Abstract
Background:
Asthma is the most common chronic illness in children and is independently impacted by obesity and by fitness. The National Youth Fitness Survey (NYFS) collected data on aerobic fitness, body composition, and health outcomes in children 6–11 years old. The goal of this study was to test hypotheses regarding relationships between asthma, aerobic fitness, and sedentary time in this uniquely studied cohort of young children.
Methods:
665 children (6-11 y/o, 49% male) were included in analyses. We explored relationships between asthma outcomes and aerobic fitness (measured by endurance time ETmax), self-reported sedentary time, and BMI categories. Fitness was categorized as unfit (lowest 25% of endurance times) or fit. Multivariate logistic regression models were created for asthma outcomes and adjusted for race, age, sex, poverty status, and overweight/obesity.
Results:
17.9% of participants had a previous history of asthma and 11.4% had current asthma. 37.3% of participants were overweight or obese. Low fitness was significantly associated with increased odds of past asthma, current asthma, asthma attacks, wheeze with exercise, and wheeze with activity limitations in multivariate models. Increased sedentary time was significantly associated with increased odds of previous asthma, current asthma, asthma attacks, and activity limitations due to wheezing.
Conclusion:
Decreased aerobic fitness and increased sedentary time were associated with worse asthma outcomes in this group of children (6-11 years old). This data suggests that fitness and sedentary time, both modifiable factors, each have an independent effect on asthma and should be included in assessments and management of asthma health.
Table of Contents Summary:
Data from a nationally representative sample of young children were used to study relationships between aerobic fitness, sedentary time, and asthma outcomes.
INTRODUCTION
Asthma, the most common chronic disease of childhood, continues to cause substantial morbidity among the approximately 6 million children affected in the United States.1 The underlying etiology of asthma is likely multifactorial and is influenced by lifestyle factors including physical activity/exercise and sedentary time.2
Increased physical activity is associated with improved cardiovascular outcomes, psychological health, and plays an important role in the growth and development of children.3 Yet, rates of physical activity have declined over the past 50+ years, while rates of obesity have increased, with almost 1 in 5 school age children currently affected.4,5 The 2018 United States Report Card on Physical Activity for Children and Youth reported that only ~24% of children 6-17 years old meet the recommended guidelines for 60 min of physical activity daily.6 Children with asthma tend to be less physically active than their healthy peers.7,8 While the mechanisms responsible for the reduced PA are not entirely clear, negative self-efficacy, child and parental health beliefs, and poor asthma control play a role.9,10 Improving physical activity and fitness is associated with improved asthma outcomes including improved asthma control, airway hyper-responsiveness, and airway inflammation.11
The combination of obesity, decreased physical activity, and increased sedentary time, each of which can stimulate inflammation, can lead to a vicious cycle in the obese child with asthma, making treatment challenging. A few studies have evaluated the relationship between asthma, obesity, and fitness, primarily in adolescents or adults.12,13 In a previous study, we found that higher fitness was associated with decreased asthma morbidity in adolescent males.14 Understanding relationships between asthma, aerobic fitness, and sedentary time in young children is critical because the clinical trajectory of childhood asthma has important implications for management, prognosis, and overall health.15,16 To our knowledge, there are currently no studies evaluating the relationships between asthma, obesity, and aerobic fitness in children less than 9 years of age. Additionally, the evidence on sedentary time in children with asthma and asthma-related outcomes is lacking.
Testing aerobic fitness in young children is challenging, requiring motivation and coordination, as well as skilled technicians and specialized equipment.17 However, physiologic measurements of fitness are needed as increasing evidence shows that aerobic fitness in children tracks across the lifespan and is an important biomarker of health and disease, including cardiovascular disease risk.18,19 The National Youth Fitness Survey (NYFS) was conducted in 2012 by the Centers for Disease Control and Prevention to obtain nationally representative data on the physical activity and fitness levels of children and adolescents. NYFS collected data on aerobic fitness (endurance time (ETmax)), sedentary time, BMI and asthma outcomes in children 6–11 years old. The goals of our study were to evaluate relationships between asthma, aerobic fitness, and sedentary time in this unique cohort of young children. We hypothesized that lower levels of fitness or increased sedentary time would be associated with worse asthma outcomes and could moderate the relationship between asthma and obesity in young children.
PATIENTS AND METHODS
The NYFS was conducted alongside the 2011-2012 National Health and Nutrition Examination Survey (NHANES). The NYFS used the same complex, stratified, multistage probability cluster sampling design as NHANES, and conducted a home interview followed by physical assessments in a mobile examination center, including cardiorespiratory fitness assessments and body measurements. The survey protocol was approved by the Research Ethics Review Board at the NCHS and written informed consent was obtained from each participant’s parent or legal guardian. Participants aged 7-11 years provided additional signed assent to participate.
Asthma Outcomes
All participants were asked (by proxy if under 16 years) whether” a doctor or other health professional ever told you that you have asthma” (past asthma). Those who answered “yes” were asked a series of additional questions, including whether they “still have asthma” (current asthma), and whether they had experienced an asthma attack in the past 12 months (asthma attack). The primary outcomes for these analyses are a report of past asthma and current asthma.
A separate set of questions were asked about wheezing. Wheeze outcomes used in these analyses include a report of wheezing related to exercise or physical activity (yes/no) and limiting of usual activities due to wheezing or whistling (yes/no).
Body Composition
Height and weight were measured using a portable stadiometer and a portable digital weight scale. Body mass index was expressed as weight in kilograms divided by height in meters squared (kg/m2). Weight status was classified into underweight, normal weight, overweight, and obese based on the age-and gender-specific 5th, 85th, and 95th percentiles of the 2000 CDC growth charts cutoff points.20 In the current study, overweight and obese were combined as overweight/obese (OW/OB).
Calf and triceps skinfolds were measured to the nearest 0.1 millimeter using a skinfold caliper. Arm and calf measurements were made on the right side of the body. Predicted percent body fat was calculated using the Slaughter-Lohman equation.21 Percent fat categories were calculated based on body fat reference curves for children.22 The 2nd, 85th, and 95th percentiles define the cut-off points for underfat, overfat, and obese.
Cardiorespiratory Fitness (CRF) Testing
Aerobic fitness was evaluated by an age-specific treadmill protocol designed to obtain the participant’s maximal endurance time (ETmax) as the key exercise outcome variable. Full details of the protocol can be found at www.cdc.gov/nchs/data/nnyfs/treadmill.pdf. Participants were assigned to one of three age-specific treadmill testing protocols varying in grade and speed (ages 6–7, 8–9, and 10–11). The treadmill test protocol was designed to find ETmax values between 5 and 12 minutes. The protocols include: a 1-minute warmup, seven 2-minute stages designed to maximally exercise most children, three 1-minute additional stages for exceptionally fit children, and a 2-minute recovery stage.23 Heart rate was monitored throughout the exercise tests by electrodes attached to the participant’s chest.
Participants who did not complete CRF testing were excluded from our analysis, including those who were excluded from fitness testing based on medical conditions, certain medications, physical limitations, and safety limitations per discretion of the nurse practitioner. This included participants who had untreated asthma or were prescribed a medication for asthma before participating in exercise or sports but had not brought the inhalant to the mobile examination center.
Physical Activity and Sedentary Time
Proxy-reported physical activity was obtained from the physical activity questionnaire using the question: “During the past 7 days, on how many days was [child’s name] physically active for a total of at least 60 minutes per day? Add up all the time [child’s name] spent in any kind of physical activity that increased [his/her] heart rate and made [him/her] breathe hard some of the time.” Responses ranged from 0–7 days.
Screen time, defined as hours of TV watching plus computer use, was estimated from responses to 2 separate questions: 1) “Over the past 30 days, on average how many hours per day did [child’s name] sit and watch TV or videos?” and 2) “Over the past 30 days, on average about how many hours per day did [child’s name] use a computer or play computer games outside of school?”. For both questions, response options were as follows: none, less than 1 hour, 1 hour, 2 hours, 3 hours, 4 hours, and 5 or more hours. Screen time was calculated by adding the time contributed from both questions. Children were categorized as meeting screen-time recommendations if the combined screen time was 2 hours or less per day.24
Statistical Analyses
Of 762 children who were eligible for the treadmill protocol, 665 children (6–11 y/o, 49% male) completed the NYFS age-specific maximal treadmill protocol and were included in our analyses. Underweight participants (BMI < 5 %ile) were excluded from analyses as they were too few in number (n = 17) to analyze as a group. We evaluated relationships between asthma outcomes (past and current asthma, asthma attacks, wheeze with exercise, and activity limitations due to wheezing) with endurance times, BMI categories, and self-reported sedentary time. Fitness was categorized as low fit (lowest 25% of ETmax). Multivariate logistic regression models were created for asthma outcomes and adjusted for race, age, sex, poverty status (ratio of family income to poverty), and BMI category. Interactions between aerobic fitness/screen time and sex, race, poverty status, and BMI categories on asthma outcomes were tested. All analyses used the examination sample weights to account for NHANES sampling methods. Analyses were performed with STATA 11.2 (StataCorp, College Station, TX). A p value < 0.05 was considered statistically significant for main effects.
RESULTS
Baseline demographics are presented in Table 1. The mean age was 8.4 years, 49.4% of participants were male, and 64.4% were of normal weight. Approximately 12% of participating children had current asthma. Approximately 42.5% of children reported more than 2 hrs of screen time daily, and 57.5% of children reported physical activity of at least 60 mins daily. Sociodemographic and physiologic characteristics of participants with and without current asthma are shown in supplement Table 1.
Table 1.
Baseline Demographics
| Analytic sample (n = 665) |
All NYFS participants (n = 762) |
|
|---|---|---|
| Age in years, mean (SE) | 8.4 (0.05) | 8.4 (0.04) |
| Sex | ||
| Male, % | 50.2 | 51.1 |
| Race/Ethnicity % | ||
| Non-Hispanic White | 52.9 | 52.1 |
| Non-Hispanic Black | 13.4 | 13.4 |
| Hispanic | 13.2 | 13.4 |
| Other race or multi-racial | 20.5 | 21.0 |
| Fitness testing, % | ||
| Done | 100 | 93.2 |
| Stopped early | --1 | 2.3 |
| Not done | --1 | 4.5 |
| BMI categories, % (SE) | ||
| Underweight | --1 | 2.4 |
| Normal weight | 64.3 | 62.8 |
| Overweight | 17.4 | 16.3 |
| Obese | 18.3 | 18.5 |
| Asthma outcomes, % | ||
| Past asthma | 17.4 | 16.9 |
| Current asthma | 12.1 | 11.9 |
| Asthma attacks | 5.3 | 5.5 |
| Wheeze with exercise | 8.4 | 8.2 |
| Wheeze with activity limitations | 8.4 | 8.3 |
| Screen time | ||
| Reported >2 hrs per day, % (SE) | 42.1 | 43.6 |
| Physical activity ≥60 min daily: | ||
| Mean days/week (SE) | 5.7 (0.1) | 5.7 (0.1) |
| Percent reporting ≥ 60mins every day | 57.5 | 57.5 |
Analytic sample excludes underweight participants (n=17), those that did not complete fitness test (n = 50), and those that did not complete the examination (n=30).
Asthma, Fitness, and Obesity
Mean ETmax values were significantly lower in children with past asthma, current asthma, and those with asthma attacks compared to controls (Figure 1). Mean ETmax values were also significantly lower in overweight and obese children compared to normal weight children: 626.5s (SD ± 106.7), 552.4s (SD ± 105.4), and 688.8s (SD ± 134.5), respectively (p < 0.01).
Figure 1.
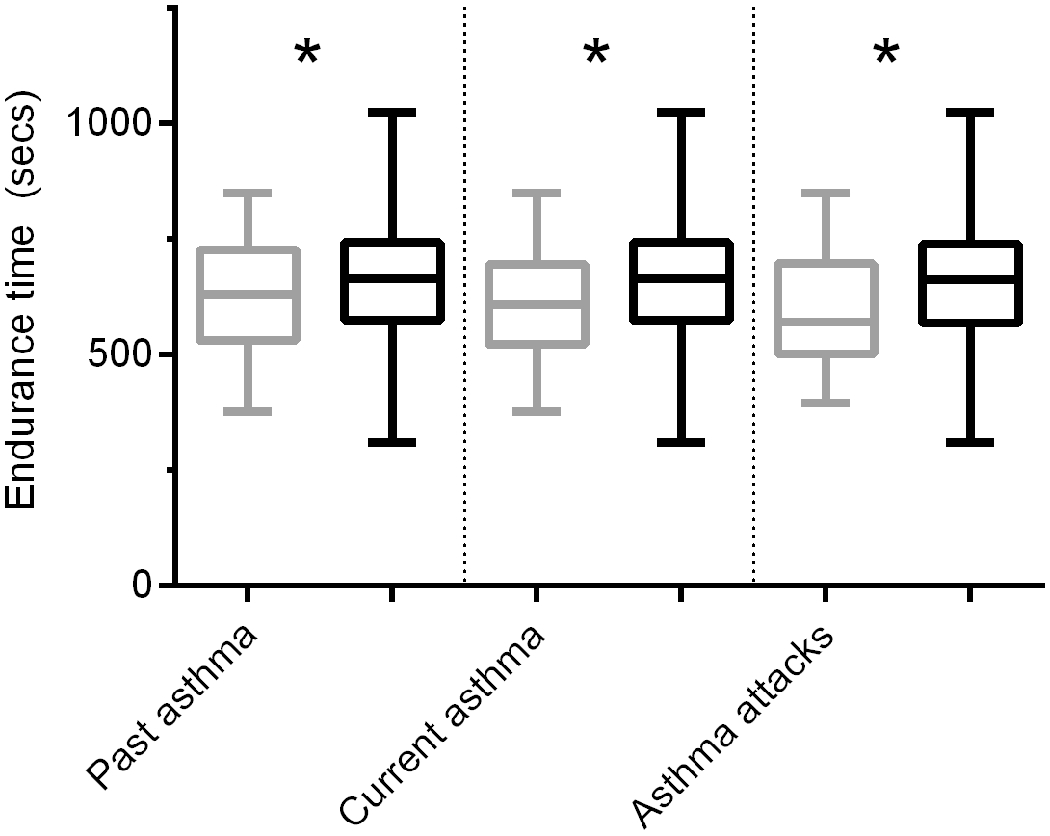
Mean endurance times are significantly lower in children with previous history of asthma, current asthma, and those with asthma attacks (gray bars) compared to controls (black bars). *Asterisks indicate p value < 0.05.
Mean ETmax values by healthy controls vs asthma and normal weight vs overweight/obese are illustrated in Figure 2; Ow/Ob children with asthma had significantly shorter endurance times compared to normal weight controls for all of the asthma outcomes. For example, Ow/Ob children reporting asthma attacks had a mean endurance time of 533.8 s (95% CI 483.8, 583.9) compared to normal weight controls with a mean endurance time of 698.4 s (95% CI 686.6, 710.2), p < 0.05.
Figure 2.
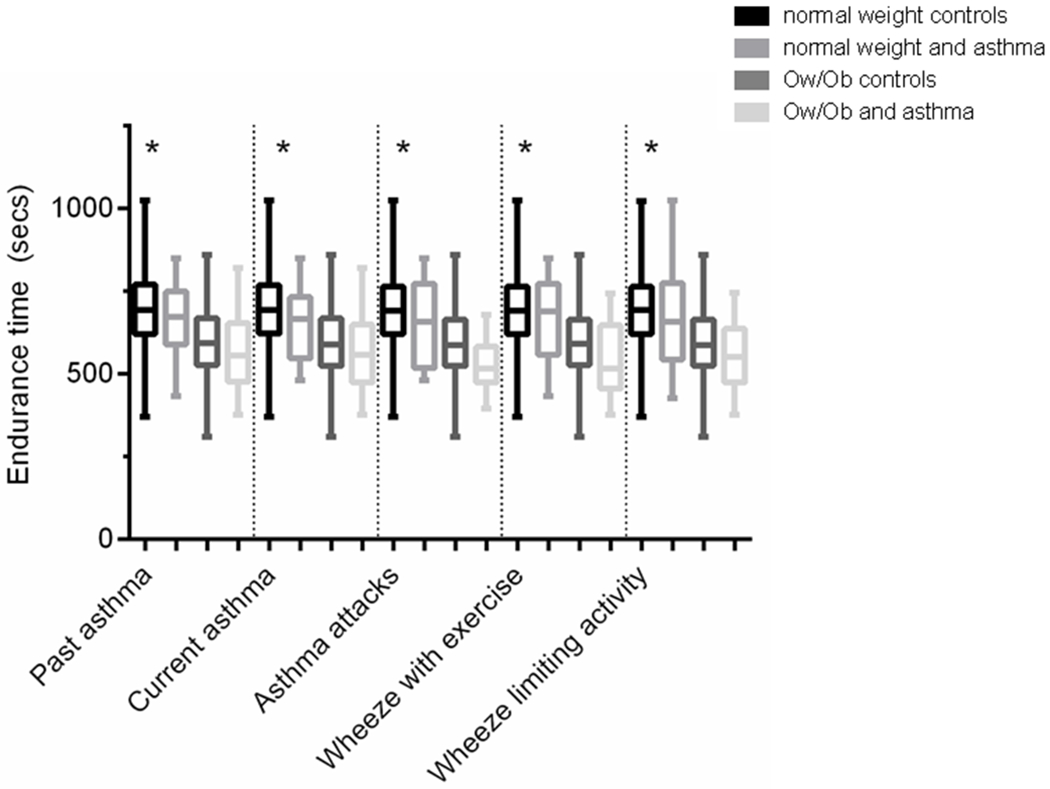
Mean endurance times (95% CI) by healthy controls vs asthma and normal weight vs overweight/obese. Ow/Ob children with asthma have the shortest endurance times compared to normal weight controls for all of the asthma outcomes. For asthma attacks, controls include those with current asthma but no asthma attacks as well as healthy controls. *Asterisks indicates significant difference between Ow/Ob children with asthma outcome and normal weight controls (p value < 0.05).
ETmax values were weakly correlated with reported days of physical activity (at least 60 mins) with r = 0.219, p < 0.001.
Odds of asthma outcomes in lower fit children are shown in Table 2. Overall, lower aerobic fitness was significantly associated with increased odds of past asthma, current asthma, asthma attacks, wheeze with exercise, and wheeze with activity limitations, after adjusting for age, sex, race, poverty status, and BMI category. There were no significant interactions between aerobic fitness and obesity status for any of the asthma outcomes analyzed (all p > 0.05). Being overweight/overfat or obese (based on BMI categories or body fat) was not significantly associated with worse asthma outcomes (see Table 3 and supplement Table 2).
Table 2.
Odds of asthma outcomes in low-fit compared to higher-fit children
| OR (95% CI) Unadjusted |
OR (95% CI) Adjusted |
|
|---|---|---|
| Past asthma | 2.34 (1.34, 4.06) | 2.45 (1.27, 4.72) |
| Current asthma | 2.50 (1.16, 5.39) | 3.09 (1.15, 8.33) |
| Asthma attacks | 3.05 (1.09, 8.49) | 4.50 (1.57, 12.92) |
| Wheeze with exercise | 3.13 (1.28, 7.64) | 4.73 (1.55, 14.45) |
| Wheeze with activity limitations | 2.57 (1.14, 5.81) | 4.53 (1.85, 11.16) |
Models are adjusted for age, sex, race, poverty status, and BMI category.
Significant ORs are bolded (p <0.05).
Table 3.
Odds of asthma outcomes for normal weight versus overweight/obese children.
| OR (95% CI) Unadjusted |
OR (95% CI) Adjusted |
|
|---|---|---|
| Past asthma | 1.41 (0.82, 2.41) | 1.02 (0.55, 1.89) |
| Current asthma | 1.26 (0.66, 2.39) | 0.84 (0.39, 1.82) |
| Asthma attacks | 0.94 (0.30, 2.99) | 0.60 (0.20, 1.82) |
| Wheeze with exercise | 1.25 (0.54, 2.88) | 0.85 (0.34, 2.10) |
| Wheeze with activity limitations | 0.75 (0.31, 1.84) | 0.47 (0.17, 1.28) |
Adjusted for sex, age, race, poverty, and fitness.
Asthma and Sedentary Time
Approximately 42.5% of children reported more than 2 hrs of screen time daily. Children reporting greater than 2 hrs of screen time daily had increased rates of past asthma, current asthma, asthma attacks, and wheeze with activity limitations compared to children reporting 2 hrs or less of screen time daily. Odds of asthma outcomes in children reporting 2 hrs or more of screen time daily compared to children reporting 2 hrs or less of screen time daily are shown in Table 4. Increased sedentary time remained significantly associated with increased odds of past asthma, current asthma, asthma attacks, and wheeze with activity limitations, after adjusting for multiple confounders.
Table 4.
Odds of asthma outcomes in children reporting more than 2 hrs of screen time daily compared to children reporting 2 hrs or less of screen time daily
| OR (95% CI) Unadjusted |
OR (95% CI) Adjusted |
|
|---|---|---|
| Past asthma | 1.79 (1.07, 2.99) | 1.84 (1.18, 2.87) |
| Current asthma | 1.82 (1.05, 3.17) | 1.98 (1.32, 2.96) |
| Asthma attacks | 1.70 (1.08, 2.66) | 1.87 (1.04, 3.36) |
| Wheeze with exercise | 1.48 (0.74, 2.97) | 1.63 (0.84, 3.16) |
| Wheeze with activity limitations | 2.25 (1.00, 5.06) | 2.71 (1.00, 7.33) |
Adjusted for sex, age, race, poverty, BMI category, and fitness.
Significant ORs are bolded (p <0.05).
DISCUSSION
In this unique cohort of children ages 6–11 years in the United States, we found that lower levels of aerobic fitness were seen in children with past asthma, current asthma, asthma attacks, wheeze with exercise, and wheeze with activity limitations. We also found that increased sedentary time was associated with worse asthma outcomes including past asthma, current asthma, asthma attacks, and wheeze with activity limitations. To our knowledge, this is one of the first studies to test the associations of aerobic fitness, sedentary time, and asthma outcomes in young children.
Guldberg-Moller and colleagues followed 1360 children longitudinally in the Odense schoolchild study with cardiorespiratory fitness testing at 9 years of age with follow-up up through 29 years of age.25 The authors found that lower physical fitness at age 9 was significantly associated ever having asthma. Mcnarry and colleagues studied 20577 children 9–12 years old in the U.K. who completed a 20 m shuttle run test and found that children with asthma had lower shuttle run performance compared to healthy controls.26 They also found a significant sex effect and sex/asthma interaction on shuttle run performance. Our findings are consistent with these earlier reports while at the same time we extend these associations to children as young as 6 years old.
Our findings are in contrast to those of Chen and colleagues who followed 2758 children in Taiwan from 4th to 6th grade who completed an 800 m sprint test to determine cardiorespiratory fitness. Children were categorized into low and high fit based on sex and age corrected z-score.12 They found that low fitness was not associated with active or physician-diagnosed asthma. The authors concluded that central obesity was most associated with asthma, and low fitness and high screen time increased the risk for central obesity. In our analysis, increased weight was associated with poor aerobic fitness; however, we did not find a statistically significant relationship between obesity and asthma outcomes. While the majority of studies have shown associations between overweight/obesity and asthma in children, there have been studies showing no association between obesity and asthma including a few large studies in younger children similar in age to this cohort27–30. Additionally, obesity did not moderate the relationship between asthma and aerobic fitness. The discrepancies may be due to differences in the patient population, the definition of ‘fitness,’ or the testing administered (i.e., a field sprint test administered at the schools compared to the NYFS progressive maximal treadmill test).
The increase in sedentary time, particularly the excessive screen time in children over the past several years, is concerning and has prompted updated guidelines from the American Academy of Pediatrics and the American Heart Association.31,32 Increased sedentary time, independent of aerobic fitness, is associated with poor health outcomes including obesity, type 2 diabetes, as well as all-cause and cardiovascular disease mortality.33,34 A recent systematic review by Cordova-Rivera et al. found that in adults with asthma, physical activity is reduced and is associated with worse asthma outcomes.35 These investigators also reported that sedentary time did not differ between adults with asthma and controls though only 4 studies were included. While there is increasing number of studies using objective measures of physical activity such as actigraphy, few studies have studied sedentary time in children with asthma. Rota and colleagues found that children with asthma engaged in significantly more screen time compared to healthy controls (median 35 vs 26 hr/week, respectively) in an urban environment.36 Proudjer and colleagues found that high screen time (>1hr/day) was associated with greater odds of asthma, and this relationship was more pronounced in overweight youth.37 We found that children with an average reported screen time greater than 2 hrs daily had significantly increased odds of current asthma, with an adjusted odds ratio of 1.93. Increased screen time was also associated with increased odds of past asthma, asthma attack, and wheezing with activity limitations.
Understanding relationships between aerobic fitness and sedentary time, both modifiable factors, on asthma risk and morbidity, particularly in young children, provides potential opportunities for intervention. There is mounting data that fitness in children tracks across the lifespan and may prove to be an early, modifiable indicator of cardiovascular disease risk later in life.38 Large longitudinal studies have shown that childhood asthma is associated with reduced pulmonary function that tracks into adulthood;16 however, how fitness and sedentary time in young children affect asthma outcomes, including lung function in adults, in unknown. While the underlying mechanism(s) of improving fitness on asthma is unknown, a growing number of animal models and human studies suggest that aerobic exercise reduces airway remodeling, pro-inflammatory cytokines/responses including glucocorticoid receptor expression, and enhances regulatory T cell responses.39–41 Interventions targeting increased physical activity and decreasing sedentary time may be more effective in younger children, who have less autonomy and control of their external environment.42
A major strength of our findings is the objective measure of fitness in a large group of young children using a maximal exercise test, which is extremely challenging in this age group. Further, we did not find a significant association between overweight/obesity and asthma outcomes highlighting the importance of fitness independently and beyond the role of obesity in young children. Additionally, the population was sampled to be representative of the U.S. Most recently, Proudfoot and colleagues measured physical activity and fitness in a very young cohort (3–5 years old) and found that higher levels of fitness, even in young children, were associated with better cardiovascular health indicators including decreased arterial stiffness.43 One limitation of the study is the cross-sectional design and thus we are unable to establish temporal relationships between aerobic fitness, sedentary time, and asthma outcomes. While no objective measures of physical activity were collected, children with asthma reported fewer days of physical activity compared to controls, which likely contributes to their aerobic fitness. However, it is unclear whether young children who are less active are more likely to develop asthma or whether their asthma limits their activity levels. Another limitation is the asthma diagnoses or morbidity were based subjectively on questionnaires and, unfortunately, no objective measures of asthma were captured, including pulmonary function testing. Studies evaluating relationships between lung function and aerobic fitness have shown mixed results.44,45 Lastly, other potential confounders including family history (i.e. asthma or allergy) or environmental exposures (i.e. tobacco smoke, air pollution) or reliable medication use were not available in this cohort.
Our data also suggest some intriguing possible biological mechanisms, namely, the validity of the “hygiene hypothesis”—the notion that early life exposure to immune system mediators could influence the later development of asthma.46 In the hygiene hypothesis, asthma later in life is more frequent in children who grew up in “hygienic,” typically urban, environments. Children raised in agricultural settings in rural environments are more likely to be exposed to a variety of antigens that stimulate the immune system. This early exposure downregulates the immune system and reduces the risk of asthma later in life. Like the non-hygienic environment, exercise stimulates both pro- and anti-inflammatory immune mechanisms,47 and studies in the murine model suggest that exercise-induced bronchoconstriction can result even if the original immune stimulus is unrelated specifically to physical activity.48 Our observation of generally greater asthma severity in physical inactive young children leads to the speculation that physical inactivity early in life might limit immune downregulation enhancing the possibility of asthma later in life.
CONCLUSION
In summary, this data suggests that aerobic fitness, a modifiable factor, has an independent effect on asthma outcomes. We also found that increased sedentary time is associated with worse asthma outcomes. Both aerobic fitness and sedentary time should be included in assessments and management of asthma health in children.
Supplementary Material
What This Study Adds:
In young children, 6–11 years old, low fitness and increased sedentary time are associated with increased asthma prevalence and worse asthma symptoms.
Acknowledgments
Funding Source: UCI CTSI grant UL1-TR00141, U01TR002004 (project REACH)
Dr. Forno’s contribution was partly funded by NIH grant HL125666.
Abbreviations:
- BMI
Body mass index
- ETmax
Endurance time
- NHANES
National Health and Nutrition Examination Survey
- NYFS
National Youth Fitness Survey
- OW/OB
Overweight/Obese
Footnotes
Financial Disclosure: none.
Conflict of Interest: none.
References
- 1.CDC - Asthma - Most Recent Asthma Data - Current Asthma Prevalence 2016. https://www.cdc.gov/asthma/most_recent_data.htm Accessed August 1, 2018.
- 2.Lucas SR, Platts-Mills TA. Paediatric asthma and obesity. PaediatrRespirRev 2006;7(1526-0542 (Print)):233–238. [DOI] [PubMed] [Google Scholar]
- 3.Hallal PC, Victora CG, Azevedo MR, Wells JCK. Adolescent Physical Activity and Health. Sport Med 2006;36(12):1019–1030. [DOI] [PubMed] [Google Scholar]
- 4.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity Among Adults and Youth: United States, 2015-2016. NCHS Data Brief 2017;(288):1–8. [PubMed] [Google Scholar]
- 5.Boreham C, Riddoch C. The physical activity, fitness and health of children. J Sports Sci. 2001;19(12):915–929. [DOI] [PubMed] [Google Scholar]
- 6.National Physical Activity Plan Alliance. The 2018 United States Report Card on Physical Activity for Children and Youth. National Physical Activity Plan Alliance; https://www.physicalactivityplan.org/projects/reportcard.html Published 2018. Accessed May 8, 2019. [Google Scholar]
- 7.Groth SW, Rhee H, Kitzman H. Relationships among obesity, physical activity and sedentary behavior in young adolescents with and without lifetime asthma. J Asthma 2015;(1532-4303 (Electronic)):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams B, Powell A, Hoskins G, Neville R. Exploring and explaining low participation in physical activity among children and young people with asthma: a review. BMCFamPract 2008;9(1471-2296 (Electronic)):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glazebrook C, McPherson AC, Macdonald IA, et al. Asthma as a barrier to children’s physical activity: implications for body mass index and mental health. Pediatrics. 2006;118(1098-4275 (Electronic)):2443–2449. [DOI] [PubMed] [Google Scholar]
- 10.Leinaar E, Alamian A, Wang L. A systematic review of the relationship between asthma, overweight, and the effects of physical activity in youth. Ann Epidemiol. 2016;26(7):504–510. e6. [DOI] [PubMed] [Google Scholar]
- 11.Eichenberger PA, Diener SN, Kofmehl R, Spengler CM. Effects of exercise training on airway hyperreactivity in asthma: a systematic review and meta-analysis. Sport Med 2013;43(1179-2035 (Electronic)):1157–1170. [DOI] [PubMed] [Google Scholar]
- 12.Chen YC, Tu YK, Huang KC, Chen PC, Chu DC, Lee YL. Pathway from central obesity to childhood asthma. Physical fitness and sedentary time are leading factors. AmJRespirCrit Care Med. 2014;189(1535-4970 (Electronic)):1194–1203. [DOI] [PubMed] [Google Scholar]
- 13.Shim YM, Burnette A, Lucas S, et al. Physical deconditioning as a cause of breathlessness among obese adolescents with a diagnosis of asthma. PLoSOne 2013;8(1932-6203 (Electronic)):e61022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu KDKD, Billimek J, Bar-Yoseph R, Radom-Aizik S, Cooper DMDM, Anton-Culver H. Sex Differences in the Relationship between Fitness and Obesity on Risk for Asthma in Adolescents. 2016;176:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang AL, Datta S, Weiss ST, Tantisira KG. Remission of persistent childhood asthma: Early predictors of adult outcomes. J Allergy Clin Immunol. 2019;143(5):1752–1759. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGeachie MJ, Yates KP, Zhou X, et al. Patterns of Growth and Decline in Lung Function in Persistent Childhood Asthma. N Engl J Med. 2016;374(19):1842–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper DM. Exercise Science and Child Health: A Tale of Many Journeys. Pediatr Exerc Sci. 2019;31(2):164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt MD, Magnussen CG, Rees E, Dwyer T, Venn AJ. Childhood fitness reduces the long-term cardiometabolic risks associated with childhood obesity. Int J Obes. 2016;40(7):1134–1140. [DOI] [PubMed] [Google Scholar]
- 19.Shah RV, Murthy VL, Colangelo LA, et al. Association of Fitness in Young Adulthood With Survival and Cardiovascular Risk. JAMA Intern Med. 2016;176(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11 2002;(246):1–190. http://www.ncbi.nlm.nih.gov/pubmed/12043359. [PubMed] [Google Scholar]
- 21.Slaughter MH, Lohman TG, Boileau RA, et al. Skinfold equations for estimation of body fatness in children and youth. Hum Biol. 1988;60(5):709–723. [PubMed] [Google Scholar]
- 22.McCarthy HD, Cole TJ, Fry T, Jebb SA, Prentice AM. Body fat reference curves for children. Int J Obes. 2006;30(4):598–602. [DOI] [PubMed] [Google Scholar]
- 23.Borrud L, Chiappa MM, Burt VL, et al. National Health and Nutrition Examination Survey: national youth fitness survey plan, operations, and analysis, 2012. Vital Health Stat 2 2014;(163):1–24. [PubMed] [Google Scholar]
- 24.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120 Suppl(1098-4275 (Electronic)):S164–S192. [DOI] [PubMed] [Google Scholar]
- 25.Guldberg-Møller J, Hancox B, Mikkelsen D, et al. Physical fitness and amount of asthma and asthma-like symptoms from childhood to adulthood. ClinRespirJ 2014;9(1752-699X (Electronic)):314–321. [DOI] [PubMed] [Google Scholar]
- 26.McNarry MA, Boddy LM, Stratton GS. The relationship between body mass index, aerobic performance and asthma in a pre-pubertal, population-level cohort. Eur J Appl Physiol. 2014;114(2):243–249. [DOI] [PubMed] [Google Scholar]
- 27.To T, Vydykhan TN, Dell S, Tassoudji M, Harris JK. Is obesity associated with asthma in young children? J Pediatr. 2004;144(2):162–168. [DOI] [PubMed] [Google Scholar]
- 28.Liu PC, Kieckhefer GM, Gau BS. A systematic review of the association between obesity and asthma in children. J Adv Nurs 2013;69(1365-2648 (Electronic)):1446–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartz J, Gold D, Dockery DW, Weiss ST, Speizer FE. Predictors of asthma and persistent wheeze in a national sample of children in the United States. Association with social class, perinatal events, and race. Am Rev Respir Dis. 1990;142(3):555–562. [DOI] [PubMed] [Google Scholar]
- 30.Chinn S, Rona RJ. Can the increase in body mass index explain the rising trend in asthma in children? Thorax 2001;56(0040-6376 (Print)):845–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnett TA, Kelly AS, Young DR, et al. Sedentary Behaviors in Today’s Youth: Approaches to the Prevention and Management of Childhood Obesity: A Scientific Statement From the American Heart Association. Circulation. 2018;138(11). [DOI] [PubMed] [Google Scholar]
- 32.COUNCIL ON COMMUNICATIONS AND MEDIA COCA. Media Use in School-Aged Children and Adolescents. Pediatrics. 2016;138(5):e20162592. [DOI] [PubMed] [Google Scholar]
- 33.Biswas A, Oh PI, Faulkner GE, et al. Sedentary Time and Its Association With Risk for Disease Incidence, Mortality, and Hospitalization in Adults. Ann Intern Med. 2015;162(2):123. [DOI] [PubMed] [Google Scholar]
- 34.Proper KI, Singh AS, van Mechelen W, Chinapaw MJM. Sedentary Behaviors and Health Outcomes Among Adults. Am J Prev Med 2011;40(2):174–182. [DOI] [PubMed] [Google Scholar]
- 35.Cordova-Rivera L, Gibson PG, Gardiner PA, McDonald VM. A Systematic Review of Associations of Physical Activity and Sedentary Time with Asthma Outcomes. J Allergy Clin Immunol Pract. 2018;6(6):1968–1981. e2. [DOI] [PubMed] [Google Scholar]
- 36.Rota AP, Bacharier LB, Jaffee K, et al. Screen Time Engagement Is Increased in Urban Children With Asthma. Clin Pediatr (Phila) 2017;56(11):1048–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Protudjer J, Kozyrskyj AL, McGavock JM, Ramsey CD, Becker AB. High Screen Time Is Associated with Asthma in Overweight Manitoba Youth. J Asthma 2012;49(9):935–941. [DOI] [PubMed] [Google Scholar]
- 38.Pahkala K, Laitinen TT, Heinonen OJ, et al. Association of Fitness With Vascular Intima-Media Thickness and Elasticity in Adolescence. Pediatrics. 2013;132(1):e77–e84. [DOI] [PubMed] [Google Scholar]
- 39.Lu KDKD, Cooper DM, Haddad F, Zaldivar F, Kraft M, Radom-Aizik S. Glucocorticoid receptor expression on circulating leukocytes in healthy and asthmatic adolescents in response to exercise. Pediatr Res. 2017;82(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lowder T, Dugger K, Deshane J, Estell K, Schwiebert LM. Repeated bouts of aerobic exercise enhance regulatory T cell responses in a murine asthma model. Brain Behav Immun. 2010;24(1):153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silva RA, Almeida FM, Olivo CR, Saraiva-Romanholo BM, Martins MA, Carvalho CR. Exercise reverses OVA-induced inhibition of glucocorticoid receptor and increases anti-inflammatory cytokines in asthma. Scand J Med Sci Sport. 2016;26(1600-0838 (Electronic)):82–92. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt ME, Haines J, O’Brien A, et al. Systematic Review of Effective Strategies for Reducing Screen Time Among Young Children. Obesity 2012;20(7):1338–1354. [DOI] [PubMed] [Google Scholar]
- 43.Proudfoot NA, King-Dowling S, Cairney J, Bray SR, MacDonald MJ, Timmons BW. Physical Activity and Trajectories of Cardiovascular Health Indicators During Early Childhood. Pediatrics. June 2019:e20182242. [DOI] [PubMed] [Google Scholar]
- 44.Wong TW, Yu TS, Wang XR, Robinson P. Predicted maximal oxygen uptake in normal Hong Kong Chinese schoolchildren and those with respiratory diseases. Pediatr Pulmonol. 2001;31(2):126–132. [DOI] [PubMed] [Google Scholar]
- 45.Berntsen S, Lødrup Carlsen KC, Anderssen SA, Mowinckel P, Carlsen K-H. Factors associated with aerobic fitness in adolescents with asthma. Respir Med. 2013;107(8):1164–1171. [DOI] [PubMed] [Google Scholar]
- 46.Stein MM, Hrusch CL, Gozdz J, et al. Innate Immunity and Asthma Risk in Amish and Hutterite Farm Children. N Engl J Med. 2016;375(5):411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nimmo MA, Leggate M, Viana JL, King JA. The effect of physical activity on mediators of inflammation. Obe Metab. 2013;15:51–60. [DOI] [PubMed] [Google Scholar]
- 48.Kodesh E, Zaldivar F, Schwindt C, et al. A rat model of exercise-induced asthma: a nonspecific response to a specific immunogen. AmJ Physiol RegulIntegrComp Physiol 2011;300(1522-1490 (Electronic)):R917–R924. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


