Abstract
Observational studies have reported an association between underlying cardiovascular diseases (CVD) and worse prognosis in COVID-19 patients, but this still remains unclear. We conducted a meta-analysis of recent studies that reported the association of CVD with worse prognosis and increased mortality in COVID-19 patients. Literature search through PubMed, the Cochrane Library, and Embase was completed by 2 reviewers from November 1, 2019 to April 20, 2020. Inclusion criteria were observational case-control or cohort studies on COVID-19 patients with a history of CVD included, which reported outcomes of COVID-19 infection severity, clearly outlined the definition of “severe disease” and with sample size >10. Data were abstracted independently by 2 authors. Studies were divided into 2 separate cohorts for analysis: severity (severe vs nonsevere) and mortality (nonsurvivors vs survivors). Data was pooled into a meta-analysis to estimate pooled odds ratio (OR) with 95% confidence interval (95% CI) for each outcome. A total of 18 studies (n = 4858 patients) were included. Sixteen studies were from China, while 2 were from the United States. Pre-existing CVD was associated with a significantly increased risk of a severe form of COVID-19 (OR = 3.14; 95% CI 2.32-4.24; I2 = 0%; Q = 8.68, P= 0.73) and overall risk of COVID-19 all-cause mortality (OR = 11.08; 95% CI: 2.59-47.32; I2 = 55%; P = 0.11). However, this study did not find a significant association between previous history of CVD and mortality in severe COVID-19 disease (OR = 1.72; 95% CI: 0.97-3.06, I2 = 0%, P = 0.46). Pre-existing CVD is associated with worse outcomes among patients with COVID-19. Clinicians and policymakers need to take account of these findings in implementing risk stratification models.
Introduction
The ongoing pandemic of coronavirus disease 2019 (COVID-19) has been associated with high rates of morbidity and mortality. Clinical and biological predictors are needed for identifying the severity of COVID-19 infection and helping in judicious allocation of limited resources. Cardiovascular disease (CVD) is the leading cause of morbidity and mortality in the United States, and has also been associated with worse outcomes in patients with influenza and bacterial pneumonias.1, 2, 3 Emerging data have linked the presence of CVD with a worse prognosis in COVID-19 patients.4, 5, 6 Likewise, COVID-19 is associated with a high inflammatory burden, the so called inflammatory or “cytokine storm,” inducing vascular inflammation, myocarditis, and cardiac arrhythmias,5 which in turn can aggravate the damage to the heart.4 However, a few and scattered studies, with widely varying results, have been published so far. The small sample size of patients with a previous CVD history in these studies further limits its applicability. Thus, we aimed to compile and analyze all results recently published on the influence of CVD in the severity of COVID-19 infection. To this end, we carried out a pooled analysis of current studies and evaluated the association between underlying or previous history of CVD conditions and outcomes of infection severity in COVID-19 patients.
Methods
Study Protocol and Registration
This systematic review and meta-analysis were conducted in strict conformity with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.7 The protocol for this study has been reported in the International Prospective Register of Systematic Reviews (PROSPERO identifier: CRD42020180086).
Literature Search Strategy
A comprehensive and systematic search of literature from November 1, 2019 to April 20, 2020 was conducted on the electronic databases Pubmed, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) to identify studies eligible for inclusion. The electronic search was carried out using the strategy as follows: (1) “COVID-19,” OR “SARS-CoV-2,” OR “coronavirus disease 2019”; (2) “cardiovascular disease,” OR “coronary artery disease,” OR “hypertension”; (3) 2 AND 3. No language restriction was made. When the articles were published by the same study group and there was an overlap of the search period, only the most recent article was included to avoid duplication of data. The PubMed function "related articles" was used to extend the search. Also, we searched major infectious disease, cardiovascular and general medicine journals reporting articles about COVID-19 infection to look for additional studies. We then performed hand-search of the bibliography of included studies, to detect other potentially eligible investigations.
Eligibility Criteria
All studies were screened and assessed for eligibility by 4 independent reviewers (G.A., S.A., B.M.H, and I.C). Search results were screened by title and abstract, with those of potential relevance evaluated by full text. Studies were deemed eligible for inclusion if they fulfilled the following criteria: (1) observational case-control or cohort studies (2) COVID-19 patients with a history of CVD included; (3) outcomes of COVID-19 infection severity reported; (4) clearly outlined the definition of “severe disease” and (5) sample size >10. Severe disease was defined in this analysis as a composite of: (1) Respiratory distress, respiratory rate ≥30 per minute; (2) Oxygen saturation on room air at rest ≤93%; (3) Partial pressure of oxygen in arterial blood/fraction of inspired oxygen ≤300 mmHg; (4) Patients requiring mechanical ventilation/vital life support/intensive care unit admission; (5) Death. Cardiovascular disease was defined as any cardiac pathology with the exception of hypertension. Reviews and studies with incomplete or irrelevant data were excluded. Any disagreements between reviewers arising during the eligibility assessment were settled through a consensus.
Data Extraction and Quality Assessment
Data extraction was conducted by 4 independent reviewers (G.A., S.A., B.M.H, and I.C). For each study, the following information was extracted: the surname of the first author and the year of publication, the geographical region where the study was performed, the type of study (cohort or case-control), sample size, baseline demographic characteristics, proportion of patients with severe and nonsevere COVID-19, proportion of patients with underlying CVD, and mortality from COVID-19. Any variances were resolved by a consensus. Quality assessment and analysis of risk of bias of all selected full-text articles was performed using the Newcastle-Ottawa Scale for nonrandomized studies.
Outcomes of Interest
The primary outcome of interest was the association between pre-existing CVD and COVID-19 severity. The secondary outcome was the association between underlying CVD and COVID-19 mortality. A tertiary outcome was the association between underlying CVD and mortality in patients with severe COVID-19 disease. Thus, a total of 3 separate meta-analyses were performed.
Statistical Analysis
The statistical analysis was carried out using MetaXL (software Version 5.3, EpiGear International Pty Ltd., Sunrise Beach, Australia) and the Comprehensive Meta-analysis software (Version 3.3.070, Biostat, New Jersey). The strength of association between pre-existing CVD and COVID-19 severity and mortality was estimated using odds ratio (OR). A random-effects model (DerSimonian and Laird) was applied due to the heterogeneity in definition of CVD amongst the studies. The magnitude of heterogeneity among the included studies was assessed using the chi-squared test (Chi2) and I-squared statistic (I2). For the Chi2 test, a Cochrane's Q P value of <0.10 was considered significant. The values of the I2 statistic were interpreted as follows at a 95% confidence interval: 0%-40% might not be important, 30%-60% might indicate moderate heterogeneity, 50%-90% may represent substantial heterogeneity, and 75%-100% may represent significant heterogeneity (37). Subgroups were used to compare cohort vs case-control studies. Publication bias was assessed by a funnel plot analysis. A random effects meta-regression using log OR was performed to evaluate the impact of baseline characteristics (age and sex) on association of CVD with disease severity in patients with COVID-19. Additionally, leave-one out sensitivity analysis was performed to assess the robustness of the results, and to further probe the sources of inter-study heterogeneity.
Results
Study Identification
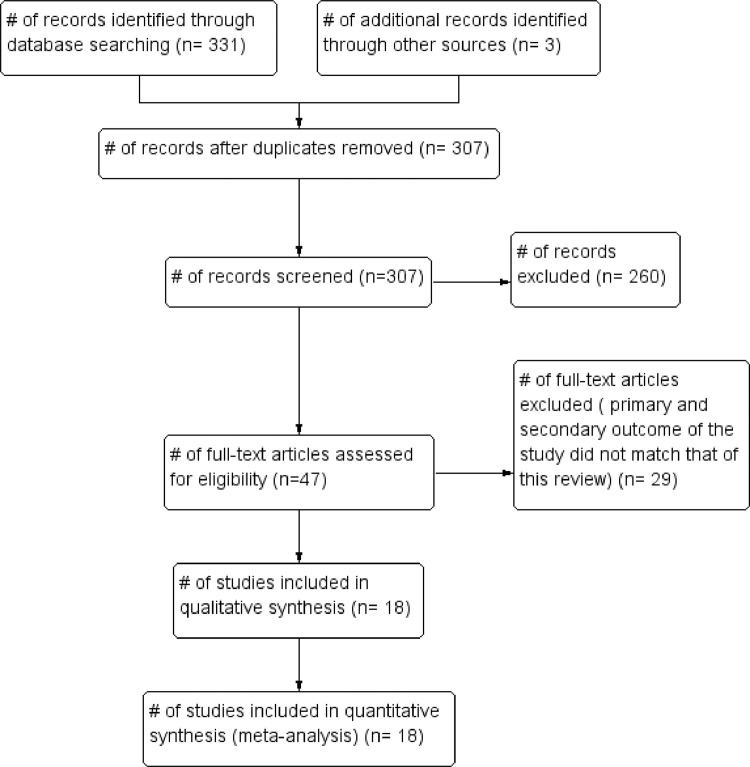
The initial search produced 331 potentially relevant articles. Following the removal of duplicates and primary screening, 47 articles were assessed by full text for eligibility in the meta-analysis. Of these, 29 were excluded because the primary and secondary outcome of the study did not match that of this review. Thus, a total of 18 articles were included in this systematic review and meta-analysis (Fig 1 ; Tables 1 and 2 ).
FIG 1.
Flow of studies through the systematic review.
TABLE 1.
Characteristics of patients included in the severity analysis cohort
| Study | Country | Sample Size | Severe patients |
Non-severe patients |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | Age (yrs)* | Women (%) | CVD n (%) |
n (%) | Age (yrs)* | Women (%) | CVD n (%) |
|||
| Aggarwal et al. (2020) | Iowa, USA | 16 | 8 (50%) | 67 (38-70) | 3 (38%) | 5 (63%) | 8 (50%) | 68.5 (41-95) | 1 (13%) | 2 (26%) |
| Li et al. (2020) | Wuhan, China | 548 | 269 (49.1%) | 65 | 116 (43.1%) | 28 (10.4%) | 279 (50.9%) | 56 | 153 (54.8%) | 6 (2.2%) |
| Goyal et al. (2020) | New York, USA | 393 | 130 (33.1%) | 64.5 | 32 (28.2%) | 25 (19.2%) | 263 (66.9%) | 61.5 | 117 (44.5%) | 29 (11%) |
| Guan et al. (2020) | Outside Hubei, China | 1099 | 173 (15.7%) | 52 (40–65) | 73 (42%) | 10 (5.8%) | 926 (84.3%) | 45 (34-57) | 386 (42%) | 17 (1.8%) |
| Huang et al. (2020) | Wuhan, China | 41 | 13 (31.7%) | 49 (41-61) | 2 (15%) | 3 (23%) | 28 (68.3%) | 49 (41-57.5) | 9 (32%) | 3 (11%) |
| Liu et al. (2020) | Shenzen, China | 12 | 6 (50%) | 64 | 3 (50%) | 3 (50%) | 6 (50%) | 43.3 | 1 (16%) | 1 (16%) |
| Qin et al. (2020) | Wuhan, China | 452 | 286 (63.3%) | 61 (51-69) | 131 (45.8%) | 24 (8.4%) | 166 (36.7%) | 53 (41.25-62) | 86 (51.8%) | 3 (1.8%) |
| Wan et al. (2020) | Chongqing, China | 135 | 40 (29.6%) | 56 (52-73) | 19 (47.5%) | 6 (15%) | 95 (70.4%) | 44 (33-49) | 43 (45.3%) | 1 (1%) |
| Wang D et al. (2020) | Wuhan, China | 138 | 36 (26.1%) | 66 (57-78) | 14 (39%) | 9 (25%) | 102 (73.9%) | 51 (37-62) | 49 (48%) | 11 (10.8%) |
| Wu et al. (2020) | Wuhan, China | 201 | 84 (41.7%) | 58.5 (50-69) | 24 (28.6%) | 5 (6%) | 117 (58.3%) | 48 (40-54) | 49 (41.9%) | 3 (2.6%) |
| Zhang et al. (2020) | Wuhan, China | 140 | 58 (41.4%) | 64 (25-87) | 25 (43%) | 4 (6.9%) | 82 (58.6%) | 52 (26-78) | 44 (54%) | 3 (3.7%) |
| Feng et al. (2020) | Wuhan, China | 476 | 124 (26.1%) | 58 (48-67) | 43 (34.7%) | 17 (13.7%) | 352 (73.9%) | 51 (37-63) | 162 (46%) | 21 (6%) |
| Zheng et al. (2020) | Changsha, China |
161 | 30 (18.6%) | 57 (46.5-66) | 16 (53.3%) | 2 (6.7%) | 131 (81.4%) | 40 (31-51) | 65 (49.6%) | 2 (1.5%) |
TABLE 2.
Characteristics of patients included in the mortality analysis cohort
| Study | Country | Outcome | Sample Size | Non-survivors |
Survivors |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | Age (yrs)* | Women (%) | CVD n (%) |
n (%) | Age (yrs)* | Women (%) | CVD n (%) |
||||
| Deng et al. (2020) | Wuhan, China | In-hospital mortality | 225 | 109 (48.5%) | 69 (62-74) | 36 (33%) | 13 (11.9%) | 116 (51.5%) | 40 (33-57) | 65 (56%) | 4 (3.4%) |
| Ruan et al. (2020) | Wuhan, China | In-hospital mortality | 150 | 68 (45.3%) | 67 (15-81) | 19 (28%) | 13 (19%) | 82 (54.6%) | 50 (44-81) | 29 (35%) | 0 (0%) |
| Wu et al. (2020) | Wuhan, China | In-hospital mortality | 84 | 44 (52.3%) | 68.5 (59.3-75) | 15 (34.1%) | 4 (9.1%) | 40 (47.7%) | 50 (40.3-56.8) | 9 (22,5%) | 4 (10%) |
| Yang et al. (2020) | Wuhan, China | 28-day mortality after ICU admission | 52 | 32 (61.5%) | 64.6 (11.2) | 11 (34%) | 3 (9%) | 20 (38.5%) | 51.9 (12.9) | 6 (30%) | 2 (10%) |
| Zhou et al. (2020) | Wuhan, China | In-hospital mortality or discharge | 191 | 54 (28.3%) | 69 (63-76) | 16 (30%) | 13 (24%) | 137 (71.7%) | 52 (45-58) | 56 (41%) | 2 (1%) |
| Wang Y et al. (2020) | Shanghai, China | 28-day mortality after ICU admission | 334 | 211 | 69 (62-74) | 36 (33%) | 13 (11.9%) | 133 (34.7%) | 40 (33-57) | 65 (56%) | 4 (3.4%) |
Characteristics of the Included Studies and Quality Assessment
A total of 18 included studies (n = 4858 patients) were included. Sixteen studies were from China, while 2 were from the United States. Thirteen studies reported data on pre-existing cardiovascular diseases in patients with and without severe COVID-19,4 , 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 5 studies reported data on mortality in COVID-19 with and without pre-existing CVD,20, 21, 22, 23, 24 while 1 study reported both.16 Essential characteristics of the included studies are outlined in Tables 1 and 2. Summary of the Newcastle-Ottawa Scale for the included studies is provided in supplementary material.
Meta-Analysis of the Association Between CVD and COVID-19 Severity
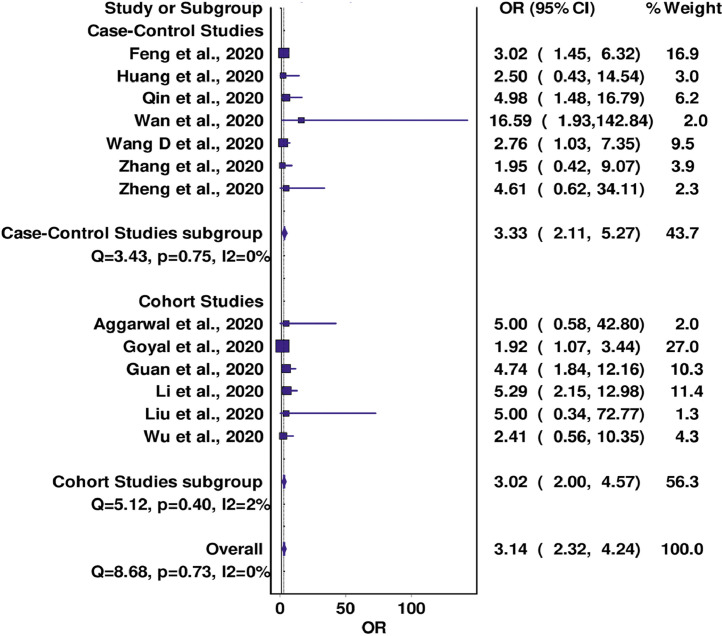
A total of 13 studies reported data on the association between pre-existing CVD and COVID-19 severity. In pooled analysis, CVD was found to be associated with a significantly increased risk of a severe form of COVID-19 in both case-control (OR = 3.33; 95% CI 2.11-5.27; I2 = 0%; Q = 3.43; P = 0.75) and cohort studies (OR = 3.02; 95% CI 2.00-4.57; I2 = 2%; Q = 5.12; P = 0.40), with the overall analysis revealing very low evidence of inter-study heterogeneity (Cochran's Q = 8.68, P = 0.73, I2 = 0%). No significant change in the OR was seen in the leave-one-out sensitivity analysis (Fig 2 ; supplementary material).
FIG 2.
Forest plot for association between CVD and COVID-19 severity.
Meta-Analysis of the Association Between CVD and Mortality in Severe COVID-19 Disease
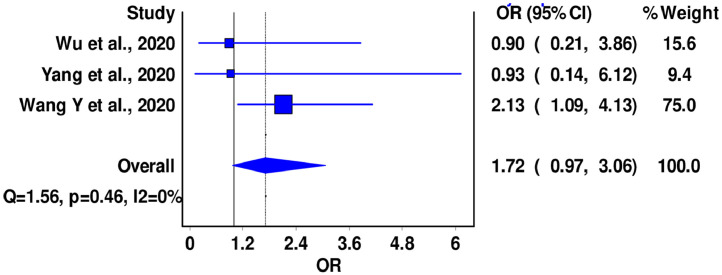
A total of three (n = 480) studies reported data on mortality in patients with severe COVID-19 disease and pre-existing history of CVD. Pooled analysis of these studies did not find a significant association between previous history of CVD and mortality in severe COVID-19 disease (OR = 1.72; 95% CI: 0.97-3.06, I2 = 0%, Cochran's Q = 1.56, P = 0.46). No heterogeneity was seen among the studies (Fig 4; supplementary material).
FIG 4.
Forest plot for association between CVD and COVID-19 mortality in patients with severe disease.
Meta-Analysis of the Association Between CVD and Overall COVID-19 Mortality
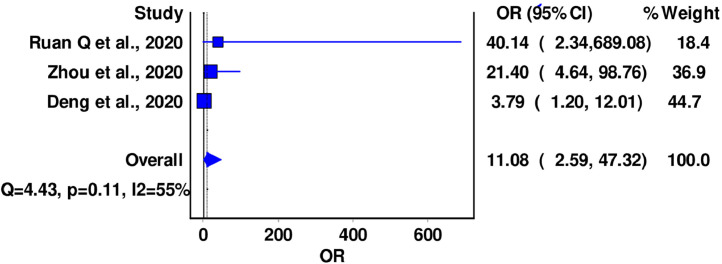
A total of 3 (n = 566) studies reported data on mortality in all hospitalized patients COVID-19 disease and pre-existing history of CVD. In the pooled analysis, previous history of CVD was associated with an ∼11-fold increase in mortality (OR = 11.08; 95% CI: 2.59-47.32), with moderate level of inter-study heterogeneity (I2 = 55%, Cochran's Q = 4.43, P = 0.11). No significant differences were noted in the leave-one-out sensitivity analysis (Fig 3 ; supplementary material).
FIG 3.
Forest plot for association between CVD and COVID-19 mortality.
Bias and Meta-Regression
The potential for publication bias was evaluated in funnel plots (supplementary material). Only very mild asymmetry was noted in funnel plot for CVD and severity. The limited studies for both mortality analyses limited any determination of potential for publication bias in these studies. Importantly, in meta-regression of odds of severe disease with underlying CVD, the age of patients in the severe group had no significant influence on calculated pooled odds ratio (P = 0.34). However, interestingly, as the percentage of women in the severe group increased, so did the odds ratio of severe disease and CVD association (P = 0.02).
Discussion
Our results demonstrate that underlying or previous history of CVD is directly associated with both worse prognosis and severity outcomes in COVID-19 patients. CVD was found to be associated with around 3-fold increased odds of severe COVID-19 infection, and an 11-fold increase in all-cause mortality among COVID-19 patients.
There are several possible explanations for these findings. First, coexistence of COVID-19 may considerably enhance the severity of an underlying CVD, has already proven in some of the respiratory infections.1, 2, 3 Symptomatology of fever, cough, and imaging findings may divert clinicians’ attention away from possible cardiovascular injury in patients with confirmed COVID-19. Several drugs being currently used to treat COVID-19 infection have been shown to have deleterious cardiovascular effects.25 Chloroquine/hydroxychloroquine and azithromycin have been associated with prolonged QTc interval and risk of arrhythmias.26 , 27 Conduction defects, ventricular arrhythmias, and heart failure have been reported with azithromycin and remdesivir therapy.28 , 29 Lopinavir/ritonavir and interleukin therapies have been shown to be associated with ischemic heart disease and abnormalities in lipid profile.28 , 30 Last but not the least, patients deemed to be either relatively stable or those with guarded prognosis may not be considered for invasive strategies to preserve healthcare resources and prevent spread of infection.
Our study has several limitations. First, since most studies were from Wuhan China, the potential of overlap, in which 1 patient could be the included more than 1 study, is high. Further, we found limited number of studies with relatively small sample size during literature search. However, at this stage of ongoing pandemic, our findings may provide early insights into building models for risk stratification and help with judicious use of limited healthcare resources. Careful evaluation of the patients was performed in order to avoid an overlap between studies. We did not use any exclusion criteria due to the small number of studies. Sensitivity and publication bias analyses were performed to assess for heterogeneity. Since age and sex can be significant confounding variables, we performed a meta-regression to assess their impact on disease severity and mortality in COVID-19 patients.
The presence of CVD was found to be associated with COVID-19 severity and mortality. However, larger studies are needed to confirm these findings. Individuals with previous history of CVD, especially older individuals or those with other co-morbidities, should be aware that if they develop COVID-19 related symptoms, such as fever cough or dyspnea, or were previously exposed to persons with known or suspected COVID-19 infection, they should immediately refer to their healthcare service for receiving specific health and safety advices, or for being timely managed to prevent worsening of their clinical conditions. Finally, the general population should strongly avoid contact with people at higher risk for severe illness, such as those with underlying CVD.
Acknowledgment
Fabian Sanchis-Gomar is supported by a postdoctoral contract granted by “Subprograma Atracció de Talent - Contractes Postdoctorals de la Universitat de València.”
Footnotes
Conflict of Interest: The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Funding: None.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.cpcardiol.2020.100617.
Appendix. Supplementary materials
References
- 1.Dhainaut J.F., Claessens Y.E., Janes J., Nelson D.R. Underlying disorders and their impact on the host response to infection. Clin Infect Dis. 2005;41(Suppl 7):S481–S489. doi: 10.1086/432001. [DOI] [PubMed] [Google Scholar]
- 2.Peiris J.S., Chu C.M., Cheng V.C. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viasus D., Cillóniz C., Cardozo C.G., Puerta P., Garavito A., Torres A. Early, short and long-term mortality in community-acquired pneumonia. Ann Res Hospitals. 2018;2 [Google Scholar]
- 4.Li B., Yang J., Zhao F. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 6.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liberati A., Altman D.G., Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Aggarwal S., Garcia-Telles N., Aggarwal G., Lavie C.J., Lippi G., Henry B.M. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with Coronavirus Disease 2019 (COVID-19): first report from the United States. Diagnostics. 2020;7:91–96. doi: 10.1515/dx-2020-0046. [DOI] [PubMed] [Google Scholar]
- 9.Goyal P., Choi J.J., Pinheiro L.C. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020 doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of Coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y., Yang Y., Zhang C. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin C., Zhou L., Hu Z. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wan S., Xiang Y., Fang W. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020 doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel Coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J.J., Dong X., Cao Y.Y. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 18.Feng Y., Ling Y., Bai T. COVID-19 with different severity: a multi-center study of clinical features. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng F., Tang W., Li H., Huang Y.X., Xie Y.L., Zhou Z.G. Clinical characteristics of 161 cases of corona virus disease 2019 (COVID-19) in Changsha. Eur Rev Med Pharmacol Sci. 2020;24:3404–3410. doi: 10.26355/eurrev_202003_20711. [DOI] [PubMed] [Google Scholar]
- 20.Deng Y., Liu W., Liu K. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China: a retrospective study. Chin Med J (Engl) 2020 doi: 10.1097/CM9.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. pii: S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y., Lu X., Chen H. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202003-0736LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Driggin E., Madhavan M.V., Bikdeli B. Cardiovascular considerations for patients, health care workers, and health systems during the Coronavirus Disease 2019 (COVID-19) pandemic. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.03.031. pii: S0735-1097(20)34637-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Owens R.C., Jr., Nolin T.D. Antimicrobial-associated QT interval prolongation: pointes of interest. Clin Infect Dis. 2006;43:1603–1611. doi: 10.1086/508873. [DOI] [PubMed] [Google Scholar]
- 27.Stas P., Faes D., Noyens P. Conduction disorder and QT prolongation secondary to long-term treatment with chloroquine. Int J Cardiol. 2008;127:e80–e82. doi: 10.1016/j.ijcard.2007.04.055. [DOI] [PubMed] [Google Scholar]
- 28.Limsreng S., Marcy O., Ly S. Dyslipidemias and elevated cardiovascular risk on lopinavir-based antiretroviral therapy in Cambodia. PLoS One. 2016;11 doi: 10.1371/journal.pone.0160306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trac M.H., McArthur E., Jandoc R. Macrolide antibiotics and the risk of ventricular arrhythmia in older adults. CMAJ. 2016;188:E120–E129. doi: 10.1503/cmaj.150901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bacchiega B.C., Bacchiega A.B., Usnayo M.J., Bedirian R., Singh G., Pinheiro G.D. Interleukin 6 inhibition and coronary artery disease in a high-risk population: a prospective community-based clinical study. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.005038. pii: e005038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.