Abstract
Problem
Preterm, premature rupture of membranes (PPROM) is a dire pregnancy outcome that is frequently associated with infection by the genital mycoplasmas, Mycoplasma hominis, Ureaplasma parvum, and U. urealyticum. One potential mechanism by which these microorganisms may cause PPROM is by increasing the concentration of matrix metalloproteinases (MMPs) in the membranes and amniotic fluid. We tested this hypothesis in a well‐defined model system of genital infection with M. pulmonis, a natural reproductive pathogen of rats.
Method of study
Timed‐pregnant, specific pathogen‐free, Sprague–Dawley rats were infected with 107 CFU M. pulmonis at gestation day (gd) 14. Controls received an equivalent volume (100 μL) of sterile medium. At gd 18, rats were euthanized, and membranes and amniotic fluids were harvested and stored at −70°C until analysis. Proteinase activity of amniotic fluid and membranes was resolved on discontinuous 7.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gelatin zymography gels. Band intensity was determined using a digital gel documentation system and the manufacturer's software (Kodak).
Results
Gelatinolytic activity associated with a band similar in molecular weight to ProMMP‐9 (92 kDa, the inactive precursor of MMP‐9) was significantly increased in amniotic fluids and membranes harvested from M. pulmonis‐treated pups at gd 18 when compared with tissues harvested from control pups. Both ProMMP‐9 and ProMMP‐2 (72 kDa, the inactive precursor of MMP‐2) were increased in infected animals at gd 21.
Conclusion
Our study suggests that the genital mycoplasmas can increase MMP‐9 production in vivo.
Keywords: Matrix metalloproteinases, Mycoplasma pulmonis, preterm premature rupture of membranes, Ureaplasma urealyticum
Introduction
Preterm premature rupture of membranes (PPROM) is a common pregnancy complication that may lead to chorioamnionitis, preterm labor, neonatal sepsis, and fetal death. 1 Infection is frequently associated with PPROM, and genital mycoplasmas such as Mycoplasma hominis, Ureaplasma parvum, and U. urealyticum are often isolated from the reproductive tract of women with this condition. 2 , 3
Mycoplasma hominis and Ureaplasma sp. are thought to cause damage to the membranes by increasing the production of proinflammatory cytokines at the maternal–fetal interface through interactions with immune cells or direct interactions with trophoblast cells themselves. Indeed, both M. hominis and U. urealyticum increased the production of tumor necrosis factor‐alpha (TNF‐α) and nitric oxide by murine macrophages in vitro, 4 and both microorganisms can cause preterm labor when administered by intra‐amniotic infusion into rhesus monkeys. 5 , 6 TNF‐α and other proinflammatory cytokines can disrupt pregnancy in a number of ways, including upregulation of the production of matrix metalloproteinases (MMPs).
Matrix metalloproteinases comprise a family of over 20 members and can be divided into groups based on their substrates (e.g. stromalysins, gelatinases, collagenases, etc). MMP‐9 is particularly important in remodeling of the membranes and cervix during parturition. MMP‐9 is upregulated in the membranes at the time of labor in the human, monkey, and rat. 7 , 8 , 9 This enzyme is also increased in the amnion by group B streptococci 8 or bacterial cell wall components such as lipopolysaccharide (LPS). 10 MMP‐2 has also been shown to be increased during preterm rupture of membranes. 11 Whether genital mycoplasmas that lack LPS can increase MMP‐9 or MMP‐2 production in this tissue is unknown.
Our laboratory has been investigating the pathophysiology of genital mycoplasmosis using Sprague–Dawley rats and M. pulmonis. This animal model system has several advantages for investigations in reproductive pathology because it uses a natural pathogen of rodents that is associated with increased production of proinflammatory cytokines, low birthweight and fetal wastage. 12 In this study, we tested the hypothesis that genital mycoplasmosis causes increased production of MMP‐2 and MMP‐9 in the membranes and amniotic fluid.
Materials and methods
Materials
Coomassie Blue R‐250 and acrylamide‐bis acrylamide solution were purchased from Bio‐Rad (Hercules, CA, USA). All other chemical reagents were purchased from either Sigma (St Louis, MO, USA) or Fisher Scientific (Atlanta, GA, USA). Mycoplasma pulmonis strain X1048 used for this project was generously provided by Dr Maureen Davidson (West Lafayette, IN, USA). A stock culture was grown in Frey's broth, stored in aliquots at −85°C, and a single vial from this stock culture was thawed just prior to use. The thawed culture contained 108 CFU/mL when thawed, and the CFU was confirmed for each experiment.
Animals and Treatments
All experiments were performed in accordance with University of Florida Institutional Animal Care and Use Committee‐approved protocols. Timed‐pregnant, specific pathogen‐free (SPF) Sprague–Dawley rats were purchased from Harlan (Indianapolis, IN, USA) and delivered to the University of Florida at gestation day (gd) 11 or 12 and maintained under SPF conditions at all times. Rats were monitored by the commercial vendor and were presumed SPF for the following: Sendai virus, H‐1 virus, rat coronavirus, sialodacryoadenitis virus, reovirus type 3, Kilham rat virus, Hantaan virus, M. pulmonis, respiratory and enteric pathogens, endoparasites and ectoparasites and housed in Microisolator® (Lab Products, Inc., Maywood, NJ, USA) cages. In order to maintain SPF conditions, all animals were handled within a laminar airflow hood. All food, water and caging were autoclaved prior to use. Control rats were always handled first and were housed separately from inoculated rats.
On gd 14, rats were anesthetized with an intraperitoneal dose of ketamine (25 mg; Ketaject, Phoenix Pharmaceutical Inc., St Joseph, MO, USA) mixed with xylazine (0.375 mg; Xylazine‐20, The Butler Co., Columbus, OH, USA) to produce 40 min of anesthesia. Animals then received either 107 CFU M. pulmonis via intracardiac injection, or an equivalent volume of sterile Frey's broth. This method results in nearly a 100% infection rate for the placenta and amniotic fluid by gd 18, 13 severe histological placentitis, and increased production of proinflammatory cytokines at the maternal–fetal interface. 13 , 14
At necropsy, gd 18 or 21, rats were anesthetized with an overdose of sodium pentobarbital (180 mg; Veterinary Laboratories Inc., Lenexa, TX, USA) injected intraperitoneally. After deep anesthesia was obtained, rats were exsanguinated by transection of the femoral artery and vein. Fetal rat pups died in utero upon death of the dam. Samples of maternal and fetal tissues were collected to determine the infection status of the rats and their pups. Membranes and amniotic fluid were collected from the fetal units, snap‐frozen in liquid nitrogen and stored at −85°C until zymography.
Zymography of Rat Amniotic Fluid and Membranes
Amniotic fluid was thawed and clarified by centrifugation at 10,000 × g for 15 min at 4°C. The clarified amniotic fluid was mixed 1:3 with zymography loading buffer and loaded onto discontinuous 7.5% (w/v) sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels that contained 0.5 mg/mL gelatin. Amniotic fluid obtained at gd 18 was analyzed for MMP‐2 (1.6 μL loaded per lane) and for MMP‐9 (3.4 μL amniotic fluid loaded per lane). After electrophoresis, gels were incubated in 2.5% Triton X‐100 for 1 hr and then incubated in zymography development solution for 18–20 hr at 37°C. Gels were incubated in 0.5% Coomassie Blue in 40% methanol, 7% acetic acid, 53% water R‐250 for 1 hr, destained for 1 hr in 40% methanol, 7% acetic acid, 53% water, and photographed using a digital gel analysis system. With this method, bands of proteolytic activity appear as clear bands on a blue background. Band intensities at approximately 72 kDa (ProMMP‐2) and 92 kDa (ProMMP‐9) were quantified using the Kodak 1D digital gel analysis and documentation system (Rochester, NY, USA).
Membranes were homogenized in 5 mL lysis buffer (50 mm Tris–HCl, 2 m urea, 1 g/L Brij 35, and 0.1 mm phenylmethylsulfonyl fluoride) and the proteins were clarified by centrifugation at 15,000 × g for 15 min. Total protein concentration in the supernatant was measured using the Bio‐Rad protein reagent with bovine serum albumin as standard. Protein (100 μg) was then precipitated by adding ice‐cold acetone to 90% (v/v) and separated by centrifugation at 10,000 × g for 15 min at 4°C. Samples were then prepared by resuspending the proteins in loading buffer, and 6 μL (30 μg) was analyzed by zymography as described above.
Real‐Time RT‐PCR for MMP‐9
Membranes were homogenized in 1 mL Trizol reagent (Gibco, Grand Island, NY, USA) and total RNA was extracted and precipitated according to the manufacturer's instructions. Complementary cDNA was prepared from 2 μg total RNA using a cDNA archive kit (Applied Biosystems International, Foster City, CA, USA). Real‐time polymerase chain reaction (PCR) was performed in duplicate reactions on 2 μL cDNA using master‐mix, PCR primers and probes for MMP‐9 and 18S rRNA using the TaqManTM system and associated software as directed by the manufacturer (Applied Biosystems, Foster City, CA, USA).
Statistics
Potential differences in ProMMP‐9 and ProMMP‐2 band intensity were evaluated by generalized estimating equations using the generalized linear models procedure of sas (SAS Institute, Cary, NC, USA). Data for MMP‐9 gene expression (cycles to amplification) were analyzed in a similar manner except that 18S rRNA was used as a covariate to correct for potential differences in RNA loading. Differences were considered significant at the P < 0.05 level, and data are shown as least‐squares mean ± S.E.M.
Results
Culture results for the samples used for these analyses are shown in Table I. Level of infection was generally higher for amniotic fluid and placenta than for the spleen–liver. Gelatinolytic activity was consistently observed at ∼92 and ∼72 kDa, corresponding to ProMMP‐9 and ProMMP‐2. The gelatinolytic activity of these bands could be inhibited by addition of 10 mm ethylenediaminetetraacetic acid to the development solution suggesting that the bands were MMPs (data not shown).
Table I.
Culture Data for all Samples Analyzed by Zymography for MMP‐2 and MMP‐9 and Real‐Time Polymerase Chain Reaction for MMP‐9
| Samples (method of analysis) | Culture resultsa | ||
|---|---|---|---|
| Amniotic fluid (%) | Placenta (%) | Fetal spleen–liver (%) | |
| gd 18 Amniotic fluid (zymography) | 46/46 (100) | 22/22 (100) | 21/22 (95.4) |
| gd 18 Membranes (zymography) | 41/41 (100) | 38/38 (100) | 36/38 (94.7) |
| gd 18 Membranes (RT‐PCR) | 16/16 (100) | 14/14 (100) | 13/14 (92.8) |
| gd 21 Membranes (zymography) | ND | 19/20 (100) | 14/24 (58.3) |
ND, not done; RT‐PCR, reverse transcription‐polymerase chain reaction; MMP, matrix metalloproteinase; gd, gestation day.
aNumber of positive samples/samples randomly selected for the analyses of this study (%).
Significantly more activity (band intensity) was observed in amniotic fluid (Fig. 1) and membranes (Fig. 2) for the band corresponding to ProMMP‐9 in animals that received M. pulmonis than in control animals at gd 18. Real‐time reverse transcription (RT)‐PCR demonstrated that fewer cycles of amplification were required for MMP‐9 in the membranes collected from control animals than in tissues harvested from infected animals (Fig. 3). No significant differences were detected for gelatinolytic activity for the band corresponding to ProMMP‐2 in amniotic fluid or membranes at gd 18. At gd 21, however, more activity for both ProMMP‐9 and ProMMP‐2 was present in membranes of M. pulmonis‐infected dams than control dams (Fig. 4).
Figure 1.
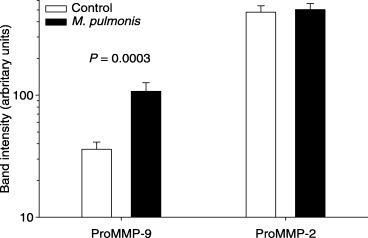
Pro‐matrix metalloproteinase‐9 (ProMMP‐9), is increased in membranes of pups at gestational age 18 that were harvested from dams infected with Mycoplasma pulmonis at gestation day 14. Shown are the least‐squares mean ± S.E.M. for pixel intensity for each group (note that data are shown on a logarithmic scale).
Figure 2.
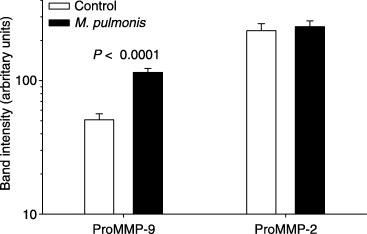
Pro‐matrix metalloproteinase‐9 (ProMMP‐9), is increased in amniotic fluid of pups at gestation day 18 from dams infected with Mycoplasma pulmonis at gestation day 14. Shown are the least‐squares mean ± S.E.M. for pixel intensity for each group (note that data are shown on a logarithmic scale).
Figure 3.
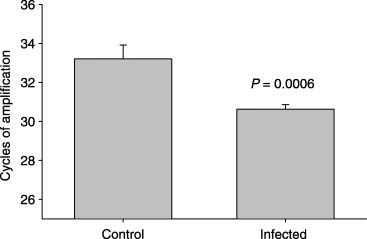
mRNA for matrix metalloproteinase‐9 (MMP‐9) is increased in membranes of gestation day 18 pups harvested from Mycoplasma pulmonis‐infected pregnancies. Shown is the least‐squares mean ± S.E.M. of the number of cycles to threshold, which is inversely and geometrically proportional to the level of mRNA.
Figure 4.
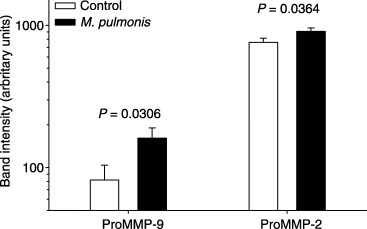
ProMMP‐9 and ProMMP‐2 are increased in the membranes of gestation day 21 pups harvested from dams infected with Mycoplasma pulmonis at gestation day 14. Shown are the least‐squares mean ± S.E.M. for pixel intensity for each group (note that data are shown on a logarithmic scale).
Discussion
Preterm premature rupture of membranes accompanies about 50% of all preterm deliveries and is frequently associated with infection. 15 The genital mycoplamas M. hominis and Ureaplasma sp. are most common pathogens of the reproductive tract. These organisms have been isolated from amniotic fluid harvested from women with intact membranes. 16 It has been reported that vaginal colonization or amniotic infection with this organism significantly increases the likelihood of PPROM. 2 , 3
Infection with Ureaplasma sp. in women is associated with increased concentrations of TNF‐α in amniotic fluid 16 and experimental infection of Rhesus monkeys with either U. urealyticum or M. hominis increases the production of TNF‐α in amniotic fluid. 5 , 6 This cytokine has been shown in vitro to stimulate MMP‐9 production by amnion 10 or amniochorion. 17 Mycoplasma pulmonis has previously been shown to increase TNF‐α concentrations in amniotic fluid in experimentally infected rats. 12 Therefore, we hypothesized that genital mycoplasmas would increase in vivo production of MMP‐9 in a well‐defined system of intra‐amniotic infection.
We found that MMP‐9 was significantly increased in the membranes and amniotic fluids of M. pulmonis‐infected dams. Although M. pulmonis is a different species than the genital pathogens affecting humans (U. parvum, U. urealyticum, M. genitalium, and M. hominis), there are many similarities in the pathophysiology of these organisms with regard to their consequences on infertility and fetal wastage. 12 Although no overt clinical effects of infection on pregnancy are observed with this model, intravenous injection of M. pulmonis into pregnant rats results in high levels of bacterial infection in amniotic fluid, placenta and endometrium as well as histological placentitis. 14 Future studies comparing the pathophysiology of infection by the intravaginal route with the intravenous route may be valuable for separating clinical from subclinical aspects of intrauterine infection. This is particularly relevant as the clinical course of genital mycoplasmosis in humans is most closely linked to clinically silent chorioamnionitis rather than febrile intrauterine infections. 18 This study suggests that increased production of proinflammatory cytokines and MMP‐9 occurs during genital mycoplasmosis but this is not sufficient for induction of PPROM, preterm labor or low birthweight in this model system. During spontaneous labor in monkeys, ProMMP‐9 increases as labor approaches followed by increased production of activated MMP‐9 (83 kDa). 8 It is also possible that MMP‐9 must be transformed to its active form in order for it to digest membrane collagen and that the enzymes which activate proMMP‐9 are not increased in this model.
References
- 1. Garite TJ: Premature rupture of the membranes In Maternal–Fetal Medicine, Creasy RK, Resnik R. (eds). Philadelphia, PA, Saunders, 2003, pp 723–739. [Google Scholar]
- 2. Grattard F, Soleihac B, De Barbeyrac B, Bebear C, Seffert P, Pozzetto B: Epidemiologic and molecular investigations of genital mycoplasmas from women and neonates at delivery. Pedatr Infect Dis J 1995; 14:853–858. [DOI] [PubMed] [Google Scholar]
- 3. Gravett MG, Eschenbach DA: Possible role of Ureaplasma urealyticum in preterm premature rupture of the fetal membranes. Pediatr Infect Dis J 1986; 5 (Suppl.): S253–S257. [DOI] [PubMed] [Google Scholar]
- 4. Crouse DT, English BK, Livingston L, Meals EA: Genital mycoplasmas stimulate tumor necrosis factor‐alpha and inducible nitric oxide synthase production from a murine macrophage cell line. Pediatr Res 1998; 44:785–790. [DOI] [PubMed] [Google Scholar]
- 5. Sadowsky DW, Duffy LB, Axthelm MK, Cook MJ, Witkin SS, Gravett MG, Cassell GH, Novy MJ: Experimental primate model for Mycoplasma hominis chorioamnionitis and preterm labor. J Soc Gyn Invest 2002; 9:206A. [Google Scholar]
- 6. Novy MJ, Duffy L, Axthelm MK, Cook MJ, Haluska GJ, Witkin S, Gerber S, Gravett MG, Sadowsky D, Cassell GH: Experimental primate model for ureaplasma chorioamnionitis and preterm labor. J Soc Gynecol Investig 2001; 8(Suppl.):48A. [Google Scholar]
- 7. Vadillo Ortega F, Gonzalez‐Avila G, Furth EE, Lei H, Muschel RJ, Stetler‐Stevenson WG, Strauss JF, III : 92‐kd type IV collagenase (matrix metalloproteinase‐9) activity in human amniochorion increases with labor. Am J Pathol 1995; 146:148–156. [PMC free article] [PubMed] [Google Scholar]
- 8. Vadillo‐Ortega F, Sadowsky DW, Haluska GJ, Hernandez‐Guerrero C, Guevara‐Silva R, Gravett MG, Novy MJ: Identification of matrix metalloproteinase‐9 in amniotic fluid and amniochorion in spontaneous labor and after experimental intrauterine infection or interleukin‐1 beta infusion in pregnant rhesus monkeys. Am J Obstet Gynecol 2002; 186:128–138. [DOI] [PubMed] [Google Scholar]
- 9. Lei H, Vadillo‐Ortega F, Paavola LG, Strauss JF, III : 92‐kDa gelatinase (matrix metalloproteinase‐9) is induced in rat amnion immediately prior to parturition. Biol Reprod 1995; 53:339–344. [DOI] [PubMed] [Google Scholar]
- 10. Arechavaleta‐Velasco F, Ogando D, Parry S, Vadillo Ortega F: Production of matrix metalloproteinase‐9 in lipopolysaccharide‐stimulated human amnion occurs through an autocrine and paracrine proinflammatory cytokine‐dependent system. Biol Reprod 2002; 67:1952–1958. [DOI] [PubMed] [Google Scholar]
- 11. Fortunato SJ, Menon R: Distinct molecular events suggest different pathways for preterm labor and premature rupture of membranes. Am J Obstet Gynecol 2001; 184:1399–1405. [DOI] [PubMed] [Google Scholar]
- 12. Brown MB, Peltier M, Hillier M, Crenshaw B, Reyes L: Genital mycoplasmosis in rats: a model for intrauterine infection. Am J Reprod Immunol 2001; 46:232–241. [DOI] [PubMed] [Google Scholar]
- 13. Peltier MR, Brown MB: Experimental genital mycoplasmosis causes increased levels of mRNA for IL‐6 and TNF‐alpha in the placenta. Am J Reprod Immunol 2005; 53:189–198. [DOI] [PubMed] [Google Scholar]
- 14. Peltier MR, Richey L, Brown MB: Placental lesions caused by experimental infection of Sprague–Dawley rats with Mycoplasma pulmonis . Am J Reprod Immunol 2003; 50:254–262. [DOI] [PubMed] [Google Scholar]
- 15. Goldenberg RL: Prevention of pretmature birth. N Engl J Med 1998; 339:313–320. [DOI] [PubMed] [Google Scholar]
- 16. Yoon BH, Romero R, Park JS, Chang JW, Kim YA, Kim JC, Kim KS: Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol 1998; 179:1254–1260. [DOI] [PubMed] [Google Scholar]
- 17. Fortunato SJ, Menon R, Lombardi SJ: Role of tumor necrosis factor‐alpha in the premature rupture of membranes and preterm labor pathways. Am J Obstet Gynecol 2002; 187:1159–1162. [DOI] [PubMed] [Google Scholar]
- 18. Shurin PA, Alpert S, Bernard Rosner BA, Driscoll SG, Lee YH: Chorioamnionitis and colonization of the newborn infant with genital mycoplasmas. N Engl J Med 1975; 293:5–8. [DOI] [PubMed] [Google Scholar]


