To the Editor
GNA11 and GNAQ are highly homologous genes encoding different Gα subunits of the Gαq subfamily of heterotrimeric G-proteins. GNAQ-mutation mosaicism has previously been found to cause Sturge-Weber syndrome (SWS) and isolated capillary malformations (Shirley et al., 2013). We recently described postzygotic activating mutations in GNA11 or GNAQ as causes of phakomatosis pigmentovascularis (PPV) (Thomas et al., 2016), a group of conditions defined by the co-occurrence of pigmentary and vascular birthmarks (Happle, 2005, Ota et al., 1947), and GNAQ mosaicism as a cause of extensive or atypical dermal melanocytosis (EDM) (Thomas et al., 2016). Subsequently, GNA11 mosaicism was identified as a cause of capillary malformation and overgrowth in a limb (Couto et al., 2017). Systematic phenotypic description and phenotype-genotype studies have not yet been performed across this disease spectrum. Of note, somatic pathogenic variants in GNAQ and GNA11 have been described in melanocytic tumors (Van Raamsdonk et al., 2010, Van Raamsdonk et al., 2009) and in congenital hemangiomas (Ayturk et al., 2016).
In this international cohort study of 45 patients, of whom 44 were children and 39 were recruited sequentially and prospectively from a single center, we used deep phenotyping and DNA sequencing of skin biopsies to characterize the clinical spectrum, analyze phenotype-genotype correlations, and delineate predictors of adverse outcomes. Inclusion criteria were a clinical diagnosis of SWS, PPV, or EDM. Cutaneous features recorded were the presence or absence of types of capillary malformation (port-wine stain, reticulate patterning, or nevus anemicus), types of pigmented lesion (dermal melanocytosis, café-au-lait macule, or nevus spilus), and involvement of the forehead area (as previously defined [Waelchli et al., 2014]) by vascular and/or pigmentary lesions. EDM was specifically defined to distinguish it from common Mongolian blue spots as (i) extending outside the lumbosacral area and (ii) either not visibly fading by the age of one year, and/or having very clearly delineated edges, and/or being very deeply pigmented.
DNA was extracted directly from affected skin from all 45 patients, with informed written consent under local Research Ethics Committee approval. All samples were sequenced for GNAQ and GNA11 hotspot mutations affecting codons 183 and 209 in both genes, as previously described (Thomas et al., 2016)—briefly, initially by Sanger sequencing with restriction enzyme digest and, if wild-type, by deep targeted next generation sequencing with a sensitivity of 1% mutant allele detection. Statistical modeling of two adverse clinical outcomes - any neurological or ophthalmological abnormalities - was by multiple logistic regression with independent variables being phenotypic and genotypic data as above.
Clinical phenotyping data and outcome frequencies are shown in Table 1. Certain features are of note. First, most patients with PPV, independent of the subtype, developed multiple café-au-lait macules over time. This was also seen in EDM but not in SWS. Some of these café-au-lait macules would be considered typical, but most were atypical, with irregular or broken up edges and/or large in size (Fois et al., 1993). Second, macrocephaly was seen in 9% of cases where head circumference had been documented, all of whom had capillary malformations as part of the phenotype. Third, asymmetry of growth was seen in 35%. Fourth, two patients had renal hypertension. The first of these presented incidentally following a routine blood pressure measurement but later developed severe headaches and proteinuria and was found to have an enlarged edematous left kidney with a renal cyst. The second was diagnosed during investigation for recurrent acute life-threatening events from central apnea, and was found to have stenosis of the superior right renal artery. Fifth, one patient with PPV developed hypopigmented mycosis fungoides, and one patient with EDM developed a dermatofibromasarcoma protuberans. Finally, two patients with PPV type II or cesioflammea died, however as both cases were referred to us for genotyping due to the severity of clinical disease, this is likely to be a higher incidence than a true prospective cohort figure. One of these died in infancy, with recurrent apnea and classical changes of SWS on magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) (recently reported in detail; Sliepka et al., 2019), and the second died in adulthood, after sudden onset of multiple intracranial hemorrhages with no discoverable underlying MRI/MRA vascular abnormality and no hypertension.
Table 1.
Demographic and Phenotypic Features and Outcomes of the Cohort of 45 Patients by Genotype
| Feature, diagnosis, or outcome | GNAQ mutant | GNA11 mutant | Double wild type | Total GNAQ or GNA11 mutant/total cohort (%) |
|---|---|---|---|---|
| Male | 9 | 5 | 4 | 18/45 (40%) |
| White Caucasian | 6 | 2 | 5 | 13/42 (31%) |
| Diagnosis of SWS | 4 | 1 | 2 | 7/45 (16%) |
| Diagnosis of PPV | 8 | 7 | 9 | 24/45 (69%) |
| Diagnosis of EDM | 2 | 0 | 12 | 14/45 (31%) |
| Presence of any capillary malformation | 11 | 8 | 10 | 29/45 (64%) |
| Presence of port-wine stain (nevus flammeus) | 9 | 3 | 4 | 16/45 (36%) |
| Presence of a pale pink capillary malformation (nevus roseus) | 1 | 0 | 3 | 4/45 (9%) |
| Presence of reticulate or telangiectatic vascular patterning | 5 | 5 | 1 | 11/45 (24%) |
| Presence of nevus anemicus | 1 | 2 | 0 | 3/45 (7%) |
| Presence of pigmentary abnormalities of any type | 10 | 7 | 18 | 35/43 (81%) |
| Presence of dermal melanocytosis | 9 | 4 | 14 | 27/42 (64%) |
| Presence of café-au-lait macular pigmentation | 5 | 3 | 7 | 15/36 (42%) |
| Forehead involvement, vascular | 6 | 4 | 4 | 14/39 (36%) |
| Forehead involvement, pigmentary | 3 | 2 | 3 | 8/41 (20%) |
| Forehead involvement, either vascular or pigmentary | 7 | 5 | 7 | 19/40 (48%) |
| Epidermal nevus | 0 | 0 | 0 | 0/45 (0%) |
| Hypertrophy | 6 | 2 | 3 | 11/37 (28%) |
| Hypotrophy | 0 | 1 | 1 | 2/37 (5%) |
| Macrocephaly | 2 | 1 | 0 | 3/34 (9%) |
| Ophthalmological abnormalities | 7 | 4 | 2 | 13/28 (46% of those tested) |
| Glaucoma | 6 | 3 | 2 | 11/30 (37% of those tested) |
| Heterochromia irides | 0 | 1 | 0 | 1/36 (3%) |
| Any neurological abnormality | 8 | 3 | 4 | 15/41 (37%) |
| Neurodevelopmental abnormalities | 7 | 2 | 2 | 11/41 (27%) |
| Seizures | 6 | 0 | 2 | 8/42 (19%) |
| MRI/MRA head abnormalities | 6 | 1 | 4 | 11/20 (55% of the 20 scanned) |
| All other internal organ anomalies (including renal) | 0 | 3 | 0 | 3 (most not screened) |
| Renal or renovascular anomalies | 0 | 2 | 0 | 2 (most not screened) |
| Hypertension | 0 | 2 | 0 | 2 (most not measured) |
Abbreviations: EDM, extensive dermal melanocytosis; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging; PPV, phakomatosis pigmentovascularis; SWS, Sturge-Weber syndrome.
Where the cohort totals do not equal 45, this is because of missing data. This cohort includes eight patients from our previous publication (Thomas et al., 2016). In 21 of 22 cases, these mutations affected codons 183 of GNAQ (p.(R183Q)) or GNA11 (p.(R183C)) in all except one (p.(R183S) [Thomas et al., 2016]), and in one case of EDM, the mutation affected codon 209 (p.(Q209P) [Thomas et al., 2016]). We have subdivided capillary malformations into port-wine stain/nevus flammeus and pale pink telangiectatic nevi to reflect recent publications suggesting clinical subdivision may be helpful.
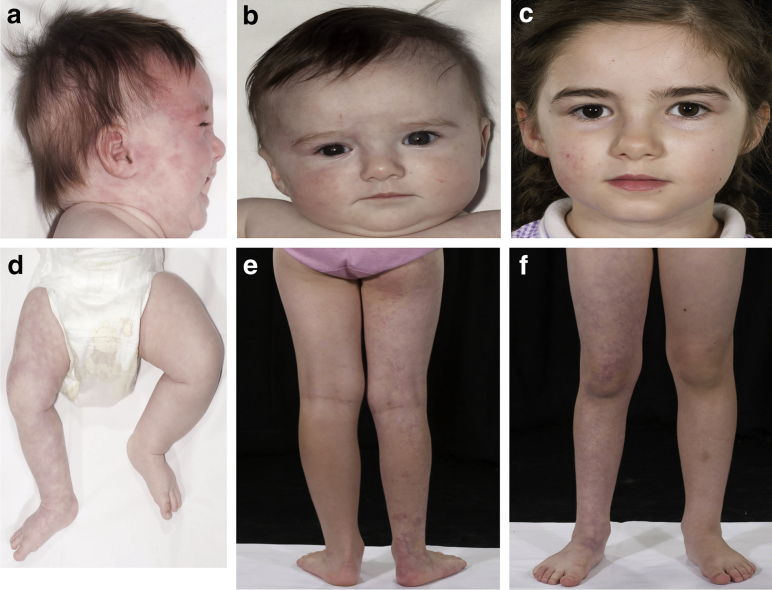
GNAQ or GNA11 mutations were found in 49% of patients with these clinical diagnoses (Table 1). Those with a diagnosis of SWS or PPV had a higher percentage of mutation positivity (71% and 63%, respectively) than those with EDM (14%). Notably, one patient with SWS was GNA11 mosaic, presenting with bilateral extensive (including forehead) reticulate vascular capillary malformation, glaucoma, hypotrophy of the right face and right leg, and a normal MRI/MRA (Figure 1). In addition, a GNAQ-mutant patient with SWS could also have been classified as nevus vascularis mixtus (Hamm and Happle, 1986), bringing this diagnosis also into this genotypic spectrum.
Figure 1.
Clinical features of GNA11-variant Sturge-Weber syndrome. (a–c) Clinical features of the patient with GNA11-variant Sturge-Weber syndrome at birth and with a congenital telangiectatic capillary malformation on the right side of the face extending onto the forehead, subtle facial asymmetry, and iris heterochromia. (d–f) There is extension of the capillary malformation onto the right arm and leg with associated right leg undergrowth. Pictures a, b, and d were taken at 4 months of age and pictures c, e, and f at 6 years of age. The parent/guardian of this patient consented in writing to publication of images.
A cutaneous vascular lesion in the previously delineated forehead area was confirmed to be a strong predictor of neurological and ophthalmological abnormalities as previously described (Waelchli et al., 2014) (odds ratio of having neurological abnormalities with a forehead area lesion 8.5, 95% confidence interval 1.8–39.7, P = 0.006; and odds ratio of ophthalmological abnormalities with a forehead lesion 60, 95% confidence interval 4.7–763.0, P = 0.002). There were no cases of neurological abnormalities or glaucoma in SWS or PPV where there was no vascular involvement of the forehead area; however, there was one case of abnormal vasculature on MRI/MRA in a child with a clinical diagnosis of EDM without cutaneous vascular lesions. Pigmentary lesions in the forehead area without accompanying vascular lesions were not used as an independent variable as the numbers were too low; however, there are known associations of oculodermal melanocytosis with glaucoma and ocular melanoma (Shields et al., 2011, Teekhasaenee et al., 1990).
Genotype did not affect adverse outcome measures in childhood. Phenotypic modeling by genotype was not undertaken because of small numbers in each phenotypic variable; however, these data hint that GNA11 mosaicism may be associated with telangiectatic capillary malformation, nevus vascularis mixtus, and hypotrophy/undergrowth. Our recommendations for urgent ophthalmological assessment and for routine central nervous system MRI/MRA after birth for children with SWS and PPV remain for any child with a capillary malformation involving any part of the defined forehead area (Waelchli et al., 2014) and for anyone with relevant symptoms. This enables early detection and treatment of glaucoma and early detection of abnormal neurovasculature and consideration for prophylaxis with aspirin and/or anticonvulsants. Our in-house recommendations for EDM currently are for any child with this diagnosis as defined here to have a screening ophthalmological examination after birth or if any symptoms arise. For EDM, currently we do not perform MRI/MRA in the absence of neurological symptoms, but will continue to audit this practice. Although the risk of cutaneous or ocular melanoma arising in patients with PPV and EDM appears to be relatively low in the literature, it is important that families and ophthalmologists are made aware of this as a possibility. Subsequent to this cohort study, we recommend that clinicians are alert to the possibilities of cafe-au-lait macules, macrocephaly, hypertension, and asymmetric growth in children from birth and throughout childhood.
Data availability statement
No datasets were generated or analyzed during the current study.
ORCIDs
Satyamaanasa Polubothu: http://orcid.org/0000-0001-7195-5670
Lara Al-Olabi: http://orcid.org/0000-0001-9947-6989
Alisha Chacko: http://orcid.org/0000-0002-3596-4462
George Eleftheriou: http://orcid.org/0000-0001-5674-9086
Maria Carmen del Boente: http://orcid.org/0000-0001-9390-6079
Mary Glover: http://orcid.org/0000-0002-6789-7241
David Jiménez-Gallo: http://orcid.org/0000-0003-1161-6698
Elizabeth A. Jones: http://orcid.org/0000-0002-0059-8829
Debra Lomas: http://orcid.org/0000-0003-4490-0038
Regina Fölster-Holst: http://orcid.org/0000-0001-9114-2351
Samira Syed: http://orcid.org/0000-0002-3872-5870
Monika Tasani: http://orcid.org/0000-0002-8139-3058
Anna Thomas: http://orcid.org/0000-0001-6586-465X
Antonio Torrelo: http://orcid.org/0000-0002-5940-6916
Martin Tisdall: http://orcid.org/0000-0001-8880-8386
Sarah Aylett: http://orcid.org/0000-0001-9630-3222
Veronica A. Kinsler: http://orcid.org/0000-0001-6256-327X
Conflict of Interest
The authors state no conflict of interest.
Acknowledgments
The authors gratefully acknowledge the participation of all patients and families in this study and research coordination by Jane White. VAK, AT, and the work presented in this study were funded by the Wellcome Trust (Grant WT104076MA). SP was funded by Caring Matters Now Charity and by the Newlife Foundation. The work was supported by the GOSHCC Livingstone Skin Research Centre, the UK National Institute for Health Research through the Biomedical Research Centre at Great Ormond St Hospital for Children NHS Foundation Trust, and the UCL GOS Institute of Child Health.
Author Contributions
Conceptualization: VAK; Formal Analysis: VAK, SP; Investigation: SP, LA-O, ACT; Resources: VAK, SP, MCB, AC, GE, MG, DJ-G, EAJ, DL, RF-H, SS, MTa, MTi, AT, SA; Writing - Original Draft Preparation: VAK, SP; Writing - Review and Editing: SP, LA-O, MCB, AC, GE, MG, DJ-G, EAJ, DL, RF-H, SS, MTa, ACT, MTi, AT, SA, VAK
Accepted manuscript published online 12 December 2019; corrected proof published online 3 February 2020
References
- Ayturk U.M., Couto J.A., Hann S., Mulliken J.B., Williams K.L., Huang A.Y. Somatic activating mutations in GNAQ and GNA11 are associated with congenital hemangioma. Am J Hum Genet. 2016;98:789–795. doi: 10.1016/j.ajhg.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto J.A., Ayturk U.M., Konczyk D.J., Goss J.A., Huang A.Y., Hann S. A somatic GNA11 mutation is associated with extremity capillary malformation and overgrowth. Angiogenesis. 2017;20:303–306. doi: 10.1007/s10456-016-9538-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fois A., Calistri L., Balestri P., Vivarelli R., Bartalini G., Mancini L. Relationship between café-au-lait spots as the only symptom and peripheral neurofibromatosis (NF1): a follow-up study. Eur J Pediatr. 1993;152:500–504. doi: 10.1007/BF01955059. [DOI] [PubMed] [Google Scholar]
- Hamm H., Happle R. [Mixed vascular nevus. Report of 4 cases] Hautarzt. 1986;37:388–392. [in German] [PubMed] [Google Scholar]
- Happle R. Phacomatosis pigmentovascularis revisited and reclassified. Arch Dermatol. 2005;141:385–388. doi: 10.1001/archderm.141.3.385. [DOI] [PubMed] [Google Scholar]
- Ota M., Kawamura T., Ito N. Phakomatosis pigmentovascularis. Jpn J Dermatol. 1947;52:1–31. [Google Scholar]
- Shields C.L., Kligman B.E., Suriano M., Viloria V., Iturralde J.C., Shields M.V. Phacomatosis pigmentovascularis of cesioflammea type in 7 patients: combination of ocular pigmentation (melanocytosis or melanosis) and nevus flammeus with risk for melanoma. Arch Ophthalmol. 2011;129:746–750. doi: 10.1001/archophthalmol.2011.135. [DOI] [PubMed] [Google Scholar]
- Shirley M.D., Tang H., Gallione C.J., Baugher J.D., Frelin L.P., Cohen B. Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N Engl J Med. 2013;368:1971–1979. doi: 10.1056/NEJMoa1213507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliepka J.M., McGriff S.C., Rossetti L.Z., Bizargity P., Streff H., Lee Y.S. GNA11 brain somatic pathogenic variant in an individual with phacomatosis pigmentovascularis. Neurol Genet. 2019;5:e366. doi: 10.1212/NXG.0000000000000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teekhasaenee C., Ritch R., Rutnin U., Leelawongs N. Ocular findings in oculodermal melanocytosis. Arch Ophthalmol. 1990;108:1114–1120. doi: 10.1001/archopht.1990.01070100070037. [DOI] [PubMed] [Google Scholar]
- Thomas A.C., Zeng Z., Rivière J.B., O'Shaughnessy R., Al-Olabi L., St-Onge J. Mosaic activating mutations in GNA11 and GNAQ are associated with phakomatosis pigmentovascularis and extensive dermal melanocytosis. J Invest Dermatol. 2016;136:770–778. doi: 10.1016/j.jid.2015.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk C.D., Bezrookove V., Green G., Bauer J., Gaugler L., O'Brien J.M. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009;457:599–602. doi: 10.1038/nature07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk C.D., Griewank K.G., Crosby M.B., Garrido M.C., Vemula S., Wiesner T. Mutations in GNA11 in uveal melanoma. N Engl J Med. 2010;363:2191–2199. doi: 10.1056/NEJMoa1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waelchli R., Aylett S.E., Robinson K., Chong W.K., Martinez A.E., Kinsler V.A. New vascular classification of port-wine stains: improving prediction of Sturge-Weber risk. Br J Dermatol. 2014;171:861–867. doi: 10.1111/bjd.13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during the current study.