Abstract
Electronically delivered health promotion programs that are aimed primarily at educated, health-literate individuals have proliferated, raising concerns that such trends could exacerbate health disparities in the United States and elsewhere. The efficacy of a culturally and linguistically adapted virtual advisor that provides tailored physical activity advice and support was tested in low-income older adults. Forty inactive adults (92.5% Latino) 55 years of age and older were randomized to a 4-month virtual advisor walking intervention or a waitlist control. Four-month increases in reported minutes of walking/week were greater in the virtual advisor arm (mean increase = 253.5 ± 248.7 minutes/week) relative to the control (mean increase = 26.8 ± 67.0 minutes/week; p = .0008). Walking increases in the virtual advisor arm were substantiated via objectively measured daily steps (slope analysis p = .002). All but one intervention participant continued some interaction with the virtual advisor in the 20-week poststudy period (mean number of poststudy sessions = 14.0 ± 20.5). The results indicate that a virtual advisor delivering culturally and linguistically adapted physical activity advice led to meaningful 4-month increases in walking relative to control among underserved older adults. This interactive technology, which requires minimal language and computer literacy, may help reduce health disparities by ensuring that all groups benefit from e-health opportunities.
Emerging communication technologies offer a promising means of improving key health-promoting behaviors affecting a range of chronic diseases (Echt & Morrell, 2003; King & Guralnik, 2010). While electronically delivered health promotion (e-health) programs have proliferated, few have specifically targeted the needs and preferences of older adults (Norman et al., 2007). Older adults are at particular risk for the chronic diseases that can be positively influenced by reasonably modest improvements in physical activity (Physical Activity Guidelines Advisory Committee, 2008). Many older adults lack the computer capabilities needed to utilize the often visually dense and complex health promotion guidance offered on-line (Freudenthal, 2001). This may be particularly true for socioeconomically disadvantaged and ethnic minority populations who may have reduced access to computers and lower levels of language, computer, and health literacy (Bodie & Dutta, 2008). For example, few e-health programs have been designed for persons with lower levels of education (Viswanath & Kreuter, 2007), and few e-health programs incorporate cultural factors in their health communications (Kreuter & McClure, 2004). Such barriers, including reduced quality of health information websites that have been developed for non-English speakers (Cardelle & Rodriguez, 2005), may serve to further widen the nation’s health disparities gap (Berland et al., 2001; Bodie & Dutta, 2008).
At least some of these potential barriers to electronically delivered health promotion access and use may be diminished through utilizing communication technologies that require fewer advanced reading and computer skills. One such communication technology is the embodied conversational agent (ECA). ECA is an interactive, animated computer character that simulates face-to-face counseling using simple speech, hand gestures, facial cues, and other nonverbal behaviors to maximize message comprehension (Bickmore, Gruber, & Picard, 2005; Bickmore et al., 2010). Individuals interact with the ECA through simply touching one of several brief conversation-based responses being shown on the computer screen throughout the interaction. The conversation boxes appearing on the screen are aimed at less than an 8th grade education level, and ECA-based interventions have been shown to be effective in changing behavior among individuals with little or no computer experience and inadequate health literacy (Bickmore, Caruso, Clough-Gorr, & Heeren, 2005; Bickmore et al., 2010). From a communication theory perspective, ECA has the ability to simulate natural elements of human communication, including the dynamic flow of spoken language in conjunction with visual cues, and the ability to develop a personalized focus in a one-on-one format (Webster & Trevino, 1995). It can provide a virtual counseling session with a simulated health educator, offering an accessible, ongoing source of information.
The purpose of this study was to evaluate, using a randomized controlled design, the effects of a community center-based ECA virtual advisor, culturally and linguistically tailored for a low-income, bilingual, inactive older adult population, on physical activity adoption. It is the first e-health intervention study (to our knowledge) that was aimed specifically at a low-literate, non–English-speaking older adult population.
Method
Participants
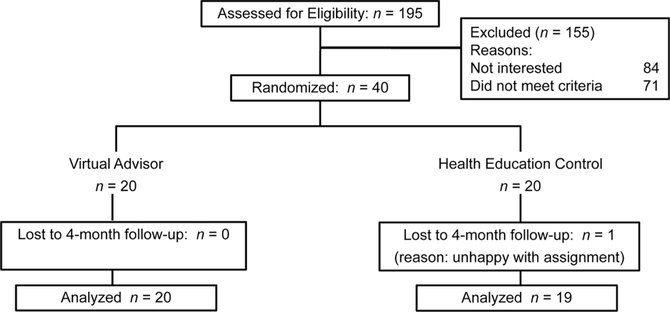
We recruited participants in 2009 from the neighborhoods surrounding a community center serving a primarily Latino population in San Jose, California. We used a combination of community outreach and printed media (e.g., brochures, targeted mailings) to recruit participants. Eligible participants were 55 years of age or older and were inactive (i.e., not engaged in moderate-intensity or more vigorous activity at least 60 minutes per week over t he past 6 months). They also had no medical conditions limiting participation in moderate-intensity physical activity based on the Physical Activity Readiness Questionnaire (Thomas, Reading, & Shephard, 1992). Participants had to be stable on all medications over the past 3 months. Subject recruitment flow is summarized in Figure 1.
Figure 1.
Subject flow.
Design
The Stanford University School of Medicine institutional review board approved the study protocol. All study materials, including informed consent and recruitment, intervention, and assessment forms, were produced in English and Spanish using professional translation services (Ubiqus, Irvine, California). Participants provided written consent upon reviewing the consent form with a bilingual staff member. All assessments occurred in a private area of the community center. After they completed the baseline assessment, participants were randomly assigned, using the Efron procedure (Efron, 1971), to one of two study arms: (a) 20 to a computerized, individually tailored walking program delivered by a bilingual virtual advisor at the community center; and (b) 20 to a waitlist control arm. Participants were reassessed at 2 and 4 months.
Virtual Advisor Intervention
The virtual advisor program was based on a successful 2-month embodied conversational agent (ECA) physical activity intervention aimed at an older low-income, largely African American population in the northeastern United States (Bickmore, Caruso, et al., 2005; Bickmore, Gruber, & Picard, 2005). It incorporated the same theoretically derived, empirically validated behavior change strategies emanating from social cognitive theory (Bandura, 2001) and the transtheoretical model (Marcus & Simkin, 1994; e.g., self-assessment, motivationally tailored goal-setting, individualized feedback, positive reinforcement and support, knowledge enhancement related to benefits of a physically active lifestyle) that have been used successfully in physical activity interventions with a range of older adults (Brawley, Rejeski, & King, 2003; King, 2001).
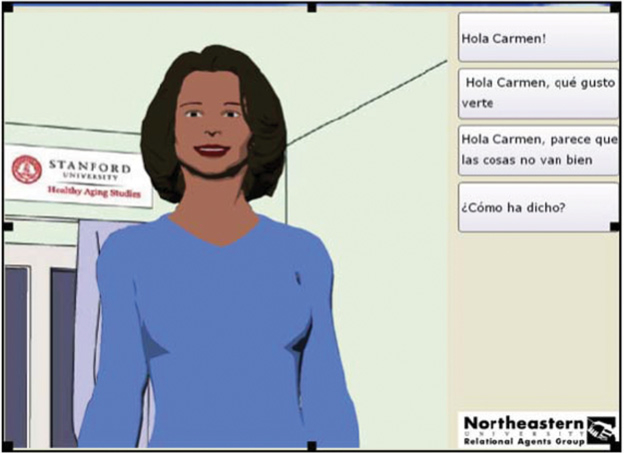
Twelve months of participatory formative research and evaluation were undertaken to enhance ECA cultural and linguistic congruity for Latino and, to some extent, Filipino older adults who represented the next largest group of center users (Hawkins, Kreuter, Resnicow, Fishbein, & Dijkstra, 2008; Munoz & Mendelson, 2005; see Figure 2, which shows the culturally tailored interaction screen). On the basis of these program adaptation activities, additional evidence-based behavioral strategies were added to the program (e.g., problem solving around barriers to physical activity that are particularly salient to Latino older adults); participants could choose to have their program interactions in English or Spanish; relevant references to the local Latino culture were added; and program length was expanded from 2 to 4 months. In contrast with the original 2-month ECA physical activity program, which was delivered by computers located in participants’ homes (Bickmore, Caruso, et al., 2005), the current ECA program was delivered through a computer in a local community senior center. This was done to expand the potential reach and efficiency of program delivery for population segments, such as the current one, which often lack access to home computers.
Figure 2.
Virtual advisor screen. (Color figure available online.)
The primary goal of this first-generation study was to test the efficacy of the culturally adapted program in promoting initial increases in walking, relative to a control, in the targeted group of older adults. If this investigation indicated initial program efficacy, subsequent dismantling research could be undertaken to systematically examine different program components to further understand which adaptations may have had the greatest influence on intervention success (Czaja, Schulz, Lee, & Belle, for the REACH Investigators, 2003; Mohr et al., 2009).
As part of the social cognitive theory-based intervention (Bandura, 2001), participants were trained in the use of a simple pedometer (Omron Healthcare, Inc., model HJ-720ITC, Bannockburn, Illinois) to track daily steps and provide individualized behavioral feedback throughout the walking program. This device has been found in prior investigations to provide a valid and reliable recording of step counts under prescribed and self-paced walking conditions in normal-weight and overweight adults (Holbrook, Barreira, & Kang, 2009). Participants were instructed to wear the pedometer at the waist on a daily basis and to download it on the virtual advisor computer via USB port each time they accessed the virtual advisor (participants did not have to manually record their pedometer steps on paper; they simply plugged the pedometer into the computer’s USB port for automatic download to the system). The virtual advisor program used the pedometer information to provide tailored feedback and advice to each intervention participant throughout the program.
The senior center virtual advisor program was housed on a dedicated computer located in a small partitioned cubicle at the community center. Intervention participants were introduced to the ECA program in a postrandomization introductory session with a project staff member. During that session, and as a standard part of the behaviorally based intervention (Brawley et al., 2003), participants were instructed in the use of the pedometer and engaged in a brief (approximately 5-minute) walk with the staff member so that the individual’s number of steps per minute could be determined. This was used to help establish personalized goals related to pedometer steps per day. Participants also completed a brief questionnaire in which they shared individual information (e.g., favorite entertainment, names of supportive relatives/friends) that could be programmed into the computer to help individualize participant–computer interactions. Each participant accessed the program through a personal identification number and wore headphones during ECA interactions to ensure privacy. The virtual advisor sessions, which lasted an average of 7 minutes (SD = 2.3), consisted of a salutation, individualized social dialogue, progress review based on downloaded pedometer information, and personalized feedback, problem solving, goal setting, and educational information based on current progress (Umstattd, Wilcox, Saunders, Watkins, & Dowda, 2008). Educational information and progress graphs could be printed if desired. Participants were encouraged to interact with the virtual advisor program weekly or whenever they came to the community center. To encourage regular use of the virtual advisor program, raffles of inexpensive (i.e., US$10) items took place approximately every 4 weeks. Participants received raffle tickets for using the virtual advisor on a regular basis, downloading their pedometers on a regular basis, and achieving their physical activity goals.
Three community center staff members and two center volunteers were trained in the basics of the virtual advisor program (which took less than 1 hour), so that they could assist participants with any problems that might occur in accessing the program.
Waitlist Control Arm
Participants randomized to the waitlist control arm were asked to complete study assessments during the four-month study period, and were told that they would be offered a 4-month virtual advisor program upon completion of the study. During the formative research development phase preceding the start of the study, members of the target population noted how making relevant general health information available to persons who ended up being randomized to the waitlist arm would facilitate study retention during the 4-month study period. Therefore, during the waitlist period for this group, control participants were offered a general health promotion program at the community center that we have found to be acceptable for this population (Pahor et al., 2006). Bilingual health educators delivered the health education information using a combination of English and Spanish to ensure participants’ understanding. Although the original plan was to offer health education sessions on a weekly basis, participants opted for such sessions to be available monthly, for 1 hour, throughout the waitlist period. As part of these sessions, participants received information on a variety of non–physical activity topics of interest to midlife and older adults in this community, such as nutrition and home safety. Monthly participation in this type of general health education program has been shown to be effective in maintaining study engagement across a diverse range of older adults for extended time periods (12 months or longer) without significantly changing physical activity behavior (Pahor et al., 2006). Similar to the virtual advisor arm, monthly raffles of inexpensive items occurred at the health education meetings. At the end of the 4-month period, participants in this arm were offered the 4-month virtual advisor program, in which all but one participated.
Dependent Measures
Physical Activity Assessment
The primary dependent measure was change in walking behavior at 4 months, with an interim assessment occurring at 2 months. Walking changes were assessed using the intervention-relevant walking items from the validated Community Healthy Activities Model Program for Seniors (CHAMPS) questionnaire (i.e., walking fast or briskly for exercise; walking leisurely for exercise or pleasure), which were summed (Stewart et al., 2001). The CHAMPS assesses usual weekly amounts of walking over the previous 4 weeks in minutes per week. The CHAMPS has been extensively validated and shown to have acceptable test–retest reliability (intraclass correlation coefficients [ICCs] = 0.56 to 0.70) in midlife and older adults (Hekler, Buman, Haskell, et al., 2012; Stewart et al., 2001), as well as demonstrating sensitivity to change with a moderate intensity physical activity program in more than a dozen different community samples of older adults (Castro & King, 2002; King, Baumann, O’Sullivan, Wilcox, & Castro, 2002; King et al., 2007; King et al., 2004; King et al., 2000; Wilcox et al., 2008). Prior research on the CHAMPS has indicated that it consistently correlates with objective measures of physical activity, including a Mini-Logger 2000 (a device that measures activity and heart rate; Harada, Chiu, King, & Stewart, 2001) and accelerometry (Hekler, Buman, Haskell, et al., 2012). Studies have found a good correlation between the sum of all items and the walking items of the CHAMPS (rs = 0.76 to 0.82), suggesting acceptable concurrent validity (Hekler, Buman, Dunton, Atienza, & King, 2012). Omron pedometer readings across the 4-month period were used as a process measure in verifying reported walking changes among intervention participants. During the formative development period before study initiation, the potential use of pedometers to ascertain daily steps in the control arm was also evaluated. Given, however, that persons in the target population were found to have insufficient numeracy skills to manually log daily or weekly pedometer steps in a reliable manner on paper, this assessment method could not be used in the study.
Two trained bilingual study assessors with extensive experience in administering the CHAMPS questionnaire collected this measure, and were supervised by an assessment expert blinded to participant arm assignment. At posttest, both assessors were blinded to participant baseline values. One assessor was blinded to participant arm assignment. Because the second assessor also served as a resource for participant questions pertaining to use of the virtual advisor computer program, she could not be blinded to participant arm assignment. There were no statistically significant differences found between the two assessors in the amount of change observed on the CHAMPS in either study arm or across the two arms combined (ps > .10).
Motivational and Attitudinal Measures
To assess whether the self-regulatory strategies being taught in the virtual advisor program were being learned, reported use of these cognitive and behavioral strategies was assessed in both arms at baseline and 4 months using an abbreviated version of a motivational processes of change inventory that has been frequently associated with physical activity change in other populations (Marcus, Rakowski, & Rossi, 1992; Napolitano et al., 2008). The seven motivational strategies of particular relevance to the intervention were understanding the benefits of a physically active lifestyle, understanding the risks of an inactive lifestyle, committing oneself to being active, substituting more active alternatives, enlisting social support for physical activity, rewarding oneself for being physically active, and reminding oneself to be physically active.
To evaluate ease of use and acceptability of the virtual advisor program, intervention participants completed several measures used in previous ECA research. To explore participants’ reactions to the virtual advisor program, a 19-item computer program acceptability scale (Bickmore, Caruso, et al., 2005) and 6-item computer credibility scale (Fogg et al., 2001), used in Dr. Bickmore’s prior ECA studies, were completed at the end of the 4-month intervention period. The 12-item bonding subscale of the Working Alliance Inventory (Horvath & Greenberg, 1989) was also completed at 4 months. Test–retest reliability for this scale is reported to be from .66 to .74 (Horvath & Greenberg, 1994). At baseline and 4 months, participants completed the short form (4 items) of the Computer Attitude Scale (Rainer & Miller, 1996), which measures favorable and unfavorable attitudes toward the use of computers. Test–retest reliability for the Computer Attitudes Scale has been reported to be 0.86 (Rainer & Miller, 1996).
Statistical Analysis
Assuming an alpha level of .05, power of 80%, and a pooled standard deviation estimate of 140 minutes, a sample size of 18–20 per arm was deemed necessary to detect a 130–135-minutes-per-week walking difference between arms. To detect changes in minutes/week of walking across the 4-month period, analysis of covariance was conducted with baseline values as the covariate (SAS, version 9.2; SAS Institute Inc., 2009). We used an analysis of covariance also in evaluating 4-month changes in the motivational processes of change variables. Because preliminary analysis of those variables indicated a correlation with age, the age main effect was included in those analyses of covariance. All reported p values are two-sided.
Results
Preliminary analyses showed that there were no between-arm differences for variables of interest at baseline (Table 1). All participants, except one, were retained at 4 months (Figure 1). The average age for the sample was 68.3 years (SD = 8.2years), and 72% were women. Of the participants, 37 self-identified as Latino and 3 as Filipino (n = 2) or Asian (n = 1). Of the participants, 81% reported an annual household income of less than $50,000, placing them within the federal definition of low income (defined as below 80% of an area’s median income, which for San Jose, California, has been reported as $70,243; from www.bayareacensus.ca.gov/cities/SanJose.htm). A substantial proportion of participants (92%) reported a history of chronic conditions, with an average of 2.5 (SD = 1.6) chronic conditions per participant. Common health conditions included high blood pressure (59.0%), high cholesterol (51.3%), arthritis (46.2%), diabetes (25.6%), and osteoporosis (18.0%). At baseline, participants’ ratings on the Computer Attitudes Scale averaged 3.1 (SD = 1.3) out of 5, equaling undecided in relation to feelings about computers.
Table 1.
Demographic Characteristics by Arm
| Virtual advisor (20) |
Control (20) |
Total |
||||
|---|---|---|---|---|---|---|
| Variable | n | % | n | % | n | % |
| Age (year), M (SD) | 72.1 (8.2) | 64.5 (6.5) | 68.3 (8.2) | |||
| Education (year), M (SD) | 11.1 (3.5) | 10.0 (4.3) | 10.5 (3.9) | |||
| Gender | ||||||
| Female | 13 | 65.0 | 16 | 80.0 | 29 | 72.5 |
| Male | 7 | 35.0 | 4 | 20.0 | 11 | 27.5 |
| Education (yr) | ||||||
| ≤8 | 6 | 31.6 | 7 | 35.0 | 13 | 33.3 |
| 9–11 | 3 | 15.8 | 2 | 10.0 | 5 | 12.8 |
| High school | 4 | 21.0 | 6 | 30.0 | 10 | 25.7 |
| Some college + | 6 | 31.6 | 5 | 25.0 | 11 | 28.2 |
| Occupation | ||||||
| Professional or manager | 7 | 35.0 | 5 | 25.0 | 12 | 30.0 |
| Clerical | 4 | 20.0 | 5 | 25.0 | 9 | 22.5 |
| Service | 1 | 5.0 | 3 | 15.0 | 4 | 10.0 |
| Skilled craft | 4 | 20.0 | 1 | 5.0 | 5 | 12.5 |
| Laborers | 4 | 20.0 | 6 | 30.0 | 10 | 25.0 |
| Income | ||||||
| Less than $15,000 | 6 | 30.0 | 6 | 30.0 | 12 | 30.0 |
| $15,000–$24,999 | 4 | 20.0 | 3 | 15.0 | 7 | 17.5 |
| $25,000–$34,999 | 5 | 25.0 | 4 | 20.0 | 9 | 22.5 |
| $35,000–$49,999 | 0 | 0.0 | 1 | 5.0 | 1 | 2.5 |
| $50,000–$74,999 | 2 | 10.0 | 3 | 15.0 | 5 | 12.5 |
| $75,000 or greater | 0 | 0.0 | 2 | 10.0 | 2 | 5.0 |
| Refused | 3 | 15.0 | 1 | 5.0 | 4 | 10.0 |
| Marital status | ||||||
| Married | 9 | 45.0 | 8 | 40.0 | 17 | 42.5 |
| Divorced | 3 | 15.0 | 5 | 25.0 | 8 | 20.0 |
| Widowed | 6 | 30.0 | 5 | 25.0 | 11 | 27.5 |
| Never married | 2 | 10.0 | 2 | 10.0 | 4 | 10.0 |
| Country born | ||||||
| United States | 8 | 40.0 | 11 | 55.0 | 19 | 47.5 |
| Mexico | 8 | 40.0 | 7 | 35.0 | 15 | 37.5 |
| Other | 4 | 20.0 | 2 | 10.0 | 6 | 15.0 |
| Language spoken at home | ||||||
| English | 4 | 20.0 | 7 | 35.0 | 11 | 27.5 |
| Spanish and English | 6 | 30.0 | 4 | 20.0 | 10 | 25.0 |
| Spanish | 7 | 35.0 | 8 | 40.0 | 15 | 37.5 |
| Other | 3 | 15.0 | 1 | 5.0 | 4 | 10.0 |
| Number of health conditions, M (SD) | 2.6 (1.3) | 2.4 (1.9) | 2.5 (1.6) | |||
| Body mass index, M (SD) | 30.0 (6.9) | 29.0 (3.8) | 29.5 (5.5) | |||
| Baseline fast & leisure walking (min), M (SD) | 36.0 (67.0) | 63.8 (160.9) | 49.9 (122.4) | |||
Note. Values reported as n and % except where noted as M (SD).
Virtual advisor participants logged into the computer program a mean of 1.56 (SD = 0.65) sessions per week (range = 0.35–2.31) and a mean of 27 (SD = 11) total sessions across the 4-month period (range = 6–40). Of the participants, 55% chose to interact with the virtual advisor in Spanish. Mean attendance at monthly health education sessions by control participants was 2.2 sessions across the 4-month period.
Changes in Walking
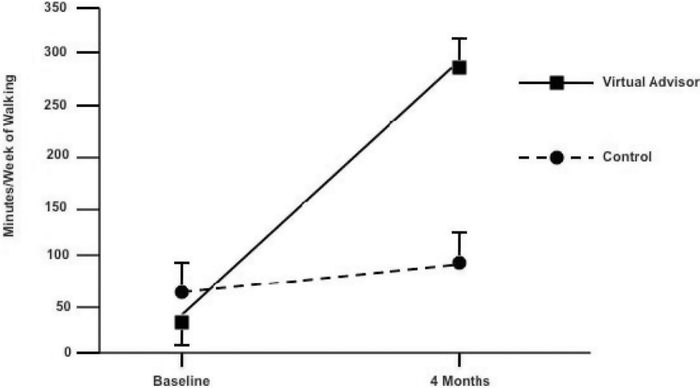
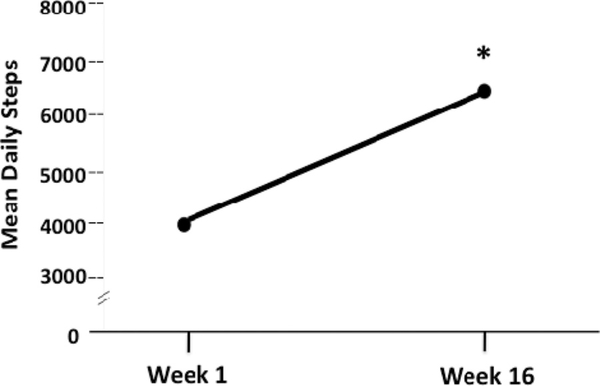
Reported 4-month changes in minutes of walking per week (the primary intervention target) were significantly greater in the virtual advisor arm (M change = 253.5 minutes, SD = 248.7 minutes) relative to the control arm (M change = 26.8, SD = 67.0). For between-group difference of 226.7, 95% CI = 107.0, 346.4, F(1, 38) = 13.6, p = .0008, effect size = 1.2 (Figure 3). Significant between-group differences were also observed in this variable at the interim 2-month intervention point (p = .01, effect size = 0.9). Walking increases reported in the virtual advisor arm were reflected in significant 4-month increases in objectively measured daily steps (slope analysis to test difference from zero across the 4-month period, t = 3.7, p = .002, effect size = 0.8; mean increase from first 2 weeks of program to last 2 weeks = 2029.9 ± 2303.8 steps per day; see Figure 4). The correlation between 4-month changes in CHAMPS-reported walking and pedometer steps in the virtual advisor arm also was significant (Spearman’s rho = 0.47, p = .04).
Figure 3.
Mean baseline and 4-month reported minutes/week of walking, by arm.
Figure 4.
Four-month change in daily steps (Omron pedometer) among intervention participants (Weeks 1–3: n = 20; Weeks 4–9 and Weeks 19–20: n = 19; Weeks 10–14: n = 18). *Slope analysis: p = .002.
Because the primary outcome variable, reported minutes of walking per week, comprised two forms of walking—walking fast or briskly for exercise and walking leisurely for exercise or pleasure—we conducted secondary analyses to better understand the effects of the intervention on these two walking types. Analyses of covariance revealed a significant between-arm difference across 4 months for walking fast or briskly favoring the virtual advisor arm: M increase = 147.0 (SD = 167.1) in virtual advisor arm; M increase = 12.6 (SD = 33.3) in control arm; F(1, 38) = 10.8, p = .002, effect size = 1.1. There was a nonsignificant trend for walking leisurely across 4 months: M increase = 106.5 (SD = 211.5) in virtual advisor arm, M increase = 14.2 (SD = 51.4) in control arm; F(1, 38) = 3.0, p = .09, effect size = 0.6.
Changes in Strategies to Promote Physical Activity Behavior Change
Of the seven motivational behavior change strategies reflected in the computer intervention, five increased significantly across 4 months in the virtual advisor arm relative to control. These included understanding the risks of inactivity (p = .03, effect size = 0.6), committing oneself to being physically active (p = .006, effect size = 0.9), substituting more active alternatives (p = .0004, effect size = 1.2), rewarding oneself for being physically active (p = .03, effect size = 0.7), and reminding oneself to be physically active (p = .009, effect size = 0.9).
Safety of the Virtual Advisor Program
No significant physical activity-related injuries or health problems were reported across the 4-month study period. Four participants reported some mild soreness in their legs during walking that did not affect their walking routine. One additional participant reported that sore leg muscles led to a temporary halt in her walking routine, but she was able to resume her walking program after a week.
Continued Use of the Virtual Advisor Following the Study Period
Because a number of participants assigned to the virtual advisor arm requested that they be allowed to continue accessing the computer program following the end of their 4-month intervention, the computer program remained in the senior center across an additional 20-week period. During this poststudy period, virtual advisor participants accessed the program a mean of 14 ± 20.5 additional times, which translates into a mean of 0.68 sessions per week during this period (range = 0 to 4.5). Of the intervention participants, 95% (19/20) interacted with the virtual advisor at some point during the poststudy period. The mean daily pedometer steps downloaded to the virtual advisor program for intervention participants who interacted with the virtual advisor during this additional period ranged from 4350.2 ± 4095.8 steps per day (postintervention week 9, n = 14) to 7129.7 ± 4740.4 steps per day (postintervention week 16, n = 9). In general, there was a gradual dropoff of participant use over the 5 months of the postintervention period (i.e., monthly participation percentages equaled 96%, 84%, 66%, 53%, and 42% for postintervention months 1 to 5, respectively).
Ease of Use and Acceptability of the Virtual Advisor Program
Problems that participants experienced during the virtual advisor intervention period were reasonably minor, consisting mostly of forgetting login information or experiencing problems in trying to print educational tips. These problems, experienced by 25% of virtual advisor program participants (n = 5) were readily corrected (i.e., requiring less than 5 minutes) by project staff or community center staff or volunteers.
Four-month ratings by intervention participants indicated generally positive responses to the program. Participants had a mean of 5.7 ± 0.67 (out of 7) across the 19 program acceptability scale items, which included how much participants perceived that the virtual advisor cared (mean ± SD = 6.2 ± 0.83) and how close their relationship was to the advisor (mean = 6.0 ± 1.2). Participants had a mean of 6.0 ± 0.78 out of 7 across the six computer credibility scale items related to the trustworthiness of the information being delivered. Participants rated the length of their talks with the virtual advisor as “just about right” (mean rating of 4.4 ± 0.59 out of 7), and rated their interest in continuing to work with the virtual advisor as reasonably high (mean of 5.9 ± 1.8 out of 7). The average 4-month ratings on the Working Alliance Inventory bonding scale were also high (mean of 6.0 ± 0.84 out of 7).
After Study End, Virtual Advisor Use by Participants Originally in the Waitlist Arm
As noted earlier, 19 of 20 participants (95%) randomized to the waitlist control arm chose to use the virtual advisor program when it was offered to them after their 4-month study participation. During this period, their mean number of program logins per week (1.1 ± 0.81) and mean pedometer steps per week were similar to those observed in those originally randomized to the virtual advisor program (p values >.10).
Discussion
A major challenge facing the e-health communication field concerns the development of computer-delivered health promotion interventions that are appropriate for socioeconomically disadvantaged populations. A computerized virtual advisor that d elivered culturally adapted, individually tailored walking advice in a neighborhood setting led to meaningful 4-month increases in walking relative to control among a largely Latino, initially inactive older adult sample. Participants receiving the virtual advisor intervention had an eight-fold increase in walking relative to modest changes among control participants across the 4-month intervention period. The reported increases in walking among intervention participants were verified through participant pedometer readings throughout the intervention period. The daily pedometer steps for intervention participants during the last month of the intervention averaged 6,670—an amount commensurate with meeting the national physical activity recommendations in this age group (Jordan, Jurca, Locke, Church, & Blair, 2005). Although it is difficult to map precisely the reported minutes of walking from the CHAMPS with the downloaded pedometer step information, estimates based on Omron calculations are intriguing. The Omron Corporation’s definition of brisk walking is estimated to be at least 60 steps per minute (Omron Healthcare, 2007). Dividing the mean number of pedometer steps per day downloaded by intervention participants by this number suggests that by approximately Week 8 of the 16-week intervention, participants were walking roughly 33 minutes per day—a number close to that being reported on the CHAMPS questionnaire by the end of the 16-week period.
It is noteworthy that 95% of intervention participants accessed the virtual advisor on their own during the 20-week period following the formal end of their program, suggesting the potential for extended use if the program was located in community settings which older adults visit regularly. Primary care clinics represent one such setting. The virtual advisor program potentially could be situated in clinic waiting rooms, or used by nutritionists and other health care staff as an adjunct to dietary counseling in patients with obesity-related diseases or conditions. Depending on the population of older adults, other settings could include pharmacies, libraries, or other community venues. Community staff and volunteers receiving basic training in using the virtual advisor program were able to readily resolve the minor program difficulties that occurred during the study period. These program-related problems generally took less than 5 minutes to resolve. This suggests the general feasibility of introducing the virtual advisor program in other community settings. Although not explicitly included in this first-generation study, additional low-cost strategies for promoting continued use of the virtual advisor program could be formally included as part of the program (Bickmore & Picard, 2005).
The results also provide evidence that the virtual advisor intervention influenced specific cognitive and behavioral skills that have been associated with positive health behavior change in other populations (Marcus, Rossi, Selby, Niaura, & Abrams, 1992). Such skills have been linked with longer-term physical activity maintenance in more affluent groups (Marcus, Rossi, et al., 1992), although they have been studied rarely in older, ethnic minority samples.
Difficulties in using the virtual advisor were minimal and easily remedied, with postintervention questionnaires indicating that participants were reasonably comfortable using the virtual advisor. The 4-month intervention was found to be safe for this sample, which was screened for eligibility using reliable tools that can be readily collected in a community or clinic setting (Thomas et al., 1992).
Study strengths include a socioeconomically disadvantaged ethnic minority population that rarely has been targeted for e-health interventions; an easy to use and engaging bilingual computer intervention that requires minimal amounts of language, computer, and health literacy skills; and the potential to place the program in community settings in which diverse groups of older adults visit regularly. Among the limitations of this first-generation study are the small sample size and short-term intervention period. Subsequent investigations should evaluate the virtual advisor program across longer time periods and a greater array of community settings. Similarly, the waitlist control arm, while commensurate with recommendations for first generation, early efficacy studies in an emerging field such as this one (Mohr et al., 2009), does not allow for evaluation of the effects of specific intervention components. A potential next step in this line of research could entail the systematic dismantling of the ECA intervention, using a specific treatment component control group design, to better understand the effects of its different components (e.g., delivery in a senior center as opposed to within participants’ homes; specific effects of the pedometer) on physical activity changes (Mohr et al., 2009; Strath et al., 2011). Although some studies have indicated that pedometer use can promote physical activity increases in generally younger populations (although most of these studies included additional counseling as well; Bravata et al., 2007), the original Bickmore et al. study testing an ECA intervention in low-income older adults found that pedometer use alone had minimal 8-week effects on physical activity levels in the absence of personalized ECA counseling (Bickmore, Caruso, et al., 2005).
Last, of note, enhancing the waitlist control arm, based on community members’ advice gathered during the prestudy phase of the research, with periodic health education sessions of interest to this underserved population may have helped in achieving the 95% study retention rate in that arm.
To emulate, as much as possible, intervention delivery and evaluation methods promoting external validity as well as meet budgetary requirements, more burdensome and costly measurement protocols, including accelerometry, were not included. Reliance on self-report, however, can be a less accurate means for capturing less intensive forms of physical activity, such as walking, that can be prone to misreporting (Hekler, Buman, Haskell, et al., 2012; Neilson, Robson, Friedenreich, & Csizmadi, 2008). Inclusion of accelerometry would have provided additional verification of the reported difference observed between study arms in brisk or faster-paced walking for exercise, relative to more leisurely forms of walking—a distinction that cannot be reliably made using pedometers (Le Masurier, Lee, & Tudor-Locke, 2004). This limitation notwithstanding, the Omron and similar pedometers have been reasonably correlated with accelerometry-derived estimates of walking (Le Masurier et al., 2004). The ease of use, inexpensiveness, and practicality of this kind of pedometer, including its c omputer download capability, make it a good choice for a wide range of populations across the socioeconomic spectrum. This is particularly the case for populations, such as the current one, which may have reduced numeracy skills that can make manual (written) recording of daily or weekly pedometer steps by participants challenging. Additionally, research has indicated that highlight intensity activities such as leisurely walking appear to have positive health benefits on physical health and psychosocial wellbeing after controlling for moderate-intensity physical activity and sedentary behaviors (Buman et al., 2010). This finding suggests that interventions among older adults may not need to exclusively promote moderate-to-vigorous intensity activity but could gain useful health benefits by targeting highlight intensity activity as well (Hekler, Buman, Haskell, et al., 2012).
In conclusion, this interactive technology, which requires minimal language, health, and computer literacy, may help to ensure that all segments of the population benefit from e-health opportunities and deserves expanded evaluation.
Acknowledgments
The authors thank the senior citizens and staff of the Eastside Neighborhood Center, San Jose, California, including Milton Cadena, Center Director, for support and participation. Dulce Garcia’s assistance in data collection was greatly appreciated. The authors also thank Juan Fernandez, Dharma Cortez, and Donna Byron, Northeastern University, for programming work, Dr. David Ahn for contributions in the area of statistical analysis, Drs. Cynthia Castro, Stanford Prevention Research Center, and Guido Urizar, Jr., California State University-Long Beach, California, for input during project development, and Randall Stafford, M.D., Ph.D., Stanford University School of Medicine, for helpful advice in reviewing earlier drafts of this article.
This research was supported by PHS grant # R21CA127511 from the National Cancer Institute awarded to Dr. King. The clinicaltrials.gov registry number for this study is NCT01144767.
Contributor Information
ABBY C. KING, Division of Epidemiology, Department of Health, Research and Policy, and Stanford Prevention Research, Program, Department of Medicine, Stanford University, School of Medicine, Stanford, California, USA
TIMOTHY W. BICKMORE, College of Computer and Information Science, Northeastern University, Boston, Massachusetts, USA
MARIA INES CAMPERO, Stanford Prevention Research Center, Department of Medicine, Stanford University School of Medicine, Stanford, California, USA.
LESLIE A. PRUITT, Stanford Prevention Research Center, Department of Medicine, Stanford University School of Medicine, Stanford, California, USA
JAMES LANGXUAN YIN, College of Computer and Information Science, Northeastern University, Boston, Massachusetts, USA.
References
- Bandura A (2001). Social cognitive theory: An agentic perspective. Annual Reviews of Psychology, 52, 1–26. [DOI] [PubMed] [Google Scholar]
- Berland GK, Elliott MN, Morales LS, Algazy JI, Kravitz RL, Broder MS, … McGlynn EA (2001). Health information on the Internet: Accessibility, quality, and readability in English and Spanish. JAMA, 285, 2612–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickmore T, Caruso L, Clough-Gorr K, & Heeren T (2005). “It’s just like you talk to a friend”—Relational agents for older adults. Interacting with Computers, 17, 711–735. [Google Scholar]
- Bickmore T, Gruber A, & Picard R (2005). Establishing the computer-patient working alliance in automated health behavior change interventions. Patient Education & Counseling, 59, 21–30. [DOI] [PubMed] [Google Scholar]
- Bickmore T, Pfeifer LM, Byron D, Forsythe S, Henault LE, Jack BW, … PaascheOrlow MK (2010). Usability of conversational agents by patients with inadequate health literacy: Evidence from two clinical trials. Journal of Health Communication, 15(Suppl 2), 197–210. [DOI] [PubMed] [Google Scholar]
- Bickmore T, & Picard R (2005). Establishing and maintaining long-term human-computer relationships. ACM Transactions on Computer Human Interaction (ToCHI), 12, 293–327. [Google Scholar]
- Bodie GD, & Dutta MJ (2008). Understanding health literacy for strategic health marketing: eHealth, literacy, health disparities, and the digital divide. Health Marketing Quarterly, 25, 175–203. [DOI] [PubMed] [Google Scholar]
- Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, … Sirard JR (2007). Using pedometers to increase physical activity and improve health: A systematic review. JAMA, 298, 2296–2304. [DOI] [PubMed] [Google Scholar]
- Brawley LR, Rejeski WJ, & King AC (2003). Promoting physical activity for older adults: The challenges for changing behavior. American Journal of Preventive Medicine, 25, 172–183. [DOI] [PubMed] [Google Scholar]
- Buman MP, Hekler EB, Haskell WL, Pruitt L, Conway TL, Cain KL, … King AC (2010). Objective light-intensity physical activity associations with rated health in older adults. American Journal of Epidemiology, 172, 1155–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardelle AJF, & Rodriguez EG (2005). The quality of Spanish health information websites: An emerging disparity. Journal of Prevention & Intervention in the Community, 29, 85–102. [Google Scholar]
- Castro CM, & King AC (2002). Telephone-assisted counseling for physical activity. Exercise and Sport Sciences Reviews, 30, 64–68. [DOI] [PubMed] [Google Scholar]
- Czaja SJ, Schulz R, Lee CC, & Belle SH, for the REACH Investigators. (2003). A methodology for describing and decomposing complex psychosocial and behavioral interventions. Psychology and Aging, 18, 385–395. [DOI] [PubMed] [Google Scholar]
- Echt KV, & Morrell RW (2003). Promoting health literacy in older adults: An overview of the promise of interactive technology. Washington, DC: National Institutes of Health. [Google Scholar]
- Efron B (1971). Forcing a sequential experiment to be balanced. Biometrika, 58, 403–417. [Google Scholar]
- Fogg BJ, Marshall JA, Kameda T, Solomon J, Rangnekar A, Boyd J, … Brown B (2001). Web credibility research: A method for online experiments and early study results. Paper presented at the Proceedings of ACM CHI 2001, Seattle, WA, USA, March 31– April 5. [Google Scholar]
- Freudenthal D (2001). Age differences in the performance of information retrieval tasks. Behaviour & Information Technology, 20, 9–22. [Google Scholar]
- Glasgow RE, Davidson KW, Dobkin PL, Ockene J, & Spring B (2006). Practical behavioral trials to advance evidence-based behavioral medicine. Annals of Behavioral Medicine, 31, 5–13. [DOI] [PubMed] [Google Scholar]
- Harada ND, Chiu V, King AC, & Stewart AL (2001). An evaluation of three self-report physical activity instruments for older adults. Medicine and Science in Sports and Exercise, 33, 962–970. [DOI] [PubMed] [Google Scholar]
- Hawkins RP, Kreuter M, Resnicow K, Fishbein M, & Dijkstra A (2008). Understanding tailoring in communicating about health. Health Education Research, 23, 454–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hekler EB, Buman MP, Dunton GF, Atienza AA, & King AC (2012). Are daily fluctuations in perceived environment associated with walking after controlling for implementation intentions? Psychology & Health, 27, 1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hekler EB, Buman MP, Haskell WL, Conway TL, Cain KL, Sallis JF, … King AC (2012). Reliability and validity of CHAMPS self-reported sedentary to vigorous intensity physical activity in older adults. Journal of Physical Activity and Health, 9, 225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook EA, Barreira TV, & Kang M (2009). Validity and reliability of Omron pedometers for prescribed and self-paced walking. Medicine and Science in Sports and Exercise, 41, 669–673. [DOI] [PubMed] [Google Scholar]
- Horvath A, & Greenberg L (1989). Development and validation of the Working Alliance Inventory. Journal of Counseling Psychology, 36, 223–233. [Google Scholar]
- Horvath A, & Greenberg L (1994). The Working Alliance: Theory, research and practice. New York, NY: Wiley. [Google Scholar]
- Jordan AN, Jurca GM, Locke CT, Church TS, & Blair SN (2005). Pedometer indices for weekly physical activity recommendations in postmenopausal women. Medicine and Science in Sports and Exercise, 37, 1627–1632. [DOI] [PubMed] [Google Scholar]
- King AC (2001). Interventions to promote physical activity in older adults. Journal of Gerontology: Biological Sciences and Medical Sciences, 56A(Special Issue II), 36–46. [DOI] [PubMed] [Google Scholar]
- King AC, Baumann K, O’Sullivan P, Wilcox S, & Castro C (2002). Effects of moderate-intensity exercise on physiological, behavioral, and emotional responses to family caregiving: A randomized controlled trial. Journal of Gerontology: Medical Sciences, 57A, M26–M36. [DOI] [PubMed] [Google Scholar]
- King AC, Friedman R, Marcus BH, Castro C, Napolitano M, Ahn D, … Baker L (2007). Ongoing physical activity advice by humans versus computers: The Community Health Advice by Telephone (CHAT) Trial. Health Psychology, 26, 718–727. [DOI] [PubMed] [Google Scholar]
- King AC, Friedman R, Marcus BH, Napolitano MA, Castro C, & Forsyth L (2004). Increasing regular physical activity via humans or automated technology: 12-month results of the CHAT trial. Annals of Behavioral Medicine, 27(Suppl), S044. [Google Scholar]
- King AC, & Guralnik JM (2010). Maximizing the potential of an aging population through shaping an active and vital old age. JAMA, 304, 1944–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Pruitt LA, Phillips W, Oka R, Rodenburg A, & Haskell WL (2000). Comparative effects of two physical activity programs on measured and perceived physical functioning and other health-related quality of life outcomes in older adults. Journal of Gerontology: Medical Sciences, 55A, M74–M83. [DOI] [PubMed] [Google Scholar]
- Kreuter MW, & McClure SM (2004). The role of culture in health communication. Annual Review of Public Health, 25, 439–455. [DOI] [PubMed] [Google Scholar]
- Le Masurier GC, Lee SM, & Tudor-Locke C (2004). Motion sensor accuracy under controlled and free-living conditions. Medicine and Science in Sports and Exercise, 36, 905–910. [DOI] [PubMed] [Google Scholar]
- Marcus BH, Rakowski W, & Rossi JS (1992). Assessing motivational readiness and decision making for exercise. Health Psychology, 11, 257–261. [DOI] [PubMed] [Google Scholar]
- Marcus BH, Rossi JS, Selby VC, Niaura RS, & Abrams DB (1992). The stages and processes of exercise adoption and maintenance in a worksite sample. Health Psychology, 11, 386–395. [DOI] [PubMed] [Google Scholar]
- Marcus BH, & Simkin LR (1994). The transtheoretical model: Applications to exercise behavior. Medicine and Science in Sports and Exercise, 26, 1400–1404. [PubMed] [Google Scholar]
- Mohr DC, Spring B, Freedland KE, Beckner V, Arean P, Hollon SD, … Kaplan R (2009). The selection and design of control conditions for randomized controlled trials of psychological interventions. Psychotherapy and Psychosomatics, 78, 275–284. [DOI] [PubMed] [Google Scholar]
- Munoz RF, & Mendelson T (2005). Toward evidence-base interventions for diverse populations: The San Francisco General Hospital Prevention and Treatment Manuals. Journal of Consulting and Clinical Psychology, 73, 790–799. [DOI] [PubMed] [Google Scholar]
- Napolitano MA, Papandonatos GD, Lewis BA, Whiteley JA, Williams DM, King AC, … Marcus BH (2008). Mediators of physical activity behavior change: A multivariate approach. Health Psychology, 27, 409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson HK, Robson PJ, Friedenreich CM, & Csizmadi I (2008). Estimating activity energy expenditure: How valid are physical activity questionnaires? American Journal of Clinical Nutrition, 87, 279–291. [DOI] [PubMed] [Google Scholar]
- Norman GJ, Zabinski MF, Adams MA, Rosenberg DE, Yaroch AL, & Atienza AA (2007). A review of eHealth interventions for physical activity and dietary behavior change. American Journal of Preventive Medicine, 33, 336–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omron Healthcare. (2007). Omron instruction manual for pocket pedometer model HJ-720ITC. Bannockburn, IL: Author. [Google Scholar]
- Pahor M, Blair SN, Espeland M, Fielding R, Gill TM, Guralnik JM, … Studenski S (2006). Effects of a physical activity intervention on measures of physical performance: Results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. Journal of Gerontology Series A: Biological Sciences and Medical Sciences, 61, 1157–1165. [DOI] [PubMed] [Google Scholar]
- Physical Activity Guidelines Advisory Committee. (2008). Report of the Physical Activity Guidelines Advisory Committee, 2008. Washington, DC: U.S. Department of Health and Human Services. [Google Scholar]
- Rainer J, R. K., & Miller MD (1996). An assessment of the psychometric properties of the Computer Attitude Scale. Computers in Human Behavior, 12, 93–105. [Google Scholar]
- SAS Institute Inc. (2009). SAS/STAT 9.2 user’s guide (2nd ed.). Cary, NC: Author. [Google Scholar]
- Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, & Ritter PL (2001). CHAMPS physical activity questionnaire for older adults: Outcomes for interventions. Medicine and Science in Sports and Exercise, 33, 1126–1141. [DOI] [PubMed] [Google Scholar]
- Strath SJ, Swartz AM, Parker SJ, Miller NE, Grimm EK, & Cashin SE (2011). A pilot randomized controlled trial evaluating motivationally matched pedometer feedback to increase physical activity behavior in older adults. Journal of Physical Activity and Health, 8(Suppl. 2), S267–S274. [DOI] [PubMed] [Google Scholar]
- Thomas S, Reading J, & Shephard RJ (1992). Revision of the Physical Activity Readiness Questionnaire (PAR-Q). Canadian Journal of Sports Science, 17, 338–345. [PubMed] [Google Scholar]
- Umstattd MR, Wilcox S, Saunders R, Watkins K, & Dowda M (2008). Self-regulation and physical activity: The relationship in older adults. American Journal of Health Behavior, 32, 115–124. [DOI] [PubMed] [Google Scholar]
- Viswanath K, & Kreuter MW (2007). Health disparities, communication inequalities, and eHealth. American Journal of Preventive Medicine, 32(5 Suppl), S131–S133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster J, & Trevino LK (1995). Rational and social theories as complementary explanations of communication media choices: Two policy-capturing studies. Academy of Management Journal, 38, 1544–1572. [Google Scholar]
- Wilcox S, Dowda M, Leviton LC, Bartlett-Prescott J, Bazzarre T, Campbell-Voytal K, … Wegley S (2008). Active for Life: Final results from the translation of two physical activity programs. American Journal of Preventive Medicine, 35, 340–351. [DOI] [PubMed] [Google Scholar]