Abstract
Right ventricular (RV) function and autonomic dysfunction are important determinants of morbidity and mortality in patients with pulmonary arterial hypertension (PAH). Although successful in animal studies, effects of beta-blocker therapy on RV function in clinical trials were disappointing. To understand this discrepancy, we studied whether beta-blocker therapy changes RV sympathetic activity. Idiopathic PAH (IPAH) patients received beta-blocker therapy (uptitrated to a maximal tolerated dose) and underwent cardiac magnetic resonance imaging, right heart catheterization, and a [11C]-hydroxyephedrine positron emission tomography ([11C]HED PET) scan at baseline to determine, respectively, RV ejection fraction (RVEF), RV pressures, and sympathetic activity. [11C]HED, a norepinephrine analogue, allows determination of sympathetic innervation of the RV. [11C]HED retention index reflects norepinephrine transporter activity. As a consequence of excessive catecholamine levels in the synaptic cleft, this transporter may be downregulated. Therefore, low [11C]HED retention index indicates high sympathetic activity. 13 IPAH patients underwent [11C]HED PET scans at baseline and after bisoprolol treatment. Although heart rate was reduced, systemic modulation of autonomic activity by bisoprolol did not affect local RV sympathetic nerve activity, RV function, or RV wall tension. In PAH patients, RV [11C]HED retention index was lower compared to LV tracer uptake (p<0.01) and was related to systolic wall tension (R2 = 0.4731, p<0.01) and RV function (R2 = 0.44, p = 0.01). In RV failure, the tolerated dosage of bisoprolol did not result in an improvement of RV function nor in a reduction in RV sympathetic activity.
Keywords: pulmonary hypertension, right ventricular failure, sympathetic activity, beta-blocker, nuclear imaging
Introduction
Autonomic imbalance has been implicated in the development and progression of right ventricular (RV dysfunction) in pulmonary arterial hypertension (PAH). Parasympathetic activity is suppressed, whereas the sympathetic nervous system is upregulated.1–3 Increased sympathetic activity is associated with clinical deterioration and mortality, which may cause further compromise RV function.3–5 Consequently, therapies that restore autonomic balance by inhibiting sympathetic activity, such as beta-blocker therapy, have been of interest for the past decades. Despite promising results in animal models,6–8 the effects in PAH patients have been disappointing.9,10 To understand this discrepancy between preclinical success and clinical failure, we need to better delineate the relation between sympathetic regulation and RV dysfunction and the effect of beta-blocker therapy on sympathetic activity in the human right ventricle. Previously, we were limited to investigate changes in local cardiac sympathetic activity in end-stage disease only.11 With the development of in vivo imaging techniques, we are able to determine alterations in local sympathetic activation already at an earlier stage of the disease.
The [11C]Hydroxyephedrine positron emission tomography ([11C]HED PET) tracer is a norepinephrine analogue. Re-uptake of this tracer by the norepinephrine transporter (NET) from the synaptic cleft into the synapse reflects sympathetic nerve activity.12 Consequently, this tracer has been of interest to obtain information on cardiac sympathetic nervous system in several diseases affecting the left ventricle.13 [11C]HED has been extensively validated in animal models and patients with left heart failure.14–18
To understand why beta-blocker therapy did not result in improvement of RV function in patients with pulmonary arterial hypertension, we aimed to clarify the effect of bisoprolol treatment on RV sympathetic activity and to elucidate the relation between RV sympathetic activity and RV function and, taking advantage of [11C]-HED PET imaging.
Methods
Study population
This study is a substudy of a previously published randomized, placebo-controlled, double-blind, cross-over study.10 Patients with idiopathic PAH (IPAH) were included when they were stable on PAH-specific treatment, in New York Heart Association (NYHA) functional class II or III, and in sinus rhythm. Stability on PAH-specific treatment was defined as no change in PAH medication and NYHA functional class together with a <10% change in 6-minute walking distance during the past six months. Inclusion and exclusion criteria have been previously described in detail.10 The study was approved by the Medical Ethics Review Committee of the VU University Medical Center, in accordance with the Declaration of Helsinki, and written informed consent was obtained from all patients.
Study design
The study design has been described in detail previously.10 In short, patients were randomly assigned to either placebo or bisoprolol therapy (starting dose of 1.25 mg daily) in a 1:1 ratio for a period of six months. Every two weeks, patients were evaluated and dosage was increased with 1.25 mg steps when tolerated with a maximum dosage of 10 mg daily. After six months, all patients crossed over to the other treatment group and were treated for another six months. At the time of randomization, at cross-over, and after six months after cross-over, all patients underwent right heart catheterization, cardiovascular magnetic resonance (CMR) imaging, and [11C]HED PET.
PET image acquisition and analysis
All PET studies were performed using a Gemini TF 64 PET/CT scanner (Philips Healthcare, Best, The Netherlands). The scanning protocol consisted of a dynamic 60-min emission scan, which was started simultaneously with the injection of a 5 mL bolus (0.8 mL·s−1) of 370 MBq [11C]HED followed by a 35 mL saline flush (2 mL·s−1). During this scan, 7 × 8 mL venous blood samples were collected manually at 2.5, 5, 10, 20, 30, 40, and 60 min to determine plasma and whole blood activity concentrations, together with radiolabeled plasma [11C]HED metabolites. The dynamic scan was followed immediately by a respiration averaged low-dose CT scan to correct for attenuation. Images were reconstructed into 36 frames (1 × 10, 8 × 5, 4 × 10, 3 × 20, 5 × 30, 5 × 60, 4 × 150, 4 × 300, and 2 × 600 s) using the 3D row action maximum likelihood algorithm with application of all appropriate corrections.
PET image analysis was performed using in-house developed software. Image derived input functions were obtained by placing 1 cm diameter regions of interest (ROIs) over the ascending aorta in at least five transaxial planes of the frame that showed the first pass of the injected bolus. These ROIs were combined in volumes of interest (VOI) for the ascending aorta. In addition, a right ventricular VOI was obtained by drawing a second set of ROIs in at least three trans axial planes over the RV and at a distance of at least 1 cm from the myocardial wall. Subsequently, both VOIs were transferred to the full dynamic image set to obtain arterial and right ventricular whole blood time-activity curves (TAC). Ratios of plasma/whole blood concentrations and parent fractions derived from the manual blood samples were fitted to a sigmoid function after a venous-to-arterial blood transformation was applied.19 Subsequently, the arterial whole blood TAC was multiplied by both the fitted plasma/whole blood ratio and parent fraction curves to obtain the metabolite corrected arterial plasma TAC. Next, segmental VOIs were defined manually in all short axis images of the final frame of the dynamic scan. Myocardial [11C]HED uptake was expressed using the retention index (RI) which was calculated as the uptake in the last frame (50–60 min) divided by the integral of the arterial plasma corrected TAC. Since the [11C]HED retention index is calculated by normalizing the HED tissue concentration in the RV to the integral of the HED time activity curve in the blood, myocardial mass should not affect this index. [11C]HED RI was regionally calculated in the RV free wall, interventricular septum, and left ventricular (LV) free wall.
CMR image acquisition
The CMR image acquisition and analysis protocol has been described previously.20 In short, CMR studies were performed on a 1.5-Tesla Sonato whole body scanner (Siemens Medical Solutions, Erlangen, Germany) using a dedicated phased-array body coil. After survey scans, cine imaging was performed using a retrospectively ECG-gated, steady-state free precession sequence during breath holds in mild expiration. Standard four-, three-, and two-chamber orientations were obtained, and subsequently, a stack of 10 to 12 consecutive short-axis slices was acquired, fully covering both the LV and RV from base to the apex.
Images were analyzed using the software package MASS (MR Analytical Software System, Medis, Leiden, The Netherlands). Epicardial and endocardial borders of the RV and LV were outlined manually in both end diastolic and end systolic phases in all short axis cine images. Papillary muscles were excluded from the ventricular volumes. Ventricular volumes, mass, and ejection fraction were computed for the RV and LV using this software.
Wall tension
Systolic right ventricular wall tension was calculated according to the following equation21
where systolic PAP denotes systolic pulmonary artery pressure and RVESV denotes right ventricular end systolic volume.
Analysis and statistics
We determined the effect of bisoprolol on RV sympathetic activity by comparing RV [11C]HED RI at baseline and after six months of bisoprolol therapy. [11C]HED RI represents re-uptake of the tracer, which is an epinephrine analogue, by the norepinephrine transporter (NET) (Fig. 1a).14 In addition, we investigated whether RV sympathetic activity was related to ventricular function and how this activity is regulated. To investigate whether RV pre synaptic sympathetic activity is systemically or locally driven, we first compared RV free wall [11C]HED RI with left ventricular (LV) free wall [11C]HED RI. In order to study whether this RV sympathetic nerve upregulation is systemically regulated via baroreceptor activation by forward failure, we tested the relation between systolic blood pressure, which is the main baroreceptor stimulator,22 and the RV [11C]HED RI.
Fig. 1.
Right ventricular [11C]HED retention in pulmonary arterial hypertension. (a) [11C]HED retention index (RI) reflects NET activity. As a consequence of excessive catecholamine levels in the synaptic cleft, this transporter may be downregulated. Therefore, low [11C]-HED RI indicates high presynaptic sympathetic nerve activity.12,14 (b) Positron emission tomography scan of a study patient illustrating lower [11C]HED RI in the right ventricle when compared to the left ventricle. (c) Paired T-test revealed a significant difference in baseline [11C]HED RI between RV and LV free wall. No changes in [11C]HED uptake after bisoprolol or placebo were found with two-way repeated measures ANOVA. [11C]HED: [11C]-hydroxyephedrine; NET: norepinephrine transporter; RV: right ventricular, LV: left ventricular; DOPA: 3,4-dihydroxyphenylalanine; NE: norepinephrine.
Continuous data are presented as means with standard deviations (SD), whereas categorical data are expressed as frequencies with percentages. Medians with interquartile rage (IQR) are presented for non-parametric data. Histograms were used to evaluate whether continuous data were normally distributed. Paired continuous data were compared using the paired T-test. The interrelations between PET and CMR variables were evaluated using the Pearson's correlation coefficient when data were normally distributed, for non-normally distributed data Spearman's correlation coefficient was used. All tests were performed two-sided and were considered statistically significant when p-value < 0.05. All statistical analyses were performed using the SPSS software package (SPSS 20.0, IBM Corporation, Chicago, IL, USA) and GraphPad software (Prism version 7.02 for Windows, La Jolla, CA, USA).
Results
Study population
In the initial study, 18 patients with IPAH in NYHA functional class II and III were included.10 Of these 18 patients, 13 patients had a representative [11C]HED PET scan at baseline, and 5 scans could not be analyzed due to technical issues. Baseline characteristics of these 13 patients are listed in Table 1. Mean age was 43 (37–65.5) years, mean pulmonary artery pressure (MPAP) was 49 ± 8.9 mmHg, and heart rate was 80 ± 8.8 beats per minute (bpm).
Table 1.
Baseline characteristics.
| Characteristics N (%), mean ± SD, or median (IQR) | N = 13 |
|---|---|
| Clinical characteristics | |
| Age (years) | 43 (37–65.5) |
| Gender | |
| Male | 1 (8%) |
| Female | 12 (92%) |
| Heart failure | |
| NYHA II | 7 (54%) |
| NYHA III | 6 (46%) |
| Heart rate (bpm) | 80 ± 8.8 |
| Systolic blood pressure (mmHg) | 111 ± 19 |
| NT-proBNP (ng/L) | 340 (243–1089) |
| Treatment | |
| Mono therapy (n) | 4 |
| Combination therapy (n) | 7 |
| Triple therapy (n) | 2 |
| Hemodynamics | 49 ± 8.9 |
| MPAP (mmHg) | 8 ± 3.5 |
| PCWP (mmHg) | 3.1 ± 0.73 |
| CI (L/min) | 589.9 ± 218.6 |
| PVR (dynes·s·cm−5) | 69 ± 6.7 |
| SVO2 (%) | |
| Cardiac magnetic resonance imaging characteristics | |
| LVEDV (mL) | 95.5 ± 16.5 |
| LVESV (mL) | 36.5 ± 17.1 |
| LVEF (%) | 62.8 ± 13.5 |
| LV mass (g) | 97.5 ± 27.2 |
| RVEDV (mL) | 133.0 (104.5–189.5) |
| RVESV (mL) | 72.0 (51.5–141.0) |
| RVEF (%) | 42 ± 18.0 |
| RV mass (g) | 85.0 (60.0–131.5) |
CI: cardiac index; IQR: interquartile range; LV: left ventricular; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVESV: left ventricular end-systolic volume; MPAP: mean pulmonary artery pressure; NT-proBNP: N-terminal pro-brain natriuretic peptide; NYHA: New York Heart Association; PCWP: pulmonary capillary wedge pressure; PVR: pulmonary vascular resistance; RV: right ventricular; RVEDV: right ventricular end-diastolic volume; RVEF: right ventricular ejection fraction; RVESV: right ventricular end-systolic volume; SD: standard deviation; SVO2: mixed venous oxygen saturation.
Bisoprolol
NET density is decreased in case of exaggerated sympathetic activation. Therefore, low [11C]HED RI can be interpreted as a sign of upregulated pre synaptic sympathetic activity.12 To determine whether an intervention directed to lowering systemic sympathetic activation would affect the local sympathetic activity, we compared RV [11C]HED RI at baseline and after bisoprolol treatment (Fig. 1c).10 Although bisoprolol caused a significant reduction in heart rate from 80 ± 8.8 bpm at baseline to 69 ± 6.3 bpm (p<0.0001), no change was observed in local RV sympathetic activity (RV [11C]HED RI was at baseline 3.15 ± 0.80 and 3.20 ± 1.04 after bisoprolol). In addition, RV wall stress remained unchanged after bisoprolol treatment. These results suggest that local RV sympathetic activation is not affected by an intervention on systemic sympathetic tone.
RV sympathetic activity
[11C]HED retention in the RV was inversely related to serum norepinephrine levels, suggesting that a low tracer level reflects high sympathetic activity (Fig. 2). RV [11C]HED RI was lower when compared with LV [11C]HED RI, representing enhanced sympathetic nerve activity in the RV (Fig. 1b and c). This suggests that RV sympathetic activity can be activated independently from the left ventricle. To further investigate the possible triggers of increased RV sympathetic activation, we performed correlation analyses with markers of systemic hypotension, wall stress, and RV function.
Fig. 2.
Relation between systemic and RV sympathetic activity. RV [11C]HED retention was related to serum norepinephrine. [11C]HED: [11C]-hydroxyephedrine; RV: right ventricular; LN: log transformed.
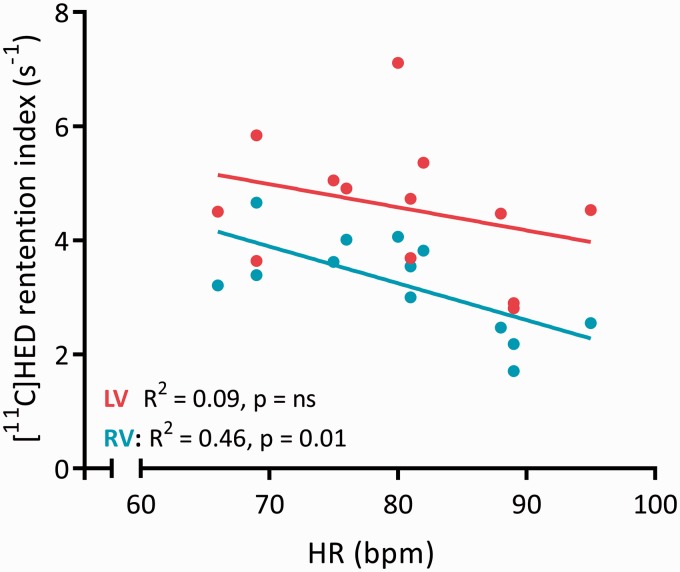
Interestingly, we found no relation between systolic blood pressure and RV [11C]HED RI (R2 = 0.005, p = 0.821). This indicates that RV sympathetic activity might not be systemically, but mainly locally stimulated. Therefore, we subsequently investigated the relation between RV sympathetic activation and RV wall stress (Fig. 3a). We observed a strong relation between right ventricular wall tension and RV [11C]HED RI (Fig. 3b), which could further be corroborated by correlations with N-terminal pro B type natriuretic peptide (NT-proBNP) levels and RV ejection fraction (Fig. 3c and d). Interestingly, heart rate was only associated with RV [11C]HED RI and not with LV[11C]HED uptake (Fig. 4), suggesting that differences in RV wall tension play an essential role in the activation of the sympathetic system. RV [11C]HED RI was also significantly related to hemodynamic parameters; right atrial pressure, pulmonary vascular resistance and cardiac output (Fig. 5).
Fig. 3.
[11C]HED retention is related to right ventricular wall tension. (a) Schematic illustration of the right ventricle demonstrating wall tension (stress) calculation.21 (b) Regression analysis demonstrating a negative relation between wall tension and right ventricular [11C]HED retention index at baseline and indicating that high wall tension is related to increased sympathetic nerve activity. (c) Regression analysis demonstrating a positive correlation between RVEF and right ventricular [11C]HED retention index, indicating that poor RV function is related to increased sympathetic nerve activity. (d) Regression analysis demonstrating a non-significant relation between logtransformed NT-proBNP and RV [11C]HED retention index. [11C]HED: [11C]-hydroxyephedrine; RVESV: right ventricular end systolic volume; RV: right ventricular; RVEF: right ventricular ejection fraction; NT-proBNP: N-terminal pro-brain natriuretic peptide.
Fig. 4.
Regression analysis demonstrating a relation between heart rate and RV [11C]HED retention index, but no relation with LV [11C]HED retention index.
Fig. 5.
Relations between hemodynamics and right ventricular [11C]HED retention. RV [11C]HED retention is significantly related to hemodynamic parameters: CO, MPAP, RAP, and PVR. Not normally distributed data (RAP) are log-transformed. [11C]HED: [11C]-hydroxyephedrine; RV: right ventricular, LV: left ventricular; CO: cardiac output; MPAP: mean pulmonary artery pressure; RAP: right atrial pressure; PVR: pulmonary vascular resistance.
Discussion
Therapies that are able to restore the autonomic imbalance by inhibiting sympathetic activity have been of interest during the past decade. Although promising results were obtained in several animal models for PAH-induced right heart failure, clinical implementation of beta-blocker therapy revealed to be more difficult and unsuccessful. Therefore, we aimed to study the effect of bisoprolol on RV sympathetic activity.
By using PET imaging of the norepinephrine analogue [11C]HED, we were able to demonstrate that:
Bisoprolol treatment could not alter local RV sympathetic activity in patients with PAH, albeit a change in heart rate was observed.
RV sympathetic activity was associated with RV wall stress, RV function, and NT-proBNP levels, but not with systemic hypotension.
Local sympathetic activity differs between the right and left ventricle in patients with stable PAH (NYHA II/III).
Only a relation between heart rate and local RV sympathetic activity was observed and not between LV sympathetic activity.
In a PAH animal model, it was possible to improve RV function and increase beta-adrenergic receptor density by bisoprolol.7 In the current study, however, bisoprolol treatment resulted in a decrease in heart rate, but did not alter RV sympathetic activity. As RV function and RV sympathetic activity were strongly related, the unchanged RV function could be a consequence of persistent high RV sympathetic activity and wall tension. Bisoprolol dosage was uptitrated depending on systemic blood pressure, heart rate, and side effect. A heart rate ≤ 60 bpm was a contraindication for further dose escalation in the study protocol. The mean maximum dose reached in this study would be considered a low dose in the left heart failure trial with bisoprolol.23 Therefore, it might be that patients will not tolerate the drug dosage required to achieve a change in RV sympathetic activity and functional improvement.
[11C]HED positron emission tomography
The [11C]HED tracer has been extensively validated in the LV. It was applied to show a relation between sympathetic innervation and contractile function in the cardiomyopathy,24 to uncover regional patterns of myocardial sympathetic denervation in dilated cardiomyopathy,13 and to investigate myocardial sympathetic innervation in unaffected myocardium of patients with prior myocardial infarction.25 It was also shown that HED was superior to another PET tracer for assessing regional abnormalities in patients with LV dysfunction.26
To the best of our knowledge, this is the first study that evaluated both LV and RV sympathetic activity in patients with PAH in non-end-stage disease. Compared with previously published [11C]HED RI data using the RI in healthy control patients, [11C]HED RI in patients with PAH was markedly reduced.13 Animal studies have demonstrated that the impaired re-uptake of norepinephrine in heart failure is not mediated by a loss of nerve terminals and suggest downregulation of the norepinephrine re-uptake transporter.27 In addition, higher sympathetic tone accompanied with higher firings of sympathetic nerves and insufficient re-uptake may result in a faster tracer washout and decreased [11C]HED retention.14,26,28 Exact discrimination of different pathophysiological pathways using non-invasive [11C]HED PET imaging is difficult. Nevertheless, the relatively lower uptake within the RV suggests a regional presynaptic nerve re-uptake dysfunction rather than overall myocardial downregulation. Few studies have assessed sympathetic nerve function using [123I]MIBG SPECT in patients with PAH and right heart disease.29,30 Due to the limited resolution of SPECT imaging, however, detailed information on regional sympathetic nerve function of the RV is lacking.
In this study, we could confirm local RV pre synaptic sympathetic activation, which is in line with a previous research reporting beta-adrenergic receptor downregulation in explanted RVs of PAH patients undergoing cardiac transplantation.11 Beta-adrenergic receptor downregulation is a result of increased post synaptic sympathetic activation. Bistow et al. demonstrated lower RV beta-adrenergic receptor density in PAH patients compared with controls.11 Our results add to these findings that sympathetic activation is not only altered in end-stage disease, but that local RV sympathetic activity is increased, when compared to the LV, already in earlier stages of RV dysfunction (NYHA functional classes II and III). Moreover, as hemodynamics and RV function parameters were obtained almost simultaneously with the RV [11C]HED PET scans, we could relate local RV sympathetic activity to RV functional parameters.
We were unable to demonstrate the exact mechanism of local activation of the sympathetic system by changes in RV wall stress. However, there is evidence that the heart has mechanoreceptors sensing myocardial stretch resulting in afferent sympathetic stimulation. Such mechanoreceptors can, for example, be found in the right atrium and where they are responsible for the increase in heart rate following right atrial pressure elevation, the so-called Bainbridge effect.31 Pressure sensitive mechanoreceptors can not only be found in atrial tissue, but also in the endocardium and the ventricular wall.32, 33
Clinical relevance
The findings of our study suggest that increased wall tension is an important activator of RV sympathetic activity. The correlations between RV sympathetic activity and high NT-proBNP levels and lower RVEF are in line with this finding, as both RVEF and NT-proBNP reflect RV wall tension.34–36 Absence of a change in wall tension after bisoprolol treatment might therefore explain why this beta-blocker did not improve RV function in an earlier study.10
Furthermore, the finding that wall tension may be an important regulator of RV sympathetic activity emphasizes the relevance of afterload reduction by PAH-specific therapy in the management of PAH.37 Hence, there might still be a role for less selective beta blocking agents with vasodilative properties in the treatment of PAH, as these agents might decrease RV wall tension. Treatment with the beta-blocker agent nebivolol, which has vasodilative properties, in an animal model of PAH, decreased pulmonary vascular resistance (PVR) and resulted in an improvement in cardiac output.8 Carvedilol, a non-selective beta-blocker was beneficial for RV function in a pilot study in patients.38 Whether these non-selective beta adrenergic receptor inhibitors can lead to a reduction in wall tension and thereby result in a decrease in RV sympathetic activity needs to be further elaborated.
Limitations
Several limitations need to be acknowledged. First, [11C]HED images only presynaptic sympathetic activity; this tracer does not represent post-sympathetic activity at the cardiomyocyte level. Second, the study population was relatively small for adequate evaluation of beta-blocking therapy on sympathetic innervation. However, an earlier study in patients with chronic left heart failure was able to demonstrate a significant difference in LV [11C]HED RI after six months of exercise training in 13 patients.18 Furthermore, no control groups were included in this study which made it impossible to compare [11C]HED RI in PAH with normal subjects. Unfortunately, obtaining reliable control scans is not possible, due to the low spatial resolution of the [11C]HED tracer and the thin-walled RVs in these subjects. To date, no previous study has evaluated sympathetic innervation in the RV of PAH patients by PET. The evaluation of the [11C]HED uptake in the RV specifically may be affected by partial volume effects, particularly within healthy thin-walled myocardium of the RV free wall. However, patients with PAH often show RV hypertrophy to some extent; therefore, PET assessment of RV [11C]HED uptake is feasible. Nonetheless, partial volume effects may have affected the [11C]HED RI in these patients.
In conclusion, in patients with RV failure due to pulmonary arterial hypertension, RV pre synaptic nerve activity is increased. Local sympathetic activity was not changed after bisoprolol treatment, whereas a significant drop in heart rate was observed. In this study, local sympathetic activity is related to RV systolic wall tension and RVEF rather than systemic triggers of sympathetic activity. Therefore, future studies assessing the effect of lowering wall stress on neurohormonal activation are warranted.
Supplemental Material
Supplemental material, sj-pdf-1-pul-10.1177_2045894019873548 for Bisoprolol therapy does not reduce right ventricular sympathetic activity in pulmonary arterial hypertension patients by Mischa T. Rijnierse, Joanne A. Groeneveldt, Jasmijn S.J.A. van Campen, Karin de Boer, Cathelijne E.E. van der Bruggen, Hendrik J. Harms, Pieter G. Raijmakers, Adriaan A. Lammertsma, Paul Knaapen, Harm Jan Bogaard, Berend E. Westerhof, Anton Vonk Noordegraaf, Cornelis P. Allaart and Frances S. de Man in Pulmonary Circulation
Supplemental material, sj-pdf-2-pul-10.1177_2045894019873548 for Bisoprolol therapy does not reduce right ventricular sympathetic activity in pulmonary arterial hypertension patients by Mischa T. Rijnierse, Joanne A. Groeneveldt, Jasmijn S.J.A. van Campen, Karin de Boer, Cathelijne E.E. van der Bruggen, Hendrik J. Harms, Pieter G. Raijmakers, Adriaan A. Lammertsma, Paul Knaapen, Harm Jan Bogaard, Berend E. Westerhof, Anton Vonk Noordegraaf, Cornelis P. Allaart and Frances S. de Man in Pulmonary Circulation
Supplemental material, sj-pdf-3-pul-10.1177_2045894019873548 for Bisoprolol therapy does not reduce right ventricular sympathetic activity in pulmonary arterial hypertension patients by Mischa T. Rijnierse, Joanne A. Groeneveldt, Jasmijn S.J.A. van Campen, Karin de Boer, Cathelijne E.E. van der Bruggen, Hendrik J. Harms, Pieter G. Raijmakers, Adriaan A. Lammertsma, Paul Knaapen, Harm Jan Bogaard, Berend E. Westerhof, Anton Vonk Noordegraaf, Cornelis P. Allaart and Frances S. de Man in Pulmonary Circulation
Acknowledgments
We thank Dr Joost van den Aardweg, Amsterdam UMC, University of Amsterdam, Pulmonary Medicine, Amsterdam Cardiovascular Sciences, Amsterdam, the Netherlands for the inspiring and enlightening discussions.
Conflicts of interest
The author(s) declare that there is no conflict of interest.
Funding
This research was financially supported by a ZonMW grant (number: 95110079). Drs Groeneveldt and Westerhof were supported by NWO-VICI (918.16.610). Dr Bogaard was supported by The Netherlands CardioVascular Research Initiative (CVON-2012-08 PHAEDRA and CVON-2017-10 DOLPHIN-GENESIS). Drs Vonk Noordegraaf and de Man were supported by The Netherlands CardioVascular Research Initiative (CVON-2012-08 PHAEDRA and CVON-2017-10 DOLPHIN-GENESIS) and The Netherlands Organization for Scientific Research (NWO-VENI: 916.14.099, NWO-VIDI: 917.18.338, and NWO-VICI: 918.16.610).
References
- 1.Ciarka A, Doan V, Velez-Roa S, et al. Prognostic significance of sympathetic nervous system activation in pulmonary arterial hypertension. Am J Respir Crit Care Med 2010; 181: 1269–1275. [DOI] [PubMed] [Google Scholar]
- 2.da Silva Goncalves Bos D, Van Der Bruggen CEE, Kurakula K, et al. Contribution of impaired parasympathetic activity to right ventricular dysfunction and pulmonary vascular remodeling in pulmonary arterial hypertension. Circulation 2018; 137: 910–924. [DOI] [PubMed] [Google Scholar]
- 3.Velez-Roa S, Ciarka A, Najem B, et al. Increased sympathetic nerve activity in pulmonary artery hypertension. Circulation 2004; 110: 1308–1312. [DOI] [PubMed] [Google Scholar]
- 4.Mak S, Witte KK, Al-Hesayen A, et al. Cardiac sympathetic activation in patients with pulmonary arterial hypertension. Am J Physiol Regul Integr Comp Physiol 2012; 302: R1153–R1157. [DOI] [PubMed] [Google Scholar]
- 5.Nootens M, Kaufmann E, Rector T, et al. Neurohormonal activation in patients with right ventricular failure from pulmonary hypertension: relation to hemodynamic variables and endothelin levels. J Am Coll Cardiol 1995; 26: 1581–1585. [DOI] [PubMed] [Google Scholar]
- 6.Bogaard HJ, Natarajan R, Mizuno S, et al. Adrenergic receptor blockade reverses right heart remodeling and dysfunction in pulmonary hypertensive rats. Am J Respir Crit Care Med 2010; 182: 652–660. [DOI] [PubMed] [Google Scholar]
- 7.de Man FS, Handoko ML, van Ballegoij JJ, et al. Bisoprolol delays progression towards right heart failure in experimental pulmonary hypertension. Circ Heart Fail 2012; 5: 97–105. [DOI] [PubMed] [Google Scholar]
- 8.Perros F, Ranchoux B, Izikki M, et al. Nebivolol for improving endothelial dysfunction, pulmonary vascular remodeling, and right heart function in pulmonary hypertension. J Am Coll Cardiol 2015; 65: 668–680. [DOI] [PubMed] [Google Scholar]
- 9.Farha S, Saygin D, Park MM, et al. Pulmonary arterial hypertension treatment with carvedilol for heart failure: a randomized controlled trial. JCI Insight 2017; 2: pii:95240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Campen JS, de Boer K, van de Veerdonk MC, et al. Bisoprolol in idiopathic pulmonary arterial hypertension: an explorative study. Eur Respir J 2016; 48: 787–796. [DOI] [PubMed] [Google Scholar]
- 11.Bristow MR, Minobe W, Rasmussen R, et al. Beta-adrenergic neuroeffector abnormalities in the failing human heart are produced by local rather than systemic mechanisms. J Clin Invest 1992; 89: 803–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raffel DM, Chen W, Sherman PS, et al. Dependence of cardiac 11C-meta-hydroxyephedrine retention on norepinephrine transporter density. J Nucl Med 2006; 47: 1490–1496. [PMC free article] [PubMed] [Google Scholar]
- 13.Hartmann F, Ziegler S, Nekolla S, et al. Regional patterns of myocardial sympathetic denervation in dilated cardiomyopathy: an analysis using carbon-11 hydroxyephedrine and positron emission tomography. Heart 1999; 81: 262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bengel FM. Imaging targets of the sympathetic nervous system of the heart: translational considerations. J Nucl Med 2011; 52: 1167–1170. [DOI] [PubMed] [Google Scholar]
- 15.Munch G, Nguyen NT, Nekolla S, et al. Evaluation of sympathetic nerve terminals with [(11)C]epinephrine and [(11)C]hydroxyephedrine and positron emission tomography. Circulation 2000; 101: 516–523. [DOI] [PubMed] [Google Scholar]
- 16.Nomura Y, Fuchigami H, Kii H, et al. Quantification of size distribution of restriction fragments in mitochondrial genome using fluorescence correlation spectroscopy. Exp Mol Pathol 2006; 80: 275–278. [DOI] [PubMed] [Google Scholar]
- 17.Tipre DN, Fox JJ, Holt DP, et al. In vivo PET imaging of cardiac presynaptic sympathoneuronal mechanisms in the rat. J Nucl Med 2008; 49: 1189–1195. [DOI] [PubMed] [Google Scholar]
- 18.Pietila M, Malminiemi K, Vesalainen R, et al. Exercise training in chronic heart failure: beneficial effects on cardiac (11)C-hydroxyephedrine PET, autonomic nervous control, and ventricular repolarization. J Nucl Med 2002; 43: 773–779. [PubMed] [Google Scholar]
- 19.Harms HJ, Huisman MC, Rijnierse MT, et al. Noninvasive quantification of myocardial 11C-meta-hydroxyephedrine kinetics. J Nucl Med 2016; 57: 1376–1381. [DOI] [PubMed] [Google Scholar]
- 20.van de Veerdonk MC, Kind T, Marcus JT, et al. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol 2011; 58: 2511–2519. [DOI] [PubMed] [Google Scholar]
- 21.Arts T, Bovendeerd PH, Prinzen FW, et al. Relation between left ventricular cavity pressure and volume and systolic fiber stress and strain in the wall. Biophys J 1991; 59: 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertinieri G, Di Rienzo M, Cavallazzi A, et al. Evaluation of baroreceptor reflex by blood pressure monitoring in unanesthetized cats. Am J Physiol 1988; 254: H377–H383. [DOI] [PubMed] [Google Scholar]
- 23.Simon T, Mary-Krause M, Funck-Brentano C, et al. Bisoprolol dose-response relationship in patients with congestive heart failure: a subgroup analysis in the cardiac insufficiency bisoprolol study (CIBIS II). Eur Heart J 2003; 24: 552–559. [DOI] [PubMed] [Google Scholar]
- 24.Bengel FM, Permanetter B, Ungerer M, et al. Relationship between altered sympathetic innervation, oxidative metabolism and contractile function in the cardiomyopathic human heart; a non-invasive study using positron emission tomography. Eur Heart J 2001; 22: 1594–1600. [DOI] [PubMed] [Google Scholar]
- 25.Aoki H, Matsunari I, Nomura Y, et al. Myocardial sympathetic innervation, function, and oxidative metabolism in non-infarcted myocardium in patients with prior myocardial infarction. Ann Nucl Med 2013; 27: 523–531. [DOI] [PubMed] [Google Scholar]
- 26.Matsunari I, Aoki H, Nomura Y, et al. Iodine-123 metaiodobenzylguanidine imaging and carbon-11 hydroxyephedrine positron emission tomography compared in patients with left ventricular dysfunction. Circ-Cardiovasc Imag 2010; 3: 595–603. [DOI] [PubMed] [Google Scholar]
- 27.Backs J, Haunstetter A, Gerber SH, et al. The neuronal norepinephrine transporter in experimental heart failure: evidence for a posttranscriptional downregulation. J Mol Cell Cardiol 2001; 33: 461–472. [DOI] [PubMed] [Google Scholar]
- 28.Thackeray JT, Bengel FM. Assessment of cardiac autonomic neuronal function using PET imaging. J Nucl Cardiol 2013; 20: 150–165. [DOI] [PubMed] [Google Scholar]
- 29.Morimitsu T, Miyahara Y, Sinboku H, et al. Iodine-123-metaiodobenzylguanidine myocardial imaging in patients with right ventricular pressure overload. J Nucl Med 1996; 37: 1343–1346. [PubMed] [Google Scholar]
- 30.Sakamaki F, Satoh T, Nagaya N, et al. Correlation between severity of pulmonary arterial hypertension and 123I-metaiodobenzylguanidine left ventricular imaging. J Nucl Med 2000; 41: 1127–1133. [PubMed] [Google Scholar]
- 31.Bainbridge FA. The influence of venous filling upon the rate of the heart. J Physiol 1915; 50: 65–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armour JA. Physiological behavior of thoracic cardiovascular receptors. Am J Physiol 1973; 225: 177–185. [DOI] [PubMed] [Google Scholar]
- 33.Paintal AS. Vagal sensory receptors and their reflex effects. Physiol Rev 1973; 53: 159–227. [DOI] [PubMed] [Google Scholar]
- 34.Blyth KG, Groenning BA, Mark PB, et al. NT-proBNP can be used to detect right ventricular systolic dysfunction in pulmonary hypertension. Eur Respir J 2007; 29: 737–744. [DOI] [PubMed] [Google Scholar]
- 35.Quaife RA, Chen MY, Lynch D, et al. Importance of right ventricular end-systolic regional wall stress in idiopathic pulmonary arterial hypertension: a new method for estimation of right ventricular wall stress. Eur J Med Res 2006; 11: 214–220. [PubMed] [Google Scholar]
- 36.Vonk Noordegraaf A, Westerhof N. Right ventricular ejection fraction and NT-proBNP are both indicators of wall stress in pulmonary hypertension. Eur Respir J 2007; 29: 622–623. [DOI] [PubMed] [Google Scholar]
- 37.Westerhof BE, Saouti N, van der Laarse WJ, et al. Treatment strategies for the right heart in pulmonary hypertension. Cardiovasc Res 2017; 113: 1465–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grinnan D, Bogaard HJ, Grizzard J, et al. Treatment of group I pulmonary arterial hypertension with carvedilol is safe. Am J Respir Crit Care Med 2014; 189: 1562–1564. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-pul-10.1177_2045894019873548 for Bisoprolol therapy does not reduce right ventricular sympathetic activity in pulmonary arterial hypertension patients by Mischa T. Rijnierse, Joanne A. Groeneveldt, Jasmijn S.J.A. van Campen, Karin de Boer, Cathelijne E.E. van der Bruggen, Hendrik J. Harms, Pieter G. Raijmakers, Adriaan A. Lammertsma, Paul Knaapen, Harm Jan Bogaard, Berend E. Westerhof, Anton Vonk Noordegraaf, Cornelis P. Allaart and Frances S. de Man in Pulmonary Circulation
Supplemental material, sj-pdf-2-pul-10.1177_2045894019873548 for Bisoprolol therapy does not reduce right ventricular sympathetic activity in pulmonary arterial hypertension patients by Mischa T. Rijnierse, Joanne A. Groeneveldt, Jasmijn S.J.A. van Campen, Karin de Boer, Cathelijne E.E. van der Bruggen, Hendrik J. Harms, Pieter G. Raijmakers, Adriaan A. Lammertsma, Paul Knaapen, Harm Jan Bogaard, Berend E. Westerhof, Anton Vonk Noordegraaf, Cornelis P. Allaart and Frances S. de Man in Pulmonary Circulation
Supplemental material, sj-pdf-3-pul-10.1177_2045894019873548 for Bisoprolol therapy does not reduce right ventricular sympathetic activity in pulmonary arterial hypertension patients by Mischa T. Rijnierse, Joanne A. Groeneveldt, Jasmijn S.J.A. van Campen, Karin de Boer, Cathelijne E.E. van der Bruggen, Hendrik J. Harms, Pieter G. Raijmakers, Adriaan A. Lammertsma, Paul Knaapen, Harm Jan Bogaard, Berend E. Westerhof, Anton Vonk Noordegraaf, Cornelis P. Allaart and Frances S. de Man in Pulmonary Circulation