Abstract
Study Design.
Whole bovine coccygeal discs were cultured under static load, with or without vertebral endplates (VEPs), and assessed for cell viability, biochemical stability, biosynthetic activity, and biosynthetic responsiveness to changes in mechanical load.
Objectives.
To assess the effects of VEPs on biochemical and cellular stability of disc cells during in vitro culture of large disc explants. To determine whether cultured discs could respond to mechanical perturbation.
Summary of Background Data.
Previous methods for culturing the intervertebral disc have focused on rabbit and rat discs, but the small size of these discs limits the relevance of these culture systems to the human condition. Bovine coccygeal discs have similar dimensions to the human lumbar disc (i.e., similar size and nominal stresses), but long-term culture of these discs has not been reported.
Methods.
Bovine coccygeal discs were harvested with or without VEPs, cultured under static load (5 kg, ~0.25 MPa, in situ swelling pressure) for up to 1 week, and evaluated for changes in hydration, glycosaminoglycan content, cell viability, and biosynthetic activity. Additionally, the biochemical and biosynthetic response of discs cultured without VEP to increasing the load to a 20-kg (~1 MPa, the estimated stress in human lumbar disc during heavy lifting) static load for 6 hours was assessed.
Results.
During the first 24 hours, culturing discs with endplates was moderately better with regards to maintaining in situ anulus hydration and nucleus glycosaminoglycan levels. The endplates, however, obstructed media flow to the disc, resulting in a marked decrease in cell viability after 1 week of culture. Nucleus pulposus cell viability was maintained in discs cultured without endplates, but there was a significant drop in biosynthetic activity within 2 days of culture. Despite this drop, the disc cells in the discs without VEP remained biosynthetically responsive to changes in mechanical loading.
Conclusions.
It is possible to maintain cell viability and the biosynthetic responsiveness of large discs for up to 1 week in vitro when the discs are cultured under static load and without VEP.
Keywords: intervertebral disc, explant culture, bovine coccygeal disc, vertebral endplate, static compression, biochemistry, metabolism, cell viability
Low back pain affects up to 80% of adults, with annual estimated direct and indirect costs upwards of $90 billion in the United States alone.1 A widely recognized contributor to low back pain is degeneration of the intervertebral disc, the soft tissue between the bony vertebrae that absorbs and distributes loads and lends flexibility to the spine. While there is strong evidence that genetic factors can influence disc degeneration (see recent reviews by Ala-Kokko2 and Battie et al3), it is also clear that mechanical exposure (particularly that associated with upright postures3) and limited nutrient supply4 – 6 of this large, avascular, aneural organ make the disc particularly susceptible to irreparable damage. Despite the huge clinical problem of low back pain and the common association with intervertebral disc pathology, the biologic and mechanical pathways contributing to disc degeneration remain poorly understood.
One of the main limitations in the study of intervertebral disc biology is the lack of a suitable in vitro culture system. Investigations of the effects of pH, osmolarity, nutrition, and mechanical stress on isolated intervertebral disc cells in culture7–11 lend important insight into how the cells respond to specific stimuli, but removal of the cells from their native, highly specialized, extracellular environment undoubtedly affects the behavior of the cells. In contrast, in vivo studies maintain a physiologic environment for the cells. Various animal models have been used to gain insight into the effects of the mechanical environment on the composition and structure of the disc. For example, Hutton et al35–36 have investigated the effects of long-term static overloading on the biochemistry of the dog lumbar intervertebral disc; Lotz et al12–15 and Iatridis et al16–18 have used mouse and rat tail models, respectively, to investigate short-and long-term biochemical, biomechanical, and cellular responses to various static and dynamic compressive loading protocols. In vivo studies, however, are expensive, labor- intensive, involve uncontrolled physiologic factors, and their relevance to the human condition limits their wide-spread use. An important link between cell culture and in vivo studies is an in vitro explant model, in which controlled in vitro conditions can be applied to cells maintained in their native extracellular matrix.
In recent years, various explant models have been developed with promising, though limited, success. One of the first challenges of disc explant culture is the marked swelling of the tissue when placed in standard culture medium.19 To minimize tissue swelling, Chiba et al cultured rabbit lumbar intervertebral discs, void of endplates, in alginate gel for up to 4 weeks.20 These discs maintained higher levels of proteoglycan and collagen content and higher rates of biosynthesis compared with discs cultured without the gel, but more than 75% of the proteoglycans were lost. More recently, Risbud et al cultured rat lumbar intervertebral discs (with endplates), in high osmolarity culture medium to minimize tissue swelling and maintain disc morphology.21 This system, however, failed to maintain nucleus cell viability or gene expression of the major matrix proteins (collagens I and II, aggrecan, and decorin). Kim et al cultured rabbit lumbar discs (with endplates) under dynamic compression and maintained disc DNA and proteoglycan content for up to 2 weeks but did not report on cell viability or metabolic activity.22 All three of these studies used discs (rabbit and rat) that are much smaller than human lumbar discs; therefore, any diffusion limitations, due to the presence of the alginate gel in the first study or due to the presence of the vertebral endplates (VEPs) in the latter two studies, that would be apparent in the culture of larger discs have been minimized in these systems.
Ohshima et al cultured bovine coccygeal discs under 5-kg static load in order to maintain in vivo hydration levels and measured changes in metabolic activity by varying the load magnitude.23 That study was limited to 12 hours and used discs void of endplates, but such a system appears to be promising, especially in terms of its applicability for mechano-biology studies of the intervertebral disc.
The purpose of the current study was to evaluate a similar system for longer-term culture and explore the possibility of culturing large disc explants with intact endplates. We hypothesized that transport through the VEP route would be necessary to maintain cell viability in the nucleus pulposus. Second, we hypothesized that our organ culture system would be able to maintain metabolically active cells that would be responsive to mechanical perturbations for up to 1 week. To test these hypotheses, we cultured bovine discs with and without VEPs for up to 1 week under a 5-kg (~0.25 MPa) static load and evaluated biochemical stability (hydration and glycosaminoglycan [GAG] content), cell viability, and metabolic activity (sulfated proteoglycan synthesis). Additionally, we measured the biosynthetic response of discs cultured without endplates to changes in the mechanical environment.
Materials and Methods
Tissue Harvest.
Intervertebral discs were harvested either with or without VEPs from young (3- to 6-month old) bovine tails obtained from the local abattoir within 3 hours of death. Discs with VEP were removed using a band saw (Exakt Apparatebau, GmbH, Germany) to make parallel cuts through the bone on either side of the intervertebral disc. Cuts were made as close to the disc as possible while maintaining intact bone across the entire surface of the nucleus pulposus. Bone thickness varied from a minimum of approximately 0.5 mm (near the periphery) to up to approximately 3 mm (at the center). The bony surfaces were flushed out with Tyrrode’s Balanced Salt Solution (TBSS) to try to remove cutting debris and blood clots in the endplates. Discs without VEP were removed using a sharp scalpel blade to cut along the proximal and distal disc-cartilage endplate boundaries. All excised discs were briefly rinsed in a solution of TBSS with a 10× concentration of penicillin-streptomycin (P/S; 10× = 1,000 U/mL penicillin, 1,000 μg/mL streptomycin), followed by another rinse in TBSS with a 1× concentration of P/S before placing in the chambers (see below). A total of four to six discs were harvested from each tail. The harvested discs were approximately circular with average “diameters” ranging from 14 to 18 mm. The thickness of the discs (without endplates) ranged from 5.5 to 8 mm.
Chamber.
The chamber design (Figure 1) was based on that of Ohshima et al.23 The upper and lower blocks of the PEEK (polyetheretherketone) chamber were machined with channels to allow media to flow to the top and bottom of the disc. The intervertebral disc was placed between two porous polyethylene platens (pore diameter 40–100 μm; POREX Technologies) through which the media could flow over the entire top and bottom surfaces of the disc. Dialysis membrane (Spectrums/Por 4, 12-kDa molecular weight cutoff, Sochochim, Lausanne, Switzerland) was placed between the intervertebral disc and the platens to prevent the disc (when cultured without VEP) from sticking to the porous platens. A 5-kg weight was placed on top of the chamber to obtain ~0.25 MPa of pressure on the disc (diameter, 14–18 mm). This load was chosen based on previous reports that the in vivo swelling pressure of the bovine coccygeal disc is 0.25 to 0.3 MPa.24
Figure 1.
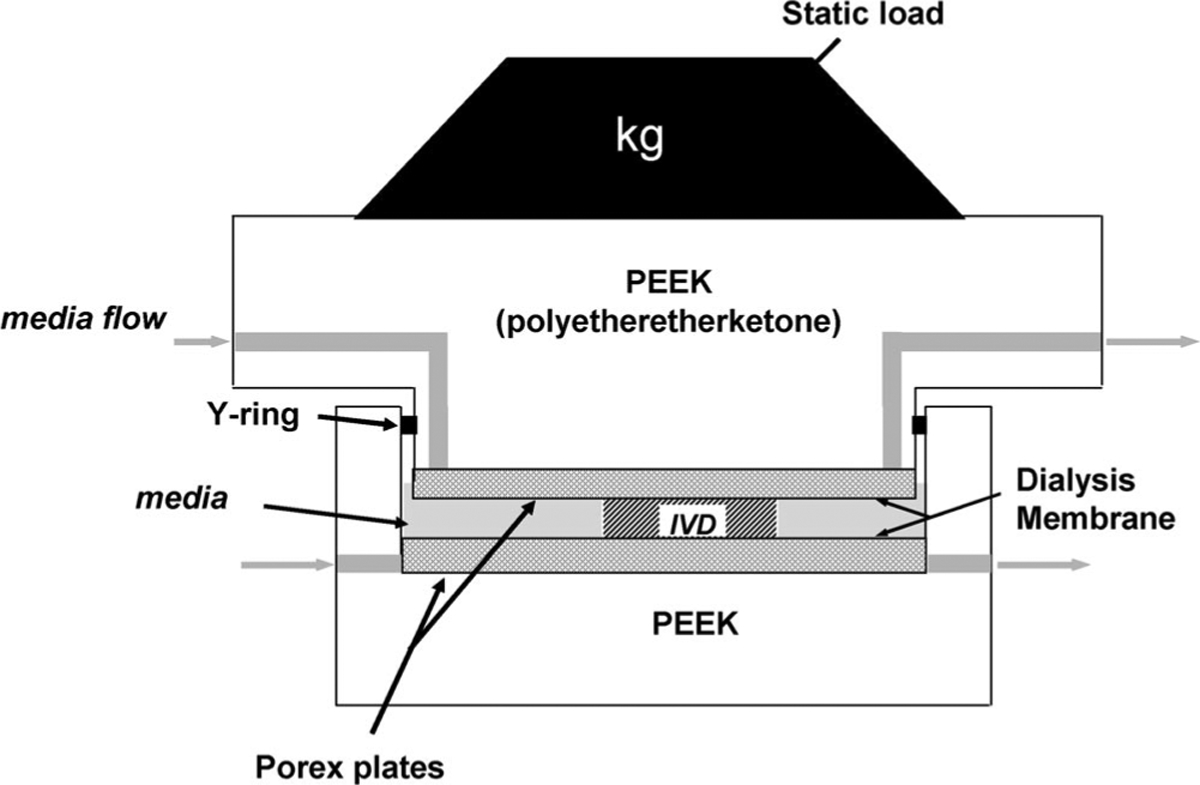
Schematic of chambers used for culture of bovine coccygeal intervertebral disc. Design based on that of Ohshima et al.23
The chambers were connected to media reservoirs via Pharmed tubing. Media (DMEM with 10% fetal bovine serum and 1× P/S) was pumped through the system at a rate of 0.3 mL/min (approximately twice the flow rate of blood to the disc in vivo25) using a peristaltic pump (MCP Process, IP65, Ismatec SA, Switzerland). The entire system was maintained in a 37°C, 5% CO2, and 95% humidity incubator and medium was refreshed every 2 to 3 days.
Part 1: Short-term Evaluation of the Effects of the Vertebral Endplate
Biochemical Analysis.
To determine the effects of disc harvest and to characterize the initial adaptation to the culture system, the first experiment evaluated biochemical changes during the first 24 hours of culture. Discs were cultured either with or without VEP for 2 hours (to evaluate effects of harvest) or 24 hours, and pieces of nucleus pulposus (“nucleus”) and anulus fibrosus (“anulus”) were removed and analyzed for hydration and GAG content. Collected samples were weighed wet and dry (after overnight lyophilization) to determine hydration (water weight/wet tissue weight). After dry weights were measured, samples were digested with proteinase K (0.5 mg/mL, overnight at 60 C) and digests were analyzed for total sulfated-GAG content using the dimethylmethylene blue (DMMB) as-say.26 GAG content was normalized to tissue dry weight and compared with “fresh” samples taken at the time of harvest. This experiment was conducted with discs from four tails, with one disc from each tail randomly assigned to one of the four culture groups and two discs from each tail (most proximal and most distal) analyzed as fresh samples.
Cell Viability.
A second set of discs was evaluated for cell viability and metabolic activity. Pieces (~4 × 4 × 4 mm) of nucleus, inner anulus, and outer anulus were dissected out from freshly harvested or cultured discs (1 or 7 days, with or without VEP; n = 6, i.e., one disc from each of six tails for each condition and time point) discs. Samples were placed in individual wells of a 24-well culture plate and stained for cell viability with 1 μmol/L calcein green (green fluorescence, 495 nm/515 nm excitation/emission, when metabolized by live cells) and 1 μmol/L ethidium homodimer (red fluorescence, 495 nm/635 nm excitation/emission, when bound to DNA in dead cells) in 1 mL of serum-free medium for 3.5 to 5 hours. Stained samples were visualized on an inverted confocal microscope (Zeiss) and images were scanned at 5.74-μm intervals.
Biosynthetic Activity.
From the same discs used to evaluate cell viability, separate pieces of nucleus, inner anulus, and outer anulus were removed and incubated for 6 hours in 1 mL of medium (“free-swelling” conditions) supplemented with 2.5 μCi 35S-sulfate to determine biosynthetic activity. After radiolabeling, samples were lyophilized, weighed dry, and digested in proteinase K, as described above. Aliquots of the proteinase K digest were dialyzed exhaustively against distilled water (Microdialysis chamber, LifeTechnologies with Spectr/Por 3, 3-kDa molecular weight cutoff dialysis membrane, Sochochim) to remove unincorporated 35S-sulfate. The radioactivity of the dialyzed samples was measured by scintillation counting (CPM = counts per minute) and normalized to tissue dry weight. Selected dialyzed samples were run through desalting PD-10 columns (Amersham Biosciences) and scintillation counts of 0.5 mL fractions were measured to verify complete removal of unincorporated 35S-sulfate.
Part 2: Longer-term Evaluation of Disc Biochemistry and Metabolic Activity in Loaded Discs Cultured With-out Vertebral Endplate.
These experiments evaluated longer-term biochemical changes, biosynthetic activity of discs while under load in the chambers, and the biosynthetic response to changes in load magnitude. Since the results of Part 1 showed that discs cultured with VEP had very low cell viability, this part of the study only included discs cultured without VEP. Discs were cultured for 1, 2, or 7 days and analyzed for hydration, GAG content, and biosynthetic activity while under load (n = 6–10 for each time point). To measure biosynthesis under load, discs were kept in the chambers, and 2.5 μCi/mL of 35S-sulfate was added to the medium circulating through the system 6 hours before the cultures were terminated. Pilot studies confirmed that the radiolabel reached equilibrium in the center of the disc within 6 hours. After the 6-hour radiolabeling period, discs were removed and pieces (~4 × 4 × 4 mm) of nucleus, inner anulus, and outer anulus were dissected out and hydration, GAG content, and radiolabel incorporation were determined as described above.
To evaluate the biosynthetic response of the discs to changes in mechanical load, freshly harvested discs and discs cultured without endplate for 7 days (under 5-kg static load) were radiolabeled for 6 hours while under 5-kg (normal load during culture) or 20-kg static load (n = 5 for each loading condition and time point). Nucleus, inner anulus, and outer anulus samples were dissected out, digested, dialyzed, and scintillation counted as described above.
Statistics.
In the first part of the study, evaluating the changes in hydration and GAG content during short-term culture, discs were separated only into nucleus and anulus. In the second part of the study, evaluating the longer-term culture of the discs, the additional distinction between inner and outer anulus was made.
The number of discs used for the various measurements are summarized in Table 1. Each disc in an experimental group was harvested from a separate animal (tail) except for the biochemical evaluation of the fresh discs where two discs were taken from each animal. Data are presented as mean ± standard error of the mean (SEM). Significant differences between culture conditions were determined by ANOVA and Fisher PLSD post hoc testing (P < 0.05).
Table 1.
Summary of the No. of Discs Used for the Various Experiments
| Condition | Length of Culture | Hydration | GAG | Viability | Sulfate Incorporation | ||
|---|---|---|---|---|---|---|---|
| Free-Swelling | Loaded | ||||||
| 5 kg | 20 kg | ||||||
| Fresh VEP | 2 hours | 8* | 8* | 6 | 6 | 10† | 5† |
| 1 day | 4 | 4 | 6 | 6 | |||
| 7 days | 4 | 4 | 6 | 6 | |||
| No VEP | 2 hours | 4 | 3 | ||||
| 1 day | 4 | 4 | 6 | 6 | 5 | ||
| 2 days | 4 | 8 | 5 | ||||
| 7 days | 4 | 8 | 6 | 6 | 8 | 5 | |
Unless otherwise noted, not more than 1 disc per animal was used in a given group (i.e., n = 4 means that 4 tails were used and 1 disc from each of the 4 different tails was allocated to that experiment/condition).
Two discs from each of 4 different animals.
These samples were cultured for the 6-hour radiolabel period only.
Results
General Observations
Discs cultured without VEPs (no VEP) were stained pink by the phenol red in the culture medium along proximal and distal surfaces as well as around the periphery. Discs cultured with VEPs were pink only around the periphery of the anulus, indicating that the media did not fully penetrate the bony endplate. Inspection of a cross section of the VEP of two discs under the dissecting microscope confirmed the presence of blood in the endplate after the harvesting and flushing procedure.
Short-term Biochemical Analysis
There were no significant differences in hydration or GAG content/dry weight of tissue from freshly harvested proximal and distal discs so the data have been combined and subsequent studies used only a single disc/tail as a “fresh” control. Freshly harvested discs had hydration values of 63.0% ± 1.6% (mean ± SEM, water weight/tissue wet weight) in the anulus and 79.8% ± 1.9% in the nucleus. The average GAG content of the anulus from the fresh discs was 2.4% ± 0.5% (GAG/dry weight) and of the nucleus was 36.2% ± 2.1%. After 24 hours of culture, anulus hydration of no VEP discs dropped to 45.8% ± 8.8% (a 17% decrease) and nucleus hydration of VEP discs dropped to 70.0% ± 1.5% (a 12% decrease; both P < 0.05, Figure 2a). There were no significant changes in GAG content of the anulus, but there was an approximate 30% decrease in GAG content of the no VEP disc nucleus during 24 hours of culture (Figure 2b). Although these changes were significant relative to the fresh tissue, there were no significant changes in the hydration or GAG content of VEP and no VEP cultured discs.
Figure 2.
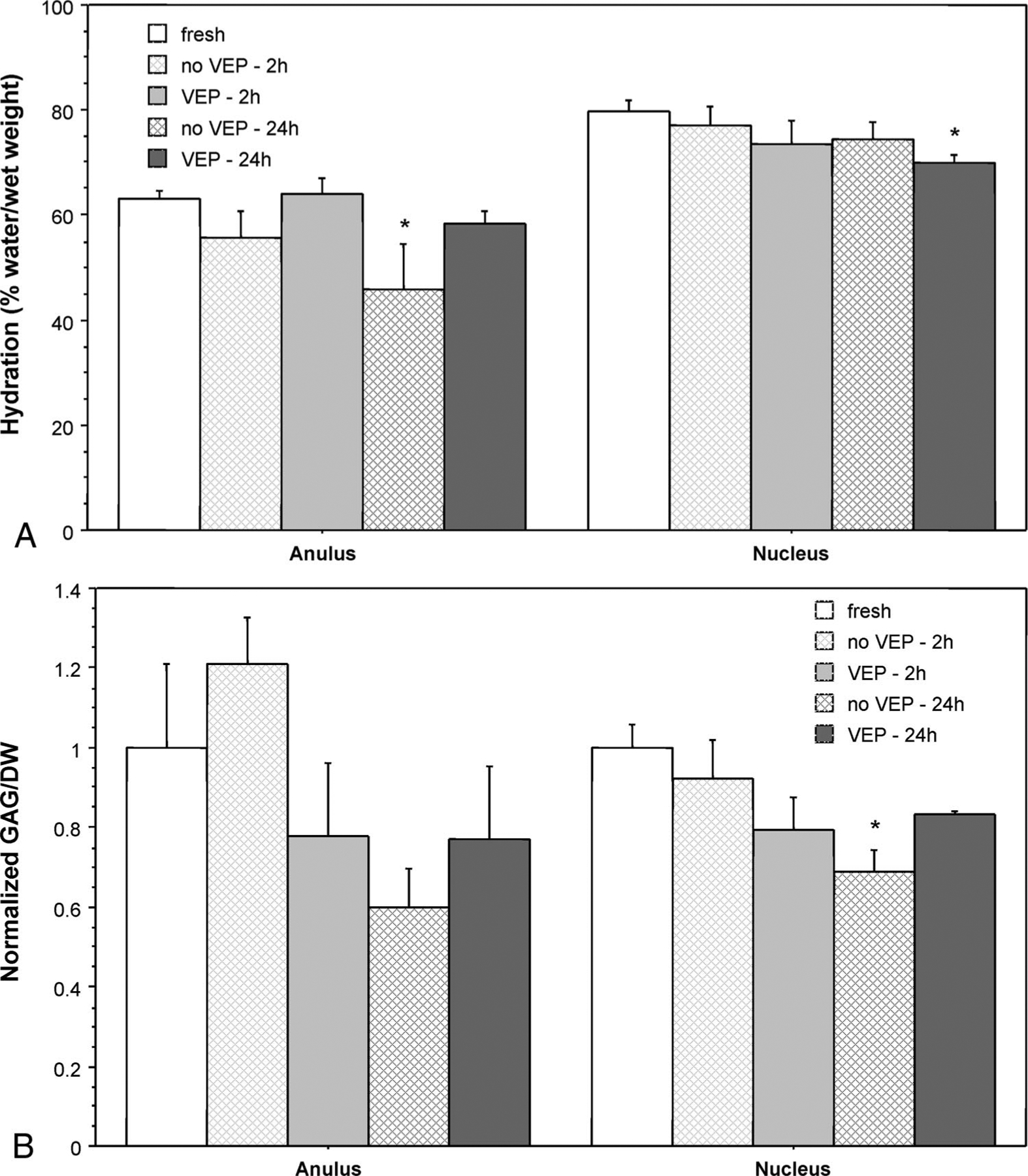
a, Hydration of anulus and nucleus of fresh and cultured bovine coccygeal discs (mean ± SEM; n = 4 for cultured discs and n = 8 for fresh discs. *P < 0.05 vs. fresh discs. b, GAG content of anulus and nucleus tissue of fresh and cultured bovine coccygeal discs. Data are mean ± SEM, normalized to the average GAG content of freshly harvested tissue; n = 3 or 4 for cultured discs and n = 8 for fresh discs. *P < 0.05 vs. fresh discs.
Cell Viability and Biosynthetic Activity
Staining of live and dead cells in the anulus, especially the outer anulus, was very difficult to see on most of the samples due to the large amount of auto-fluorescence of the collagen matrix. In the nucleus, red and green fluorescing cells could be seen at least through depths of 500 μm from the exposed surface, indicating that the fluorophores penetrated at least this deep during the staining period. However, to minimize bias by altering the detector gain and offset settings, observations were limited to the cells within 200 μm of the surface. Approximately the first 30 μm contained primarily red (dead) cells, likely the result of necrosis due to cutting of the tissue pieces in preparation for staining. The images shown in Figure 3 represent a stack of nine images (collected over a 51.7-μm tissue thickness) captured between 30 and 200 μm from the exposed surface.
Figure 3.
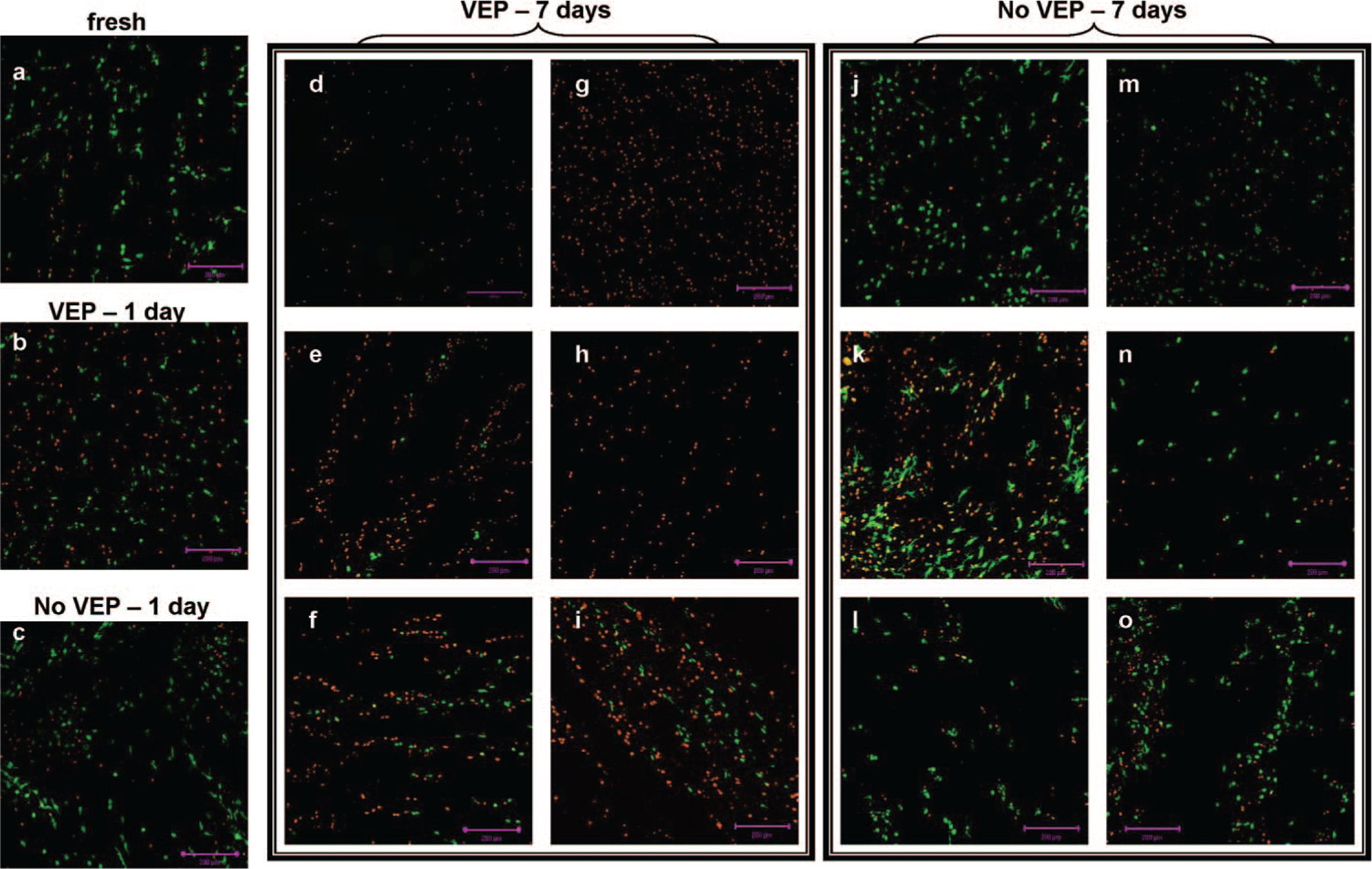
Nucleus pulposus cell viability staining of representative areas of (a) fresh disc and discs cultured for 1 (b, c) or 7 days (d–o). The six images in d–i are from the six different discs cultured with vertebral endplate (VEP). Likewise, the six images in j–o are from the six different discs cultured without VEP. Live cells fluoresce green, and dead cells fluoresce red. Images show cells in a 50-μm slice of the tissue, starting at a minimum of 50 μm from the surface. Scale bar = 200 μm.
There were no remarkable differences in nucleus cell viability of fresh discs (Figure 3a) and discs cultured for 1 day (VEP and no VEP, Figures 3b and 3c, respectively) or for 7 days without VEP (Figures 3j–o). For discs cultured for 7 days with VEP, however, there was an obvious decrease in nucleus cell viability (Figures 3d–i).
Although there was no obvious decrease in nucleus cell viability in no VEP discs, biosynthetic activity decreased for all cultured discs (Figure 4). The 35S-incorporation (measured under free-swelling conditions) of samples from the outer anulus was significantly lower after only 1 day in culture, with or without VEP. For samples from the inner anulus and nucleus, the decrease was only significant on day 7. Nucleus biosynthetic activity was significantly lower for samples from VEP discs than from no VEP discs on both days 1 and 7.
Figure 4.
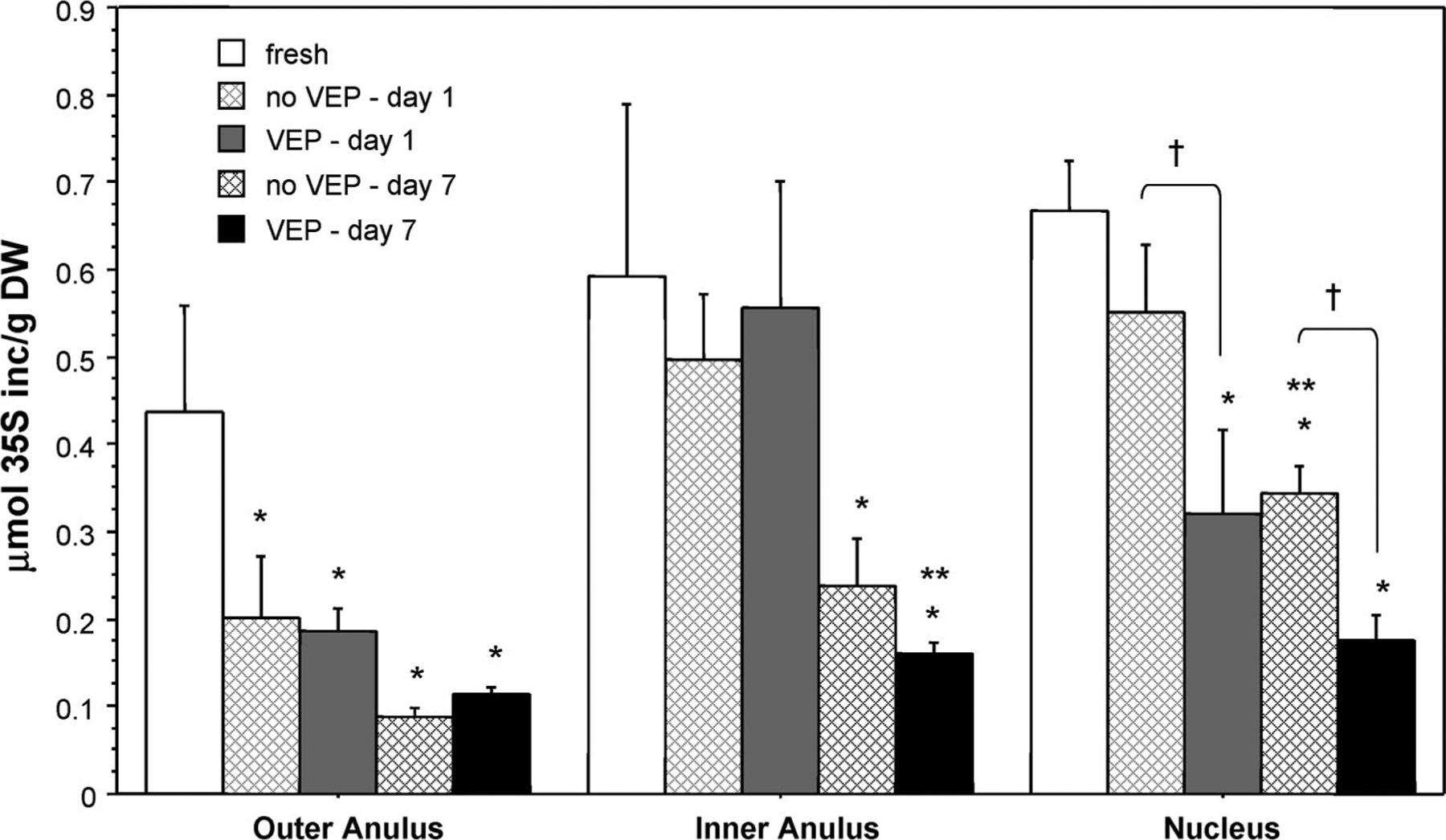
Radiolabel incorporation measured during 6 hours of free-swelling culture. Data are mean ± SEM; n = 5. *P < 0.05 vs. fresh discs. **P < 0.05 vs. day 1. †P < 0.05, no VEP vs. VEP.
Long-term Evaluation of Disc Biochemistry and Biosynthetic Activity
There were no significant changes in disc hydration or GAG content from the first to seventh day of no VEP culture (data not shown). There was, however, a significant decrease in biosynthesis during the 7-day culture period (Figure 5). The rate of proteoglycan synthesis, as measured by 35S-incorporation of discs in the chambers, was approximately 50% lower by day 2 compared with the rate of incorporation during the first 6 hours of culture (day 0). This decrease in biosynthesis was seen in all regions on both days 2 and 7.
Figure 5.
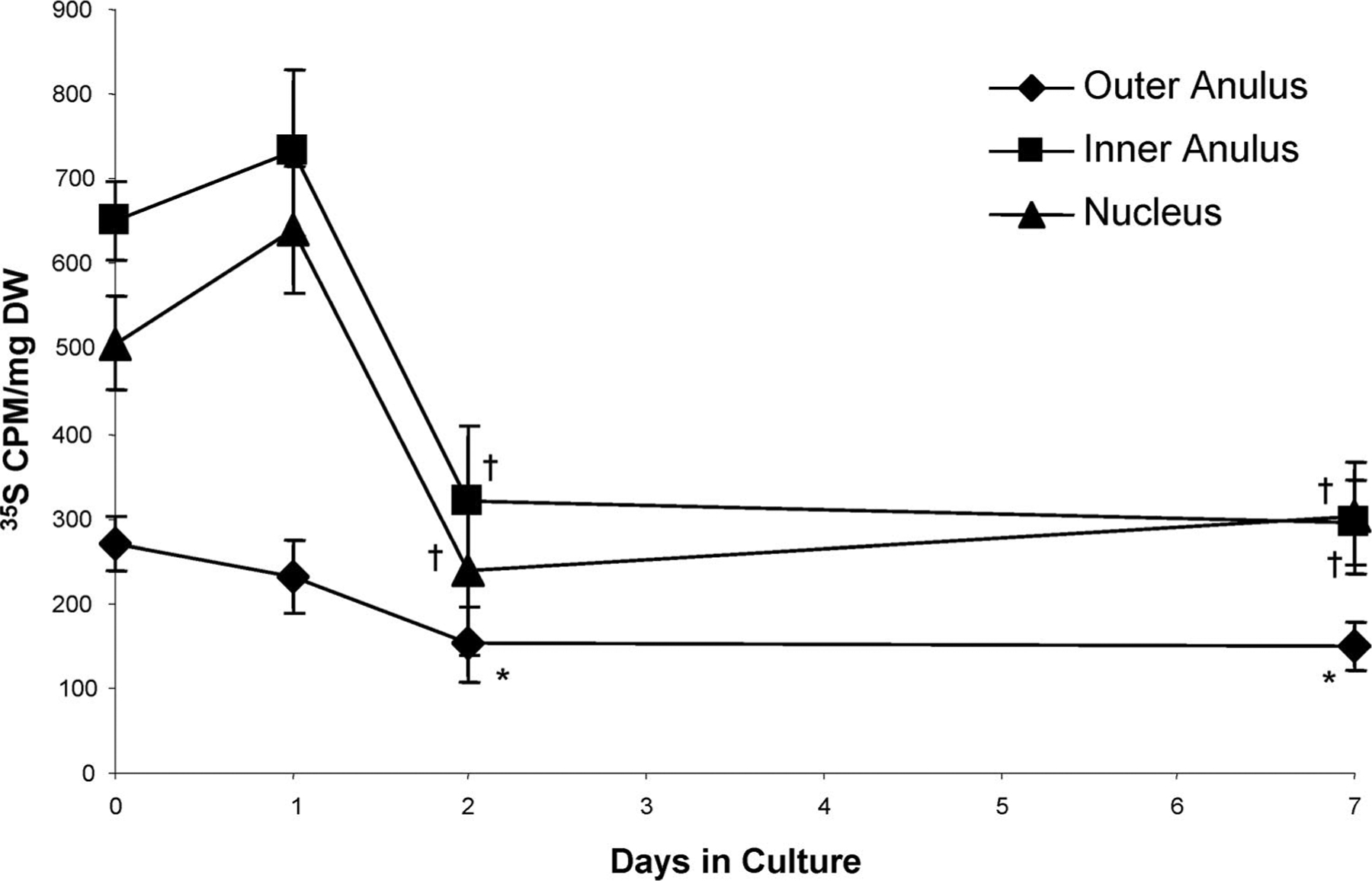
Biosynthetic activity of no VEP discs radiolabeled in chambers during the last 6 hours of culture (under 5-kg static load). Data are mean ± SEM; day 0, n = 10; days 1, 2, n = 5; day 7, n = 8. *P < 0.05 vs. day 0. †P < 0.05 vs. days 0 and 1.
Increasing the static load on the discs from 5 kg to 20 kg for the last 6 hours of culture resulted in a moderate (5%–7%), but significant, decrease in hydration of the tissue (Figure 6a) and a more substantial decrease (20%–55%) in biosynthetic activity (Figure 6b) under 20 kg compared with 5 kg. These changes (hydration and 35S-incorporation) were consistent for both freshly harvested discs (i.e., cultured only for the 6-hour radiolabeling period under 5-kg or 20-kg load) and for discs culturedunder5-kgstaticloadfor7days(before radiolabeling under the 5-kg or 20-kg load).
Figure 6.
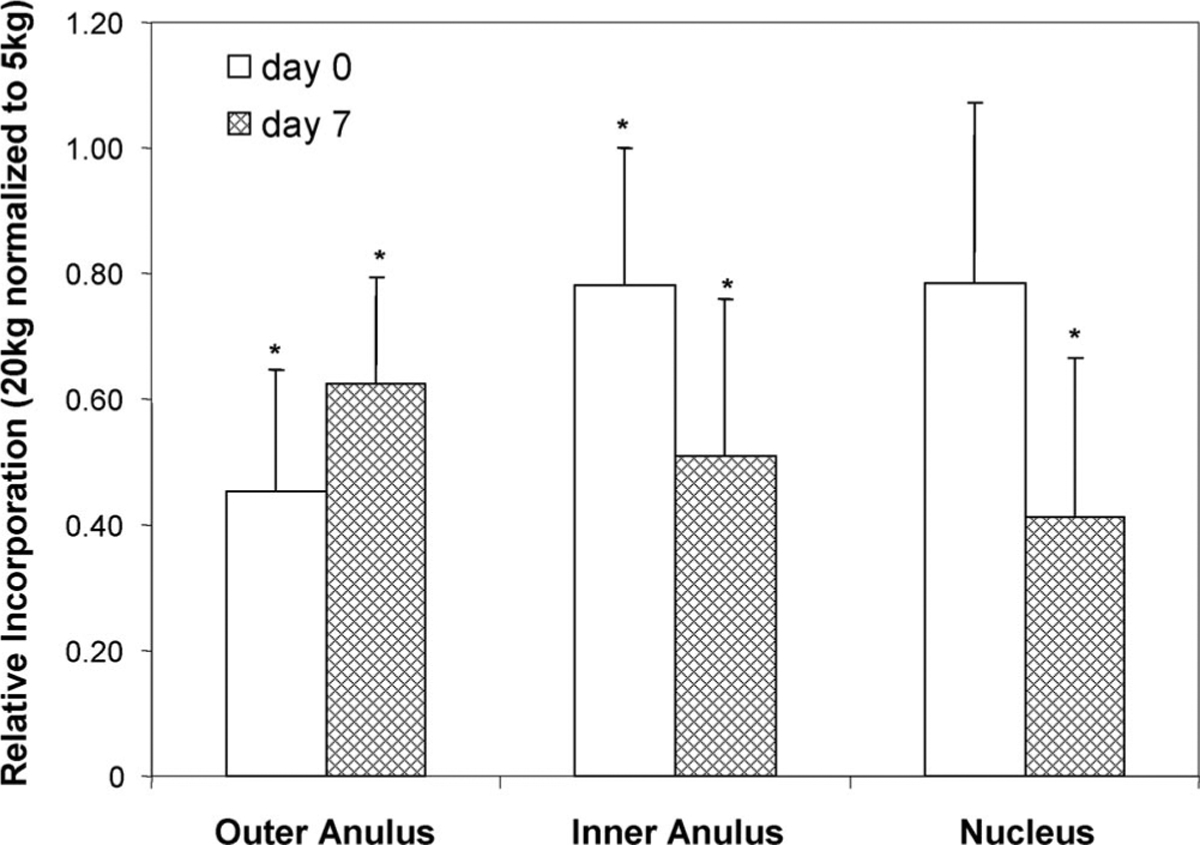
Change in 35S incorporation of no VEP discs cultured under 20-kg static load for the last 6 hours of culture compared with discs cultured under 5-kg static load. Data are mean ± SEM and are presented relative to incorporation levels measured in 5-kg cultured discs; n = 5. *P < 0.05 vs. 5 kg.
Discussion
The initial objective of this study was to evaluate the potential to maintain whole intervertebral disc (intact nucleus, anulus, and VEPs) in culture. During the first 24 hours, culturing discs with VEPs was moderately better with regards to maintaining in situ anulus hydration and nucleus GAG levels. It was clear, however, that there was no (or at least drastically reduced) nutrient transport to the superior and inferior surfaces of the discs cultured with VEP, and a marked decrease in nucleus pulposus cell viability of these discs after 7 days of culture. In contrast, when discs were cultured without VEP, allowing free diffusion of nutrients to all surfaces of the disc, there was no obvious decrease in nucleus cell viability up to 7 days in culture.
Although there were moderate decreases in anulus hydration and nucleus GAG content of discs cultured without VEP for 24 hours compared with fresh tissue, there were no significant biochemical differences between discs cultured with or without VEP for 24 hours. The moderate decreases in VEP nucleus and non-VEP anulus hydration relative to the fresh tissue suggests that the 5-kg load was slightly “too high” (or at least larger than the day 0 postmortem state). The decreased GAG content of the non-VEP nucleus without a corresponding decrease in hydration, however, might suggest that the 5-kg load was “too low.” It is likely these changes are due to both culture conditions and mechanical loading.
Despite the high level of cell viability for discs with endplates removed, there was an approximately 50% drop in proteoglycan synthesis in the cultured discs. The decrease in biosynthetic activity was measured both in free-swelling conditions (in which pieces of cultured disc were dissected out and radiolabeled in normal culture well plates) and for intact discs radiolabeled in the chambers. From the current study, it is not possible to determine whether the decrease in biosynthetic activity is due to a general decrease in biosynthesis of all the cells or due to a decrease in the number of cells that are active. The additional downregulation of proteoglycan synthesis with increased load magnitude is consistent with previous studies of articular cartilage explants showing a decrease in chondrocyte biosynthesis with increasing static load.27–29 Although not attempted in this study, the potential to up-regulate biosynthesis seems promising given the fact that the cells are still viable and did respond to the change in mechanical load, with a similar decrease in biosynthetic activity on days 0 and 7 to a fourfold increase in load. We concluded that discs in culture remained responsive to mechanical loading and note that future work should evaluate if growth factors (including increased serum concentration) and mechanical stimulation are capable of up-regulating metabolic activity.
As this was the first study to jointly address the issues of culturing large discs with endplates and harvesting discs from nonexperimental animals, there were several limitations to the current study. Tails were obtained from the local abattoir and, in accordance with regulations governing the slaughter of animals for human consumption, it was not possible to treat the animals with anticlotting agents before slaughter. Although efforts were made to clean the endplates of any debris and blood clots, we did see blood in the endplates when the VEPs were viewed under a dissecting microscope, indicating that the nutritional pathway through the endplate marrow channels was likely compromised. That the removal of the VEP permitted the maintenance of nucleus pulposus cell viability suggested the clotted VEP was a limiting factor in maintaining cell viability due to a lack of nutrient transport and/or waste product removal. This finding is consistent with the hypothesis that an important pathway of nutrients to the disc is via the superior and inferior disc surfaces and that blockage of this nutrient pathway can lead to cell death.30–32 Removal of the endplates, however, disrupts the integrity of the anulus and compromises the mechanical function of the disc as a load-bearing structure. Future work is necessary to develop harvesting techniques (i.e., administration of anticlotting agents such as heparin before donor death to prevent blood clots from forming if nonslaughterhouse animals are used, or postmortem treatment of the endplates to remove blood clots) that would enable culture of the discs with intact endplates.
A further limitation of the current work is the use of static loading. The 5-kg static load prevented tissue swelling, and the maintenance of cell viability (in no VEP discs) with static loading was expected, given that transport of nutrients vital for cell viability occurs primarily through diffusion.33 It is known, however, that static loading causes a decrease in metabolic activity of articular chondrocytes in vitro27–29 and can lead to alterations in the histologic and biochemical properties of the intervertebral disc in vivo.13,14,16,34–36 Therefore, we propose to use cyclic loading in future studies. It is hoped that the mechanical stimulation provided by cyclic loading will serve to increase/maintain biosynthetic activity as well as providing a “pumping” action to increase convective transport of larger solutes to, from, and through the disc. Increasing nutrient transport by mechanical stimulation may be as simple as applying a diurnal load. It has been observed in humans that there is substantial fluid influx (during sleeping) and efflux (during waking hours).37 Experimentally, it has recently been shown that simple diurnal loading significantly increases transport of a 3-kDa protein through the VEPs,38 and computer simulations have predicted that convective transport due to the diurnal fluid flow increases nutrient transport within the disc.39
Despite these limitations, this culture system compares favorably with previous studies involving intervertebral disc culture. All systems that have been used for multiday cultures have been able to maintain in situ tissue hydration near physiologic levels. Although the decrease in anulus hydration of discs cultured without endplates noted in this study was significant, it was less than a 20% change. The mechanical systems used here and by Kim et al22 were better able to maintain disc GAG content, while the alginate-embedding of Chiba et al20 reported large (>75%) decreases in GAG levels. Of the three systems that investigated metabolic activity, Chiba et al reported that biosynthetic activity was maintained at initial levels for up to 4 weeks of culture, but in our system and that used by Risbud et al,21 there was a drop in activity within the first week. The success of Chiba et al20 may be attributed to their use of rabbit discs without endplates, which allowed better diffusion of nutrients to (no endplates, as compared with the study of Risbud et al21) and through the disc (short diffusion distances through the small discs, as compared with the present study), and/or to their use of 20% FBS (as compared with the 10% used in the present study). The main difference between our system and the previous systems is that ours has been developed for and tested with the larger bovine discs, which we anticipate will make this system more directly applicable for studies of the human disc.
It should be emphasized that this study used bovine coccygeal discs to evaluate the potential and limitations of a system for the culture of large intervertebral disc explants, and we do not claim the bovine coccygeal disc explant culture system is a model for human lumbar discs. All animal models have similarities and differences with human lumbar discs and are only relevant for our ability to test specific hypotheses and evaluate techniques. In the context of this development, the bovine coccygeal disc was appropriate for a number of reasons. First, the size of the bovine coccygeal disc (5.5- to 8-mm- thick, 14- to 18-mm diameter for the calf discs used in this study) approximates that of the human lumbar disc (8- to 14-mm-thick, 18- to 26-mm “diameter”) better than rat or rabbit discs that have been used in previous explant models, meaning that the diffusion times and distances are more comparable. Second, bovine coccygeal discs are readily available from the local abattoir free of charge, such that no animals had to be killed for this study. Third, although the in vivo loading conditions are different in the tail and in the lumbar spine, and differences between the mechanical behavior of mouse tail and lumbar discs have been documented,40 bovine coccygeal discs and human lumbar discs do have similar in situ swelling pressures and are subjected to similar nominal stresses (0.1–0.3 MPa).23,24,41,42 Finally, a recent study has shown that the biochemical composition and cellularity of bovine coccygeal discs are similar to that of young (<40 years old) human lumbar discs.42
The development of an in vitro system for the study of intervertebral disc biology and mechano-biology is essential to furthering the treatment of disc degeneration and low back pain. Of the conditions tested, we found culturing caudal discs with endplates removed under 5-kg static load maintained hydration and GAG levels at near physiologic levels while retaining good cell viability and the cells’ ability to respond to changes in the mechanical environment. Our results provide encouraging signs for the future of large disc explant models while demonstrating a need for future studies to optimize mechanical stimulation, the development of harvesting methods that will preserve nutritional pathways to the nucleus, and the potential addition of growth factors to the culturing media.
Key Points.
Bovine coccygeal discs were maintained for up to 1 week in a simple culture system that allows for compressive loading of the intervertebral disc.
Cell viability was maintained when discs were cultured without vertebral endplates, but biosynthetic activity fell by the second day in culture.
Despite the overall drop in biosynthetic activity, the cells in discs cultured without vertebral endplates remained responsive to changes in mechanical load.
Acknowledgments
The authors thank Urs Schlegel for help with design of the culture chamber and Christoph Sprecher for assistance with confocal imaging.
Supported by the 3R Research Foundation Switzerland, National Institutes of Health #1F32AR0496664–01, and the Whitaker Foundation. The manuscript submitted does not contain information about medical device(s)/drug(s).
Federal and Foundation funds were received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
References
- 1.Luo X, Pietrobon R, Sun SX, et al. Estimates and patterns of direct health care expenditures among individuals with back pain in the United States. Spine 2004;29:79–86. [DOI] [PubMed] [Google Scholar]
- 2.Ala-Kokko L. Genetic risk factors for lumbar disc disease. Ann Med 2002; 34:42–7. [DOI] [PubMed] [Google Scholar]
- 3.Battie MC, Videman T, Parent E. Lumbar disc degeneration: epidemiology and genetic influences. Spine 2004;29:2679–90. [DOI] [PubMed] [Google Scholar]
- 4.Maroudas A, Stockwell RA, Nachemson A, et al. Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro. J Anat 1975;120:113–30. [PMC free article] [PubMed] [Google Scholar]
- 5.Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine 2004;29:2700–9. [DOI] [PubMed] [Google Scholar]
- 6.Urban JP, Holm S, Maroudas A, et al. Nutrition of the intervertebral disk: an in vivo study of solute transport. Clin Orthop 1977;170:101–14. [PubMed] [Google Scholar]
- 7.Chen J, Baer AE, Paik PY, et al. Matrix protein gene expression in intervertebral disc cells subjected to altered osmolarity. Biochem Biophys Res Commun 2002;293:932–8. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Yan W, Setton LA. Static compression induces zonal-specific changes in gene expression for extracellular matrix and cytoskeletal proteins in intervertebral disc cells in vitro. Matrix Biol 2004;22:573–83. [DOI] [PubMed] [Google Scholar]
- 9.Handa T, Ishihara H, Ohshima H, et al. Effects of hydrostatic pressure on matrix synthesis and matrix metalloproteinase production in the human lumbar intervertebral disc. Spine 1997;22:1085–91. [DOI] [PubMed] [Google Scholar]
- 10.Hutton WC, Elmer WA, Bryce LM, et al. Do the intervertebral disc cells respond to different levels of hydrostatic pressure? Clin Biomech 2001;16: 728–34. [DOI] [PubMed] [Google Scholar]
- 11.Razaq S, Wilkins RJ, Urban JP. The effect of extracellular pH on matrix turnover by cells of the bovine nucleus pulposus. Eur Spine J 2003;12: 341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Court C, Colliou OK, Chin JR, et al. The effect of static in vivo bending on the murine intervertebral disc. Spine J 2001;1:239–45. [DOI] [PubMed] [Google Scholar]
- 13.Lotz JC, Colliou OK, Chin JR, et al. Compression-induced degeneration of the intervertebral disc: an in vivo mouse model and finite-element study. Spine 1998;23:2493–506. [DOI] [PubMed] [Google Scholar]
- 14.Lotz JC, Chin JR. Intervertebral disc cell death is dependent on the magnitude and duration of spinal loading. Spine 2000;25:1477–83. [DOI] [PubMed] [Google Scholar]
- 15.Walsh AJ, Lotz JC. Biological response of the intervertebral disc to dynamic loading. J Biomech 2004;37:329–37. [DOI] [PubMed] [Google Scholar]
- 16.Iatridis JC, Mente PL, Stokes IA, et al. Compression-induced changes in intervertebral disc properties in a rat tail model. Spine 1999;24:996–1002. [DOI] [PubMed] [Google Scholar]
- 17.MacLean JJ, Lee CR, Alini M, et al. Anabolic and catabolic mRNa levels of the intervertebral disc vary with the magnitude and frequency of in vivo dynamic compression. J Orthop Res 2004;22:1193–200. [DOI] [PubMed] [Google Scholar]
- 18.Maclean JJ, Lee CR, Grad S, et al. Effects of immobilization and dynamic compression on intervertebral disc cell gene expression in vivo. Spine 2003; 28:973–81. [DOI] [PubMed] [Google Scholar]
- 19.Urban JP, Maroudas A. Swelling of the intervertebral disc in vitro. Connect Tissue Res 1981;9:1–10. [DOI] [PubMed] [Google Scholar]
- 20.Chiba K, Andersson GB, Masuda K, et al. A new culture system to study the metabolism of the intervertebral disc in vitro. Spine 1998;23:1821–7. [DOI] [PubMed] [Google Scholar]
- 21.Risbud MV, Izzo MW, Adams CS, et al. An organ culture system for the study of the nucleus pulposus: description of the system and evaluation of the cells. Spine 2003;28:2652–8. [DOI] [PubMed] [Google Scholar]
- 22.Kim JG, Lim T, Kim KW, et al. A novel biomechanical culture system for the studies of the intervertebral disc. Trans Orthop Res Soc, 2002. [Google Scholar]
- 23.Ohshima H, Urban JP, Bergel DH. Effect of static load on matrix synthesis rates in the intervertebral disc measured in vitro by a new perfusion technique. J Orthop Res 1995;13:22–9. [DOI] [PubMed] [Google Scholar]
- 24.Oshima H, Ishihara H, Urban JP, et al. The use of coccygeal discs to study intervertebral disc metabolism. J Orthop Res 1993;11:332–8. [DOI] [PubMed] [Google Scholar]
- 25.Hirano N, Tsuji H, Ohshima H, et al. Analysis of rabbit intervertebral disc physiology based on water metabolism: I. Factors influencing metabolism of the normal intervertebral discs. Spine 1988;13:1291–6. [DOI] [PubMed] [Google Scholar]
- 26.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta 1986;883:173–7. [DOI] [PubMed] [Google Scholar]
- 27.Sah RL, Kim YJ, Doong JY, et al. Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res 1989;7:619–36. [DOI] [PubMed] [Google Scholar]
- 28.Bonassar LJ, Grodzinsky AJ, Srinivasan A, et al. Mechanical and physicochemical regulation of the action of insulin-like growth factor-I on articular cartilage. Arch Biochem Biophys 2000;379:57–63. [DOI] [PubMed] [Google Scholar]
- 29.Li KW, Williamson AK, Wang AS, et al. Growth responses of cartilage to static and dynamic compression. Clin Orthop 2001;391(suppl):34–48. [DOI] [PubMed] [Google Scholar]
- 30.Benneker LM, Heini PF, Alini M, et al. 2004 Young Investigator Award winner: vertebral endplate marrow contact channel occlusions and intervertebral disc degeneration. Spine 2005;30:167–73. [DOI] [PubMed] [Google Scholar]
- 31.Holm S, Maroudas A, Urban JP, et al. Nutrition of the intervertebral disc: solute transport and metabolism. Connect Tissue Res 1981;8:101–19. [DOI] [PubMed] [Google Scholar]
- 32.Rajasekaran S, Babu JN, Arun R, et al. ISSLS prize winner. A study of diffusion in human lumbar discs: a serial magnetic resonance imaging study documenting the influence of the endplate on diffusion in normal and degenerate discs. Spine 2004;29:2654–67. [DOI] [PubMed] [Google Scholar]
- 33.Urban JP, Holm S, Maroudas A, et al. Nutrition of the intervertebral disc: effect of fluid flow on solute transport. Clin Orthop 1982;296–302. [PubMed] [Google Scholar]
- 34.Ching CT, Chow DH, Yao FY, et al. The effect of cyclic compression on the mechanical properties of the inter-vertebral disc: an in vivo study in a rat tail model. Clin Biomech 2003;18:182–9. [DOI] [PubMed] [Google Scholar]
- 35.Hutton WC, Ganey TM, Elmer WA, et al. Does long-term compressive loading on the intervertebral disc cause degeneration? Spine 2000;25:2993–3004. [DOI] [PubMed] [Google Scholar]
- 36.Hutton WC, Toribatake Y, Elmer WA, et al. The effect of compressive force applied to the intervertebral disc in vivo: a study of proteoglycans and collagen. Spine 1998;23:2524–37. [DOI] [PubMed] [Google Scholar]
- 37.Malko JA, Hutton WC, Fajman WA. An in vivo magnetic resonance imaging study of changes in the volume (and fluid content) of the lumbar intervertebral discs during a simulated diurnal load cycle. Spine 1999; 24:1015–22. [DOI] [PubMed] [Google Scholar]
- 38.Gruenhagen T, Van Donkelaar CC, Lee CR, et al. An in vitro culturing system for the study of solute transport and mechanical loading in intact intervertebral discs. Trans Eur Ortho Res Soc, 2004. [Google Scholar]
- 39.Ferguson SJ, Ito K, Nolte LP. Fluid flow and convective transport of solutes within the intervertebral disc. J Biomech 2004;37:213–21. [DOI] [PubMed] [Google Scholar]
- 40.Sarver JJ, Elliott DM. Mechanical differences between lumbar and tail discs in the mouse. J Orthop Res 2005;23:150–5. [DOI] [PubMed] [Google Scholar]
- 41.Urban J, McMullin J. Swelling pressure of the lumbar intervertebral discs: influence of age, spinal level, composition, and degeneration. Spine 1988; 13 2:179–87. [DOI] [PubMed] [Google Scholar]
- 42.Demers CN, Antoniou J, Mwale F. Value and limitations of using the bovine tail as a model for the human lumbar spine. Spine 2004;29:2793–9. [DOI] [PubMed] [Google Scholar]


