Abstract
Background
Despite 2.5 million infections and 169,000 deaths worldwide (as of April 20, 2020), no maternal deaths and only a few pregnant women afflicted with severe respiratory morbidity have been reported to be related to COVID-19 disease. Given the disproportionate burden of severe and fatal respiratory disease previously documented among pregnant women following other coronavirus-related outbreaks (SARS-CoV in 2003 and MERS-CoV in 2012) and influenza pandemics over the last century, the absence of reported maternal morbidity and mortality with COVID-19 disease is unexpected.
Objective
To describe maternal and perinatal outcomes and death in a case series of pregnant women with COVID-19 disease.
Study Design
We describe here a multiinstitution adjudicated case series from Iran that includes 9 pregnant women diagnosed with severe COVID-19 disease in their second or third trimester. All 9 pregnant women received a diagnosis of SARS-CoV-2 infection by reverse transcription polymerase chain reaction nucleic acid testing. Outcomes of these women were compared with their familial/household members with contact to the affected patient on or after their symptom onset. All data were reported at death or after a minimum of 14 days from date of admission with COVID-19 disease.
Results
Among 9 pregnant women with severe COVID-19 disease, at the time of reporting, 7 of 9 died, 1 of 9 remains critically ill and ventilator dependent, and 1 of 9 recovered after prolonged hospitalization. We obtained self-verified familial/household cohort data in all 9 cases, and in each and every instance, maternal outcomes were more severe compared with outcomes of other high- and low-risk familial/household members (n=33 members for comparison).
Conclusion
We report herein maternal deaths owing to COVID-19 disease. Until rigorously collected surveillance data emerge, it is prudent to be aware of the potential for maternal death among pregnant women diagnosed as having COVID-19 disease in their second or third trimester.
Key words: coronavirus disease in pregnancy, COVID-19, lower respiratory infections in pregnancy, maternal death, maternal mortality, maternal respiratory morbidity, pregnancy, respiratory failure with COVID-19, SARS CoV-2 virus
Over the last several decades, it has been shown that emerging novel strains of influenza and coronaviruses that cause severe respiratory disease1, 2, 3, 4 typically disproportionately affect pregnant women, in part owing to adaptive immunology and cardiopulmonary physiology of pregnancy.5, 6, 7 During 3 of the major influenza pandemics of the last 100 years (1918, 1957–1958, and 2009), pregnant women in their second or third trimester were considerably more likely to be hospitalized or die compared with the general population.3 , 8 For example, in the 1918 H1N1 pandemic, the case fatality proportion among pregnant women was 27%.3 In the most recent influenza pandemic (2009 H1N1), pregnant women in the United States accounted for 6.4% of all hospitalizations and 4.3%–5.7% of all deaths even though they generally represent only 1% of the population.6 , 8 In the severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) outbreak, the general population mortality rate was 10.5%, whereas that of pregnant women approximated 25%, with 33% requiring mechanical ventilation.1 The risks of morbidity with severe lower respiratory tract infections are not limited to maternal outcomes because there is a known increased occurrence of preterm birth, fetal demise, and delivery of low birthweight infants with nearly all maternal severe lower respiratory tract viral infections.1, 2, 3, 4 , 8
AJOG at a Glance.
Why was this study conducted?
Given the disproportionate burden of severe and mortal respiratory disease previously documented among pregnant women following other related coronavirus outbreaks (severe acute respiratory syndrome coronavirus in 2003 and Middle East respiratory syndrome coronavirus) and influenza pandemics over the last century, the absence of reported maternal morbidity and mortality with coronavirus disease 2019 (COVID-19) is unexpected. The purpose of this study was to describe maternal and perinatal outcomes and death in a case series of pregnant women with severe COVID-19 disease in an adjudicated case series.
Key findings
Among 9 pregnant women with severe COVID-19 disease, at the time of reporting 7 of 9 died, 1 of 9 remains critically ill and ventilator dependent, and 1 of 9 recovered after prolonged hospitalization.
What does this add to what is known?
Our case series is in contrast to prior reports suggesting no known mortality among pregnant women infected by severe acute respiratory syndrome coronavirus 2. Whether the COVID-19 disease maternal case fatality rate or maternal morbidity estimates will ultimately be the same, less, or greater than that of other populations is as yet unknown but will require extensive population-based surveillance data to accurately determine. However, the fatal cases reported herein demonstrate it is not zero, and should inspire caution against complacency and guide restraint in rushing estimates of relative or attributable risk with pregnancy.
However, based on initial reports largely from China, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) does not appear to follow these historical patterns of worsened disease risk in pregnancy. We identified 16 reports of SARS-CoV-2 infection or coronavirus disease 2019 (COVID-19) in pregnancy or in neonates (as of April 8, 2020).10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 9 Unexpectedly, although these case reports or retrospective case series include some information on a total of 154 pregnant women and 118 live-born neonates (1 set of twins, 1 second trimester termination, 1 fetal death, 34 undelivered), we identified only a few gravidae reported as having experienced morbidity requiring respiratory support and critical care.10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 9 For example, in a detailed case series of 9 gravida from Wuhan, China, all delivering at 36 to 40 weeks’ gestation with symptomatic COVID-19 viral pneumonia, Chen and colleagues10 reported that none required mechanical ventilation or respiratory support. Of note, however, the upper confidence interval limit for zero mortality in a series of 9 women would be 33%. The sole reported severe cardiopulmonary maternal morbidity reported from China was experienced by a gravida admitted at 34 weeks’ gestation with severe COVID-19 pneumonia.12 Subsequent to this patient’s cesarean delivery for a stillborn fetus while in septic shock, her cardiopulmonary status acutely deteriorated with multiple organ system dysfunction requiring extracorporeal membrane oxygenation (ECMO). At the time her care was reported, she remained on ECMO heart-lung bypass.12 In a recently published 2-week surveillance cohort from paired clinical affiliate institutions in New York City, Breslin and colleagues18 reported that among 43 confirmed cases of SARS-CoV-2 infection during pregnancy, the estimated rate of severe maternal disease approximated that of the nonpregnant population at 9.3%. Interestingly, 2 gravidae who progressed to critical disease (4.3%) were among the 14 of 43 (32.6%) women who were initially asymptomatic, and both required intensive care unit (ICU) admission in the postpartum period.18
SARS-CoV-1 (the viral pathogen of the 2003 SARS epidemic) and SARS-CoV-2 are both enveloped virions containing 1 positive-strand RNA genome belonging to the Betacoronavirus genus of the Coronaviridae family. SARS-CoV-2 uses the same angiotensin-converting enzyme 2 (ACE2) as its putative cell entry host receptor as SARS-CoV-1 and bears 80%–85% nucleotide homology to SARS-CoV-1.24 , 25 Although both SARS-CoV-1 and SARS-CoV-2 bind to ACE2 through the viral surface spike glycoprotein (S protein, 76% protein identity), there are some suggested distinctions regarding the role of specific serine and cysteine proteases in cleavage of the S protein in priming for enhanced cell entry.24, 25, 26 Specifically, while the S protein of both SARS-CoV-1 and SARS-CoV-2 is cleaved by the same transmembrane protease serine 2 (TMPRSS2) to facilitate efficiency of entry and viral replication, there is emerging evidence that SARS-CoV-2 co-opts and recruits additional host proteases for transmissibility.26 , 27 Nonetheless, given the overall phylogenetic and functional similarities between the viruses, the suggestion of zero pregnant fatalities is unexpected and further inconsistent with data documenting severe disease and death among similarly aged adults who are not pregnant and of low risk.28
Although accurate case fatality rates and attributable and relative risk of maternal mortality following SARS-CoV-2 infection will be reported in the future, 1 of the critical immediate questions faced by providers caring for pregnant women in the midst of the current pandemic is straightforward: are pregnant women at risk of death with COVID-19? We detail herein 7 maternal deaths in a case series of 9 women with severe COVID-19 and compare these deaths to self-verified outcomes among their familial/household members.
Methods
Study design
The intent of this retrospective case series was to document maternal death and describe maternal, fetal, neonatal, and familial self-reported characteristics among 9 patients known to have experienced severe maternal cardiopulmonary morbidity or mortality after admission to any 1 of 7 level III maternity hospitals in Iran over a 30-day period of time (mid-February to mid-March, 2020; precise dates of admission gated to protect patient identity).
This case series and its detailed reporting were approved by the Ethics Committee of Tehran University of Medical Sciences (IRB IR.TUMS.VCR.1398.1082; IRB PI S.H.) and Baylor College of Medicine (IRB H-47407); a data use agreement (DUA) between Baylor College of Medicine and Tehran University of Medical Sciences was executed for the purpose of this reporting. Subject consent was waived by both review boards, and all familial data was voluntarily self-reported and no familial medical records were reviewed. Additional protection for participants beyond no disclosure of exact dates of admission or death included gating maternal age in 5-year increments and using controlled-access encrypted electronic records for data transfer of primary source data, including digital images of patients’ medical records. Index case subjects were assigned as case 1 to 9 for the purposes of publication and communication of nonidentifying information and reflect neither the order of their care nor presentation of first symptoms. The hospital in which each patient received her care is similarly not reported in an effort to protect subject identity.
Cases were not selected by any form of systematic surveillance but rather arose from a voluntary reporting of maternal cases with known morbidity or mortality attributable to COVID-19. Our definition of severe disease was comparable with that of others (see further definitions below).28 Severe cardiopulmonary disease was defined as need for ventilator support and/or cardiopulmonary collapse. With IRB approval from the Ethics Committee of Tehran University of Medical Sciences, starting in February 2020, the Iranian Perinatology Society generated a secure reporting structure to share cases, outcomes, and management of pregnant women with severe morbidity or death. For the purposes of this case series, all 9 known cases with severe COVID-19 over a 30-day span of time at any of the 7 hospitals or centers are reported herein. Severe disease was classified by provision of having met inclusion criteria of (1) severe morbidity (dyspnea, blood oxygen saturation [SaO2] ≤93% on room air, or partial pressure of arterial oxygen to fraction of inspired oxygen <300), (2) an available death certificate, and (3) at least 1 positive SARS-CoV-2 nucleic acid testing (NAT) result. Centers were not selected for participation a priori, and all reporting of cases was voluntary. Ultimately, all 9 cases arose from 7 centers that provide high-level maternity care in Iran and are staffed by perinatology (maternal-fetal medicine) consultant specialists and critical care specialists. These 7 centers included Shariati Hospital and Imam Khomeini Hospital at Tehran University of Medical Sciences, Tehran, Iran; Shohada Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran; Baqiyatallah Hospital, Baqiyatallah University of Medical Sciences, Tehran, Iran; Mousavi Hospital, Zanjan University of Medical Sciences, Zanjan, Iran; Kamkar-Arabnia Hospital, Qom University of Medical Science, Qom, Iran; and Alzahra Hospital, Guilan University of Medical Sciences, Rasht, Iran. Of these 9 known cases of severe COVID-19 from this 30-day time period, 7 resulted in maternal death (as of April 20, 2020).
General case management
All pregnant women in this series had a positive result for SARS-CoV-2 by reverse transcription polymerase chain reaction (RT-PCR) NAT on nasopharyngeal, with or without oropharyngeal and sputum specimens; some subjects were tested multiple times. All collected specimens were tested for SARS-CoV-2 using TIB-Molbiol (Germany) and/or Sansure Biotech 2019-nCov (China) reagents and protocols, complying with the World Health Organization’s (WHO) guidelines for use. According to the COVID-19 national guidelines issued by Iran’s National Ministry of Health and Medical Education on March 8, 2020, a 3-drug regimen for all COVID-19 pneumonia was recommended and allowed extension for up to 14 days. This included oral administration of 75-mg oseltamivir every 12 hours for 5 days; 400-mg hydroxychloroquine sulfate daily, or 1000-mg chloroquine sulfate tablet as a single dose, and 400-100-mg lopinavir/ritonavir every 12 hours for 5 days. Changing to a 4-drug regimen was recommended with the presence of any of the following signs indicative of severe disease: loss of consciousness, tachypnea (respiratory rate >24, hypotension [blood pressure <90/60 mm Hg], multilobar lung infiltration with consolidation by chest imaging [computed tomography [CT] or radiograph], or hypoxemia [SaO2 <90%]). The 4-drug regimen added 1200-mg ribavirin PO twice daily for 5 days. Antibiotic administration (choice, dose, and duration) was deferred at the attending physician’s best clinical judgment, and corticosteroids were not recommended. All but 1 patient in this series was first admitted before March 8. In addition, all subjects received either enoxaparin (40 mg subcutaneous, daily) or heparin (5000 units subcutaneous, twice daily) for thromboprophylaxis.
Case ascertainment and adjudication of outcomes
After IRB and DUA approval, digital and electronic images of all available patient information from the time of admission to discharge, death, or reporting was securely sent to a single investigator (A.A.S.) and encrypted. All records were converted to paper form, subsequently stripped of any identifiers, and assigned a unique study code. A systematic process of independent interpretation/translation to distill essential elements of clinical care and outcomes from Farsi to English was undertaken by 2 bilingual investigators boarded in obstetrics and gynecology (A.A.S., S.E.A.); this included conversion of dates from the Solar Hijri calendar (Iranian chronology) to the Gregorian calendar (Western chronology). The deidentified and translated data were then independently adjudicated by 2 boarded and practicing maternal-fetal medicine clinicians (K.M.A., A.A.S.). A series of clarifying questions generated by any of the 3 case interpreters/adjudicators (K.M.A., A.A.S., S.E.A.) were communicated to the coauthors in Farsi through 3 means: video (Skype/WhatsApp/FaceTime), e-mail, and/or direct verbal communication by phone. Once all coauthors and the primary adjudicators reached consensus of all known and reportable outcomes, the case was considered fully adjudicated.
Results
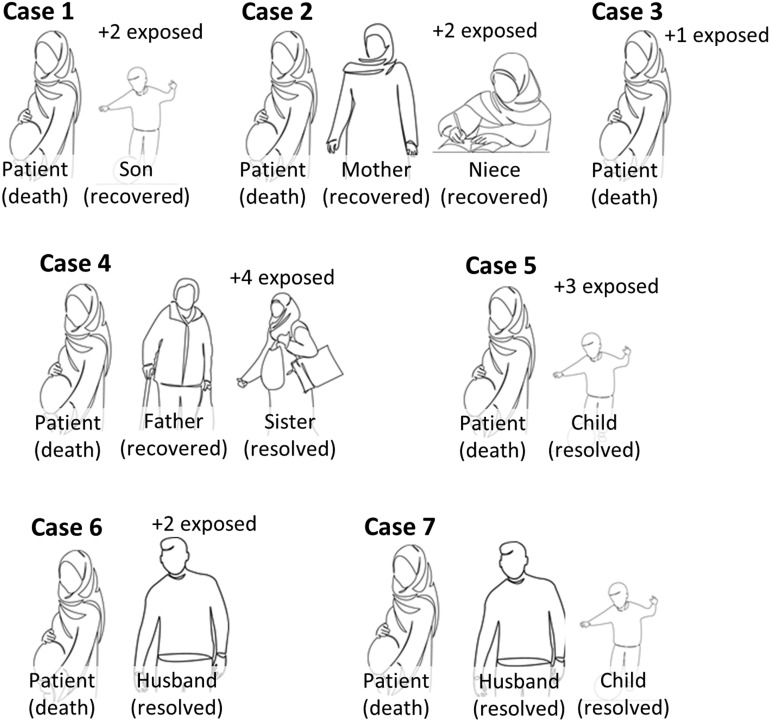
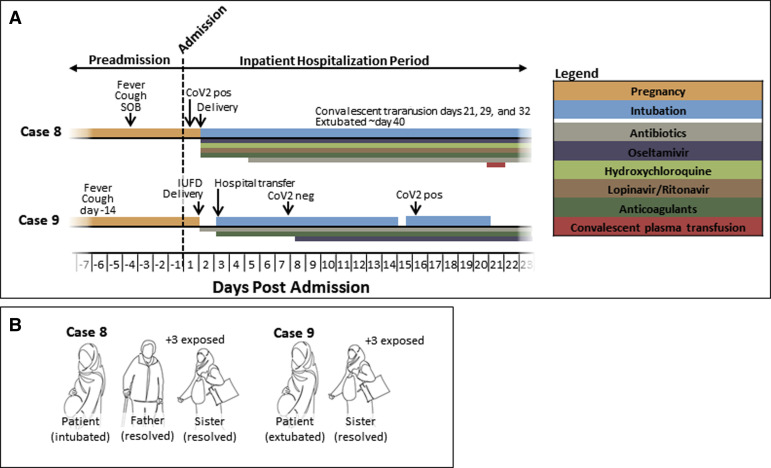
A total of 9 pregnant women are reported in this case series: 7 died and 2 survived and were alive at the time of reporting (April 20, 2020). A schematic summary of each of the 7 fatal adjudicated cases (cases 1–7) and comparative outcomes relative to their familial/household members (self-reported) are provided in Figures 1 and 2 , respectively; more granular details are presented in Tables 1 and 2 . The 2 adjudicated cases of severe morbidity without death (cases 8 and 9) are documented in Supplemental Figure 1 and Supplemental Tables 1 and 2. Key clinical aspects relative to the interpretation and findings of each case are as follows:
Figure 1.
Summary timeline of patients’ events, procedures, and medications before death
Narrative summaries are provided in the text, and further details are in Tables 1 and 2. The order of cases does not represent chronology or site of care. No patient was placed in prone position, either while pregnant or in the postpartum interval. Dosages of medications are provided in methods, timing is indicated by bars, and constituent drug therapies are detailed in each case report narrative. For all cases, anticoagulation therapy comprised enoxaparin (cases 1–7 and 9 at 40 mg subcutaneous daily) or heparin (case 8, heparin 5000 units subcutaneous twice daily).
DCDA, dichorionic diamniotic; DFM, decreased fetal movement; IUFD, intrauterine fetal death; pos, positive; resp. distress, respiratory distress; RT-PCR NAT, reverse transcription polymerase chain reaction nucleic acid testing; SOB, shortness of breath.
Hantoushzadeh et al. Maternal death due to coronavirus disease 2019. Am J Obstet Gynecol 2020.
Figure 2.
Outcomes among familial and household members of the 7 pregnant patients who died following SARS-CoV-2 infection
All of our pregnant patients had available self-reported data, and the only member who died was the pregnant patient. All occurrences of prolonged exposure occurred as a result of duration of symptoms before patient admission.
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Hantoushzadeh et al. Maternal death due to coronavirus disease 2019. Am J Obstet Gynecol 2020.
Table 1.
Maternal characteristics and outcomes among pregnant patients with SARS-CoV-2 infection and death
| Characteristics and outcomes | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 |
|---|---|---|---|---|---|---|---|
| Maternal characteristics | |||||||
| Maternal age (y)a | 25–29 | 25–29 | 40–44 | 30–34 | 30–34 | 35–39 | 45–49 |
| Gravida, para | G2 P1001 | G1 P0 | G2 P1001 | G3 P0020 | G2 P1001 | G2 P0010 | G2 P1001 |
| Comorbidities | None | Obesity | Subclinical hypothyroid AMA |
None | GDMA2 (metformin) | AMA | AMA Underweight |
| Blood type (Rh) | A (+) | B (+) | A (+) | B (+) | A (+) | O (+) | A (-) |
| Influenza vaccinated | Nob | Yes | No | No | Yes | Unknown | No |
| Admission BMI (kg/m2) | 23 | 36 | 26 | unk | 23 | 24 | 18 |
| Presenting symptoms | |||||||
| Fever | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Cough | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Dyspnea | Yes | No | No | Yes | No | Yes | Yes |
| Myalgia | Yes | Yes | No | Yes | No | Yes | No |
| Medications | |||||||
| Antivirals | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Antibiotics | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Anticoagulants | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Other | None | None | HCQ | HCQ | None | HCQ, IVIG | HCQ |
| Laboratory or relevant clinical valuesc | |||||||
| SARS-CoV-2 NAT | Positive | Positive | Positive | Positive | Positive | Positive | Positive |
| Hemoglobin (g/dL) | 9.6 (9.2, 9.6) | 9.0 (8.5, 10) | 11.6 (10.8, 11.8) | 10.8 (10.2, 14.3) | 9.9 (8.2, 10) | 8.1 (8, 10.2) | 12.3 (9.9, 12.5) |
| Platelets (× 103/μL) | 51 (48,43.4) | 68 (62,280) | 224 (220,265) | 206 (206,333) | 305 (265,328) | 177 (122,188) | 380 (172,380) |
| WBC (x 109/L) | 3.8 (3.2, 7.8) | 8 (7.2,8.2) | 7 (4.2, 13.3) | 13.3 (13, 35.6) | 20.3 (13.7, 26) | 7 (7, 8.6) | 16.4 (8.8, 18) |
| Lymphocyte (% 109/L)d | 6.8% (5.5, 7.8) | Unknown | 5% (5, 6.8) | 7.7% (unk) | 8.5% (7.5, 8.8) | 9% (8.8, 9) | 7% (6.2, 8.4) |
| CRP (mg/L)e | 41 (38, 87) | 18 (18, 22) | 25 (20, 25) | 56 (unk) | 64 (60, 68) | 117.5 (37, 12) | 81.9 |
| AST (U/L) | 52 (47, 58) | 60 (52, 76) | 160 (152, 220) | 28 (unk) | 40 (32, 48) | 29 (22, 29) | 66 (52, 68) |
| ALT (U/L) | 68 (62, 78) | 40 (32, 65) | 143 (123, 148) | 26 (unk) | 17 (15, 40) | 18 (14, 22) | 38 (34, 62) |
| Cr (mg/dL) | 0.8 (0.8–1.6) | 0.5 (0.5–1.1) | 0.6 (0.6–1.4) | 0.7 (0.6–6.0) | 0.8 (0.8–1.3) | 0.9 (0.9–1.4) | 0.7 (0.6–1.5) |
| O2 Sat, % (SaO2)f | 85 | 70 | 50–60 | 83 | 70–75 | 65 | 60–65 |
| Maternal status (as of April 20, 2020) | |||||||
| Death, intubated, or inpatient recovery | Death | Death | Death | Death | Death | Death | Death |
For antiviral and antibiotic regimens, please see Methods and case narratives.
AMA, advanced maternal age; Cr, serum creatinine; GDM, gestational diabetes mellitus; HCQ, hydroxychloroquine; IFN, interferon-alpha nebulizers; plasma, under a separate IRB, received immunotherapy through convalescent plasma transfusion from a recovered COVID-19 donor with known seropositivity; RT-PCR NAT, reverse transcription polymerase chain reaction nucleic acid testing; unk, unknown values; WBC, white blood cell count.
Hantoushzadeh et al. Maternal death due to coronavirus disease 2019. Am J Obstet Gynecol 2020.
For protection of patient identification, maternal age was gated in inclusive 5-year blocks
Case 1 did not receive seasonal influenza vaccination, but did have negative influenza testing during hospitalization for SARS-CoV-2
For all laboratory values, the initial value at the time of admission is provided, with trough and peak from the hospitalization interval (trough, peak)
Lymphopenia was defined as 10%
elevated C-reactive protein (CRP) was defined as >10 mg/L
SaO2 values are as reported at the time of diagnosis of ARDS and intubation.
Table 2.
Perinatal outcomes among pregnant patients with SARS-CoV-2 infection and death
| Outcome | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 |
|---|---|---|---|---|---|---|---|
| Fetal death | Yes | No | No | Yesa | No | Yesa | No |
| Gestational age (wk) | 30 3/7 | 38 3/7 | 30 5/7 | 24 0/7 (undelivered) | 36 0/7 | 24 0/7 (undelivered) | 28 0/7 |
| Neonatal demise | n/a | No | No | n/a | No | n/a | Yes (twins) |
| Mode of delivery | NSVD | Cesarean | Cesarean | n/a | Cesarean | n/a | Cesarean |
| Birthweight (g) | 1700 | 2800 | 2100 | n/a | 3200 | n/a | 1180; 1340 |
| Apgar score (1.5 min) | 0, 0 | 8, 9 | 9, 10 | n/a | 7, 9 | n/a | 8, 9; 7, 9 |
| DCDA twin gestation | No | No | No | Yes | No | Yes | Yes |
| SARS-CoV-2 NAT | n/a | Negative | Negativeb | n/a | Negative | n/a | Negative |
| Neonatal pneumonia | n/a | No | Yes | n/a | No | n/a | No; no |
| Neonatal lymphopenia | n/a | No | Yes | n/a | No | n/a | No; no |
In no instance was magnesium sulfate given intrapartum nor antenatal for the purpose of neuroprotection, and no patient in the series had preeclampsia.
DCDA, dichorionic diamniotic; n/a, not applicable; NSVD, normal spontaneous vaginal delivery; RT-PCR NAT, reverse transcription polymerase chain reaction nucleic acid testing; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Hantoushzadeh et al. Maternal death due to coronavirus disease 2019. Am J Obstet Gynecol 2020.
24-week singleton (case 4) or DCDA twin gestation (case 6) in utero at the time of maternal death, undelivered
As detailed in the case description, case 3 was negative on day of life 1 but converted to positive on day of life 7.
Supplemental Figure 1.
Summary data of cases of severe morbidity, but without death (as of April 20, 2020).
A, Summary timeline of patients’ events, procedures, and medications in cases of severe morbidity but without death (as of April 20, 2020). Narrative summaries are provided in the text, and further details are in Supplemental Tables 1 and 2. The order of cases does not represent chronology or site of care. No patient was positioned in the prone position, either while pregnant or in the postpartum interval. Dosages of medications are provided in Methods, timing is indicated by bars, and constituent drug therapies are detailed in each case report narrative. For all cases, anticoagulation therapy comprised enoxaparin (case 9, at 40 mg subcutaneous daily) or heparin (case 8, heparin 5000 units subcutaneous twice daily). B, Outcomes among familial and household members of the 2 pregnant patients with severe morbidity but did not die (as of April 20, 2020). All of our pregnant patients had available self-reported data, and the only member with severe cardiopulmonary morbidity was the pregnant patient. All occurrences of prolonged exposure occurred as a result of duration of symptoms before patient admission.
COV2, coronavirus 2, IUFD, intrauterine fetal death; neg, negative; pos, positive SOB, shortness of breath.
Hantoushzadeh et al. Maternal death due to coronavirus disease 2019. Am J Obstet Gynecol 2020.
Case 1
A 25- to 29-year-old previously healthy gravida at 30 3/7 weeks’ gestation was seen in the outpatient antenatal clinic the day before admission with a complaint of sore throat and rhinorrhea with dry cough, but she was afebrile and did not have dyspnea. She was admitted the next day after becoming febrile (39°C) and experiencing shortness of breath and was normotensive (125/80 mm Hg). On the day of admission, chest CT had features of viral pneumonia, laboratory values were significant for lymphopenia and pancytopenia, and antepartum testing for fetal well-being was reassuring. The SARS-CoV-2 testing was done, but her test results were not positive until the day of death. Nonetheless, she was initiated on oseltamivir, azithromycin, and ceftazidime at the time of admission. She experienced acute respiratory distress syndrome (ARDS) 24 hours later and was transferred to the ICU and intubated; linezolid and amantadine were added. Later that evening, onset of spontaneous labor accompanied persistent oxygen desaturations (SaO2 88% on ventilator support), with spontaneous vaginal delivery of an intrauterine fetal death (IUFD, stillbirth) the following morning. With persistent postpartum ARDS (SaO2 85%–90% on maximal ventilator support), meropenem, vancomycin, and lopinavir/ritonavir were added. She experienced acute hypotension and bradycardia 8 hours later and died despite cardiopulmonary resuscitative efforts. There are 3 other household members in her familial cohort: her son was diagnosed as having mild COVID-19 by clinical symptoms, and her father and husband had prolonged exposure. He recovered, and all are alive and well.
Case 2
A 25- to 29-year-old previously healthy primigravid at 38 3/7 weeks’ gestation was admitted with a 24-hour history of fever, dyspnea, and dry cough and was initiated on oseltamivir, azithromycin, and ceftriaxone; fetal well-being was reassuring. She was normotensive on admission (110/70 mm Hg). On HD2, a chest radiograph revealed bilateral patchy features typical of a viral pneumonia, her SARS-CoV-2 test result was positive, and antepartum fetal monitoring was reassuring. Within 48 hours of admission, she developed acute hypoxemia, spontaneous uterine contractions were noted, and the fetal heart rate tracing regressed to nonreassuring. Following a cesarean delivery of a viable neonate, she acutely decompensated and was transferred to the ICU and intubated for ARDS. Despite maximal ventilator support, she had cardiopulmonary collapse and died within 24 hours after failed resuscitative efforts. Her neonate’s pharyngeal swab was SARS-CoV-2 negative. There are 4 other adults in her familial/household cohort, and her mother and niece were positive for mild symptoms consistent with COVID-19. All recovered and are alive and well.
Case 3
A 40- to 44-year-old gravida with a history of subclinical hypothyroidism (normal free T4 ng/dL and negative anti-TPO antibody studies) at 30 5/7 weeks’ gestation was admitted with a 1-week history of intermittent fever, dyspnea, and persistent dry cough; she was normotensive and fetal well-being on admission was reassuring (biophysical profile 8 of 8). Her chest CT on admission had bilateral patchy ground-glass features, and her SARS-CoV-2 test result was positive. Within 36 hours, she reported decreased fetal movement and acutely decompensated (SaO2 50%). An emergency cesarean delivery was performed, and she was intubated after delivery with ARDS. After 24 hours of no improvement, oseltamivir, vancomycin, and meropenem were added. Despite a normal echocardiogram on HD4/PPD2, within 24 hours, she had persistent hypoxia despite maximal ventilator support with end organ failure; she died 1 day later after unsuccessful cardiopulmonary resuscitative efforts. Her husband is the only other member of her cohort and was asymptomatic and not tested for SARS-CoV-2 and did not have contact with the neonate at birth or during the first week of life. Her preterm neonate had a negative test result by nasopharyngeal swab at day of life 1 but presumptively acquired SARS-CoV-2 postnatally and subsequently had a positive test result at day of life 7 with an accompanying lymphopenia (nadir white blood cell 8.9, with 26% lymphocytes). The neonate was intubated for prematurity, developed pneumonia at day of life 2, and remained intubated but stable in the newborn unit.
Case 4
A 30- to 34-year-old gravida at 24 0/7 weeks’ gestation was admitted with a suspected COVID-19 pneumonia with dyspnea (respiratory rate 30 breaths/min), tachycardia (heart rate 130 beats/min), normotensive, and fever (39.1°C). She had experienced mild dyspnea with dry cough several days before, and her chest CT on admission had bilateral patchy ground-glass features; nasopharyngeal swab for SARS-CoV-2 had a positive result. She was initiated on hydroxychloroquine, oseltamivir, azithromycin, lopinavir/ritonavir, and ceftriaxone. Over the next several hours, she had dyspnea with acute hypoxemia (SaO2 88%) requiring exogenous O2 supplementation; fetal death occurred within 72 hours of admission. She acutely decompensated with ARDS, was intubated, and arrested 24 hours later but was successfully resuscitated albeit complicated by a pneumothorax requiring chest tube placement. She required hemodialysis for acute renal failure (serum creatinine 6 mg/dL) and had cardiopulmonary collapse and died after failed resuscitation within 24 hours. There are 6 other household members in her familial cohort; her father was admitted to the hospital with COVID-19 pneumonia, and her sister had clinical disease that was managed outpatient. They recovered, and all are alive and well.
Case 5
A 30- to 34-year-old previously healthy gravida at 36 0/7 weeks’ gestation was admitted to the ICU with a diagnosis of COVID-19 pneumonia and initiated on ceftriaxone, oseltamivir, and lopinavir/ritonavir. She had experienced mild cold symptoms for 2 weeks before admission and reported being febrile with new onset dyspnea and dry cough on the day before admission. Her medical history was significant for type A2 gestational diabetes and managed on low-dose metformin (500 mg, twice daily). With concern for impending cardiopulmonary collapse, she underwent cesarean delivery of a viable neonate. With persistent hypoxia (SaO2 70%), tachypnea, and impending respiratory collapse, she was intubated 24 hours later, and her antibiotic regimen was changed to vancomycin and meropenem. Over the next 5 days, her cardiopulmonary status worsened despite placement of chest tube, and despite maximal ventilator support, she died 1 day later and 3 hours after an initially successful resuscitation. Her neonate’s pharyngeal swab was negative for SARS-CoV-2. There are 4 other household members in her familial cohort; her 6-year-old son received a diagnosis of clinical COVID-19, and 3 others had prolonged exposure. He recovered, and all are alive and well.
Case 6
A 35- to 39-year-old previously healthy gravida with a current dichorionic diamniotic (DCDA) twin gestation at 24 0/7 weeks’ gestation was admitted for a 2-day history of fever, dyspnea, and persistent dry cough. She had experienced mild cold symptoms for 2 weeks before admission, and her medical history was significant only for infertility requiring in vitro fertilization (IVF) for the current pregnancy. Her chest CT on admission was significant only for bilateral patchy ground-glass features. She had a positive test result for SARS-CoV-2 with a nasopharyngeal swab and was immediately initiated on hydroxychloroquine, oseltamivir, and lopinavir/ritonavir; fetal well-being was reassessed reassuring. She acutely decompensated from ARDS with profound hypoxemia (SaO2 65%) 24 hours later. She was intubated, and ceftriaxone, azithromycin, vancomycin, and meropenem were added sequentially over the next 4 days given the concern for secondary bacterial pneumonia. On HD4, with an ongoing viable twin gestation but clinical worsening, she was provided empiric intravenous immunoglobulin (IVIG) for 3 days and initially improved enough to be extubated with documentation of an ongoing viable twin gestation while remaining inpatient for monitoring. However, 2 weeks later (HD20), her ARDS acutely recurred (SaO2 77%), and she had septic shock and disseminated intravascular coagulopathy and required reintubation with an IUFD of both twins within 24 hours. She progressed to left heart failure with an ejection fraction of 25%, had cardiopulmonary arrest 18 hours later, and died after failed resuscitative efforts. There are 4 household members in her cohort, but only her husband received a diagnosis of clinical COVID-19. He recovered, and all remain alive and well.
Case 7
A 45- to 49-year-old previously healthy gravida at 28 0/7 weeks’ gestation with a DCDA twin gestation was admitted with a 14-day history of fever and persistent dry cough. Her history was significant only for age-related infertility requiring IVF with donor oocytes for the current pregnancy. As anticipated for maternal age of more than 45 years, she was tested with a normal echocardiogram by a cardiology before IVF therapy. On the day of admission, she complained of worsening dyspnea over 2 days, and a CT study revealed a bilateral patchy ground-glass pneumonia; she was normotensive (120/80 mm Hg). Her SARS-CoV-2 testing on admission returned a positive result, and she was initiated on hydroxychloroquine, oseltamivir, and lopinavir/ritonavir. Empiric IVIG for 3 days was added 24 hours later. On HD2, she developed intermittent hypoxemia, with concerns for fetal well-being. She underwent a cesarean delivery of 2 viable but premature neonates. She continued on exogenous oxygen support until HD5/PPD3 when she acutely decompensated, was transferred to the ICU, and was intubated, and vancomycin and meropenem were added. Both neonates’ pharyngeal specimens were negative for SARS-CoV-2, and neither twin had evidence of COVID-19 after delivery (absence of lymphopenia, thrombocytopenia, and normal chest radiographs), but they experienced complications of premature birth and both died at day of life 3. She died after 18 days of maximal ventilator support after a failed cardiopulmonary resuscitation. Her husband and their surviving child are the only other members of her familial/household cohort, and he was positive for COVID-19 with mild symptoms. He recovered, and both are alive and well.
Case 8
A 35- to 39-year-old previously healthy primigravid at 33 5/7 weeks’ gestation was admitted for inpatient care due to suspected COVID-19 pneumonia (CT with bilateral patchy ground-glass features) with accompanying lymphopenia, tachypnea, and hypoxemia (SaO2 88%) with hemoptysis. Four days before admission, she reported dry cough and fever, with intermittent dyspnea. Despite being normotensive on admission (130/80 mm Hg), she acutely worsened within 24 hours and underwent cesarean delivery for nonreassuring fetal status (fetal tachycardia) and breech presentation. She was initiated on hydroxychloroquine, oseltamivir, and lopinavir/ritonavir and intubated after delivery for ARDS. With absence of improvement despite maximal ventilator support, meropenem, vancomycin, azithromycin, and levofloxacin were added over the following 72 hours. Her condition necessitated tracheotomy on HD6/PPD5 and remained conscious but on ventilator support as of HD35, despite having received immunotherapy (convalescent plasma transfusion) under a separate IRB approval from a recovered COVID-19 donor with known seropositivity. Her neonate’s pharyngeal swab was negative for SARS-CoV-2. There are 5 other members in her household/familial cohort; her father and sister received a diagnosis of clinical COVID-19, and 3 others had prolonged exposure. All recovered, and remain alive and well.
Case 9
A 35- to 39-year-old gravida with gestational diabetes (diet controlled, type A1) at 36 0/7 weeks’ gestation was admitted with fever and persistent dry cough. Her presentation to the hospital was prompted by preterm premature rupture of membranes (PPROM) and decreased fetal movement. On arrival to labor and delivery, an IUFD in breech presentation was diagnosed and cesarean delivery was performed. She was normotensive on admission, and a chest CT showed bilateral patchy ground-glass features. She decompensated within 18 hours of cesarean delivery for her IUFD and was transferred to the ICU with a diagnosis of coagulopathy and received fresh-frozen plasma and cryoprecipitate, and a second-generation cephalosporin was added. On HD3/PPD2, with persistent hypoxia, tachypnea, and impending respiratory collapse, she was intubated and her antibiotic regimen was changed to vancomycin and meropenem. Forty-eight hours later (HD5/PPD4), after negative influenza A and B testing with an initial SARS-CoV-2 NAT negative test but with persistent ventilator dependence, she was initiated on oseltamivir. Her condition gradually improved by HD14/PPD13, when she was briefly extubated but reintubated within 8 hours for acute worsening of ARDS; on that same day, 2 repeat SARS-CoV-2 NAT tests returned positive. She later experienced gastrointestinal bleeding requiring endoscopy, and colistimethate sodium was started and meropenem was held. With persistent pulmonary disease, atazanavir and piperacillin/tazobactam were added. She experienced slow recovery and was extubated after 20 days. She remained in the hospital and was the only patient who recovered and extubated at the time of reporting. There are 4 other adults in her familial household cohort, and despite prolonged exposure to the patient before admission, only her sister received a diagnosis of COVID-19 by symptoms. She recovered, and all are alive and well.
Discussion
Principal findings
Potentially consistent with reported outcomes from other severe viral lower respiratory tract infections,1, 2, 3, 4, 5, 6 , 8 we found that pregnant women with SARS-CoV-2 infection and COVID-19 in their second or third trimester of pregnancy may experience cardiopulmonary complications and die. Our reported outcomes are significantly different from outcomes observed in 104 pregnant women from China,9, 17 , 19, 20, 21, 22, 23 but are consistent with outcome in 1 woman (gravida experiencing stillbirth and remaining on ECMO at the time of the reporting12) and potentially consistent with respect to some level of respiratory support but with recovery in 2 others.9 , 14 Because our study is a case series and not a surveillance cohort, we cannot report on the rate of occurrence in any of our hospitals or centers, although our report demonstrating any occurrence of morbidity or death generally aligns with the reported trend of severe maternal outcomes (9.3% of the surveillance cohort) and critical morbidity (4.7%) in the study by Breslin et al.18 When considering our maternal deaths relative to these and other reports,9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 our series has 2 key distinguishing features. First, ours is a case series reporting death of pregnant women in their latter second or third trimester presenting with severe COVID-19 over a 30-day interval in Iran. In contrast, Breslin et al18 reported all outcomes (n=43) of SARS-CoV-2–positive gravida over 2 weeks from their paired affiliate hospitals, one-third of whom were asymptomatic and diagnosed using universal surveillance testing on routine obstetrical admission. Second, all of our subjects delivered (or died with their periviable fetuses in utero); other case series totaled 22% undelivered gravida at the time of reporting and retained only sparse maternal outcomes data.10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 9 Longer-term follow-up may be important in revealing instances of severe maternal outcomes because most of our patients died days to weeks after onset of initial symptoms and often in the postpartum interval.
There are additional characteristics of the patients in our case series that are distinct but unlikely to be confounding our reported SARS-CoV-2–associated mortality. Five of our gravidae were 35 years of age or older, with 2 of these 5 being of elder advanced maternal age (>40 years). However, the 2 of 9 still alive were aged 35 years or older (Table 1). We observed a statistically significant distinction in the mean maternal age in our case series compared with all others (average maternal age 36.7±7.3 years vs 30.3±3.6 years, P<.001); the clinical significance of this difference is unknown.10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 9 No patient in our series had preexisting comorbidities above baseline population risk (ie, gestational diabetes mellitus and subclinical hypothyroidism), and none had hypertension, cardiovascular disease, asthma, or renal disease. Finally, all women on admission were normotensive, excluding a comorbid diagnosis of preeclampsia. Similarly, we believe it is unlikely that the quality of delivered obstetrical care is the source of these outcome discrepancies, as the WHO-reported maternal mortality ratio in Iran is lower than that of China (16 vs 19.6 per 100,000, 2017 normalized data; see https://www.who.int/gho/maternal_health/countries/irn.pdf and https://www.who.int/china/health-topics/maternal-health). Maternity care delivered in Iran during the pandemic has remained a high national and regional priority, and ICU capacity at all centers was nonlimiting and in no instance was admission, ICU transfer, delivery, intubation, or medication delayed for lack of availability of resources.
Rather, we believe it more probable that delays in reporting or underreporting, alongside nonrandom selection bias, may be contributing to these first-pass differences. Assessment of epidemiologic characteristics including case to fatality ratios during the course of a pandemic may be affected by right (type I) censoring and ascertainment bias.29 As recently demonstrated by Mizumoto et al,29 breakdowns in an overwhelmed healthcare delivery system with the SARS-CoV-2 pandemic may result in an underestimated death risk in epidemic epicenters even within a given country (right censoring). We emphasize that large upper limit confidence intervals will always accompany small case series with zero mortality, and this could additionally lead to a false reassurance regarding the risk of death in early reporting.10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 9
Strengths and limitations
We acknowledge that our series is limited by lack of surveillance data and is prone to adverse outcome ascertainment bias. Therefore, we are not attempting to quantify risk or estimate rates in our small series and explicitly discourage others from doing so. Surveillance data will ultimately define the impact of pregnancy among women who died or experienced severe morbidity attributed to COVID-19. Our case series lends critical information to the current narrative based on previously published reports, which presently suggests zero mortality among pregnant women.10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 9 Determining the proportionate case fatality rate and risk of severe morbidity in pregnancy will require rigorous population-wide surveillance data from many countries, inclusive of data identifying potential modifiers and comorbidities adjusting risk. However, the cases we reported herein demonstrated that COVID-19 maternal mortality is not zero and suggested caution against complacency and early assumptions of protection with pregnancy.9, 17 , 19, 20, 21, 22, 23
Despite these limitations, there are a number of strengths to our report. First, we took a rigorous approach to collecting primary source data and adjudicating each case and its outcomes. Second, we compared our outcomes to familial/household members as a proxy for comparative risk (Figures 2 and Supplementary Figure 1, panel B). Given the R0 estimate of SARS-CoV-2 ranging from 2.1 to 3.11,24, 25, 26, 27, 28, 29 it would be anticipated that the same viral strain infected the case and her familial/household cohort and access to quality care and baseline demographic variances would be anticipated to be small. We recognize that ideally we would compare our outcomes with all severely ill nonpregnant subjects over the same period. However, the level and rigor of surveillance data that such a study would require are not feasible at this time. Thus, our conclusions are best summated in a simple statement with clinically practical implications: in this case series documenting 7 deaths among 9 pregnant women with severe COVID-19, when compared with their spouses, children, or family members living in the same household, the pregnant patients were the only reported deaths. We emphasize the risk for ascertainment bias in our case series, and we make statements neither regarding either relative or attributable risk for severe morbidity nor death among pregnant women compared with nonpregnant women from the population at large.
Interpretation of our findings in the context of other studies
Our report on a number of adverse perinatal and neonatal outcomes, including preterm birth, fetal death, and neonatal death, is consistent with several other adverse outcome reports from China and New York10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 9 and prior adverse perinatal outcomes of fetal death, fetal growth restriction, and placental abruption seen with SARS-CoV-1 in 2003 and MERS-CoV in 2012.1, 2, 3, 4 Of note, the intent of our series was not to determine whether or not neonates can vertically acquire SARS-CoV-2 through intrauterine maternal transmission. However, in 1 instance (case 3), the premature neonate initially had a negative result for SARS-CoV-2 testing by nasopharyngeal swab and a positive result later at day of life 7 while intubated in the neonatal ICU. Evidence regarding perinatal outcomes, including vertical mother-to-child transmission of SARS-CoV-2, is presently unclear, with 13 of 16 publications suggesting no evidence of transmission.10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 9 In contrast, Wang et al15 provided a single case report from generally the same region (Wuhan) of a pregnant woman living approximately 1.2 km from the Huanan Seafood Wholesale Market. In that instance, the patient wore an N95 mask during the delivery, and there was neither maternal-neonatal contact nor breastmilk feeding.15 Nevertheless, at 36 hours of life, the neonate’s nasopharyngeal swab was positive for SARS-CoV-2.15 Three other groups have recently reported on rare occasions of possible vertical transmission,19 , 22 , 23 albeit inclusive of serologic testing with immunoglobulin M antibodies with yet unproven SARS-CoV-2 specificity that unexpectedly declined in their levels within the first 2 weeks of postnatal life.19
We acknowledge that when considering the potential for vertical transmission, both the placenta and stool/meconium may be of importance, as a less lethal common Coronaviridae (229E) is known to be transplacentally transmitted but its use of the same or different cell entry receptors as SARS-CoV-2 (ie, ACE2) is unknown.30 , 31 Moreover, although ACE2 mRNA is more highly expressed in early gestation human placental syncytiotrophoblasts and ACE protein localizes to fetal endothelium, the placental expression of host proteases (such as TMPRSS2 and others) necessary for cleavage of the S protein and receptor priming is unknown but has generally only been described in lung and airway cells or their progenitors.25, 26, 27 As a result, whether the necessary and sufficient host molecular machinery to enable efficient transplacental vertical transmission is present or absent in the second or third trimester human placenta is presently unknown, and our current case series offers no further insight. With regard to fecal-oral transmission, we were struck by a recent report suggesting that as great as 23.2% of nonpharyngeal detected SARS-CoV-2 may be detected by RT-PCR NAT in the stool.32 In this case series of 73 patients with SARS-CoV-2 infection with aged ranging from 10 months to 78 years, 53.4% persistently had positive test results in the stool for as great as 12 days, and immunofluorescence visualization of biopsy specimens was consistent with viral uptake in the glandular cells of the gastric, duodenal, and rectal epithelia.32 This raises concern for the possibility of fecal-oral transmission, which would have potential implications in obstetrical practice and risk of vertical transmission if women were known to harbor infectious virions in their stool or vagina at the time of vaginal delivery. However, infectious SARS-CoV-2 viral load in stool or vagina is not known, and demonstration of fecal-oral transmission remains purely speculative. In our series, case 3 was delivered by cesarean.
We make no conclusions regarding the relative likelihood (or not) that pregnant women may be at higher risk for infection with SARS-CoV-2. Although it is frequently stated that pregnant women are “immunosuppressed,” such assumptions are incorrect.7 Rather, human pregnancy represents highly adaptive immunity, allowing the mother to become tolerant to her fetus yet remain immunocompetent to ward off pathogenic infection. This includes competency of B-cell–mediated humoral immune responses, as well as innate and T-cell–mediated responses to intracellular pathogens.7 Ours and others’ general finding of lymphopenia in both pregnant10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 9 and nonpregnant24, 25, 26, 27 patients with SARS-CoV-2 infection is a result of viral infection and not pregnancy per se. As evident by comparison with our referencing to familial/household members, our patients were never alone in their occurrence of infection or symptomatic disease. Rather, once they were infected and with the development of COVID-19 pneumonia, they experienced severe respiratory and/or cardiopulmonary morbidity and death. It is likewise important to refrain from conclusions regarding safety, harm, or efficacy of any medications or treatment decisions made in the care of these patients. Hydroxychloroquine and chloroquine are cellular autophagy modulators that interfere with endosome-mediated viral entry and latter stages of replication of enveloped viruses, including retroviruses, coronaviruses, and flaviviruses.33, 34, 35, 36 However, HIV-infected patients receiving hydroxychloroquine in the absence of other antiretroviral therapy showed increased viral replication and poorer outcomes in a randomized controlled trial.36 Its use among the patients in our case series was consistent with regional practice and did include concomitant antiviral agents.
Clinical implications
These 7 maternal deaths due to severe COVID-19 should prompt reexamination of any current guidelines and recommendations by professional societies that might be potentially construed as providing yet unproven reassurance of the absolute absence of death among pregnant women with COVID-19. Our case series is in contrast to previous reports suggesting no known mortality among pregnant women infected by SARS-CoV-2.10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 9 Whether the maternal case fatality rate or maternal morbidity estimates will ultimately be the same, less, or greater than that of other populations is yet unknown. However, the fatal cases reported herein suggest that it is not zero and should inspire caution against complacency and guide restraint in rushing estimates of relative or attributable risk with pregnancy.
Acknowledgments
This study was supported in part by National Institutes of Health (NIH) grants R01-HD091731, R21-ES029462, and R01-DK089201 to K.M.A. KMA holds the Henry & Emma Meyer Endowed Chair at Baylor College of Medicine. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors are grateful to Drs Calla Holmgren (University of Utah) and Melissa Suter, Catherine Eppes, and James Versalovic (Baylor College of Medicine and Texas Children’s Hospital) for critical review of the manuscript. The authors report no conflict of interest. We are forever indebted to our coauthors for their trust and partnership as we combined efforts to share the mortality and severe morbidity experienced by pregnant patients, their neonates, and their families in the midst of the clinical demands accompanying the SARS-CoV-2 pandemic in March 2020. We are further indebted to the willingness of the patients and their household members to voluntarily share available or self-reported familial data. It is our hope that our words and analysis describing the outcomes of these women and their neonates have done honor and justice to their lives and the high level of compassionate and dedicated care that was delivered by their providers under the trying times and difficult circumstances in the early months of the COVID-19 pandemic.
Footnotes
The authors report no conflicts of interest.
Cite this article as: Hantoushzadeh S, Shamshirsaz AA, Aleyasin A, et al. Maternal death due to COVID-19. Am J Obstet Gynecol 2020;223:109.e1-16.
Supplementary Materials
Supplemental Table 1.
Maternal characteristics and outcomes among pregnant patients with SARS-CoV-2 experiencing severe morbidity, but not death (as of April 20, 2020)
| Characteristics and outcomes | Case 8 | Case 9 |
|---|---|---|
| Maternal characteristics | ||
| Maternal age (y)a | 35–39 | 35–39 |
| Gravida, para | G1 P0 | G2 P0010 |
| Comorbidities | AMA Obesity |
GDMA1 (diet controlled) AMA Obesity |
| Blood type (Rh) | O (+) | O (+) |
| Influenza vaccinated | Nob | Yes |
| Admission BMI (kg/m2) | 32 | 31 |
| Presenting symptoms | ||
| Fever | Yes | Yes |
| Cough | Yes | Yes |
| Dyspnea | Yes | Yes |
| Myalgia | No | No |
| Medications | ||
| Antivirals | Yes | Yes |
| Antibiotics | Yes | Yes |
| Anticoagulants | Yes | Yes |
| Other | HCQ, plasma | HCQ |
| Laboratory valuesc | ||
| SARS-CoV-2 NAT | Positive | Positive |
| Hemoglobin (g/dL) | 8 (8, 10) | 7.6 (7.2, 7.8) |
| Platelets (× 103/μL) | 275 (262, 284) | 145 (122, 270) |
| WBC (× 109/L) | 9.4 (8, 9.8) | 26 (16, 32) |
| Lymphocyted (% 109/L) | 9% (8.5, 9.4) | 8.5% (8.2, 8.8) |
| CRPe, mg/L | 45 (38, 47) | 210 (120, 235) |
| AST (U/L) | 80 (66, 94) | 172 (88, 178) |
| ALT (U/L) | 6 2 (26, 68) | 126 (48, 132) |
| Cr (mg/dL) | 0.6 (0.6–1.2) | 0.6 (0.5–1.7) |
| O2 Satf, % (SaO2) | 60 | 85 |
| Maternal status (as of April 20, 2020) | ||
| Intubated or inpatient recovery | Extubated, inpatient | Discharged |
For antiviral and antibiotic regimens, please see methods and case narratives.
ALT, alanine transaminase; AST, aspartate transaminase; AMA, advanced maternal age; BMI, body mass index; Cr, serum creatinine; CRP, C-reactive protein; GDM, gestational diabetes mellitus; HCQ, hydroxychloroquine; IFN, interferon-alpha nebulizers; plasma, under a separate IRB, received immunotherapy through convalescent plasma transfusion from a recovered coronavirus disease 2019 donor with known seropositivity; RT-PCR NAT, reverse transcription polymerase chain reaction nucleic acid testing; SARS-Cov-2, severe acute respiratory syndrome coronavirus 2; unk, unknown values; WBC, white blood cell count.
Hantoushzadeh et al. Maternal death due to coronavirus disease 2019. Am J Obstet Gynecol 2020.
For protection of patient identification, maternal age was gated in inclusive 5-year blocks
Case 1 did not receive seasonal influenza vaccination, but did have negative influenza testing during hospitalization for SARS-CoV-2
For all laboratory values, the initial value at the time of admission is provided, with trough and peak from the hospitalization interval (trough, peak)
Lymphopenia was defined as 10%
elevated C-reactive protein (CRP) was defined as >10 mg/L
SaO2 values are as reported at the time of diagnosis of ARDS and intubation.
Supplemental Table 2.
Perinatal outcomes among pregnant patients with SARS-CoV-2 experiencing severe morbidity, but not death (as of April 20, 2020)
| Perinatal outcome | Case 8 | Case 9 |
|---|---|---|
| Fetal or neonatal outcome (as of April 20, 2020) | ||
| Fetal death | No | Yes |
| Gestational age (wk) | 33 6/7 | 36 0/7 |
| Neonatal demise | No | n/a |
| Mode of delivery | Cesarean | Cesarean |
| Birthweight (g) | 1800 | 3000 |
| Apgar score (1.5 min) | 6, 7 | 0, 0 |
| DCDA twin gestation | No | No |
| SARS-CoV-2 NAT | Negative | n/a |
| Neonatal pneumonia | No | n/a |
| Neonatal lymphopenia | No | n/a |
DCDA, dichorionic diamniotic; n/a, not applicable; NAT, nucleic acid testing; SARS-Cov-2, severe acute respiratory syndrome coronavirus 2.
Hantoushzadeh et al. Maternal death due to coronavirus disease 2019. Am J Obstet Gynecol 2020.
References
- 1.Wong S.F., Chow K.M., Leung T.N., et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191:292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfaraj S.H., Al-Tawfiq J.A., Memish Z.A. Middle East Respiratory Syndrome coronavirus (MERS-CoV) infection during pregnancy: report of two cases & review of the literature. J Microbiol Immunol Infect. 2019;52:501–503. doi: 10.1016/j.jmii.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasmussen S.A., Jamieson D.J., Uyeki T.M. Effects of influenza on pregnant women and infants. Am J Obstet Gynecol. 2012;207(3 Suppl):S3–S8. doi: 10.1016/j.ajog.2012.06.068. [DOI] [PubMed] [Google Scholar]
- 4.Alserehi H., Wali G., Alshukairi A., Alraddadi B. Impact of Middle East Respiratory Syndrome coronavirus (MERS-CoV) on pregnancy and perinatal outcome. BMC Infect Dis. 2016;16:105. doi: 10.1186/s12879-016-1437-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aagaard-Tillery K.M., Silver R., Dalton J. Immunology of normal pregnancy. Semin Fetal Neonat Med. 2006;11:279–295. doi: 10.1016/j.siny.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Littauer E.Q., Skountzou I. Hormonal regulation of physiology, innate immunity and antibody response to H1N1 influenza virus infection during pregnancy. Front Immunol. 2018;9:2455. doi: 10.3389/fimmu.2018.02455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ACOG practice bulletin no. 211: critical care in pregnancy. Obstet Gynecol. 2019;133:e303–e319. doi: 10.1097/AOG.0000000000003241. [DOI] [PubMed] [Google Scholar]
- 8.Mosby L.G., Rasmussen S.A., Jamieson D.J. 2009 Pandemic influenza A (H1N1) in pregnancy: a systematic review of the literature. Am J Obstet Gynecol. 2011;205:10–18. doi: 10.1016/j.ajog.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 9.Lei D., Wang C., Li C., et al. Clinical characteristics of pregnancy with the 2019 novel coronavirus disease (COVID-19) infection. Chin J Perinat Med. 2020;23:226–231. [Google Scholar]
- 10.Chen H., Guo J., Wang C., et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu D., Li L., Wu X., et al. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. AJR Am J Roentgenol. 2020 doi: 10.2214/AJR.20.23072. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Liu Y., Chen H., Tang K., Guo Y. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J Infect. 2020 doi: 10.1016/j.jinf.2020.02.028. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu H., Wang L., Fang C., et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9:51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y., Peng H., Wang L., et al. Infants born to mothers with a new conoravirus (COVID-19) Front Pediatr. 2020;8:104. doi: 10.3389/fped.2020.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang S., Guo L., Chen L., et al. A case report of neonatal COVID-19 infection in China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa225. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X., Zhou Z., Zhang J., Zhu F., Tang Y., Shen X. A case of 2019 novel coronavirus in a pregnant woman with preterm delivery. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa200. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y., Zhao R., Zheng S., et al. Lack of vertical transmission of severe acute respiratory syndrome coronavirus 2, China. Emerg Infect Dis. 2020 doi: 10.3201/eid2606.200287. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breslin N., Baptiste C., Gyamfi-Bannerman C., et al. COVID-19 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM. 2020 doi: 10.1016/j.ajogmf.2020.100118. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong L., Tian J., He S., et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020 doi: 10.1001/jama.2020.4621. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu N., Li W., Kang Q., et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30176-6. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wen R., Sun Y., Xing Q.S. A patient with SARS-CoV-2 infection during pregnancy in Qingdao, China. J Microbiol Immunol Infect. 2020 doi: 10.1016/j.jmii.2020.03.004. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng H., Xu C., Fan J., et al. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA. 2020 doi: 10.1001/jama.2020.4861. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng L., Xia S., Yuan W., et al. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.0878. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou P., Yang X.L., Wang X.G., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lukassen S., Chua R.L., Trefzer T., et al. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020 doi: 10.15252/embj.20105114. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin Z., Du X., Xu Y., et al. Structure of Mpro from COVID-19 virus and discovery of its inhibitors. Nature. 2020;582:289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 28.CDC COVID-19 Response Team Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mizumoto K., Chowell G. Emerg Infect Dis; 2020. Estimating risk for death from 2019 novel coronavirus disease, China, January-February 2020. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng W.F., Wong S.F., Lam A., et al. The placentas of patients with severe acute respiratory syndrome: a pathophysiological evaluation. Pathology. 2006;38:210–218. doi: 10.1080/00313020600696280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gagneur A., Dirson E., Audebert S., et al. Materno-fetal transmission of human coronaviruses; a prospective pilot study. Eur J Clin Microbiol Infect Dis. 2008;27:863–866. doi: 10.1007/s10096-008-0505-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pringle K.G., Tadros M.A., Callister R.J., Lumbers E.R. The expression and localization of the human placental prorenin/renin-angiotensin system throughout pregnancy: roles in trophoblast invasion and angiogenesis? Placenta. 2011;32:956–962. doi: 10.1016/j.placenta.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 33.Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.02.055. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: an old drug against today’s diseases? Lancet Infect Dis. 2003;3:722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vincent M.J., Bergeron E., Benjannet S., et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paton N.I., Goodall R.L., Dunn D.T., et al. Effects of hydroxychloroquine on immune activation and disease progression among HIV-infected patients not receiving antiretroviral therapy: a randomized controlled trial. JAMA. 2012;308:353–361. doi: 10.1001/jama.2012.6936. [DOI] [PMC free article] [PubMed] [Google Scholar]