Abstract
Objective
Acute stroke remains a medical emergency even during the COVID-19 pandemic. Most patients with COVID-19 infection present with constitutional and respiratory symptoms; while others present with atypical gastrointestinal, cardiovascular, or neurological manifestations. Here we present a series of four patients with COVID-19 that presented with acute stroke.
Methods
We searched the hospital databases for patients that presented with acute stroke and concomitant features of suspected COVID-19 infection. All patients who had radiographic evidence of stroke and PCR-confirmed COVID-19 infection were included in the study. Patients admitted to the hospital with PCR- confirmed COVID-19 disease whose hospital course was complicated with acute stroke while inpatient were excluded from the study. Retrospective patient data were obtained from electronic medical records. Informed consent was obtained.
Results
We identified four patients who presented with radiographic confirmation of acute stroke and PCR-confirmed SARS-CoV-2 infection. We elucidate the clinical characteristics, imaging findings, and the clinical course.
Conclusions
Timely assessment and hyperacute treatment is the key to minimize mortality and morbidity of patients with acute stroke. Stroke teams should be wary of the fact that COVID-19 patients can present with cerebrovascular accidents and should don appropriate personal protective equipment in every suspected patient. Further studies are urgently needed to improve current understandings of neurological pathology in the setting of COVID-19 infection.
Keywords: COVID-19, Stroke, SARS-CoV2
1. Introduction
In December 2019, the first reports of the Corona Virus Disease 2019 (COVID-19) – an illness caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – emerged from Wuhan, Hubei Province, China (Li et al., 2020). Since then, this disease has become a worldwide pandemic, with over two-hundred thousand deaths to date (WHO, 2020, COVID-19, 2020). Symptoms of SARS-Cov-2 infection range from asymptomatic disease to life-threatening acute respiratory distress syndrome (ARDS), severe pneumonia, acute kidney injury (AKI), myocarditis, eventual multi-organ failure, and death (Huang et al., 2020, Inciardi et al., 2020). Recent literature reported multiple neurological manifestations including cerebrovascular accidents in patients with severe infection (Mao et al., 2020). So far, there are no reported cases of COVID-19 presenting with strokes in the literature. Here, we report a case series of four patients that presented with ischemic stroke in the setting of PCR-confirmed SARS-CoV-2 infection.
2. Methods
We searched the hospital database for patients that presented with radiographic confirmation of acute stroke and concomitant features of suspected COVID-19 infection. All patients who had radiographic evidence of stroke and PCR-confirmed COVID-19 infection were included in the study. Patients admitted to the hospital with PCR-confirmed COVID-19 disease whose hospital course was complicated with acute stroke while inpatient were excluded from the study. Retrospective patient data was obtained from electronic medical records. Informed consent was obtained.
3. Results
We identified four patients who presented with radiographic evidence of acute stroke and PCR-confirmed COVID-19 disease. We elucidate the clinical characteristics, imaging findings, and clinical course. Laboratory data are presented in Table 1 .
Table 1.
Pertinent laboratory findings.
| Laboratory Findings | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|---|---|---|---|
| White blood cell count (4.8–10.8 K/μL) | 12.32 | 4.95 | 18.89 | 7.5 |
| Neutrophils (1.4–6.5 K/μL) | 10.95 | 3.41 | 16.36 | 5.3 |
| Lymphocytes (1.2–3.4 K/μL) | 0.67 | 1.07 | 1.15 | 1.5 |
| Platelet count (130–400 per mm3) | 182 | 138 | 380 | 176 |
| Hemoglobin (12–16 g/dL) | 15.5 | 15.1 | 12.9 | 11.6 |
| Albumin (3.5–5.2 g/dL) | 2.7 | 4.1 | 3.2 | 2.9 |
| Alanine aminotransferase (0–41 U/L) | 55 | 25 | 18 | 14 |
| Aspartate aminotransferase (0–41 U/L) | 47 | 31 | 34 | 18 |
| Lactate dehydrogenase (50–242 U/L) | NA | NA | 712 | 200 |
| Creatinine (0.7–1.5 mg/dL) | 1.1 | 1 | 1.6 | 1.8 |
| Cardiac Troponin T (<0.01 pg/ml) | <0.01 | <0.01 | 0.11 | 0.14 |
| Prothrombin Time (9.95–12.87 s) | 19.70 | 13.5 | 15.2 | 13.5 |
| Activated partial thromboplastin time (27–39.2 s) | 27.6 | 27.2 | 38.2 | 27.7 |
| D-Dimer (0–230 ng/mL) | NA | NA | 13,966 | 3442 |
| Ferritin (15–150 ng/L) | NA | NA | 891 | 135.9 |
| Procalcitonin (0.02–0.10 ng/mL) | 14 | NA | 0.49 | 0.08 |
| C-reactive protein (0.00–0.40 mg/dL) | 26.22 | NA | 16.24 | 12.7 |
| IL-6 (0–6.3 pg/mL) | NA | NA | NA | 8.5 |
Notes: The first available laboratory data are presented here. NA: Not available.
3.1. Patient 1
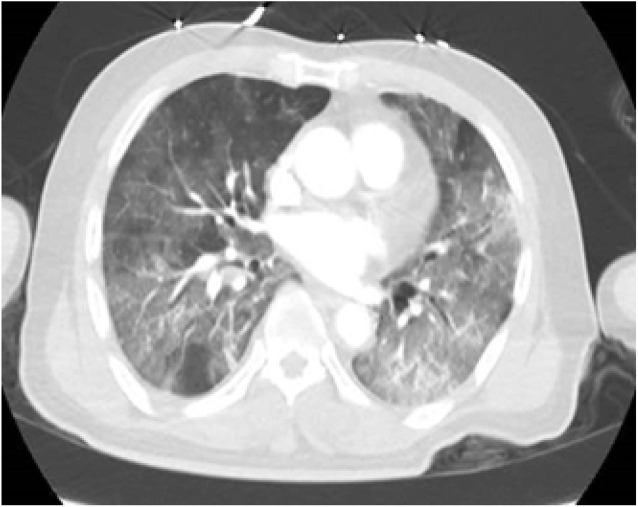
73-year-old male with a past medical history of hypertension, dyslipidemia, and carotid stenosis presented to the emergency department(ED) with fever, respiratory distress, and altered mental status. Review of systems was positive for dyspepsia, nausea, vomiting, reduced oral intake for two days, and negative for fevers or chills at home. He had a sick contact at home. Vital signs on presentation were fever of 101° F (38.3° C), tachycardia with heart rate (HR) of 102, hypoxemia with saturation of 85% on 100% non-rebreather mask. The patient was intubated in the ED for hypoxemic respiratory failure. A computed tomography (CT) scan of the head was performed for altered mental status, which demonstrated loss of gray-white differentiation at the left occipital and parietal lobes, consistent with acute infarct (Fig. 1a ). CT of the chest demonstrated bilateral peripheral patchy airspace opacities and diffuse ground-glass opacities, characteristic for atypical pneumonia/viral infection from COVID-19 (Fig. 1b ). Electrocardiogram (EKG) was within normal limits. Blood work was significant for leukocytosis with lymphopenia. Urine analysis was within normal limits. C-reactive protein was elevated to 26 mg/dl (0–0.4 mg/dl). D-Dimer was not checked during the hospital course. COVID-19 polymerase chain reaction (PCR) detected the virus. Blood and urine cultures did not yield any growth. A repeat CT of the head demonstrated progression toward a large acute infarct of the left MCA territory with hyperdense appearance of left MCA vessels - consistent with an acute thrombus. The patient was deemed not a candidate for thrombolysis or neuro-intervention due to poor functional status. The patient was treated with aspirin and other supportive measures. No abnormal heart rhythm was noted on telemetry monitoring. The family eventually decided to pursue comfort measures and terminally extubated the patient.
Fig. 1a.

A CT of the head demonstrating loss of gray-white differentiation at the left occipital and parietal lobes, consistent with acute infarct.
Fig. 1b.
CT chest demonstrating bilateral peripheral dominant patchy airspace opacities and diffuse ground glass opacities, characteristic for atypical pneumonia/viral infection from COVID-19.
3.2. Patient 2
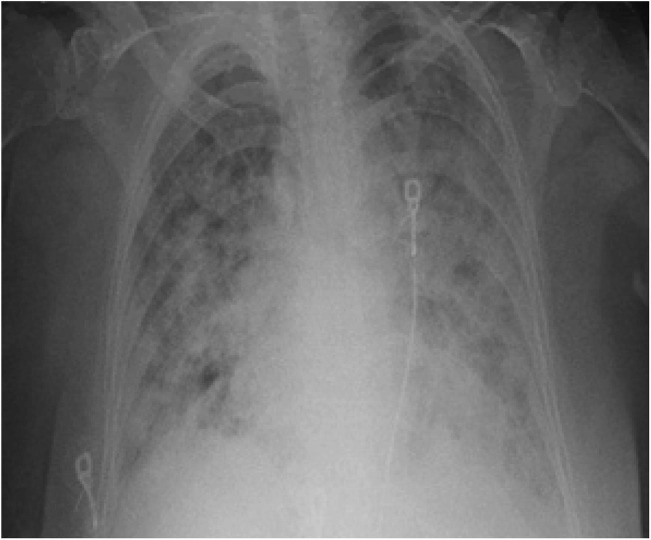
83-year-old female with a past medical history of frequent urinary tract infections, hypertension, hyperlipidemia, diabetes mellitus type 2, and neuropathy presented to the ED with fever, facial droop, slurred speech, and reduced oral intake. Initial vital signs were significant for temperature of 101.4° F (38.6° C), HR of 94, blood pressure (BP) of 172/64, saturating 94% on room air. The examination was significant for left facial droop and slurred speech with no other significant neurological deficits. National Institute of Health Stroke Scale (NIHSS) was calculated to be 2. Blood work was significant for leucopenia with lymphopenia. Urine analysis was within normal limits, chest X-Ray showed bilateral peripheral opacities. CT head was negative for any acute changes. CTA of head and neck demonstrated no large vessel occlusion, with focal moderate stenosis of right MCA (Fig. 2a ), and incidental pulmonary ground-glass opacities in bilateral apices. COVID-19 was suspected given the fevers and CT findings. COVID-19 PCR detected the virus. D-Dimer and other inflammatory markers were not checked. CXR progressively worsened with bilateral infiltrates (Fig. 2b ). Urine and blood cultures did not yield any growth. No arrhythmias were noted during the hospitalization. On day 3 of hospitalization, the patient developed left-sided hemineglect, worsening left-sided facial droop with left hemiparesis. The speech was normal. NIHSS was calculated to be 16. Repeat CT of the head demonstrated a new moderate hypodensity in the right frontal lobe representing acute infarct (Fig. 2c ). The patient was deemed too high risk for thrombolysis or neuro-intervention. A CT angiogram was planned prior to starting Integrellin, however her respiratory status worsened, requiring intubation and mechanical ventilation. Soon after, the family decided to withdraw care.
Fig. 2a.
CTA of head and neck demonstrated no large vessel occlusion, focal moderate stenisis of right MCA.
Fig. 2b.
CXR demonstrating worsening bilateral opacities.
Fig. 2c.
CT of the head demonstrated new moderate hypodensity in the right frontal lobe representing acute infarct.
3.3. Patient 3
80 year-old-female with a history of hypertension was brought to the ED for a chief complaint of altered mental status and left-sided weakness. The family denied history of fever or cough, but reported that the patient has been falling frequently in the past week. The patient was intubated for airway protection and a code stroke was activated. Vital signs in the ED were significant for Temp of 100.2° F (37.9° C), HR 101, BP 130/77, Examination was significant for left hemiplegia and aphasia. NIHSS was calculated to be 36. CT head revealed an acute right MCA stroke (Fig. 3a ). CTA of the head and neck demonstrated occlusion of the right internal carotid artery at origin and incidental bilateral patchy apical lung opacities (Fig. 3b ). CT perfusion demonstrated a 305 cc core infarct in the right MCA distribution and a surrounding 109 cc ischemic penumbra (Fig. 3c ). The patient was deemed not a suitable candidate for any acute neuro-intervention due to the large core infarct. Considering these characteristic CT findings, the patient was tested for COVID-19 infection with PCR and was positive. Laboratory data on admission demonstrated leukocytosis with lymphopenia, elevated d-dimer (13966 ng/ml DDU), along with elevated lactate dehydrogenase (712 U/L) and elevated C – reactive protein (16.24 mg/dl). The patient’s hospital course was complicated by acute kidney injury and progressively increasing oxygen requirements. On the third day of admission, her family chose for terminal extubation with comfort measures.
Fig. 3a.

CT head demonstrating acute right MCA stroke.
Fig. 3b.

CTA of the head and neck demonstrating occlusion of the right internal carotid artery at origin.
Fig. 3c.
CT perfusion demonstrating large core infarct.
3.4. Patient 4
An 88-year-old female with a past medical history of hypertension, chronic kidney disease, and hyperlipidemia presented to the ED with a transient 15-minute episode of right arm weakness and numbness along with word-finding difficulty. Vital signs on presentation were within normal limits. The initial neurological examination was normal. Code stroke was activated. CT head did not show any acute findings. EKG showed normal sinus rhythm. Thrombolysis was deferred, and she was admitted with a diagnosis of transient ischemic attack. As the patient complained of mild shortness of breath with dry cough, Covid-19 PCR was sent, which detected the virus. D-Dimer (<880 ng/ml) was elevated to 3,442 ng/ml, and other inflammatory markers were elevated as well. Magnetic Resonance Imaging (MRI) showed an acute infarct in the left medial temporal lobe (Fig. 4a ). Magnetic resonance angiogram (MRA) of the head and neck revealed mild stenosis of the right M1 segment (Fig. 4b ). No arrhythmias were noted on telemetry. Patient was treated with aspirin, statins, and was discharged to a rehab facility with an event monitor.
Fig. 4a.

MRI of the brain demonstrating acute infarct in the left medial temporal lobe.
Fig. 4b.
MRA of the head and neck demonstrating mild stenosis of right M1 segment.
4. Discussion
To our knowledge, this is the first series of cases with PCR confirmed COVID-19 infection presenting with a cerebrovascular accident. In the study of Mao et al., 5.7% of patients with severe infection developed cerebrovascular disease later in the course of illness (Mao et al., 2020). In a study by Li Y et al., the incidence of stroke in COVID-19 patients was about 5% with a median age of 71.6 years (Li et al., 2020). These patients were associated with severe disease and had a higher incidence of risk factors like hypertension, diabetes, coronary artery disease, and previous cerebrovascular disease (Li et al., 2020). Average time of onset of stroke after COVID-19 diagnosis was 12 days (Li et al., 2020). Elevated levels of CRP and D-dimer, indicating a high inflammatory state and abnormalities with the coagulation cascade, respectively, might play a role in the pathophysiology of stroke in the setting of COVID-19 infection (Li et al., 2020). Despite these reports, all four cases here presented with a cerebrovascular accident in early stages of their illness.
The SARS-CoV-2 virus is the seventh known variant of coronavirus that infects humans (Corman et al., 2019). SARS-CoV-2 is genetically similar to SARS-CoV-1 (Wu et al., 2020). SARS-CoV-1 in 2003 affected about 8000 patients with few reports of neurological manifestations - mostly peripheral neuropathy and encephalitis (Tsai et al., 2005). Similar to SARS-CoV-1, the current SARS-CoV-2 affects the neurological system in about 36.7% of patients as per Mao et al. (Mao et al., 2020). Most coronaviruses are neurotropic, and others speculate that SARS-CoV-2 is neurotropic too (Steardo et al., 2020). Furthermore, there are reports of SARS-CoV-2 being identified in cerebrospinal fluid by PCR (Moriguchi et al., 2020). Angiotensin converting enzyme-2 (ACE) receptors are the major entry points for SARS-Cov-2 and other coronaviruses (Hoffmann et al., 2020). Although ACE-2 receptors are present in the nervous system, alternate pathways have been proposed to explain the entry of SARS-CoV-2 into the nervous system, including direct injury to the blood and blood brain barrier, hypoxic injury, and immune-related injury (Steardo et al., 2020, Wu et al., 2020).
The exact pathophysiology behind these cerebrovascular accidents is still to be determined. Recent bacterial or viral infections have been known to cause strokes by increasing the risks for cardioembolic as well as arterio-arterial embolic events (Grau et al., 1998). A recent study from the Netherlands by Klock et al. demonstrated that 31% of critically ill ICU patients develop thrombotic complications (Kloka et al., 2020). Another study looking at activated partial thromboplastin time-based clot waveform analysis (CWA) in COVID-19 patients concluded that CWA parameters demonstrate hypercoagulability that precedes or coincides with severe illness (Tan et al., 2020). Multiple reports of pulmonary emboli are currently available in the literature (Danzi et al., 2020). Autopsy and pathology findings are scarcely available, but recent autopsy findings suggest thrombotic microangiopathy in multiple organs especially in the lungs and kidney (Fox et al., 2020, Barton et al., 2020, Yao et al., 2020). No autopsy reports of the brain are available at the time of this writing. With this evidence, the likely mechanism of early cerebrovascular accidents could be hypercoagulability leading to macro and micro thrombi formation in the vessels. Other pathophysiology could be directly related to the infection or hypoxia. Further studies are urgently needed for a comprehensive understanding of the neurological pathology of COVID-19 and its effects on the nervous system.
5. Treatment implications
The COVID-19 pandemic necessitates that extra measures be taken to provide and care for stroke patients, along with measures aimed at minimizing the spread of infection. Some of the challenges we encounter with acute stroke patients include their inability to effectively communicate due to speech problems, altered mental status, inadequate history (because of the limitations on visitors by hospital policies), etc.
Khosravani. et al. proposed a Protected Code Stroke (PCS) concept during this pandemic which provides a framework for key elements like screening guidelines, PPE, and crisis resource management (Khosravani et al., 2020). Based on previous studies, recommendations for PCS include: Paramedics should develop an infectious screening policy on all patients with stroke-like presentations, before bringing them to the hospital (Khosravani et al., 2020). Outside transfers should be minimized, and even the ones that need transfer should have an infectious screening before the transfer (Khosravani et al., 2020). A dedicated neurology hot-spot along with a mobile CT unit for COVID-19 patients with stroke-like symptoms is beneficial (Umapathi et al., 2004). Clinically stable patients after thrombolysis can be monitored on non-intensive care units (Umapathi et al., 2004).
6. Conclusion
Stroke teams should be wary of the fact that COVID-19 patients can present with cerebrovascular accidents and dawn appropriate personal protective equipment in every suspected patient. Plans should be developed not to neglect the management of acute cerebrovascular accidents, even though the control of COVID-19 infection is our biggest priority. More research is needed to identify the neurological implications of COVID-19 disease.
Funding
None.
Disclosures
None.
Conflict of interest
None.
References
- Li X., Zai J., Zhao Q. Evolutionary history, potential intermediate animal host, and cross-species analyses of SARS-CoV-2. J. Med. Virol. 2020 doi: 10.1002/jmv.25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Director-General's opening remarks at the media briefing on COVID-19 – 11 March 2020 [online]. Available at: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020.
- COVID-19 CORONAVIRUS PANDEMIC [online]. Available at: https://www.worldometers.info/coronavirus/.
- Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inciardi R.M., Lupi L., Zaccone G. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang M, Zhou Y, Chang J. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Available at SSRN: https://ssrn.com/abstract=3550025 March 3, 2020. [DOI] [PMC free article] [PubMed]
- Corman V.M., Lienau J., Witzenrath M. Coronaviruses as the cause of respiratory infections. Internist (Berl.) 2019;60:1136–1145. doi: 10.1007/s00108-019-00671-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A., Peng Y., Huang B. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27:325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai L.K., Hsieh S.T., Chang Y.C. Neurological manifestations in severe acute respiratory syndrome. Acta Neurol. Taiwan. 2005;14:113–119. [PubMed] [Google Scholar]
- Steardo L., Zorec R., Verkhratsky A. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19. Acta Physiol. (Oxf.) 2020 doi: 10.1111/apha.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T., Harii N., Goto J. A first Case of Meningitis/Encephalitis associated with SARS-Coronavirus-2. Int. J. Infect Dis. 2020 doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Xu X., Chen Z. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau A.J., Buggle F., Becher H. Recent bacterial and viral infection is a risk factor for cerebrovascular ischemia: clinical and biochemical studies. Neurology. 1998;50:196–203. doi: 10.1212/wnl.50.1.196. [DOI] [PubMed] [Google Scholar]
- Kloka F.A., Kruipb M.J.H.A., van der Meerc N.J.M., Arbousd MS. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020 doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C.W., Low J.G.H., Wong W.H., Chua Y.Y., Goh S.L., Ng H.J. Critically Ill COVID-19 infected patients exhibit increased clot waveform analysis parameters consistent with hypercoagulability. Am. J. Hematol. 2020 doi: 10.1002/ajh.25822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzi G.B., Loffi M., Galeazzi G., Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur. Heart J. 2020 doi: 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox SE, Akmatbekov A, Harbert JL, Li G, Brown JQ, Vander Heide RS. Pulmonary and cardiac pathology in Covid-19: the first autopsy series from new orleans. medRxiv 2020:2020.2004.2006.20050575. [DOI] [PMC free article] [PubMed]
- Barton L.M., Duval E.J., Stroberg E., Ghosh S., Mukhopadhyay S. COVID-19 autopsies, Oklahoma, USA. Am. J. Clin. Pathol. 2020 doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X.H., Li T.Y., He Z.C. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49:E009. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- Khosravani H., Rajendram P., Notario L., Chapman M.G., Menon B.K. Protected code stroke: hyperacute stroke management during the coronavirus disease 2019 (COVID-19) pandemic. Stroke. 2020 doi: 10.1161/STROKEAHA.120.029838. STROKEAHA120029838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umapathi T., Kor A.C., Venketasubramanian N. Large artery ischaemic stroke in severe acute respiratory syndrome (SARS) J. Neurol. 2004;251:1227–1231. doi: 10.1007/s00415-004-0519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]