Abstract
Peptide secondary and tertiary structure motifs frequently serve as inspiration for the development of protein–protein interaction (PPI) inhibitors. While a wide variety of strategies have been used to stabilize or imitate α helices, similar strategies for β-sheet stabilization are more limited. Synthetic scaffolds that stabilize reverse turns and cross-strand interactions have provided important insights into β-sheet stability and folding. However, these templates occupy regions of the β-sheet that might impact the β-sheet’s ability to bind at a PPI interface. Here, we present the hydrogen bond surrogate (HBS) approach for stabilization of β-hairpin peptides. The HBS linkage replaces a cross-strand hydrogen bond with a covalent linkage, conferring significant conformational and proteolytic resistance. Importantly, this approach introduces the stabilizing linkage in the buried β-sheet interior, retains all side chains for further functionalization, and allows efficient solid-phase macrocyclization. We anticipate that HBS stabilization of PPI β-sheets will enhance the development of β-sheet PPI inhibitors and expand the repertoire of druggable PPIs.
Graphical Abstract
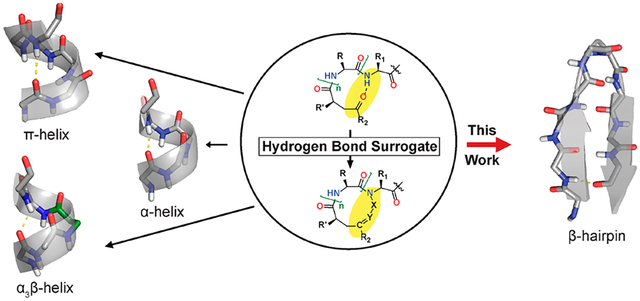
Peptides that fold into stable secondary and tertiary structures have become powerful tools to mimic molecular recognition epitopes and modulate protein–protein interactions (PPIs) to probe biological pathways.1–4 A range of stabilization strategies have been validated for common secondary (α-helix,5–13 β-strand14,15) and tertiary structures (coiled coils,16 β-sheets17–22) as well as nonregular structures.4,23,24
The hydrogen bond surrogate (HBS) approach is unique among these strategies in that the resulting peptides retain all side chains to confer desirable properties. In the HBS approach, a main chain hydrogen bond is replaced with an isosteric covalent linkage.5,25,26 Because only a main chain hydrogen bond is replaced, all side chains can be manipulated to control binding affinity and specificity. The HBS approach was originally developed to nucleate the α-helical conformation.5,25–28 For HBS α-helix stabilization, compatible functional groups replace atoms involved in the N-terminal main-chain hydrogen bond, namely, the carbonyl of position i and the amide hydrogen of position i + 4. Reaction of these functional groups produces a 13-membered macrocycle positioned to nucleate an α-helix. HBS linkers containing olefin,27 thioether,29 and disulfide30 groups have been validated for their ability to nucleate the α-helical conformation and inhibit a variety of α-helix-mediated PPIs.2,31–37
Exploratory studies have suggested that the HBS approach can also be used to stabilize other macrocycle sizes through main chain hydrogen bond mimicry.26,27,38,39 For instance, peptides containing 14- and 16-membered HBS macrocycles have been synthesized. The former incorporates β-amino acids at every fourth amino acid (α3β) to promote peptides’ proteolytic stability while maintaining a helical conformation.40 The latter yields a macrocycle that is predicted to mimic a π helix—a high energy conformation rarely seen in proteins.41
We envisioned that the HBS approach could be generalized to mimic macrocycles with diverse hydrogen bonding patterns. Specifically, we hypothesized that the HBS approach could be used to stabilize β-sheet structures, which also feature prominently at PPI interfaces.42 Many strategies have been evaluated to generate stable macrocyclic β-sheet peptide mimics. These seminal efforts include various stabilized turn motifs (e.g., D-Pro/L-Pro,17,18 D-Pro/Gly,43 L-ornithine44) and covalent and noncovalent side chain bridges (e.g., cystine disulfide,45 1,2,3-triazole,22,46,47 diphenylacetylene,21 tryptophan zipper19) to stabilize the folded conformation (Figure 1). The salient feature of the HBS strategy is that natural sequences can be stabilized using a minimalist template by converting a key hydrogen bond to its covalent mimic. To test the hypothesis that replacement of a single hydrogen bond with a covalent bond is enough to provide a stable β-sheet conformation, we examined HBS stabilization of β-hairpins, which are the smallest unit of antiparallel β-sheets.
Figure 1.
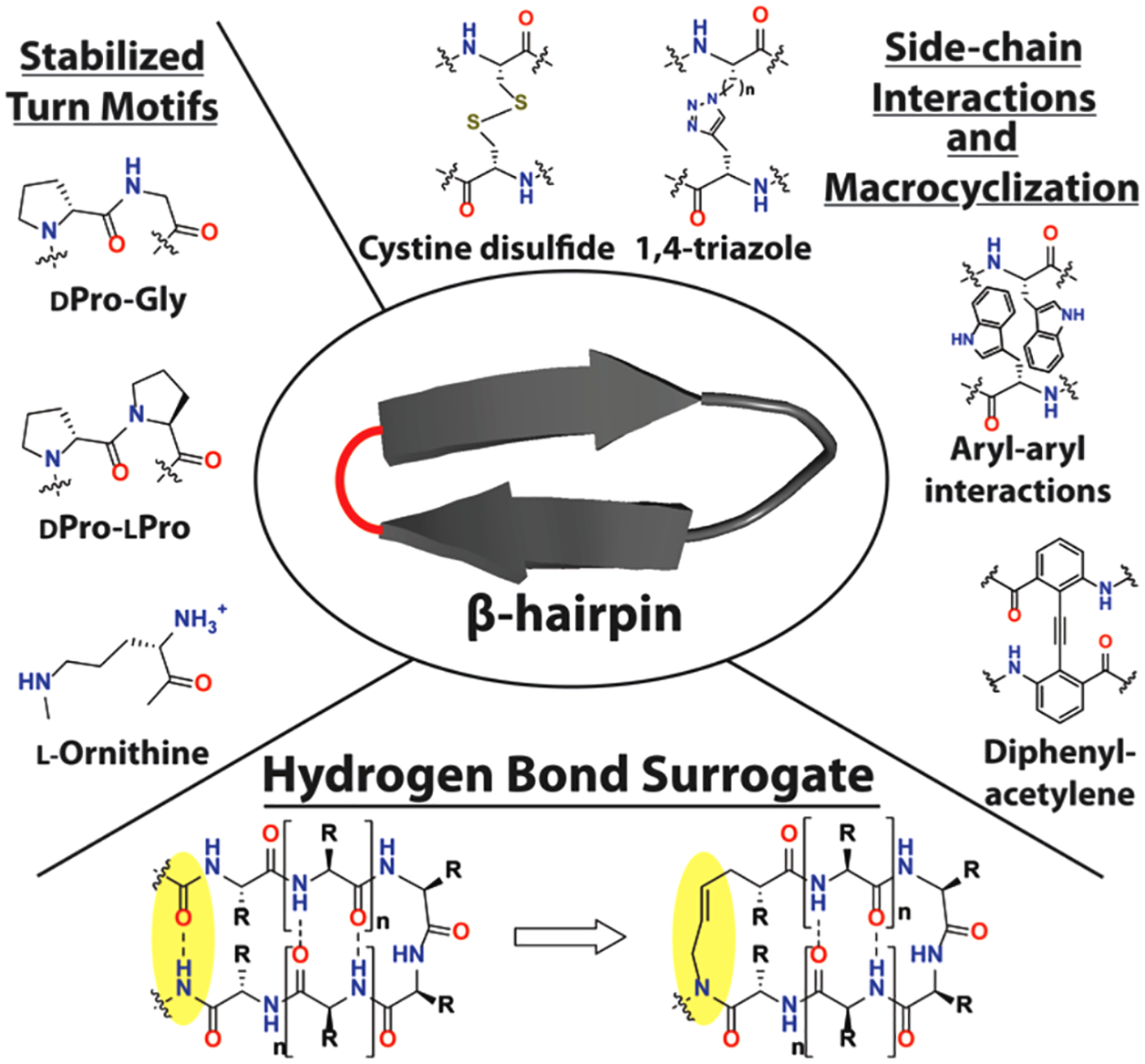
Strategies for stabilizing β-hairpins by macrocyclization. Previous templates to stabilize β-hairpin macrocycles include stabilized turn motifs (left) and side-chain interactions. The hydrogen bond surrogate replaces a cross-strand hydrogen bond (bottom), retaining all side chains for functionalization.
We chose the HP7 β-hairpin sequence as a model system. HP7 is a minimal β-hairpin designed by Andersen et al. and is one of the smallest stable β-hairpin sequences.48 Importantly, destabilized HP7 variants have also been reported, allowing us to determine if HBS macrocyclization could rescue the conformational stability of a destabilized variant.
We began by replacing the N-to-C-terminal salt bridge, which occupies a hydrogen bonding position, with a hydrocarbon mimic enabled by a ring closing metathesis reaction (Figure S1). The olefin HBS stabilizes tertiary interactions consistent with β-hairpin formation. Figure 2A–D shows structures and circular dichroism (CD) spectra for HP7, its destabilized variant HP7Δ, and HP7Δ with an HBS linking the N- and C-termini (HP7Δ-HBS). Characteristic features of HP7 include a maximum at 228 nm resulting from excition coupling between interacting cross-strand Trp residues arranged in an edge-to-face orientation,19,49 a minimum at 215 nm consistent with β-sheet formation, an inflection point near 201 nm, and a local maximum at 190 nm. Importantly, the 228 nm peak is a strong indicator of β-hairpin formation in HP7, as it requires interaction between the tryptophan residues on opposite strands. Compared to HP7, peptides that replace the terminal salt bridge with a hydrogen bond or remove the terminal Lys/Glu residues (HP7Δ)48 show decreased stability across the whole wavelength range (Figures 2D, S2).
Figure 2.
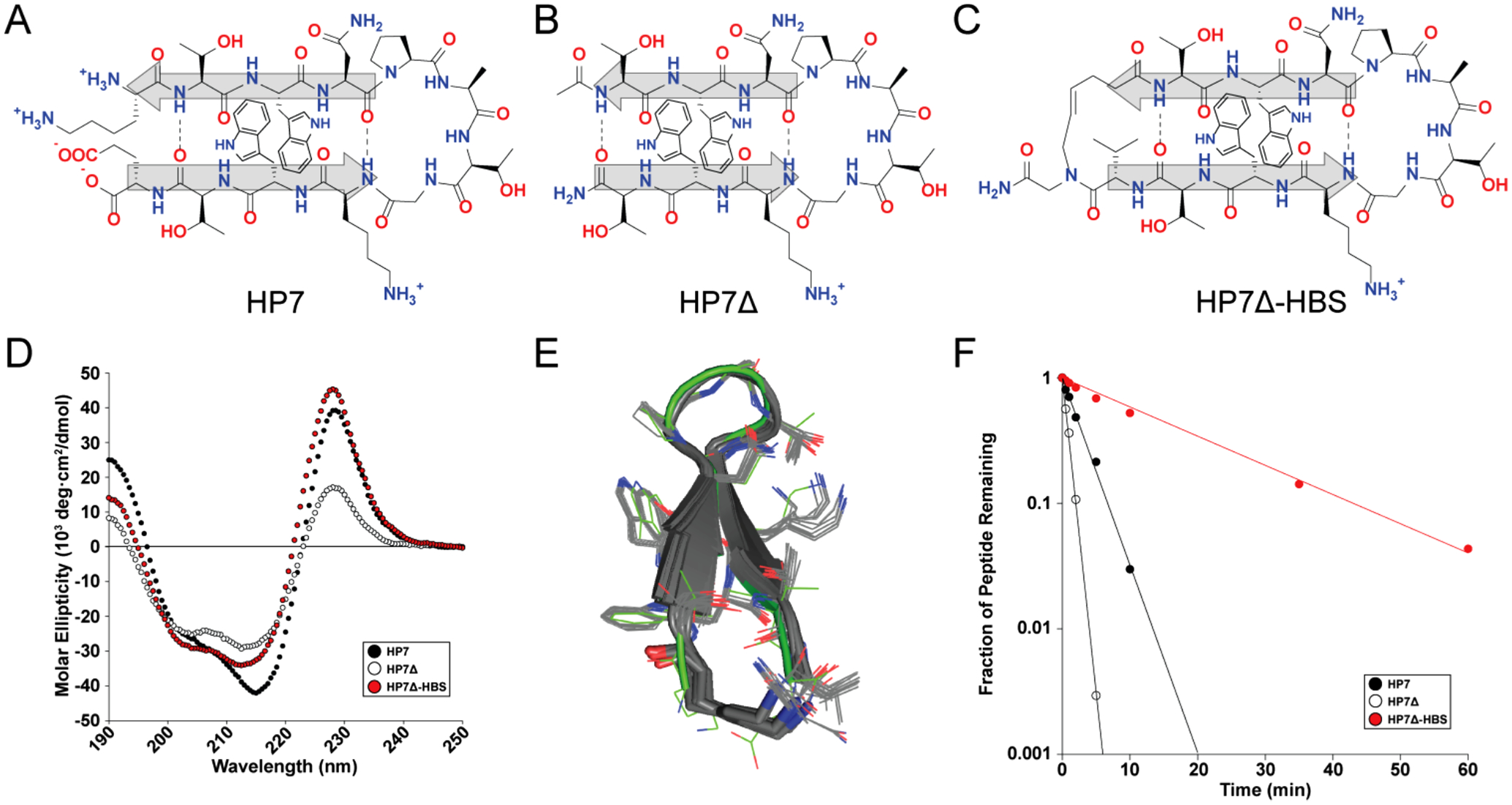
HP7 β-hairpin conformational and proteolytic stability using an olefin HBS linkage. (A–C) Structures of the model β-hairpin peptide HP7, the destabilized variant HP7Δ, and HP7Δ with an olefin HBS linkage (HP7Δ-HBS), respectively. (D) Circular dichroism spectra of HP7 (black), HP7Δ (white), and HP7Δ-HBS (red). (E) An ensemble of the 20 lowest-energy HP7Δ-HBS conformations derived from Monte Carlo simulations with NMR-derived torsion angle and NOE constraints (gray) overlaid with the HP7 structure (green). (F) Resistance of HP7 peptides to proteinase K digestion.
The CD spectrum of HP7Δ-HBS shows intensity recovery across the spectrum, with complete recovery of the 228 nm Trp–Trp interaction peak intensity. Significantly, the spectrum is nearly identical to the spectrum for an HP7 cystine disulfide macrocycle that serves as a traditional model for full β-hairpin folding (Figure S2C). Temperature-dependent CD spectra (Figures S2, S3) further support the hypothesis that incorporating the covalent HBS linkage into HP7Δ improves β-hairpin stability to a greater extent than a terminal hydrogen bond or even a strong salt bridge interaction.
NMR spectroscopy studies confirmed formation of a stable β-hairpin formation in HP7Δ-HBS. Two-dimensional TOCSY and NOESY spectra were acquired (Figures 2E, S4), showing excellent agreement with the previously reported HP7 spectra (except for protons within and adjacent to the HBS). 3JNHCαH coupling constants and NOE data support the formation of the expected β-hairpin structure. Simulations using NOE and φ angle constraints (derived from 3JNHCαH coupling constants) produced a structural ensemble that overlays very well with the original HP7 β-hairpin (Figure 2E).
One of the weaknesses of many peptides in clinical applications is their susceptibility to protease degradation, which limits their effective dose. We hypothesized that HBS macrocyclization of the HP7Δ peptide would improve its stability relative to HP7Δ, as observed with HBS stabilization of α-helices.33 We measured the amount of peptide remaining at various times after incubation with high (100 μg/mL) concentrations of the broad-specificity proteinase K. This stringent assay allowed us to calculate half-lives of each peptide. While HP7Δ has a half-life of 36 s and HP7 has a half-life of 120 s under these conditions, HP7Δ-HBS has a half-life of 770 s, a >20-fold improvement over HP7Δ from which it is derived (Figure 2F).
Next, we investigated the effects of different HBS linker chemistry on β-hairpin folding and stability to determine if a thioether bridge, which does not require a metal catalyst for synthesis, can be installed in place of the hydrocarbon bridge (Figures 3, S5). We also explored a disulfide bridge to develop a strategy for reversibly probing β-hairpin formation. Previous studies with HBS stabilization of α-helices examined these three chemistries—olefin, thioether, and disulfide—and showed that all three HBS linkers stabilize the α-helix to promote PPI inhibition.29,32,50 In the olefin HBS, the C–C double bond mimics the N-terminal amide carbonyl, providing linker rigidity. The thioether and disulfide HBS linkers lack this rigidifying double bond (though disulfide bonds show specific conformational preferences).51 CD spectra of HP7Δ peptides with each HBS linker reveal that all three chemistries fully restore the Trp–Trp interaction peak at 228 nm (Figure 3). Surprisingly, both thioether (HP7Δ-teHBS) and disulfide (HP7Δ-dsHBS) HBS peptides show increased spectral intensity in regions of the spectra (212–218 nm and 190–195 nm) that are characteristic of β-sheets. These data suggest that the more rigid olefin HBS may stabilize a strained β-sheet while the more flexible thioether and disulfide HBS linkers may relax any distortion.
Figure 3.
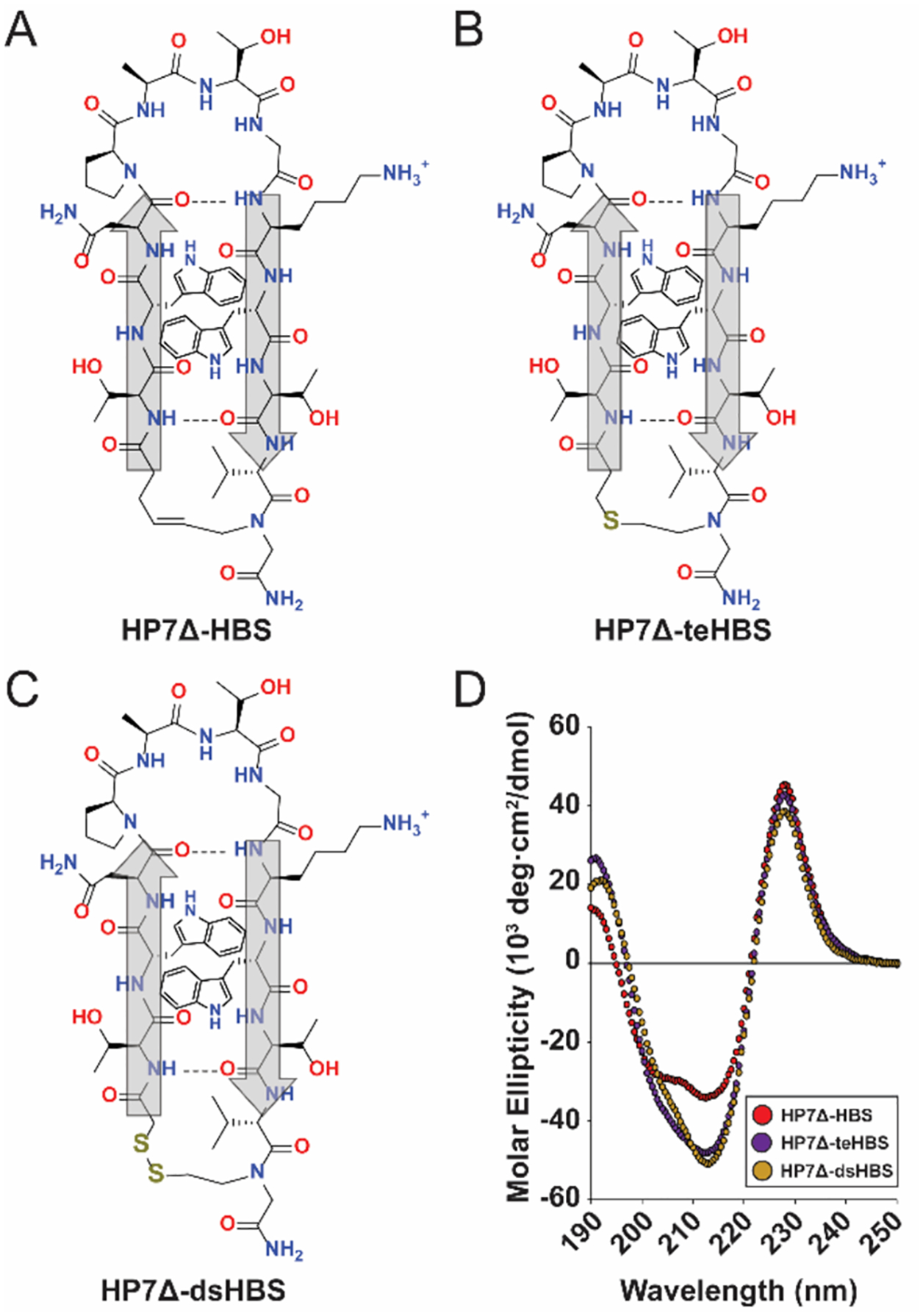
Comparison of β-hairpin conformational stability using different HBS linkages. (A–C) Structures of HP7Δ-HBS peptides with olefin (HP7Δ-HBS), thioether (HP7Δ-teHBS), and disulfide (HP7Δ-dsHBS) HBS linkages, respectively. (D) Circular dichroism spectra of olefin (red), thioether (purple), and disulfide (yellow) HBS peptides.
We also examined the potential of the disulfide macrocycle HP7Δ-dsHBS to serve as a redox-sensitive β-hairpin. Reduction of HP7Δ-dsHBS to the bis-thiol peptide (HP7Δbt) using tris(2-carboxyethyl)phosphine (TCEP) showed significant loss of structure (Figure S6). The loss of structure upon reduction mirrors the difference between the structured HP7 β-hairpin and the destabilized HP7Δ variant (Figure 1). Temperature-dependent CD shows that HP7Δ-bt forms a stable β-hairpin at 5°C but rapidly loses structure, displaying ~50% loss of initial 5°C structure at physiological temperature. In contrast, HP7Δ-dsHBS retains >50% of its original 5°C structure even at 95°C.
Finally, we evaluated the generality of the β-hairpin HBS strategy for stabilizing protein-derived β-hairpins. We selected a β-hairpin from the Ras-binding NS1 monobody designed by Koide et al.52 as a prototypical PPI β-hairpin featuring a common type II′ turn rather than the optimized turn in HP7. Comparison of the CD spectra for the unconstrained and HBS peptides shows dramatic stabilization of the HBS peptide in the β-hairpin conformation compared to the unconstrained peptide (Figure 4). In addition to the characteristic β-sheet minimum at 215 nm, we observed spectral signatures of cross-strand interactions between Trp and Tyr at 230 and 199 nm.49,53 Even at 95°C, we observed only a 25% decrease in spectral intensity at 215 nm. A cystine disulfide macrocycle of the same NS1 sequence shows similar intensity at 215 nm, suggesting that the NS1 HBS is well-folded (Figure S7).
Figure 4.
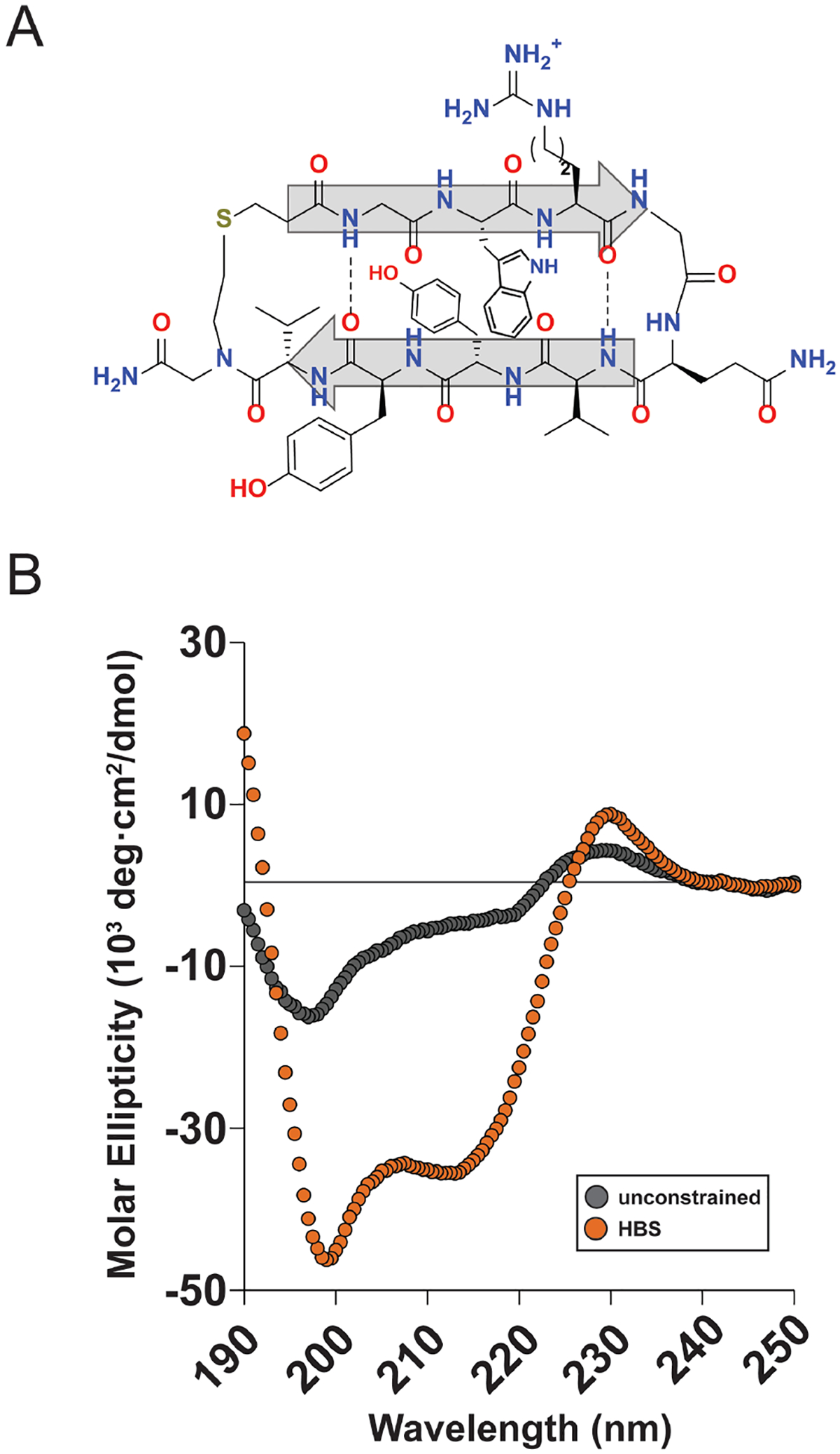
HBS stabilization of a protein-derived β-hairpin. (A) Structure of the NS1-derived HBS. (B) Circular dichroism spectra for NS1 unconstrained (gray, Ac-GGWKGQVYYVG-CONH2) and HBS (orange) peptides.
Overall, we found that the hydrogen bond surrogate approach can be generalized to stabilize nonhelical peptide conformations, specifically the β-hairpin. Using the HP7 sequence, we confirmed β-hairpin stabilization by CD and NMR spectroscopy. Conformational stabilization also confers significant protease resistance, which is promising for designing β-hairpin HBS PPI inhibitors with potential in vivo applications. Comparison of the hydrocarbon, thioether, and disulfide HBS linkers suggests that the thioether and disulfide bridges provide more β-hairpin stability. This finding is consistent with earlier findings with HBS-stabilized α-helices, which showed that rigidity and the hydrogen bond isostere geometry provide subtle control over the overall peptide conformation.25 Taking synthetic ease into account as well, the thioether β-hairpin HBS is preferred for generating metabolically stable β-hairpins while the disulfide β-hairpin HBS provides a redox trigger for reversible β-hairpin formation.54
The β-hairpin HBS approach provides a generalizable scaffold for targeting β-hairpin or β-sheet-mediated PPIs. Computational analysis of the protein complexes in the Protein Data Bank (PDB) demonstrated that interfacial β-strands are key elements in thousands of PPIs.42 These interfacial β-strands interact with their partners in various binding modes that involve complex combinations of main- and/or side-chain interactions using one or both faces of the β-strand. Because the β-hairpin HBS approach described here replaces only an internal hydrogen bond that cannot participate in molecular recognition, all main- and side-chain functional groups from the original β-hairpin can be incorporated and/or optimized for binding affinity and specificity.
An increasing number of designed proteins that use β-hairpins or β-sheets for molecular recognition of targets may facilitate further development of β-hairpin PPI inhibitors.52,55–59 As compared to native PPIs that are optimized for overall function in their biological context (not strictly binding) and constrained by their evolutionary history, designed proteins undergo focused optimization for target protein binding affinity and specificity. It is therefore likely that binding epitopes extracted from these proteins will have higher initial affinities and require less optimization than epitopes from native PPIs. Studies to generate β-hairpin HBS peptides from both native and designed β-hairpin binding proteins are underway and will be reported in due course.
Supplementary Material
ACKNOWLEDGMENTS
N.S. is supported by a Ruth L. Kirschstein National Research Service Award (NRSA) postdoctoral fellowship from NIGMS. P.S.A. thanks the National Intitutes of Health (R01GM073943) for financial support of this work
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acschembio.8b00641.
Methods, additional tables, and figures (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Chang YS, Graves B, Guerlavais V, Tovar C, Packman K, To K-H, Olson KA, Kesavan K, Gangurde P, Mukherjee A, Baker T, Darlak K, Elkin C, Filipovic Z, Qureshi FZ, Cai H, Berry P, Feyfant E, Shi XE, Horstick J, Annis DA, Manning AM, Fotouhi N, Nash H, Vassilev LT, and Sawyer TK (2013) Stapled α–helical peptide drug development: A potent dual inhibitor of MDM2 and MDMX for p53-dependent cancer therapy. Proc. Natl. Acad. Sci. U. S. A 110, E3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Kushal S, Lao BB, Henchey LK, Dubey R, Mesallati H, Traaseth NJ, Olenyuk BZ, and Arora PS (2013) Protein domain mimetics as in vivo modulators of hypoxia-inducible factor signaling. Proc. Natl. Acad. Sci. U. S. A 110, 15602–15607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Moellering RE, Cornejo M, Davis TN, Bianco CD, Aster JC, Blacklow SC, Kung AL, Gilliland DG, Verdine GL, and Bradner JE (2009) Direct inhibition of the NOTCH transcription factor complex. Nature 462, 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Peraro L, Zou Z, Makwana KM, Cummings AE, Ball HL, Yu H, Lin Y-S, Levine B, and Kritzer JA (2017) Diversity Oriented Stapling Yields Intrinsically Cell-Penetrant Inducers of Autophagy. J. Am. Chem. Soc 139, 7792–7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Chapman RN, Dimartino G, and Arora PS (2004) A highly stable short α-helix constrained by a main-chain hydrogen-bond surrogate. J. Am. Chem. Soc 126, 12252–12253. [DOI] [PubMed] [Google Scholar]
- (6).Blackwell HE, Sadowsky JD, Howard RJ, Sampson JN, Chao JA, Steinmetz WE, O’Leary DJ, and Grubbs RH (2001) Ring-Closing Metathesis of Olefinic Peptides: Design, Synthesis, and Structural Characterization of Macrocyclic Helical Peptides. J. Org. Chem 66, 5291–5302. [DOI] [PubMed] [Google Scholar]
- (7).Schafmeister CE, Po J, and Verdine GL (2000) An AllHydrocarbon Cross-Linking System for Enhancing the Helicity and Metabolic Stability of Peptides. J. Am. Chem. Soc 122, 5891–5892. [Google Scholar]
- (8).Jackson DY, King DS, Chmielewski J, Singh S, and Schultz PG (1991) General approach to the synthesis of short.alpha.-helical peptides. J. Am. Chem. Soc 113, 9391–9392. [Google Scholar]
- (9).Leduc A-M, Trent JO, Wittliff JL, Bramlett KS, Briggs SL, Chirgadze NY, Wang Y, Burris TP, and Spatola AF (2003) Helix-stabilized cyclic peptides as selective inhibitors of steroid receptor–coactivator interactions. Proc. Natl. Acad. Sci. U. S. A 100, 11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).de Araujo AD, Hoang HN, Kok WM, Diness F, Gupta P, Hill TA, Driver RW, Price DA, Liras S, and Fairlie DP (2014) Comparative α-Helicity of Cyclic Pentapeptides in Water. Angew. Chem., Int. Ed 53, 6965–6969. [DOI] [PubMed] [Google Scholar]
- (11).Kawamoto SA, Coleska A, Ran X, Yi H, Yang C-Y, and Wang S (2012) Design of Triazole-Stapled BCL9 α-Helical Peptides to Target the β-Catenin/B-Cell CLL/lymphoma 9 (BCL9) Protein–Protein Interaction. J. Med. Chem 55, 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Kumita JR, Smart OS, and Woolley GA (2000) Photocontrol of helix content in a short peptide. Proc. Natl. Acad. Sci. U. S.A 97, 3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Lau YH, Wu Y, de Andrade P, Galloway WRJD, and Spring DR (2015) A two-component ‘double-click’ approach to peptide stapling,. Nat. Protoc 10, 585. [DOI] [PubMed] [Google Scholar]
- (14).Phillips ST, Piersanti G, and Bartlett PA (2005) Quantifying amino acid conformational preferences and side-chain side-chain interactions in β-hairpins. Proc. Natl. Acad. Sci. U. S. A 102, 13737–13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Nowick JS, Chung DM, Maitra K, Maitra S, Stigers KD, and Sun Y (2000) An unnatural amino acid that mimics a tripeptide β-strand and forms β-sheetlike hydrogen-bonded dimers. J. Am. Chem. Soc 122, 7654–7661. [Google Scholar]
- (16).Wuo MG, Mahon AB, and Arora PS (2015) An effective strategy for stabilizing minimal coiled coil mimetics. J. Am. Chem. Soc 137, 11618–11621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Nair CM, Vijayan M, Venkatachalapathi YV, and Balaram P (1979) X-Ray crystal structure of pivaloyl-D-Pro-L-Pro-L-Ala-Nmethylamide; observation of a consecutive [small beta]-turn conformation. J. Chem. Soc., Chem. Commun, 1183–1184. [Google Scholar]
- (18).Fasan R, Dias RLA, Moehle K, Zerbe O, Vrijbloed JW, Obrecht D, and Robinson JA (2004) Using a β-hairpin to mimic an α-helix: Cyclic peptidomimetic inhibitors of the p53-HDM2 protein-protein interaction. Angew. Chem., Int. Ed 43, 2109–2112. [DOI] [PubMed] [Google Scholar]
- (19).Cochran AG, Skelton NJ, and Starovasnik MA (2001) Tryptophan zippers: Stable, monomeric β-hairpins. Proc. Natl. Acad. Sci. U. S. A 98, 5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Hughes RM, and Waters ML (2005) Influence of N-Methylation on a Cation–π Interaction Produces a Remarkably Stable β-Hairpin Peptide. J. Am. Chem. Soc 127, 6518–6519. [DOI] [PubMed] [Google Scholar]
- (21).Lingard H, Han JT, Thompson AL, Leung IKH, Scott RTW, Thompson S, and Hamilton AD (2014) Diphenylacetylene-linked peptide strands induce bidirectional β-sheet formation. Angew. Chem., Int. Ed 53, 3650–3653. [DOI] [PubMed] [Google Scholar]
- (22).Park JH, and Waters ML (2013) Positional effects of click cyclization on [small beta]-hairpin structure, stability, and function. Org. Biomol. Chem 11, 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Cromm PM, Wallraven K, Glas A, Bier D, Fürstner A, Ottmann C, and Grossmann TN (2016) Constraining an Irregular Peptide Secondary Structure through Ring-Closing Alkyne Metathesis. ChemBioChem 17, 1915–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Siegert TR, Bird MJ, Makwana KM, and Kritzer JA (2016) Analysis of Loops that Mediate Protein–Protein Interactions and Translation into Submicromolar Inhibitors. J. Am. Chem. Soc 138, 12876–12884. [DOI] [PubMed] [Google Scholar]
- (25).Joy ST, and Arora PS (2016) An optimal hydrogen-bond surrogate for α-helices. Chem. Commun 52, 5738–5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Wang D, Chen K, Dimartino G, and Arora PS (2006) Nucleation and stability of hydrogen-bond surrogate-based [small alpha]-helices. Org. Biomol. Chem 4, 4074–4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Chapman RN, and Arora PS (2006) Optimized synthesis of hydrogen-bond surrogate helices: surprising effects of microwave heating on the activity of Grubbs catalysts. Org. Lett 8, 5825–5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Dimartino G, Wang D, Chapman RN, and Arora PS (2005) Solid-Phase Synthesis of Hydrogen-Bond Surrogate-Derived α-Helices. Org. Lett 7, 2389–2392. [DOI] [PubMed] [Google Scholar]
- (29).Mahon AB, and Arora PS (2012) Design, synthesis and protein-targeting properties of thioether-linked hydrogen bond surrogate helices. Chem. Commun 48, 1416–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Miller SE, Kallenbach NR, and Arora PS (2012) Reversible α-helix formation controlled by a hydrogen bond surrogate. Tetrahedron 68, 4434–4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Henchey LK, Kushal S, Dubey R, Chapman RN, Olenyuk BZ, and Arora PS (2010) Inhibition of hypoxia inducible factor 1-transcription coactivator interaction by a hydrogen bond surrogate α-helix. J. Am. Chem. Soc 132, 941–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Henchey LK, Porter JR, Ghosh I, and Arora PS (2010) High specificity in protein recognition by hydrogen-bond-surrogate α-helices: selective inhibition of the p53/MDM2 complex. Chem- BioChem 11, 2104–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Wang D, Liao W, and Arora PS (2005) Enhanced metabolic stability and protein-binding properties of artificial α helices derived from a hydrogen-bond surrogate: application to Bcl-xL. Angew. Chem., Int. Ed 44, 6525–6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Wang D, Lu M, and Arora PS (2008) Inhibition of HIV-1 fusion by hydrogen-bond-surrogate-based α helices. Angew. Chem., Int. Ed 47, 1879–1882. [DOI] [PubMed] [Google Scholar]
- (35).Patgiri A, Yadav KK, Arora PS, and Bar-Sagi D (2011) An orthosteric inhibitor of the Ras-Sos interaction. Nat. Chem. Biol 7, 585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Xie X, Piao L, Bullock BN, Smith A, Su T, Zhang M, Teknos TN, Arora PS, and Pan Q (2014) Targeting HPV16 E6-p300 interaction reactivates p53 and inhibits the tumorigenicity of HPV-positive head and neck squamous cell carcinoma. Oncogene 33, 1037–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Rooklin D, Modell AE, Li H, Berdan V, Arora PS, and Zhang Y (2017) Targeting Unoccupied Surfaces on Protein–Protein Interfaces. J. Am. Chem. Soc 139, 15560–15563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Chapman R, Kulp JL III, Patgiri A, Kallenbach NR, Bracken C, and Arora PS (2008) Trapping a Folding Intermediate of the α-Helix: Stabilization of the π-Helix. Biochemistry 47, 4189–4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Patgiri A, Joy ST, and Arora PS (2012) Nucleation Effects in Peptide Foldamers. J. Am. Chem. Soc 134, 11495–11502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Horne WS, Johnson LM, Ketas TJ, Klasse PJ, Lu M, Moore JP, and Gellman SH (2009) Structural and biological mimicry of protein surface recognition by alpha/beta-peptide foldamers. Proc. Natl. Acad. Sci. U. S. A 106, 14751–14756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Cooley RB, Arp DJ, and Karplus PA (2010) Evolutionary Origin of a Secondary Structure: π-Helices as Cryptic but Widespread Insertional Variations of α-Helices That Enhance Protein Functionality. J. Mol. Biol 404, 232–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Watkins AM, and Arora PS (2014) Anatomy of βstrands at protein–protein interfaces. ACS Chem. Biol 9, 1747–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Haque TS, and Gellman SH (1997) Insights on beta-hairpin stability in aqueous solution from peptides with enforced type I’ and type II’ beta-turns. J. Am. Chem. Soc 119, 2303–2304. [Google Scholar]
- (44).Nowick JS, and Brower JO (2003) A New Turn Structure for the Formation of β-Hairpins in Peptides,. J. Am. Chem. Soc 125, 876–877. [DOI] [PubMed] [Google Scholar]
- (45).Cochran AG, Tong RT, Starovasnik MA, Park EJ, McDowell RS, Theaker JE, and Skelton NJ (2001) A Minimal Peptide Scaffold for β-Turn Display: Optimizing a Strand Position in Disulfide-Cyclized β-Hairpins,. J. Am. Chem. Soc 123, 625–632. [DOI] [PubMed] [Google Scholar]
- (46).Holland-Nell K, and Meldal M (2011) Maintaining Biological Activity by Using Triazoles as Disufide Bond Mimetics. Angew. Chem., Int. Ed 50, 5204–5206. [DOI] [PubMed] [Google Scholar]
- (47).Celentano V, Diana D, De Rosa L, Romanelli A, Fattorusso R, and D’Andrea LD (2012) [small beta]-Hairpin stabilization through an interstrand triazole bridge. Chem. Commun 48, 762–764. [DOI] [PubMed] [Google Scholar]
- (48).Andersen NH, Olsen KA, Fesinmeyer RM, Tan X, Hudson FM, Eidenschink LA, and Farazi SR (2006) Minimization and Optimization of Designed β-Hairpin Folds. J. Am. Chem. Soc 128, 6101–6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Wu L, McElheny D, Takekiyo T, and Keiderling TA (2010) Geometry and Efficacy of Cross-Strand Trp/Trp, Trp/Tyr, and Tyr/Tyr Aromatic Interaction in a β-Hairpin Peptide. Biochemistry 49, 4705–4714. [DOI] [PubMed] [Google Scholar]
- (50).Miller SE, Watkins AM, Kallenbach NR, and Arora PS (2014) Effects of side chains in helix nucleation differ from helix propagation. Proc. Natl. Acad. Sci. U. S. A 111, 6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Richardson JS (1981) The Anatomy and Taxonomy of Protein Structure, in Advances in Protein Chemistry (Anfinsen CB, Edsall JT, and Richards FM, Eds.), pp 167–339, Academic Press. [DOI] [PubMed] [Google Scholar]
- (52).Spencer-Smith R, Koide A, Zhou Y, Eguchi RR, Sha F, Gajwani P, Santana D, Gupta A, Jacobs M, Herrero-Garcia E, Cobbert J, Lavoie H, Smith M, Rajakulendran T, Dowdell E, Okur MN, Dementieva I, Sicheri F, Therrien M, Hancock JF, Ikura M, Koide S, and O’Bryan JP (2017) Inhibition of RAS function through targeting an allosteric regulatory site. Nat. Chem. Biol 13, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Honda S, Yamasaki K, Sawada Y, and Morii H (2004) 10 Residue Folded Peptide Designed by Segment Statistics. Structure 12, 1507–1518. [DOI] [PubMed] [Google Scholar]
- (54).Wang X, Bergenfeld I, Arora PS, and Canary JW (2012) Reversible Redox Reconfiguration of Secondary Structures in a Designed Peptide. Angew. Chem., Int. Ed 51, 12099–12101. [DOI] [PubMed] [Google Scholar]
- (55).Koide A, Wojcik J, Gilbreth RN, Hoey RJ, and Koide S (2012) Teaching an Old Scaffold New Tricks: Monobodies Constructed Using Alternative Surfaces of the FN3 Scaffold. J. Mol. Biol 415, 393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Kauke MJ, Traxlmayr MW, Parker JA, Kiefer JD, Knihtila R, McGee J, Verdine G, Mattos C, and Wittrup KD (2017) An engineered protein antagonist of K-Ras/B-Raf interaction. Sci. Rep 7, 5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Mouratou B, Schaeffer F, Guilvout I, Tello-Manigne D, Pugsley AP, Alzari PM, and Pecorari F (2007) Remodeling a DNA-binding protein as a specific in vivo inhibitor of bacterial secretin PulD. Proc. Natl. Acad. Sci. U. S. A 104, 17983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Kalichuk V, Béhar G, Renodon-Cornière A, Danovski G, Obal G, Barbet J, Mouratou B, and Pecorari F (2016) The archaeal “7 kDa DNA-binding” proteins: extended characterization of an old gifted family. Sci. Rep 6, 37274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Traxlmayr MW, Kiefer JD, Srinivas RR, Lobner E, Tisdale AW, Mehta NK, Yang NJ, Tidor B, and Wittrup KD (2016) Strong Enrichment of Aromatic Residues in Binding Sites from a Charge-neutralized Hyperthermostable Sso7d Scaffold Library. J. Biol. Chem 291, 22496–22508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


