Abstract
Bilateral acoustic hearing in cochlear implant (CI) recipients with hearing preservation may allow access to binaural cues. Sensitivity to acoustic binaural cues has been shown in some listeners combining electric and acoustic stimulation (EAS), yet remains poorly understood and may be subject to limitations imposed by the electrical stimulation and/or amplification asymmetries. The purpose of this study was to investigate the effect of stimulus level, frequency-dependent gain, and the addition of unilateral electrical stimulation on sensitivity to low-frequency binaural cues. Thresholds were measured for interaural time and level differences (ITD and ILD) carried by a low-frequency, bandpass noise (100–800 Hz). 16 adult CI EAS listeners (mean age = 50.2 years) each participated in three listening conditions: acoustic hearing only at 90 dB SPL, acoustic hearing only at 60 dB SPL with frequency-dependent gain, and acoustic hearing plus unilateral CI at 60 dB SPL with frequency-dependent gain applied to the acoustic channels only. Results revealed thresholds within the ecologically relevant ITD and/or ILD range for most EAS listeners. No significant effects of presentation level, frequency-dependent gain, or the addition of unilateral electrical stimulation on the resultant thresholds for ITDs or ILDs were observed at the group level. Correlational analyses related ITD and ILD thresholds to the degree of EAS benefit (i.e., advantage of acoustic hearing in the implanted ear) for speech recognition in diffuse noise. There was a significant relationship between EAS benefit and ITD thresholds, but no statistically significant relationship between EAS benefit and ILD thresholds. In summary, the results of this study are not consistent with our previous data obtained with simulated EAS in normal-hearing listeners, which showed significant binaural interference by a unilateral electrical “distractor” (Van Ginkel et al., 2019). The difference between studies suggests that chronic exposure to unilateral electrical stimulation combined with bilateral acoustic stimulation may reduce interference effects, perhaps because listeners adapt to the presence of the constant but binaurally incongruous CI stimulus. These results are consistent with past studies that demonstrated no interference in spatial hearing tasks due to the addition of a unilateral CI in adult EAS listeners.
Keywords: Hearing preservation, Cochlear implant, Binaural cues, Binaural interference, Electric and acoustic stimulation (EAS)
1. Introduction
Cochlear implant (CI) criteria have evolved in recent years such that there are now FDA-labeled indications for combined Electric and Acoustic Stimulation (EAS) systems allowing adults with as good as normal low-frequency thresholds and precipitously sloping high-frequency hearing loss to pursue cochlear implantation. By adding acoustic hearing to electrical stimulation, CI recipients can derive nonlinear gains in speech understanding and basic auditory function. This adjunctive acoustic stimulation can be added via the non-implanted ear, the implanted ear in cases of hearing preservation, or both ears with binaural acoustic hearing. For CI users with binaural acoustic hearing, a number of studies have demonstrated the efficacy of EAS by showing the additive benefit of low-frequency acoustic hearing in the implanted ear for speech understanding in complex listening environments (Dunn et al., 2010; Gifford et al., 2013, 2017; Plant and Babic, 2016) as well as significantly improved horizontal-plane localization (Dunn et al., 2010; Gifford et al., 2014; Plant and Babic, 2016). However, binaural-cue sensitivity in EAS listeners remains poorly understood and may be subject to limitations imposed by the electrical stimulation and/or amplification asymmetries. This paper aims to quantify EAS listeners’ sensitivity to low-frequency interaural time and level difference (ITD and ILD) cues carried by acoustic stimuli in the presence and absence of unilateral electrical stimulation.
EAS listeners with binaural hearing in the low-to-mid frequencies are theoretically expected to access ITD cues, which are known to be most salient for frequencies below 1500 Hz (Klumpp and Eady, 1956; Zwislocki and Feldman, 1956; Brughera et al., 2013). In contrast, interaural level difference (ILD) cues are largest above 1500 Hz (Macaulay et al., 2010) and thus good access to ILDs requires high-frequency audibility in both ears. Given that most EAS listeners are unilaterally implanted and have sloping high-frequency hearing loss, high-frequency ILDs are generally not available, as bilateral acoustic hearing for most adult EAS patients is limited up to approximately 750–1000 Hz (Dillon et al., 2015; Gifford et al., 2017, 2013; Pillsbury et al., 2018; Skarzynski et al., 2012). There are three reports in the literature demonstrating that CI users with hearing preservation have significantly better localization abilities in listening conditions including bilateral HA and best-aided EAS (CI + bilateral HA) as compared to the bimodal condition (CI + contralateral HA) (Dunn et al., 2010; Gifford et al., 2014; Plant and Babic, 2016). Further research has demonstrated that EAS patients can access ITD cues and that ITD thresholds for low-frequency stimuli are significantly correlated with EAS localization abilities (Gifford et al., 2014). Though this seems to suggest that ITD sensitivity is the primary mechanism for EAS-related benefit in localization, another possibility is that individuals with hearing preservation can use both ITDs and ILDs that are present in the low-frequency region. Though ILDs are typically considered high-frequency cues, low-frequency ILDs are present at smaller but detectable magnitudes, typically ranging from 2 to 10 dB (Macaulay et al., 2010). Indeed, Macpherson and Middlebrooks (2002) demonstrated a small, but non-zero contribution of low-frequency ILDs for horizontal-plane localization of complex sounds.
Up to this point, ITD testing in the EAS population has been completed under headphones without acoustic amplification (Gifford et al., 2013, 2014). EAS patients generally have higher acoustic hearing thresholds in the CI ear. Thus, measuring ITD sensitivity using unamplified fixed-level stimuli may introduce biases due to the ILD-like effects of hearing asymmetry at low frequencies. Consequently, it is possible that ITD (and ILD) thresholds may appear larger for unaided testing than for conditions using frequency-dependent amplification as would be experienced in everyday EAS listening. Consistent with that idea, individuals with greater interaural asymmetry in unaided audiometric thresholds tended to exhibit poorer ITD sensitivity and less benefit from binaural acoustic hearing (as compared to the bimodal hearing configuration). Gifford et al. (2014) found significant correlations between acoustic ITD thresholds and both EAS benefit for speech recognition in semi-diffuse noise and degree of asymmetry in audiometric thresholds for 14 adult EAS patients. A similar effect was reported by Bronkhorst and Plomp (1988) demonstrating a 2.0- to 2.5-dB deficit in ITD-related benefit in the speech reception threshold (SRT) for individuals with asymmetric hearing losses.
Another factor influencing binaural cue sensitivity for EAS listeners is the presence of unilateral electrical stimulation, which is typical of most CI recipients with preserved acoustic hearing. Specifically, EAS listeners receive conflicting information across ears and even across frequencies as binaural envelope-based cues provided by the CI can conflict with cues for higher and lower frequency acoustic inputs. Indeed, for listeners with normal hearing, such conflicts result in binaural interference (impaired binaural sensitivity for a distant-frequency target), even for binaural targets considerably above or below the distracting frequency (McFadden and Pasenan, 1976; Heller and Trahoitis, 1995; Heller and Richards, 2010; Bibee and Stecker, 2016). van Ginkel et al. (2019) used acoustic simulations of EAS simulations to assess binaural interference as might occur with unilateral and bilateral electrical stimulation. EAS simulation was achieved with a high frequency filtered click train presented either unilaterally or bilaterally (diotic). The frequency content of the click train was varied across three conditions to represent various degrees of spectral overlap with the low-frequency target, as commonly occurs with EAS arising from the processing provided by the hearing aid and CI in the implanted ear. Acoustic ITD and ILD thresholds were significantly impaired by the addition of a unilateral high-frequency distracter, but significantly less binaural interference was observed when the high-frequency distracter was presented diotically. Binaural interference was also reduced when there was a spectral gap between the acoustic and simulated CI stimuli. The results suggest that sensitivity to acoustic binaural cues may depend significantly on the lateral and channel configuration of EAS fitting.
1.1. Current study
The primary purpose of this study was to quantify low-frequency ITD and ILD sensitivity in adult EAS listeners and to investigate the effects of unilateral electrical stimulation on binaural cue sensitivity. Though adult EAS listeners have exhibited no evidence of binaural interference for horizontal plane localization in the free field (Dunn et al., 2010; Gifford et al., 2014; Plant and Babic, 2016), no previous studies have investigated the impact of a unilateral CI under direct connect conditions. Based on previous reports with normal-hearing listeners demonstrating binaural interference with unilateral distracters, we hypothesized that the addition of a unilateral CI would significantly impair binaural cue sensitivity. Because binaural sensitivity may also be impacted by interaural asymmetry in auditory detection, a further purpose was to compare psychophysical measures of ITD and ILD sensitivity obtained with and without frequency-dependent gain applied to the acoustic stimulation.
2. Material and methods
2.1. Participants
Sample size justification was completed via power analysis based on the trends in our data as reported in Van Ginkel et al. (2019). Based on the effect size and standard deviations across listening conditions in our previous study, we would need to recruit 10 participants to be able to reject the null hypothesis that this response difference between listening conditions is zero with probability (power) 0.8. The Type I error probability associated with this test of this null hypothesis was set at 0.05. Thus we recruited sixteen adult EAS listeners (13 female, 3 male) ranging in age from 27 to 78 years (mean 50.2 years) to participate in this study (to account for attrition). All listeners were long-time users of their EAS system (>6 months) and had postlingual onset of severe-to-profound sensorineural hearing loss. Table 1 displays participant information regarding age at experimentation, CI type and electrode array, duration of EAS experience, low-frequency pure tone average (LFPTA; 125, 250, and 500 Hz) in each ear, and low frequency (LF) CI cutoff (in Hz) for testing. Fig. 1 displays individual and mean audiograms for the implanted and non-implanted ears in panels A and B, respectively. All experimental procedures were approved by the Vanderbilt University Medical Center Institutional Review Board.
Table 1.
Participant demographics including age at testing (years), CI manufacturer and electrode array, duration of EAS experience (years), LFPTA for the CI ear and non-CI ear (dB HL), as well as AzBio sentence recognition at +5 dB SNR (S0N45–315) for the CI alone, CI + HA (bimodal) and CIHA + HA (bilaterally aided EAS). − indicates that the condition was not assessed.
| ID | Age | Electrode array | EAS experience (years) | LFPTA CI ear (dB HL) | LFPTA Non-CI ear (dB HL) | LF CI cutoff (Hz) | AzBio +5 dB SNR S0N45–315 CI only, CI + HA, CIHA + HA (% correct) | AzBio +5 EAS benefit (percentage points) | AzBio +5 EAS normalized benefit (percent) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 41 | Cochlear CI422 | 3.5 | 63.8 | 47.5 | 313 | 57, 62, 72 | 10 | 26% |
| 2 | 27 | AB slim J | 0.6 | 32.5 | 37.5 | 383 | 43, 45, 84 | 39 | 71% |
| 3 | 62 | Cochlear Hybrid L24 | 0.9 | 53.8 | 31.9 | 438 | 28, 52, 76 | 24 | 51% |
| 4 | 57 | AB slim J | 1.0 | 26.3 | 26.3 | 383 | 41, 56, 66 | 10 | 23% |
| 5 | 36 | AB mid-scala | 0.9 | 62.5 | 21.3 | 383 | 27, 49, 55 | 6 | 12% |
| 6 | 42 | AB mid-scala | 2.8 | 62.5 | 42.5 | 383 | -, 72, 81 | 9 | 32% |
| 7 | 66 | AB mid-scala | 1.0 | 66.3 | 47.5 | 383 | 83, 71, 75 | 4 | 14% |
| 8 | 33 | Cochlear Hybrid L24 | 1.1 | 45.0 | 38.1 | 563 | -,-, - | − | − |
| 9 | 48 | Cochlear Hybrid L24 | 1.3 | 61.3 | 30.0 | 563 | 33, 47, 85 | 38 | 72% |
| 10 | 38 | Cochlear Hybrid L24 | 2.3 | 56.3 | 21.9 | 563 | 30, 29, 74 | 45 | 64% |
| 11 | 63 | MED-EL FLEX24 |
1.0 | 66.3 | 31.9 | 386 | 20, 34, 27 | −8 | −12% |
| 12 | 60 | Cochlear CI522 |
1.0 | 61.3 | 36.9 | 313 | 46, 84, 87 | 3 | 21% |
| 13 | 58 | Cochlear CI522 |
0.8 | 52.5 | 30.6 | 313 | -, 70, 85 | 14 | 49% |
| 14 | 53 | Cochlear CI512 |
2.2 | 62.5 | 32.5 | 438 | -, 68, 67 | −1 | −3% |
| 15 | 78 | MED-EL FLEX28 |
0.5 | 75.0 | 39.3 | 125 | 50, 55, 52 | −3 | −7% |
| 16 | 41 | Cochlear CI422 |
2.7 | 86.3 | 42.9 | 188 | 31,43, 71 | 28 | 49% |
| Mean 50.2 | N/A | 1.5 | 58.4 | 34.9 | 382.4 | 41, 56, 71 | 14.7 | 31% | |
Fig. 1.

Individual audiometric thresholds for the CI and non-CI ear for the 16 participants.
2.2. Stimuli
Bandpass noise stimuli (100–800 Hz) were used for ITD and ILD threshold estimation. Stimuli were presented at either a fixed level (90 dB SPL) or amplified at 60 dB SPL. For the amplified stimulus at 60 dB SPL, we applied frequency-dependent acoustic amplification according to the frequency-gain prescription for a 60-dB-SPL input as dictated by NAL-NL1 (Keidser and Grant, 2001). The outputs of the acoustic stimuli were verified to match NAL-NL1 targets for a 60-dB-SPL input measured in a 2-cc coupler to verify target audibility of the stimuli across ears–similar to what would be encountered with typical hearing aid settings.
2.3. Procedure
To quantify the degree of EAS benefit derived for speech recognition, AzBio sentence recognition was assessed at +5 dB SNR (S0N45–315) with speech at 67 dB SPL (A weighted). AzBio sentence recognition in noise was assessed in the CI-alone condition (n = 12), bimodal condition (CI + HA; n = 15), EAS condition (CIHA + HA; n = 15) Prior to speech recognition testing, all participants’ hearing aids were verified to be providing NAL-NL1 (Keidser and Grant, 2001) target audibility for 60- and 65-dB-SPL input via real-ear measures using an Audioscan Verifit system. Because these participants had been followed carefully by both the clinic and the laboratory for previous experiments, all participants’ HAs were confirmed to be providing NAL-NL1 target audibility. CI-aided thresholds to frequency modulated pure tones presented in the free field were between 20 and 30 dB HL from 250 through 6000 Hz for all participants. Speech recognition scores for all tested conditions and degree of EAS benefit is displayed in Table 1.
ITD and ILD thresholds were obtained for the low-frequency, bandpass noise (100–800 Hz) in three listening conditions: acoustic hearing only at 90 dB SPL, acoustic hearing only at 60 dB SPL with NAL-NL1 frequency-dependent gain, and acoustic hearing plus unilateral CI at 60 dB SPL with NAL-NL1 gain applied to the acoustic channels only. The testing order for all listening conditions was randomly determined for each participant prior to each experimental session. We used a 3-channel experimental paradigm written in MATLAB to deliver the amplified acoustic stimuli to the left and right ER-1 earphones. A third DAC channel was routed to deliver unamplified stimuli directly to the CI processor via direct audio input port. An adaptive 2-interval forced-choice procedure was used to determine threshold in which the bandpass stimulus was presented bilaterally in each of the two intervals, separated by 400 ms. In the first interval, an ITD or ILD was presented favoring one side, and in the second interval an ITD or ILD of the same magnitude favored the other side, with the order randomized across trials. Each run had a maximum of 80 trials. Participants responded by selecting from a graphical user interface the direction in which the sound image changed across intervals (“left-to-right” or “right-to-left”). Correct answer feedback was provided on the computer monitor. A two-down one-up stepping rule was used to track 70.7% correct performance (Levitt, 1971) with threshold calculated from the last 6 reversals in each run. For ILD threshold measurements, stimulus level was roved over an 8-dB range (±4 dB) to discourage the use of monaural level cues. All listeners completed 2 practice runs for each of the tasks before data collection commenced. Two listeners (12 and 14) were unable to complete all testing due to time constraints. For those listeners, ITD thresholds were not measured for the 60-dB-SPL stimulus for either listening condition (acoustic only or EAS).
As shown in Table 1, the 100–800 Hz stimulus used to obtain ITD and ILD thresholds was within the passband of acoustic hearing audibility and within the range of CI stimulation. Note, however, that there were no participants for whom the entire 100–800 Hz stimulus band was encompassed by the CI; LF CI cutoff ranged from 125 Hz (for participant 15) to 563 Hz (participants 8, 9, and 10). For each participant, several LF CI cutoffs had been assessed as described in a previous paper (Gifford et al., 2017). The LF CI cutoff yielding best outcomes for tasks of speech recognition in diffuse noise was used for each listener. In most cases, this resulted in some, but not complete, EAS overlap in the LF region. Thus the full stimulus bandwidth may not have been transmitted by both the acoustic and electric stimulation in all cases. The everyday EAS overlap conditions tested here were most similar to “meet” and “complete overlap” as described by Van Ginkel et al. (2019).
3. Results
3.1. Speech recognition in noise (S0N45–315)
Speech recognition in noise scores, in percent correct (Table 1), were converted to rationalized arcsine units (RAU) (Studebaker, 1985) prior to statistical analysis. Statistical analysis was then completed via repeated measures analysis of variance (ANOVA) to investigate the effect of listening configuration (CI + HA vs. CIHA + HA) on speech recognition in diffuse noise (S0N45–315). Note that we did not include the CI-alone condition in the analysis as we were not concerned with the addition of contralateral acoustic hearing to the CI-alone condition, but were rather concerned with the additional benefit afforded by acoustic hearing in the implanted ear. Statistical analysis revealed a significant effect of listening condition (F(1, 27.84) = 6.73, p = 0.015) for speech recognition in noise. That is, unilateral CI stimulation combined with bilateral acoustic hearing (CIHA + HA) yielded significantly higher speech recognition performance in diffuse noise as compared to the CI plus unilateral (contralateral) acoustic hearing (CI + HA).
3.2. ITD and ILD thresholds
Individual and mean ITD and ILD threshold data are displayed for all three listening conditions as shown in panels A and B, respectively, of Fig. 2. The horizontal dashed line in each panel indicates the approximate upper limit for ITDs and ILDs present in ecologically valid listening scenarios for the stimuli used here (Kuhn, 1977). All but 3 listeners (5, 8, and 15) exhibited ITD thresholds within the ecological range in the EAS (CIHA + HA) listening condition and all but 2 listeners (13 and 14) exhibited ILD thresholds within the ecological range in the EAS (CIHA + HA) condition. Friedman repeated measures ANOVA on ranks was completed as the data were not normally distributed. In each analysis, ITD or ILD thresholds were the dependent variable and the 3 listening conditions were the independent variables. We found no effect of listening condition for either ITD thresholds Х2(2) 2.0, p = 0.37 or ILD thresholds Х2(2) = 2.6, p = 0.27. That is, for this group of adult EAS users, binaural cue sensitivity was not affected by stimulus presentation level, application of frequency-dependent gain, or by the addition of unilateral electrical stimulation.
Fig. 2.

Individual and mean ITD and ILD thresholds. The horizontal dashed line in each panel represents the approximate upper limit for ITDs and ILDs present in ecologically valid listening scenarios. Error bars represent +1 SEM.
Fig. 3 displays ILD thresholds plotted against ITD thresholds obtained in the EAS condition (CIHA + HA) with a 60-dB-SPL stimulus and frequency-dependent gain applied acoustically. The range for ecologically relevant ITD and ILD cues is shaded in gray. Nine listeners demonstrated access to ecologically relevant ITDs and ILDs. The remaining 5 listeners either had access to ecologically relevant ILDs (n = 4) or ITDs (n = 1).
Fig. 3.

Individual ILD thresholds are plotted against ITD thresholds as obtained in the EAS condition (CIHA + HA) with a 60-dB-SPL stimulus and frequency-dependent gain applied acoustically. The shaded region represents the range of ecologically relevant ITD and ILD cues.
Fig. 4 plots EAS benefit for AzBio sentence recognition at +5 dB SNR in a semi-diffuse noise configuration (S0N45–315, Table 1) as a function of ITD and ILD thresholds obtained in the EAS condition. EAS benefit was calculated using two methods. The first method is a simple difference score, in percentage points, between the EAS condition (CIHA + HA) and the bimodal condition (CI + HA). The second method calculates EAS normalized benefit, in percent, taking into account the listener’s bimodal (CI + HA) score and how far that score is from ceiling. The equation for EAS normalized benefit is as follows:
Fig. 4.
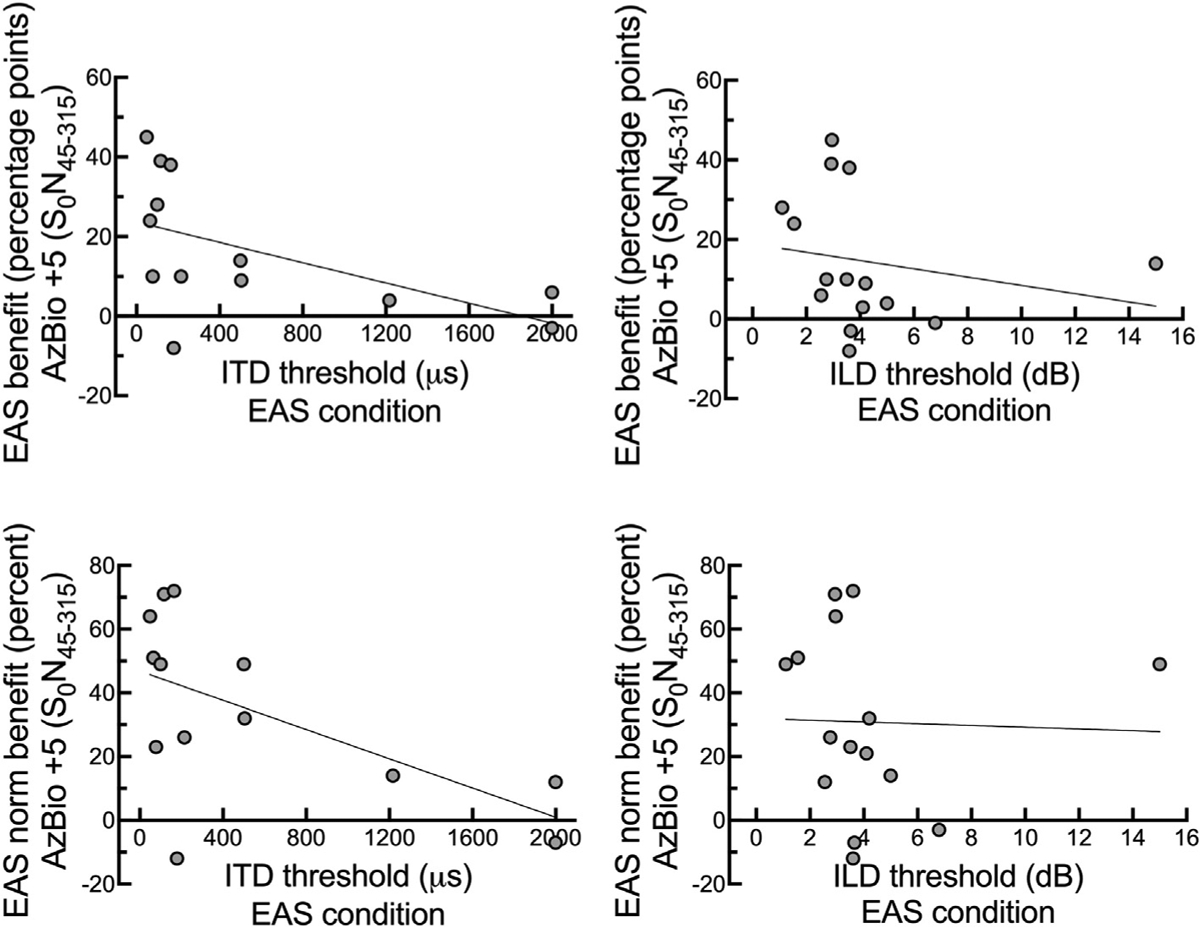
Individual EAS benefit for AzBio sentence recognition in diffuse noise (S0N45–315) as displayed in Table 1 is plotted against ITD and ILD thresholds obtained in the EAS condition (CIHA + HA).
As shown in Fig. 4, there was a significant relationship between EAS benefit and ITD thresholds (r = −0.54, p = 0.028) as well as between EAS normalized benefit and ITD thresholds (r = −0.59, p = 0.017). In contrast, there was no statistically significant relationship between EAS benefit and ILD thresholds (r = −0.21, p = 0.23) or EAS normalized benefit and ILD thresholds (r = −0.03, p = 0.45). The correlation between EAS benefit for AzBio sentences at +5 dB SNR (S0N45–315) remained significant even when applying the Bonferroni correction for multiple comparisons (α = 0.0125).
To compare the current dataset to our previous reports for a different sample of EAS listeners, we also completed correlation analyses for degree of EAS benefit and the following variables: 250- Hz threshold in CI ear (r = 0.12, p = 0.67), LFPTA (125–750 Hz) in CIear (r = −0.07, p = 0.80), interaural asymmetry in audiometric thresholds at 250 Hz (r = 0.12, p = 0.67), and interaural asymmetry in LFPTA (125–750 Hz) (r = 0.07, p = 0.80). Consistent with past studies, there was no relationship between degree of EAS benefit and audiometric thresholds in the implanted ear or degree of interaural asymmetry in audiometric thresholds.
4. Discussion
The current study investigated speech recognition in noise as well as ITD and ILD thresholds for a group of unilateral CI recipients using combined EAS (CIHA + HA). Though the primary purpose was to study the impact of stimulus level, frequency-dependent gain, and the addition of unilateral electrical stimulation on sensitivity to low-frequency binaural cues, we also assessed speech recognition in diffuse noise to quantify the degree of EAS benefit for the current sample of adult EAS listeners. As reported in multiple previous studies, bilateral acoustic hearing yielded statistically significant benefit beyond that afforded by unilateral (contralateral) acoustic hearing combined with unilateral electrical stimulation (Dunn et al., 2010; Gifford et al., 2013, 2014; 2017; Plant and Babic, 2016). The magnitude of the EAS effect was 15-percentage points, on averaged—which is consistent with the previously referenced studies.
With respect to binaural cue sensitivity, we found that the majority of EAS listeners have access to ecologically relevant ITD and/or ILD cues and that there was no effect of presentation level or the addition of unilateral electrical stimulation on the resultant ITD or ILD thresholds at the group level. The latter finding is somewhat surprising given the results of our previous study demonstrating significant binaural interference for both ITD and ILD perception in the presence of a unilateral distracter in participants with normal hearing listening to EAS/CI simulations (Van Ginkel et al., 2019). The difference between studies suggests that chronic exposure to unilateral electrical stimulation combined with bilateral acoustic stimulation may allow for listeners to adapt to the “distracter” stimulus. We completed Spearman correlation analyses between length of EAS experience (Table 1) and degree of binaural interference for ITD and ILD thresholds. We did not observe a relationship between EAS experience and binaural interference for either ITDs (r = −0.11, p = 0.69) or ILDs (r = −0.10, p = 0.70). Nevertheless, this study provides the first dataset demonstrating that the presence of a unilateral electrical stimulus does not preclude acoustic binaural sensitivity under direct-connect conditions in a group of experienced adult EAS listeners. Rather this group of adult EAS listeners largely maintained functional resolution of binaural cues in their everyday EAS listening configuration (CIHA + HA)—a result that is consistent with past studies showing no CI interference for horizontal plane localization (Dunn et al., 2010; Gifford et al., 2014; Plant and Babic, 2016).
For all participants in the current study, the low-frequency cutoff for the CI (Table 1) was well within the audible range for acoustic hearing and the target stimulus was represented both acoustically and electrically. Thus, it appears that for experienced EAS users, binaural cue sensitivity may persist despite spectral overlap between the acoustic and electric representations. This finding holds high clinical application, in that we should not expect most unilaterally implanted CI users with hearing preservation to have poor resolution of binaural cues following a period of chronic EAS use. However, 2 EAS listeners (5 & 7) exhibited considerably poorer ITD thresholds with the addition of the CI as compared to the binaural acoustic conditions. For these two listeners, the addition of unilateral electrical stimulation rendered ITD resolution ecologically non-functional. However, these same two listeners maintained ecologically functional ILD sensitivity with the addition of the CI but demonstrated minimal EAS benefit for speech recognition in diffuse noise (Table 1). Considering just ILD thresholds, two different listeners (13 & 14) demonstrated loss of functional ILD resolution with the addition of the CI as compared to the binaural acoustic conditions. Participant 13 did have functional ITD resolution with the addition of the CI and participant 14 did not complete ITD thresholds beyond that with a 90-dB-SPL acoustic presentation. These various patterns observed among a minority of participants suggest that individual differences in the weighting of electric and acoustic cues may play an important role in variation of EAS benefit for speech and spatial listening.
In the current study, we did not recruit any EAS participants with bilateral CI to investigate whether the presence of bilateral electrical stimulation may yield differential effects on ITD and ILD thresholds. Recall that Van Ginkel et al. (2019) reported a significant reduction in binaural interference with bilateral electrical simulations as compared to the unilateral CI simulation. However, given that we did not observe a significant binaural interference at the group level for either ITD or ILD resolution, it remains unclear whether we should expect to observe differences in binaural cue sensitivity with unilateral versus bilateral electrical stimulation.
Finally, the results of this study were consistent with past reports (Gifford et al., 2013, 2014) demonstrating that CI recipients with acoustic hearing preservation have access to low-frequency ITDs and that there is a significant correlation between ITD thresholds and EAS benefit for speech recognition in diffuse noise (S0N45–315). Though we did not observe a significant relationship between ILD thresholds and EAS benefit, none of the participants with ILD thresholds ≥3 dB demonstrated a significant EAS benefit based on 95% confidence interval for test-retest variability for the AzBio corpus (Spahr et al., 2012). Further we do not know whether ILD resolution influences spatial hearing abilities in this population—an investigation worthy of scientific pursuit holding clinical application for design and management of hearing aids and CI used in EAS listening configurations.
4.1. Limitations
We did not investigate whether different degrees of EAS spectral overlap may produce different effects on binaural cue sensitivity, as was the case for EAS simulations in Van Ginkel et al. (2019). For listeners with normal hearing and EAS simulations, introducing any spectral overlap between target and distracter resulted in significant decrement in ITD and ILD thresholds. Additional research is warranted to investigate whether there may be a tradeoff between EAS overlap and binaural cue sensitivity which, would likely also impact EAS benefit for speech recognition in diffuse noise. Relatedly, we did not assess the impact of the unilateral electric stimulation on ITD/ILD sensitivity longitudinally following CI activation. Without such an investigation, we cannot definitively conclude that the differences between the current study and the EAS/CI simulations presented by Van Ginkel et al. (2019) are due to chronic versus acute EAS exposure. Future studies should investigate whether EAS users adapt to a unilateral “distracter” stimulus over time or simply do not exhibit binaural interference as demonstrated by normal-hearing listeners (McFadden and Pasenan, 1976; Heller and Trahoitis, 1995; Heller and Richards, 2010; Bibee and Stecker, 2016; Van Ginkel et al., 2019). As mentioned previously in the discussion, we did not observe a relationship between duration of EAS experience and binaural interference; however, it is possible that having some duration of chronic EAS use was sufficient to allow adaptation, but further investigation is needed to define the time course. Additionally, though we provided frequency-dependent gain, the gain was linear based on a 60-dB-SPL input without the presence of interaural asymmetries in phase and/or level-dependent gain (i.e. input-based compression). With many hearing aids, the interaural phase and level characteristics may not be as well controlled. Thus it is possible that EAS listeners’ sensitivity to ITD/ILD cues may not be as useful while wearing their hearing technology. We are pursuing further investigation in this area.
5. Conclusions
A group of 16 adult CI recipients with binaural low-frequency acoustic hearing exhibited significant EAS benefit for speech recognition in diffuse noise which can be largely attributed to preservation of low-frequency ITD and/or ILD cues. Contrary to our previous report with EAS simulations, adult EAS listeners did not exhibit binaural interference with the addition of unilateral electrical stimulation. These data suggest that chronic exposure to unilateral electrical stimulation combined with bilateral acoustic stimulation may reduce interference, perhaps because listeners adapt to the presence of the constant but binaurally incongruous CI stimulus. Additional research is warranted to investigate whether the presence of amplification-based asymmetries in phase and/or level-dependent gain may influence sensitivity to binaural cues.
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grant Nos. R01 DC009404 (R.H.G.), R01 DC011548 (G.C.S), and R01 DC016643 (G.C.S.). We would like to thank Linsey Sunderhaus, AuD for her assistance with participant recruitment and data collection.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heares.2020.107929.
References
- Bibee JM, Stecker GC, 2016. Spectrotemporal weighting of binaural cues: effects of a diotic interferer on discrimination of dynamic interaural differences. J. Acoust. Soc. Am 140, 2584–2592. 10.1121/1.4964708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronkhorst AW, Plomp R, 1988. The effect of head-induced interaural time and level differences on speech intelligibility in noise. J. Acoust. Soc. Am 83, 1508–1516. [DOI] [PubMed] [Google Scholar]
- Brughera A, Dunai L, Hartmann WM, 2013. Human interaural time difference thresholds for sine tones: the high-frequency limit. J. Acoust. Soc. Am 133, 2839–2855. 10.1121/1.4795778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon MT, Buss E, Adunka OF, Buchman CA, Pillsbury HC, 2015. Influence of test condition on speech perception with electric-acoustic stimulation. Am. J. Audiol 24, 520–528. 10.1044/2015_AJA-15-0022. [DOI] [PubMed] [Google Scholar]
- Dunn CC, Perreau A, Gantz B, Tyler RS, 2010. Benefits of localization and speech perception with multiple noise sources in listeners with a short-electrode cochlear implant. J. Am. Acad. Audiol 21, 44–51. 10.3766/jaaa.21.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford RH, Davis TJ, Sunderhaus LW, Menapace C, Buck B, Crosson J, O’Neill L, Beiter A, Segel P, 2017. Combined electric and acoustic stimulation with hearing preservation: effect of cochlear implant low-frequency cutoff on speech understanding and perceived listening difficulty. Ear Hear. 38, 539–553. 10.1097/AUD.0000000000000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford RH, Dorman MF, Skarzynski H, Lorens A, Polak M, Driscoll CLW, Roland P, Buchman CA, 2013. Cochlear implantation with hearing preservation yields significant benefit for speech recognition in complex listening environments. Ear Hear. 34, 413–425. 10.1097/AUD.0b013e31827e8163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford RH, Grantham DW, Sheffield SW, Davis TJ, Dwyer R, Dorman MF, 2014. Localization and interaural time difference (ITD) thresholds for cochlear implant recipients with preserved acoustic hearing in the implanted ear. Hear. Res 312, 28–37. 10.1016/j.heares.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller LM, Richards VM, 2010. Binaural interference in lateralization thresholds for interaural time and level differences. J. Acoust. Soc. Am 128, 310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller LM, Trahiotis C, 1995. Interference in detection of interaural delay in a sinusoidally amplitude-modulated tone produced by a second, spectrally remote sinusoidally amplitude-modulated tone. J. Acoust. Soc. Am 97, 1808–1816. [DOI] [PubMed] [Google Scholar]
- Klumpp RB, Eady HR, 1956. Some measurements of interaural time difference threshold. J. Acoust. Soc. Am 28, 859–860. [Google Scholar]
- Keidser G, Grant F, 2001. The preferred number of channels (one, two, or four) in NAL-NL1 prescribed wide dynamic range compression (WDRC) devices. Ear Hear. 22 (6), 516–527. [DOI] [PubMed] [Google Scholar]
- Kuhn GF, 1977. Model for the interaural time differences in the azimuthal plane. J. Acoust. Soc. Am 62, 157–167. [Google Scholar]
- Levitt H, 1971. Transformed up-down methods in psychoacoustics. J. Acoust. Soc. Am 49, 467–477. [PubMed] [Google Scholar]
- Macaulay EJ, Hartmann WM, Rakerd B, 2010. The acoustical bright spot and mislocalization of tones by human listeners. J. Acoust. Soc. Am 127, 1440–1449. 10.1121/1.3294654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson EA, Middlebrooks J, 2002. Listener Weighting of Cues for Lateral Angle: the Duplex Theory of Sound Localization Revisited, vol. 111, pp. 2219–2236, 5 Pt 1. [DOI] [PubMed] [Google Scholar]
- McFadden D, Pasanen EG, 1976. Lateralization of high frequencies based on interaural time differences. J. Acoust. Soc. Am 59, 634–639. [DOI] [PubMed] [Google Scholar]
- Pillsbury HC, Dillon MT, Buchman CA, Staecker H, Prentiss SM, Ruckenstein MJ, Bigelow DC, Telischi FF, Martinez DM, Runge CL, Friedland DR, Blevins NH, Larky JB, Alexiades G, Kaylie DM, Roland PS, Miyamoto RT, Backous DD, Warren FM, El-Kashlan HK, Slager HK, Reyes C, Racey AI, Adunka OF, 2018. Multicenter US clinical trial with an electric-acoustic stimulation (EAS) system in adults: final outcomes. Otol. Neurotol 39, 299–305. 10.1097/MAO.0000000000001691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant K, Babic L, 2016. Utility of bilateral acoustic hearing in combination with electrical stimulation provided by the cochlear implant. Int. J. Audiol 55 (Suppl. 2), S31–S38. 10.3109/14992027.2016.1150609. [DOI] [PubMed] [Google Scholar]
- Skarzynski H, Lorens A, Matusiak M, Porowski M, Skarzynski PH, James CJ, 2012. Partial deafness treatment with the nucleus straight research array cochlear implant. Audiol. Neurotol 17, 82–91. 10.1159/000329366. [DOI] [PubMed] [Google Scholar]
- Spahr AJ, Dorman MF, Litvak LL, Van Wie S, Gifford RH, Loizou PC, Loiselle LM, Oakes T, Cook S, 2012. Development and validation of the AzBio sentence lists. Ear Hear. 33, 112–117. 10.1097/AUD.0b013e31822c2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studebaker GA, 1985. A ‘rationalized’ arcsine transform. J. Speech Hear. Res 28 (3), 455–462. [DOI] [PubMed] [Google Scholar]
- Van Ginkel C, Gifford RH, Stecker GC, 2019. Binaural interference with simulated electric acoustic stimulation. J. Acoust. Soc. Am 145 10.1121/1.5098784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwislocki J, Feldman RS, 1956. Just noticeable differences in dichotic phase. J. Acoust. Soc. Am 28, 860–864. [Google Scholar]


