329. [DOI] [PubMed] [Google Scholar]
- 17. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 18. Beck AT, Steer RA, Brown GK. Manual for the Beck depression inventory, 2nd ed. San Antonio, TX: The Psychological Corporation, 1996. [Google Scholar]
- 19. Kokmen E, Smith GE, Petersen RC, et al. The short test of mental status. Correlations with standardized psychometric testing. Arch Neurol 1991;48:725–728. [DOI] [PubMed] [Google Scholar]
- 20. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Int Med 2004;256:183–194. [DOI] [PubMed] [Google Scholar]
- 21. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, 4th ed Washington, DC: American Psychiatric Association, 1994. [Google Scholar]
- 22. McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 23. Jack CR Jr, Wiste HJ, Weigand SD, et al. Defining imaging biomarker cut points for brain aging and Alzheimer's disease. Alzheimers Dement. 2017;13:205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with pittsburgh compound‐B. Ann Neurol 2004;55:306–319. [DOI] [PubMed] [Google Scholar]
- 25. Klunk WE, Koeppe RA, Price JC, et al. The Centiloid Project: standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement 2015;11:1–15.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jack CR Jr, Wiste HJ, Therneau TM, et al. Associations of amyloid, tau, and neurodegeneration biomarker profiles with rates of memory decline among individuals without dementia. JAMA. 2019;321:2316–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tractenberg RE, Weiner MF, Cummings JL, et al. Independence of changes in behavior from cognition and function in community‐dwelling persons with Alzheimer's disease: a factor analytic approach. J Neuropsychiatry Clin Neurosci 2005;17:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vassilaki M, Aakre JA, Kremers WK, et al. Association between functional performance and Alzheimer's disease biomarkers in individuals without dementia. J Am Geriatr Soc 2018;66:2274–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 30. Marshall GA, Fairbanks LA, Tekin S, et al. Neuropathologic correlates of activities of daily living in Alzheimer disease. Alzheimer Dis Assoc Disord 2006;20:56–59. [DOI] [PubMed] [Google Scholar]
- 31. Overdorp EJ, Kessels RP, Claassen JA, Oosterman JM. The Combined Effect of neuropsychological and neuropathological deficits on instrumental activities of daily living in older adults: a systematic review. Neuropsychol Rev 2016;26:92–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gure TR, Langa KM, Fisher GG, et al. Functional limitations in older adults who have cognitive impairment without dementia. J Geriatr Psychiatry Neurol 2013;26:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weintraub S, Carrillo MC, Farias ST, et al. Measuring cognition and function in the preclinical stage of Alzheimer's disease. Alzheimers Dement (N Y) 2018;4:64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sikkes SAM, Hooghiemstra AM, Pijnenburg YAL, Scheltens P. The past, present, and future of instrumental activities of daily living assessments in Alzheimer's disease. Alzheimer's Dement 2016;12:P372–P3. [Google Scholar]
- 35. Lau KM, Parikh M, Harvey DJ, et al. Early cognitively based functional limitations predict loss of independence in instrumental activities of daily living in older adults. J Int Neuropsychol Soc 2015;21:688–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tomaszewski Farias S, Giovannetti T, Payne BR, et al. Self‐perceived difficulties in everyday function precede cognitive decline among older adults in the ACTIVE study. J Int Neuropsychol Soc 2018;24:104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fieo R, Zahodne L, Tang MX, et al. The historical progression from ADL scrutiny to IADL to advanced ADL: assessing functional status in the earliest stages of dementia. J Gerontol A Biol Sci Med Sci 2018;73:1695–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mortamais M, Ash JA, Harrison J, et al. Detecting cognitive changes in preclinical Alzheimer's disease: a review of its feasibility. Alzheimers Dement 2017;13:468–492. [DOI] [PubMed] [Google Scholar]
- 39. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sperling R, Mormino E, Johnson K. The evolution of preclinical Alzheimer's disease: implications for prevention trials. Neuron 2014;84:608–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Petersen RC, Lundt ES, Therneau TM, et al. Predicting Progression to Mild Cognitive Impairment. Ann Neurol 2019;85:155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jack CR Jr, Wiste HJ, Weigand SD, et al. Age‐specific and sex‐specific prevalence of cerebral beta‐amyloidosis, tauopathy, and neurodegeneration in cognitively unimpaired individuals aged 50–95 years: a cross‐sectional study. Lancet Neurol 2017;16:435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rog LA, Park LQ, Harvey DJ, et al. The independent contributions of cognitive impairment and neuropsychiatric symptoms to everyday function in older adults. Clin Neuropsychol 2014;28:215–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lim YY, Kalinowski P, Pietrzak RH, et al. Association of beta‐Amyloid and Apolipoprotein E epsilon4 with memory decline in preclinical Alzheimer disease. JAMA Neurol 2018;75:488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Classon E, Fallman K, Wressle E, Marcusson J. Relations between concurrent longitudinal changes in cognition, depressive symptoms, self‐rated health and everyday function in normally aging octogenarians. PLoS One 2016;11:e0160742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hsiao JJ, Lu PH, Grill JD, Teng E. Longitudinal declines in instrumental activities of daily living in stable and progressive mild cognitive impairment. Dement Geriatr Cogn Disord 2015;39:12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Verlinden VJA, van der Geest JN, de Bruijn R, et al. Trajectories of decline in cognition and daily functioning in preclinical dementia. Alzheimers Dement 2016;12:144–153. [DOI] [PubMed] [Google Scholar]


