Abstract
Lung transplant recipients present an increased risk for severe complications associated with respiratory infections. We conducted a review of the literature examining the clinical relationship between viral respiratory infection and graft complications. Thirty‐four studies describing the clinical impact of influenza, respiratory syncytial virus, parainfluenza, human metapneumovirus, rhinovirus, enterovirus, coronavirus, bocavirus or adenovirus were identified. The detection rate of respiratory viral infection ranged from 1.4% to 60%. Viruses were detected five times more frequently when respiratory symptoms were present [odds ratio (OR) = 4.97; 95% CI = 2.11–11.68]. Based on available observations, we could not observe an association between respiratory viral infection and acute rejection (OR = 1.35; 95% CI = 0.41–4.43). We found a pooled incidence of 18% (9/50) of bronchiolitis obliterans syndrome (BOS) in virus‐positive cases compared to 11.6% (37/319) in virus‐negative cases; however, limited number of BOS events did not allow to confirm the association. Our review confirms a causal relationship between respiratory viruses and respiratory symptoms, but cannot confirm a link between respiratory viruses and acute lung rejection. This is related in part to the heterogeneity and limitations of available studies. The link with BOS needs also to be reassessed in appropriate prospective studies.
Keywords: Acute rejection, bronchiolitis obliterans, influenza, lung transplantation, viral infection
Short abstract
The authors find, through a review of the literature, a causal relationship between respiratory viral infections and respiratory symptoms among lung transplant recipients.
Abbreviations:
- OR
odds ratio
- BOS
bronchiolitis obliterans syndrome
- RSV
respiratory synctial virus
- PIV
parainfluenza virus
- HMpV
human metapneumovirus
- LTRs
lung transplant recipients
- PCR
polymerase chain reaction
- CI
confidence interval
- ICU
intensive care unit
- BAL
bronchoalveolar lavage
- FEV1
forced expiratory volume in 1 sec.
- OB
obliterative bronchiolitis
Introduction
Respiratory viruses comprise different viruses, such as influenza, respiratory synctial virus (RSV), parainfluenza (PIV), human metapneumovirus (HMpV), rhinovirus, enterovirus, bocavirus and adenovirus. Although most respiratory viral infections cause self‐limited upper respiratory diseases, lung transplant recipients (LTRs) are particularly prone to develop complications (1, 2, 3, 4, 5, 6). This is related to the immunosuppressive therapy that could promote protracted infection, but also to the direct exposure of the graft to the infectious agent together with an impaired mucociliary function and lymphatic drainage, and the absence of cough reflex. Apart from the direct infection‐related morbidity, it is commonly accepted that these infections could promote rejection and subsequently lead to bronchiolitis obliterans syndrome (BOS), the main limitation to long‐term survival. However, this association is based on reports that have focused mainly on paramyxoviruses or on influenza and adenovirus to a lesser extent (7). These studies are heterogeneous and have several technical limitations in terms of design, case selection and diagnostic procedures (1, 8).
Over the years the improvement of molecular tools, including real‐time PCR technology, has contributed to increase the sensitivity of our diagnostic procedures and new species (HMpV, coronavirus NL63 and HKU1, bocavirus, rhinovirus C) have also emerged. To assess appropriately the evidence supporting a role of respiratory viruses as a cause of symptoms and graft complications in LTRs, we conducted a systematic review of the published literature (9).
Methods
We searched the MEDLINE database from 1 January 1985 to 31 March 2010 using the following key words: ‘lung transplant recipients or immunocompromised hosts’ and ‘influenza, parainfluenza, RSV, metapneumovirus, coronavirus, bocavirus, adenovirus and respiratory viruses’, respectively. In addition, reference lists from review articles and selected papers were hand‐searched and matched to our database. Only peer‐reviewed original articles reporting at least three lung transplant cases with a description of virological methods, design and clinical end‐points were included.
Data were collected in standardized report forms with the following information: year of the screening period; design (cohort, case series, retrospective, prospective); age and size of the population screened; number and type of specimens/viruses tested; number of virus‐positive episodes analyzed; type of assays used; clinical conditions; association with acute rejection/chronic rejection/BOS and histopathological results; antiviral treatment and survival rate. The potential limitations and any other comments considered as relevant were noted.
We calculated confidence intervals (CI) around proportions for studies on viral frequency using the Agresti and Coull method. Odds ratios (OR) were calculated for each study to determine the association of respiratory viruses with acute rejection or respiratory symptoms. Due to significant heterogeneity between studies, we used random effect models to calculate meta‐analytic summaries of the association between respiratory viruses and acute rejection or respiratory symptoms. All analyses were performed with STATA 11 (77845; College Station, TX, USA).
Results
Main study characteristics
We identified 34 studies; 26 focused on LTRs only and eight analyzed also other immunocompromised populations. Viruses considered in our review cover influenza A, B and C, RSV A and B, PIV 1–4, HMpV, rhinovirus, enterovirus, coronavirus 229E, OC43, NL63 and HKU1, bocavirus and adenovirus, but not herpes viruses. The main characteristics of the 34 studies (1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34) are presented in Table 1 and it can be estimated that more than 4000 specimens from LTRs have been screened for the presence of at least one of the above‐mentioned respiratory viruses. In approximately one‐third of studies (29%), screening was within the frame of prospective cohort studies investigating the cause and/or the clinical impact of acute viral respiratory tract infections; all others were retrospective or case series.
Table 1.
Main characteristics of 34 studies exploring the role of respiratory viruses in lung transplant recipients
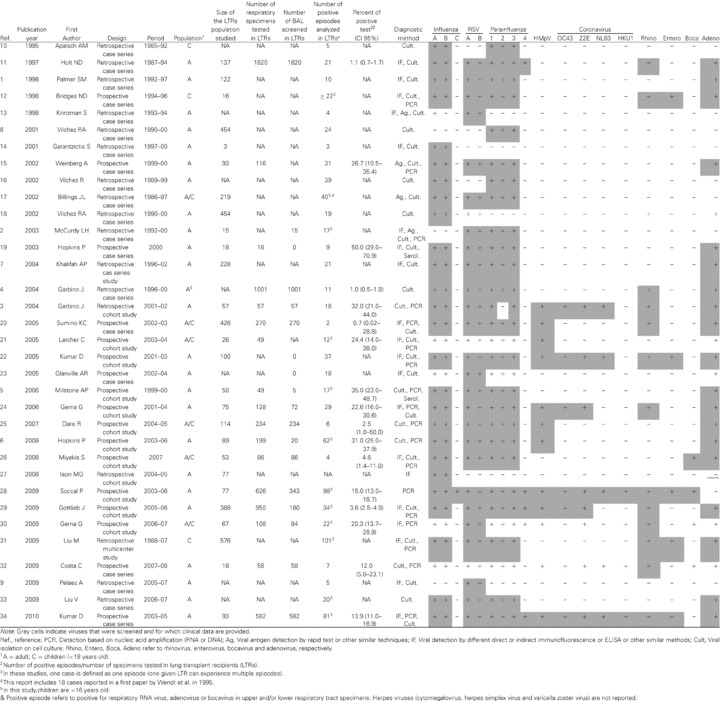
In 21 (61.7%) studies, patients were recruited from outpatient clinics; in six (17.6%), patients were hospitalized; one recruited both in‐ and outpatients and the recruitment setting could not be determined precisely for the remaining six (2, 11, 12, 20, 24, 34). Diagnostic procedures were performed only in symptomatic patients in 12 (35.2%) studies, and in 20 (58.8%) they were also performed as routine posttransplant surveillance or as a control procedure after treatment of an acute rejection. Reasons for the procedure were not identifiable in two retrospective studies (2, 27). An 8.8% of studies concerned children only, 17.6% enrolled both children and adults and 73.5% were in adults. Clinical conditions analyzed ranged from uncomplicated upper respiratory tract infection to severe pneumonia requiring intensive care unit (ICU) admission.
Viral investigations
Overall, it was possible to identify that viral investigations were performed in upper respiratory specimens (nasopharyngeal swabs or aspirates) in 8.8% of studies and in bronchoalveolar lavage (BAL) specimens in 38.2%. Both types of specimens were used in the remaining 52.9% studies, but in 61.1% of these it was not possible to clearly establish the respective proportion of upper versus lower respiratory specimens. We identified only one study that compared systematically upper versus lower respiratory tract viral screening performed simultaneously in a given individual (28). Thus, we were unable to compare the respective sensitivity and role of viral screening in the upper versus the lower respiratory tract.
As expected, there was a significant heterogeneity of the different diagnostic procedures used. At least one molecular assay was used in 53% of investigations and only five studies used a large panel to target at least 12 of the above‐mentioned 18 viruses. In the older studies, classical methods, such as immunofluorescence or viral culture, were the sole diagnostic tools. The type of technique used (immunofluorescence‐based, culture or nucleic acid detection) and the completeness of the screening performed in each of the available studies selected are shown in Table 1. In terms of viral screening, influenza was screened in most studies (91% and 88% for influenza A and B, respectively, but only 3% for influenza C), followed by RSV (85%), PIV 1–3 (82–85%, but only 15% for PIV 4) and adenovirus (71%). Other respiratory viruses that require mainly molecular assays to be detected were less frequently screened; 41% for rhinovirus (rhinovirus C screened in only one study); 35% for HMpV; 11–24% for the different subtypes of coronaviruses; 18% for enterovirus and 12% for bocavirus. Overall, only 15% of studies screened at least 75% of the 18 respiratory viruses listed in Table 1. When assessable, the overall detection rate of respiratory viral infection in the screened population varied from 1.4% to 60%. This wide range can be explained in part by the heterogeneity of the population enrolled (asymptomatic cases versus subjects with limited upper respiratory symptoms versus patients hospitalized with complications). Table S1 depicts the prevalence of virus positivity for each individual study and as a pooled prevalence according to diagnostic method, number of viruses screened and sample size. As expected, the virus positivity rate was higher for studies with small sample size using PCR technique and screening for numerous viruses. For example, studies using PCR techniques had a higher detection rate (12.0%) compared to those not using PCR (1.4%). This can be explained in part by the greater number of viruses searched for by PCR techniques (8.4% for studies identifying nine or more viruses; 3.0% for those identifying eight or less viruses).
The respective contribution of each species in positive cases was available in 82% of studies. When a large panel of molecular tools was used, viruses most frequently detected were rhinovirus and coronavirus. In the three studies that screened at least 14 respiratory viruses (22, 28, 34), rhinovirus represented 35–55% of all positive cases and coronaviruses 13–27%. When including three supplementary studies screening up to 12 or 13 viruses (3, 24, 29), the most frequent virus detected was still rhinovirus (8.8–55.5%; Table S1). Of note, for some targets, such as coronaviruses, not all species (OC43, E229, NL63, HKU1) were included.
Respiratory symptoms and lung function
Ten of 34 studies compared the rate of viral infections observed in symptomatic cases versus those without respiratory symptoms. We found that smaller studies tended to include more symptomatic patients and that larger studies were associated with a lower virus detection rate (22.3% for studies with less than 150 specimens versus 0.6% for studies with 150 specimens or more; Table S1).
Figure 1A highlights that in all but one study, the association between laboratory‐proven respiratory viruses and symptoms was present. We found that viruses were detected five times more frequently when respiratory symptoms were present (OR = 4.97; 95% CI = 2.11–11.68). In terms of objective assessment of the graft function during the acute phase, lung function assessment was available in 53% of studies and showed a forced expiratory volume (FEV1) decline that ranged from –5% to –30% for the overall enrolled population. The FEV1 decline was usually similar or even more important among symptomatic patients, but very few studies provided a specific comparison of FEV1 variability according to the presence or absence of a viral illness (28), which prevented further analysis.
Figure 1.
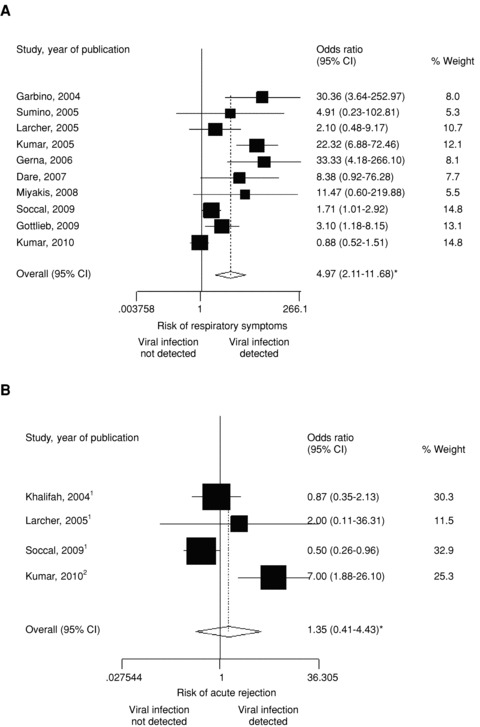
Odds ratio of (a) respiratory symptoms and (B) acute graft rejection according to the presence or absence of respiratory viral infections in lung transplant recipients. 1Biopsy‐proven. 2Biopsy‐proven or FEV decline >/= 20%. *Random effect.
Outcome and antiviral interventions
Short‐term crude mortality rate was evaluated in 52.9% of studies and ranged from 0% to 25%. Antiviral treatment was used in 53% of studies, mostly ribavirin for RSV infection, but also neuraminidase inhibitors and amantadine for influenza infection. Of these studies, 72% discussed treatment efficacy, but only 28% considered treatment efficacy as an end‐point. Based on the clinical outcome of treated subjects, it is reported that early antiviral therapy might be associated with a reduction of complications and mortality. Nevertheless, given the small number of cases, the lack of randomization and appropriate control groups, and the absence of analysis reporting a precise rate of reduction in mortality and/or morbidity, these trends could only considered as non evidence‐based conclusions.
Graft rejection
Twenty‐five of 34 studies representing more than 2900 LTR specimens reported that transbronchial lung biopsies had been performed and a total of 923 pathological examinations were potentially available. However, the presence of acute rejection or obliterative bronchiolitis (OB) was reported only in 68% and 2.6% of cases, respectively. Among a total of 282 virus‐positive and 553 virus‐negative cases, 21 (61.8%) studies reported histopathological results and an acute rejection rate. Three studies were not suitable for the present analysis, thus leaving 19 studies reporting a total of 267 acute rejection events graded ≥ A2. In these 19 investigations, the frequency of acute rejections ≥ A2 ranged between 5.9% and 47.6% (Table S4). The association with acute rejection can only be estimated by comparing the rate observed in virus‐positive cases with the one observed in virus‐negative cases; this was available in only four studies (7, 21, 28, 34). One study suggests a significant positive association (34), which could not be confirmed in the three others (Figure 1B). Overall, we found no statistically significant association between respiratory viruses and acute rejection (OR = 1.35; 95% CI = 0.41–4.43). OB/BOS incidence following respiratory viral infections was reported for a period of time ranging from a few months to 1 year. In 11 studies (32.5%), all except one (1) used either biopsy‐proven chronic rejection (defined by the presence of OB) or a sustained FEV1 decline of 20% according to the International Society for Heart and Lung Transplantation guidelines (35). BOS incidence (Table 2) following a respiratory viral infection ranged from 5.4% to 62.5% in virus‐positive cases and was reported in only three studies for virus‐negative cases (5, 21, 29) with a rate ranging from 9.1% to 52.9%. Pooled incidence rates of these three studies revealed a BOS incidence of 18% (9/50) in virus‐positive cases compared to 11.6% (37/319) in virus‐negative cases. The low number of BOS events analyzed in these three investigations limited our ability to provide any meta‐analytic summary that could be considered as relevant. Four of the 11 studies (Table 2) provided a statistical analysis testing the potential association with BOS, but two without providing clearly the BOS rate in virus‐negative cases. One (17) failed to show any significant association and three (7, 12, 29) described a significant higher rate of BOS in subjects experiencing a respiratory viral infection.
Table 2.
Summary of studies analyzing the potential association between new onset of bronchiolitis obliterans syndrome and/or obliterans bronchiolitis and respiratory viral infections in lung transplant recipients
| Reference | Virus‐positive cases (n = 201) | Virus‐negative cases (n = 757) | Type or number of viruses considered for this analysis | Statistical analysis if available | ||
|---|---|---|---|---|---|---|
| Total cases | No. (%) with BOS | Total no. of cases | Number (%) with BOS | |||
| 1 | 10 | 4 (40.0) | NA | NA | 8 | NA |
| 12 | 9 | 4 (44.4) | NA | NA | Adenovirus only | Cox proportional hazards p <0.0001 |
| 8 | 22 | 7 (32.0) | NA | NA | PIV only | NA |
| 14 | 3 | 3 (100.0) | NA | NA | Influenza only | NA |
| 7 | 21 | 13 (62.0) | 207 | NA | 8 | p = 0.27, 0.02 and 0.01 for BOS 1, 2 and 3, respectively |
| 21 | 9 | 2 (22.2) | 17 | 9 (52.9) | HMpV only | NA |
| 5 | 15 | 1 (6.7) | 28 | 3 (10.7) | 8 | p value = non significant |
| 6 | 37 | 2 (5.4) | NA | NA | HMpV and RSV only | NA |
| 29 | 26 | 6 (23.0) | 274 | 25 (9.1) | 13 | Rate of BOS higher among CARV‐positive group (Kaplan‐Meier curve; p = 0.01) |
| 34 | 161 | 10 (62.5) | 45 | NA | 16 | NA |
| Pooled cases | 50 | 9 (18) | 319 | 37 (11.6) | p = 0.242 | |
| 172 | 33 | NA | 186 | NA | 7 | Previous CARV infection does not predispose to OB/BOS (relative risk 1.1; 95% CI 0.52–2.3)3 |
CARV, community‐acquired respiratory viruses; BOS, broncholitis obliterans syndrome; OB, obliterans bronchiolitis.
1The analysis focuses on 16 virus‐positive cases initially diagnosed with acute rejection at 3 months.
2Statistical analysis performed, but number of BOS cases not provided.
3In a subset analysis, lower CARV infection predisposes to BOS3 (Cox proportional hazards regression model; RR 2.3, 95% CI 1.1–4.9).
Discussion
During seasonal peaks, LTRs living in the community are exposed to RNA and DNA respiratory viruses. Given the concomitant presence of a significant immunosuppression and impaired protective mechanisms of the grafted lung, these viral infections will promote complications and graft rejection (7, 21, 22, 34). In the present review of 34 studies, our goal was to assess the strength and the characteristics of this association in available clinical reports and whether this translates into an observable association in real‐life conditions. Incomplete microbiological investigations or insensitive diagnostic tools limited the completeness of viral investigations; only the most recent reports have used a large panel of molecular tests and can provide a less biased image of the respective role of each viral agent. In the early 1990s, studies used mainly viral culture or direct immunofluorescence and, if available, PCR was limited to influenza, RSV or parainfluenza viruses. The recent emergence of new viruses such as HMpV, coronavirus NL63, coronavirus, HKU1, bocavirus and human rhinovirus C need to be included in any modern molecular panel; these new agents have been systematically studied in two studies only. Interestingly, when tested, the so‐called ‘common cold’ viruses like rhinoviruses and coronavirus revealed to be the most frequent compared to others such as influenza or paramyxoviruses, an observation consistent with other hospital‐based studies. Depending on the type and number of technique used, the size of the study or enrolment criteria, the observed frequency of viral infections can dramatically change—ranging for each individual virus from less than 1% to more than 20% in our pooled analysis (Table S1).
The clinical significance of a positive viral nucleic acid detection result is a critical point that needs to be confronted with the presence or absence of respiratory symptoms. This type of analysis has been done in at least 11 studies (Table S3 and Figure 1A) in which LTRs submitted to a routine respiratory screening for graft follow‐up were used as controls and compared to symptomatic cases. It was consistently shown that in the presence of a viral infection, the likelihood of respiratory symptoms was five times higher. This observation could guide clinicians in their interpretation of microbiological results in an era where increasingly sensitive molecular diagnostic panels are available. Even if a background positivity rate is expected, for example, following unnoticed or asymptomatic infection, or when seasonal outbreaks are ongoing in the community, these viruses likely contributed to symptom production in most cases and cannot be regarded as innocent bystanders (3, 22, 28, 29).
Although mainly expected in the upper respiratory tract, viral infections are also present in lower respiratory specimens. This raises several issues such as the respective ability of each respiratory virus to infect the lower respiratory tract and whether all of them should be considered as equally able to cause graft complications in LTRs. Despite being expected, our pooled analysis was unable to confirm a positive association between acute rejection and a previous viral infection. However, this conclusion needs to be considered carefully since the three largest studies (7, 28, 34) representing 96% of all cases brought discordant results; two of these failed to observe a positive association (7, 28), whereas a third (34) reported a 33.3% rejection rate in 48 virus‐positive cases compared to 6.7% in virus‐negative cases (p value = 0.001). Of note, this latter study considered not only biopsy‐proven cases as rejection criteria, but also a FEV1 decline of 20% or more. Another potential limitation of our pooled analysis is related to the heterogeneity of the design of each study: some reported an acute rejection rate during the acute phase of the viral infection and others during a follow‐up period of 3 months. Although the present report focuses on respiratory viruses, it must also be kept in mind that these agents could be associated, or promote other bacterial or fungal infections that subsequently could lead to graft complications.
With regard to chronic rejection, a relationship between a previous respiratory viral infection and the subsequent development of BOS was reported as statistically significant in three studies (14, 24,41). In at least seven other studies in which BOS incidence was evaluated or discussed, the risk could not be linked to respiratory viruses or was not evaluable. The median number of virus‐positive cases in the 10 studies in which BOS was analyzed was five (range 1–13). Four of these studies compared the rate in virus‐positive versus negative cases for a total of only nine BOS events in those virus‐positive cases (Table 2). The low number of events, incompleteness, heterogeneity and the retrospective design of published reports, did not allow us to conduct any appropriate statistical analysis. Of note, some studies have suggested that selected viruses, such as RSV, PIV, influenza and possibly (7, 21, 22, 34) HMpV (6, 21), are particularly prone to trigger graft rejection. In most of these studies, control groups were incomplete and thus again the clinical relationship between one specific respiratory viral family and graft rejection needs to be reconsidered carefully.
In conclusion, our review confirms a causal relationship between respiratory viral infections and respiratory symptoms, even when these infections are documented by molecular assays. However, the respective role of each respiratory virus, especially with respect to picorna‐ and coronavirus, needs to be reconsidered. Although it is certain that lower respiratory viral infection will promote graft complication, we highlight that the clinical link between respiratory viruses and acute lung rejection or BOS needs to be characterized in prospective and appropriately designed cohort studies.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting information
Table S1: Frequency of viral infections observed in lung transplant recipients according to individual study characteristics
Table S2: Range of virus detection per family in the 6 studies where at least 12 viruses were screened
Table S3: Relative proportion of virus‐positive cases according to the presence of respiratory symptoms in lung transplants recipients screened for respiratory viruses
Table S4: Summary of studies analyzing the potential association between acute rejection and respiratory viral infections in lung transplant recipients
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Acknowledgments
This work was supported by a grant of the Swiss National Science Foundation attributed to L. Kaiser (3200B‐101670).
References
- 1. Palmer SM, Henshaw NG, Howell DN et al Community respiratory viral infection in adult lung transplant recipients. Chest 1998; 113: 944–950. [DOI] [PubMed] [Google Scholar]
- 2. McCurdy LH, Milstone A, Dummer S. Clinical features and outcomes of paramyxoviral infection in lung transplant recipients treated with ribavirin. J Heart Lung Transplant 2003; 22(7):745–753. [DOI] [PubMed] [Google Scholar]
- 3. Garbino J, Gerbase MW, Wunderli W et al Lower respiratory viral illnesses: Improved diagnosis by molecular methods and clinical impact. Am J Respir Crit Care Med 2004; 170: 1197–1203. [DOI] [PubMed] [Google Scholar]
- 4. Garbino J, Gerbase MW, Wunderli W et al Respiratory viruses and severe lower respiratory tract complications in hospitalized patients. Chest 2004; 125: 1033–1039. [DOI] [PubMed] [Google Scholar]
- 5. Milstone AP, Brumble LM, Barnes J et al A single‐season prospective study of respiratory viral infections in lung transplant recipients. Eur Respir J 2006; 28: 131–137. [DOI] [PubMed] [Google Scholar]
- 6. Hopkins P, McNeil K, Kermeen F et al Human Metapneumovirus in lung transplant recipients and comparison to respiratory syncytial virus. Am J Respir Crit Care Med 2008; 178: 876–881. [DOI] [PubMed] [Google Scholar]
- 7. Khalifah AP, Hachem RR, Chakinala MM et al Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. Am J Respir Crit Care Med 2004; 170: 181–187. [DOI] [PubMed] [Google Scholar]
- 8. Vilchez RA, McCurry K, Dauber J et al The epidemiology of parainfluenza virus infection in lung transplant recipients. Clin Infect Dis 2001; 33: 2004–2008. [DOI] [PubMed] [Google Scholar]
- 9. Pelaez A, Lyon M, Force SD et al Efficacy of oral ribavirin in lung transplant patients with respiratory syncytial virus lower respiratory tract infection. J Heart Lung Transplant 2008; 28: 67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Apalsch AM, Green M, Ledesma‐Medina J et al Parainfluenza and influenza virus infections in pediatric organ transplant recipients. Clin Infect Dis 1995; 20: 394–399. [DOI] [PubMed] [Google Scholar]
- 11. Holt ND, Gould FK, Taylor CE et al Incidence and significance of noncytomegalovirus viral respiratory infection after adult lung transplantation. J Heart Lung Transplant 1997; 16: 416–419. [PubMed] [Google Scholar]
- 12. Bridges ND, Spray TL, Collins MH et al Adenovirus infection in the lung results in graft failure after lung transplantation. J Thorac Cardiovasc Surg 1998; 116: 617–623. [DOI] [PubMed] [Google Scholar]
- 13. Krinzman S, Basgoz N, Kradin R et al Respiratory syncytial virus‐associated infections in adult recipients of solid organ transplants. J Heart Lung Transplant 1998; 17: 202–210. [PubMed] [Google Scholar]
- 14. Garantziotis S, Howell DN, McAdams HP et al Influenza pneumonia in lung transplant recipients: Clinical features and association with bronchiolitis obliterans syndrome. Chest 2001; 119: 1277–1280. [DOI] [PubMed] [Google Scholar]
- 15. Weinberg A, Zamora MR, Li S et al The value of polymerase chain reaction for the diagnosis of viral respiratory tract infections in lung transplant recipients. J ClinVirol 2002; 25: 171–175. [DOI] [PubMed] [Google Scholar]
- 16. Vilchez R, McCurry K, Dauber J et al Influenza and parainfluenza respiratory viral infection requiring admission in adult lung transplant recipients. Transplantation 2002; 73: 1075–1078. [DOI] [PubMed] [Google Scholar]
- 17. Billings JL, Hertz MI, Savik K, Wendt CH. Respiratory viruses and chronic rejection in lung transplant recipients. J Heart Lung Transplant 2002; 21: 559–566. [DOI] [PubMed] [Google Scholar]
- 18. Vilchez RA, McCurry K, Dauber J et al Influenza virus infection in adult solid organ transplant recipients. Am J Transplant 2002; 2: 287–291. [DOI] [PubMed] [Google Scholar]
- 19. Hopkins PM, Plit ML, Carter IW et al Indirect fluorescent antibody testing of nasopharyngeal swabs for influenza diagnosis in lung transplant recipients. J Heart Lung Transplant 2003; 22: 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sumino KC, Agapov E, Pierce RA et al Detection of severe human Metapneumovirus infection by real‐time polymerase chain reaction and histopathological assessment. J Infect Dis 2005; 192: 1052–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Larcher C, Geltner C, Fischer H et al Human metapneumovirus infection in lung transplant recipients: Clinical presentation and epidemiology. J Heart Lung Transplant 2005; 24: 1891–1901. [DOI] [PubMed] [Google Scholar]
- 22. Kumar D, Erdman D, Keshavjee S et al Clinical impact of community‐acquired respiratory viruses on bronchiolitis obliterans after lung transplant. Am J Transplant 2005; 5: 2031–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Glanville AR, Scott AIR, Morton JM et al Intravenous ribavirin is a safe and cost‐effective treatment for respiratory syncytial virus infection after lung transplantation. J Heart Lung Transplant 2005; 24: 2114–2119. [DOI] [PubMed] [Google Scholar]
- 24. Gerna, G , Vitulo P, Rovida F et al Impact of human metapneumovirus and human cytomegalovirus versus other respiratory viruses on the lower respiratory tract infections of lung transplant recipients. J Med Virol 2006; 78: 408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dare R, Sanghavi S, Bullotta A et al Diagnosis of human metapneumovirus infection in immunosuppressed lung transplant recipients and children evaluated for pertussis. J Clin Microbiol 2007; 45: 548–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miyakis S, van Hal SJ, Barratt J et al Absence of human bocavirus in bronchoalveolar lavage fluid of lung transplant patients. J Clin Virol 2009; 44: 179–180. [DOI] [PubMed] [Google Scholar]
- 27. Ison MG, Sharma A, Shepard JA et al Outcome of influenza infection managed with oseltamivir in lung transplant recipients. J Heart Lung Transplant 2008; 27: 282–288. [DOI] [PubMed] [Google Scholar]
- 28. Soccal PM, Aubert JD, Bridevaux PO et al Upper and lower respiratory viral infections and acute graft rejection in lung transplant recipients. Clin Infect Dis 2010; 51: 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gottlieb J, Schulz TF, Welte T et al Community‐acquired respiratory viral infections in lung transplant recipients: A single season cohort study. Transplantation 2009; 87: 1530–1537. [DOI] [PubMed] [Google Scholar]
- 30. Gerna G, Piralla A, Rovida F et al Correlation of rhinovirus load in the respiratory tract and clinical symptoms in hospitalized immunocompetent and immunocompromised patients. J Med Virol 2009; 81: 1498–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu M, Worley S, Arrigain P et al Respiratory viral infections within one year after pediatric lung transplant. Transplant Infect Dis 2009; 11: 304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Costa C, Bergallo M, Sidoti F et al What role for human rhinovriuses in the lower respiratory tract?. New Microbiol 2009; 32: 115–117. [PubMed] [Google Scholar]
- 33. Liu V, Dhillon GS, Weill D. A multi‐drug regimen for respiratory syncytial virus and parainfluenza virus infections in adult lung and heart‐lung transplant recipients. Transplant Infect Dis 2009; 12: 38–44. [DOI] [PubMed] [Google Scholar]
- 34. Kumar D, Husain S, Chen MH et al A prospective molecular surveillance study evaluating the clinical impact of community‐acquired respiratory viruses in lung transplant recipients. Transplantation 2010; 89: 1028–1033. [DOI] [PubMed] [Google Scholar]
- 35. Estenne M, Maurer JR, Boehler A et al Bronchiolitis obliterans syndrome 2001: An update of the diagnostic criteria. J Heart Lung Transplant 2002; 21: 297–310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Frequency of viral infections observed in lung transplant recipients according to individual study characteristics
Table S2: Range of virus detection per family in the 6 studies where at least 12 viruses were screened
Table S3: Relative proportion of virus‐positive cases according to the presence of respiratory symptoms in lung transplants recipients screened for respiratory viruses
Table S4: Summary of studies analyzing the potential association between acute rejection and respiratory viral infections in lung transplant recipients
Supporting info item
Supporting info item
Supporting info item
Supporting info item


