Abstract
Asian hair is known for its straightness, dark pigmentation, and large diameter. The cuticle layer in Asians is thicker with more compact cuticle cells than that in Caucasians. Asian hair generally exhibits the strongest mechanical properties, and its cross-sectional area is determined greatly by genetic variations, particularly from the ectodysplasin A receptor gene. However, knowledge on Asian hair remains unclear with limited studies. This article aimed to review and summarize the characteristics and properties of Asian hair. It also aimed to discuss hair disorders including linear lupus panniculitis and pseudocyst of the scalp that occur distinctively in Asian populations.
Keywords: alopecia, biogeographic population, ethnicity, hair loss, hair shaft disorders
Introduction
Human hair is one of the significant parts of the human body that reflects an individual’s appearance and identity. The diversity of human hair is the result of different genetics and demographic backgrounds together with hair grooming and cultural practices. Understanding the differences in hair morphologies, structures, and properties among various populations is important for clinicians to provide a correct diagnosis and treatment as hair disorders present and progress differently. Human hair is generally categorized into three major groups according to ethnic origins, ie, Asian, African, and Caucasian. Each ethnic group possesses different hair features including hair thickness, curvature, pigmentation, and mechanical properties.
Currently, knowledge on Asian hair remains unclear due to limited studies. This review aimed to summarize the characteristics and properties of Asian hair and present hair disorders exclusively described in Asian population. It is based on a systemic literature search of published articles from the MEDLINE database via PubMed using specific search terms “Asian,” “race,” “ancestry,” and “ethnic” combined with “hair” and/or “alopecia.” We conducted a manual research of each article’s reference list to ensure a more complete sample of existing literature.
Characteristics of Asian Hair
Hair in Asian population shows a distinctive appearance of being straight, round, and having black or brown pigmentation.1 Compared with Caucasian and African hairs, Asian hair shows multiple unique structures and properties. The comparisons of hair characteristics among the three major ethnic groups are summarized in Table 1.2–8
Table 1.
Comparisons of Hair Characteristics Among Three Major Ethnic Groups
| Asian | African | Caucasian | |
|---|---|---|---|
| Hair density |
|
|
|
| Cross-sectional area |
|
|
|
| Hair curvature and shape |
|
|
|
| Hair pigmentation |
|
|
|
| Hair growth rate |
|
|
|
| Lipid content and hydration |
|
|
|
| Integrity |
|
|
|
Note: Information provided in Table 1 is summarized from references 2–8.
Hair Shaft Structure
Hair shaft, mainly made up of keratin materials, consists of three layers, namely, the cuticle, cortex, and occasionally medulla. The medulla is located at the center, surrounded by the cortex, which is the principal structure of the hair shaft. The cuticle is the outermost layer that functions as a barrier against physical and chemical damage.
Cuticle
The cuticle layers are composed of multiple sheaths of flat overlapping cells and cell membrane complex (CMC) between the cuticle cells. Cuticle cells are small keratinized cells with a mean length of 60 µm and a thickness of about 0.5 µm.9 They are more compact with a higher number of cell layers. A study found that the Asian’s cuticle has a statistically significantly greater number of cuticle layers in one transverse section compared with the Caucasian’s cuticle.10,11 Although the total cuticle layers are significantly thicker in Asians, the thickness of one layer is particularly similar between the two ethnicities.11 A recent study with larger samples agreed that Asian hair has a higher median cuticle thickness than Caucasian and African hair.12 The cuticular inclination of Asian hair is steeper than that of Caucasian hair, while the interval between each cuticle is wider. The Caucasian’s cuticle is more fragile and tends to collapse into small pieces when exposed to extension stress.8,10 By contrast, the Asian’s cuticle has the ability to maintain its original shape and peels off as large fragments under stress or during the stretching process.8,10 Among the three biogeographic populations, the cuticle layers closely attached more in Asian and Caucasian hairs, while instances of cuticle layer separation occur in African hair. Moreover, the African hair shows a higher incidence of cuticle damage.12
Cortex
With regard to the cortex of the hair shaft, cortical cell structures generally comprise macrofibrils, remnants, and pigment granules. Macrofibrils are the major components made up of intermediate filaments (IF) or microfibrils, which are cross-linked with keratin-associated proteins and embedded in the amorphous materials called matrix.13 With regard to the gross diversity of hair among the three ethnicities, keratin filament structures are consistent.14 Low-sulfur proteins and amino acid composition establishing keratin filaments are invariable.15,16 Studies with X-ray scattering analysis did not observe any differences in the structures and configurations of the proteins (α-helical arrangements, coiled-coil structures, IF, and macrofibrils) across major biogeographic origins.3,14 In addition, the differences between wet and dry states among hair types observed from the lipid diffraction via X-ray scattering analysis indicated a variation of crystalline lipid structures among different hair origins.14
Cross-Sectional Area
Asian hair has the greatest cross-sectional area among the three human hair types. African and Caucasian hairs have relatively equal cross-sectional areas that are smaller than Asian hair.1,3 Asian hair possesses the most circular cross-sectional shape and the greatest mean ellipticity, calculated based on small diameter and large diameter ratio, indicating that it is more oval than African and Caucasian hairs.3
Genetic studies found that hair thickness in Asian populations is linked to genetic variations. Fujimoto et al observed a correlation between a nonsynonymous single nucleotide polymorphism (SNP) in ectodysplasin A receptor (EDAR) gene and hair thickness in Asians in a genome-wide analysis.17 The SNP (rs3827760) is located at the 1540th nucleotide from the transcription site (1540T/C) in the EDAR gene causing the substitution between valine and alanine (p.Val370Ala) and shows a strong positive selection in Asians in several studies.17,18 The 1540C allele has high frequency only in Chinese and Japanese, but not in Nigerian or European ancestry.17 The 1540T/C shows a significant association with the cross-sectional area and hair diameter in Thais and Indonesians, whereas Melanesians, whose hair diameter is similar to African and European hairs, show a lesser 1540C allele frequency.19 The cross-sectional area of hair is associated with the diversity of genotypes rather than the individual’s ethnicity, and EDAR gene is a strong determinant of hair thickness in Asians.17,19 CC genotype was reported with the highest values of mean area, followed by TC and TT genotypes, respectively. Subsequent research confirmed that EDAR gene is a genetic contributor of hair thickness as the EDAR370A (1540C)-derived cells demonstrate a superior ability to activate NF-κB expression and enhancement of signal potency compared with the EDAR370V (1540T)-derived cells.20 In vivo, transgenic mice with EDAR370A expression produce their hair phenotype mimicking the Asian hair fiber. The high output from EDAR370A may influence the hair morphology in Asian populations. Besides, the hair thickness in Asian populations is also linked to the FGFR2 polymorphism. Multiple regression analysis when considering the effect of EDAR revealed the significant associations of both hair diameter and cross-sectional area, and the SNP in FGFR2 (rs4752566).21
Hair Curvature
Hair curvature is greatly diverse among different ethnic populations. In contrast to African hair that is more flattened and often has twists and turns, Asian hair tends to be round in shape, straight, and more cylindrical.1,22 The low values of average curvature in Asian hair suggest a relatively straight hair. There are multiple proposed mechanisms of hair curvature: uneven expression of hair keratins in the precortex; asymmetrical distribution of proliferating cells and unbalanced proliferative compartment of the hair follicle; uneven bilateral distribution of cortical cell types that are determined by the different arrangements of IF inside the cortical cells; inner root sheaths incorporated in shaping hair fiber; and differences in dermal papilla shape that influences hair curl.23–25
Hair Straightness
Hair straightness is one of the morphological features that are diverse among races, implying an influential genetic linkage. A genome-wide scan exhibited a significant association between EDAR370A variant and hair straightness in East Asian populations.26 EDAR370A was attributable to 3.66% of the total variance.27 On the other hand, a genome-wide analysis in Europeans found that the trichohyalin (TCHH) gene variant is accounted for their straight hair phenotypes, although it explained only around 6% of the variance.28 The TCHH gene does not show high frequency in East Asians. Likewise, the EDAR variant cannot explain the hair straightness in Europeans. A genome-wide analysis in admixed individuals with eastern and western ancestries (Uyghur population) revealed that EDAR can explain hair straightness variation better and has a greater effect than TCHH. No interaction was found between these two genes, indicating their effects on hair straightness through different mechanisms.27 In addition, recent genome-wide association studies reported the links of curl formation in African individuals to polymorphic variation in trichohyalin, a copper transporter protein CUTC, and keratin 74 of the inner root sheath.23
Hair Pigmentation
The diversity of hair pigmentation, as well as skin color, mainly results from various amounts of admixed eumelanin and pheomelanin. Dark hair contains a high level of eumelanin and a small level of pheomelanin. Blond hair contains a low level of eumelanin and a trace of pheomelanin, whereas red hair has low to medium levels of both eumelanin and pheomelanin.5,6 Follicular melanogenesis in the hair follicles is characteristically cyclic as the melanogenic activity of follicular melanocytes is mainly coupled to the anagen phase of the hair cycle.29 The biosynthetic mechanism is regulated by several regulators including hormones, neurotransmitters, cytokines, growth factors, eicosanoids, cyclic nucleotides, nutrients, and the physicochemical milieu.30 Positive regulators include melanocyte-stimulating hormones, adrenocorticotrophic hormone, β-endorphin, estrogens, androgens, prostaglandins, leukotrienes, endothelin 1 and 3, histamine, stem cell factor, bone morphogenic proteins, and vitamin D3.30,31 The nutritional factors L-tyrosine and L-dihydroxyphenylalanine that serve as substrates and intermediates of melanogenesis also act as positive regulators of melanocyte functions.32 Besides, hair follicles and skin produce various substances and neuromediators including corticotropin-releasing hormone, proopiomelanocortin, melatonin, serotonin, and steroids that can affect hair physiology and pathology.33–36
The dark pigmentation is mostly determined by an eumelanin production from melanocytes residing in the basement membrane encircled by the dermal papillae. Losing a melanocortin 1 receptor (MC1R) function from the mutation leads to pheomelanin overproduction and red hair color phenotype. The high degree of polymorphisms in human MC1R had been reported, but an Arg163Gln variant was detected only in Asian, Indian, and African-Indian populations with great differences in frequency.37,38 The extraordinary high frequencies were observed in East and Southeast Asians.38 Although the supportive evidence is not clearly elucidated, there is a presumption that the Arg163Gln variant may be involved in pheomelanin production in these populations.38
Size and density of melanosomes in a hair fiber contribute to hair pigmentation. Black hair has the largest melanosome and tightly packed eumelanin content. Brown hair has a smaller melanosome with ellipsoid shape.4 Hairs from individuals with African descendants display larger melanosome size and higher melanosome density than those of Caucasians and Asians.12 A study demonstrated that human hair becomes darker with age.39 The finding was considerably affected by the increase in total melanin amount correlating to the enlargement of the melanosome size.40 Further research regarding melanosome is advocated to identify genetic markers and improve knowledge of human hair pigmentation.
Hair Growth Rate
Asian hair shows the fastest growth rate in comparison to the other two hair types. African hair has the lowest growth rate.2,41 Hair growth rate and hair diameter were reported to be associated with cuticle interscale distance. Hairs with larger cross-sectional area grow faster and have a shorter cuticle interscale distance, and vice versa. This relationship was reported in Asians and Caucasians with straight or semi-straight hairs.42 The cuticle interscale distance of the wavy hair shafts exhibits high variations among convex and concave regions. Hence, it can be inferred that a curly hair would grow with a different rate correspondent to various cellular division rates in the convex and concave parts.42
Lipid Content and Hydration
In 2003, Franbourg et al found that African hair has a lower radial swelling percentage in water compared with Asian and Caucasian hairs, which leads to a possibility of lipid differences among ethnic hair types.3 To date, few studies have evaluated the differentiation on hair lipids among human populations. Hair lipid components can be separated into internal and external lipids according to their origins. Internal lipids are biosynthesized inside the hair matrix cells and mostly comprise free fatty acid (FFA), cholesterol, and polar components, such as ceramide, cholesterol ester, and cholesterol sulfate. External lipids, including sterol esters and squalene, are known to arise from the surface sebaceous lipids.7,43
Experimental results showed that among the three ethnicities, African hair has the largest amount of total hair lipid extractions, followed by Caucasian and Asian hairs.43 The lipids in African hair mainly come from the sebaceous lipids, and those in Asian and Caucasian hairs mainly come from the internal lipids.43 However, African hair still has 1.7 times greater internal lipid contents than the other two ethnic groups.13 With regard to percentages over weight of whole fibers, contents of FFA, sterol, and polar lipids are the highest in African hair.7,13 The internal lipids were postulated to intercalate the keratin dimers and influence the organization of keratin fibers, which may lead to various hair morphologies.13,14 In relation to the external lipids, African fiber presents the greatest proportion of apolar lipids, and Asian and Caucasian extracts show a higher amount of FFA and polar lipids.7 The high amount of apolar lipids with hydrophobic property in African fiber is associated with lesser swelling in water as it blocks the entrance of water into the hair.3,13
Integral hair lipids located in the CMC of hair cuticle layers are accountable for hair integrity, maintenance of hydrophobicity, moisture, and hair stiffness. All three hair origins similarly show a smaller amount of lipids in the cortex compared with the cuticles.7 Asian hair demonstrates a greater amount of integral lipids than the other two ethnic hairs, which exhibit lesser damage to ultraviolet irradiation. In addition, Takahashi and Yoshida detected glycoside-like lipids (particularly N-acetyl glycoside) in Japanese female hairs at the interface between the cuticle and the cortex producing an adhesive effect between the two layers.44 They also found unsaturated fatty acids, such as linoleic acid and alpha-linoleic acid, that reside in the hair bulb and melanin granules. The alpha-linoleic acid-derived oxidative metabolites incorporate tightly in the melanin granules, implying the possibility of their participation during melanosome synthesis and melanogenesis.44
Among human races, Caucasian fiber is the most hydrated one, which is different from African and Asian hairs that relatively have similar moisture content.43 Asian and Caucasian hairs demonstrate lower permeability due to lower diffusion coefficients compared with African hair.7,43 Optimum permeability is important in preserving humidity retention of the fiber from being modified by a rapid change of water uptake and desorption. When the lipid was extracted, Caucasian hair was shown to have the greatest decreased hydration despite higher contents of lipid extraction in African fiber. Asian and African hairs share the same reduction level in moisture content. The diminution of water was speculated to be related to lipid saturation degree or the internal lipid content rather than the total amount of lipids.7,43
As the lipids are involved in keratin conformation, they may contribute to hair tensile strength. Lipid extraction does not affect African fiber’s mechanical properties, but decreases deformation at the break of Asian hair and increases the break tenacity of Caucasian hair.7 Lipid diminution may decrease matrix plasticity leading to the lower general mechanical property of Asian hair.43
Mechanical Properties
Mechanical properties vary among different ethnicities. Asian hair demonstrates the highest hardness and elastic modulus, followed by Caucasian and African hairs, respectively. All three hair types that were studied in this research presented a decrease of both properties at the hair tip due to cuticle damage.45 Compared with Asian and Caucasian hairs, African hair is more fragile, which is associated with lower breaking stress and lower breaking elongation of fibers. It requires the earliest breaking time and a lower stress at breaking. With regard to the influence of hair size, Asian and Caucasian hairs exhibit similar response to stress and strain even though Asian hair has a bigger cross-sectional area.3 The fragility of African hair accompanied by traumatic hairstyles leads to a high prevalence of hair shaft abnormality in the African population.22 By contrast, the hair shaft disorder is less problematic and uncommon in Asians.
Stretching of hair can distort hair structure as well as its properties. A study conducted on Asian and Caucasian hair samples observed the elevated level of cysteine residues, indicating disulfide bond ruptures from cysteine oxidation during the stretching process.8 As the stretching ratio increases, the α-helix conformations in the fibers transform into β-pleated sheets, β-turn structures, and disordered content. The β-turn structures are easier to retract against the increased stretching ratios and may have a secondary transformation to the β-pleated sheets.8,46 The disordered content represents the destroyed macromolecular structures of fibers. Asian hair has greater stability than Caucasian hair due to its larger diameter and its ability to easily transfer to β-turn structures. The disordered content in Caucasian hair is raised at lower stretching ratios compared with that in Asian hair.8
Hair Damage from Chemical Products
Chemical products, such as hair bleaching and hair dye, can alter hair shaft morphology and its properties. The cortex from the three major ethnic groups shows a similar response to straightening and coloring treatment, but African hair exhibits greater tolerance to combination treatment (straightening and coloring). Hairs from all studied ethnic groups demonstrate similar patterns of CMC damage after straightening and combination treatment, but Asian hair displays a bulged CMC and has lesser endurance than the others after treatment of coloring products.47 However, the cuticle’s strength against chemical treatments is inconclusive due to disagreeing outcomes in existing studies.47,48 Therefore, more research focusing on hair strength and hair change from the styling is warranted to provide a better understanding of Asian hair.
Hair Disorders in Asian Populations
Diagnosis and management of hair and scalp disorders have always been a challenge for dermatologists. Increasing evidence suggests that the prevalence, as well as clinical presentation and severity of diseases, vary greatly among different ethnicities. This section discusses the different characteristics of common and distinct hair disorders in Asian populations.
Non-Scarring Alopecias in Asians
Androgenetic alopecia (AGA) and female pattern hair loss (FPHL) are common non-scarring alopecia in Asian population. Several studies performed on Asians reported a lower prevalence of AGA and FPHL compared with the Europeans.49–52 The development of AGA in Japanese men is approximately 10 years slower than that in Europeans, with 1.4-times lower in prevalence in each decade.51 By contrast, the prevalence of AGA in Thais is higher and relatively similar to Caucasians, suggesting that the prevalence may be influenced by various genetic backgrounds.53
Alopecia areata affects 0.1–0.2% of the population worldwide.54 Most Asian patients experience the onset during their first four decades of life. The mean age of onset is similar to Caucasians. Family history and vitiligo in Asians are lesser associated compared with the Westerners, but the percentages of autoimmune thyroid diseases are comparable.55,56
Tinea capitis is another common hair problem worldwide and is caused by Trichophyton and Microsporum spp. Etiologic agents diversely depend on the regions. In Asians, the most common causative organisms are Trichophyton ferrugineum and Trichophyton violaceum.57 The prevalence of tinea capitis in African populations is higher compared with that in Asian populations. The triglyceride-rich sebum production in adults was hypothesized to have a fungistatic effect creating a greater innate protection against ecthotrix fungal infections and a lower prevalence compared with that in children.57 Therefore, it may be plausible that the lower prevalence in Asians is associated with different types and a lower amount of lipid content. Other contributing factors to fungal infection are hair care practices. A low frequency of shampooing links to decreased spore disposal. The traction hairstyle in Africans allows easier access to fungus.57–59
Scarring Alopecias in Asians
Scarring or cicatricial alopecia results from irreversible injury of follicular stem cells and replacement of hair follicles with fibrous tissues or hyalinization of collagen. Primary scarring alopecia (PSA) is characterized by an idiopathic folliculocentric inflammation that leads to a permanent hair follicular destruction. Secondary scarring alopecia is caused by inflammatory infiltrates of scalp skin that damage their nearby hair follicles.60
According to the North American Hair Research Society, PSA can be classified into three groups: lymphocytic, neutrophilic, and mixed inflammation.61 Caucasians including Hispanics have high proportions of lymphocytic PSA compared with other ethnic groups, with PSA from 4:1 to 8.2:1.62,63 Diseases with neutrophilic or mixed PSAs are known to predominantly afflict African populations.22 A recent retrospective study of PSA in Taiwanese patients reported that the ratio of lymphocytic to neutrophilic PSA is 1.2:1. Lymphocytic PSA has a female predominance, and neutrophilic PSA mainly affects males with younger age of onset.64 By contrast, a study from China reported a ratio of lymphocytic to neutrophilic PSA of 1:1.3. Neutrophilic PSA also occurs more in men, but mostly in their middle age.65 Altogether, we observed a trend of lymphocytic PSA in older females, and neutrophilic PSA in young males. Unlike Asians, Caucasians are dominated with lymphocytic PSA, eg, lichen planopilaris and chronic cutaneous lupus erythematosus.
The curly and frizzy hair of African populations, together with markedly curved hair follicles and manipulation cause hairs to curl back into the dermis, which then leads to a foreign-body reaction. Therefore, pseudofolliculitis barbae and acne keloidalis nuchae, which are characterized by an “ingrown hair” inflammation, have the highest prevalence in African populations, followed by the Hispanic and Asian populations.22,66 Moreover, androgen-dependent follicular occlusion diseases, such as dissecting cellulitis of the scalp (DCS), are significantly found more in African populations than in other populations.22 We speculate that the genetic variations may play a role in androgen level alteration, unequal lipid contents of the hair and scalp among different ethnicities, and pathophysiology of diseases.
Given the lower hair tensile strength combined with the high degree of curl and dryness, African hair fibers are more vulnerable to damage from daily traction-producing hair cares compared with Asian hair. These properties are speculated to be the causes of central centrifugal cicatricial alopecia (CCCA) and traction alopecia.22,66,67 Excessive hair care practice also leads to a higher incidence of hair shaft abnormalities in Africans including acquired trichorrhexis nodosa and bubble hair.22
Exclusively Described Hair Disorders in Asians
Linear Lupus Panniculitis of the Scalp
Linear lupus panniculitis of the scalp (LLPS) or linear and annular lupus panniculitis was firstly described by Nagai et al.68 It is a rare entity that manifests as chronic non-scarring alopecic patches that follow the Blaschko’s lines. The morphologies of LLPS lesion are linear, annular, and arch-shaped (Figure 1), and LLPS can be found in any area of the scalp. Erythema, atrophic change, and follicular plugging may accompany the alopecic area.69,70 The LLPS has a predilection for Asians with few reports in Caucasians.69,70
Figure 1.
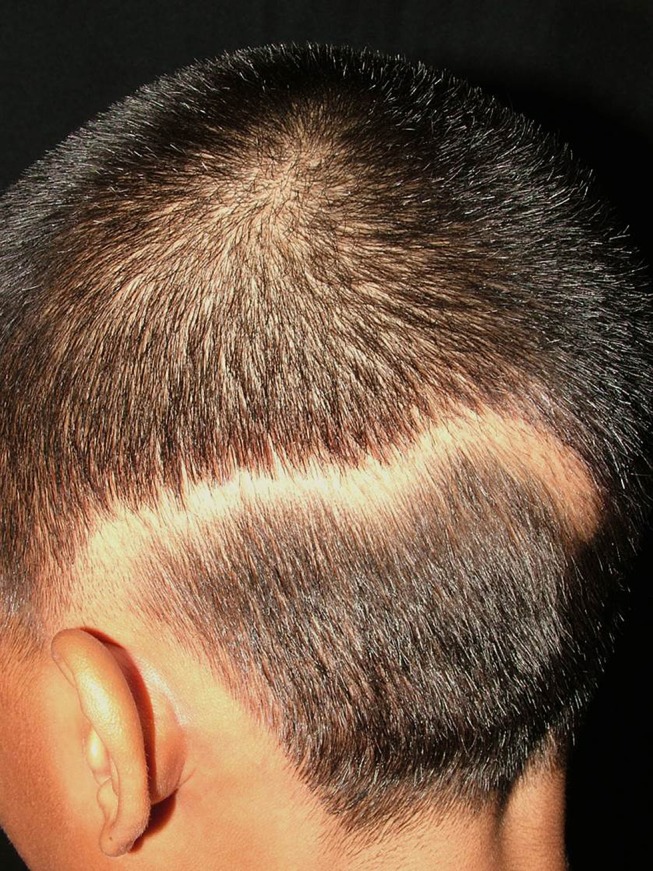
Linear lupus panniculitis of the scalp: non-scarring alopecic patch in a linear configuration.
Genetic predisposition may be associated with this disorder that is predominantly found in Asians. Histopathologically, the lesion is characterized by a lobular panniculitis with mainly lymphocytic infiltrates, mucin deposition, hyaline fat degeneration, and fat necrosis. The dermis shows abnormality with lymphocytic infiltration around the follicles, eccrine glands, and vessels. Granular deposition of IgM, IgG, and C3 can be observed at the peribulbar area and along the basement membrane by direct immunofluorescence in some cases. Patients with LLPS generally have a good prognosis and a complete recovery after treating with oral hydroxychloroquine and oral/intralesional corticosteroids. Only a few patients have partial response or recurrence.69,70 The disease rarely develops systemic involvement. Half of the cases have positive antinuclear Antibody titer, but none of the reported cases show any sign of systemic lupus erythematosus.69
Pseudocyst of the Scalp
In 1992, Iwata and Niimura described 19 Japanese patients with pseudocyst of the scalp (PCS) represented as a solitary reddish nodule that might be associated with pain then gradually evolved into a dome-shaped mass with alopecia. Some lesions are bloody, yellowish-fluid, or purulent discharge.71 Abdennader et al later proposed another name called “alopecic and aseptic nodules of the scalp (AANS),” suggesting that it was more suitable for describing the condition. PCS predominantly affects young Asian men with unknown prevalence.72,73 Patients are clinically present with one or few, often asymptomatic, nodules that are implicated with non-scarring alopecia and surrounded by an area of normal skin (Figure 2). In contrast to DCS, AASN lesions are usually non-fluctuating.72 Some authors believed that PCS and AANS are two different diseases; however, most of them agreed that both conditions have the same entity with a varied histopathologic spectrum.72,74 A well-defined subcutaneous hypoechoic nodule was observed using ultrasonography.75 A positive puncture can be found in both conditions with varied material. The content inside the lesions is always sterile with negative microbiological cultures. Histopathologically, a pseudocyst formation is not always present, but it is always observed in the PCS.72 The presence of pseudocyst was suspected to be influenced by the severity and duration of the inflammatory process and developed as a secondary change.74
Figure 2.
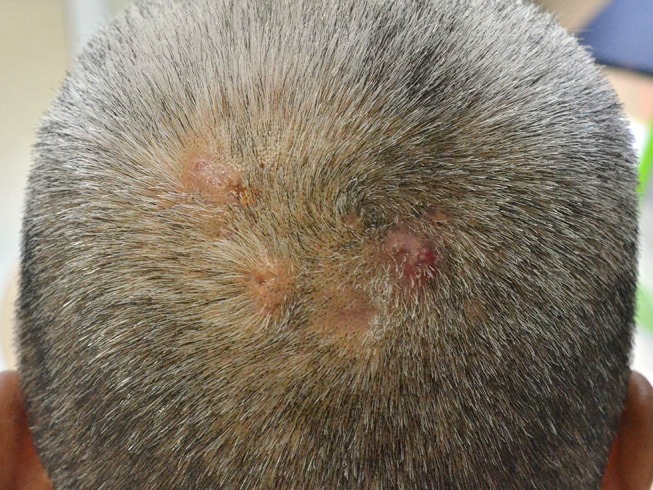
Pseudocyst of the scalp/alopecic and aseptic nodules of the scalp: soft and dome-shaped alopecic nodules, slightly erythematous and pustules on surface, surrounded by an area of normal skin.
The etiology remains questionable. Some authors assumed that it was a spectrum of follicular occlusions that cause deep folliculitis, leading to non-scarring alopecia and eventually a pseudocyst formation. Some believed that the follicular occlusion was only a predisposing factor as it was not observed in every case. In our opinion, the PCS/AASN could be a minor severity spectrum of DCS with milder inflammation and less noticeable follicular occlusion. We hypothesized that the different racial or genetic backgrounds may be associated with the variation as Africans tend to have neutrophilic PSA with higher severity compared with Asians. Meanwhile, the inflammation targeting the hair follicles may be induced by an immune process and the reaction may secondarily develop from a follicular modification, a foreign body or an unknown factor.72,76 PCS/AASN shows a good response with a spontaneous resolution after treatments of doxycycline 100 mg/d for at least 3 months, repetitive aspiration, or surgical excision, and other alternatives including intralesional corticosteroids and topical 3% indomethacin.71,72,76
Conclusion
Asian hair presenting as a cylindrical and straight fiber with black or brown coloration is one of the renowned characteristics that distinguish among the three major ethnic origins. This review provides the currently available knowledge and presents various interesting points of view of Asian hair, including characteristics, morphological features, and distinct hair disorders involving Asian populations. However, much remains to be explained regarding differences in hair microstructures, genetics and diseases. Therefore, further clinical research is encouraged to help gain better knowledge on the nature of Asian hair and hair disorders.
Ethics and Consent Statement
The patients provided written informed consent to perform all necessary investigations, to take clinical photographs, and to use them for research purposes and publication.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Richards GM, Oresajo CO, Halder RM. Structure and function of ethnic skin and hair. Dermatol Clin. 2003;21(4):595–600. doi: 10.1016/S0733-8635(03)00081-0 [DOI] [PubMed] [Google Scholar]
- 2.Loussouarn G, El Rawadi C, Genain G. Diversity of hair growth profiles. Int J Dermatol. 2005;44(Suppl 1):6–9. doi: 10.1111/j.1365-4632.2005.02800.x [DOI] [PubMed] [Google Scholar]
- 3.Franbourg A, Hallegot P, Baltenneck F, Toutain C, Leroy F. Current research on ethnic hair. J Am Acad Dermatol. 2003;48(6 Suppl):S115–S119. doi: 10.1067/mjd.2003.277 [DOI] [PubMed] [Google Scholar]
- 4.Maronas O, Sochtig J, Ruiz Y, Phillips C, Carracedo A, Lareu MV. The genetics of skin, hair, and eye color variation and its relevance to forensic pigmentation predictive tests. Forensic Sci Rev. 2015;27(1):13–40. [PubMed] [Google Scholar]
- 5.Ito S, Wakamatsu K. Diversity of human hair pigmentation as studied by chemical analysis of eumelanin and pheomelanin. J Eur Acad Dermatol Venereol. 2011;25(12):1369–1380. doi: 10.1111/j.1468-3083.2011.04278.x [DOI] [PubMed] [Google Scholar]
- 6.Ito S, Nakanishi Y, Valenzuela RK, Brilliant MH, Kolbe L, Wakamatsu K. Usefulness of alkaline hydrogen peroxide oxidation to analyze eumelanin and pheomelanin in various tissue samples: application to chemical analysis of human hair melanins. Pigment Cell Melanoma Res. 2011;24(4):605–613. doi: 10.1111/j.1755-148X.2011.00864.x [DOI] [PubMed] [Google Scholar]
- 7.Coderch L, Oliver MA, Carrer V, Manich AM, Marti M. External lipid function in ethnic hairs. J Cosmet Dermatol. 2019;18(6):1912–1920. doi: 10.1111/jocd.12899 [DOI] [PubMed] [Google Scholar]
- 8.Zhou AJ, Liu HL, Du ZQ. Secondary structure estimation and properties analysis of stretched Asian and Caucasian hair. Skin Res Technol. 2015;21(1):119–128. doi: 10.1111/srt.12169 [DOI] [PubMed] [Google Scholar]
- 9.Garcia ML, Epps JA, Yare RS, Hunter LD. Normal cuticle-wear patterns in human hair. J Soc Cosmet Chem. 1978;29(3):155–175. [Google Scholar]
- 10.Takahashi T, Hayashi R, Okamoto M, Inoue S. Morphology and properties of Asian and Caucasian hair. J Cosmet Sci. 2006;57(4):327–338. [PubMed] [Google Scholar]
- 11.Kim BJ, Na JI, Park WS, Eun HC, Kwon OS. Hair cuticle differences between Asian and Caucasian females. Int J Dermatol. 2006;45(12):1435–1437. doi: 10.1111/j.1365-4632.2006.03094.x [DOI] [PubMed] [Google Scholar]
- 12.Koch SL, Shriver MD, Jablonski NG. Variation in human hair ultrastructure among three biogeographic populations. J Struct Biol. 2019;205(1):60–66. doi: 10.1016/j.jsb.2018.11.008 [DOI] [PubMed] [Google Scholar]
- 13.Cruz CF, Fernandes MM, Gomes AC, et al. Keratins and lipids in ethnic hair. Int J Cosmet Sci. 2013;35(3):244–249. doi: 10.1111/ics.12035 [DOI] [PubMed] [Google Scholar]
- 14.Wade M, Tucker I, Cunningham P, et al. Investigating the origins of nanostructural variations in differential ethnic hair types using X-ray scattering techniques. Int J Cosmet Sci. 2013;35(5):430–441. doi: 10.1111/ics.12061 [DOI] [PubMed] [Google Scholar]
- 15.Dekio S, Jidoi J. Hair low-sulfur protein composition does not differ electrophoretically among different races. J Dermatol. 1988;15(5):393–396. doi: 10.1111/j.1346-8138.1988.tb04075.x [DOI] [PubMed] [Google Scholar]
- 16.Gold RJ, Scriver CG. The amino acid composition of hair from different racial origins. Clin Chim Acta. 1971;33(2):465–466. doi: 10.1016/0009-8981(71)90510-9 [DOI] [PubMed] [Google Scholar]
- 17.Fujimoto A, Kimura R, Ohashi J, et al. A scan for genetic determinants of human hair morphology: EDAR is associated with Asian hair thickness. Hum Mol Genet. 2008;17(6):835–843. doi: 10.1093/hmg/ddm355 [DOI] [PubMed] [Google Scholar]
- 18.Sabeti PC, Varilly P, Fry B. Genome-wide detection and characterization of positive selection in human populations. Nature. 2007;449(7164):913–918. doi: 10.1038/nature06250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujimoto A, Ohashi J, Nishida N, et al. A replication study confirmed the EDAR gene to be a major contributor to population differentiation regarding head hair thickness in Asia. Hum Genet. 2008;124(2):179–185. doi: 10.1007/s00439-008-0537-1 [DOI] [PubMed] [Google Scholar]
- 20.Mou C, Thomason HA, Willan PM, et al. Enhanced ectodysplasin-A receptor (EDAR) signaling alters multiple fiber characteristics to produce the East Asian hair form. Hum Mutat. 2008;29(12):1405–1411. doi: 10.1002/humu.20795 [DOI] [PubMed] [Google Scholar]
- 21.Fujimoto A, Nishida N, Kimura R, et al. FGFR2 is associated with hair thickness in Asian populations. J Hum Genet. 2009;54(8):461–465. doi: 10.1038/jhg.2009.61 [DOI] [PubMed] [Google Scholar]
- 22.Lindsey SF, Tosti A. Ethnic hair disorders. Curr Probl Dermatol. 2015;47:139–149. [DOI] [PubMed] [Google Scholar]
- 23.Westgate GE, Ginger RS, Green MR. The biology and genetics of curly hair. Exp Dermatol. 2017;26(6):483–490. doi: 10.1111/exd.13347 [DOI] [PubMed] [Google Scholar]
- 24.Bernard BA. Hair shape of curly hair. J Am Acad Dermatol. 2003;48(6 Suppl):S120–S126. doi: 10.1067/mjd.2003.279 [DOI] [PubMed] [Google Scholar]
- 25.Bryson WG, Harland DP, Caldwell JP, et al. Cortical cell types and intermediate filament arrangements correlate with fiber curvature in Japanese human hair. J Struct Biol. 2009;166(1):46–58. doi: 10.1016/j.jsb.2008.12.006 [DOI] [PubMed] [Google Scholar]
- 26.Tan J, Yang Y, Tang K, Sabeti PC, Jin L, Wang S. The adaptive variant EDARV370A is associated with straight hair in East Asians. Hum Genet. 2013;132(10):1187–1191. doi: 10.1007/s00439-013-1324-1 [DOI] [PubMed] [Google Scholar]
- 27.Wu S, Tan J, Yang Y, et al. Genome-wide scans reveal variants at EDAR predominantly affecting hair straightness in Han Chinese and Uyghur populations. Hum Genet. 2016;135(11):1279–1286. doi: 10.1007/s00439-016-1718-y [DOI] [PubMed] [Google Scholar]
- 28.Medland SE, Nyholt DR, Painter JN, et al. Common variants in the trichohyalin gene are associated with straight hair in Europeans. Am J Hum Genet. 2009;85(5):750–755. doi: 10.1016/j.ajhg.2009.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slominski A, Paus R. Melanogenesis is coupled to murine anagen: toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J Invest Dermatol. 1993;101(1 Suppl):90S–97S. doi: 10.1016/0022-202X(93)90507-E [DOI] [PubMed] [Google Scholar]
- 30.Slominski A, Wortsman J, Plonka PM, Schallreuter KU, Paus R, Tobin DJ. Hair follicle pigmentation. J Invest Dermatol. 2005;124(1):13–21. doi: 10.1111/j.0022-202X.2004.23528.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84(4):1155–1228. [DOI] [PubMed] [Google Scholar]
- 32.Slominski A, Zmijewski MA, Pawelek J. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012;25(1):14–27. doi: 10.1111/j.1755-148X.2011.00898.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80(3):979–1020. doi: 10.1152/physrev.2000.80.3.979 [DOI] [PubMed] [Google Scholar]
- 34.Slominski AT, Zmijewski MA, Zbytek B, Tobin DJ, Theoharides TC, Rivier J. Key role of CRF in the skin stress response system. Endocr Rev. 2013;34(6):827–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slominski AT, Manna PR, Tuckey RC. On the role of skin in the regulation of local and systemic steroidogenic activities. Steroids. 2015;103:72–88. doi: 10.1016/j.steroids.2015.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slominski AT, Hardeland R, Zmijewski MA, Slominski RM, Reiter RJ, Paus R. Melatonin: a cutaneous perspective on its production, metabolism, and functions. J Invest Dermatol. 2018;138(3):490–499. doi: 10.1016/j.jid.2017.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harding RM, Healy E, Ray AJ, et al. Evidence for variable selective pressures at MC1R. Am J Hum Genet. 2000;66(4):1351–1361. doi: 10.1086/302863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rana BK, Hewett-Emmett D, Jin L, et al. High polymorphism at the human melanocortin 1 receptor locus. Genetics. 1999;151(4):1547–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Commo S, Wakamatsu K, Lozano I, et al. Age-dependent changes in eumelanin composition in hairs of various ethnic origins. Int J Cosmet Sci. 2012;34(1):102–107. doi: 10.1111/j.1468-2494.2011.00691.x [DOI] [PubMed] [Google Scholar]
- 40.Itou T, Ito S, Wakamatsu K. Effects of aging on hair color, melanosome morphology, and melanin composition in japanese females. Int J Mol Sci. 2019;20(15):15. doi: 10.3390/ijms20153739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loussouarn G, Lozano I, Panhard S, Collaudin C, El Rawadi C, Genain G. Diversity in human hair growth, diameter, colour and shape. An in vivo study on young adults from 24 different ethnic groups observed in the five continents. Eur J Dermatol. 2016;26(2):144–154. doi: 10.1684/ejd.2015.2726 [DOI] [PubMed] [Google Scholar]
- 42.Baque CS, Zhou J, Gu W, et al. Relationships between hair growth rate and morphological parameters of human straight hair: a same law above ethnical origins? Int J Cosmet Sci. 2012;34(2):111–116. doi: 10.1111/j.1468-2494.2011.00687.x [DOI] [PubMed] [Google Scholar]
- 43.Marti M, Barba C, Manich AM, Rubio L, Alonso C, Coderch L. The influence of hair lipids in ethnic hair properties. Int J Cosmet Sci. 2016;38(1):77–84. doi: 10.1111/ics.12261 [DOI] [PubMed] [Google Scholar]
- 44.Takahashi T, Yoshida S. Distribution of glycolipid and unsaturated fatty acids in human hair. Lipids. 2014;49(9):905–917. doi: 10.1007/s11745-014-3937-0 [DOI] [PubMed] [Google Scholar]
- 45.Wei G, Bhushan B, Torgerson PM. Nanomechanical characterization of human hair using nanoindentation and SEM. Ultramicroscopy. 2005;105(1–4):248–266. doi: 10.1016/j.ultramic.2005.06.033 [DOI] [PubMed] [Google Scholar]
- 46.Byler DM, Susi H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers. 1986;25(3):469–487. doi: 10.1002/bip.360250307 [DOI] [PubMed] [Google Scholar]
- 47.Lee Y, Kim YD, Pi LQ, Lee SY, Hong H, Lee WS. Comparison of hair shaft damage after chemical treatment in Asian, White European, and African hair. Int J Dermatol. 2014;53(9):1103–1110. doi: 10.1111/ijd.12247 [DOI] [PubMed] [Google Scholar]
- 48.Galliano A, Saint Olive Baque C, Marty G, et al. Resistance of human hair cuticle after a shaking process in wet conditions: comparison between Chinese and Caucasian hair. Int J Cosmet Sci. 2010;32(5):356–368. doi: 10.1111/j.1468-2494.2009.00563.x [DOI] [PubMed] [Google Scholar]
- 49.Rojhirunsakool S, Suchonwanit P. Parietal scalp is another affected area in female pattern hair loss: an analysis of hair density and hair diameter. Clin Cosmet Investig Dermatol. 2018;11:7–12. doi: 10.2147/CCID.S153768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suchonwanit P, Srisuwanwattana P, Chalermroj N, Khunkhet S. A randomized, double-blind controlled study of the efficacy and safety of topical solution of 0.25% finasteride admixed with 3% minoxidil vs. 3% minoxidil solution in the treatment of male androgenetic alopecia. J Eur Acad Dermatol Venereol. 2018;32(12):2257–2263. doi: 10.1111/jdv.15171 [DOI] [PubMed] [Google Scholar]
- 51.Lee WS, Lee HJ. Characteristics of androgenetic alopecia in asian. Ann Dermatol. 2012;24(3):243–252. doi: 10.5021/ad.2012.24.3.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suchonwanit P, Iamsumang W, Rojhirunsakool S. Efficacy of topical combination of 0.25% finasteride and 3% minoxidil versus 3% minoxidil solution in female pattern hair loss: a randomized, double-blind, controlled study. Am J Clin Dermatol. 2019;20(1):147–153. doi: 10.1007/s40257-018-0387-0 [DOI] [PubMed] [Google Scholar]
- 53.Pathomvanich D, Pongratananukul S, Thienthaworn P, Manoshai S. A random study of Asian male androgenetic alopecia in Bangkok, Thailand. Dermatol Surg. 2002;28(9):804–807. doi: 10.1046/j.1524-4725.2002.02036.x [DOI] [PubMed] [Google Scholar]
- 54.Perera E, Yip L, Sinclair R. Alopecia areata. Curr Probl Dermatol. 2015;47:67–75. [DOI] [PubMed] [Google Scholar]
- 55.Sriphojanart T, Khunkhet S, Suchonwanit P. A retrospective comparative study of the efficacy and safety of two regimens of diphenylcyclopropenone in the treatment of recalcitrant alopecia areata. Dermatol Reports. 2017;9(2):7399. doi: 10.4081/dr.2017.7399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tan E, Tay YK, Goh CL, Chin Giam Y. The pattern and profile of alopecia areata in Singapore–a study of 219 Asians. Int J Dermatol. 2002;41(11):748–753. doi: 10.1046/j.1365-4362.2002.01357.x [DOI] [PubMed] [Google Scholar]
- 57.Elewski BE. Tinea capitis: a current perspective. J Am Acad Dermatol. 2000;42(1 Pt 1):1–20. (). doi: 10.1016/S0190-9622(00)90001-X [DOI] [PubMed] [Google Scholar]
- 58.Silverberg NB, Weinberg JM, DeLeo VA. Tinea capitis: focus on African American women. J Am Acad Dermatol. 2002;46(2Suppl Understanding):S120–S124. doi: 10.1067/mjd.2002.120793 [DOI] [PubMed] [Google Scholar]
- 59.Ahn CS, Suchonwanit P, Foy CG, Smith P, McMichael AJ. Hair and scalp care in African American women who exercise. JAMA Dermatol. 2016;152(5):579–580. doi: 10.1001/jamadermatol.2016.0093 [DOI] [PubMed] [Google Scholar]
- 60.Harnchoowong S, Suchonwanit P. PPAR-gamma agonists and their role in primary cicatricial alopecia. PPAR Res. 2017;2017:2501248. doi: 10.1155/2017/2501248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Olsen EA, Bergfeld WF, Cotsarelis G, et al. Summary of North American Hair Research Society (NAHRS)-sponsored workshop on cicatricial alopecia, duke university medical center, february 10 and 11, 2001. J Am Acad Dermatol. 2003;48(1):103–110. doi: 10.1067/mjd.2003.68 [DOI] [PubMed] [Google Scholar]
- 62.Tan E, Martinka M, Ball N, Shapiro J. Primary cicatricial alopecias: clinicopathology of 112 cases. J Am Acad Dermatol. 2004;50(1):25–32. doi: 10.1016/j.jaad.2003.04.001 [DOI] [PubMed] [Google Scholar]
- 63.Moure ER, Romiti R, Machado MC, Valente NY. Primary cicatricial alopecias: a review of histopathologic findings in 38 patients from a clinical university hospital in Sao Paulo, Brazil. Clinics (Sao Paulo). 2008;63(6):747–752. doi: 10.1590/S1807-59322008000600007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Su HJ, Cheng AY, Liu CH, et al. Primary scarring alopecia: a retrospective study of 89 patients in Taiwan. J Dermatol. 2018;45(4):450–455. doi: 10.1111/1346-8138.14217 [DOI] [PubMed] [Google Scholar]
- 65.Qi S, Zhao Y, Zhang X, Li S, Cao H, Zhang X. Clinical features of primary cicatricial alopecia in Chinese patients. Indian J Dermatol Venereol Leprol. 2014;80(4):306–312. doi: 10.4103/0378-6323.136833 [DOI] [PubMed] [Google Scholar]
- 66.Madu P, Kundu RV. Follicular and scarring disorders in skin of color: presentation and management. Am J Clin Dermatol. 2014;15(4):307–321. doi: 10.1007/s40257-014-0072-x [DOI] [PubMed] [Google Scholar]
- 67.Suchonwanit P, Hector CE, Bin Saif GA, McMichael AJ. Factors affecting the severity of central centrifugal cicatricial alopecia. Int J Dermatol. 2016;55(6):e338–e343. doi: 10.1111/ijd.13061 [DOI] [PubMed] [Google Scholar]
- 68.Nagai Y, Ishikawa O, Hattori T, Ogawa T. Linear lupus erythematosus profundus on the scalp following the lines of Blaschko. Eur J Dermatol. 2003;13(3):294–296. [PubMed] [Google Scholar]
- 69.Udompanich S, Chanprapaph K, Suchonwanit P. Hair and scalp changes in cutaneous and systemic lupus erythematosus. Am J Clin Dermatol. 2018;19(5):679–694. doi: 10.1007/s40257-018-0363-8 [DOI] [PubMed] [Google Scholar]
- 70.Udompanich S, Chanprapaph K, Suchonwanit P. Linear and annular lupus panniculitis of the scalp: case report with emphasis on trichoscopic findings and review of the literature. Case Rep Dermatol. 2019;11(2):157–165. doi: 10.1159/000500848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Iwata THT, Niimura M. A pseudocyst with inflammatory granulation tissue on scalp-Pseudocyst of scalp. Jpn J Clin Dermatol. 1992;46:9–16. [Google Scholar]
- 72.Abdennader S, Vignon-Pennamen MD, Hatchuel J, Reygagne P. Alopecic and aseptic nodules of the scalp (pseudocyst of the scalp): a prospective clinicopathological study of 15 cases. Dermatology. 2011;222(1):31–35. doi: 10.1159/000321475 [DOI] [PubMed] [Google Scholar]
- 73.Eisenberg EL. Alopecia-associated pseudocyst of the scalp. J Am Acad Dermatol. 2012;67(3):e114–e116. doi: 10.1016/j.jaad.2011.09.028 [DOI] [PubMed] [Google Scholar]
- 74.Seol JE, Park IH, Kim DH, et al. Alopecic and aseptic nodules of the scalp/pseudocyst of the scalp: clinicopathological and therapeutic analyses in 11 Korean patients. Dermatology. 2016;232(2):165–170. doi: 10.1159/000441219 [DOI] [PubMed] [Google Scholar]
- 75.Garrido-Colmenero C, Arias-Santiago S, Aneiros Fernandez J, Garcia-Lora E. Trichoscopy and ultrasonography features of aseptic and alopecic nodules of the scalp. J Eur Acad Dermatol Venereol. 2016;30(3):507–509. doi: 10.1111/jdv.12903 [DOI] [PubMed] [Google Scholar]
- 76.Rodriguez-Lobato E, Morgado-Carrasco D, Giavedoni P, Ferrando J. Alopecic and aseptic nodule of the scalp in a girl. Pediatr Dermatol. 2017;34(6):697–700. doi: 10.1111/pde.13293 [DOI] [PubMed] [Google Scholar]


