Abstract
In response to the coronavirus disease 2019 (COVID-19) pandemic, many jurisdictions and gastroenterological societies around the world have suspended nonurgent endoscopy. Subject to country-specific variability, it is projected that with current mitigation measures in place, the peak incidence of active COVID-19 infections may be delayed by over 6 months. Although this aims to prevent the overburdening of healthcare systems, prolonged deferral of elective endoscopy will become unsustainable. Herein, we propose that by incorporating readily available point-of-care tests and conducting accurate clinical risk assessments, a safe and timely return to elective endoscopy is feasible. Our algorithm not only focuses on the safety of patients and healthcare workers, but also assists in rationalizing the use of invaluable resources such as personal protective equipment.
Abbreviations: COVID-19, coronavirus disease 2019; CRISPR, clustered regularly interspaced short palindromic repeat; FDA, U.S. Food and Drug Administration; HCW, healthcare worker; iNAAT, isothermal nucleic acid amplification test; POC, point of care; PPE, personal protective equipment; RT-PCR, reverse transcriptase polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome–coronavirus 2
COVID-19 and Endoscopy
In December 2019 a novel coronavirus termed severe acute respiratory syndrome–coronavirus 2 (SARS-CoV-2) emerged from a suspected zoonotic source in Wuhan, China. Driven by its ability to spread through respiratory droplets, including by asymptomatic individuals, SARS-CoV-2 has rapidly traversed international borders to infect over 1.5 million people in over 200 countries.1 Now termed coronavirus disease 2019 (COVID-19) by the World Health Organization, it is the first coronavirus to be declared a global pandemic and carries a mortality rate of 1% to 10%.1 , 2 To curtail the spread of COVID-19, restrictive measures have been implemented worldwide. This has included the closure of international borders, countrywide lockdowns, limitations on gatherings, social distancing, and the quarantining of any suspected or confirmed COVID-19 cases.3 The overarching intention of these measures is to “flatten the curve,” that is, reduce the peak incidence of active COVID-19 infections and hospitalizations so that healthcare systems are not overburdened. Unfortunately, healthcare workers (HCWs) remain up to 3 times more likely to contract COVID-19 than the general population,4 with up to 20% having contracted the disease within certain geographic regions.5
Accordingly, jurisdictions and gastroenterological societies around the world have recommended the suspension of nonurgent endoscopy.6, 7, 8, 9 In this article, we discuss the risk of COVID-19 transmission associated with endoscopy and the implications of a reduced endoscopy service. We propose that by incorporating readily available point-of-care (POC) tests and conducting accurate clinical risk assessments, a safe and timely return to elective endoscopy is feasible. Our algorithm not only focuses on the safety of patients and HCWs, but also assists in rationalizing the use of invaluable resources such as personal protective equipment (PPE).
Risk of COVID-19 Transmission during Endoscopy
Endoscopy is currently limited to emergency or urgent procedures including the treatment of GI bleeding, foreign body removal, acute luminal obstruction, and cholangitis. Furthermore, the endoscopic diagnosis, staging, or resection of advanced lesions and malignancy may be performed on a case-by-case basis. However, as peak SARS-CoV-2 viral loads are reached in the presymptomatic phase of disease, there are concerns that upper GI procedures including gastroscopy, ERCP, and EUS may aerosolize virus particles that are shed from the nasopharynx of infected individuals.10 This risk may be further enhanced if a patient dry retches, sneezes, coughs, or requires endotracheal intubation. Although data on SARS-CoV-2 transmission via aerosol-generating procedures are lacking, prior studies on SARS-CoV revealed that HCWs exposed to such procedures were 4.66 times (95% confidence interval, 3.13-6.94) more likely to become infected than nonexposed HCWs.11 With the detection of live SARS-CoV-2 virus in stool surpassing that of respiratory samples in up to 23% of patients,12, 13, 14, 15, 16, 17, 18, 19 the risk of fecal–oral transmission during colonoscopy is also plausible. This concern is not unfounded, with tissue samples from the esophagus, stomach, duodenum, and rectum of COVID-19 patients all demonstrating the presence of SARS-CoV-2 RNA.16 Additionally, because microbial dissemination can occur up to 6 feet away from a patient undergoing endoscopy20 and bodily fluids may splatter when manipulating devices in and out of the working channel of an endoscope, there is also a risk of fomite and environmental transmission. This risk is extended to clerical and cleaning staff because SARS-CoV-2 has been demonstrated to easily contaminate a patient’s surroundings, including sinks, light switches, and doors,21 and is viable on plastics and stainless steel for hours.22
Thus, because endoscopy is viewed as a high-risk procedure for COVID-19 transmission, current guidelines recommend the use of PPE for all emergency and urgent procedures, including a full-sleeve gown, eye protection, hairnet, gloves, and respirator mask.23 Although there was an initial concern over a potential shortage of PPE in the United States, with over 500,000 cases by mid-April 2020, this is looking less likely because of a smaller than projected caseload and increased PPE procurement.1 , 24 , 25 Another byproduct of current mitigation measures is the delay of the projected peak by a further 6 months.24 , 25 It should also be noted that the active caseload will take time to subside, and the eventual relaxation of mitigation measures may also result in disease resurgence.26 These additional challenges may result in a further delay to the reinstitution of elective endoscopy.
Consequences of Reduced Endoscopy
The importance of recommencing routine endoscopy is reflected by its economic and health impacts. In the United States, 17.7 million endoscopic procedures are performed annually, accounting for 5.6% of the population.27 Furthermore, over 136 billion U.S. dollars is spent on GI disease annually, exceeding that of heart disease, trauma, and mental health.27 Similar trends exist in less-populous countries such as Australia, where over 850,000 endoscopic procedures are performed annually, accounting for 3.5% of the population, 13.0% of all same-day separations from healthcare facilities, and 7.2% (or 5 billion Australian dollars) of all public and private hospital expenditure.28 , 29 In the United States alone, a hypothetical suspension of elective endoscopy for 6 months is predicted to result in the delayed diagnosis of over 2800 colorectal cancers and 22,000 high-grade adenomatous polyps with malignant potential.27 The 6-month mortality rate for those eventually diagnosed with colorectal cancer would increase by 6.5%.30 Just as ominously, with over 600,000 cirrhotic patients in the United States, over 1500 may have a terminal variceal bleed that may have been otherwise prevented by endoscopic surveillance.31, 32, 33, 34 Thus, clearly the long-term suspension of routine endoscopy is unsustainable, and it is therefore imperative that we resume elective endoscopy as early and safely as possible. A deeper understanding of available screening tools and the host-immune response to SARS-CoV-2 is valuable in working toward achieving this goal.
SARS-CoV-2: Immunity, Testing, and Implications on the Return to Elective Endoscopy
Is immunity to SARS-CoV-2 possible?
An animal study using a COVID-19–recovered rhesus macaque model raised the possibility of immunity to SARS-CoV-2 after the virus remained undetected in nasopharyngeal and anal swabs after an intratracheal rechallenge with SARS-CoV-2.35 Furthermore, a promising study on the plasma of recovered patients identified the presence of neutralizing antibodies, the activity of which was transferred to recipients after plasma infusion.36 In contrast, epidemiologic data from China suggest that COVID-19 reinfection or reactivation may be possible, with some recovered HCWs who experienced symptom resolution and had 2 consecutive negative polymerase chain reaction (PCR) results subsequently yielding positive PCR results up to 13 days later.37 Moreover, these recovered HCWs were only rescreened because of their need to recommence healthcare work. However, because the quality of screening tests used is unclear, the negative PCR results may have been false negatives. This is reflected in other studies that revealed despite a median seroconversion time of 7 days and rising antibody titers, the clearance of SARS-CoV-2 RNA from sputum and stool could take up to 3 weeks, including in asymptomatic individuals.15 , 38 , 39 Hence, given the limited body of knowledge pertaining to SARS-CoV-2 immunity, it would be prudent to currently assume that reactivation, reinfection, and viral shedding can occur despite seroconversion.
Testing for COVID-19
With the aforementioned concerns of aerosol generation, spread by asymptomatic individuals, and the possibility of reinfection, we believe that rapid POC tests are a vital component of any algorithm proposing a return to routine endoscopy. Current testing methods for COVID-19 include reverse transcriptase (RT)-PCR, isothermal nucleic acid amplification tests (iNAATs), clustered regularly interspaced short palindromic repeat (CRISPR) assays, enzyme-linked immunosorbent assays, and lateral flow immunoassays.
Although lab-based RT-PCR performed on nasopharyngeal swabs is limited by a complex and expensive protocol that can take up to 4 hours to yield a result, the U.S. Food and Drug Administration (FDA) recently approved a POC test that can yield a result within 45 minutes.40 Now commercially available, it carries a 95% sensitivity for diagnosing acute infection, although it is unreliable beyond week 1 of disease because nasopharyngeal viral loads may reach undetectable levels.15 , 40 Although RT-PCRs can detect SARS-CoV 2 RNA in blood, this usually occurs in the setting of clinically severe disease14 and thus unlikely to be useful in assessing asymptomatic patients presenting for endoscopy.
Both iNAAT and CRISPR can also be performed on nasopharyngeal swabs and are highly specific (>95%) to SARS-CoV-2.41, 42, 43, 44 Unlike RT-PCR, iNAAT does not require multiple heating cycles and therefore can provide results within 15 minutes with a sensitivity of >95%.45 An FDA-approved iNAAT POC test is readily available and has already been procured by clinics and hospitals across the United States. CRISPR relies on the Cas13a protein to form a complex with amplified RNA product, which then cleaves a fluorophore-quencher probe to produce a fluorescent light, signaling disease. Although it can yield a result in 60 minutes with a sensitivity of 90% and specificity of 100%, there is currently no POC test available.43 , 44 , 46, 47, 48
Enzyme-linked immunosorbent assays are inadequate for detecting early infection, with a sensitivity of 38.3% at day 7 of the disease.49 Contrastingly, lateral flow immunoassays combine IgG and IgM within a single assay to yield a result within 15 minutes, with a sensitivity of 88.7% and specificity of 90.6%.50 A recent FDA-approved POC test only requires 2 drops of blood via fingerprick.51
Proposal for the Return to Routine Endoscopy
Important components
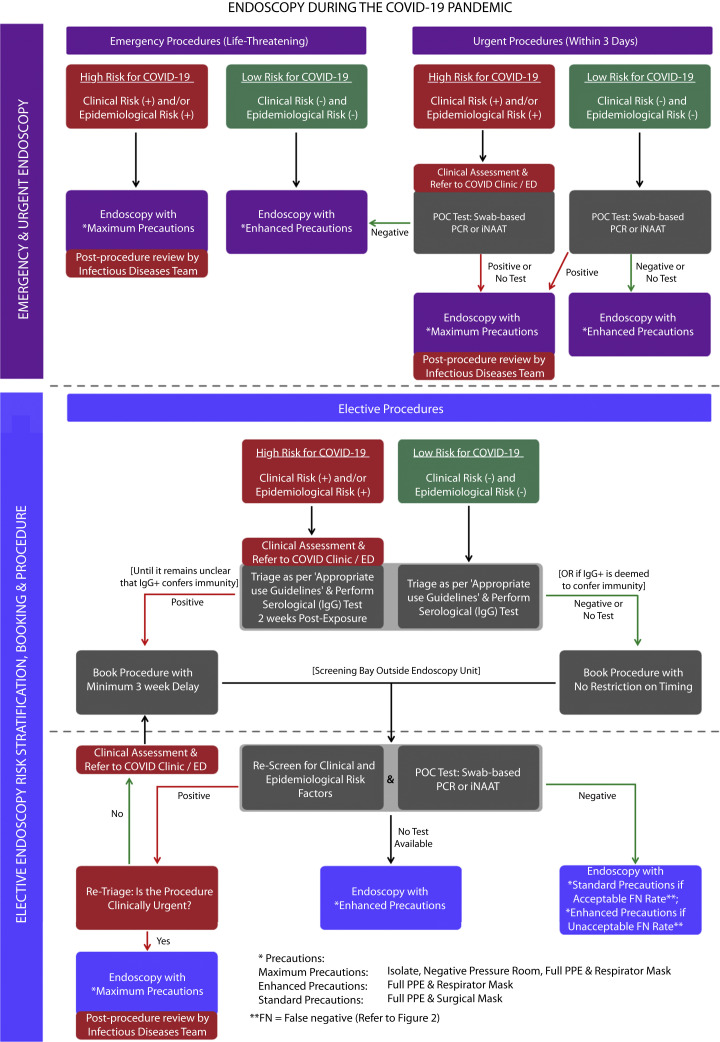
Many units are to be commended for their work on COVID-19 risk stratification for patients presenting for endoscopy. Although Repici et al52 prudently stratified risk based on clinical and epidemiologic factors, there is potential for asymptomatic individuals to be overlooked. Recently, Han et al53 introduced a laboratory-based RT-PCR test to assess risk; however, this was time-consuming, and it is unclear if it assisted in rationalizing the use of PPE. Interestingly, Lui et al54 stratified risk based on the proposed endoscopic procedure but recommend use of respirator masks in all cases. We believe that a safe return to routine endoscopy is possible by using a strict protocol that stratifies risk by combining an assessment of epidemiologic and clinical risk factors with the use of highly sensitive rapid POC tests (Fig. 1 ).
Figure 1.
Algorithm for a return to endoscopy during the COVID-19 pandemic. COVID-19, Coronavirus disease 2019; FN, false negative; iNAAT, isothermal nucleic acid amplification; PPE, personal protective equipment; POC, point of care; PCR, polymerase chain reaction.
Epidemiologic and clinical factors
Clinicians should establish the pretest probability of COVID-19 in asymptomatic patients based on epidemiologic and clinical risk factors. Although dependent on relevant locoregional factors, standard questioning can include the following:
-
1.
Epidemiologic: Have you had close contact with a suspected or confirmed case of COVID-19? Have you traveled overseas or on a cruise ship in the past 14 days? Have you been in contact with anyone who has traveled overseas in the past 14 days?
-
2.
Clinical: In the last 14 days have you had fever (>37.5°C), cough, sore throat, or respiratory problems?
POC testing
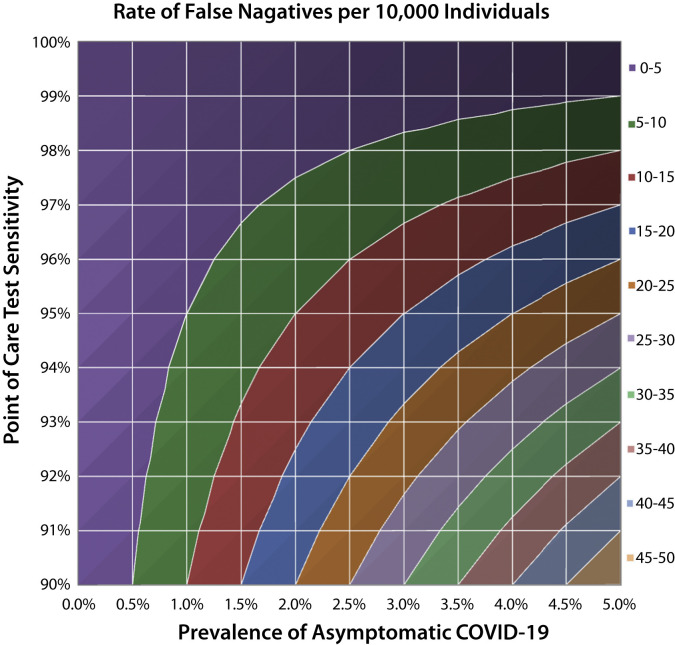
Population-screening data from Iceland suggests that up to 43% of COVID-19 patients are asymptomatic at the time of diagnosis.55 Hence, with over 500,000 cases in the United States and a current symptomatic prevalence of approximately .15%, the rate of asymptomatic disease can be estimated as .11% (or 370,000 persons). This information can be assessed against the sensitivity of available POC tests to determine the number of false-negative results expected per 10,000 asymptomatic individuals tested (Fig. 2 ). For example, in an endoscopy unit that serves 10,000 patients annually in the United States, a POC test with 95% sensitivity would result in only 1 false-negative result. Comparatively, in a higher-prevalence population of 2%, there would be 10 false-negative results per 10,000 patients. This, of course, would evolve with changes in disease prevalence and test sensitivity.
Figure 2.
The rate of false negatives per 10,000 asymptomatic individuals as determined by test sensitivity and the prevalence of asymptomatic COVID-19. COVID-19, Coronavirus disease 2019.
Precautionary measures
If each endoscopy unit establishes a false-negative threshold deemed acceptable to them, a 3-tiered system for the precautionary measures required during endoscopy can be used (Table 1 ). For example, in a low-risk patient with a negative POC result, if the false-negative threshold is satisfied, then standard precautions may be used instead of enhanced precautions. The key difference here is the use of a surgical mask over a respirator mask, which may help preserve valuable PPE. Because transmission of the small SARS-CoV-2 virus (3 μm) is through larger respiratory droplets, both masks may offer adequate protection. This is reflected in a previous study on SARS-CoV, which revealed marginally better protection by respirator masks (odds ratio, .86).56 However, studies on SARS-CoV-2 are lacking.
Table 1.
Three-tiered system for the precautionary measures required during endoscopy
| Maximum precautions | Enhanced precautions | Standard precautions | |
|---|---|---|---|
| Safety measures | |||
| Isolate patient | Yes | No | No |
| Negative pressure room | Yes | No | No |
| Head and shoe coverings | Yes | Yes | No |
| Full-sleeved gown | Yes | Yes | Yes |
| Face shield or goggles | Yes | Yes | Yes |
| Gloves | Yes | Yes | Yes |
| Mask | Respirator (N95+) | Respirator (N95+) | Surgical mask |
| Timing of procedure | |||
| Emergency endoscopy | High clinical or epidemiologic risk | Low clinical and epidemiologic risk | N/A |
| Urgent endoscopy | High clinical or epidemiologic risk, with a positive POCT (or no test) Or Low clinical and epidemiologic risk, with a positive POCT |
Low clinical and epidemiologic risk, with a negative POCT (or no test) Or High clinical or epidemiologic risk, with a negative POCT |
N/A |
| Elective endoscopy | High clinical/epidemiologic risk or positive POCT and procedure retriaged as urgent | Low clinical and epidemiologic risk with a negative POCT and unacceptable FN threshold | Low clinical and epidemiologic risk with a negative POCT and acceptable FN threshold |
N/A, not applicable; FN, false negative; POCT, point-of-care test.
Emergency and urgent endoscopy
By the very nature of emergency endoscopy, for life-threatening procedures, POC testing should not be performed. The decision regarding the level of precautionary measures required should be determined through a clinical and epidemiologic risk assessment. However, for urgent procedures, which we defined as requiring endoscopy within 3 days, POC testing (RT-PCR or iNAAT) offers the ability to further stratify risk (Table 1). For example, a patient with a low pretest probability and positive POC result will require maximum precautions, whereas a patient with a high pretest probability and negative POC result can proceed with enhanced precautions. To minimize unnecessary contact, all patients requiring maximum precautions should be kept isolated outside of the endoscopy unit and taken straight into their allocated procedure room, once endoscopy staff is ready. After the procedure, they should be moved into a dedicated COVID-19 recovery bay.
Elective endoscopy
Booking cases
For the safe and gradual reintroduction of elective endoscopy, cases should comply with guidelines for the appropriate use of endoscopy and be triaged on their clinical merits.57 Patients with a low pretest probability should proceed to a serologic IgG test to assess for previous COVID-19 exposure, whereas higher-risk patients should be isolated for further clinical assessment and only undergo serologic testing once cleared. Because viral shedding and viral RNA detection can occur up to 3 weeks after seroconversion, a positive serologic result requires deferral of endoscopy for this time period.15 In the future, with greater clarity of a patient’s immune status, this delay may no longer be required. Although we acknowledge that false-positive results may delay endoscopy by up to 3 weeks, the alternative would be no endoscopy.
Admission and discharge
On the day of endoscopy, patients should present to an independent screening bay located outside of the endoscopy unit. On arrival, a dedicated staff member using enhanced precautions should reassess patient risk factors and perform a POC test (RT-PCR or iNAAT) to rule out acute infection. Patients satisfying all criteria would be allowed to enter the unit, with accompanying individuals remaining outside. Those with newly identified risk factors or a positive result would be isolated and retriaged. If still deemed necessary to proceed, maximum precautions would be required. If deemed nonurgent, the procedure would be deferred until the patient is well and exposure to the risk factor has passed. On discharge, patients would be met by their accompanying individual at a separate exit to the unit. Follow-up should be organized with the referring physician by telehealth consultation if possible.
Intraprocedural safety
To reduce the spread of COVID-19, staff should use correct hand hygiene58 and follow local recommendations for the donning and doffing of PPE. In critical shortages, the reuse of respirator masks is possible after decontamination with ultraviolet light, hydrogen peroxide vapor, or moist heat.59, 60, 61, 62, 63, 64, 65 Although the effect of these methods on SARS-CoV-2 is yet to be established, prior studies demonstrate effective inactivation of coronaviruses.59, 60, 61, 62, 63, 64, 65 To further conserve supplies, it is possible to conduct the donning of a respirator mask up to 5 times before fit factors consistently drop to unsafe levels.4 , 66 In such cases, great care should be exercised to avoid accidental contact with the front of the mask. Anecdotally, the use of a surgical mask over a respirator mask may help preserve it for longer, although further studies are required.67 However, these measures are unlikely to be required because the FDA has taken steps to increase procurement of PPE by providing clear guidelines for importers and manufacturers to follow.68
Staffing considerations
Social distancing should be practiced by staff, with work conducted using designated chairs, computers, and phones. As a contingency measure, endoscopy staff should be split into 2 teams working nonconcurrent shifts. Each endoscopy department should have a detailed plan addressing the systematic cleaning of all surfaces in the procedure room, including chemical agents required to inactivate coronaviruses.69 , 70 If it is deemed that seroconversion confers immunity to SARS-CoV-2, then HCWs within the endoscopy unit should also be tested for COVID-19 at set intervals with serology-based tests. This may enable seroconverted staff to perform endoscopy in high-risk patients or those with confirmed COVID-19. However, at present, the duration and protective antibody thresholds after SARS-CoV-2 exposure remain unclear. Furthermore, if it is deemed that fecal–oral transmission is not viable, then colonoscopies in patients with a negative POC test result may be performed with standard precautions, irrespective of the false-negative threshold.
Conclusions
Amid the COVID-19 pandemic, to conserve resources and reduce the risk of transmission, jurisdictions across the world have suspended elective endoscopy. With mitigation measures projected to increase the duration of the pandemic, elective endoscopy may be delayed for an unsustainable period of time. Our algorithm proposes a return to elective endoscopy in a safe and timely manner through a multifaceted approach to risk stratification. This requires an assessment of epidemiologic and clinical risk factors, rapid POC testing, and evaluation of a predefined false-negative threshold based on the prevalence of asymptomatic disease in the community and the sensitivity of the POC test used. This maximizes safety for patients and HCWs, while rationalizing the use of valuable resources such as PPE. Ultimately, herd immunity or vaccination may be required to reduce risk of community transmission and enable endoscopy units to reach full capacity once again.
Acknowledgment
We thank Ms Stephanie Todd for her valuable comments.
Footnotes
DISCLOSURE: All authors disclosed no financial relationships.
References
- 1.World Health Organization . WHO; Geneva, Switzerland: Epub 2020 May 4. Coronavirus disease (COVID-2019) situation report 84. [Google Scholar]
- 2.World Health Organization . WHO; Geneva, Switzerland: 2020. Coronavirus disease (COVID-2019) situation report 51. [Google Scholar]
- 3.MacIntyre C.R., Heslop D.J. Public health, health systems and palliation planning for COVID-19 on an exponential timeline. Med J Austral. 2020 doi: 10.5694/mja2.50592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . WHO; Geneva, Switzerland: 2020. Report of the WHO–China joint mission on coronavirus disease 2019 (COVID-19) [Google Scholar]
- 5.Remuzzi A., Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020;395:1225–1228. doi: 10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiu P.W.Y., Ng S.C., Inoue H. Practice of endoscopy during COVID-19 pandemic: position statements of the Asian Pacific Society for Digestive Endoscopy (APSDE-COVID statements) Gut. 2020;69:991–996. doi: 10.1136/gutjnl-2020-321185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.British Society of Gastroenterology . BSG; London, UK: 2020. Endoscopy activity and COVID-19: BSG and JAG guidance. [Google Scholar]
- 8.Gastroenterological Society of Australia . GESA; Melbourne, Australia: 2020. Guide for triage of endoscopic procedures during the COVID-19 pandemic. [Google Scholar]
- 9.American College of Gastroenterology Gastroenterology professional society guidance on endoscopic procedures during the COVID-19 pandemic 2020. https://gi.org/2020/04/01/joint-gi-society-message-on-endoscopy-during-covid-19/ Available at:
- 10.Zou L., Ruan F., Huang M. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tran K., Cimon K., Severn M. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PloS One. 2012;7:e35797. doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan Y., Zhang D., Yang P. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020;20:411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ong J., Young B.E., Ong S. COVID-19 in gastroenterology: a clinical perspective. Gut. 2020;69:1144–1145. doi: 10.1136/gutjnl-2020-321051. [DOI] [PubMed] [Google Scholar]
- 14.Wang W., Xu Y., Gao R. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. Epub 2020 Mar 11 doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wölfel R., Corman V.M., Guggemos W. Virological assessment of hospitalized patients with COVID-2019. https://www.bsg.org.uk/covid-19-advice/endoscopy-activity-and-covid-19-bsg-and-jag-guidance/ Available at: Accessed April 10, 2020. [DOI] [PubMed]
- 16.Xiao F., Tang M., Zheng X. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holshue M.L., DeBolt C., Lindquist S. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ling Y., Xu S.B., Lin Y.X. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J (Engl) 2020;133:1039–1043. doi: 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan L., Mu M., Ren H. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;20 doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnston E.R., Habib-Bein N., Dueker J.M. Risk of bacterial exposure to the endoscopist’s face during endoscopy. Gastrointest Endosc. 2019;89:818–824. doi: 10.1016/j.gie.2018.10.034. [DOI] [PubMed] [Google Scholar]
- 21.Ong S.W.X., Tan Y.K., Chia P.Y. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323:1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Doremalen N., Bushmaker T., Morris D.H. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sultan S., Lim J.K., Altayar O. AGA Institute rapid recommendations for gastrointestinal procedures during the COVID-19 pandemic. Gastroenterology. Epub 2020 Apr 1 doi: 10.1053/j.gastro.2020.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atkeson A. National Bureau of Economic Research; 2020. What will be the economic impact of COVID-19 in the US? Rough estimates of disease scenarios. [Google Scholar]
- 25.Fox G.J., Trauer J.M., McBryde E. Modelling the impact of COVID-19 upon intensive care services in New South Wales. Med J Austral. 2020;212:1. doi: 10.5694/mja2.50606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson R.M., Heesterbeek H., Klinkenberg D. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet. 2020;395:931–934. doi: 10.1016/S0140-6736(20)30567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peery A.F., Crockett S.D., Murphy C.C. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2018. Gastroenterology. 2019;156:254–272. doi: 10.1053/j.gastro.2018.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Australian Institute of Health and Welfare . Australian Government; Canberra: 2019. Admitted patient care 2017-2018: Australian hospital, statistics. [Google Scholar]
- 29.Australian Institute of Health and Welfare . Australian Government; Canberra: 2019. Disease expenditure study. [Google Scholar]
- 30.Pita-Fernández S., González-Sáez L., López-Calviño B. Effect of diagnostic delay on survival in patients with colorectal cancer: a retrospective cohort study. BMC Cancer. 2016;16:664. doi: 10.1186/s12885-016-2717-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scaglione S., Kliethermes S., Cao G. The epidemiology of cirrhosis in the United States. J Clin Gastroenterol. 2015;49:690–696. doi: 10.1097/MCG.0000000000000208. [DOI] [PubMed] [Google Scholar]
- 32.Lay C., Tsai Y., Teg C.-Y. Endoscopic variceal ligation in prophylaxis of first variceal bleeding in cirrhotic patients with high-risk esophageal varices. Hepatology. 1997;25:1346–1350. doi: 10.1002/hep.510250608. [DOI] [PubMed] [Google Scholar]
- 33.North Italian Endoscopic Club for the Study Treatment of Esophageal Varices Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. N Engl J Med. 1988;319:983–989. doi: 10.1056/NEJM198810133191505. [DOI] [PubMed] [Google Scholar]
- 34.Brocchi E., Caletti G., Brambilla G. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. N Engl J Med. 1988;319:983–989. doi: 10.1056/NEJM198810133191505. [DOI] [PubMed] [Google Scholar]
- 35.Bao L., Deng W., Gao H. Reinfection could not occur in SARS-CoV-2 infected rhesus macaques. https://www.biorxiv.org/content/10.1101/2020.03.13.990226v1 Available at: Accessed April 10, 2020.
- 36.Shen C., Wang Z., Zhao F. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. Epub 2020 Mar 27 doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lan L., Xu D., Ye G. Positive RT-PCR test results in patients recovered from COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo L., Ren L., Yang S. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cepheid . Cepheid; 2020. Xpert® Xpress SARS-CoV-2: fact sheet for healthcare providers. [Google Scholar]
- 41.Jiang M., Pan W., Arastehfar A. Development and validation of a rapid single-step reverse transcriptase loop-mediated isothermal amplification (RT-LAMP) system potentially to be used for reliable and high-throughput screening of COVID-19. https://www.medrxiv.org/content/10.1101/2020.03.15.20036376v2 Available at: Accessed April 10, 2020. [DOI] [PMC free article] [PubMed]
- 42.Zhu X., Wang X., Han L. Reverse transcription loop-mediated isothermal amplification combined with nanoparticles-based biosensor for diagnosis of COVID-19. https://www.medrxiv.org/content/10.1101/2020.03.17.20037796v1 Available at: Accessed April 10, 2020. [DOI] [PMC free article] [PubMed]
- 43.Hou T., Zeng W., Yang M. Development and evaluation of a CRISPR-based diagnostic for 2019-novel coronavirus. https://www.medrxiv.org/content/10.1101/2020.02.22.20025460v2 Available at: Accessed April 10, 2020.
- 44.Broughton J.P., Deng X., Yu G. Rapid detection of 2019 novel coronavirus SARS-CoV-2 using a CRISPR-based DETECTR lateral flow assay. https://www.medrxiv.org/content/10.1101/2020.03.06.20032334v2 Available at: Accessed April 10, 2020.
- 45.Abbott ID NOW™ COVID-19 2020. https://www.alere.com/en/home/product-details/id-now-covid-19.html Available at:
- 46.Kellner M.J., Koob J.G., Gootenberg J.S. SHERLOCK: nucleic acid detection with CRISPR nucleases. Nat Protocols. 2019;14:2986–3012. doi: 10.1038/s41596-019-0210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang F., Abudayyeh O.O., Jonathan S.G. Broad Institute; 2020. A protocol for detection of COVID-19 using CRISPR diagnostics. [Google Scholar]
- 48.O'Connell M.R. Molecular mechanisms of RNA Targeting by Cas13-containing type VI CRISPR–Cas systems. J Mol Biol. 2019;431:66–87. doi: 10.1016/j.jmb.2018.06.029. [DOI] [PubMed] [Google Scholar]
- 49.Zhao J., Yuan Q., Wang H. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.BioMedomics . BioMedomics; 2020. Rapid IgM-IgG combined antibody test for coronavirus. [Google Scholar]
- 51.Li Z., Yi Y., Luo X. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol. 2020 doi: 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Repici A., Maselli R., Colombo M. Coronavirus (COVID-19) outbreak: what the department of endoscopy should know. Gastrointest Endosc. 2020 doi: 10.1016/j.gie.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han J., Wang Y., Zhu L. Preventing the spread of COVID-19 in digestive endoscopy during the resuming period: meticulous execution of screening procedures. Gastrointest Endosc. 2020 doi: 10.1016/j.gie.2020.03.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lui R.N., Wong S.H., Sánchez-Luna S.A. Overview of guidance for endoscopy during the coronavirus disease 2019 (COVID-19) pandemic. J Gastroenterol Hepatol. 2020 doi: 10.1111/jgh.15053. [DOI] [PubMed] [Google Scholar]
- 55.Gudbjartsson D.F., Helgason A., Jonsson H. Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med. 2020 doi: 10.1056/NEJMoa2006100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Offeddu V., Yung C.F., Low M.S.F. Effectiveness of masks and respirators against respiratory infections in healthcare workers: a systematic review and meta-analysis. Clin Infect Dis. 2017;65:1934–1942. doi: 10.1093/cid/cix681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Early D.S., Ben-Menachem T., Decker G.A. Appropriate use of GI endoscopy. Gastrointest Endosc. 2012;75:1127–1131. doi: 10.1016/j.gie.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 58.Longtin Y., Sax H., Allegranzi B. Hand hygiene. N Engl J Med. 2011;364:e24. doi: 10.1056/NEJMvcm0903599. [DOI] [PubMed] [Google Scholar]
- 59.Lindsley W.G., Martin S.B., Jr., Thewlis R.E. Effects of ultraviolet germicidal irradiation (UVGI) on N95 respirator filtration performance and structural integrity. J Occupat Environ Hyg. 2015;12:509–517. doi: 10.1080/15459624.2015.1018518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heimbuch B.K., Wallace W.H., Kinney K. A pandemic influenza preparedness study: use of energetic methods to decontaminate filtering facepiece respirators contaminated with H1N1 aerosols and droplets. Am J Infect Control. 2011;39:e1–e9. doi: 10.1016/j.ajic.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 61.Mills D., Harnish D.A., Lawrence C. Ultraviolet germicidal irradiation of influenza-contaminated N95 filtering facepiece respirators. Am J Infect Control. 2018;46:e49–e55. doi: 10.1016/j.ajic.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holmdahl T., Walder M., Uzcátegui N. Hydrogen peroxide vapor decontamination in a patient room using feline calicivirus and murine norovirus as surrogate markers for human norovirus. Infect Control Hosp Epidemiol. 2016;37:561–566. doi: 10.1017/ice.2016.15. [DOI] [PubMed] [Google Scholar]
- 63.Rudnick S.N., McDevitt J.J., First M.W. Inactivating influenza viruses on surfaces using hydrogen peroxide or triethylene glycol at low vapor concentrations. Am J Infect Control. 2009;37:813–819. doi: 10.1016/j.ajic.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kariwa H., Fujii N., Takashima I. Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions, and chemical reagents. Jpn J Vet Res. 2004;52:105–112. [PubMed] [Google Scholar]
- 65.Yunoki M., Urayama T., Yamamoto I. Heat sensitivity of a SARS-associated coronavirus introduced into plasma products. Vox Sang. 2004;87:302–303. doi: 10.1111/j.1423-0410.2004.00577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Centers for Disease Control and Prevention CDC recommended guidance for extended use and limited reuse of N95 filtering facepiece respirators in healthcare settings 2020. https://www.cdc.gov/niosh/topics/hcwcontrols/recommendedguidanceextuse.html Available at:
- 67.Roberge R.J. Effect of surgical masks worn concurrently over N95 filtering facepiece respirators: extended service life versus increased user burden. J Public Health Manage Pract. 2008;14:E19–E26. doi: 10.1097/01.PHH.0000311904.41691.fd. [DOI] [PubMed] [Google Scholar]
- 68.Hahn S.M. FDA; United States: 2020. Coronavirus (COVID-19) update: FDA takes action to increase U.S. supplies through instructions for PPE and device manufacturers. [Google Scholar]
- 69.Calderwood A.H., Day L.W., Muthusamy V.R. ASGE guideline for infection control during GI endoscopy. Gastrointest Endosc. 2018;87:1167–1179. doi: 10.1016/j.gie.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 70.Kampf G., Todt D., Pfaender S. Persistence of coronaviruses on inanimate surfaces and its inactivation with biocidal agents. J Hosp Infect. 2020 doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]