Abstract
Macrophages act as scavengers, modulating the immune response against pathogens, and maintaining tissue homeostasis. Metabolism governs macrophage differentiation, polarization, mobilization, and the ability to mount an effective anti-tumor response. In cancer, however, the tumor microenvironment can actively reprogram macrophage metabolism either by direct exchange of metabolites or through cytokines and other signaling mediators. Thus, metabolic reprogramming holds great potential for modulating macrophages and developing new therapeutic approaches. In this review, we give an overview of macrophage metabolism as it relates to macrophage function and plasticity in cancer.
Keywords: M1-M2 macrophage polarization, tumor-associated macrophages, immunometabolism, metabolic reprogramming, MUC1, tumor microenvironment, cancer
Metabolic Activity Regulates Immune Functions of Macrophages
Immune escape, characterized by the inability of the immune system to remove transformed cells, is a hallmark of cancer. T cells mount an effective defense against foreign pathogens but often fail to do the same against developing tumors. This ineffective T cell function is in part due to the presence of deficient antigen presenting cells in the tumor microenvironment (TME). While macrophages are abundant in TME, they serve as weak antigen presenters and thus contribute to immune suppression [1, 2]. They also serve phagocytic functions in the TME. Importantly, signaling cues in the TME alter the metabolism of macrophages, which has widespread implications in their biology and function far beyond an antigen-presenting role (Figure 1).
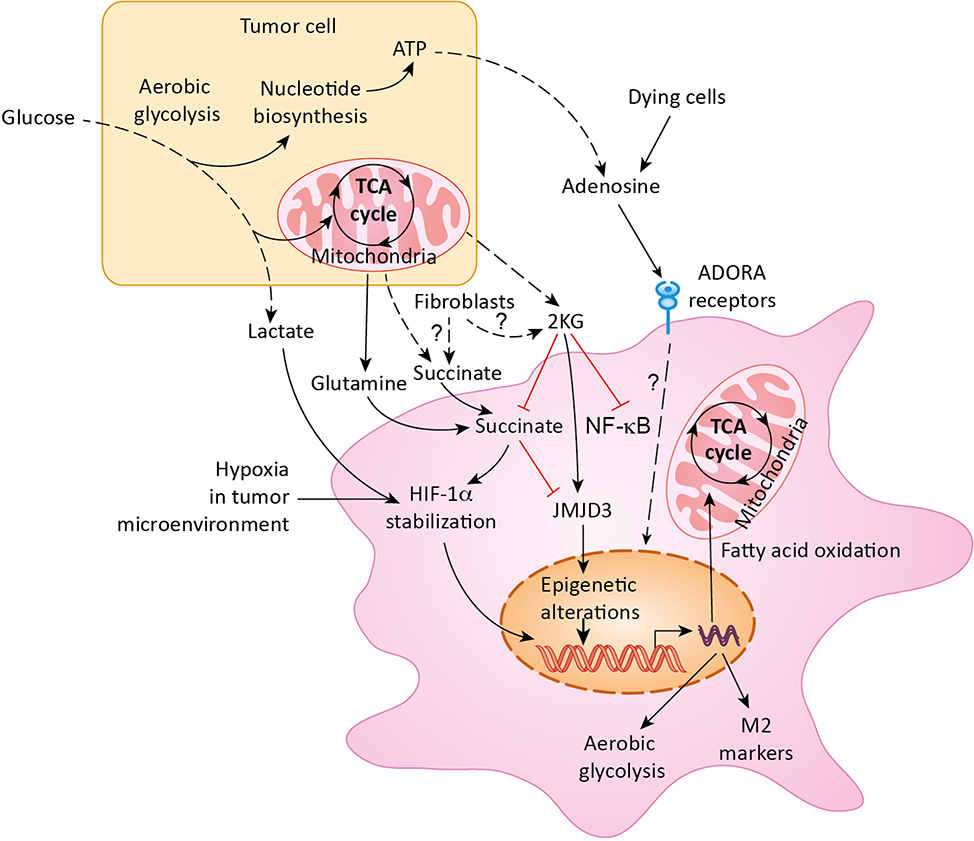
Figure 1. Polarization of macrophages in the tumor microenvironment.
Metabolic reprogramming of macrophages in the tumor microenvironment is mediated by multiple metabolites secreted by tumor cells as well as by fibroblasts and dying tumor cells. Lactate, glutamine, succinate, α-ketoglutarate (2KG), and adenosine are key metabolites regulating macrophage polarization. ADORA: Adenosine receptor; HIF-1α: Hypoxia-inducible factor-1 alpha; NF-κB: Nuclear factor kappa-light-chain-enhancer of activated B cells; JMJD3: Jumonji domain containing 3; TCA cycle: Tricarboxylic acid cycle; ATP: Adenosine triphosphate.
Metabolism regulates macrophage differentiation, polarization, mobilization, and their anti-tumor response. The phagocytic activity of macrophages clears dead and dying cells, and in turn, the clearing of cell debris provides macrophages with nutrients. Tumors, however, reprogram macrophage metabolism to prevent macrophage-mediated inflammation and killing of tumor cells. Macrophages undergo a switch in their metabolic pathways that leads to differentiation into either inflammatory (M1) or regulatory (M2) subtypes in response to various cytokine stimulations (Figure 2). While tumor-associated macrophages (TAMs) do not completely follow the M1 and M2 subtypes, they are in general M2-like and facilitate tumor growth by inducing immune suppression. However, the exact nature of the metabolic pathways of macrophages in tumors is not fully understood. What is known is that these metabolic alterations not only directly contribute to the function of macrophages, they also facilitate a change in the state of transcriptional activation of functionally critical cytokines. The pronounced role of macrophages in cancer suppression or progression offers an avenue for manipulating macrophages through metabolic reprogramming in order to generate new therapeutic approaches. However, macrophage metabolism is increasingly seen as a complex, tightly-regulated phenomenon affecting and/or influenced by various features of tumor cells and TME. Therefore, it appears insufficient to focus future research as well as therapeutic efforts on macrophage metabolism without considering the complex regulation of macrophage metabolism by internal and external factors.
Figure 2. Metabolic distinctions of M1 and M2 macrophages.
M1-polarized macrophages primarily depend on glucose and the flux of glucose into lactate, reactive oxygen species (ROS) production, and nitric oxide (NO) generation for tumor killing. M2-polarized macrophages primarily depend on beta-oxidation of fatty acids and TCA cycle, while inducing production of polyamines and L-proline to support tumor growth. PPP: Pentose phosphate pathway; NADPH: Nicotinamide adenine dinucleotide phosphate- reduced form; NADPH ox: NADPH oxidase; iNOS: Inducible nitric oxide synthase; TCA cycle: Tricarboxylic acid cycle: O2: oxygen.
This review describes recent developments in characterizing macrophage metabolism in the context of cancer. We describe the alterations and complex regulations of metabolic pathways that contribute to macrophage polarization. We also identify key macrophage-intrinsic or TME-produced metabolites that regulate macrophage polarization and function. Lastly, we discuss recent developments in therapeutic avenues to reprogram macrophage metabolism for mounting effective anti-tumor response.
Metabolic Pathways Driving Macrophage Polarization
Glycolysis
Glycolysis is the anaerobic breakdown of glucose into pyruvate. Under anaerobic conditions, pyruvate is converted into lactate, while under aerobic conditions, pyruvate is instead converted to acetyl coenzyme A (acetyl-CoA) and enters the tricarboxylic acid (TCA) cycle. Malignant cells use glycolysis despite the presence of oxygen (referred to as aerobic glycolysis, or the Warburg effect). Aerobic glycolysis is operational in cells with high demand for rapid energy production and biosynthesis, since glycolysis provides ATP at a much faster pace than the TCA cycle, at the expense of inefficient and increased consumption of glucose. Macrophage activity against pathogens and tumor cells requires aerobic glycolysis. Lipopolysaccharide (LPS) stimulation, which results in macrophage M1 polarization, causes increased aerobic glycolysis [3]. Glycolysis also facilitates routing the carbon flux into the oxidative pentose phosphate pathway (PPP), which produces NADPH for generating reactive oxygen species (ROS; BOX1) via NADPH oxidases. ROS production is essential for the phagocytic activity of macrophages in M1 subtype. Inhibition of aerobic glycolysis by activation of pyruvate kinase M2 (PKM2), or by inhibition of pyruvate dehydrogenase kinase 1 (PDK1), diminishes the polarization of LPS-challenged macrophages towards the M1 phenotype [4, 5]. Glycolysis is required for not only M1 but also M2 polarization state of macrophages [6]. Production of cytokines by M2 macrophages after LPS-stimulation needs glycolysis [7]. Furthermore, tumor-derived soluble factors, including hyaluronan fragments, can facilitate glycolysis in tumor-associated macrophages (TAMs) by inducing the expression of a key glycolytic enzyme, PFKFB3 [8]. Inhibition of glycolysis with 2-deoxy-d-glucose (2-DG) or dichloroacetate during macrophage differentiation significantly reduces the production of interleukin-10 (IL-10) and other differentiation-associated genes, including macrophage colony-stimulating factor (M-CSF) and endogenous matrix metalloproteinase (MMP-9). Of note, glycolytic flux-regulated IL-10 production plays a key role in nitric oxidize production and maintenance of M1 phenotype via an autocrine loop that facilitates glycolytic production via NO-mediated inhibition of OXPHOS [9]. In in vitro systems, macrophages that differentiate in the presence of glycolytic inhibitors retain plasticity and can revert to the M1 polarization state upon removal of inhibitors [6]. In tumor-bearing mice with Ehrlich ascites, treatment with 2-DG and radiation restored the M1 phenotype of spleen macrophages, which correlated with diminished tumor burden [10]. Collectively, glycolysis can be induced by multiple factors in TME and is critical for both pro- and anti-tumorigenic functions of macrophages; targeting glycolysis in tumors may revert pro-tumorigenic functions of macrophages. However, most of the studies investigating the role of glycolysis in macrophage polarization rely upon glycolytic inhibitors, which are not very specific. A recent study suggests that 2-DG is also not very specific and affects OXPHOS in a differential manner, increasing OXPHOS at lower doses (<1.25 mM) but decreasing it at higher doses [11]. Hence, further studies are needed to decipher the effect of targeting glycolysis specifically in TAMs on the tumor burden.
Text BOX1. ROS-mediated regulation of macrophages.
Role of ROS in macrophage biology
ROS actively participates in intracellular signaling in response to extracellular cues and plays a critical role in macrophage polarization and function. The primary function of ROS is regulating macrophage phagocytosis [27, 95]. ROS also regulates macrophage polarization.
ROS generation in macrophages
The major source of ROS production in mammalian cells is the metabolic flux through the TCA cycle that subsequently feeds into the mitochondrial electron transport chain, which is the main site of ROS production. Accumulation of citrate in M1 macrophages contributes to generation of nitric oxide (NO) and ROS [96].
NADPH oxidase (NOX) and ROS
Citrate generates nicotinamide adenine dinucleotide phosphate (NADPH) via the malic enzyme and pyruvate [96]. NADPH can be utilized for NO synthesis by inducible nitric oxide synthase (iNOS). NADPH is also utilized by the NOX to generate ROS, which is critical for M1 polarization. NOX1 and NOX2 are also critical for monocyte differentiation into macrophages, M2 polarization, and the occurrence of tumor-associated macrophages [97]. Deletion of both NOX1 and NOX2 causes a dramatic decrease in ROS generation in macrophages and results in impaired monocyte-to-macrophage differentiation and M2 polarization, and reduced tumor growth and metastasis [97]. Another study demonstrated that NOX2-dependent ROS production activates ataxia telangiectasia mutated (ATM) kinase, which is required for ionizing radiation-elicited macrophage activation and M1 polarization [98]. Specifically, this study showed that inhibition of NOX2 impaired M1 polarization and caused a poor tumor response to preoperative radiotherapy in locally advanced rectal cancer [98]. While discrepancies exist, ROS is generally considered to plan an important role in M1 polarization [99].
NADPH production and macrophage polarization
As an alternative to malic enzyme-dependent generation of the reducing agent NADPH, NADPH is also generated by the pentose phosphate pathway (PPP), which is induced in macrophages upon LPS stimulation [13] and regulated at multiple levels. In macrophages, sedoheptulose kinase CARKL represents the regulatory step in PPP that determines M1 versus M2 polarization. CARKL expression is downregulated by LPS in M1 macrophages and it is induced by IL2 in M2 macrophages [13]. Thus, regulation of NADPH production can also regulate macrophage polarization.
The TCA Cycle
To maintain anti-pathogen phagocytic activity, mitochondria switch their mitochondrial metabolism from ATP production to primarily generate ROS. However, the M2 subtype macrophages sustain ATP production through the TCA cycle due to slower rates of aerobic glycolysis. In eukaryotic systems, the TCA cycle is an amphibolic system (participates in opposing reaction types – cataplerotic and anaplerotic) that is crucial for efficient energy production. Cataplerotic reactions utilize and drain TCA cycle intermediates for the biosynthesis of lipids and amino acids; while anaplerotic reactions replenish intermediates by oxidation of lipids and breakdown/transamination/deamination of amino acids.
M2-polarized macrophages have a higher number of mitochondria and increased oxygen consumption rates. The expression of mitochondrial transcription factor A (TFAM), a regulator of mitochondrial biogenesis; and cytochrome c oxidase subunit 1 (Cox-1), a component of the electron transport chain, increase significantly during M2 macrophage polarization [12]. M1 macrophages have multiple interruptions in the TCA cycle, that result in the accumulation of TCA metabolites, including citrate and succinate [13]. Citrate accumulation occurs after inhibition of isocitrate dehydrogenase (Idh) and leads to lipid biosynthesis and production of itaconic acid (via the immunoresponsive gene 1 [14]). Itaconic acid can, in turn, inhibit the activity of succinate dehydrogenase, another enzyme in the TCA cycle that regulates ROS production [15, 16]. Inhibition of the TCA cycle enzymes is often compensated by other metabolic pathways. 13C-tracer studies suggest the presence of an active variant of the aspartate-arginosuccinate shunt that compensates for Idh blockage in M1 macrophages [13]. In M1 macrophages, inhibition of aspartate-aminotransferase, a key enzyme of the shunt, blocks the production of nitric oxide and interleukin-6 (IL-6), while promoting mitochondrial respiration [13]. These mechanisms ensure decreased dependence of M1 polarized macrophages on the TCA cycle for ATP production. Thus, while the M2-polarized macrophages have an increased dependence on the TCA cycle for energy, the M1-polarized macrophages have increased dependence on the TCA cycle for ROS production and various metabolites that sustain M1 macrophage functions.
The TCA cycle in macrophages can be fed by both glucose and glutamine. Unlike M1-polarized macrophages, M2-polarized macrophages are highly dependent on the flux of glutamine into the TCA cycle [13]. A recent study demonstrates that activation of anti-tumor functions of macrophages by CpG oligodeoxynucleotide, a Toll-like receptor 9 agonist, promotes a shift away from complete utilization of carbon from glucose and toward glutamine anaplerosis for generating TCA cycle intermediates such as succinyl-CoA [17]. These results suggest that the choice of nutrients may be a factor in determining the polarization status of TAMs. Of note, plasticity in macrophage phenotypes is in part due to carbon flux through the TCA cycle. Due to increased numbers of functional mitochondria, M2 macrophages demonstrate a great deal of plasticity; however, due to mitochondrial dysfunction, M1-polarized macrophages cannot repolarize to the M2 phenotype [18]. Thus, M1 polarization cannot be reverted. Overall, the TCA cycle prevalent in TAMs may contribute to pro-tumorigenic bioenergetic functions and plasticity in macrophage phenotypes but the plasticity to anaplerotic flux in the TCA cycle can be utilized to facilitate anti-tumorigenic functions.
Fatty Acid Metabolism
Fatty acid oxidation (FAO) provides a crucial energy source for macrophage polarization towards the M2 phenotype. For FAO, M2 polarized macrophages internalize triacylglycerol substrates via the scavenger receptor CD36 (i.e., cluster of differentiation 36) and the substrates subsequently undergo lipolysis by lysosomal acid lipases [19]. Specifically, stimulation of macrophages with interleukin-4 (IL-4), which induces M2 polarization, facilitates fatty acid uptake and oxidation, and enhances mitochondrial biogenesis [20]. Inhibition of FAO in TAMs promotes anti-tumorigenic differentiation of TAMs and inhibits tumor growth [21]. The mechanistic basis of upregulation of FAO in macrophages is well known. Both FAO and mitochondrial biogenesis are transcriptionally induced by the peroxisome proliferator-activated receptor-gamma (PPARγ) and downstream activation of the PPARγ-coactivator-1β (PGC-1β) [20, 22]. PPARγ is required for M2 or pro-tumorigenic polarization of macrophages in vitro and in vivo [20, 21]. Similarly, PGC-1β is both necessary and sufficient to polarize macrophages to the M2 phenotype [22]. Further, PGC-1β-mediated regulation of FAO is, at least in part, mediated by activation of the signal transducer and activator of transcription 6 (STAT6) [22]. STAT6-null macrophages are unable to induce FAO in response to IL-4 stimulation. Thus, targeting PPARγ, PGC-1β, or STAT6 may prevent/revert M2 polarization of macrophages by inhibiting FAO. Lipids also have signaling functions in macrophage biology. Short-chain saturated fatty acids act as endogenous activators of toll-like receptor 4 (TLR4) signaling, which can in turn regulate macrophage activation [23]. Hence, FAO supports pro-tumorigenic macrophage polarization.
While FAO supports pro-tumorigenic potential of macrophages, when coupled with fatty acid biosynthesis it can also facilitate anti-tumorigenic macrophage polarization. Simultaneous induction of fatty acid biosynthesis and FAO can facilitate overriding of inhibitory signals used by cancer cells to evade elimination by innate immunity [17]. CpG oligodeoxynucleotide facilitate antitumor activity, including engulfment of CD47+ cancer cells by facilitating FAO and shunting of the TCA cycle intermediates for de novo lipid biosynthesis [17]. Carnitine palmitoyltransferase 1A and adenosine tri-phosphate citrate lyase, which, together, facilitate integration of FAO and lipid biosynthesis to induce antitumor potential in macrophages, allowing them to overcome inhibitory CD47 on cancer cells [17]. The de novo lipid biosynthesis can facilitate biosynthesis of cholesterol and other lipids that can regulate membrane fluidity for anti-tumorigenic phagocytic functions [17]. Of note, while induction of de novo cholesterol biosynthesis facilitates antitumorigenic functions, membrane cholesterol efflux by tumor cells can impart pro-tumorigenic role in macrophages in metastatic ovarian cancer [24]. Thus, while FAO supports pro-tumorigenic functions, when coupled with de novo lipid and cholesterol biosynthesis, it may support anti-tumorigenic functions of macrophages. However, the relative contributions of general lipid biosynthesis versus cholesterol biosynthesis in reverting FAO-mediated pro-tumorigenic support of macrophages is not known.
Metabolites that Regulate Macrophage Polarization
Succinate
Succinate is a proinflammatory metabolite that stabilizes HIF-1α by inhibiting prolyl hydroxylase activity and enhances generation of ROS [25]. Succinate-mediated stabilization of HIF-1α enhances glycolysis. Metabolomic analysis of LPS-activated macrophages shows upregulation of aerobic glycolysis and downregulation of TCA cycle intermediates, which correlates directly with the expression profiles of altered metabolites, including succinate [26]. Prolyl hydroxylases utilize alpha-ketoglutarate (α-KG) as a substrate, and succinate is the reaction product which can cause feed-back inhibition of reaction, resulting in HIF-1α stabilization. Succinate is derived from glutamine through glutamine anaplerosis, as well as the “gamma-aminobutyric acid (GABA) shunt” pathway. LPS induces expression of glutamine transporter solute carrier family 3 member 2 (SLC3A2), as well as GABA transporters SLC6A13 and SLC6A12, and enhances glutamine uptake, anaplerosis, and GABA production. Glutamine and GABA then increase succinate levels to stabilize HIF-1α that regulates glycolysis and macrophage polarization. Additionally, succinate regulates ROS production [16], which is a key mechanism for maintaining a pro-inflammatory state in macrophages [27] [28]. Mitochondria maintain a high membrane potential in M1-polarized macrophages due to a parallel increase in glycolytic flux [16]. The M1-polarized macrophages enhance succinate dehydrogenase-mediated oxidation of succinate. The increased oxidation of succinate and elevated mitochondrial membrane potential collectively drive ROS production. The increased ROS levels facilitate pro-inflammatory gene expression. Notably, inhibition of succinate oxidation with dimethyl malonate (DMM) can diminish the inflammatory phenotype in macrophages. The inflammatory phenotype is also diminished by inhibition of ROS production by mitochondrial uncoupling with rotenone or by expressing the alternative oxidase (AOX), which can oxidize excess electrons that build up in the ubiquinone (CoQ). The metabolic alterations that occur upon activation of macrophages, therefore, repurpose mitochondria from ATP synthesis to ROS production in order to promote a pro-inflammatory state. Thus, succinate acts as a key metabolite regulating macrophage polarization via enhancing aerobic glycolysis as well as ROS production.
α-ketoglutarate
Another key metabolite in the TCA cycle that induces macrophage polarization to an M2 phenotype is α-KG [25]. Inhibiting glutaminase, which provides glutamate as a source for α-ketoglutarate production, decreases M2 polarization, while increasing M1 polarization. By acting as the substrate for prolyl hydroxylases, α-ketoglutarate can destabilize HIF-1α, which is critical for M1 polarization. In addition to its role in stabilizing HIF-1α, α-KG is important for the activation of M2 macrophages, by facilitating FAO and jumonji domain-containing protein 3 (Jmjd3)-dependent epigenetic regulation of M2 gene expression [25]. M2 macrophages operate a functional TCA cycle for ATP production that is, in part, fed by FAO [19]. The increased glycolytic and glutaminolytic carbon metabolism facilitates M2 activation by providing ATP and acetyl-CoA for epigenetic reprogramming [29]. However, α-KG-mediated M2 polarization is primarily a result of glutaminolysis [25]. M2 activation increases the expression of the FAO rate-limiting enzyme carnitine palmitoyltransferase 1a (Cpt1a) and correspondingly enhances the fatty acid uptake in an αKG-dependent fashion. Glutaminolysis-dependent production of α-KG regulates trimethylation of histone H3 lysine 27 (H3K27me3) on promoter regions of genes that define the M2 phenotype. While α-KG serves as a co-stimulator for Jmjd3, succinate serves as an inhibitor. Hence, the ratio of α-KG: succinate determines the macrophage polarization status. While an increased α-KG:succinate ratio causes an M2 polarization, a decreased α-KG:succinate ratio induces the proinflammatory M1 macrophage phenotype. Also, α-KG can restrict M1 activation by downregulating the activation of the NF-κB pathway. Hence, α-KG can regulate macrophage polarization by destabilizing HIF-1α, enhancing FAO and glutaminolysis, and by inducing epigenetic reprogramming.
Itaconate
Itaconic acid (ITA), or methylenesuccinic acid, is synthesized by macrophages in response to activation by LPS and interferon gamma (IFN-γ) [30]. Specifically, ITA is synthesized by decarboxylation of cis-aconitate by the immunoresponsive gene 1, which is also induced in activated macrophages [31]. Itaconate exerts anti-inflammatory effects by regulating macrophage metabolism. Itaconate inhibits oxidation of succinate by succinate dehydrogenase and thus, inhibits ROS production and inflammasome priming [15] [32]. The increased succinate can also stabilize HIF-1α-mediated glycolytic flux. Interestingly, macrophages from mice that are knockout for immunoresponsive gene 1 cannot produce itaconate and do not maintain high succinate levels in response to activation. By diminishing succinate levels, itaconate also regulates mitochondrial respiration and inflammatory cytokine production during macrophage activation [15]. Itaconate can also inhibit substrate-level phosphorylation and thus regulate the overall bioenergetics of macrophages [14]. Hence, itaconate facilitates an anti-inflammatory state in macrophages by directly regulating the activation of succinate dehydrogenase.
Amino acids
The activation and function of macrophages are regulated by multiple amino acids. For example, LPS-activated macrophages consume glutamine and serine at increased rates, while producing increased amounts of glycine, glutamate, alanine, citrulline, and histidine [33]. Glycine causes inhibition of LPS-induced nitric oxide production and macrophage activation [34]. Glutamine contributes to anaplerotic replenishment of the TCA cycle, providing carbon and nitrogen for the synthesis of amino acids, proteins, nucleotides, and lipids [35]. Glutamine also contributes to the synthesis of glutamate, α-KG, glutathione, and the hexosamine biosynthesis pathway. Chiefly, glutamine regulates the production of inflammatory cytokines and nitric oxide in macrophages [36], and also participates in TCA cycle anaplerosis, which plays an important role in macrophage polarization and activation [13]. Integrated high-throughput transcriptional-metabolic profiling and analysis studies demonstrate that M2 polarization activates glutamine catabolism and the hexosamine biosynthesis pathway [13]. Along the same line, depriving cells of glutamine or inhibiting N-glycosylation negatively impacts M2 polarization and expression of C-C motif chemokine 22 (CCL22) and other M2-specific markers. However, glutamine is not needed for the development of LPS-stimulated M1 macrophages [13].
Several other amino acids, including L-arginine, tryptophan, and branched-chain amino acids, also regulate the activation states of macrophages. L-arginine is a major amino acid regulator of macrophage activation/polarization and can be derived from glutamine via a citrulline intermediate. Previous work suggests that macrophages are capable of maintaining high rates of arginine production from glutamine [37]. The metabolic fate of arginine regulates polarization of M1 and M2. While the arginine flux into nitric oxide production facilitates M1 polarization, its flux into the arginase pathway regulates M2 polarization [38]. Tryptophan is another key amino acid that regulates macrophage activation. Notably, tryptophan catabolism in macrophages inhibits adaptive immune system activity. Indoleamine-2,3-dioxygenase, which catalyzes the rate-limiting step in tryptophan catabolism, is stimulated by LPS, IFNγ, and activation of TLRs, thus facilitating flux along the kynurenine pathway [39–41]. Kynurenine release by macrophages induces signaling via the aryl-hydrocarbon receptor. Likewise, tryptophan consumption by macrophages and dendritic cells expressing indoleamine-2,3-dioxygenase induces signaling via amino-acid sensors GCN2 and mTOR. Together, these actions robustly modulate the responses of T cells and T regulatory cells (Treg) to inflammation and antigens [42]. Branched-chain amino acids also contribute to a state of macrophage activation. Specifically, branched chain amino acids like leucine can provide acetoacetate, acetyl-CoA, and glutamine, which can in turn feed into the TCA cycle or provide metabolites like α-KG, which can directly reprogram macrophage activation [43]. Further, BCAT1, the predominant branched-chain aminotransferase (BCAT) isoform in human primary macrophages, regulates oxygen consumption, glycolysis, immunoresponsive gene 1 levels, and itaconate synthesis [44]. Thus, glutamine, arginine, tryptophan, and branched-chain amino acids regulate macrophage functions at multiple levels.
TME Metabolism and macrophage polarization
Adenosine
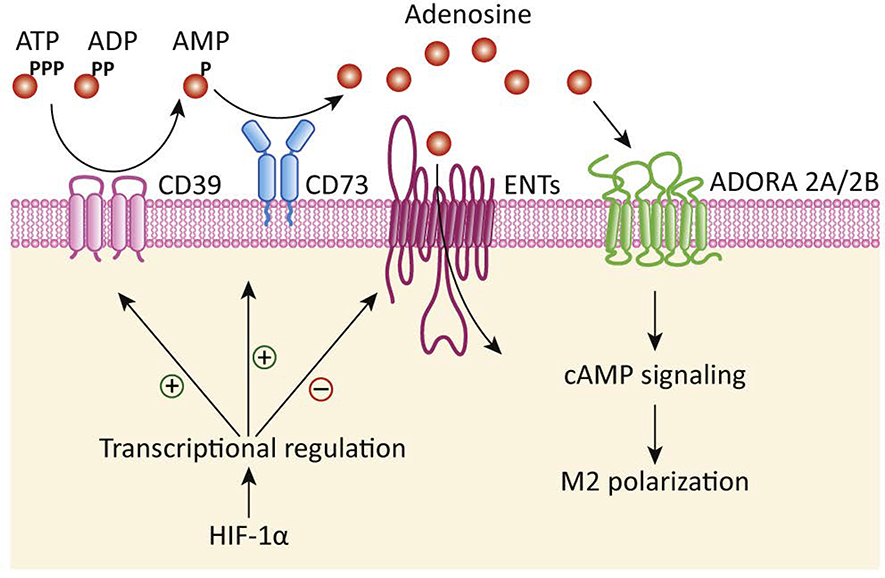
Adenosine nucleoside is released from a variety of cell types in the tumor microenvironment. Adenosine regulates the phagocytic activity of mononuclear cells, including macrophages, via adenosine receptors A1, A2A, A2B, and A3, which are G-protein–coupled transmembrane receptors [45]. Adenosine binding to A2A receptors diminishes M1 polarization, while adenosine-mediated activation of A2B receptor induces M2 macrophage polarization. In culture conditions, adenosine facilitates migration and recruitment of monocytes to tumors [46]. Tumor-associated macrophages also express ectonucleotidases CD39 and CD73, which can further catalyze adenosine production and signaling downstream adenosine receptors. Accordingly, knockout of the A2A receptor in myeloid cells results in inhibition of melanoma tumor growth and metastasis due to the increased cytotoxic activity of natural killer (NK) and T cells [47]. A2A receptor knockout in myeloid cells also diminishes M2 polarization, while increases M1 polarization in tumor-associated macrophages [47]. Thus, adenosine in tumor microenvironments leads to immunosuppression. Importantly, inhibition of CD39 or CD73 can reverse tumor-associated macrophage-mediated inhibition of T-cell proliferation [46]. Furthermore, expressions of CD39 and CD73 are induced by HIF-1α [48, 49]. Hypoxia also induces expression of A2A andA2B receptors [50, 51]. Additionally, hypoxia can diminish the expression of adenosine kinase and equilibrative nucleoside transporters [52, 53]. Thus, hypoxic microenvironments or mTOR signaling in tumors that stabilize/induce HIF-1α expression result in further enhancements in adenosine levels in tumor microenvironments. Hence, metabolic alterations in TME that facilitate adenosine accumulation in the interstitial spaces cause immunosuppression by facilitating M2 polarization (Figure 3).
Figure 3. Adenosine signaling regulates macrophage polarization.
Ectonucleoside triphosphate diphosphohydrolase 1 (CD39) expressed at the cell surface of macrophages, cancer cells, and other cells in the tumor microenvironment catalyzes the production of adenosine monophosphate (AMP). Ecto-5’-nucleotidase (CD73) further converts AMP into adenosine, which can either enter the cells through equilibrative subclass of nucleoside transporters (ENTs) or act on adenosine receptors 2A (ADORA 2A) and 2B (ADORA 2B) to induce M2 polarization of macrophages. Hypoxia-inducible factor-1 alpha (HIF-1α) further facilitates these processes by transcriptional induction of CD39/CD73 and transcriptional downregulation of ENTs.
Lactate
Signaling functions and polarization of M2 macrophages, in particular, can be regulated by lactate in the tumor microenvironment. Lactate is an end-product of aerobic glycolysis, which is actively carried out by tumor cells [54, 55]. Lactate production is further facilitated by stabilization of HIF-1α under conditions of hypoxia and signaling by oncogenes like the mucin MUC1 [56–59]. Aerobic glycolysis in macrophages regulates macrophage polarization, as well as tumor growth and metastasis due to immunosuppression [60–63]. Like the case of aerobic glycolysis, lactate also serves as a direct regulator of macrophage polarization [64, 65]. Tumor cell-secreted lactate stabilizes HIF-1α and induces expression of vascular endothelial growth factor (VEGF) and M2 polarization of tumor-associated macrophages [64]. Tumor cell-secreted lactate is sufficient to stimulate expression of VEGF and M2 polarization markers, including Arg1, Fizz1, Mgl1, and Mgl2. While HIF-1α is also critical for M1 polarization, the same regulator may play a role in both M1 and M2 polarizations, depending on the cues from the microenvironment. These activities of lactate are dependent on HIF-1α expression, and these functions of lactate are cytokine independent. Experiments with microfluidic systems have demonstrated that lactate, by virtue of its small size, can penetrate faster and deeper into the tumor microenvironments compared to the large cytokine molecules [65]. Sensing of lactate by macrophages for M2 polarization is mediated by G protein-coupled receptor 132 (Gpr132) on macrophages, and loss of Gpr132 in mice inhibits breast cancer metastasis [66]. Also, decreased Gpr132 expression in breast cancer patients correlates with improved metastasis-free survival. Studies demonstrate that secreted metabolites like lactate form concentration gradients and relay spatial information about tumor metabolism that impacts macrophage polarization. Such metabolite gradients also induce differential activation of signaling pathways, including the Kras/MAPK pathway, in tumor-associated macrophages [67]. Thus, lactate levels in tumor microenvironments regulate signaling functions and polarization of M2 macrophages.
Therapeutic potential of targeting macrophage metabolism in cancer
Macrophage-targeted therapies are increasingly being utilized for cancer. These therapies target macrophage reprogramming (anti-MARCO, PI3Kγ antagonist, and HDAC inhibitor), mobility, and immunosuppressive phenotype (CD40 agonists, anti-CTLA4, and anti-PD1) as well as their absolute numbers in the tumor microenvironment (anti-CSFR1 and anti-CCR2) [68–77]. Macrophage-targeted approaches, in combination with chemotherapies, have shown reduced tumor burden and improved survival in tumor-bearing mice. Interestingly, the efficacy of these therapeutic strategies varies in terms of generating an anti-tumor response. A recent study highlights that reprogramming but not depletion of tumor-associated macrophages reduces tumor burden by allowing the accumulation of TCR-engineered T cells (mesothelin-targeted T cells) in the pancreatic tumors [78]. Also, another study documents that macrophage depletion triggers the compensatory replacement of TAMs with CXCR2+ granulocytes in anti-CCR2 and treated pancreatic tumor-bearing mice [68]. Together these data suggest that macrophage reprogramming but not depletion may provide superior benefits in triggering anti-tumor response and controlling tumor growth.
Since tumor-derived metabolites dictate macrophage reprogramming that is integral to macrophage function, metabolic reprogramming presents an attractive therapeutic candidate for repolarization of macrophages in cancer. At present lactate as well as adenosine-targeted interventions are currently being explored in preclinical models of cancer. Lactate modulates macrophage metabolism via Gpr132. Gpr132, a pH sensor, is highly expressed in macrophages and regulates macrophage function in acidic tumor-microenvironment of breast cancer [66]. Pharmacological inhibition of Gpr 132 has shown a significant reduction of tumor weight and volume of breast tumor-bearing mice. Of note, Gpr132 is primarily expressed in lymphocytes (www.gtexportal.org) and hence, may serve as an optimal target with minimal toxicity. Similarly, another study showed that tumor-derived lactate favor M2 polarization by up-regulating ERK/STAT3 axis in a murine model of breast cancer [79]. Moreover, inhibition of this axis with MEK/ STAT3 inhibitors (selumetinib/ static) attenuates lactate-induced macrophage polarization. These data are encouraging the identification of lactic acid regulators (glycolytic regulators) for controlling aerobic glycolysis in tumors and hence tumor growth.
Extracellular adenosine, another key tumor-metabolite alters macrophages functions, including phagocytosis and cytokine production via different adenosine receptors (A1, A2A, A2B, and A3) [80–84]. Emerging pieces of evidence reveal that adenosine also governs VEGF production by macrophages [85]. VEGF is an important factor for tumor growth as it drives tumor angiogenesis [86, 87]. Hence, modulation of macrophage function by adenosine or adenosine receptor-targeted therapies will benefit cancer treatment.
A recent report suggests that fasting can control cancer growth by modulating M2 polarization [88]. Alternate day fasting-induced autophagy of tumor cells inhibits intra-tumoral adenosine levels and reduces M2 polarization of macrophages in tumor-bearing mice [88]. Similarly, another study highlights the effect of intermittent fasting on the metabolic reprogramming of macrophages in mice models of obesity [89]. The fasting-mimicking diets (FMDs) can benefit in cancer treatment [88, 90–92]. These diets can induce metabolic alteration not only in the tumor cells but also in immunosuppressive immune cells such as macrophages and MDSCs while preserving normal cells [93]. Through its effect on macrophage polarization, FMD can improve the therapeutic benefits of chemotherapies/radiation therapies in cancer. Of note, FMD, in combination with chemotherapies, is currently being tested in multiple clinical trials (NCT03595540, NCT03340935, NCT03709147, NCT03700437).
Glutamine synthase (GS) is another key enzyme that primes macrophage polarization toward M2-Like phenotype by increasing glutamine levels to facilitate tumor metastasis and can be exploited for cancer therapy [94]. GS also helps in providing nucleotide pools that are necessary for building blocks and glycosylation of key M2-marker such as mannose receptor. GS inhibition by methionine sulfoximine (MSO) inhibits the pro-inflammatory cytokine secretion by murine macrophages and recuse M2-like polarization skews M2 macrophages toward an M1-like phenotype in IL10-treated macrophages [94]. Methionine sulfoximine shunts glucose instead of glutamine into TCA and up-regulates the expression inflammatory cytokines such as TNF-α, NOS2, CXCL9 and CXCL10 (M1-associated cytokines) with a concomitant decrease of CD163 expression (M2-marker) in IL10-treated macrophages. Furthermore, genetic deletion of GS in macrophages repolarize macrophages toward M1-like phenotype, favors intra-tumoral CTL (cytotoxic T cells) accumulation and attenuates metastasis in the Lewis lung carcinoma model [94].
In addition to macrophage reprogramming by lactate and glutamine synthase, macrophage-specific targeting of mTOR signaling reverses angiogenic properties of M2 macrophage, induces M1-like phenotype, and diminishes metastasis in Lewis lung carcinoma [62]. Hence, metabolic rewiring of macrophages from M2 to M1 type can trigger anti-tumor effector T cells responses in tumor-bearing mice. Overall, these studies highlight that macrophage-specific metabolic alterations can enhance anti-tumor responses for robust control of tumor growth and metastatic spread.
Concluding Remarks
Overall, metabolic alterations in macrophages regulate their survival, differentiation, polarization, and recruitment into tumor microenvironments. Such responses are mediated, at least in part, by direct changes in metabolite flux or by metabolite-mediated regulation of transcription factors like HIF-1α. Secreted metabolites such as adenosine and lactate also fine-tune the response of macrophages to tumor cells in the tumor microenvironment. However, due to the complex interplay of cytokines and metabolites, much remains to be deciphered regarding the physiological response of macrophages resulting from metabolic alterations/secreted metabolite levels. Also, it is not clear how such responses differ in primary tumors versus metastatic niches (see Outstanding Questions). To what extent metabolic alterations in macrophages drive the evolution of tumor cells, remains yet to be fully deciphered. Furthermore, only a few tumor cell-secreted metabolites have been investigated in driving macrophage plasticity and polarization. Roles of other tumor- or stroma-secreted metabolites remain largely unknown. While the metabolic perturbations significantly modulate macrophage polarization and responses, the potential for therapeutics for cancer patients remains to be investigated. Of note, the same shared pathway used by tumor cells and other cells in the microenvironment may have opposing functions depending on the cellular context. Hence, the most effective metabolic pathway for targeted therapy would have synergistic effects on tumor cells and TME. Nonetheless, the studies discussed above clearly show that metabolic alterations are a major driving force in the suppressive or activating roles of macrophages in the tumor microenvironment and hold great potential for immune-responsive cancer treatment.
Acknowledgements
This work was supported in part by funding from the National Institutes of Health grant (R01 CA163649, R01 CA210439, and R01 CA216853, NCI) to PKS; P01 CA217798 to PKS; the Specialized Programs of Research Excellence (SPORE, 2P50 CA127297, NCI) to PKS. NCI-Career development award (SPORE, 2P50 CA127297, NCI) to KM.
References
- 1.Muraoka D et al. (2019) Antigen delivery targeted to tumor-associated macrophages overcomes tumor immune resistance. J Clin Invest 129 (3), 1278–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broz ML et al. (2014) Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 26 (5), 638–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van den Bossche J et al. (2015) Metabolic Characterization of Polarized M1 and M2 Bone Marrow-derived Macrophages Using Real-time Extracellular Flux Analysis. J Vis Exp (105). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palsson-McDermott EM et al. (2015) Pyruvate kinase M2 regulates Hif-1alpha activity and IL-1beta induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab 21 (1), 65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan Z et al. (2015) Pyruvate dehydrogenase kinase 1 participates in macrophage polarization via regulating glucose metabolism. J Immunol 194 (12), 6082–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki H et al. (2016) Glycolytic pathway affects differentiation of human monocytes to regulatory macrophages. Immunol Lett 176, 18–27. [DOI] [PubMed] [Google Scholar]
- 7.Chiba S et al. (2017) Glycolysis regulates LPS-induced cytokine production in M2 polarized human macrophages. Immunol Lett 183, 17–23. [DOI] [PubMed] [Google Scholar]
- 8.Chen DP et al. (2019) Glycolytic activation of peritumoral monocytes fosters immune privilege via the PFKFB3-PD-L1 axis in human hepatocellular carcinoma. J Hepatol. [DOI] [PubMed] [Google Scholar]
- 9.Baseler WA et al. (2016) Autocrine IL-10 functions as a rheostat for M1 macrophage glycolytic commitment by tuning nitric oxide production. Redox Biol 10, 12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farooque A et al. (2016) Polarization of macrophages towards M1 phenotype by a combination of 2-deoxy-d-glucose and radiation: Implications for tumor therapy. Immunobiology 221 (2), 269–81. [DOI] [PubMed] [Google Scholar]
- 11.Wang F et al. (2018) Glycolytic Stimulation Is Not a Requirement for M2 Macrophage Differentiation. Cell Metab 28 (3), 463–475 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tavakoli S et al. (2013) Bioenergetic profiles diverge during macrophage polarization: implications for the interpretation of 18F-FDG PET imaging of atherosclerosis. J Nucl Med 54 (9), 1661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jha AK et al. (2015) Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 42 (3), 419–30. [DOI] [PubMed] [Google Scholar]
- 14.Nemeth B et al. (2016) Abolition of mitochondrial substrate-level phosphorylation by itaconic acid produced by LPS-induced Irg1 expression in cells of murine macrophage lineage. FASEB J 30 (1), 286–300. [DOI] [PubMed] [Google Scholar]
- 15.Lampropoulou V et al. (2016) Itaconate Links Inhibition of Succinate Dehydrogenase with Macrophage Metabolic Remodeling and Regulation of Inflammation. Cell Metab 24 (1), 158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mills EL et al. (2016) Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell 167 (2), 457–470 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu M et al. (2019) Metabolic rewiring of macrophages by CpG potentiates clearance of cancer cells and overcomes tumor-expressed CD47-mediated ‘don’t-eat-me’ signal. Nat Immunol 20 (3), 265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van den Bossche J et al. (2016) Mitochondrial Dysfunction Prevents Repolarization of Inflammatory Macrophages. Cell Rep 17 (3), 684–696. [DOI] [PubMed] [Google Scholar]
- 19.Huang SC et al. (2014) Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol 15 (9), 846–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Odegaard JI et al. (2007) Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 447 (7148), 1116–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niu Z et al. (2017) Caspase-1 cleaves PPARgamma for potentiating the pro-tumor action of TAMs. Nat Commun 8 (1), 766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vats D et al. (2006) Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab 4 (1), 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi H et al. (2006) TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116 (11), 3015–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goossens P et al. (2019) Membrane Cholesterol Efflux Drives Tumor-Associated Macrophage Reprogramming and Tumor Progression. Cell Metab 29 (6), 1376–1389 e4. [DOI] [PubMed] [Google Scholar]
- 25.Liu PS et al. (2017) alpha-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat Immunol. [DOI] [PubMed] [Google Scholar]
- 26.Tannahill GM et al. (2013) Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature 496 (7444), 238–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan HY et al. (2016) The Reactive Oxygen Species in Macrophage Polarization: Reflecting Its Dual Role in Progression and Treatment of Human Diseases. Oxid Med Cell Longev 2016, 2795090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Formentini L et al. (2017) Mitochondrial ROS Production Protects the Intestine from Inflammation through Functional M2 Macrophage Polarization. Cell Rep 19 (6), 1202–1213. [DOI] [PubMed] [Google Scholar]
- 29.Covarrubias AJ et al. (2016) Akt-mTORC1 signaling regulates Acly to integrate metabolic input to control of macrophage activation. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strelko CL et al. (2011) Itaconic acid is a mammalian metabolite induced during macrophage activation. J Am Chem Soc 133 (41), 16386–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michelucci A et al. (2013) Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc Natl Acad Sci U S A 110 (19), 7820–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cordes T et al. (2016) Immunoresponsive Gene 1 and Itaconate Inhibit Succinate Dehydrogenase to Modulate Intracellular Succinate Levels. J Biol Chem 291 (27), 14274–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishiyama A et al. (2010) Changes in amino acid metabolism during activation of mouse macrophage-like cell lines. In Vivo 24 (6), 857–60. [PubMed] [Google Scholar]
- 34.Hino S et al. (2016) Quest for Cells Responsible for Age-related Increase of Salivary Glycine and Proline. In Vivo 30 (2), 91–7. [PubMed] [Google Scholar]
- 35.Shanware NP et al. (2014) Glutamine deprivation stimulates mTOR-JNK-dependent chemokine secretion. Nat Commun 5, 4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bellows CF and Jaffe BM (1999) Glutamine is essential for nitric oxide synthesis by murine macrophages. J Surg Res 86 (2), 213–9. [DOI] [PubMed] [Google Scholar]
- 37.Murphy C and Newsholme P (1998) Importance of glutamine metabolism in murine macrophages and human monocytes to L-arginine biosynthesis and rates of nitrite or urea production. Clin Sci (Lond) 95 (4), 397–407. [PubMed] [Google Scholar]
- 38.Rath M et al. (2014) Metabolism via Arginase or Nitric Oxide Synthase: Two Competing Arginine Pathways in Macrophages. Front Immunol 5, 532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orhan F et al. (2016) Tryptophan Metabolism Along the Kynurenine Pathway Downstream of Toll-like Receptor Stimulation in Peripheral Monocytes. Scand J Immunol 84 (5), 262–271. [DOI] [PubMed] [Google Scholar]
- 40.Werner ER et al. (1989) Parallel induction of tetrahydrobiopterin biosynthesis and indoleamine 2,3-dioxygenase activity in human cells and cell lines by interferon-gamma. Biochem J 262 (3), 861–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Neill LA et al. (2016) A guide to immunometabolism for immunologists. Nat Rev Immunol 16 (9), 553–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Munn DH and Mellor AL (2013) Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol 34 (3), 137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hutson SM et al. (2001) Function of leucine in excitatory neurotransmitter metabolism in the central nervous system. J Nutr 131 (3), 846S–850S. [DOI] [PubMed] [Google Scholar]
- 44.Papathanassiu AE et al. (2017) BCAT1 controls metabolic reprogramming in activated human macrophages and is associated with inflammatory diseases. Nat Commun 8, 16040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hasko G and Pacher P (2012) Regulation of macrophage function by adenosine. Arterioscler Thromb Vasc Biol 32 (4), 865–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montalban Del Barrio I et al. (2016) Adenosine-generating ovarian cancer cells attract myeloid cells which differentiate into adenosine-generating tumor associated macrophages - a self-amplifying, CD39- and CD73-dependent mechanism for tumor immune escape. J Immunother Cancer 4, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cekic C et al. (2014) Myeloid expression of adenosine A2A receptor suppresses T and NK cell responses in the solid tumor microenvironment. Cancer Res 74 (24), 7250–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Synnestvedt K et al. (2002) Ecto-5’-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest 110 (7), 993–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li J et al. (2017) CD39/CD73 upregulation on myeloid-derived suppressor cells via TGF-beta-mTOR-HIF-1 signaling in patients with non-small cell lung cancer. Oncoimmunology 6 (6), e1320011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahmad A et al. (2009) Adenosine A2A receptor is a unique angiogenic target of HIF-2alpha in pulmonary endothelial cells. Proc Natl Acad Sci U S A 106 (26), 10684–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eltzschig HK et al. (2009) Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood 113 (1), 224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morote-Garcia JC et al. (2008) HIF-1-dependent repression of adenosine kinase attenuates hypoxia-induced vascular leak. Blood 111 (12), 5571–80. [DOI] [PubMed] [Google Scholar]
- 53.Eltzschig HK et al. (2005) HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J Exp Med 202 (11), 1493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Warburg O (1956) On respiratory impairment in cancer cells. Science 124 (3215), 269–70. [PubMed] [Google Scholar]
- 55.Goodwin J et al. (2017) The distinct metabolic phenotype of lung squamous cell carcinoma defines selective vulnerability to glycolytic inhibition. Nat Commun 8, 15503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Semenza GL et al. (1994) Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem 269 (38), 23757–63. [PubMed] [Google Scholar]
- 57.Shukla SK et al. (2017) MUC1 and HIF-1alpha Signaling Crosstalk Induces Anabolic Glucose Metabolism to Impart Gemcitabine Resistance to Pancreatic Cancer. Cancer Cell 32 (1), 71–87 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mehla K and Singh PK (2014) MUC1: a novel metabolic master regulator. Biochim Biophys Acta 1845 (2), 126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chaika NV et al. (2012) MUC1 mucin stabilizes and activates hypoxia-inducible factor 1 alpha to regulate metabolism in pancreatic cancer. Proc Natl Acad Sci U S A 109 (34), 13787–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Penny HL et al. (2016) Warburg metabolism in tumor-conditioned macrophages promotes metastasis in human pancreatic ductal adenocarcinoma. Oncoimmunology 5 (8), e1191731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang SC et al. (2016) Metabolic Reprogramming Mediated by the mTORC2-IRF4 Signaling Axis Is Essential for Macrophage Alternative Activation. Immunity 45 (4), 817–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wenes M et al. (2016) Macrophage Metabolism Controls Tumor Blood Vessel Morphogenesis and Metastasis. Cell Metab 24 (5), 701–715. [DOI] [PubMed] [Google Scholar]
- 63.Tawakol A et al. (2015) HIF-1alpha and PFKFB3 Mediate a Tight Relationship Between Proinflammatory Activation and Anerobic Metabolism in Atherosclerotic Macrophages. Arterioscler Thromb Vasc Biol 35 (6), 1463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Colegio OR et al. (2014) Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513 (7519), 559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao Y et al. (2015) Bladder cancer cells re-educate TAMs through lactate shuttling in the microfluidic cancer microenvironment. Oncotarget 6 (36), 39196–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen P et al. (2017) Gpr132 sensing of lactate mediates tumor-macrophage interplay to promote breast cancer metastasis. Proc Natl Acad Sci U S A 114 (3), 580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carmona-Fontaine C et al. (2017) Metabolic origins of spatial organization in the tumor microenvironment. Proc Natl Acad Sci U S A 114 (11), 2934–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nywening TM et al. (2018) Targeting both tumour-associated CXCR2(+) neutrophils and CCR2(+) macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut 67 (6), 1112–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wiehagen KR et al. (2017) Combination of CD40 Agonism and CSF-1R Blockade Reconditions Tumor-Associated Macrophages and Drives Potent Antitumor Immunity. Cancer Immunol Res 5 (12), 1109–1121. [DOI] [PubMed] [Google Scholar]
- 70.Gordon SR et al. (2017) PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 545 (7655), 495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guerriero JL et al. (2017) Class IIa HDAC inhibition reduces breast tumours and metastases through anti-tumour macrophages. Nature 543 (7645), 428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Henau O et al. (2016) Overcoming resistance to checkpoint blockade therapy by targeting PI3Kgamma in myeloid cells. Nature 539 (7629), 443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Georgoudaki AM et al. (2016) Reprogramming Tumor-Associated Macrophages by Antibody Targeting Inhibits Cancer Progression and Metastasis. Cell Rep 15 (9), 2000–11. [DOI] [PubMed] [Google Scholar]
- 74.Ries CH et al. (2015) CSF-1/CSF-1R targeting agents in clinical development for cancer therapy. Curr Opin Pharmacol 23, 45–51. [DOI] [PubMed] [Google Scholar]
- 75.Zhu Y et al. (2014) CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res 74 (18), 5057–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Strachan DC et al. (2013) CSF1R inhibition delays cervical and mammary tumor growth in murine models by attenuating the turnover of tumor-associated macrophages and enhancing infiltration by CD8(+) T cells. Oncoimmunology 2 (12), e26968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beatty GL et al. (2011) CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 331 (6024), 1612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stromnes IM et al. (2019) Differential Effects of Depleting versus Programming Tumor-Associated Macrophages on Engineered T Cells in Pancreatic Ductal Adenocarcinoma. Cancer Immunol Res 7 (6), 977–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mu X et al. (2018) Tumor-derived lactate induces M2 macrophage polarization via the activation of the ERK/STAT3 signaling pathway in breast cancer. Cell Cycle 17 (4), 428–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takenaka MC et al. (2019) Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nat Neurosci 22 (5), 729–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Desai BN and Leitinger N (2014) Purinergic and calcium signaling in macrophage function and plasticity. Front Immunol 5, 580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ernens I et al. (2015) Adenosine stimulates angiogenesis by up-regulating production of thrombospondin-1 by macrophages. J Leukoc Biol 97 (1), 9–18. [DOI] [PubMed] [Google Scholar]
- 83.Csoka B et al. (2012) Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB J 26 (1), 376–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kara FM et al. (2010) Adenosine A1 receptors (A1Rs) play a critical role in osteoclast formation and function. FASEB J 24 (7), 2325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ernens I et al. (2010) Adenosine up-regulates vascular endothelial growth factor in human macrophages. Biochem Biophys Res Commun 392 (3), 351–6. [DOI] [PubMed] [Google Scholar]
- 86.Carmeliet P (2005) VEGF as a key mediator of angiogenesis in cancer. Oncology 69 Suppl 3, 4–10. [DOI] [PubMed] [Google Scholar]
- 87.Saharinen P et al. (2011) VEGF and angiopoietin signaling in tumor angiogenesis and metastasis. Trends Mol Med 17 (7), 347–62. [DOI] [PubMed] [Google Scholar]
- 88.Sun P et al. (2017) Fasting inhibits colorectal cancer growth by reducing M2 polarization of tumor-associated macrophages. Oncotarget 8 (43), 74649–74660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim KH et al. (2017) Intermittent fasting promotes adipose thermogenesis and metabolic homeostasis via VEGF-mediated alternative activation of macrophage. Cell Res 27 (11), 1309–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lettieri-Barbato D and Aquilano K (2018) Pushing the Limits of Cancer Therapy: The Nutrient Game. Front Oncol 8, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.O’Flanagan CH et al. (2017) When less may be more: calorie restriction and response to cancer therapy. BMC Med 15 (1), 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Di Biase S et al. (2016) Fasting-Mimicking Diet Reduces HO-1 to Promote T Cell-Mediated Tumor Cytotoxicity. Cancer Cell 30 (1), 136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.de Groot S et al. (2019) Effects of short-term fasting on cancer treatment. J Exp Clin Cancer Res 38 (1), 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Palmieri EM et al. (2017) Pharmacologic or Genetic Targeting of Glutamine Synthetase Skews Macrophages toward an M1-like Phenotype and Inhibits Tumor Metastasis. Cell Rep 20 (7), 1654–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lo HM et al. (2013) The carotenoid lutein enhances matrix metalloproteinase-9 production and phagocytosis through intracellular ROS generation and ERK1/2, p38 MAPK, and RARbeta activation in murine macrophages. J Leukoc Biol 93 (5), 723–35. [DOI] [PubMed] [Google Scholar]
- 96.O’Neill LA and Pearce EJ (2016) Immunometabolism governs dendritic cell and macrophage function. J Exp Med 213 (1), 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu Q et al. (2016) NADPH Oxidases Are Essential for Macrophage Differentiation. J Biol Chem 291 (38), 20030–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu Q et al. (2017) NOX2-dependent ATM kinase activation dictates pro-inflammatory macrophage phenotype and improves effectiveness to radiation therapy. Cell Death Differ 24 (9), 1632–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Calvani M et al. (2012) Time-dependent stabilization of hypoxia inducible factor-1alpha by different intracellular sources of reactive oxygen species. PLoS One 7 (10), e38388. [DOI] [PMC free article] [PubMed] [Google Scholar]