Abstract
Extracellular vesicles (EVs), including exosomes and microvesicles, are found to play an important role in various biological processes and maintaining tissue homeostasis. Because of the protective effects, stem cell-derived EVs can be used to reduce oxidative stress and apoptosis in the recipient cells. In addition, EVs/exosomes have been used as directional communication tools between stem cells and parenchymal cells, giving them the ability to serve as biomarkers. Likewise, altered EVs/exosomes can be utilized for drug delivery by loading with proteins, small interfering RNAs, and viral vectors, in particular, because EVs/exosomes are able to cross the blood–brain barrier. In this review article, the properties of human induced pluripotent stem cell (iPSC)-derived EVs are discussed. The biogenesis, that is, how EVs originate in the endosomal compartment or from the cell layer of microvesicles, EV composition, the available methods of purification, and characterizations of EVs/exosomes are summarized. In particular, EVs/exosomes derived from iPSCs of different lineage specifications and the applications of these stem cell-derived exosomes in neurological diseases are discussed.
Impact statement
In this review, we summarized the work related to extracellular vesicles (EVs) derived from human pluripotent stem cells (hPSCs). In particular, EVs/exosomes derived from hPSCs of different lineage specifications and the applications of these stem cell-derived exosomes in neurological diseases are discussed. The results highlight the important role of cell-cell interactions in neural cellular phenotype and neurodegeneration. The findings reported in this article are significant for pluripotent stem cell-derived cell-free products toward applications in stem cell-based therapies.
Keywords: extracellular vesicles, exosomes, induced pluripotent stem cells, mesodermal cells, neurological diseases
Introduction
Extracellular vesicles (EVs) are lipid-enclosing vesicles with transmembrane proteins and cytosolic proteins in the diameter of 30–1000 nm. Exosomes are a subset of EVs with a diameter between 30 and 200 nm. EVs contain various classes of nucleic acids and soluble and transmembrane proteins, and are described based on size, cell origin, proposed functions, biogenesis, and release pathways.1,2 EVs, including exosomes and microvesicles, are secreted by most cells and can be found in body fluids (e.g., plasma). Functionally, EVs play an important role in intercellular communications, immune modulation, senescence, proliferation, and differentiation in various biological processes, and are vital in maintaining tissue homeostasis.3–6 For example, EVs are implicated in cancer, infections, neural degenerative diseases, and cardiovascular diseases.
EVs/exosomes have been proposed as therapeutic biologics (cell free) for in vivo delivery, which can promote endogenous progenitor proliferation, angiogenesis, extracellular matrix (ECM) remodeling, and regulating immune response.7–10 EVs are much less complex than cells and thus are easier to control, and can be given a more singular objective. They have protective effects and can promote cell viability by reducing cell apoptosis. In particular, induced pluripotent stem cell (iPSC)-derived EVs/exosomes are safer than iPSC-derived cells, which may generate tumor in vivo due to the residue undifferentiated iPSCs.6,11 The unlimited proliferative ability of human iPSCs (hiPSCs) is especially suitable for transplantation studies, such as ischemic heart treatment.6,11
EVs/exosomes can be modified for their cargo and used for drug delivery.12,13 Particularly, EVs can be loaded with bioactive cargo such as proteins, small interfering RNA (siRNA), and viral vectors. For example, EVs can functionally transfer siRNAs and/or microRNAs (miRNAs) to the target cells. Lipid composition enhances their stability and protein contents slow their clearance. EVs/exosomes are also able to cross the blood–brain barrier14 and then deliver exogenous therapeutic molecules (nucleic acids or other small molecules). One example is to load EVs with doxorubicin as a drug for breast cancer.
EVs/exosomes can also be used for identification of novel biomarkers, leading to early diagnostics or possible drug treatments in cancer, neurology, and immunology.15 For example, EVs were collected from body fluids (e.g., blood) and tested for the expression of G protein-coupled receptors (GPCRs) on chips to screen GPCR agonists or antagonists.6 In addition, PCA-3 and TMPRSS2: ERG in EVs were found as RNA-encoding key biomarkers for prostate cancer.16 Similarly, blood-derived EVs were used to diagnose fetal development and predict gestational age and preterm delivery.17
Several clinical trials are ongoing using dendritic cell-derived exosomes to treat different types of cancers or mesenchymal stem cell (MSC)-derived exosomes to treat graft-versus host diseases.3,9 There are several good reviews published recently about the characteristics of MSC-derived exosomes.4,6,18 For example, MSC-exosomes were reported to increase ATP in the cells and reduce oxidative stress through the phosphoinositide-3-kinase/AKT pathway to enhance cell viability. However, the properties of human pluripotent stem cell (hPSC)-derived EVs have not been well reviewed. Therefore, the focus of this article is to summarize the properties of EVs derived from hPSCs of different lineage specifications and genetic backgrounds. This literature analysis indicates that EVs derived from hPSCs are a promising therapeutic agent and provide a useful platform for identifying novel biomarkers.
Mechanism of EV Secretion and Uptake
Biogenesis and composition of EVs
EVs/Exosomes are characterized by the marker expression of CD9, CD63, CD81, ALIX, TSG101, Hsc70, and MHC class II. EVs/exosomes originate from the endosomal compartment or the microvesicles of cellular membrane, which form buds of multivesicular bodies.19,20 The formation is driven by the endosomal sorting complexes required for transport (ESCRT), which is composed of about 30 proteins assembled into four complex (ESCRT-0, -I, -II, and–III) (Fig. 1). TSG101 and ALIX are related to exosome biogenesis and tumor cell exosomes contain syndecan and syntenin. EV/exosome secretion can also occur through an ESCRT-independent mechanism (e.g., in oligodendrocyte cells), which requires the synthesis of ceramide (ceramide dependent).19–21 Reduced expression of CD63, CD81, and TSG101 would be observed upon the treatment of GW4869, a N-SMase inhibitor, to inhibit neutral sphingomyelinase.22
FIG. 1.
Biogenesis of exosome. Exosomes get compartmentalized into multivesicular bodies, which then fuse with cell membrane and are released to the extracellular space. This prevents the exosome degradation by lysosomes. The exosomes released contain proteins such as integrins CD9, CD63, and CD81. Exosomes also contain DNA fragments, miRNAs, and other noncoding RNAs from the donor cell. HSPs, heat shock proteins; miRNAs, microRNAs; mRNA, messenger RNA. Revised from Colombo et al.19 Color images are available online.
EV/exosome secretion can be increased by the treatment with Ca2+ ionophores (i.e., stimulated secretion).19,20 For cortical neurons, exosomes can be stimulated by neurotransmitters. Rab GTPases (e.g., RAB11, RAB35, RAB27A, and RAB27B) are involved in exosome secretion through vesicle budding or mobility through the interaction of cytoskeleton.23 For example, silencing RAB27A and RAB27B was found to decrease the exosomes with CD63, CD81, and MHC-II expression.
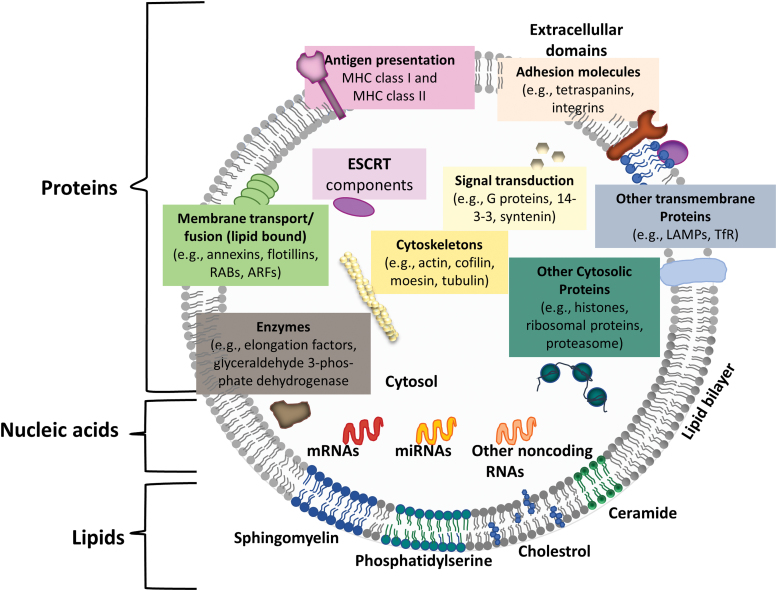
The major composition of EVs are proteins, lipids, and nucleic acids (Fig. 2).19,24 The most common proteins include those from endosomes, peripheral membrane, and the cytosol (e.g., EpCAM, TSG101, CHMP2A, and RAB11B). However, the proteins from nucleus, mitochondria, endoplasmic reticulum, and Golgi complex are excluded. Fewer studies have analyzed lipid content. In general, EVs/exosomes are enriched with sphingomyelin, cholesterol, phosphatidylserine, ceramide, and so on. Cargo nucleic acids carried by the EVs include mRNA and miRNA of various sizes. For example, dendritic cell-derived exosomes contain miR-451 and monocyte-derived EVs contain miR-223.25 For MSCs, EVs were found to be enriched with miR-16 that targets vascular endothelial growth factor (VEGF),26 miR-24 and miR-29 (cardioprotective), miR-146 that binds and suppresses epidermal growth factor receptor (EGFR) mRNA,27 miR-294 (activation of cardiac stem cells), and miR-494 (enhancing myogenesis and angiogenesis).24,28 miRNAs can be exported outside cells and affect gene expression of distant cells. EV compositions are strongly affected by inflammatory signals, for example, lipopolysaccharide (LPS), tumor necrosis factor (TNF)-α, and interferon (IFN)-γ, and hypoxia. Acidic environment also alters lipid composition in EVs.
FIG. 2.
Schematic representation of the overall composition of EVs. EVs commonly contain tetraspanins like CD63, CD81, and CD9. The EVs also contain cytosolic, cytoskeletal, and transmembrane proteins, and enzymes. Note that the listed components may represent large plasma membrane-derived EVs rather than exosomes. ARF, ADP ribosylation factor in the brain; ESCRT, endosomal sorting complexes required for transport; EVs, extracellular vesicles; MHC, major histocompatibility complex; LAMP, lysosome-associated membrane proteins; TfR, transferrin receptor; RAB, ras-related proteins. Revised from Colombo et al.19 and Deng et al.24 Color images are available online.
EV/exosome uptake
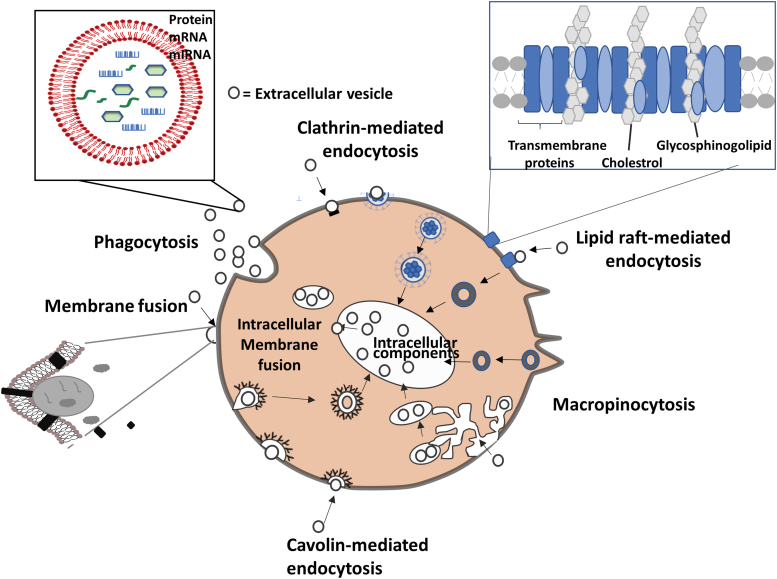
EVs/exosomes can be endocytosed and internalized, and undergo reverse fusion to release their cargo into the recipient cells. A variety of endocytic pathways are used, such as clathrin-mediated endocytosis and clathrin-independent pathways: caveolin-mediated uptake, macropinocytosis, phagocytosis, and lipid raft-mediated internalization (Fig. 3).29 Clathrin-mediated and caveolin-dependent endocytosis are similar pathways that involve pits and invaginations found on cell membranes.30,31 The membranes are deformed into a small bud that eventually pinches off and moves toward the endosomal membrane. Compounds such as hepatin, cytochalasin D, latrunculin B, and human receptor-associated protein can inhibit EV uptake.29 Proteoglycans such as heparin sulfated glycosaminoglycans (e.g., syndecan and glypican) as well as lectins facilitate the uptake of EVs.32 In addition, chelation of calcium with ethylenediaminetetraacetic acid was found to reduce EV uptake by dendritic cells.33 EV uptake may be cell specific, that is, the cell and EVs share the right combination of ligands and receptors. For example, Tspan8-containing lymph node stroma-derived EVs were more effectively internalized by endothelial cells and pancreatic cells than parental lymph node stromal cells.34
FIG. 3.
Pathways for EV uptake. EVs get internalized by cells through phagocytosis, clathrin- and caveolin-mediated endocytosis, or maybe by interactions with lipid rafts. EVs can also be internalized by macropinocytosis where membrane protrusions extend from the cell, fold backwards around the EVs, and enclose them into the lumen of a macropinosome. EVs deliver the proteins, mRNAs, and miRNAs either by fusion with the plasma membrane or by endosomal limiting membrane following endocytosis. Revised from Mulcahy et al.29 Color images are available online.
Bidirectional communication between stem cells and parenchymal cells
EVs play an important role in intercellular communications between stem cells and parenchymal cells, eliciting several important pathways in cell signaling such as Wnt and Notch (Fig. 4).6 Wnt and Wnt-binding proteins reemit and receive signals across synapses using exosomes, which may contribute to Wnt gradient during the development.6 MSC-EVs were reported to activate Wnt4, promoting the self-regulation of Wnt/β-catenin signaling and acting as accelerators for damaged tissue repair.35 Hedgehog (Hh) signaling was found in exosomes budding from microvilli of ventral node during embryonic development. Similarly, Notch-bearing EVs (e.g., Notch ligand delta-like 4 can be distributed in exosomes) transduce Notch signals through receptor-ligand internalization in EVs over long distance.6
FIG. 4.
EV-mediated paracrine effects between stem cells and parenchymal cells. Stem cells sense the injury or distress of parenchymal cells by receiving parenchymal EVs, and in turn, stem cell-EVs containing prohealing RNAs and proteins will be received by parenchymal cells, maintaining tissue homeostasis. There also exists a direct contact between cells for transfer of biomolecules through cellular cytonemes and cilia. EVs carry morphogens such as Wnt, Hh, and Notch ligands. Hh, Hedgehog; ICAM, intercellular adhesion molecule; MHC-I, major histocompatibility complex class I. Revised from Riazifar et al.6 Color images are available online.
Host-pathogen response and immunomodulatory ability
For infected cells (by virus or bacteria), EVs/exosomes contain the pathogen components (e.g., hepatitis C).36 EVs/exosomes can carry tumor antigens and promote T cell activation; therefore, the RNA contents within the exosomes need to be defined. Dendritic cell-derived exosomes, when stimulated by LPS, can release EVs to stimulate epithelial cells to secrete cytokines such as interleukin (IL)-8 and RANTES, or directly activate CD4+ or CD8+ T cells. Bacteria-infected cells release exosomes that can modify T cell and macrophage function (e.g., stimulate macrophage production of proinflammatory cytokines such as TNF-α).
Methods of Purification and Characterizations
EV production
Culture microenvironment has great impacts on EV production, such as cell density, passage number (aging), hypoxia culture condition, ECM in the culture system, and mechanical stress.37–39 Primary stem cells (e.g., MSCs) experience the aging process during serial passage, which can influence their EV production.39 In addition, the cellular differentiation process can change the miRNA cargo, for example, increased miR-21 and decreased miR-221, miR-144, and miR-31 were observed during osteogenic differentiation of MSCs.40 It was also suggested that ECM properties (e.g., topography and stiffness) in the culture systems affect EV cargo composition.37,41 For example, colon cancer organoids with basolateral and apical topography generated distinct EV populations: the basolateral and apical surfaces of cells produced A33-rich and EpCAM-rich EVs, respectively.37
Another method to increase the EV yield for clinical applications is the use of bioreactor culture.42 For example, 1L of MSC-conditioned medium from about 60 million cells can produce 1–2 mg protein content of EVs. In vivo EV administration needs 50–500 μg (protein content) per mouse.6 Therefore, producing EVs on a scale large enough to meet preclinical and clinical demands may require bioreactors. A hollow fiber bioreactor system was reported for scalable production of EVs associated with heterodimeric IL-15.42 Compared to conventional T-flask culture, the hollow fiber culture system produced about 40-fold higher EVs in the yield. This system was integrated with a current good manufacturing practice (cGMP)-compatible EV purification system to generate EVs with immunostimulatory properties.43 A three-dimensional (3D) printed scaffold perfusion bioreactor system was recently reported to enhance the EV production from human endothelial cells.44 Moreover, ethanol conditioning in combination with 3D dynamic culture promoted the provascularization bioactivity of the EVs, with increased levels of long noncoding RNA (lncRNA) HOTAIR, and MALAT1, compared to two-dimensional (2D) static culture.44
EV isolation
Methods of purifying and characterizing EVs/exosomes from cell culture supernatants are provided by Thery et al.45 and are summarized in Table 1. The most common isolation method is differential ultracentrifugation combining filtration/concentration.46 To separate EVs/exosomes from aggregates of proteins, vesicles are allowed to flow into a sucrose gradient.19 EV yields were reported to range from 1 to 10 μg/mL of culture supernatants (e.g., our study generated 2–3 μg/mL spent medium in hiPSC culture).42,47 To overcome the issues of the co-isolation of protein aggregates and incomplete separation of vesicles from lipoproteins, chromatography-based methods, such as size-exclusion chromatography, have been developed.18 Recently, a polyethylene glycol-based method was reported to yield EVs/exosomes comparable to the gold standard of differential centrifugation method and was better than commercial Total Exosome Isolation Reagent.48
Table 1.
Examples of Methods for Extracellular Vesicle Purification*
| Method | Scalable | Advantages | Disadvantages |
|---|---|---|---|
| Magnetic bead isolation | Not currently | Fast; pure product | Costly; low yield; depends on knowledge of specific surface markers; need to remove EVs from antibodies |
| Ultrafiltration | Yes | Works with large volumes | Potential losses under high pressure; impure product |
| Differential ultracentrifugation | No | Most commonly used method; best to produce large quantities; pure product | Includes contaminants; additional isolation steps necessary; difficult to resuspend the EV pellets |
| Density gradient ultracentrifugation | No | Commonly used method; highest purity products | Media components interfere with EV function; volume limitations apply; slow process |
| High-performance liquid chromatography (size exclusion) | Yes | Ideal for large scale | Shown to preserve therapeutic activity |
| Size-exclusion chromatography | Yes | Good separation, removing albumin, many lipoproteins | Postcolumn concentration may be needed |
| Tangential flow filtration; for example, a closed system of AKTATM Flux 6 tangential flow filtration50 | Yes | Ideal for industrial manufacturing; commercially available; can process the samples at a large scale | Need to purchase the device, not readily available for research |
| Precipitation or “salting out” | Yes | Does not require specialized equipment; fast PEG precipitation has been used to generate clinical-grade EVs | Relatively impure product; PEG may interfere with some downstream assays and processes |
| Asymmetric flow field-flow fractionation49 | Yes | Can isolate the EVs of different sizes | Costly; may not be commercially available yet |
| A thermophoretic aptasensor15 | Not currently | Enrich EVs conjugated with Cy5-labeled single-strand DNA aptamers; good for diagnosis | Not good for EV production; costly |
Reiner et al.18
EV, extracellular vesicle; PEG, polyethylene glycol.
A novel isolation method used a thermophoretic aptasensor to enrich EVs conjugated with Cy5-labeled single-strand DNA aptamers from plasma for the early detection and classification of cancers.15 The size-dependent accumulation of EVs relies on the interplay of thermophoresis, diffusion, and convection induced by localized laser heating. In addition, an asymmetric flow field-flow fractionation method was reported recently to separate EVs into large exosome vesicles (Exo-L, 90–120 nm), small exosome vesicles (Exo-S, 60–80 nm), and nonmembranous nanoparticles termed “exomeres” (about 35 nm).49 An enrichment in metabolic enzymes, hypoxia signaling, glycolysis, and mammalian target of rapamycin (mTOR) signaling pathways was found for exomere population.49 Exo-S and Exo-L were enriched with proteins of endosomal function and secretion pathways, such as mitotic spindle and IL-2/STAT5 signaling, respectively.49
Commercialization of EV/exosome isolation and production have been achieved by several companies, for example, Life Technologies, Qiagen, System Biosciences, and Exosome Diagnostics,9,35 using polymer-based precipitation, immunocapture by antibody-coated beads, or size-exclusion chromatography.19 A cGMP-grade method was reported for large-scale preparation of exosomes from human cardiac progenitor cells (Exo-CPC).50 Up to 8L of conditioned media was processed for exosome isolation using a closed system of AKTA™ Flux 6 instrument (GE Healthcare). The Exo-CPC (3 × 1013 particles formulated in a clinical-grade solution Plasma-Lyte A®) was evaluated by quality control test and functional tests for antiapoptotic activity and proangiogenic activity.50
EV characterizations
Quality aspects of the derived EVs include the following: size distribution (by nanoparticle tracking analysis), protein concentration (by Western blot, proteomics), transmission electron microscopy, flow cytometry (with antibody-coated beads), proteomics,51 and miRNA profiling.52 For example, small EVs were found to be enriched in proteins associated with cell−cell junctions, cell−matrix adhesion, exosome biogenesis machinery, and various signaling pathways. In contrast, large EVs were enriched in proteins associated with ribosome and RNA biogenesis, processing, and metabolism.51 As a general rule, at least three or more categories of EV-specific markers and non-EV-specific proteins should be measured semiquantitatively.53,54 For preclinical and clinical studies of EV therapy, several types of assays need to be performed: (1) fingerprint assays, to provide quality control using a narrowly defined set of surrogate markers for EVs; (2) potency assays, to determine which cells are activated by EVs and to what extent; (3) mechanistic assays, important to establish positive and negative controls for fingerprint and potency assays; and (4) safety testing, such as biodistribution patterns, cytotoxicity, and pharmacokinetics.18
To track EVs in vivo, different nanoparticles (5–20 nm) were developed to label the EVs for imaging, such as glucose-coated gold nanoparticles8,10 and ultrasmall superparamagnetic iron oxide.55 EV encapsulation in polymer hydrogels (e.g., chitosan and collage I) was also evaluated to enhance therapeutic effects in animal studies.56,57 It was found that incorporating EVs/exosomes into chitosan hydrogels can improve EV retention, stability, and release in vitro. The chitosan-nitric oxide (NO) compound was shown to significantly reduce necrosis of ischemia hind limbs in diabetic mice through enhanced NO-stimulated EV release when compared to a saline solution and just chitosan due to increased capillary density.57
EV storage and stability
The EV stability over time for miRNAs (e.g., miR-16, 21, 126, 143, 145, 150, 222, and 320) isolated from circulating plasma was evaluated, establishing a key step in the use of exosomal miRNAs as biomarkers.58 The plasma can be frozen before EV/exosome isolation or isolated EVs/exosomes can be later frozen to ensure miRNA stability. The storage temperature was found to be critical for EV stability (over 25 days) and a range of −20°C to −80°C was required to maintain exosomal marker expression.59,60 Another report showed that storage at −20°C for 90 days led to 50% loss of miRNAs, while storage at −80°C showed little change in miRNA cargo.60 A chitosan hydrogel was used to increase the stability of proteins and miRNA cargo in MSC-EVs for hindlimb ischemia treatment.56 The presence of a matrix also increased the local concentration of EVs and enhanced the retention of EVs in vivo, thereby augmenting the therapeutic effects.
iPSC-Derived EVs/Exosomes
Undifferentiated iPSC-derived EVs/exosomes
Undifferentiated iPSCs were reported to release about 2200 EVs/cell/hour in the first 12 h (with an average diameter of 122 nm) in culture, producing 16-fold more EVs than various types of MSCs in a chemically defined medium.61,62 mRNAs in iPSC-EVs were found to contain reprogramming factors Oct3/4, Nanog, Klf4, and c-Myc. Glycome of EVs derived from hiPSCs was analyzed using high-density lectin microarray,63 which found that the characteristic glycan signature of hiPSCs was captured in the derived EVs. Since glycosylation is a major post-translational modification and glycan molecules attach to membrane proteins and lipids, glycans located on EV surface play an important role in EV function. For example, podocalyxin is a glycoprotein ligand of rBC2LCN, an hiPSC-specific lectin.63 EVs/exosomes secreted by hiPSCs reprogrammed from urine exfoliated renal epithelial cells were also evaluated as the RNA interference delivery system.64 The secreted EVs/exosomes expressed CD63, TSG101, and ALIX, the typical exosomal markers. Then siRNA against ICAM-1 was introduced into EVs/exosomes through electroporation. The recipient cells exhibited selective gene silencing and the inhibition of ICAM-1 expression.64 This study indicates that iPSC-EVs can be used as natural gene delivery vectors to reduce inflammatory response of recipient cells.
iPSC-EVs are affected by the origin of the cells being reprogrammed. For example, iPSCs derived from aged donor cells (A-iPSC) and from young donor cells (Y-iPSCs) were compared for their secretion of RNA-exosome complex.65 A-iPSC-secreted exosomes had poor expression of ZSCAN10, leading to excess glutathione-mediated reactive oxygen species (ROS) scavenging activity (i.e., imbalance of ROS/glutathione homeostasis). ZSCAN10 binds to the promoters of RNA-exosome complex and can elevate glutathione peroxidase 2. Expression of ZSCAN10 in A-iPSCs can recover normal DNA damage response and apoptosis. In addition, iPSCs reprogrammed from cardiac fibroblasts were observed to secrete EVs/exosomes that can deliver cardioprotective miRNAs (e.g., miR-21 and miR-210) and protect H9C2 cells from H2O2-induced oxidative stress through caspase 3/7 inhibition.16,66 Moreover, iPSC-derived EVs exerted protective effects and affected transcriptome and proteomic profiles of the recipient cells, and enhanced endothelial cell differentiation.
The therapeutic effects of iPSC-derived EVs/exosomes have been reported in various animal models. For example, hiPSC-exosomes were reported to restore cell viability and capillary-like structure formation, and reduce senescence in human umbilical vein endothelial cells (HUVECs) exposed to high glucose,67 but have minimal effects on normal HUVECs (Table 2).68,69 hiPSC-exosomes were also reported to stimulate the proliferation and migration of human dermal fibroblasts.70 In addition, hiPSC-EVs or exosomes reduced MMP-1/-3 expression and restored the collagen I expression in senescent skin fibroblast cells, showing the potential in treating skin aging. iPSC-EVs (about 300 nm) also reduced hepatic stellate cell activation and liver fibrosis, showing the ability to decrease profibrogenic markers α-smooth muscle actin, collagen Ia1, and fibronectin, and tissue inhibitor of metalloproteinases-1.71 Genomics analysis of miRNA cargo of iPSC-EVs showed 22 highly expressed miRNAs, and miR-92a-3p was found to be the most abundant one. In particular, iPSC-EVs were reported to reduce ROS levels of senescent MSCs, improve the growth of replicatively aged MSCs, and alleviate cellular aging in a genetically induced senescent model, in part, by delivering intracellular peroxiredoxin antioxidant enzymes (e.g., PRDX1 and PRDX2).62 ALIX overexpression (using Crispr/cas9 genome editing of iPSCs) was reported to increase therapeutic function of iPSC-derived exosomes, showing their protective ability on injured endothelial cells and the rescuing of H2O2-blocked angiogenesis.72
Table 2.
Therapeutic Effects of Extracellular Vesicles from Undifferentiated Human Induced Pluripotent Stem Cells
| Cell source | EV characterization | Therapeutic effects | Reference |
|---|---|---|---|
| Urine exfoliated renal epithelial cells reprogrammed hiPSCs | EM; NTA; immunostaining | Use as a natural gene delivery vector to transport therapeutic siRNAs for alleviating inflammatory responses in recipient cells. | Ju et al.64 |
| hiPSCs generated from human dermal fibroblasts by viral transduction | EM; NTA; DLS | Ameliorate the aging of skin fibroblasts | Oh et al.70 |
| hiPSCs from adult human adipose stem cells | EM; NTA; immunoblot; mRNA sequencing | Promote cell proliferation, enhance capillary-like structure formation and reduce senescence in endothelial cells exposed to high glucose | Ding et al.67 |
| hiPSCs generated from human dermal fibroblasts by viral transduction | EM; DLS; immunoblot; mRNA and miRNA sequencing | Reduce hepatic stellate cell activation and liver fibrosis | Povero et al.71 |
| iPSCs | EM; NTA; proteomics and gene ontology analysis | Alleviate aging cellular phenotypes of senescent human cells | Liu et al.62 |
| miRNA expression profiling | iPSC-derived EVs impart cytoprotective properties to cardiac cells in vitro; induced superior cardiac repair in vivo | Adamiak et al.82 | |
| EM; immunoblot; miRNA expression profiling | Deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium | Wang et al.66 | |
| AFM; flow cytometry; proteome array miRNA analysis | Transmit RNAs and proteins to recipient mature heart cells modulating cell fate and behavior | Bobis-Wozowicz et al.68 | |
| Mouse embryonic stem cells | EM; DLS | Promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction | Khan et al.69 |
From Undifferentiated iPSC-Derived EVs/Exosomes section.
AFM, atomic force microscopy; DLS, dynamic light scattering; EM, electron microscopy; hiPSC, human-induced pluripotent stem cell; iPSCs, induced pluripotent stem cells; NTA, nanoparticle tracking analysis; siRNAs, small interfering RNAs.
Direct comparison of iPSC-EVs and MSC-EVs was performed. Proteomic analysis showed that iPSC-EVs contained proteins involved in EGFR interactions and receptor tyrosine kinase signaling, while MSC-EVs contained proteins involved in insulin-like growth factor, Janus kinase (JAK)-signal transducer and activator of transcription (STAT), and Ras-related protein 1 pathways.73 Three cytokines, FGF-2, VEGF, and IL-4, were observed to display significantly higher association with iPSC-EVs than MSC-EVs.
EVs/exosomes secreted by iPSC-derived mesoderm cells
EVs/exosomes derived from mesoderm cells (usually MSCs or cardiomyocytes) differentiated from iPSCs were found to exert protective effects in the treatment of cardiovascular diseases by regulating apoptosis (e.g., prevent cardiomyocyte apoptosis), inflammation, and fibrosis, as well as promoting angiogenesis (Table 3).16,74,75 These are achieved through intercellular communications facilitated by exosomal cargo such as miRNAs, small molecules, and proteins. For example, EV/exosome secretion regulated by neural sphingomyelinase 2 and Rab27 can package 3′-end uridylated miRNAs and shuttle RNAs between cells.
Table 3.
Therapeutic Effects of Extracellular Vesicles Derived from Mesoderm Cells Differentiated from Pluripotent Stem Cells
| Cell source | EV characterization | Therapeutic effects | Reference |
|---|---|---|---|
| iMSCs | EM; NTA; immunoblot | Promote the proliferation of skin cells by stimulating ERK1/2 | Kim et al.77 |
| TEM; immunoblot | Transplanting to wound sites resulted in accelerated reepithelialization, reduced scar widths, and the promotion of collagen maturity | Zhang et al.78 | |
| EM; NTA; immunoblot; ELISA | Protects against renal ischemia/reperfusion injury and inhibits necroptosis | Yuan et al.74 | |
| EM; NTA; immunoblot; ELISA | Protects liver against hepatic ischemia; reperfusion injury by activating sphingosine kinase and sphingosine-1-phosphate signaling pathway | Du et al.75 | |
| SMMSCs; iMSCs | TRPS; EM; immunoblot | iMSC-EVs have a greater therapeutic effect than SMMSC-EVs in the experimental mouse model of collagenase-induced osteoarthritis | Zhu et al.3 |
| iMSCs | EM; flow cytometry; LC-MS/MS Proteomics | EVs acquire a more specific set of proteins: a stromal modulatory proteomic pattern. Arguably, this might confer their therapeutic properties | La Greca et al.79 |
| Embryonic stem cell-derived cardiovascular progenitor cells | Cryotransmission electron microscopy; NTA | The secreted EVs are effective in the treatment of chronic heart failure | El Harane et al.81 |
| hiPSC-CM | EM; NTA; immunoblot | EVs isolated from hiPSC-CM enhance angiogenesis in endothelial cells | Aminzadeh et al.84 |
| EM; immunoblot; miRNA profiling | The derived exosomes provided comparable functional recovery of ischemic heart failure | Lee et al.80 | |
| Human iCM | EM; NTA; immunoblot; miRNA sequencing | Extended delivery of iCM-EVs can protect and promote recovery of the heart | Liu et al.11 |
| Cardiac progenitor cells | EM; NTA; immunoblot; flow cytometry | Reliable human therapeutic applications for acute myocardial infarction | Andriolo et al.50 |
| hiPSC-derived neural stem cells; compared to MSCs | EM; NTA | Improve tissue and functional recovery in the murine thromboembolic stroke model | Webb et al.96 |
| EM; flow cytometry; presence of integrins β1 and α2b | Improve recovery in a porcine model of ischemic stroke | Webb et al.95 |
From EVs/Exosomes Secreted by iPSC-Derived Mesoderm Cells section.
AFM, atomic force microscopy; CM, cardiomyocytes; DLS, dynamic light scattering; EM, electron microscopy; hiPSC-CM, human iPSC-derived cardiomyocytes; iCM, iPSC-derived cardiomyocytes; iMSCs, iPSC-derived mesenchymal stem cells; LC-MS/MS: liquid chromatography-tandem mass spectrometry; MSC, mesenchymal stem cells; NTA, nanoparticle tracking analysis; SMMSCs, synovial membrane mesenchymal stem cells; TRPS, tunable resistive pulse sensing.
Human iPSC-derived MSCs (iMSCs) seem to better promote cell survival, proliferation, and differentiation than adult MSCs, potentially through the secretion of iMSC-exosomes.76 EVs/exosomes derived from iMSCs were found to promote proliferation of skin cells and facilitate wound healing.77 Moreover, EVs/exosomes from iMSCs and synovial membrane-derived MSCs (SMMSCs) were compared for the treatment of osteoarthritis.3 iMSC-derived exosomes showed stronger therapeutic effects than SMMSC-exosomes because iMSC-derived exosomes can promote endothelial cell migration and tube formation, as well as ECM synthesis (e.g., collagens).78 EVs from iMSCs and undifferentiated iPSC-EVs were compared and their cargos were found to differ substantially.79 While iPSC-EVs enclose proteins that modulate RNA and miRNA stability and protein sorting, iMSC-EVs enriched proteins that organize ECM and influence cell–substrate adhesions.
iPSC-derived cardiomyocytes (iCMs) also release EVs/exosomes and their properties remain to be fully characterized. Heat shock proteins (e.g., HSP20, 27, 60, 70, and 90) were detected in iCM-exosomes. Since hypoxia or stress preconditioning may enhance the beneficial properties of exosomes,16 under stress conditions, functional angiotensin-1 receptor was detected in iCM-exosomes. The miRNA crosstalk was found between cardiac fibroblasts and iCMs through fibroblast-secreted miRNA-enriched exosomes. EVs/exosomes derived from human embryonic stem cell (ESC)-CMs and iCMs (for normoxic and hypoxic cultures) were compared for exosomal miRNA and lncRNA profiling.80 MiRNA sequencing was performed using miRCURY RNA isolation kit to isolate exosomal RNAs. Cardioprotection miRs were found, for example, miR-1, miR-21, and miR-30, which were comparable for exosomes from ESC-CMs and iCMs.
However, some studies suggest that iPSC-derived cardiac progenitors are the better sources than iCMs. EVs from iPSC-derived cardiovascular progenitors (good EV yields) and iCMs (no detectable EVs) were isolated by ultracentrifugation.81 The derived EVs were found to be enriched with miRNAs that are involved in tissue repair. In vitro, the EVs were internalized by target cells and the increased cell survival, proliferation, and endothelial cell migration were observed. In vivo, improved cardiac function and decreased left ventricular volumes were demonstrated after EV injections. The EVs injected to mice with myocardial infarction exhibited proangiogenic and cytoprotective properties, ameliorating apoptosis and hypertrophy. Apparently, the derived EVs, enriched with proteins and miRNAs, are safer (no tumor formation) than parent iPSCs for cardiac repair in vivo.82 iCM-derived EVs also showed the ability to promote angiogenesis.83 Endothelial cells treated with 100 μg/mL of the isolated EVs showed significant increases in tube formation, wound closure, and cell proliferation compared to no-EV control.
The 3D cardiospheres were also investigated for EV/exosome isolation. Sphere-derived EVs transiently restored partial expression of dystrophin for Duchenne muscular dystrophy in a mouse animal model and in an hiPSC-based in vitro human Duchenne model.84 EV/exosome treatment resulted in increased expression of dystrophin, improved mitochondrial function, enhanced myocyte proliferation, and suppression of oxidative stress, inflammation, and fibrosis. Similarly, cardioprogenitor cell-derived EVs/exosomes were found to promote H9C2 cell growth (i.e., higher 5-ethynyl-2′-deoxyuridine expression) through activation of Akt and mTOR expression.85
EVs/Exosomes in Applications of Neurological Diseases
Therapeutic effects of EVs/exosomes
EVs from neural cells have important roles in maintaining neural functions
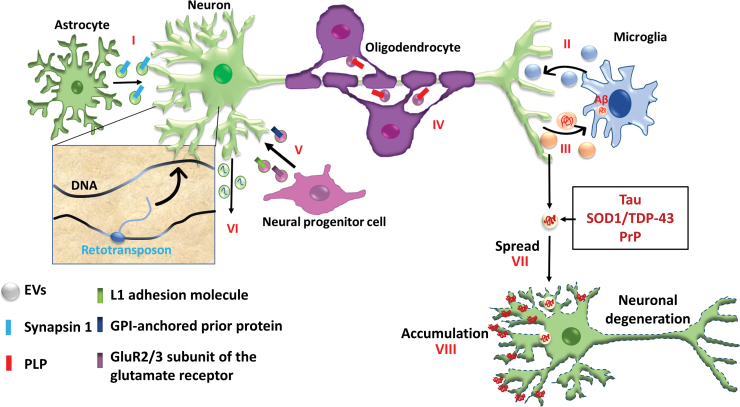
In the human brain, oligodendrocytes are responsible for myelination on axons of neurons and they respond to glutamate on N-methyl-D-aspartate receptors and α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors.3 The EVs secreted by the oligodendrocytes contain proteins that are specific to myelin proteins and RNAs that are related to myelin formation (Fig. 5). Oligodendrocyte-derived exosomes release neurotransmitters and provide glia-mediated trophic support to axons.3 Oligodendrocyte-neuron communication was found to be mediated by exosomes containing miR-219.86 For Alzheimer's disease (AD), hippocampus neuron-derived EVs/exosomes can decrease amyloid beta (Aβ) burden and rescue synaptic activities in AD mice.87,88 In particular, EVs/exosomes can trap Aβ and promote its clearance by microglia. Mouse macrophage-secreted exosomes can increase neural cell viability and decrease ROS levels.89 A similar mechanism may be suggested for microglia cells during neuroinflammation.90 EVs/exosomes can be transported to the brain and taken up by microglia cells, reducing the number of activated inflammatory microglia and suppressing neuroinflammation.
FIG. 5.
Physiology, pathology, and paracrine effects of EVs in the central nervous system. (I) Astrocyte-secreted EVs stimulate dendritic arborization of neurons through synapsin; (II) EVs from microglia increase neuronal synaptic activity; (III) EVs from neurons activate glial cell function, such as phagocytosis of inactive synapses and toxic proteins (e.g., Aβ); (IV) EVs from oligodendrocytes enhance stress tolerance of neurons and stimulate anterograde transport of signaling molecules for myelination such as PLP; (V) EVs also participate in early brain development through proteins released from immature neural progenitor cells, such as L1 adhesion molecule, GPI-anchored prion protein, and the GluR2/3 subunit of glutamate receptor; (VI) Retrotransposon transportation between cells occur through EV compartment. In neurodegenerative diseases, EVs promote (VII) cell-to-cell spreading and (VIII) accumulation of toxic proteins such as tau, SOD1, TDP-43, and prions. Aβ, amyloid beta; GPI, glycosylphosphatidylinositol; PLP, proteolipoprotein. Revised from Zappulli et al.99 Color images are available online.
MSC-EVs have shown therapeutic effects on various neurological disorders
MSC-exosomes were reported to have neuroprotective properties and suppressed 6-hydroxydopamine-induced apoptosis in dopaminergic neurons.91 MSC-EVs carry active neprelysin, an enzyme that can degrade Aβ; therefore, MSC-EVs have been shown to reduce the Aβ plaque burden and the amount of dystrophic neurites in both the cortex and hippocampus in AD mice.92 The communication of astrocytes and neurons is mediated by MSC-EVs containing miR-133.35 miR-133 was downregulated after rat brain ischemia, which can be relieved by EVs containing miR-133. miR-133 also mediates the downregulation of connective tissue growth factor and prevents glial scar formation. For stroke treatment, MSCs may communicate with brain parenchymal cells through exosome-mediated miR-133b and result in the expression of specific genes that enhance neurite outgrowth and improve functional recovery.93 MSC-EVs also were reported to increase remyelination and activate nestin-positive oligodendrocyte progenitors.94
For EVs/exosomes from iPSC-derived neural cells
EVs were also derived from human iPSC-neural stem cells (iNSCs) and used for stroke treatment.95,96 iPSC-NSC EVs were found to be smaller (<200 nm) than iMSC-EVs. iNSC-EVs promoted macrophage polarization toward an anti-inflammatory phenotype and increased the regulatory T cell population in vivo after thromboembolic stroke. iNSC-EVs also reduced lesion volume (based on T2-weighted sequences) and improved behavioral outcomes (e.g., coordination on balance beam) in aged mice. The mechanisms include antioxidative, proangiogenic, immunomodulatory, and neural plasticity regulating processes. In a porcine model of ischemic stroke,95 iNSC-EV treatment decreased cerebral lesion volume and decreased brain swelling compared to control. Long-term (14 weeks) evaluation showed the increased corpus callosum fractional anisotropy values after the EV treatment. The presence of integrins β1 and α2b in iNSC-EVs may account for maintaining the integrity of microvessels.
One of the major mechanisms of the neuroprotective role that EVs/exosomes exhibit
One of the major mechanisms of the neuroprotective role that EVs/exosomes exhibit is their protection against oxidative stress, therefore protecting neural stem cells from oxidative damage. In particular, EVs/exosomes may cross the blood–brain barrier through two possible mechanisms: (1) internalized by endothelial cells, undergo transcytosis, and are released again to be internalized by recipient cells, and (2) entering central nervous system through intercellular junctions of endothelial cells.97 For example, breast cancer-secreted exosomes (associated with miR-105) can downregulate ZO-1 expression and increase the BBB permeability.98 For neurological disorder treatments, iNSC-EVs may be more protective and therapeutically relevant than iMSC-EVs.
The physiopathology of EVs for understanding disease mechanisms and identifying potential biomarkers
The composition of EVs/exosomes depends on cell type and their physiology state.97 In addition to the role of neural protection, EVs have been shown to have two different roles in neural degeneration (Fig. 5).99 On one hand, EVs can modulate the phagocytic clearing of misfolded proteins such as Aβ. On the other hand, EVs can promote the extracellular release of toxic proteins such as tau, SOD1, TOP-43, and prions. Prion diseases originate from misfolded proteins that can travel from infected cells to healthy cells, spreading the infection.99 Recent studies suggest that exosomes may be the vehicle for protein aggregate propagation (APP, tau, a-synuclein, and prion) in AD.100–102 The evidence shows that Aβ assembly is accelerated by incubation with exosomes from the PC12 cell culture media. EVs/exosomes can mediate cell-to-cell transmission of APP.102 EVs/exosomes may act as nucleation center for amyloid plaque formation and can be used as a biomarker for disease status. For example, EVs released from astrocytes may contain synapsin I; EVs from microglia increase synaptic activity of neurons; and EVs from neurons can activate glial cell functions.99
For example, microglia-derived EVs have both beneficial and detrimental roles during AD development.101 They have neuroprotection (promoting TLR4-dependent phagocytosis and Aβ clearance) and neurotransmission ability and provide immune signaling. On the other hand, they promote Aβ deposition, neuroinflammation, and synaptic dysfunction. miR-155 is associated with inflammatory microglia and is a major driver of inflammation in innate immunity.103 Targeting miR-155 in microglia is a possible therapeutic strategy for neurodegeneration.
In particular, iPSC-derived EVs can provide a useful platform for disease modeling and identifying novel biomarkers. For example, the secretome of AD-iPSC neurons was found to contain extracellular Aβ and tau, which contribute to the dysfunction of synaptic activity.104 EVs from hiPSC-derived neurons contained more mid-region tau than full-length tau.1 In AD, tau pathology proposes that aggregated tau is passed from neuron to neuron. Since tau moves from cell to cell through EVs,105 exosomal tau may be used as a biomarker for AD. However, it still remains to be tested if EVs/exosomes containing aggregated tau are capable of seeding monomeric tau in the recipient cells.
It was reported that the apolipoprotein E4 genotype resulted in lower level of exosomes and reduced TSG101 expression in the extracellular space of the human brain and AD mice, possibly due to downregulation of exosome biogenesis and secretion from the endosomal pathway.106 In our study, we also observed little expression of ALIX and TSG101 in EVs/exosomes derived from an iPSC line (i.e., SY-UBH line) reprogrammed from the fibroblasts of an early-onset AD individual with presenilin M146V mutation compared to those derived from healthy iPSK3 cells.47 These results suggest reduced exosome production compared to other EV subpopulations secreted. Alternatively, another possible mechanism of exosome biogenesis (e.g., ceramide pathway) may be predominantly utilized by SY-UBH cells.
miRNAs and Wnt signaling
EV cargo usually contains signaling proteins such as Notch, Wnt, Hh, and TGF-β.6 In particular, miRNA cargo in EVs/exosomes is able to affect downstream signaling processes (Table 4), since EVs/exosomes can travel from one cell and be accepted by another cell where they release their miRNAs. This miRNA cargo has the same ability as endogenous miRNAs.107 It is possible that these miRNAs have the capability of reducing the amyloid plaques that are present in some neurological disorders. Therefore, there are critical needs for research on the functional qualities of the miRNAs contained in EVs/exosomes of different size, as well as the types of miRNAs in EVs from multiple cell lines and subpopulations. Methods to isolate and detect miRNAs in EVs are summarized in a recent review article.108
Table 4.
Examples of the Roles for Different MicroRNAs
| Functions of miRNAs | miRNAs | References |
|---|---|---|
| Cardiac protective | miR-1, 21, 24, 29 | Deng et al.24 |
| miR-294 | ||
| Angiogenesis | miR-16 | Deng et al.24 |
| miR-494 | ||
| Antiapoptosis, neurogenic | miR-133 | Xin et al.93 |
| miR-133b | ||
| Suppresses EGFR | miR-146 | Deng et al.24 |
| Oncogenic | miR-9, 17, 21, 105, 106b, 221 | Diana et al.113 |
| Tumor suppressive | miR-34a, 124, 137, 146a, 152 | Diana et al.113 |
| Osteogenic | miR-21 (decrease in miR-31, 144, 221) | Wang et al.40 |
| Neural protective | miR-1, 9, 16, 20a, 29a/b, 101, 124, 132/212, 135, 147, 153, 155, 181, 186, 195, 219, 298, 328, 330, 335, 449a, 455, 644, 655 | Amakiri et al.114 Reddy et al.115 |
| Neural degenerative | miR-21, 34a, 122, 126, 130b, 183, 206, 296, 329, 346 | Amakiri et al.114 Reddy et al.115 |
| Wnt activation | miR-21, 26a, 27, 31, 141, 144, 155 | Nie et al.111 Song et al.110 |
| Wnt inhibition | miR-15a, 34a, 148a, 200a, 200b, 218, 320, 493, 499, 577, 1862 | Nie et al.111 Reddy et al.115 |
| Biomechanically responsive | miR-21 | Frith et al.38 Li et al.41 |
| miR-100, miR-143 |
EGFR, epidermal growth factor receptor; miRNAs, microRNAs.
MiRNA profiling for hiPSC-derived cortical neurons and oligodendrocytes shows the importance of miRNAs in neural cell function.52,109 In particular, miRNAs regulate a majority of Wnt signaling components, which are critical regulators of development and disease.110,111 For example, miR-221 targets transcriptional factors in canonical Wnt pathway and miR-155 targets the β-catenin-interacting proteins. EV engineering can be achieved using molecular cloning and lentivirus packaging to fuse ischemic myocardium-targeting peptide with exosomal protein.112 Overexpression of miR-146 was also achieved through transfection of MSCs using plasmids with hsa-miR-146b expression.27 Another possible method is to use anti-miR molecules. Compared to normal NSCs, cancerous NSCs upregulate oncogenic miRNAs, including miR-9, 17, 21, 106b, and 221, and downregulate tumor suppressor miRNAs, including miR-34a, 124, 137, 146a, and 152.113
The relationship of Aβ and miRNAs in AD was also reviewed.114 The major miRNAs that are upregulated in AD include miR-21, 34a, 122, 126, 130b, 183, and 206 (i.e., most are neurodegenerative miRNAs). The major miRNAs that are downregulated in AD include miR-1, 9, 16, 101, 124, 135, 155, 186, 219, 328, and 455 (i.e., most are neuroprotective miRNAs).115 For example, miR-155 directly targets APP by binding to its 3′UTR, downregulating Aβ. As another example, miR-34a plays a critical role in regulating oxidative stress and its expression increases with aging.
Our study characterized the EVs derived from undifferentiated iPSK3 cells, iPSK3-derived cardiac cells (mesoderm), iPSK3-derived neural progenitors (ectoderm), and AD-associated SY-UBH cells (with presenilin 1 M146V mutation).47 miRNAs, including miR-133, miR-155, miR-221, and miR-34a, were differently expressed in the EVs isolated from distinct hiPSC lineages. Treatment of cortical spheroids with hiPSC-EVs in vitro resulted in enhanced cell proliferation (indicated by BrdU+ cells) and axonal growth (indicated by β-tubulin III staining). Furthermore, hiPSC-derived EVs exhibited neural protective abilities in Aβ42 oligomer-treated cultures by enhancing cell viability and reducing oxidative stress.
The importance of 3D architecture in iPSC-based tissue models for EV production
It was reported that 3D microenvironment (e.g., cancer organoids) promotes secretion of HSP90 and EpCAM-exosomes, a marker of cancer stem cell phenotype compared to 2D culture,116 better recapitulating the size and cargo of in vivo exosomes.117 3D culture (e.g., aggregates with 200 μm in diameter) of malignant gastric cancer cells was found to produce higher amounts of smaller EVs than 2D culture.118 Global upregulation of miRNAs and downregulation of proteins were observed in 3D culture (e.g., miR-155-5p, miR-143-3p, and miR-127-3p were exclusively present in 3D condition) in agarose microwell arrays, with significantly downregulated ADP-ribosylation factor 6 signaling pathway in 3D EVs.118 Based on these reports, it is reasonably hypothesized that the EVs released from hiPSCs (including 2D or 3D differentiation) reflect the developmental stages, tissue homeostasis, and lineage specification of the cells. These different properties are reflected by their ability to reduce oxidative stress, enhance viability, and promote cell differentiation and neurogenesis. In particular, recent brain organoid technology provides a promising platform for studying the paracrine signaling and cell–cell communications in the human brain.119–121
Conclusions
EVs contain various classes of nucleic acids as well as soluble and transmembrane proteins that need to be well characterized. Culture microenvironment has a great impact on EV production, such as cell density, passage number (aging), hypoxia, ECM, and mechanical stress. The derived EVs can be characterized by size distribution, proteomics, and miRNA profiling. The EVs/exosomes released from stem cells (e.g., MSCs and iPSCs) have been studied for their immunological and therapeutic functions. Undifferentiated iPSC-EVs can reduce ROS levels of senescent MSCs and alleviate cellular aging. The EVs from iPSC-mesoderm exert protective effects in the treatment of cardiovascular diseases by regulating apoptosis, inflammation, and angiogenesis. The EVs from iPSC-neural progenitors can be transported to the brain and taken up by microglia, reducing the number of activated inflammatory microglia and suppressing neuroinflammation. For neurodegenerative diseases, EVs/exosomes may decrease Aβ burden and rescue synaptic activities. Further studies on hiPSC-derived EVs/exosomes are necessary to find helpful intervention for neurological diseases.
Author Contributions
R.J., M.M., and Y.L. reviewed the literature, wrote the article, summarized tables, and prepared figures. J.B. summarized tables, prepared figures, and reviewed the article. Y.L. conceived the projects and revised the article.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure Statement
No competing financial interests exist.
Funding Information
This work is supported by NSF Career Award (No. 1652992 to Y.L.) and NIH R03EB020770 (Y.L.).
References
- 1. Guix F.X., Corbett G.T., Cha D.J., et al. Detection of aggregation-competent tau in neuron-derived extracellular vesicles. Int J Mol Sci 19, pii: , 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wiklander O.P.B., Brennan M.A., Lotvall J., Breakefield X.O., and El Andaloussi S.. Advances in therapeutic applications of extracellular vesicles. Sci Transl Med 11, pii: , 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhu Y., Wang Y., Zhao B., et al. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res Ther 8, 64, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Han C., Sun X., Liu L., et al. Exosomes and their therapeutic potentials of stem cells. Stem Cells Int 2016, 7653489, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fuster-Matanzo A., Gessler F., Leonardi T., Iraci N., and Pluchino S.. Acellular approaches for regenerative medicine: on the verge of clinical trials with extracellular membrane vesicles? Stem Cell Res Ther 6, 227, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Riazifar M., Pone E.J., Lotvall J., and Zhao W.. Stem cell extracellular vesicles: extended messages of regeneration. Annu Rev Pharmacol Toxicol 57, 125, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lamichhane T.N., Sokic S., Schardt J.S., Raiker R.S., Lin J.W., and Jay S.M.. Emerging roles for extracellular vesicles in tissue engineering and regenerative medicine. Tissue Eng Part B Rev 21, 45, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perets N., Betzer O., Shapira R., et al. Golden exosomes selectively target brain pathologies in neurodegenerative and neurodevelopmental disorders. Nano Lett 19, 3422, 2019 [DOI] [PubMed] [Google Scholar]
- 9. Gyorgy B., Hung M.E., Breakefield X.O., and Leonard J.N.. Therapeutic applications of extracellular vesicles: clinical promise and open questions. Annu Rev Pharmacol Toxicol 55, 439, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Betzer O., Perets N., Angel A., et al. In vivo neuroimaging of exosomes using gold nanoparticles. ACS Nano 11, 10883, 2017 [DOI] [PubMed] [Google Scholar]
- 11. Liu B., Lee B.W., Nakanishi K., et al. Cardiac recovery via extended cell-free delivery of extracellular vesicles secreted by cardiomyocytes derived from induced pluripotent stem cells. Nat Biomed Eng 2, 293, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Armstrong J.P.K., and Stevens M.M.. Strategic design of extracellular vesicle drug delivery systems. Adv Drug Deliv Rev 130, 12, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang Y., Hong Y., Nam G.H., Chung J.H., Koh E., and Kim I.S.. Virus-mimetic fusogenic exosomes for direct delivery of integral membrane proteins to target cell membranes. Adv Mater 29, 2017 [DOI] [PubMed] [Google Scholar]
- 14. Chen C.C., Liu L., Ma F., et al. Elucidation of exosome migration across the blood-brain barrier model in vitro. Cell Mol Bioeng 9, 509, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu C., Zhao J., Tian F., et al. Low-cost thermophoretic profiling of extracellular-vesicle surface proteins for the early detection and classification of cancers. Nat Biomed Eng 3, 183, 2019 [DOI] [PubMed] [Google Scholar]
- 16. Jung J.H., Fu X., and Yang P.C.. Exosomes generated from iPSC-derivatives: new direction for stem cell therapy in human heart diseases. Circ Res 120, 407, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ngo T.T.M., Moufarrej M.N., Rasmussen M.H., et al. Noninvasive blood tests for fetal development predict gestational age and preterm delivery. Science 360, 1133, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reiner A.T., Witwer K.W., van Balkom B.W.M., et al. Concise review: developing best-practice models for the therapeutic use of extracellular vesicles. Stem Cells Transl Med 6, 1730, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Colombo M., Raposo G., and Thery C.. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30, 255, 2014 [DOI] [PubMed] [Google Scholar]
- 20. van Niel G., D'Angelo G., and Raposo G.. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 19, 213, 2018 [DOI] [PubMed] [Google Scholar]
- 21. Stuffers S., Sem Wegner C., Stenmark H., and Brech A.. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic 10, 925, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Trajkovic K., Hsu C., Chiantia S., et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319, 1244, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Ostrowski M., Carmo N.B., Krumeich S., et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 12, 19; sup 1, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Deng H., Sun C., Sun Y., et al. Lipid, protein, and MicroRNA composition within mesenchymal stem cell-derived exosomes. Cell Reprogram 20, 178, 2018 [DOI] [PubMed] [Google Scholar]
- 25. Ismail N., Wang Y., Dakhlallah D., et al. Macrophage microvesicles induce macrophage differentiation and miR-223 transfer. Blood 121, 984, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee J.K., Park S.R., Jung B.K., et al. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS One 8, e84256, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Katakowski M., Buller B., Zheng X., et al. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett 335, 201, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nakamura Y., Miyaki S., Ishitobi H., et al. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett 589, 1257, 2015 [DOI] [PubMed] [Google Scholar]
- 29. Mulcahy L.A., Pink R.C., and Carter D.R.. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles 3, 24641, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fitzner D., Schnaars M., van Rossum D., et al. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci 124 (Pt 3), 447, 2011 [DOI] [PubMed] [Google Scholar]
- 31. Doherty G.J., and McMahon H.T.. Mechanisms of endocytosis. Annu Rev Biochem 78, 857, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Christianson H.C., Svensson K.J., van Kuppevelt T.H., Li J.P., and Belting M.. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci U S A 110, 17380, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hao S., Bai O., Li F., Yuan J., Laferte S., and Xiang J.. Mature dendritic cells pulsed with exosomes stimulate efficient cytotoxic T-lymphocyte responses and antitumour immunity. Immunology 120, 90, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rana S., Yue S., Stadel D., and Zoller M.. Toward tailored exosomes: the exosomal tetraspanin web contributes to target cell selection. Int J Biochem Cell Biol 44, 1574, 2012 [DOI] [PubMed] [Google Scholar]
- 35. Gimona M., Pachler K., Laner-Plamberger S., Schallmoser K., and Rohde E.. Manufacturing of human extracellular vesicle-based therapeutics for clinical use. Int J Mol Sci 18, pii: , 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schorey J.S., Cheng Y., Singh P.P., and Smith V.L.. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep 16, 24, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Patel D.B., Santoro M., Born L.J., Fisher J.P., and Jay S.M.. Towards rationally designed biomanufacturing of therapeutic extracellular vesicles: impact of the bioproduction microenvironment. Biotechnol Adv 36, 2051, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Frith J.E., Kusuma G.D., Carthew J., et al. Mechanically-sensitive miRNAs bias human mesenchymal stem cell fate via mTOR signalling. Nat Commun 9, 257, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Patel D.B., Gray K.M., Santharam Y., Lamichhane T.N., Stroka K.M., and Jay S.M.. Impact of cell culture parameters on production and vascularization bioactivity of mesenchymal stem cell-derived extracellular vesicles. Bioeng Transl Med 2, 170, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang X., Omar O., Vazirisani F., Thomsen P., and Ekstrom K.. Mesenchymal stem cell-derived exosomes have altered microRNA profiles and induce osteogenic differentiation depending on the stage of differentiation. PLoS One 13, e0193059, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li C.X., Talele N.P., Boo S., et al. MicroRNA-21 preserves the fibrotic mechanical memory of mesenchymal stem cells. Nat Mater 16, 379, 2017 [DOI] [PubMed] [Google Scholar]
- 42. Watson D.C., Bayik D., Srivatsan A., et al. Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials 105, 195, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Watson D.C., Yung B.C., Bergamaschi C., et al. Scalable, cGMP-compatible purification of extracellular vesicles carrying bioactive human heterodimeric IL-15/lactadherin complexes. J Extracell Vesicles 7, 1442088, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Patel D.B., Luthers C.R., Lerman M.J., Fisher J.P., and Jay S.M.. Enhanced extracellular vesicle production and ethanol-mediated vascularization bioactivity via a 3D-printed scaffold-perfusion bioreactor system. Acta Biomater 95, 236, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thery C., Amigorena S., Raposo G., and Clayton A.. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol Chapter 3, Unit 3.22, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Konoshenko M.Y., Lekchnov E.A., Vlassov A.V., and Laktionov P.P.. Isolation of extracellular vesicles: general methodologies and latest trends. Biomed Res Int 2018, 8545347, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Marzano M., Bejoy J., Cheerathodi M., et al. Differential effects of extracellular vesicles of lineage-specific human pluripotent stem cells on cellular behaviours of isogenic cortical spheroids. Cells 8, 993, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rider M.A., Hurwitz S.N., and Meckes D.G. Jr.. ExtraPEG: a polyethylene glycol-based method for enrichment of extracellular vesicles. Sci Rep 6, 23978, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang H., Freitas D., Kim H.S., et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol 20, 332, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Andriolo G., Provasi E., Lo Cicero V., et al. Exosomes from human cardiac progenitor cells for therapeutic applications: development of a GMP-grade manufacturing method. Front Physiol 9, 1169, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jimenez L., Yu H., McKenzie A., et al. Quantitative proteomic analysis of small and large extracellular vesicles (EVs) reveals enrichment of adhesion proteins in small EVs. J Proteome Res 18, 947, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tu J., Cao D., Li L., Cheung H.H., and Chan W.Y.. MicroRNA profiling during directed differentiation of cortical interneurons from human-induced pluripotent stem cells. FEBS Open Bio 8, 502, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lotvall J., Hill A.F., Hochberg F., et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles 3, 26913, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Thery C., Witwer K.W., Aikawa E., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7, 1535750, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Busato A., Bonafede R., Bontempi P., et al. Magnetic resonance imaging of ultrasmall superparamagnetic iron oxide-labeled exosomes from stem cells: a new method to obtain labeled exosomes. Int J Nanomedicine 11, 2481, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang K., Zhao X., Chen X., et al. Enhanced therapeutic effects of mesenchymal stem cell-derived exosomes with an injectable hydrogel for hindlimb ischemia treatment. ACS Appl Mater Interfaces 10, 30081, 2018 [DOI] [PubMed] [Google Scholar]
- 57. Du W., Zhang K., Zhang S., et al. Enhanced proangiogenic potential of mesenchymal stem cell-derived exosomes stimulated by a nitric oxide releasing polymer. Biomaterials 133, 70, 2017 [DOI] [PubMed] [Google Scholar]
- 58. Sanz-Rubio D., Martin-Burriel I., Gil A., et al. Stability of circulating exosomal miRNAs in healthy subjects. Sci Rep 8, 10306, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Park S.J., Jeon H., Yoo S.M., and Lee M.S.. The effect of storage temperature on the biological activity of extracellular vesicles for the complement system. In Vitro Cell Dev Biol Anim 54, 423, 2018 [DOI] [PubMed] [Google Scholar]
- 60. Jeyaram A., and Jay S.M.. Preservation and storage stability of extracellular vesicles for therapeutic applications. AAPS J 20, 1, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhou J., Ghoroghi S., Benito-Martin A., et al. Characterization of induced pluripotent stem cell microvesicle genesis, morphology and pluripotent content. Sci Rep 6, 19743, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Liu S., Mahairaki V., Bai H., et al. Highly purified human extracellular vesicles produced by stem cells alleviate aging cellular phenotypes of senescent human cells. Stem Cells 37, 779, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Saito S., Hiemori K., Kiyoi K., and Tateno H.. Glycome analysis of extracellular vesicles derived from human induced pluripotent stem cells using lectin microarray. Sci Rep 8, 3997, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ju Z., Ma J., Wang C., Yu J., Qiao Y., and Hei F.. Exosomes from iPSCs delivering siRNA attenuate intracellular adhesion molecule-1 expression and neutrophils adhesion in pulmonary microvascular endothelial cells. Inflammation 40, 486, 2017 [DOI] [PubMed] [Google Scholar]
- 65. Skamagki M., Zhang C., Ross C.A., et al. RNA exosome complex-mediated control of redox status in pluripotent stem cells. Stem Cell Reports 9, 1053, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wang Y., Zhang L., Li Y., et al. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int J Cardiol 192, 61, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ding Q., Sun R., Wang P., et al. Protective effects of human induced pluripotent stem cell-derived exosomes on high glucose-induced injury in human endothelial cells. Exp Ther Med 15, 4791, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bobis-Wozowicz S., Kmiotek K., Sekula M., et al. Human induced pluripotent stem cell-derived microvesicles transmit RNAs and proteins to recipient mature heart cells modulating cell fate and behavior. Stem Cells 33, 2748, 2015 [DOI] [PubMed] [Google Scholar]
- 69. Khan M., Nickoloff E., Abramova T., et al. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res 117, 52, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Oh M., Lee J., Kim Y.J., Rhee W.J., and Park J.H.. Exosomes derived from human induced pluripotent stem cells ameliorate the aging of skin fibroblasts. Int J Mol Sci 19, pii: , 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Povero D., Pinatel E.M., Leszczynska A., et al. Human induced pluripotent stem cell-derived extracellular vesicles reduce hepatic stellate cell activation and liver fibrosis. JCI Insight 5, pii: , 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sun R., Liu Y., Lu M., et al. ALIX increases protein content and protective function of iPSC-derived exosomes. J Mol Med (Berl) 97, 829, 2019 [DOI] [PubMed] [Google Scholar]
- 73. Branscome H., Paul S., Khatkar P., et al. Stem cell extracellular vesicles and their potential to contribute to the repair of damaged CNS cells. J Neuroimmune Pharmacol 2019. [Epub ahead of print]; DOI: 10.1007/s11481-019-09865-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yuan X., Li D., Chen X., et al. Extracellular vesicles from human-induced pluripotent stem cell-derived mesenchymal stromal cells (hiPSC-MSCs) protect against renal ischemia/reperfusion injury via delivering specificity protein (SP1) and transcriptional activating of sphingosine kinase 1 and inhibiting necroptosis. Cell Death Dis 8, 3200, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Du Y., Li D., Han C., et al. Exosomes from human-induced pluripotent stem cell-derived mesenchymal stromal cells (hiPSC-MSCs) protect liver against hepatic ischemia/reperfusion injury via activating sphingosine kinase and sphingosine-1-phosphate signaling pathway. Cell Physiol Biochem 43, 611, 2017 [DOI] [PubMed] [Google Scholar]
- 76. Sabapathy V., and Kumar S.. hiPSC-derived iMSCs: nextGen MSCs as an advanced therapeutically active cell resource for regenerative medicine. J Cell Mol Med 20, 1571, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kim S., Lee S.K., Kim H., and Kim T.M.. Exosomes secreted from induced pluripotent stem cell-derived mesenchymal stem cells accelerate skin cell proliferation. Int J Mol Sci 19, pii: , 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhang J., Guan J., Niu X., et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med 13, 49, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. La Greca A., Solari C., et al. Extracellular vesicles from pluripotent stem cell-derived mesenchymal stem cells acquire a stromal modulatory proteomic pattern during differentiation. Exp Mol Med 50, 119, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lee W.H., Chen W.Y., Shao N.Y., et al. Comparison of non-coding RNAs in exosomes and functional efficacy of human embryonic stem cell- versus induced pluripotent stem cell-derived cardiomyocytes. Stem Cells 35, 2138, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. El Harane N., Kervadec A., Bellamy V., et al. Acellular therapeutic approach for heart failure: in vitro production of extracellular vesicles from human cardiovascular progenitors. Eur Heart J 39, 1835, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Adamiak M., Cheng G., Bobis-Wozowicz S., et al. Induced pluripotent stem cell (iPSC)-derived extracellular vesicles are safer and more effective for cardiac repair than iPSCs. Circ Res 122, 296, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dougherty J.A., Kumar N., Noor M., et al. Extracellular vesicles released by human induced-pluripotent stem cell-derived cardiomyocytes promote angiogenesis. Front Physiol 9, 1794, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Aminzadeh M. A., Rogers, R. G., Fournier, M., et al. Exosome-mediated benefits of cell therapy in mouse and human models of duchenne muscular dystrophy. Stem Cell Reports 10, 942, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Li S., Jiang J., Yang Z., Li Z., Ma X., and Li X.. Cardiac progenitor cellderived exosomes promote H9C2 cell growth via Akt/mTOR activation. Int J Mol Med 42, 1517, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Pusic A.D., and Kraig R.P.. Youth and environmental enrichment generate serum exosomes containing miR-219 that promote CNS myelination. Glia 62, 284, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yuyama K., Sun H., Usuki S., et al. A potential function for neuronal exosomes: sequestering intracerebral amyloid-beta peptide. FEBS Lett 589, 84, 2015 [DOI] [PubMed] [Google Scholar]
- 88. Yuyama K., Sun H., Sakai S., et al. Decreased amyloid-beta pathologies by intracerebral loading of glycosphingolipid-enriched exosomes in Alzheimer model mice. J Biol Chem 289, 24488, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Haney M.J., Klyachko N.L., Zhao Y., et al. Exosomes as drug delivery vehicles for Parkinson's disease therapy. J Control Release 207, 18, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhuang X., Xiang X., Grizzle W., et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther 19, 1769, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Jarmalaviciute A., Tunaitis V., Pivoraite U., Venalis A., and Pivoriunas A.. Exosomes from dental pulp stem cells rescue human dopaminergic neurons from 6-hydroxy-dopamine-induced apoptosis. Cytotherapy 17, 932, 2015 [DOI] [PubMed] [Google Scholar]
- 92. Elia C.A., Tamborini M., Rasile M., et al. Intracerebral injection of extracellular vesicles from mesenchymal stem cells exerts reduced Abeta plaque burden in early stages of a preclinical model of Alzheimer's disease. Cells 8, pii: , 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Xin H., Li Y., Buller B., et al. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells 30, 1556, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Farinazzo A., Turano E., Marconi S., Bistaffa E., Bazzoli E., and Bonetti B.. Murine adipose-derived mesenchymal stromal cell vesicles: in vitro clues for neuroprotective and neuroregenerative approaches. Cytotherapy 17, 571, 2015 [DOI] [PubMed] [Google Scholar]
- 95. Webb R.L., Kaiser E.E., Jurgielewicz B.J., et al. Human neural stem cell extracellular vesicles improve recovery in a porcine model of ischemic stroke. Stroke 49, 1248, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Webb R.L., Kaiser E.E., Scoville S.L., et al. Human neural stem cell extracellular vesicles improve tissue and functional recovery in the murine thromboembolic stroke model. Transl Stroke Res 9, 530, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Jarmalavičiūtė A., and Pivoriūnas A.. Exosomes as a potential novel therapeutic tools against neurodegenerative diseases. Pharmacol Res 113, 816, 2016 [DOI] [PubMed] [Google Scholar]
- 98. Jiang X., Hu S., Liu Q., Qian C., Liu Z., and Luo D.. Exosomal microRNA remodels the tumor microenvironment. PeerJ 5, e4196, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zappulli V., Friis K.P., Fitzpatrick Z., Maguire C.A., and Breakefield X.O.. Extracellular vesicles and intercellular communication within the nervous system. J Clin Invest 126, 1198, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lim Y.J., and Lee S.J.. Are exosomes the vehicle for protein aggregate propagation in neurodegenerative diseases? Acta Neuropathol Commun 5, 64, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Trotta T., Panaro M.A., Cianciulli A., Mori G., Di Benedetto A., and Porro C.. Microglia-derived extracellular vesicles in Alzheimer's disease: a double-edged sword. Biochem Pharmacol 148, 184, 2018 [DOI] [PubMed] [Google Scholar]
- 102. Zheng T., Wu X., Wei X., Wang M., and Zhang B.. The release and transmission of amyloid precursor protein via exosomes. Neurochem Int 114, 18, 2018 [DOI] [PubMed] [Google Scholar]
- 103. Butovsky O., and Weiner H.L.. Microglial signatures and their role in health and disease. Nat Rev Neurosci 19, 622, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hu N.W., Corbett G.T., Moore S., et al. Extracellular forms of Abeta and Tau from iPSC models of Alzheimer's disease disrupt synaptic plasticity. Cell Rep 23, 1932, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Aulston B., Liu Q., Mante M., Florio J., Rissman R.A., and Yuan S.H.. Extracellular vesicles isolated from familial Alzheimer's disease neuronal cultures induce Aberrant Tau phosphorylation in the wild-type mouse brain. J Alzheimers Dis 72, 575, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Peng K.Y., Perez-Gonzalez R., Alldred M.J., et al. Apolipoprotein E4 genotype compromises brain exosome production. Brain 142, 163, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Chen X., Liang H., Zhang J., Zen K., and Zhang C.Y.. Secreted microRNAs: a new form of intercellular communication. Trends Cell Biol 22, 125, 2012 [DOI] [PubMed] [Google Scholar]
- 108. Sun L., and Meckes D.G. Jr. Methodological approaches to study extracellular vesicle miRNAs in Epstein–Barr virus-associated cancers. Int J Mol Sci 19, pii: , 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Letzen B.S., Liu C., Thakor N.V., Gearhart J.D., All A.H., and Kerr C.L.. MicroRNA expression profiling of oligodendrocyte differentiation from human embryonic stem cells. PLoS One 5, e10480, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Song J. L., Nigam, P., Tektas, S. S., and Selva, E. microRNA regulation of Wnt signaling pathways in development and disease. Cell Signal 27, 1380, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Nie X., Liu Y., Chen W.D., and Wang Y.D.. Interplay of miRNAs and canonical Wnt signaling pathway in hepatocellular carcinoma. Front Pharmacol 9, 657, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wang X., Chen Y., Zhao Z., et al. Engineered exosomes with ischemic myocardium-targeting peptide for targeted therapy in myocardial infarction. J Am Heart Assoc 7, e008737, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Diana A., Gaido G., and Murtas D.. MicroRNA signature in human normal and tumoral neural stem cells. Int J Mol Sci 20, pii: , 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Amakiri N., Kubosumi A., Tran J., and Reddy P.H.. Amyloid beta and MicroRNAs in Alzheimer's disease. Front Neurosci 13, 430, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Reddy P.H., Tonk S., Kumar S., et al. A critical evaluation of neuroprotective and neurodegenerative MicroRNAs in Alzheimer's disease. Biochem Biophys Res Commun 483, 1156, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Eguchi T., Sogawa C., Okusha Y., et al. Organoids with cancer stem cell-like properties secrete exosomes and HSP90 in a 3D nanoenvironment. PLoS One 13, e0191109, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Villasante A., Marturano-Kruik A., Ambati S.R., et al. Recapitulating the size and cargo of tumor exosomes in a tissue-engineered model. Theranostics 6, 1119, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Rocha S., Carvalho J., Oliveira P., et al. 3D cellular architecture affects MicroRNA and protein cargo of extracellular vesicles. Adv Sci (Weinh) 6, 1800948, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Pasca S.P. The rise of three-dimensional human brain cultures. Nature 553, 437, 2018 [DOI] [PubMed] [Google Scholar]
- 120. Kelava I., and Lancaster M.A.. Stem cell models of human brain development. Cell Stem Cell 18, 736, 2016 [DOI] [PubMed] [Google Scholar]
- 121. Lancaster M.A., Renner M., Martin C.A., et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]