Abstract
Purpose of review:
Although vulnerable populations are disproportionately exposed to synthetic chemicals with endocrine disrupting properties, few recent reviews have summarized the impact of synthetic chemicals on cardiometabolic health among these groups.
Recent findings:
Of 37 eligible epidemiological studies among vulnerable populations published between January 2018 and April 2019 in which over half were prospective, the most investigated populations were pregnant women and children. Racial/ethnic minorities, individuals of low socioeconomic status (SES), and those occupationally-exposed were studied the least. The most studied persistent organic pollutants (POPs) were per-/poly-fluoroalkyl substances (PFAS), and the most studied non-POPs were phenols. Across chemical classes, studies found certain POPs (e.g., PFAS) and non-POPs (i.e., phenols, phthalates, and parabens) to be associated with gestational diabetes and dysregulated glucose metabolism. Results for other cardiometabolic health outcomes were inconsistent but suggested certain chemicals may negatively affect cardiometabolic health.
Summary:
Synthetic chemicals likely adversely affect cardiometabolic health, but current findings were inconclusive. Few recent studies focused on racial/ethnic minorities, low SES, and occupationally-exposed populations. To address poor cardiometabolic health and related disparities, more studies across vulnerable populations are warranted.
Keywords: endocrine disruptors, metabolic diseases, cardiovascular diseases, minority health, vulnerable populations, pregnancy complications
INTRODUCTION
Considered a major public health burden in the United States (1), cardiometabolic conditions like type 2 diabetes mellitus (T2DM), chronic kidney disease (CKD), cerebrovascular disease, and cardiovascular disease (CVD) constitute four of the ten leading causes of US deaths (2). Vulnerable populations like racial/ethnic minority groups (e.g., non-Hispanic black (hereafter referred to as black) and Hispanic/Latino) as well as individuals with low socioeconomic status (SES) are disproportionately affected compared to non-Hispanic white (hereafter referred to as white) and higher-SES individuals (3–6). Health conditions like obesity and gestational diabetes mellitus (GDM) are also of concern among pregnant women due to their potential health implications across the life course for both the mother and offspring (7–9). As the prevalence of obesity and related metabolic abnormalities increase among youth, preventing poor cardiometabolic health and focusing on health promotion is increasingly important among children and adolescents (10). Although there are multifactorial causes for poor cardiometabolic health across the life course, exposure to synthetic chemicals throughout the life course is increasingly recognized as potentially contributing to both poor cardiometabolic health outcomes and cardiometabolic health disparities (11–13).
Synthetic chemicals are made by humans using non-natural methods, and synthetic chemical structures may or may not be found in nature. Synthetic chemical classes including pesticides, fungicides, herbicides, and insecticides ; industrial solvents/lubricants and their byproducts (e.g., polychlorinated biphenyls (PCBs), flame retardants such as polybrominated diphenyl ethers (PBDEs); chemicals used in the production of polycarbonate plastics and epoxy resins (e.g., bisphenol A (BPA)); plasticizers (e.g., phthalates); chemicals found in personal care products (e.g., parabens); and pharmaceutical agents (e.g., diethylstilbestrol (DES)) have several characteristics that may prove harmful to human cardiovascular health (14). Exposure to synthetic chemicals is ubiquitous and humans are often exposed at low levels (15). Synthetic chemicals are of public health importance because many are considered endocrine disruptors or endocrine-disrupting compounds (EDCs). EDCs, which are present in water, soil, food, and various consumer products (e.g., furniture, electronics, personal care products [e.g., deodorants], cosmetics [e.g., fragrances], pharmaceuticals, pesticides, and plastics [e.g., medical devices, food packaging/containers, children’s toys]), are defined by the US Environmental Protection Agency as “natural or synthetic exogenous agent[s] that interfere with synthesis, secretion, transport, metabolism, binding action, or elimination of natural blood-borne hormones that are present in the body and are responsible for homeostasis, reproduction and developmental process” (14). EDCs can disrupt the functioning of several bodily organs and systems including the hypothalamus, pituitary gland, thyroid, mammary glands, pancreas, adipose tissue, cardiovascular system, and reproductive system (14). Regarding an example of cardiometabolic health, EDCs may increase diabetes risk by interfering with glucose homeostasis regulation and insulin secretion by targeting pancreatic alpha cells that, in turn, impair molecular signaling and lead to glucagon secretion in response to low blood glucose levels (13, 14).
Recent literature highlighted the importance of conducting epidemiologic investigations explicitly among vulnerable populations (16), and we are considering vulnerable populations to be pregnant women and children, the economically disadvantaged, and racial/ethnic minorities based on the Belmont Report (17). Duran and Pérez-Stable highlighted the needed for minority health research because it is important to understand “health characteristics and attributes of racial and/or ethnic minority groups, who are socially disadvantaged due in part by being subject to potential discriminatory acts” (16). Further, a health disparity has recently been defined as “a health difference that adversely affects defined disadvantaged populations (i.e., blacks/African Americans, Hispanics/Latinos, American Indians/Alaska Natives, Asian Americans, Native Hawaiians and other Pacific Islanders, socioeconomically-disadvantaged populations, underserved rural populations, sexual and gender minorities) based on one or more health outcomes” (16). Based on this definition, cardiometabolic health outcomes – obesity, hypertension, T2DM, CKD, and CVD – meet the criteria for health disparities because of the often observed higher incidence or prevalence of disease, including earlier onset or more aggressive progression; premature or excessive mortality from specific conditions; and poorer health behaviors as well as clinical outcomes related to the health conditions (3–6, 16). Not only do cardiometabolic health outcomes meet the aforementioned criteria for minority health and health disparities research, but exposure to synthetic chemicals has been found to be higher among these groups (13, 18–24), which further underscores the importance of understanding the state of the literature regarding the relationship between synthetic chemicals and cardiometabolic health among vulnerable populations.
As illustrated in the socioecological conceptual framework, modifiable environmental and social factors may contribute to disparate exposures across populations (see Figure 1). For instance, Zota and Shamasunder described potential explanations for why women of color generally have higher body burden of beauty product-related chemicals (22). “Upstream” determinants (or the root causes) like discriminatory practices and policies related to targeted marketing of chemical products to racial/ethnic minorities can affect intermediate determinants such as manufacturing formulations (e.g., hair relaxers with lye) and product preferences to achieve a culturally desirable appearance (e.g., Eurocentric beauty standards of straight hair) across social contexts (22). These determinants may then influence “downstream” behaviors like product uptake or usage of hair relaxers and skin lighteners by individuals desiring a Eurocentric beauty standard (22). More broadly, “upstream” or fundamental determinants can also increase the likelihood of synthetic chemical exposures by, for example, racial/ethnic discrimination reducing job opportunities for racial/ethnic minorities (influencing income and residential location), residential segregation leading to closer proximity to point sources of pollution from, for example, water and air, and consumption of processed foods that contain EDCs through “food deserts” or “food swamps” with less healthy food options.
Figure 1.
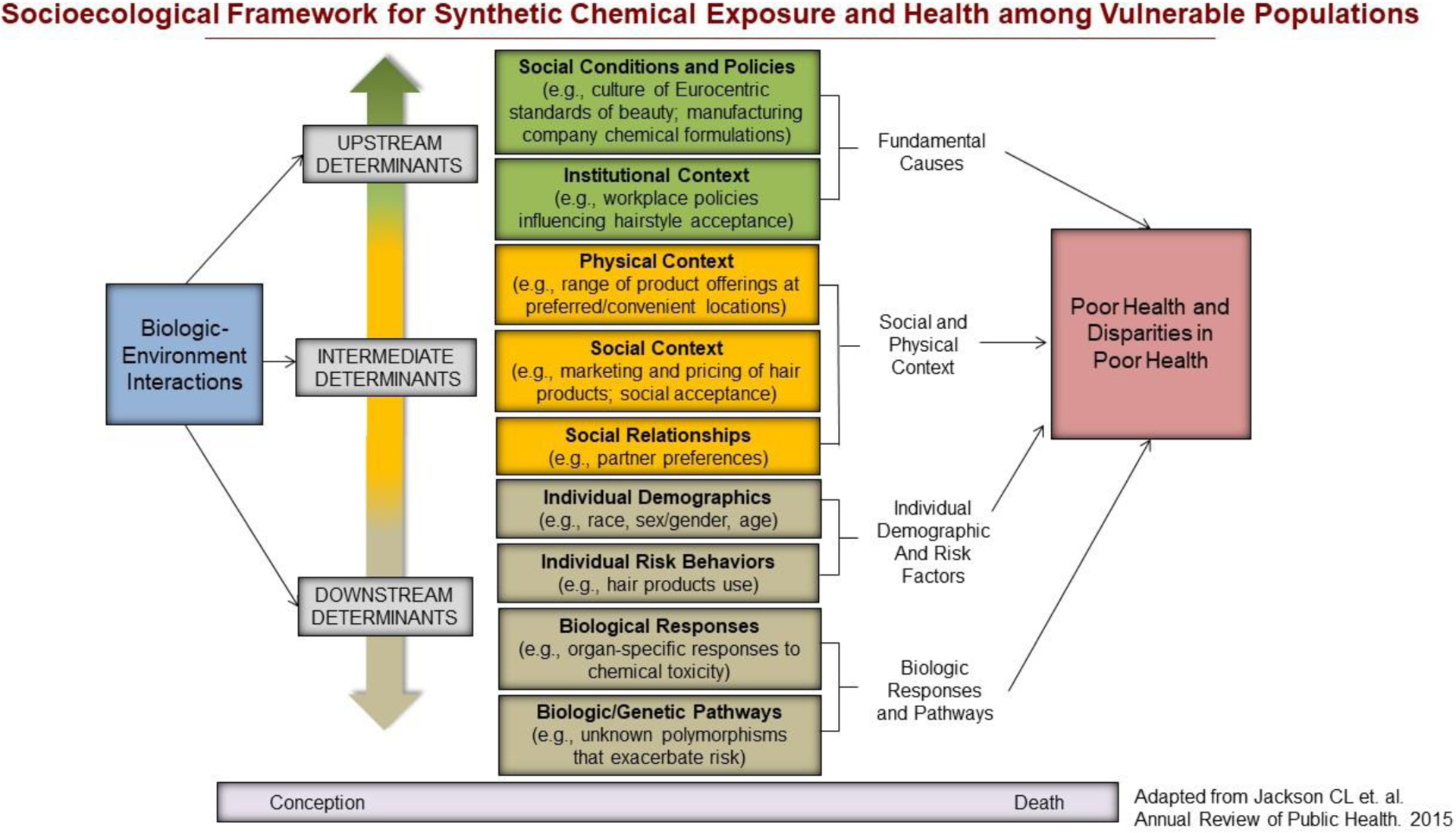
Socioecological Framework for Synthetic Chemical Exposure and Health among Vulnerable Populations
Although vulnerable populations are disproportionately exposed to synthetic chemicals with endocrine disrupting properties, few reviews have summarized studies of the impact of multiple synthetic chemicals on cardiometabolic health within these populations (13, 25). Further, few have comprehensively summarized these impacts on several prevalent cardiometabolic health outcomes across life stages and vulnerable populations in the US (7, 13, 25–27). To address this gap, the objective of this non-systematic, narrative review was to summarize the scientific literature published over a 16-month period (between Jan 2018 to April 2019). Eligible studies had to investigate associations between synthetic chemicals and cardiometabolic health within vulnerable populations in the US. Our goal is not to exhaustively discuss all chemicals that have been implicated as contributors to poor cardiometabolic health; therefore, we will not include a discussion of air pollution/related by-products or metals/metalloids.
METHODS
We searched PubMed, Web of Science, and Scopus using Medical Subject Heading (MeSH) and advanced search terms (see supplemental materials). Eligibility criteria included: (1) peer-reviewed original research among populations located in the US (i.e., no meta-analyses including studies from other countries, literature reviews, or published abstracts); (2) publications between January 1, 2018 and April 1, 2019 to capture relevant studies published from 2018 to the time we began the literature search and data extraction (April 23, 2019); and (3) focus on at least one vulnerable population (i.e., pregnant women, children, racial/ethnic minority group, low SES, or occupationally-exposed). Exposures of interest included synthetic chemicals like persistent organic pollutants (POPs) (pesticides [e.g., dichlorodiphenyl-trichloroethane/dichlorethylene (DDT/DDE)]; industrial chemicals [e.g., PCBs, PBDEs, per- and poly-fluoroalkyl substances (PFAS)]; and transformation products of these chemicals) and non-POPs (phenols; phthalates; parabens; pharmaceutical agents of concern [previously suggested to adversely affect cardiometabolic health outcomes]; acrylamide; and solvents). Cardiometabolic outcomes of a priori interest included gestational hypertension, preeclampsia, or gestational diabetes; birth weight (due to its association with later life cardiometabolic health (28)); weight or adiposity; elevated blood glucose, insulin resistance, or type 2 diabetes mellitus; elevated blood pressure or hypertension; elevated cholesterol or dyslipidemia; kidney function or kidney disease; liver function, liver disease, or non-alcoholic fatty liver disease; and cardiovascular disease.
We screened titles and abstracts of articles from the search to first determine if eligibility/inclusion criteria were met. After identification of relevant articles, we read full-text and extracted main findings using a standardized data extraction form. Relevant articles related to POPs and non-POPs are separately described. Results are additionally organized by chemical class (i.e., persistent pesticides, fungicides, herbicides, and insecticides; industrial chemicals and transformation by-products; phenols; phthalates, parabens; pharmaceutical agents; acrylamide; and non-specified solvents). Within each class, we present results for each vulnerable population in the following order (if applicable): pregnant women, children, race/ethnicity, low SES, and occupationally-exposed. Within each vulnerable population, we then present cardiometabolic health outcomes in the following order (if applicable): obesity, hypertension, diabetes, dyslipidemia, kidney functioning/disease, liver disease, and cardiovascular disease. Because vulnerable populations can overlap in one study (e.g., investigations of multiple health outcomes among pregnant women also stratified by race/ethnicity, some results were concurrently discussed.
RESULTS
Thirty-seven studies met inclusion criteria. Most of the eligible studies were of pregnant women (n=9) and children (n=20), and over half used a prospective study design (mainly among pregnant women and children, n=20). Studies determined associations across different congeners, metabolites, and subtypes of chemicals, which resulted in complex findings that are difficult to summarize/synthesize. While we do not describe all specific congeners, metabolites, and subtypes in this review, we summarize the important findings of prespecified classes (Table 1).
Table 1.
Published manuscripts of synthetic chemicals and cardiometabolic health outcomes among vulnerable populations (January 2018 to April 2019)
| Author, publication year | Vulnerable population | Study population | Study design | Synthetic chemical | Cardiometabolic Health outcome | Main finding |
|---|---|---|---|---|---|---|
| Both Persistent Pesticides, Fungicides, Herbicides, and Insecticides and Industrial Chemicals/Transformation-products | ||||||
| Rahman et al., 2019 (40) | Pregnant women | 2,334 NH-White, NH-Black, Asian, and Hispanic non-obese pregnant women (8–12 weeks gestation at enrollment) | Prospective | POPs- 11 OCPs, 9 PBDEs, 44 PCBs, and 11 PFAS | GDM | Certain PBDEs, heavily chlorinated PCBs, and certain PFAS were positively associated with GDM with suggestion of effect modification by family history of T2DM and adiposity. |
| Buck Louis et al., 2018 (33) | Children | 2,106 NH-White, NH-Black, Hispanic, and Asian/Pacific Islander women with singleton pregnancies | Prospective | 11 OCPs, 1 polybrominated biphenyl, 9 PBDEs, 44 PCBs, and 11 PFAS | Birth weight | There were no consistent associations between prenatal EDCs and birthweight across any races/ethnicities. |
| Bulka et al., 2019 (34) | Occupationally-exposed | 7,404 Hispanic/Latino males and females aged 18–74 years | Cross-sectional | organic solvents, pesticides | CVD (CHD, heart failure, atrial fibrillation, cerebrovascular disease) | Solvent exposure in the primary job was not associated with any CVD outcome. Pesticide exposure at the current job was associated with two times the prevalence of CVD and CHD and six times the prevalence of cerebrovascular disease. |
| Persistent Pesticides, Fungicides, Herbicides, and Insecticides | ||||||
| Shaw et al., 2018 (41) | Pregnant women | 295,387 NH-White and Hispanic pregnant women | Case-control | 543 individual chemicals used as pesticides or as adjuvants in pesticides products or application mixtures and 69 physiochemical groupings | Preeclampsia | There was a generally no association between pesticide exposures and elevated risk of preeclampsia. |
| Ling et al., 2018 (38) | Children | 24,693 preterm and 220,297 term births and 4,412 term low birthweight and 194,732 term normal weight infants of NH-White, NH-Black, Hispanic, Asian/Pacific Islander, and other race/ethnicity | Cross-sectional | Organophosphates, pyrethroids, and carbamates | Term low birth weight | There was a positive association between maternal exposure to myclobutanil and the pyrethroid class and term low birthweight. Pesticide ORs were stronger for non-US born and US-born Hispanic mothers compared to NH-white mothers; however, OR’s were not stronger across all three chemical classes for any single racial/ethnic subgroup. |
| Hoffman et al., 2018 (36) | Children | 349 NH-White and non-NH-White mother-infant pairs | Prospective | OPEs | Birth weight | Higher prenatal OPEs were associated with lower birthweight but not after standardization for gestational age. |
| Boyle et al., 2019 (32) | Children | 784 children aged 6–19 years | Cross-sectional | OPEs | BMI z-score, WC | Certain OPEs were associated with higher odds of obesity while others were associated with lower odds, and sex modified the associations. |
| Industrial Chemicals and Transformation-products | ||||||
| Vuong et al., 2019 (42) | Children | 206 NH-White and non-NH-White children aged 8 years at follow-up | Prospective | PBDEs at 1,2,3,5, and 8 years | Weight, BMI, WC, body fat percentage | For most PBDEs, there was no evidence that postnatal PBDEs were associated with higher adiposity at age 8 years. Sex modified associations between BD3–153 and adiposity. |
| Shoaff et al., 2018 (45) | Children | 345 NH-White, NH-Black and of other race/ethnicity mother-child pairs | Prospective | PFAS | Birth weight | There were weak, non-significant associations between four measured (prenatal) PFAS and birthweight z-scores. |
| Yeung et al., 2019 (43) | Children | 1,954 singletons and 966 twins of NH-White, NH-Black, Hispanic, Asian, and other race/ethnicity | Prospective | PFAS | Obesity and weight gain | PFOS an PFOA at delivery were associated with lower BMI but not with obesity among singletons. Associations were inconsistent among twins |
| Alderete et al., 2019 (31) | Children | 40 Hispanic/Latino males and females with a mean age (SD)=11.4 (2.0) years | Prospective | PFAS | Glucose metabolism | Higher plasma PFAS concentrations were associated with dysregulated glucose levels. |
| Khalil et al., 2018 (37) | Children | 48 NH-White, NH-Black, and Hispanic/Latino obese males and females aged 8–12 years | Cross-sectional | PFAS | BMI, systolic BP, diastolic BP, lipid profiles, glucose metabolism, insulin resistance | Higher PFAS were positively associated with adverse lipid profiles and higher systolic BP. |
| Mora et al., 2018 (39) | Children | 682 NH-White, NH-Black, and other race/ethnicity pregnant women and their offspring | Prospective | PFAS | Lipid profiles and liver function/disease (alanine aminotransferase) | Associations between PFAS and both lipid profiles and liver function varied by prenatal vs. childhood concentrations and by sex. |
| Dong et al., 2019 (35) | Children | 2,987 US adolescents aged 12–19 years (NHANES) | Cross-sectional | PFAS | Lipid profiles | There were no associations between PFAS and lipids. |
| Jain et al., 2018 (44) | Children | 458 US children aged 6–11 years (NHANES) | Cross-sectional | PFAS | Lipid profiles | PFAS were associated with total cholesterol and suggestively associated with lipoproteins. |
| Non-persistent pollutants | ||||||
| 61 Bellavia et al., 2018 (58) | Pregnant women | 350 pregnant women of NH- White, NH-Black, Hispanic, Asian and other race/ethnicity | Prospective | Phenols | Glucose | First and 2nd trimester urinary BPA concentrations were associated with moderate, non-significant increases in glucose levels. BMI was an effect modifier. |
| Polinski et al., 2018 (23) | Pregnant women | 466 pregnant women aged ≥16 years of NH-White, NH-Black, Hispanic, and other race/ethnicity | Cross-sectional | Phenols | Pre-pregnancy BMI | Benzophenone-3 and triclosan concentrations were significantly higher among women with normal BMI vs. overweight/obese and who were NH-White vs. non-NH-White. |
| Messerlian et al., 2018 (62) | Children | 346 singleton male and female infants of couples who sought medically assisted reproduction of NH-White, NH-Black, Asian, and other race/ethnicity | Prospective | Phenols | Birth weight | Paternal preconception benzophenone-3 levels were positively associated with birth weight, and these association varied by BMI of the father. Higher prenatal triclosan concentrations were associated with lower birth weight. |
| Kalloo et al., 2018 (56) | Children | Males and females aged 8 years (N=220- prenatal, N=212- early childhood, N=218- age 8 years) of NH-White, NH-black, and other race/ethnicity | Prospective | Phenols | Adiposity (BMI z-score, WC, body fat percentage) | Urinary triclosan concentrations during pregnancy, early childhood, and at 8 years of age were not associated with measures of childhood adiposity at age 8 years. |
| Yeung, et al., 2019 (43) | Children | 1,954 singletons and 966 twins of NH-White, NH-Black, Hispanic, Asian, and other race/ethnicity | Prospective | Phenols | Weight gain and obesity | BPA was associated with rapid weight gain at 4, 9, and 12 months and obesity. |
| Liu et al., 2019 (61) | Children | 745 US children and adolescents aged 6–17 years (NHANES) | Cross-sectional | Phenols | Obesity | Bisphenol F concentrations were positively associated with obesity and abdominal obesity with effect modification by race/ethnicity. Associations with BPA varied by sex. There were no associations with BPS. |
| Verstraete et al., 2018 (64) | Children | 944 US adolescents aged 12–19 years (NHANES) | Cross-sectional | Phenols | Non-alcoholic fatty liver disease | The odds of suspected non-alcoholic fatty liver disease were higher in the second quartile of BPA concentrations compared to the first quartile. This association was greater in Hispanic compared to NH-White adolescents. |
| Bethea et al., 2019 (54) | Black women | 1,693 Black women aged 23–34 years | Cross-sectional | Phenols | BMI | BPA, BPS, and triclocarban concentrations were correlated with BMI. |
| James-Todd et al., 2018 (60) | Pregnant women | 245 NH-White and non-NH-White pregnant women aged 18–46 years | Prospective | Phthalates | Glucose | Second trimester MEP concentrations were positively associated with 2nd trimester glucose levels. MiBP concentrations were inversely associated with glucose levels. |
| Shaffer et al., 2019 (70) | Pregnant women | 705 pregnant women aged ≥18 years of NH-White, NH-Black, Asian, Hispanic, and other race ethnicity | Prospective | Phthalates | Impaired glucose tolerance, continuous blood glucose, and GDM | In the evaluation of associations with 11 urinary phthalate metabolites, certain phthalate metabolites were associated with glucose intolerance with possibly stronger associations among racial/ethnic subgroups. Several phthalates were associated with elevated blood glucose only among Asians. |
| Polinski et al., 2018 (23) | Pregnant women | 466 pregnant women aged ≥16 years of NH-White, NH-Black, Hispanic, and other race/ethnicity | Cross-sectional | Phthalates | Pre-pregnancy BMI | Concentrations of high molecular weight phthalates were higher among women who were overweight vs. normal weight prior to pregnancy. |
| Wenzel et al., 2018 (66) | Pregnant Women | 378 NH-White and NH-Black pregnant women at 18–22 weeks gestation who were aged ≥18 years | Cross-sectional | Phthalates | BMI | After stratifying by race: age, BMI, education, and income were significantly associated with phthalate concentrations in NH-Black women, while only marital status was significantly associated with phthalate concentrations in NH-White women. |
| Zhou et al., 2018 (67) | Pregnant women | 115 Hispanic/Latina pregnant women aged ≥18 years | Cross-sectional | Phthalates | Metabolomic profiles | There were positive associations between higher phthalate concentrations and poor metabolomic profiles that suggested changes in lipid biosynthesis and inflammation. |
| Chiu et al., 2018 (65) | Children | 300 mother (aged 18–45 years)-infant pairs of NH-White, NH-Black, Asian, and other race/ethnicity | Prospective | Phthalates | Birth weight | There were no significant associations between prenatal individual phthalates or phthalate mixtures and birth weight. |
| Gaston et al., 2019 (68) | Children | 918 NH-White, NH-Black, and Hispanic/Latino adolescents aged 12–19 years (NHANES) | Cross-sectional | Phthalates | MetS | There was a suggestive positive association between MnBP and MiBP and MetS among adolescents. |
| Noor et al., 2019 (69) | Children | 350 mother-infant pairs of NH-White, NH-Black, Asian, and Hispanic/other race/ethnicity | Prospective | Phthalates | Birthweight, LGA | There were generally no associations between phthalate metabolite concentrations and birthweight or LGA after stratification by maternal glucose tolerance. |
| Bellavia et al., 2019 (59) | Pregnant women | 241 NH-White and non-NH-White pregnant women aged 18–45 years | Prospective | Parabens | Glucose | Measured at the first and second trimesters, butylparaben was positively associated and propyl paraben was negatively associated with glucose levels. |
| Messerlian et al., 2018 (62) | Children | 346 singleton male and female infants of couples who sought medically assisted reproduction of NH-White, NH-Black, Asian, and other race/ethnicity | Prospective | Parabens | Birth weight | Among males but not females, higher prenatal propylparaben concentrations were associated with lower birth weight. |
| Bethea et al., 2019 (54) | Black women | 1,693 Black women aged 23–34 years | Cross-sectional | Parabens | BMI | Butyl paraben and methyl paraben concentrations were correlated with BMI. |
| Quiros-Alcala et al., 2018 (57) | Children | 1,324 children aged 6–19 years of NH-White, NH-Black, Mexican, and other race/ethnicity (NHANES) | Cross-sectional | Parabens | BMI z-score, WC | Children/adolescents with higher methylparaben concentrations were less likely to be obese, especially females. |
| Troisi et al., 2018 (71) | Children | 3,941 DES-prenatally exposed and 1,705 DES-unexposed women | Prospective | Pharmaceutical agents of concern | CVD | Women who were prenatally exposed DES were twice as likely to develop coronary heart disease and myocardial infarction compared to unexposed women. |
| Litvak et al., 2018 (72) | Black women | 216 NH-White and NH-Black women with HER2-positive, invasive breast cancer | Retrospective chart review | Pharmaceutical agents of concern | Cardiotoxicity | Compared to NH-Whites, NH-Black women had higher rates of cardiotoxicity, resulting in incomplete cancer treatment therapy. |
| 78 Huang et al., 2018 (75) | Racial/ethnic minority groups | 8,364 US adults aged 20–85+ years (NHANES) | Cross-sectional | Acrylamide | Overweight, obesity, abdominal obesity | Two out of three measures of acrylamide were positively associated with adiposity markers (i.e., overweight, obesity, and abdominal obesity); conversely, one acrylamide measure was negatively associated with the adiposity markers. Associations did not vary by race/ethnicity. |
| Huang et al., 2018 (74) | Racial/ethnic minority groups | 5,504 US adults aged ≥25 years (NHANES) | Prospective | Acrylamide | CVD mortality | Two out of three measures of acrylamide were negatively associated with CVD mortality among non-smokers; conversely, one acrylamide measure was not associated with CVD morality. Associations did not vary by race/ethnicity. |
| Callahan et al., 2019 (76) | Occupationally-exposed | 5,369 NH-White and non-NH-White male and female dry-cleaning workers in a union | Prospective | Non-specified solvents | CVD mortality | High exposure was associated with higher risk of mortality from heart disease |
| DeBono et al., 2019 (77) | Occupationally-exposed | 4,396 United Autoworkers members of NH-White, NH-Black, Hispanic, and other/missing race/ethnicity | Prospective | Non-specified solvents | CVD mortality | Compared to other workers, those who were more likely to be exposed to adverse environmental conditions (because of poor ventilation, solvent exposure in closed areas, and possible asbestos exposure) had higher risk of CVD mortality. |
POPs were studied the most. Suggestive associations between both POPs and non-POPs with altered glucose metabolism, GDM, and T2DM or associated risk factors were most consistently studied and, therefore, observed. Despite prior literature revealing disproportionate burdens of both synthetic chemical exposure and poor cardiometabolic health by race/ethnicity and socioeconomic status, each sociodemographic characteristic was rarely investigated as a moderator or an effect modifier. As a result, we briefly identify and discuss studies of POPs/non-POPs and cardiometabolic health that included these groups as illustrative examples, although they did not meet inclusion criteria but are relevant to minority health research.
Persistent Organic Pollutants (POPs)
POPs represent a group of toxic chemicals that do not easily break down in the environment (29). Certain POPs also bioaccumulate in the fat tissue of both animals and humans (29) while others are hypothesized to accumulate by binding to proteins (30). As a result, POPs can circulate through the food chain and persist in the environment. There are detectable levels of POPs in water, food (e.g., fish), air, and a variety of consumer products, including commercially available pesticides, insecticides, and paints (29). Therefore, humans are commonly exposed through ingestion, inhalation, and dermal routes. In 2001, the Stockholm Convention on POPs identified 12 of greatest concern, an additional 16 were added to this list in 2017 (29), and several more have been recently proposed. Subcategories of POPs included in this review are pesticides (e.g., dichlorodiphenyl-trichloroethane/dichlorethylene (DDT/DDE)), industrial chemicals (e.g., PCBs, PBDEs, per- and poly-fluoroalkyl substances (PFAS)), and transformation products of these chemicals. POPs are of concern due to their persistence, exposure likelihood, and previous findings of endocrine disrupting properties (26, 27).
Eligible studies of POPs recently reported inconsistent associations with birthweight, had mixed results regarding obesity and lipid dysregulation that varied by sex/gender, and several studies suggested that exposure to individual pesticides, PBDEs, PCB congeners, and PFAS may be associated with poor cardiometabolic health outcomes across the life course, including GDM, glucose metabolism, liver disease, and CVD (31–45).
Both Persistent Pesticides, Fungicides, Herbicides, and Insecticides and Industrial Chemicals/Transformation-products
Below, we discuss three studies that met inclusion criteria and two other studies with multiethnic adult samples.
Pregnant Women.
In a prospective investigation of POPs in early pregnancy (11 organochlorine pesticides, 9 PBDEs, 44 PCBs, and 11 PFAS) and GDM among 2,334 white, black, Asian, and Hispanic non-obese women enrolled in the NICHD Fetal Growth Study, Singletons (2009–2013) across 12 clinical sites in the US (40), several congeners of PCBs, PFAS, and PBDEs were positively associated with GDM, and there was a suggestion of effect modification by family history of T2DM and adiposity (40). Among all participants, the specific PCB congeners positively associated with GDM were PCBs 170, 172_192, 177, 180, 183, 194, 196_203, and 199; non-dioxin like PCBs; and total PCB. After stratification by family history of T2DM, the specific PCB congeners positively associated with GDM among women with family history of T2DM were PCBs 138_158, 146_161, 153, 156, 167, 170, 172_192, 177, 180, 182_187, 183, 194, 196_203, 199, 202, and 206. Among women with normal pre-pregnancy BMI, the specific PCB congeners positively associated with GDM were PCBs 153, 170, 172_192, 177, 180, 183, 194, 196_203, 199, 202, and 206. Among all women, the specific PFAS positively associated with GDM were perfluoroheptanoic acid (PFHpA), perfluorododecanoic acid (PFDoDA), and perfluorononaoic acid (PFNA). Among women with family history of T2DM, perfluorooctanoic acid (PFOA) was positively associated with GDM. Lastly, BDE 47 and BDE 154 were positively associated with GDM among women without family history of T2DM.
Children.
Also observed in the NICHD Fetal Growth Study, Singletons, associations were inconsistent between maternal concentrations of the assessed POPs and infant birthweight (33). These findings were similar across races/ethnicities.
Racial/ethnic Minorities, Low SES, and Occupationally-exposed.
No studies on this topic assessed variation in associations by race/ethnicity or SES. However, studies with multiethnic adult samples adjusted for race/ethnicity and SES in statistical models. Two studies of multiple POPs found that only PCBs and not pesticides were associated with cardiometabolic health outcomes. Plasma concentrations of certain PCBs were positively associated with T2DM among middle-to-older aged white (95%) and black (5%) female cases (n=793) and controls (n=793) enrolled in the Nurses’ Health Study II (46). In the Coronary Artery Risk Development in Young Adults (CARDIA) Study, a prospective study of 180 black and white young adults, serum PCBs measured in young adulthood were positively associated with alterations in blood lipid levels over 23 years of follow-up (47).
Among 7,404 adult Hispanic/Latino participants of the Hispanic Community Health Study/Study of Latinos, self-reported occupational exposure to both pesticides and solvents with CVD was the one cross-sectional study (34). Occupational solvent exposure was not associated with any CVD outcome after robust adjustment for sociodemographic characteristics, health behaviors, health care access, and CVD risk factors (34). However, occupational pesticide exposure was associated with a 2-foldhigher prevalence of CVD and coronary heart disease as well as 6 times the prevalence of cerebrovascular disease (34).
Persistent Pesticides, Fungicides, Herbicides, and Insecticides
Below, we discuss four studies that met inclusion criteria and one study of a multiethnic adult sample.
Pregnant Women.
Based on our literature search, one case-control study of pesticide exposure, alone, and cardiometabolic health among a multiethnic population of pregnant women met inclusion criteria. In this study of 295,387 non-Hispanic white and Hispanic pregnant women living in the San Joaquin Valley, California, Shaw et al. reported that women with various preeclampsia phenotypes and preterm delivery were no more likely than controls to be exposed to residential pesticides (including 543 individual chemical and 69 physiochemical pesticide groupings) (41).
Children.
In the same data source as Shaw et al., Ling et al. examined potential prenatal residential exposure to 17 pesticides representing three chemical classes (i.e., organophosphates; pyrethroids; and carbamates) in relation to term low birthweight (38). The authors compared 4,412 term low birthweight infants to term normal birthweight infants to estimate odds ratios associated with ever versus never exposure at the first, second, and third trimesters, separately. For individual pesticides, only ever exposure to the fungicide, myclobutanil, in the second and third trimester was positively associated with term low birthweight. Beyond marginal associations with exposure to ≥2 pyrethroids, no pesticide classes were associated with term low birthweight.
Similarly, after adjustment for potential confounders and stratification by infant sex, higher prenatal exposures to organophosphate esters (OPEs) were not associated with lower birthweight after standardizing birthweight by gestational age in the prospective Pregnancy, Infection and Nutrition Study (2001–2006) among a multiethnic population of 349 mother-child pairs in North Carolina (36). In another study of five OPE metabolites, Boyle et al. used National Health and Nutrition Examination Survey (NHANES) 2013–2014 cross-sectional data to determine overall and sex-specific associations with adiposity markers including body mass index (BMI) z-score and waist circumference among 784 US children aged 6–19 years (32). Results were mixed: log2-transformed concentrations of the OPE metabolite, dibutyl phosphate, was associated with lower odds of obesity, but bis(2-chloroethyl) phosphate concentrations were associated with higher odds of overweight among both sexes/genders. However, sex was a modifier of the association between bis(1-chloro-2-propyl) phosphate (BCPP) and BMI z-scores because detectable BCPP was negatively associated with BMI z-score among males, and there was no association among females.
Racial/ethnic Minorities, Low SES, and Occupationally-exposed.
We did not identify other eligible studies that investigated relationships between pesticide exposure, alone, and cardiometabolic health outcomes while stratifying by race/ethnicity, SES, or occupation among US adults. However, it is important to mention one nationally-representative study. After adjustment for race/ethnicity, education, poverty-to-income ratio, and other confounders using pooled cross-sectional NHANES 2007–2010 data, the urinary pyrethroid metabolite, 3-phenoxybenzoic acid (3-PBA) was associated with higher odds of diabetes among US 2,796 adults aged 20–79 years (48).
Industrial Chemicals and Transformation-products
In this section, we discuss eight studies that met inclusion criteria and six other studies among racially/ethnically- and socioeconomically- diverse sample of adults.
Pregnant Women.
Our literature review yielded no studies that investigated industrial chemicals and by-products, alone, in relation to cardiometabolic health among pregnant women.
Children.
In a prospective investigation of repeated measures of serum PBDE congeners at ages 1, 2, 3, 5, and 8 years and adiposity (i.e., weight, BMI, WC, body fat percentage) at age 8 years among 206 white and non-white children in the Health Outcomes and Measures of the Environment (HOME) Study, there was no evidence of associations with any PBDE congeners except BDE-153 (42). For BDE-153, associations were sex-specific in that the negative association between BDE-153 and body fat percentage among males was not observed among females (42).
PFAS was heavily studied among children during this time frame with seven studies meeting inclusion criteria. In a prospective investigation of prenatal PFAS exposure and infant growth from age 4 weeks to 2 years among 345 mother-child pairs in the HOME study, there were weak, non-significant associations between PFAS and birthweight (45). In the prospective Upstate KIDS Study (New York state, 2008–2010) that followed 1,954 singletons and 966 twins of white, black, Hispanic, Asian, and other race/ethnicity, higher concentrations of perfluorooctane sulfonic acid (PFOS) and PFOA measured from blood spots at delivery were associated with lower BMI at age 2 years among singletons and higher BMI (PFOA only) among twins (43). Further, higher PFOA and perfluorohexanesulphonic acid (PFHxS) but not PFOS concentrations were associated with dysregulated glucose metabolism in a longitudinal study of 40 overweight and obese Hispanic/Latino children (mean age [SD]= 11.5 [2.0] years at enrollment) living in Los Angeles, California (31). Only higher PFNA (out of four PFAS- PFOA, PFOS, PFHxS, and PFNA) was positively associated with adverse cardiometabolic profiles including higher systolic blood pressure, low density lipoprotein cholesterol (LDL-C), and total cholesterol (TC) in a cross-sectional study of 48 black, white, and Hispanic obese children aged 8–12 years in Dayton, Ohio (37).
Of three studies, one prospective (39) and two cross-sectional studies (35, 44) reported mixed findings related to PFAS and lipid dysregulation, but the two studies among children in middle childhood suggested associations varied by sex/gender (39, 44). Among a multiethnic population of 682 mother-child pairs enrolled in Project Viva, prenatal and mid-childhood PFAS concentrations were associated with TC, triglycerides (TG), and liver function as measured by alanine aminotransferase among offspring, and associations appeared to vary by sex (39). Specifically, among girls, higher prenatal PFOS and PFOA were associated with generally better lipoprotein profiles, which included higher high density lipoprotein cholesterol (HDL-C), lower triglycerides (TG) and lower TC/HDL-C ratios, and better liver function at median age 7.7 years (39). However, in both overall and sex-stratified analyses, higher mid-childhood PFAS concentrations were associated with both worse (i.e., higher TC and LDL-C) and better lipoprotein profiles (i.e., higher high density lipoprotein (HDL-C) and lower TG) (39). In a cross-sectional analysis of NHANES 2013–2014 data collected from 458 children aged 6–11 years, Jain and Ducatman reported that higher PFOS concentrations were associated with higher TC, and associations varied by sex/gender and race/ethnicity (44).. Conversely, there were no associations between PFAS and lipid profiles among 2,987 adolescents enrolled in NHANES 2003–2014 (35).
Racial/ethnic Minorities, Low SES, and Occupationally-exposed.
Our search resulted in no publications that investigated race/ethnicity or SES as potential modifiers among adult populations. However, studies of multiethnic and various SES populations yielded mixed results regarding associations between PFAS and adiposity markers and suggested that various PFAS may be positively associated with higher risk of T2DM, worse lipid profiles particularly among women, and reduced kidney functioning among US adults (35, 49–52). Also, in the cross-sectional Anniston Community Health Survey (2003) among 738 white and black adults aged >18 years, 15 of 35 measured PCBs were positively associated with liver disease biomarkers (53). No occupational studies met inclusion criteria.
Non-Persistent Pollutants (non-POPs)
Non-POPs do not bioaccumulate and are rapidly excreted from the body (14), which presents methodological issues for researchers related to measurement. Exposure is ubiquitous because these chemicals are found in food, consumer products, and personal care products (14, 15). Therefore, an individual can be exposed through ingestion, inhalation, and dermal absorption routes. Subcategories of non-persistent pollutants that we discuss in this review include phenols (i.e., BPA, benzophenone-3, triclosan, bisphenol F (BFP), bisphenol S (BPS), triclocarban, 2,5-dichlorophenol, and 2,4-dichlorophenol), phthalates, parabens, pharmaceutical agents, acrylamide, and solvents. Studies of non-POPs generally reported mixed results related to birthweight and adiposity markers (23, 54–57); however, non- POPs may affect glucose metabolism among pregnant women (58–60). Eligible studies of other cardiometabolic health outcomes were sparse.
Phenols
Eight articles met inclusion criteria and two notable studies included racial/ethnic minority populations (23, 43, 54–56, 58, 61–64). Although results were mixed, most studies suggested phenols may be associated with adiposity.
Pregnant Women.
In the LIFECODES Study (2006–2008) of 350 white, black, Hispanic, Asian, and other pregnant women in Boston, MA, urinary BPA measured at 4 time points and second trimester glucose levels were prospectively investigated while considering BMI as a modifier (58). Associations were non-significant in the overall population; however, higher BPA concentrations were associated with higher glucose levels only among women who were overweight or obese (58). Among a multiethnic cohort of 446 pregnant women aged 16 years and older who were enrolled in the Healthy Start Study in Colorado (2009–2014), benzophenone-3 and triclosan concentrations were significantly higher among women who were normal weight versus overweight pre-pregnancy and who were NH-white vs. non-NH-white (23).
Children.
The Environment and Reproductive Health (EARTH Study) is a multiethnic prospective cohort of male and female couples seeking medically assisted reproduction in Boston, MA from 2004–2016. In the EARTH Study, paternal preconception and maternal prenatal phenol concentrations were associated with infant birthweight (62). Specifically, higher paternal preconception benzophenone-3 concentrations were associated with higher birthweight, and these associations were stronger if men were overweight or obese (62). Prenatal triclosan and propylparaben concentrations were associated with lower birthweight among male offspring (62). However, there were no associations between prenatal and childhood triclosan concentrations and adiposity markers (i.e., BMI z-score, WC, body fat percentage) at age 8 years among white, black, and other races/ethnicities of children in the HOME Study (56). In Upstate KIDS Study, higher BPA concentrations measured from infant blood spots were associated with rapid weight gain at 4, 9, and 12 months as well as obesity at age 2 years and older (43).
Two studies used NHANES data. Among 745 children aged 6–17 years in the 2013–2014 cycles, higher urinary concentrations of BPA and BPF were associated with obesity and abdominal obesity, particularly among boys, and there were null associations for BPS (61). Furthermore, the BPF-abdominal obesity association was stronger among non-whites compared to whites (61). Among 944 adolescents aged 12–19 years in the 2003–2010 cycles, those with BPA concentrations in the second quartile versus the first had higher odds of non-alcoholic fatty liver disease (64). Further, the association was stronger among Hispanic/Latino adolescents compared to white adolescents (64).
Racial/ethnic Minorities, Low SES, and Occupationally-exposed.
No eligible studies stratified by SES or focused on occupational subgroups; however, one study focused on black women. Two studies included racial/ethnic minorities and are worthy of mention but did not investigate racial/ethnic-specific associations. In a cross-sectional study using data from the Study of Environment, Lifestyle and Fibroids cohort of black women aged 23–34 years at enrollment (N=1,693), BMI was positively correlated with BPA, BPS, and triclocarban (54). Though not stratified by race/ethnicity, a prospective investigation among black and white participants found that urinary BPA levels were inversely correlated with weight loss and insulin levels after weight loss surgery (55). Also, a cross-sectional study of two phenols (2,5-dichlorophenol and 2,4-dichlorophenol) using NHANES data (2007–2010) among 3,617 adults aged 20 years and older reported that higher urinary 2,5-dichlorophenol was associated with higher prevalence of CVD (63).
Phthalates
Eight published studies met inclusion criteria (23, 60, 65–70). Five studies investigated either correlates, metabolomics, or glucose levels and phthalate metabolite concentrations among pregnant women (23, 60, 66, 67, 70). Two studies investigated prenatal phthalate metabolites and birthweight (65, 69), and the one remaining study focused on cardiometabolic health among adolescents (68).
Pregnant women.
The two cross-sectional investigations of urinary phthalate metabolite concentrations reported that correlates of exposure varied by race/ethnicity (23, 66). Among a multiethnic cohort of 446 pregnant women aged 16 years and older who were enrolled in the Healthy Start Study in Colorado (2009–2014), concentrations of high molecular weight phthalates (ΣHMWP) were higher among women who were overweight prior to pregnancy (body mass index (BMI) ≥25 kg/m2 vs. BMI <25 kg/m2) (23). Further, white women had the lowest concentrations of di(2-ethylhexyl) ΣDEHP and di-n-butyl ΣDBP compared to black, Hispanic, and other non-white women. Similarly, six of nine investigated urinary phthalate metabolite concentrations were higher among NH-black compared to NH-white women in a cohort of 378 pregnant women aged 18 years and older in Charleston, South Carolina (66). Correlates of phthalate exposure also varied by race in this study (66). Among black women, older age, higher BMI, and lower income were associated with higher concentrations of certain phthalate metabolites (66).
Two prospective studies investigated glucose levels among pregnant women (60, 69). In the EARTH Study of 245 white and non-white pregnant women aged 18–46 years who were seeking medically-assisted reproduction, associations between seven urinary phthalate metabolites and second trimester pregnancy glucose levels varied by metabolite (60). Second trimester mono-ethyl phthalate (MEP) was positively associated with glucose levels; however, mono-isobutyl (MiBP) was negatively associated with glucose levels. Among a multiethnic cohort of 705 pregnant women aged 18 years and older in The Infant Development and Environment Study (TIDES, 2010–2012), associations between 11 urinary phthalate metabolites measured at each trimester and gestation diabetes mellitus, impaired glucose tolerance, and continuous blood glucose also varied by phthalate metabolite (69). Average MEP concentrations were positively associated with GDM (69). Associations between phthalate metabolites and glucose intolerance as well as continuous glucose varied by race/ethnicity, with marginal associations between several metabolites and blood glucose among only Asians (69).
In a cross-sectional study of enrollment data collected from 115 Hispanic/Latina pregnant women enrolled in the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS, 1999–2000) cohort in California, higher urinary phthalate metabolites collected at a mean of 26.4 weeks gestation were mostly positively associated with metabolomic profiles suggestive of inflammation and changes in lipid biosynthesis as well as metabolism in relation to phthalate exposure (67).
Children.
Both prospective studies of prenatal phthalate exposure and infant birthweight were conducted among multiethnic cohorts in Massachusetts (65, 69). Among both 300 mother-child pairs in the EARTH Study and 350 mother-child pairs in LIFECODES (2006–2008), there were null or non-significant associations between repeatedly measured phthalate metabolites and infant birthweight even after stratification by maternal glucose levels in LIFECODES (65, 69).
The one remaining study among adolescents was a cross-sectional study of multiple metabolic abnormalities indicative of possible metabolic syndrome (i.e., at least 3 abnormalities- abdominal obesity, prehypertension or hypertension, dyslipidemia, prediabetes or T2DM) using pooled NHANES data from 2003–2014 (68). Suggestive positive associations between both mono-n-butyl (MnBP) and MiBP and metabolic syndrome varied by sex but not economic adversity among 918 adolescents after adjustment for race/ethnicity, socioeconomic characteristics, and other covariates (68).
Racial/ethnic Minorities, Low SES, and Occupationally-exposed.
No eligible studies stratified by race/ethnicity or SES, nor did studies focus on occupational subgroups
Parabens
Four studies met inclusion criteria (54, 57, 59, 62). One was a prospective analysis among pregnant women, one was a prospective study among couples and their singleton infants, and two were cross-sectional investigations of paraben exposure correlates. Based on results, paraben exposure may affect glucose metabolism in pregnant women (59). Further, prenatal exposure to parabens may contribute to low birth weight among male offspring (62). Findings related to parabens and obesity were mixed and varied by study participant demographic characteristics (e.g., life stage) (54, 57).
Pregnant Women.
In the prospective EARTH Study of 241 pregnant white and non-white women who used fertility clinics, a higher urinary concentration of butylparaben (BP) was associated with elevated blood glucose levels when assessed individually and as a chemical mixture with two other parabens. Propylparaben and blood glucose were negatively associated (59).
Children.
In a separate analysis using EARTH Study data, higher prenatal propylparaben concentrations were associated with lower birth weight among singleton male but not female infants (62). Using pooled NHANES data (2007–2012) collected from 1,324 children aged 6–19 years of all races/ethnicities, only methylparaben (out of four measured parabens) was associated with obesity as children/adolescents with higher methylparaben concentrations were less likely to be obese (especially females) (57). Notably, in the same NHANES study, higher concentrations of all four measured parabens were negatively associated with adiposity markers among adults (57).
Racial/ethnic Minorities, Low SES, and Occupationally-exposed.
Although there were no eligible studies for low SES, and occupationally-exposed, Bethea et al. cross-sectionally evaluated correlates of parabens among black women in the SELF Study (54). BMI was positively correlated with BP and methylparaben concentrations (54).
Pharmaceutical Agents of Concern
Two studies of pharmaceutical agents that have been implicated as having adverse effects on cardiometabolic health met eligibility criteria, and both found positive associations with adverse cardiometabolic health.
Pregnant Women.
There were no studies among pregnant women that met inclusion criteria.
Children.
Troisi et al. investigated the association between prenatal exposure to diethylstilbestrol (DES) and adulthood CVD risk (71); therefore, we included this study in the children category. In this prospective investigation of 3,941 DES-prenatally exposed and 1,705 DES-unexposed women in the Combined DES Cohort Follow-up Study from1994–2013, exposed women were twice as likely to develop coronary heart disease and myocardial infarction during follow-up (71).
Racial/ethnic Minorities, Low SES, and Occupationally-exposed.
Although our search yielded no recent publications investigating low SES populations in the US, one study investigated whether breast cancer treatment-induced cardiotoxicity varied by race/ethnicity among 59 black and 157 white women (72). Findings of this retrospective chart review (2005–2015) were consistent with prior studies and demonstrated that black women had higher rates of cardiotoxicity, resulting in incomplete therapy compared to whites (72).
Acrylamide
Acrylamide, a monomer of polyacrylamide, can be found in small amounts in final products and substances treated with polyacrylamide (i.e., paper products, wastewater, and personal care as well as grooming products) and in cooked carbohydrate-rich foods (e.g., french fries) (73). Despite evidence of toxicity in animal models, recent studies, including two eligible cross-sectional studies among adults in the general population, have not consistently identified associations between acrylamide and poor cardiometabolic health outcomes (73–75).
Pregnant Women.
There were no studies among pregnant women that met inclusion criteria.
Children.
There were no studies among children that met inclusion criteria.
Racial/ethnic Minorities, Low SES, and Occupationally-exposed.
Huang et al. used NHANES 2003–2006 data to investigate associations between acrylamide and adiposity measures as well as CVD mortality in two separate studies among US adults overall and stratified by race/ethnicity (74, 75). Black and Mexican adults in this sample usually had lower acrylamide concentrations in hemoglobin compared to whites, but interaction terms by race/ethnicity were non-significant. Two out of three measures of acrylamide were positively associated with adiposity markers (i.e., overweight, obesity, and abdominal obesity) in one cross-sectional study but were also negatively associated with CVD mortality among non-smokers in the other study; conversely, one acrylamide measure was negatively associated with the adiposity markers and not associated with CVD morality (74, 75).
Non-specified Solvents
Pregnant Women.
There were no studies among pregnant women that met inclusion criteria.
Children.
There were no studies among children that met inclusion criteria.
Racial/ethnic Minorities, Low SES, and Occupationally-exposed.
Two occupational cohort studies met inclusion criteria. One study reported that high exposure to solvents in the dry cleaning industry (based on a solvent exposure score created from previously published monitoring studies and applied to job titles) was associated with elevated mortality from heart disease among a multiethnic population of 5,369 union members (76). Relatedly, after discovery of surrounding soil and ground water contamination by chlorinated solvents, an occupational cohort study ascertained mortality data for 4,396 automotive workers in Huntsville, Alabama who were employed for any duration of time from 1972 to 1993 (77). Although investigators could not identify specific chemical exposures, they found that compared to other workers, those who were more likely to be exposed to adverse environmental conditions (because of poor ventilation, solvent exposure in closed areas, and possible asbestos exposure) had higher risk of CVD mortality at follow-up in 2016 (77).
DISCUSSION
In this non-systematic, narrative review of recent literature, we found that recent studies among vulnerable populations generally found suggestive positive associations between synthetic chemicals and poor cardiometabolic health throughout the life course; however, results were inconsistent for certain chemicals and cardiometabolic health outcomes. Most of the studies were among the vulnerable populations of pregnant women and children; and the most consistent observations centered around both persistent and non-persistent pollutants being associated with altered glucose metabolism, GDM, and T2DM or associated risk factors. Very few studies considered race/ethnicity and socioeconomic status as effect modifiers despite prior literature suggesting greater burdens of both synthetic chemical exposure and poor cardiometabolic health among these populations compared to white and higher SES populations (3–6, 13, 18–24). The POP, PFAS, and the non-POP, phenols, were highly studied during this period; however, like other synthetic chemicals under investigation, results were inconclusive. Nonetheless, there is evidence that synthetic chemicals should continue to be investigated in relation to cardiometabolic health as minimal data exist among racial/ethnic minorities and low-SES populations.
While results of most studies in this limited time period were mixed and inconclusive for most cardiometabolic health outcomes, several studies consistently suggested that exposure to pesticides, PBDEs, PCB congeners, PFAS phenols, phthalates, and parabens may be associated with alterations in glucose metabolism, and higher risk of GDM as well as T2DM (31, 40, 46, 48, 51, 58–60). Our findings are consistent with prior reviews suggesting that although further research is warranted, EDCs are likely contributors to the rise in these health conditions across several vulnerable populations including pregnant women, racial/ethnic minorities, and low SES individuals (13, 78). Although biological mechanisms remain poorly understood, several reviews and studies to date offer potential mechanisms by which synthetic chemicals may impact glucose metabolism, where the associations were more evident. For instance, EDCs can interfere with insulin secretion and regulation of glucose homeostasis through pathways like targeting pancreatic alpha cells and impairing the molecular signaling that leads to secretion of glucagon in response to low blood glucose levels (13, 14). Furthermore, although inconsistent associations were observed for adiposity-related measures, synthetic chemicals may also negatively affect glucose metabolism indirectly through promoting adipogenesis and increasing obesity risk (11, 14). Additional obesity-related health outcomes remain warranted because prior evidence also suggest plausible mechanisms by which synthetic chemicals contribute to renal development and functioning, liver injury, and cardiovascular system dysfunction by interfering with hormonal and inflammatory pathways (11, 14, 79, 80).
Several eligible studies also suggested that either concentrations of synthetic chemicals were higher or associations between synthetic chemicals and cardiometabolic health were stronger among racial/ethnic minority populations compared to white populations (23, 44, 61, 64, 66, 69, 72), which is consistent with prior literature (13, 20, 22). Adverse upstream (fundamental), intermediate, and downstream modifiable environmental and social factors disproportionately experienced by racial/ethnic minority groups compared to whites likely drive these observations (see Figure 1). For example, the upstream determinant (or root cause) of racial/ethnic residential segregation has caused closer proximity of racial/ethnic minority neighborhoods to point sources of pollution while also contributing to concentrated poverty or socioeconomic disadvantage in these communities (13, 81). Socioeconomic disadvantage and lack of resources in surrounding environments can contribute to individual behaviors like greater consumption of processed foods due to low cost and lack of healthy options and purchase of cheaper consumer and personal care products. Greater consumption of such processed foods, consumer products, and personal care products likely contribute to disparate exposure to synthetic chemicals with endocrine-disrupting properties, which may partially explain disparities in poor cardiometabolic health (13). While this serves as one illustrative example, there are several social pathways that contribute to racial/ethnic disparities in exposure to synthetic chemicals, which warrant further investigation.
Recent literature as well as the current review have noteworthy limitations. Limitations of the reviewed studies include inability to distinguish which specific chemicals may be driving associations and infrequent use of chemical mixtures approaches that examine synergistic effects. Many of the studies used data from the same data source, which could increase the likelihood that results may be due to chance. Furthermore, use of the same populations in the same geographic regions limited generalizability. Few studies investigated synthetic chemicals and cardiometabolic health among racial/ethnic minority, low-SES individuals, or occupationally-exposed populations. Furthermore, studies that included racial/ethnic minority or low-SES populations rarely presented stratified analyses. In addition to the lack of race/ethnicity- and SES- stratified analyses, studies also often combine Asian, Native American, Pacific Islander, multi-racial, and other populations into one heterogeneous “other” category, which does not provide insight related to exposures and associations specific to unique racial/ethnic minority groups for which data is lacking. Limitations of our review include its narrative, non-systematic approach and inclusion of only studies published over a recent 16-month time frame. Our search criteria and strategy (albeit advanced) could have missed relevant articles. We also focused on synthetic chemicals and did not include non-synthetic exposures like air pollution and heavy metals, which have also been previously shown to negatively affect cardiometabolic health (82). We also did not assess the quality of the studies in this review.
Systematic reviews and meta-analyses focused on vulnerable populations are needed. More minority health research is needed for subsequent reviews to be fruitful. Further, minority health research of synthetic chemicals should also consider multilevel physical and social environmental pathways due to potential racial/ethnic differences in exposure patterns that can be attributed to social or cultural drivers. Moreover, future original research would be strengthened by 1) employing more prospective investigations with standardized assessments among adult populations across designated vulnerable populations since most were among pregnant women and children; 2) applying complex mixtures approaches since there were many different chemicals from similar exposure sources for which the impact of their interactions are unknown; and 3) including diverse populations across different locations in the US while considering sex/gender, race/ethnicity, and SES as potential modifiers and using minority health and health disparities frameworks. While applying a mixtures approach among diverse populations, more comprehensive inclusion of multiple chemical classes in individual studies are also needed to identify chemicals driving observed associations. Results from applying such methods could ultimately inform targeted interventions to reduce the burden of synthetic chemical exposures among all individuals, and particularly among vulnerable populations who may be more susceptible to poor health burdens associated with such exposures.
Ultimately, synthetic chemicals likely adversely affect cardiometabolic health throughout the life course, but few recent studies focused on understanding these relationships within racial/ethnic minority, low SES, and occupationally-exposed groups. In order to address poor cardiometabolic health and related disparities, more studies among vulnerable populations are warranted.
Supplementary Material
Acknowledgements:
The authors wish to thank the National Institute of Environmental Health Sciences library staff, Stacy Mantooth and Erin Knight, for assistance with the literature search. The authors also wish to thank Samuel Goldstein for assistance with the literature review and data extraction.
Funding: This work was funded by the Intramural Program at the NIH, National Institute of Environmental Health Sciences (Z1AES103325-;01).
ABBREVIATIONS
- BP
Butylparaben
- BPA
Bisphenol A
- BPF
Bisphenol F
- BPS
Bisphenol S
- CKD
Chronic kidney disease
- CVD
Cardiovascular disease
- DES
Diethylstilbestrol
- DDT/DDE
Dichlorodiphenyl-trichloroethane/dichlorethylene
- EDC
endocrine disrupting compounds
- GDM
Gestational diabetes mellitus
- HDL-C
High-density lipoprotein cholesterol
- ΣHMWP
High molecular weight phthalates
- LDL-C
Low density lipoprotein cholesterol
- OPE
organophosphate ester
- PCBs
Polychlorinated biphenyls
- PBDEs
Polybrominated diphenyl ethers
- PFAS
Per- and poly-fluoroalkyl substances
- PFDoDA
Perfluorododecanoic acid
- PFHpA
Perfluoroheptanoic acid
- PFHxS
Perfluorohexanesulphonic acid
- PFNA
Perfluorononaoic acid
- PFOA
Perfluorooctanoic acid
- PFOS
Perfluorooctane sulfonic acid
- POP
Persistent organic pollutant
- SES
Socioeconomic status
- T2DM
Type 2 diabetes mellitus
- TC
Total cholesterol
- TG
Triglycerides
- US
United States
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest: SA. Gaston, LS. Birnbaum, and CL. Jackson declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
REFERENCES
- 1.Health and Economic Costs of Chronic Diseases: National Center for Chronic Disease Prevention and Health Promotion; 2019. [Available from: https://www.cdc.gov/chronicdisease/about/costs/index.htm.
- 2.Heron M Deaths: Leading causes for 2017. National Vital Statistics Reports. 2019;68. [PubMed] [Google Scholar]
- 3.Petersen R, Pan L, Blanck HM. Racial and Ethnic Disparities in Adult Obesity in the United States: CDC’s Tracking to Inform State and Local Action. Preventing chronic disease. 2019;16:E46–E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beckles GL, Chou CF. Disparities in the Prevalence of Diagnosed Diabetes - United States, 1999–2002 and 2011–2014. MMWR Morbidity and mortality weekly report. 2016;65(45):1265–9. [DOI] [PubMed] [Google Scholar]
- 5.Moore JX, Chaudhary N, Akinyemiju T. Metabolic Syndrome Prevalence by Race/Ethnicity and Sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Preventing chronic disease. 2017;14:E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137(12):e67–e492. [DOI] [PubMed] [Google Scholar]
- 7.Vrijheid M, Casas M, Gascon M, Valvi D, Nieuwenhuijsen M. Environmental pollutants and child health-A review of recent concerns. International journal of hygiene and environmental health. 2016;219(4–5):331–42. [DOI] [PubMed] [Google Scholar]
- 8.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet (London, England). 2009;373(9677):1773–9. [DOI] [PubMed] [Google Scholar]
- 9.Dassanayake M, Langen E, Davis MB. Pregnancy Complications as a Window to Future Cardiovascular Disease. Cardiology in review. 2019. [DOI] [PubMed] [Google Scholar]
- 10.Wittcopp C, Conroy R. Metabolic Syndrome in Children and Adolescents. Pediatrics in review. 2016;37(5):193–202. [DOI] [PubMed] [Google Scholar]
- 11.Shafei AE, Nabih ES, Shehata KA, Abd Elfatah ESM, Sanad ABA, Marey MY, et al. Prenatal Exposure to Endocrine Disruptors and Reprogramming of Adipogenesis: An Early-Life Risk Factor for Childhood Obesity. Childhood obesity (Print). 2018;14(1):18–25. [DOI] [PubMed] [Google Scholar]
- 12.Sanders AP, Saland JM, Wright RO, Satlin L. Perinatal and childhood exposure to environmental chemicals and blood pressure in children: a review of literature 2007–2017. Pediatric research. 2018;84(2):165–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruiz D, Becerra M, Jagai JS, Ard K, Sargis RM. Disparities in Environmental Exposures to Endocrine-Disrupting Chemicals and Diabetes Risk in Vulnerable Populations. Diabetes care. 2018;41(1):193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diamanti-Kandarakis E, Bourguignon J-P, Giudice LC, Hauser R, Prins GS, Soto AM, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30(4):293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables. Atlanta, GA: Centers for Disease Control and Prevention, Services UDoHaH; 2019. [Google Scholar]
- 16.Duran DG, Pérez-Stable EJ. Novel Approaches to Advance Minority Health and Health Disparities Research. 2019;109(S1):S8–S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Belmont report: Ethical principles and guidelines for the protection of human subjects of research. Bethesda, MD: National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research; 1978. [PubMed] [Google Scholar]
- 18.Hendryx M, Luo J. Latent class analysis of the association between polycyclic aromatic hydrocarbon exposures and body mass index. Environment international. 2018;121(Pt 1):227–31. [DOI] [PubMed] [Google Scholar]
- 19.Ye X, Kato K, Wong LY, Jia T, Kalathil A, Latremouille J, et al. Per- and polyfluoroalkyl substances in sera from children 3 to 11 years of age participating in the National Health and Nutrition Examination Survey 2013–2014. International journal of hygiene and environmental health. 2018;221(1):9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.James-Todd TM, Chiu YH, Zota AR. Racial/ethnic disparities in environmental endocrine disrupting chemicals and women’s reproductive health outcomes: epidemiological examples across the life course. Current epidemiology reports. 2016;3(2):161–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Przybyla J, Kile M, Smit E. Description of exposure profiles for seven environmental chemicals in a US population using recursive partition mixture modeling (RPMM). Journal of exposure science & environmental epidemiology. 2019;29(1):61–70. [DOI] [PubMed] [Google Scholar]
- 22.Zota AR, Shamasunder B. The environmental injustice of beauty: framing chemical exposures from beauty products as a health disparities concern. American journal of obstetrics and gynecology. 2017;217(4):418.e1-.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polinski KJ, Dabelea D, Hamman RF, Adgate JL, Calafat AM, Ye X, et al. Distribution and predictors of urinary concentrations of phthalate metabolites and phenols among pregnant women in the Healthy Start Study. Environmental research. 2018;162:308–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hendryx M, Luo JH. Children’s environmental chemical exposures in the USA, NHANES 2003–2012. Environmental Science and Pollution Research. 2018;25(6):5336–43. [DOI] [PubMed] [Google Scholar]
- 25.Wang A, Padula A, Sirota M, Woodruff TJ. Environmental influences on reproductive health: the importance of chemical exposures. Fertility and sterility. 2016;106(4):905–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evangelou E, Ntritsos G, Chondrogiorgi M, Kavvoura FK, Hernandez AF, Ntzani EE, et al. Exposure to pesticides and diabetes: A systematic review and meta-analysis. Environment international. 2016;91:60–8. [DOI] [PubMed] [Google Scholar]
- 27.Song Y, Chou EL, Baecker A, You NC, Song Y, Sun Q, et al. Endocrine-disrupting chemicals, risk of type 2 diabetes, and diabetes-related metabolic traits: A systematic review and meta-analysis. Journal of diabetes. 2016;8(4):516–32. [DOI] [PubMed] [Google Scholar]
- 28.Qiao Y, Ma J, Wang Y, Li W, Katzmarzyk PT, Chaput JP, et al. Birth weight and childhood obesity: a 12-country study. Int J Obes Suppl. 2015;5(Suppl 2):S74–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lallas PL. The Stockholm Convention on Persistent Organic Pollutants. The American Journal of International Law. 2001;95(3):692–708. [Google Scholar]
- 30.Kennedy GL Jr, Butenhoff JL, Olsen GW, O’Connor JC, Seacat AM, Perkins RG, et al. The toxicology of perfluorooctanoate. Crit Rev Toxicol. 2004;34(4):351–84. [DOI] [PubMed] [Google Scholar]
- 31.Alderete TL, Jin R, Walker DI, Valvi D, Chen Z, Jones DP, et al. Perfluoroalkyl substances, metabolomic profiling, and alterations in glucose homeostasis among overweight and obese Hispanic children: A proof-of-concept analysis. Environment international. 2019;126:445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyle M, Buckley JP, Quiros-Alcala L. Associations between urinary organophosphate ester metabolites and measures of adiposity among U.S. children and adults: NHANES 2013–2014. Environment international. 2019;127:754–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buck Louis GM, Zhai S, Smarr MM, Grewal J, Zhang C, Grantz KL, et al. Endocrine disruptors and neonatal anthropometry, NICHD Fetal Growth Studies - Singletons. Environment international. 2018;119:515–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bulka CM, Daviglus ML, Persky VW, Durazo-Arvizu RA, Lash JP, Elfassy T, et al. Association of occupational exposures with cardiovascular disease among US Hispanics/Latinos. Heart (British Cardiac Society). 2019;105(6):439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong Z, Wang H, Yu YY, Li YB, Naidu R, Liu Y. Using 2003–2014 U.S. NHANES data to determine the associations between per- and polyfluoroalkyl substances and cholesterol: Trend and implications. Ecotoxicology and environmental safety. 2019;173:461–8. [DOI] [PubMed] [Google Scholar]
- 36.Hoffman K, Stapleton HM, Lorenzo A, Butt CM, Adair L, Herring AH, et al. Prenatal exposure to organophosphates and associations with birthweight and gestational length. Environment international. 2018;116:248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khalil N, Ebert JR, Honda M, Lee M, Nahhas RW, Koskela A, et al. Perfluoroalkyl substances, bone density, and cardio-metabolic risk factors in obese 8–12 year old children: A pilot study. Environmental research. 2018;160:314–21. [DOI] [PubMed] [Google Scholar]
- 38.Ling CX, Liew Z, von Ehrenstein OS, Heck JE, Park AS, Cui X, et al. Prenatal Exposure to Ambient Pesticides and Preterm Birth and Term Low Birthweight in Agricultural Regions of California. Toxics. 2018;6(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mora AM, Fleisch AF, Rifas-Shiman SL, Baidal JAW, Pardo L, Webster TF, et al. Early life exposure to per- and polyfluoroalkyl substances and mid-childhood lipid and alanine aminotransferase levels. Environment international. 2018;111:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahman ML, Zhang CL, Smarr MM, Lee S, Honda M, Kannan K, et al. Persistent organic pollutants and gestational diabetes: A multi-center prospective cohort study of healthy US women. Environment international. 2019;124:249–58. [DOI] [PubMed] [Google Scholar]
- 41.Shaw GM, Yang W, Roberts EM, Aghaeepour N, Mayo JA, Weber KA, et al. Residential agricultural pesticide exposures and risks of preeclampsia. Environmental research. 2018;164:546–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vuong AM, Braun JM, Wang ZY, Yolton K, Xie CC, Sjodin A, et al. Exposure to polybrominated diphenyl ethers (PBDEs) during childhood and adiposity measures at age 8 years. Environment international. 2019;123:148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yeung EH, Bell EM, Sundaram R, Ghassabian A, Ma WL, Kannan K, et al. Examining Endocrine Disruptors Measured in Newborn Dried Blood Spots and Early Childhood Growth in a Prospective Cohort. Obesity. 2019;27(1):145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jain RB, Ducatman A. Associations between lipid/lipoprotein levels and perfluoroalkyl substances among US children aged 6–11 years. Environmental pollution (Barking, Essex : 1987). 2018;243(Pt A):1–8. [DOI] [PubMed] [Google Scholar]
- 45.Shoaff J, Papandonatos GD, Calafat AM, Chen A, Lanphear BP, Ehrlich S, et al. Prenatal Exposure to Perfluoroalkyl Substances: Infant Birth Weight and Early Life Growth. Environmental epidemiology (Philadelphia, Pa). 2018;2(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zong G, Valvi D, Coull B, Goen T, Hu FB, Nielsen F, et al. Persistent organic pollutants and risk of type 2 diabetes: A prospective investigation among middle-aged women in Nurses’ Health Study II. Environment international. 2018;114:334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suarez-Lopez JR, Clemesha CG, Porta M, Gross MD, Lee DH. Organochlorine pesticides and polychlorinated biphenyls (PCBs) in early adulthood and blood lipids over a 23-year follow-up. Environmental toxicology and pharmacology. 2019;66:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park J, Park SK, Choi YH. Environmental pyrethroid exposure and diabetes in U.S. adults. Environmental research. 2018;172:399–407. [DOI] [PubMed] [Google Scholar]
- 49.Liu G, Dhana K, Furtado JD, Rood J, Zong G, Liang L, et al. Perfluoroalkyl substances and changes in body weight and resting metabolic rate in response to weight-loss diets: A prospective study. PLoS medicine. 2018;15(2):e1002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blake BE, Pinney SM, Hines EP, Fenton SE, Ferguson KK. Associations between longitudinal serum perfluoroalkyl substance (PFAS) levels and measures of thyroid hormone, kidney function, and body mass index in the Fernald Community Cohort. Environmental Pollution. 2018;242:894–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun Q, Zong G, Valvi D, Nielsen F, Coull B, Grandjean P. Plasma Concentrations of Perfluoroalkyl Substances and Risk of Type 2 Diabetes: A Prospective Investigation among U.S. Women. Environmental health perspectives. 2018;126(3):037001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jain RB, Ducatman A. Roles of gender and obesity in defining correlations between perfluoroalkyl substances and lipid/lipoproteins. Science of the Total Environment. 2019;653:74–81. [DOI] [PubMed] [Google Scholar]
- 53.Clair HB, Pinkston CM, Rai SN, Pavuk M, Dutton ND, Brock GN, et al. Liver Disease in a Residential Cohort With Elevated Polychlorinated Biphenyl Exposures. Toxicological sciences : an official journal of the Society of Toxicology. 2018;164(1):39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bethea TN, Wesselink AK, Weuve J, McClean MD, Hauser R, Williams PL, et al. Correlates of exposure to phenols, parabens, and triclocarban in the Study of Environment, Lifestyle and Fibroids. Journal of exposure science & environmental epidemiology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dambkowski CL, Garcia L, Leva N, Morton JM. Does Urinary Bisphenol-A Change after Bariatric Surgery? Journal of the American College of Surgeons. 2018;227(2):232–7. [DOI] [PubMed] [Google Scholar]
- 56.Kalloo G, Calafat AM, Chen AM, Yolton K, Lanphear BP, Braun JM. Early life Triclosan exposure and child adiposity at 8 Years of age: a prospective cohort study. Environmental Health. 2018;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quiros-Alcala L, Buckley JP, Boyle M. Parabens and measures of adiposity among adults and children from the US general population: NHANES 2007–2014. International journal of hygiene and environmental health. 2018;221(4):652–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bellavia A, Cantonwine DE, Meeker JD, Hauser R, Seely EW, McElrath TF, et al. Pregnancy urinary bisphenol-A concentrations and glucose levels across BMI categories. Environment international. 2018;113:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bellavia A, Chiu YH, Brown FM, Minguez-Alarcon L, Ford JB, Keller M, et al. Urinary concentrations of parabens mixture and pregnancy glucose levels among women from a fertility clinic. Environmental research. 2019;168:389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.James-Todd TM, Chiu YH, Messerlian C, Minguez-Alarcon L, Ford JB, Keller M, et al. Trimester-specific phthalate concentrations and glucose levels among women from a fertility clinic. Environmental health : a global access science source. 2018;17(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu B, Lehmler HJ, Sun Y, Xu G, Sun Q, Snetselaar LG, et al. Association of Bisphenol A and Its Substitutes, Bisphenol F and Bisphenol S, with Obesity in United States Children and Adolescents. Diabetes & metabolism journal. 2019;43(1):59–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Messerlian C, Mustieles V, Minguez-Alarcon L, Ford JB, Calafat AM, Souter I, et al. Preconception and prenatal urinary concentrations of phenols and birth size of singleton infants born to mothers and fathers from the Environment and Reproductive Health (EARTH) study. Environment international. 2018;114:60–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rooney MR, Lutsey PL, Bhatti P, Prizment A. Urinary 2,5-dicholorophenol and 2,4-dichlorophenol concentrations and prevalent disease among adults in the National Health and Nutrition Examination Survey (NHANES). Occupational and environmental medicine. 2019;76(3):181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Verstraete SG, Wojcicki JM, Perito ER, Rosenthal P. Bisphenol a increases risk for presumed non-alcoholic fatty liver disease in Hispanic adolescents in NHANES 2003–2010. Environmental health : a global access science source. 2018;17(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chiu YH, Bellavia A, James-Todd T, Correia KF, Valeri L, Messerlian C, et al. Evaluating effects of prenatal exposure to phthalate mixtures on birth weight: A comparison of three statistical approaches. Environment international. 2018;113:231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wenzel AG, Brock JW, Cruze L, Newman RB, Unal ER, Wolf BJ, et al. Prevalence and predictors of phthalate exposure in pregnant women in Charleston, SC. Chemosphere. 2018;193:394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou M, Ford B, Lee D, Tindula G, Huen K, Tran V, et al. Metabolomic Markers of Phthalate Exposure in Plasma and Urine of Pregnant Women. Frontiers in public health. 2018;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gaston SA, Tulve NS. Urinary phthalate metabolites and metabolic syndrome in U.S. adolescents: Cross-sectional results from the National Health and Nutrition Examination Survey (2003–2014) data. International journal of hygiene and environmental health. 2019;222(2):195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Noor N, Ferguson KK, Meeker JD, Seely EW, Hauser R, James-Todd T, et al. Pregnancy phthalate metabolite concentrations and infant birth weight by gradations of maternal glucose tolerance. International journal of hygiene and environmental health. 2019;222(3):395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shaffer RM, Ferguson KK, Sheppard L, James-Todd T, Butts S, Chandrasekaran S, et al. Maternal urinary phthalate metabolites in relation to gestational diabetes and glucose intolerance during pregnancy. Environment international. 2019;123:588–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Troisi R, Titus L, Hatch EE, Palmer JR, Huo DZ, Strohsnitter WC, et al. A Prospective Cohort Study of Prenatal Diethylstilbestrol Exposure and Cardiovascular Disease Risk. Journal of Clinical Endocrinology & Metabolism. 2018;103(1):206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Litvak A, Batukbhai B, Russell SD, Tsai HL, Rosner GL, Jeter SC, et al. Racial disparities in the rate of cardiotoxicity of HER2-targeted therapies among women with early breast cancer. Cancer. 2018;124(9):1904–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shipp A, Lawrence G, Gentry R, McDonald T, Bartow H, Bounds J, et al. Acrylamide: review of toxicity data and dose-response analyses for cancer and noncancer effects. Crit Rev Toxicol. 2006;36(6–7):481–608. [DOI] [PubMed] [Google Scholar]
- 74.Huang M, Jiao J, Wang J, Chen X, Zhang Y. Associations of hemoglobin biomarker levels of acrylamide and all-cause and cardiovascular disease mortality among U.S. adults: National Health and Nutrition Examination Survey 2003–2006. Environmental pollution (Barking, Essex : 1987). 2018;238:852–8. [DOI] [PubMed] [Google Scholar]
- 75.Huang M, Zhuang P, Jiao J, Wang J, Zhang Y. Association of acrylamide hemoglobin biomarkers with obesity, abdominal obesity and overweight in general US population: NHANES 2003–2006. The Science of the total environment. 2018;631–632:589–96. [DOI] [PubMed] [Google Scholar]
- 76.Callahan CL, Stewart PA, Blair A, Purdue MP. Extended Mortality Follow-up of a Cohort of Dry Cleaners. Epidemiology (Cambridge, Mass). 2019;30(2):285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.DeBono N, Richardson D, Keil A, Kelly-Reif K, Robinson W, Troester M, et al. Employment characteristics and cause-specific mortality at automotive electronics manufacturing plants in Huntsville, Alabama. American journal of industrial medicine. 2019;62(4):296–308. [DOI] [PubMed] [Google Scholar]
- 78.Varshavsky J, Smith A, Wang A, Hom E, Izano M, Huang H, et al. Heightened susceptibility: A review of how pregnancy and chemical exposures influence maternal health. Reproductive toxicology (Elmsford, NY). 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weidemann DK, Weaver VM, Fadrowski JJ. Toxic environmental exposures and kidney health in children. Pediatric nephrology (Berlin, Germany). 2016;31(11):2043–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heindel JJ, Blumberg B. Environmental Obesogens: Mechanisms and Controversies. Annual review of pharmacology and toxicology. 2019;59:89–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Williams DR, Mohammed SA, Leavell J, Collins C. Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Annals of the New York Academy of Sciences. 2010;1186:69–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dockery Douglas W, Luttmann-Gibson H, Rich David Q, Link Mark S, Mittleman Murray A, Gold Diane R, et al. Association of Air Pollution with Increased Incidence of Ventricular Tachyarrhythmias Recorded by Implanted Cardioverter Defibrillators. Environmental health perspectives. 2005;113(6):670–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


