Abstract
Objective
Chinese pediatricians are working on the front line to fight COVID-19. They have published a great amount of first-hand clinical data. Collecting their data and forming a large sample for analysis is more conducive to the recognition, prevention and treatment of coronavirus disease 2019 in children. The epidemic prevention and control experience of Chinese pediatricians should be shared with the world.
Methods
By searching Chinese and English literature, the data of 406 children with COVID-19 in China were analyzed.
Results
It was found that the clustered incidence of children's families is a dynamic transmission feature; the incidence is low; asymptomatic infections and mild cases account for 44.8%, with only 7 cases of critical illness; laboratory examination of lymphocyte counts is not reduced, as it is for adults; chest CT findings are less severe than those for adults. These presentations are the clinical features of COVID-19 in children. Only 55 of the 406 cases were tested by anal swab for virus nucleic acid, 45 of which were positive, accounting for 81.8% of stool samples.
Conclusion
There are more children than adults with asymptomatic infections, milder conditions, faster recovery, and a better prognosis. Some concealed morbidity characteristics also bring difficulties to the early identification, prevention and control of COVID-19. COVID-19 screening is needed in the pediatric fever clinic, and respiratory and digestive tract nucleic acid tests should be performed. Efforts should be made to prevent children from becoming a hidden source of transmission in kindergartens, schools or families. Furthermore, China's experience in treating COVID-19 in children has led to faster recovery of sick children.
Keywords: Coronavirus disease 2019, Transmission Ddynamics, Clinical Characteristics, Children, China
Introduction
Starting in mid-December 2019, a novel coronavirus (SARS-CoV-2) began to wreak havoc around the world, leading to the global spread of COVID-19. As of April 29, 2020, 3098695 COVID-19 cases were diagnosed globally, and 217081 people had died, involving 212 countries and regions.
On January 30, 2020, the WHO announced that COVID-19 caused by SARS-CoV-2 infection was an international public health event.1 As early as March 11, 2020, the WHO considered SARS-CoV-2 a global pandemic.2
The International Committee on Taxonomy of Viruses (ICTV) renamed the novel coronavirus (2019-nCoV) to SARS-CoV-2 and identified this virus as a sister virus to SARS coronavirus.3 On February 11, 2020, the WHO named the disease caused by SARS-CoV-2 to COVID-19 (coronavirus disease 2019).4 Chinese pediatricians have accumulated rich experience on the front line of the epidemic and pooled their first-hand data to form a large sample analysis, which can more fully reflect the clinical and transmission dynamies characteristics of COVID-19 in children. In this way, the incidence of COVID-19 can be better understood, and it will be more beneficial to prevent and control COVID-19 in children and allow the experience of Chinese pediatricians to be shared with the world.
Materials and methods
Methods
The following search terms were used using both Chinese and English vocabulary: "2019-nCoV, Children"; SARS-CoV-2, Children "; "COVID-19, Children” to search "www.cnki.net", "www.cqvip.com", "www.Wanfangdata.com.cn", "PubMed" and other databases. From February 1, 2020 to April 3, 2020, 54 Chinese and foreign studies on COVID-19 case data in children were collected, a total of 17 articles including foreign cases, literatures lacking main data, and repeated reports from the same unit were excluded. This article analyzes the data of 406 Chinese children in 37 articles. Understanding the clinical and transmission dynamies characteristics of COVID-19 in children is beneficial to the prevention and control of COVID-19 in children.
Data collection and analysis
From the collected articles, demographic, epidemiological, clinical, laboratory and CT image data were collected in a standardized form. Descriptive statistics were used to summarize COVID-19 demographics, propagation dynamics, and clinical data. Categorical variables are expressed in numbers and proportions.
Clinical data
General clinical information
Of the 406 confirmed cases, 219 were male and 187 were female, with a male to female ratio of 1.2:1.0, with ages from 2 days to 16 years and a median age of 7 years. Among them, 67 cases (16.5%) were <1 year old, 76 cases (18.7%) were 1 to 5 years old, 94 cases (23.2%) were >5 to 10 years old, 66 cases (16.3%) were >10 to 16 years old, and the age classification was unknown in 103 cases (25.4%). The minimum age was 36 hours after birth; the mother had COVID-19, and the SARS-CoV-2 nucleic acid test was positive 36 hours after the baby is born, and chest CT showed a thickened texture of the right upper lobe of the lung.5
Characteristics of clinical symptoms
The symptoms of COVID-19 are nonspecific, ranging from no symptoms to pneumonia and death.6 Fever and cough are more common. Of the 406 cases, 206 cases (50.7%) had fever, including 47 cases (11.6%) with a body temperature of 37.5°C - 38.0°C, 76 cases (18.7%) with 38.1°C - 39.0°C, 27 cases (6.6) with > 39.0°C, and 56 cases of unknown heat (13.6%). Two hundred cases (49.3%) did not have fever. The fever lasted for 1 to 3 days, and the longest was 16 days.
There were 172 cases of cough (42.4%), 12 cases of sputum (3.0%), 23 cases of runny nose (5.7%), 128 cases of pharyngeal redness (31.5%), 22 cases of diarrhea (5.4%), 22 cases of vomiting (5.4%), and 57 cases of shortness of breath (14.0%). There are also signs of poor mental health, fatigue, myalgia, and poor appetite, which mostly disappear in approximately 1 week.
Diagnosis was based on the "Novel Coronavirus Pneumonia Diagnosis and Treatment Program (Trial Version 7)" 7; 77 cases (19.0%) were asymptomatic infections, 105 cases (25.9%) were mild (upper respiratory tract infections), 170 cases were common cases (pneumonia) (41.9%), 47 cases were severe (severe pneumonia) (11.6%), and 7 cases were critically ill (critical pneumonia) (1.7%), of which there were 5 underlying diseases (2 cases of congenital heart disease, 1 case each of hydronephrosis, leukemia and intussusception). Among the 406 cases, there were 8 underlying diseases (2.0%). The other three cases were asthma, polycystic kidney disease, and pleural effusion. In 1 case of death, he had intussusception as the underlying disease.
Laboratory inspection characteristics
In 406 cases, the laboratory examination results only slightly increased or decreased. In routine blood tests, leukocytes increased slightly in 18 cases and decreased in 92 cases; neutrophils increased slightly in 12 cases and decreased in 35 cases; lymphocytes decreased in 33 cases, and children were not as consumptive as adult patients and were related to the severity of the disease.8 Among the infectious markers, procalcitonin (PCT) increased in 115 cases, and C-reactive protein (CRP) increased in 59 cases. Although it was slightly elevated, it also showed inflammatory activation. Among the biochemical markers, lactate dehydrogenase (LDH) increased in 20 cases, glutamate aminotransferase increased slightly in 34 cases, aspartate aminotransferase increased in 21 cases, with normal renal function indexes, blood coagulation indexes and electrolyte indexes. The change has no clinical significance. Of the 406 cases, information on 26 cases is unknown.
In 406 cases, respiratory specimens were positive for viral nucleic acid. Only 55 cases of anal swab virus nucleic acid tests were performed, and 45 cases were positive for nucleic acid, accounting for 81.8% of the feces.
Imaging features
Among the 406 cases, 107 cases (26.4%) had ground-glass lesions on CT, including 13 cases with consolidation and ground-glass shadows; 59 cases (14.5%) had unilateral lung consolidation changes, which showed localized patchy high-density shadow; 58 cases (14.3%) had bilateral bronchopneumonia-like changes, showing high density of patchy uneven density in the lung lobes; 4 cases (1.0%) showed pulmonary interstitial abnormalities; and 172 cases had normal lung CT (42.4%). The data of 6 cases (1.5%) were unknown.
In China's first critically ill child, a 1-year-old 1-month-old boy's CT showed diffuse ground-glass shadow and consolidation in the right lung, showing unilateral white lung manifestations, with visible air bronchial signs 9 (see Fig. 1 ). The child was a 8-year-old female, No fever 9 (see Fig. 2 ). The male patient was 6 months old, coughed for 2 days, and had a fever for 3 hours 9 (see Fig. 3 ).
Fig. 1.
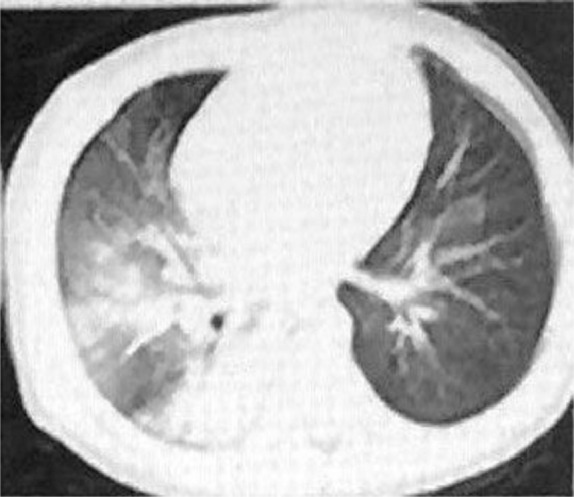
Severely ill type. Male, 1 year and 1 month. CT showed a diffuse ground-glass shadow and consolidation in the right lung, showing unilateral white lung manifestations, with air bronchial signs.
Fig. 2.
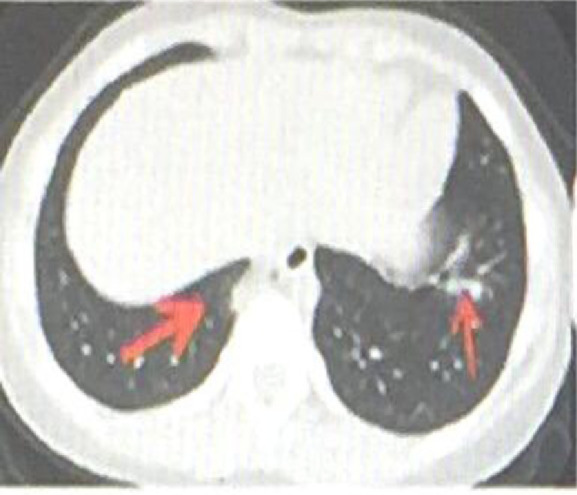
CT showed that the shape of the left lower lobe lesion was perpendicular to the chest wall and parallel to the bronchial vascular bundle (fine ↑). The basal lesions in the right lower lobe were located under the pleura, with uneven density and visible halo signs (thick ↓) .
Fig. 3.
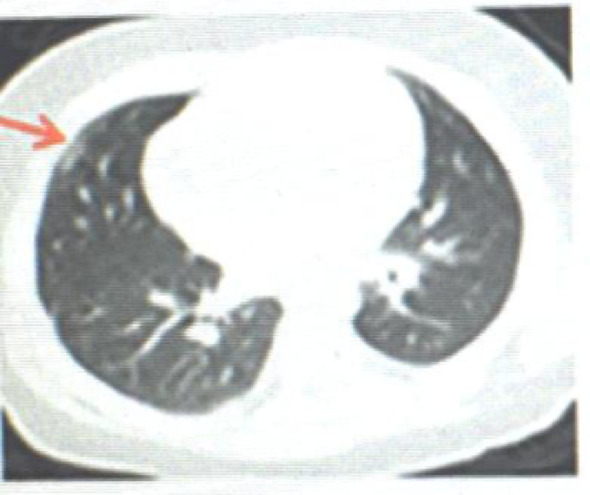
CT shows the subpleural ground glass shadow outside the middle lobe of the right lung (↑).
SARS-CoV-2 transmission dynamic characteristics
SARS-CoV-2 transmission dynamic and population susceptibility
SARS-CoV-2 is a novel β-coronavirus with a 96% total gene homology to SARS-like coronaviruses carried by bats.6 Structural analysis reveals that there is an atomic-level interaction between the SARS-CoV spike protein receptor binding domain (RBD) and its host receptor, angiotensin-converting enzyme 2 (ACE2), which regulates SARS-CoV between different species and cross communication between people. Several key residues in SARS-CoV-2 RBM (especially Gln493) provide a good interaction with human ACE2, indicating that SARS-CoV-2 has gained to the ability to spread between people.10
Everyone is susceptible to SARS-CoV-2,6 and the main source of infection is patients with SARS-CoV-2 infection. Children have less contact with the outside world, so there are fewer incidences than adults. Among the 406 cases in this article, familial cluster transmission is characteristic, accounting for 88.4% (359 cases); non familial contact transmission was found in 17 cases (4.2%), and 30 cases had unknown history (7.4%). The average incubation period is 5-6 days, ranging from 1-14 days.6
Although children are mostly infected by family members, children may also spread it to adults. A 3-month-old baby girl was admitted to the hospital on January 26, 2020 due to a fever. The blood routine was normal, and the upper respiratory tract viral nucleic acid test was positive on the second day. The texture of the CT lungs was thickened, and there were a few patchy shadows in the lower right lung field. Her diagnosis was COVID-19 (pneumonia). Her parents accompanied her without any symptoms. However, 7 days later, her father had fever and fatigue and CT left lungs with ground-glass-like shadows, and throat swabs were positive for SARS-CoV-2 nucleic acid. Although her mother was asymptomatic, the pharyngeal swab nucleic acid was positive for 2 consecutive days on February 3 and 4. On February 9th, the feces of the infant tested positive for nucleic acid. After supportive treatment, the child was discharged on February 10.11 In the first child COVID-19 case in China, a 10-year-old boy also possibly spreading the disease to adults,12 which is different from the literature report.6 There are many cases of asymptomatic and mild cases in children, which are relatively hidden. Children should be avoided as a hidden source of transmission in kindergartens, schools or families.
To date, the prevalence of children aged 18 and younger is relatively low, accounting for 2.4% of reported cases nationwide.6
Transmission dynamic mode and exploration
Airborne droplet transmission is the main mode of transmission, and it can also be transmitted through close contact. In a relatively closed environment, there is the possibility of aerosol transmission.7 It is still uncertain whether there is mother-to-child transmission and fecal-oral transmission risk.6 , 13 Especially the question of whether there is fecal-oral transmission is worth studying.
Among the 406 cases in this article, only 55 cases were tested for anal swab virus nucleic acid, and 45 cases were positive, accounting for 81.8% of the stool samples. At the beginning of the epidemic, there was insufficient understanding of the significance of anal swab nucleic acid detection. A 10-year-old boy had a history of close contact with the diagnosed patient, but he had no symptoms, and his blood routine and chest CT examination were normal. Nucleic acid samples from respiratory tract specimens were negative many times, but stool samples were positive for nucleic acids after 17 days and then turned negative after 9 days.14 There are also reports of children who were discharged from the hospital twice after a negative nucleic acid test and then returned to the hospital because of digestive tract symptoms and positive fecal nucleic acid.15 The author detected 73 cases of adult SARS-CoV-2-infected fecal virus RNA, and the positive rate accounted for 53.4%. After the respiratory viral nucleic acid was negative, 23.3% of the stools were still positive. Viral RNA was also detected in gastrointestinal tissue biopsy specimens of infected cases. Immunofluorescence staining of the ACE2 receptor and viral nucleocapsid protein in gastric, duodenal and rectal epithelium were positive, which provided evidence for gastrointestinal infection.13 Nucleic acid positivity in anal swabs mostly occurs after positive respiratory tract specimens, and the negative time is longer than that in respiratory tract specimens, indicating that the digestive tract clears viruses slower than the respiratory tract.12 Is the novel coronavirus shed from the respiratory tract and swallowed into the digestive tract? It is unclear whether the respiratory and digestive tracts are also infected. The long-term discharge of SARS-CoV-2 in feces may pollute the environment and endanger public health.16 Is there a risk of fecal-oral transmission? Should the isolation (or discharge) of the patient be terminated before the viral nucleic acid in the feces becomes negative? It is worth exploring.
Discussion
Enlightenment of clinical characteristics of COVID-19 in children
The incidence of children increases with age, which may be related to the increased range of activities of older children and increased contact with COVID-19 patients. Children with fever and cough are common, accounting for 50.7% and 42.4%, respectively. Asymptomatic infections and mild children accounted for 182 cases (44.8%), which is approximately half of them, and critical illnesses accounted for a relatively small portion (2.0% in this article). Severe cases included 2.5% of reported cases in children, 0.2% in critical cases, and a crude mortality rate of 3.8% in adults.6 One of the 406 cases in this article died.
The prevalence of COVID-19 in children is low, and the condition is mild, which may be related to the protection of children by parents and the small range of activities. ACE2 is an important binding receptor for SARS-CoV-2 infecting humans.6 , 11 The maturity and function (such as binding ability) of ACE2 receptors in children are lower than those in adults, making them less sensitive to SARS-CoV-2. It is also relevant to make the load of infected SARS-CoV-2 less. In addition, the immune function of children is still in the development process. The intensity of the excessive immune stress response (inflammatory cytokine storm) is not as strong as that of adults, which reduces damage to the body. However, these factors need to be further studied.
Treatment characteristics of COVID-19 in children and exploration
The principles of the novel Coronary Virus Pneumonia Diagnosis and Treatment Program (Trial Seventh Edition) of the National Health Commission should be followed and the importance of the experience of adult patients should be emphasized.7 Of course, each hospital also has its own rich clinical diagnosis and treatment experience.
Antiviral therapy
There are currently no effective antiviral drugs. Interferon alpha-2b atomized inhalation can be tried as follows: The ordinary type is 100,000-200,000 IU/kg each time, the severe type is 200,000-400,000 IU/kg each time, 2 times/d, and the treatment course is 5-7 days; or it can be added with ribavirin intravenous infusion, 10 mg/kg each time (maximum amount 500 mg/time), 2-3 times/d. For abidor, adults take 20 mg, 3 times a day, and the course of treatment does not exceed 10 days. Special attention should be paid to adverse drug reactions and drug interactions.7 , 17 Clinical trials have initially shown that chloroquine phosphate has a significant effect.18 The remdesivir clinical phase III study enrolled 168 patients with severe disease and 17 patients with ordinary type, but it still needs to wait for the final evaluation results. A case of COVID-19 in the United States achieved good results with the application of remdesivir.19
Glucocorticoid
Avoid routine use of corticosteroids. It is not beneficial to use early in the course of the disease and may spread the virus. Adult treatment experience is that if there are any 3 of the following 4 items, consider using it: ① Fever above 38.5°C for ≥ 3 days; ② CRP ≥ 30 mg/L; ③ serum ferritin ≥ 1000 μg/L; ④ diffuse lesions of both lungs. The above changes indicate that the lung disease of the patient is in the advanced stage. Short-term therapy with methylprednisolone is recommended. The initial dose is 1 to 2 mg / (kg • d), which is administered in 2 doses, and then decreases gradually after heat remission until the drug is stopped. The total treatment course is 3 to 5 days.20
The WHO guidelines suggest that people with SARS-CoV-2 infection cannot benefit from glucocorticoid therapy and are more likely to be injured as a result. Except for lung injury or shock caused by SARS-CoV-2, glucocorticosteroids should not be used.21
Intravenous immunoglobulin (IVIG)
IVIG for critical pediatric patients can be considered; the recommended dose is 0.2 g/(kg • d), and the treatment course is 3 to 5 days.7
Novel coronary pneumonia patients will produce specific antibodies against novel coronavirus after treatment and recovery, which can kill and eliminate the virus. Using this specific immune plasma therapy is the most effective method, which can reduce the mortality of critical patients.22 , 23 In the first phase, 3 critically ill patients and 10 subsequent critically ill patients received treatment for 12 to 24 hours. The main inflammation indicators were significantly reduced, the proportion of lymphocytes increased, blood oxygen saturation, viral load and other indicators were better, and clinical signs and symptoms improved significantly.24
Reasonable use of antibacterial drugs
Avoid blind or inappropriate use of antibiotics, especially in combination with broad-spectrum antibacterial drugs.
Chinese medicine treatment
In this fight against the epidemic, Chinese medicine has achieved very good results, and children can be treated with syndrome differentiation treatment.
There are some deficiencies in this article. First, there are fewer cases in children, and the situation may not be comprehensive. Second, the heterogeneity of the collected data has some difficulties in comprehensive analysis.Third, the few cases that cannot be classified uniformly can only be classified as unknown, which affects the accuracy of classification.
In short, COVID-19 in children has the following characteristics: (1) children are susceptible to SARS-CoV-2 at all ages, even newborns who are born soon; (2) children have family cluster characteristics; (3) the proportion of men and women is approximately equal; (4) the clinical symptoms are mainly fever and cough, and some children have no clinical symptoms; (5) there are no major abnormalities in laboratory tests, and lymphocytes are not reduced as in adults; (6) chest CT performance is lighter than adults and includes ground-glass shadow and patchy high-density shadow;(7) a single negative nucleic acid sample in the upper respiratory tract cannot rule out the diagnosis. It should be combined with clinical manifestations, especially chest CT manifestations, to make a judgment; (8) the child's condition is mild, recovery is fast, the treatment effect and prognosis are good, and one case has died so far. The above characteristics are worth paying attention to: asymptomatic, light cases are more concealed, which makes early identification and early isolation difficult in children with COVID-19, and children should be avoided as a hidden source of transmission power for kindergartens, schools or families.
Declaration of Competing Interest
The authors declare no conflicts of interest
Acknowledgments
Acknowledgments
The authors thank Dr. Guo Hai-Hong from the Institute of Medical Informatics, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences for his guidance in data analysis.
Author contribution
Professor Yang Zhen-Dong designed this study, collected materials, wrote the manuscript, and revised and translated the manuscript. Professor Zhou Gao-Jun, Professor Jin Run-Ming, Professor Liu Zhi-Sheng, Professor Dong Zong-Qi Associate professor Xie Xiong and Professor Song Guo-Wei all provided materials for the manuscript writing and contributed to the manuscript review. All authors reviewed and approved the submitted manuscript.
Funding
no
References
- 1.WHODirector-General's statement on IHR Emergency Committee on Novel Coronavirus (2019-nCoV). (2020-01-30). https://www.who.int/dg/speeches/detail/who-director-general-s-statement-on-ihr-emergency-committee-on-novel-coronavirus-(2019-ncov).
- 2.WHODirector-General's opening remarks at the media briefing on COVID-19-11March 2020. (2020-03-11). https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020.
- 3.ICTV. Naming the 2019 Coronavirus. (2020-03-02) https://talk.ictvonline.org/
- 4.WHODirector-General's remarks at the media briefing on 2019-nCoV on 11February 2020. (2020-02-11). https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020.
- 5.Wang S., Guo L., Chen L. A case report of neonatal COVID-19 infection in China [published online ahead of print, 2020 Mar 12] Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa225. ciaa225. [DOI] [Google Scholar]
- 6.The Health Committee of the People ’s Republic of China. China-WHO New Coronavirus Pneumonia (COVID-19 Joint Investigation Report. (2020-02-29). https: //www.nhc.gov.cn/.
- 7.The General Office of the National Health and Health Commission. The Office of the State Administration of Traditional Chinese Medicine. Novel Coronavirus Pneumonia Diagnosis and Treatment Program(Trial Version 7). (2020-03-04) https://www.gov.cn/zhengce/zhengceku/2020-03/04/content_5486705.htm
- 8.Wang D., Hu B., Hu C., Zhu F. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020 Feb 7 doi: 10.1001/jama.2020.1585. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huijing M., Jianbo S., Yongjiao W. High-resolution CT manifestations of children with novel coronavirus pneumonia. Chinese Journal of Radiology. 2020;54(00) doi: 10.3760/cma.j.issn.1005-1201.2020.0002. E002-E002. [DOI] [Google Scholar]
- 10.Wan Y., Shang J., Graham R., Baric R.S., Li F.Receptor recognition by novel coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS. J Virol. https://jvi.asm.org/content/early/2020/01/23/JVI.00127-20. DOI: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed]
- 11.Yuehua Zhang, Meifang Xiao, Jiachong Wang. A case of new coronavirus infection in three-month-old infants. Chin J Pediatr. 2020;58 doi: 10.3760/cma.j.issn.0578-1310.2020.0006. 2020-02-11https: //rs.yiigle.com/yufabiao/1180186.htm. [DOI] [Google Scholar]
- 12.Chan J.F., Yuan S., Kok K.H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2 [published online ahead of print, 2020 Mar 3] Gastroenterology. 2020;S0016-5085(20):30282. doi: 10.1053/j.gastro.2020.02.055. -1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang An, Tong Zhen-dong, Wang Hong-ling. Detection of novel coronavirus by RT-PCR in stool specimen from asymptomatic child, China. Emerg Infect Dis. Jun 2020 doi: 10.3201/eid2606.200301. [date cited]https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang T., Cui X., Zhao X. Detectable SARS-CoV-2 Viral RNA in Feces of Three Children during Recovery Period of COVID-19 Pneumonia [published online ahead of print, 2020 Mar 29] J Med Virol. 2020 doi: 10.1002/jmv.25795. 10.1002 / jmv.25795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He Y., Wang Z., Li F., Shi Y. Public health might be endangered by possible prolonged discharge of SARS-CoV-2 in stool [published online ahead of print, 2020 Mar 4] J Infect. 2020;S0163-4453(20):30111. doi: 10.1016/j.jinf.2020.02.031. -0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhimin Chen, Junfen Fu, Qiang Shu. Children ’s 2019 Coronary Virus Disease (COVID-19) Diagnosis and Treatment Guidelines (Second Edition) Journal of Zhejiang University (Medical Edition) February 2020 doi: 10.3785/j.issn.1008-9292.2020.02.01. https: //www.zjujournal.com/med. [DOI] [Google Scholar]
- 18.Gao J., Tian Z., Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies [published online ahead of print, 2020 Feb 19] Biosci Trends. 2020 doi: 10.5582/bst.2020.01047. 10.5582 / bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 19.Holshue M.L., DeBolt C., Lindquist S. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001191. January 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng Fang, Xiaoping Luo. Facing the major epidemic situation of 2019 new coronavirus infection: thinking of pediatricians. Chin J Pediatr. 2020;58(02):81–85. doi: 10.3760/cma.j.issn.0578-1310.2020.02.001. [DOI] [Google Scholar]
- 21.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. [Online ahead of print] Lancet. 2020 Feb 7 doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Long, Xiong Jing, Bao Lei, Shi Yuan. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30141-9. Published Online February 27https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen C., Wang Z., Zhao F. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA. 2020 doi: 10.1001/jama.2020.4783. Published online March 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.China Biology: Rehabilitation-specific plasma treatment of 11 people has a significant effect, and it is recommended that donors donate blood. (2020-02-13). https://news.ifeng.com/c/7u2ZOUAAt5w.


