Abstract
There is a renewed interest in investigating individual variation in hormone levels in relation to fitness metrics, as hormones act as mediators of life-history trade-offs. Hormone concentrations, however, are labile, responding to both internal and external stimuli, so the relationship between hormones and fitness can be non-consistent. One explanation of this inconsistent relationship is that a single hormone sample may not be representative of individual phenotypes in a free-living species. We addressed this issue by repeatedly sampling a free-living population of mountain white-crowned sparrows, Zonotrichia leucophrys oriantha, for baseline and stress-induced corticosterone (cort) and testosterone (T) across different stages of the breeding season. We measured (co)variation using three different methods, taking into account inter- and intra-individual variances, to determine whether hormone levels and the stress response are repeatable. We documented the temporal (over 3 months) and spatial (home-range) variation of individual hormone phenotypes and investigated how these components related to nesting success. At the population level, we found significant repeatability in male stress-induced cort concentrations but no repeatability in male or female baseline cort or male T concentrations. Using a new metric of intra-individual variance focusing on the stress response (profile repeatability), we found a wide range of variance scores, with most individuals showing high variation in their stress response. Similarly, we found a low level of repeatability of the reaction norm intercept and slope for the stress response across different life-history stages. Males with higher concentrations of stress-induced cort had more central home-ranges. Males with higher body condition had larger home-ranges; however, home-range size did not relate to male hormone concentrations or nesting success. We also did not find any significant relationship between variation in hormone levels and nesting success. We recommend that future studies combine both physiological and environmental components to better understand the relationship between hormones and fitness.
Keywords: corticosterone, glucocorticoids, testosterone, repeatability, home-range, white-crowned sparrow
1. Introduction
Hormones mediate many physiological and behavioral traits and, consequently, play a significant role in the trade-off between reproduction and survival (Adkins-Regan, 2005). As such, researchers have been investigating the relationship between hormone traits and fitness as a proactive measure to predict the response of populations to environmental change and to assess population health (Romero and Wikelski, 2001; Sorenson et al., 2017). Studies that correlate hormone concentrations with fitness, however, find positive, negative, or no significant relationships (Guimont and Wynne-Edwards, 2006; Cyr and Romero, 2007; Husak et al., 2007; Gesquiere et al., 2008; Romero and Reed, 2008; Maestripieri et al., 2009; Gerlach and Ketterson, 2013; Patterson et al., 2014; Ouyang et al., 2016; Cockrem et al., 2017; Gangloff et al., 2017). The reason for this inconsistency is that researchers often measure hormones at one time point, but with labile traits, this one-time measurement can be context-dependent and have varying relationships with fitness (Williams, 2008; Bonier et al., 2009a; Bonier and Martin, 2016). Natural seasonal variations in hormone levels also add to the general complexity (Casao et al., 2010; Janati et al., 2013), but an individual may have an elevated response or increased levels compared to other individuals despite life-history stage (Jachowski et al., 2015). Thus, a major goal in physiological ecology and evolution remains to describe the pattern of inter- and intra-individual variation in relation to life- history trade-offs.
Glucocorticoids (GC) and testosterone (T) are hormones that coordinate reproductive events and response to stressors, and often correlate with reproductive success and/or survival (Uller et al., 2007; Fusani, 2008; Hau et al., 2016). For example, baseline GC levels fluctuate in response to external and internal conditions, such as temperature (Ouyang et al., 2012) and body condition (Jaatinen et al., 2013). GC levels rise in response to noxious stimuli (stress-induced) and promote survival over non-essential activities (e.g., reproduction; Wingfield, 2013). Moreover, GCs are also deposited in hair (Malcolm et al., 2013), feathers (Bortolotti et al., 2008), and/or feces (Bonier at al., 2004) and can be used as long-term (days to weeks) measurements, as opposed to short-term measurements from plasma (minutes to hours). These long-term measures of GCs help account for variation in baseline GC levels, the frequency of the stress response, and the magnitude in which an individual responds to a stressor (Harris et al., 2017). T influences an individual’s mating behaviors and concentrations need to be elevated for reproductive events to occur, particularly in males (McGlothlin et al., 2007; Mokkonen et al., 2012; Raynaud et al., 2013; Apfelbeck et al., 2016). For example, higher levels of T are associated with increased attractiveness (Enstrom et al., 1997) and reproductive output (Reed et al., 2006; Edwards et al., 2015); however, individuals with higher levels of T also show a decrease in parental care (Ketterson et al., 1992; Veiga and Polo, 2008).
Although most studies focus on these two hormones separately, GCs and T are likely to interact to mediate life-history trade-offs. The dual-hormone hypothesis (DHH) posits that T’s role in aggression and status-relevant behavior should depend on GC concentrations (Mehta and Prasad, 2015). Meta-analyses of the DHH, however, suggest the relationship between GCs and T is insignificant in many studies (Hau et al., 2010; Li et al., 2017; Dekkers et al., 2019; Grebe et al., 2019). The varying results between GCs and T (e.g., Ketterson et al., 1991; Wikelski et al., 1999; Klukowski, 2011; Zito et al., 2017) suggest that the interaction between these two hormones is species specific (e.g., life-history strategy; Garamszegi et al., 2008) and/or context dependent (e.g., environmental influence; Brischoux et al., 2018). Assessing the level of (co)variation in free-living populations of these hormones can establish individual hormonal phenotypes that affect fitness outcomes.
Repeatability (R) is an index for quantifying the consistency of individual phenotypes and is measured as the ratio of phenotypic variation that is due to differences among individuals relative to differences within an individual (Lessells and Boag, 1987). To improve our interpretations of among- individual variation in hormone concentrations, information about within-individual variation is needed (Schoenemann and Bonier, 2018). The repeatability of hormones has been extensively studied with no conclusive agreement about (co)variation (Cockrem and Silverin, 2002; Love et al., 2003; Kralj-Fiser et al., 2007; Cockrem et al., 2009; Ouyang et al., 2011; Rensel and Schoech, 2011). For example, Grace and Anderson (2014) found repeatability of stress-induced GCs in the Nazca booby (Sula granti) measured over multiple years, while Baugh et al. (2014) found no repeatability in stress-induced GCs in great tits (Parus major) measured within one season. The various timescales used by different studies likely add to the conflicting results (Fanson and Biro, 2019). Although, meta-analyses do show repeatability in GCs, more so in stress-induced levels (Schoenemann and Bonier, 2018; Taff et al., 2018).
Hormone repeatability (here GC and T) is most often calculated using a metric introduced by Lessells and Boag (1987), which calculates among individual variation with respect to total variation. A recent metric proposed by Reed et al. (2019) focuses on the shape of the stress response (the rate and pattern of the elevation of GC concentrations over time), known as profile repeatability (PR). PR measures the level of intra-individual variance and can be used to assess individual variation in the stress response across time. It is calculated using the variance of multiple stress series at each time point as well as the number of crossover events (See Reed et al., 2019 Fig. 1 for further explanation). PR scores are calculated per individual and indicate the level of variance of their stress response (PR=1: high consistency; PR=0: low consistency). A limitation of PR is that it may not be comparable across studies. Lastly, Araya-Ajoy et al. (2015) use a reaction norm approach to partition among and within-individual variance. This method can give a repeatability estimate for the slope and intercept of the stress response. These three methods explore different components of individual variation in the stress response and a comparison of these methods is currently lacking for free-living organisms exposed to different abiotic factors.
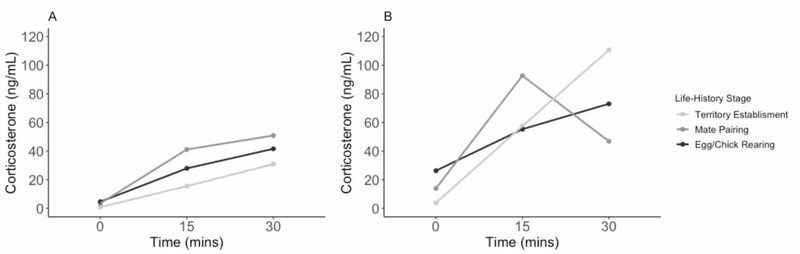
Figure 1. Profile repeatability.
Profile repeatability (PR) plots of individual male mountain white- crowned sparrows with 3 stress series collected at separate life-history stages. The individual with the highest intra-individual consistency (A) had a PR score of 0.86. The individual with the lowest intra-individual consistency (B) had a PR score of <0.0001. Mean PR score for all males with 3 stress series each (n=13) was 0.18 (95% confidence intervals: 0.00 – 0.36).
(Co)variation in hormone levels may be the direct result of environmental heterogeneity. For example, environmental variation leads to differences in territory quality, which can influence hormone levels during breeding (Navarro-Castilla and Barja, 2019). Resulting individual differences in physiology combined with territory metrics then affect breeding success (Both and Visser, 2000; Baird and Hews, 2007; Viblanc et al., 2014; Apfelbeck et al., 2017). For example, territory size can have a positive effect on offspring survival (Larus glaucescens, Hunt and Hunt, 1976; Parus major, Both and Visser, 2000). Skorupski et al. (2017), however, found smaller territories had higher habitat quality for multiple songbird species, which supports other studies relating increased food abundance with smaller territory size (Steingrímsson and Grant, 1999; Newmark and Stanley, 2016; Ippi et al., 2018). Therefore, there is likely a context dependent trade-off between small territories, with high food abundance and high competition, and large territories, with low food abundance and low competition (Johnson, 2007).
How habitat variation within a breeding area drives the spatial distribution of individuals of different physiological phenotypes is unknown. GCs and T both influence the behavior and activity of individuals (Breuner et al., 1998; Wikelski et al., 1999) and, consequently, may determine where an individual is located on the breeding grounds; or an individual’s territory quality and structure may determine their hormone concentrations. Individuals with high T levels tend to hold larger territories (Watson and Parr, 1981; Wingfield, 1984; Chandler et al., 1994) and experimentally increased GC levels have been shown to influence home-range size in the common side-blotched lizard (Uta stansburiana; DeNardo and Sinervo, 1994). One study on King Penguins, Aptenodytes patagonicus, found that individuals were spatially distributed across territories with individuals with higher GC levels in the middle of the breeding area (Viblanc et al., 2014). Further investigation on how individuals aggregate across the breeding grounds may shed light on how individuals are coping with or selecting for certain environments, and we might expect resulting associations to be paired with spatial distribution of individual physiological profiles.
Here, we collected repeated within-individual measures of GCs (corticosterone (cort) in birds) and T across different life-history stages in a free-living songbird, the mountain white-crowned sparrow (MWCS; Zonotrichia leucophrys oriantha) to answer two major questions: 1) which individual and environmental factors explain the patterns of differences among individuals given variability within individuals and 2) whether the level of hormone variation (plasma cort, feather cort, and plasma T) is related to environmental variation and nesting success. We used three different methods to characterize inter- and intra-individual variation in hormone levels: 1) inter-individual variation was measured to assess absolute hormone concentration repeatability at the population level (Lessells and Boag, 1987) for baseline cort (males and females), stress-induced cort (males), and T (males); 2) PR scores were calculated to measure intra-individual variance in the shape of male stress responses (Reed et al 2019); and 3) inter- and intra-individual plasticity was measured for male baseline and stress-induced cort using a reaction norm approach (Araya-Ajoy et al., 2015). Due to the flexibility of hormone concentrations to respond to environmental variation, we expected to find low repeatability and high intra-individual variation using any of these approaches. We used individual PR scores to test whether flexibility in cort levels related to nesting success. We predicted that more flexible males would have higher nesting success, as these males are able to suppress their stress-induced cort levels during chick-rearing but not necessarily during territory establishment, decreasing the likelihood of nest abandonment (Ouyang et al., 2012). We predicted that these males may hold more centralized territories and have higher nesting success.
2. Materials and Methods
2.1. Field methods
Data were collected from May to July 2018 in Tioga Pass Meadow, Mono County, CA, USA (37.91°N, −119.25°W). The population of MWCSs has been monitored at this field site since 1968 (Morton, 2002; Crino et al., 2011; Malisch et al., 2018). Weather conditions ranged from complete snow cover with temperatures below 0° C (early May) to no snow cover with temperatures closer to 20° C (early July; Sup. Fig. 1). Male MWCSs arrive on the breeding grounds in early May and begin establishing territories while females arrive an average of 9 days later (Morton, 2002). MWCSs are socially monogamous and mate-pairing begins shortly after females arrive. We caught male and female MWCSs across the breeding season using two-compartment potter traps baited with millet. Trapping occurred daily, beginning at 6:00 AM and ending when birds stopped entering traps, or around 12:00 PM. Traps were monitored and checked every 20 minutes. Previous research on the use of potter traps and baseline cort in white-crowned sparrows indicated that 15 minutes in a trap does not alter baseline or stress-induced cort (Romero and Romero, 2002), however, this not true for Lapland-longspurs (Calcarius lapponicus; Romero and Romero, 2002) or house sparrows (Passer domesticus; Lynn and Porter, 2008). Morphological measurements (mass and tarsus) were collected at every capture. To gain a better understanding of how the stress response varies across the breeding season for male MWCSs, a stress series was collected during territory establishment, mate-pairing, and egg/chick rearing for a maximum of 3 stress series per individual (see Sup. Fig. 2 for a breakdown of life-history stages and blood sampling during data collection). The territory establishment stage began when data collection started. The mate-pairing stage began when individual pairs were seen together (late May) and the egg/chick rearing stage began when nests were found with eggs (early June). We measured adult mass to the nearest 0.5 g and tarsus to the nearest 0.5 mm and calculated body condition using a scaled mass index (Peig and Green, 2009). We collected the outermost rectrix during the first capture of each individual to measure feather cort concentrations. All adult MWCSs were identified with a unique code on an aluminum leg band, in addition to colored leg bands for behavioral observations. For each individual, a blood sample (~100ul) was taken from the brachial vein within 3 minutes of capture (mean +/− SE: 2.35 ± 0.3), as well as 15 (mean +/− SE: 14.97 ± 0.5) and 30 (mean +/− SE: 29.88 ± 0.5) minutes after initial capture, following a standardized stress series protocol (Romero and Reed, 2005). Briefly, birds were placed in opaque bags to simulate capture and restraint stress. Blood samples were placed on ice until centrifuged at 15,300 g for 5 minutes within 5 hours of collection and placed into a −80° C freezer for later hormone analyses. T samples were only collected from baseline male blood samples. A stress series was collected on all captured MWCSs from May to June unless a blood sample had been collected from that individual within the past 14 days. Due to the critical need of females to attend their nests, we did not collect a full stress series from females once they began laying. If a female was caught during this stage, only a baseline blood sample was collected (see Table 1 for sample sizes).
Table 1. Summary of sample sizes.
Sample sizes are separated by hormone and subsequently separated by sex. Numbers outside of parentheses indicate total number of blood samples collected. Numbers inside parentheses indicate unique individuals from which these samples were collected. For example, 55 baseline corticosterone measurements were collected from 23 individual male mountain white-crowned sparrows, Zonotrichia leucophrys oriantha.
| Hormone | Males (n) | Females (n) |
|---|---|---|
| Baseline corticosterone | 53 (21) | 47 (18) |
| Stress-induced corticosterone | 53 (21) | 16 (13) |
| Testosterone | 45 (21) | 0 |
When males arrived on the breeding grounds in early May, we deployed 17 Advanced Telemetry Systems radio transmitters (< 0.5 g; Model A2455; Pulse width 16ms; Pulse rate 60ppm) during the first capture of males to map home-range size. Using epoxy, we glued an elastic string in the shape of a figure- eight to the bottom side of each transmitter. The transmitter was placed on the bird’s upper back and the loops of the string were hooked around each leg acting as a harness for a secure fit that would not impair the bird’s movement. Male MWCSs generally establish territories near their previous years’ territory. Younger males arrive later, settling in the free areas. Territories are firmly set soon after arrival to the breeding grounds (Morton, 2002). We used handheld radio telemetry (Communications Specialist Inc. Model R1000 with a Yagi antenna) to locate males and collected a GPS point (handheld Garmin GPSMAP 62st: 3m accuracy) where the male was spotted (Chandler et al., 1994). We practiced caution in tracking males as to not chase the individual and overestimate home-range size. A minimum of 5 GPS points were collected per individual (mean +/− SE: 12.35 ± 1.84). Transmitters were removed from birds at the end of data collection.
Weather conditions strongly impact the start of nest building and egg laying. Female MWCSs lay between 2–6 eggs with a mode of 4 (Morton, 2002). We began nest searching when MWCSs were seen collecting grasses and twigs and mate pairs started to form, which, due to low snowpack, started in late May. We walked line transects across the breeding grounds as close together as the vegetation would allow to flush females. Areas with vegetation too thick to walk through were monitored visually with binoculars. When nests were located, we recorded the number of eggs and marked the location with a GPS point. MWCS nests are heavily depredated by corvids and ground squirrels, so time spent at nests was kept to a minimum (<1 min). Nests were monitored daily to count the number of eggs, hatchlings, and/or fledglings. Nests were monitored until all chicks had fledged or the nest had been abandoned or depredated. A nest was deemed successful if at least one of the young fledged. We monitored MWCS pairs daily to accurately identify which male and female belonged to which nest.
2.2. Hormone assays
2.2.1. Plasma corticosterone
Plasma cort levels for male and female MWCSs were measured using an enzyme immunoassay kit (Cat. No. ADI-901–097; Enzo Life Sciences, Inc., Farmingdale, NY, USA) following standard assay protocols as modified for white-crowned sparrows (Wada et al., 2007). We used 1% steroid displacement reagent for the purpose of freeing cort bound to corticosteroid-binding globulin (CBG). Samples were diluted 1:40 in assay buffer. Samples were run in duplicate and randomly placed across the plate with the exception of keeping all samples per individual on the same plate. The intra-plate coefficient of variation was 3.55% and the inter-plate coefficient of variation was 8.30% (n=7 plates). Our levels are consistent with other published studies on this species.
2.2.2. Feather corticosterone
We used feather cort as a long-term hormone measure, compared to the short-term measurement of plasma cort. To validate a feather assay for male and female MWCS rectrices, we followed the general protocol of Lattin et al. (2011) and Bortolotti et al. (2008). We ran samples diluted by 1:300, 1:600, and 1:900. Parallel curves were generated and a dilution of 1:300 was selected due to this dilution having the highest level of parallelism. Before hormone analysis, feathers were weighed to the nearest 0.0001 g and measured to the nearest 0.5 mm before and after the calamus was removed and discarded (feathers: n=28). Feathers were then cut into small pieces (<5 mm) and placed in scintillation vials with 7 mL of methanol. All vials were placed in a sonicating water bath at room temperature for 30 minutes. Directly after, the vials were placed in a shaking water bath at 50° C overnight. We used vacuum filtration to filtrate all samples with two rinses of 2 mL of methanol. We used a vented air system to dry the methanol under 50° C. Samples were then reconstituted in 300 μl of assay buffer overnight (300 μl tested from 100, 600, and 900 μl resulted in the best sample fit along a standard curve). For hormone analysis, we used an enzyme immunoassay kit (Cat. No. 80–0045; Enzo Life Sciences, Inc., Farmingdale, NY, USA), following the manufacturer’s instructions and ran all samples on a single plate in duplicate. To validate this assay, we verified that a serially diluted sample was parallel with the cort standard curve. Intra-plate coefficient of variation was calculated from duplicated controls that were taken through the entire assay process: 9.37%. Cort values were then standardized by feather length to account for variation in cort deposition during growth (Bortolotti et al., 2009).
2.2.3. Testosterone
We quantified plasma testosterone for male MWCSs from baseline blood samples using Salimetrics (State College, PA) salivary testosterone kit (#1–2402) following the validation protocol for avian plasma (Washburn et al., 2007). We only measured T concentrations of males as this was the most relevant for our study. In brief, the slope of serial dilutions of MWCS plasma did not differ from the slope of the standard curve (ANOVA: F1, 8=0.097, p=0.76) and the optimal plasma dilution was determined to be 1:10. Plasma samples were run on one plate in duplicate and the intra-assay coefficient of variation was 4.03% and plate sensitivity was 1 pg/mL.
2.3. Statistical analyses
All analyses were conducted in R v3.6 (R Core Team, 2018). Two female cort samples were marked as statistical outliers (greater than 3 standard deviations from the mean) and were removed from all analyses. We kept one male cort sample in the PR analysis (even though it was marked as a statistical outlier) because the metric does not use exact measures of cort concentrations, but instead measures the shape of the stress response. Removing this male from analyses did not change our conclusions. One male testosterone sample was marked as a statistical outlier and was removed from all analyses. Stress-induced cort was measured from blood samples collected 30 minutes after capture. Samples collected 15 minutes after capture were only included in PR and reaction norm analyses.
2.3.1. Repeatability: Inter-individual
We used the R package “rptR” v0.9.21 (Stoffel et al., 2017) to measure intra-class correlation coefficients to calculate cort and T repeatability at the population level. We calculated the R score (repeatability score) for male and female baseline cort, male stress-induced cort, and male T concentrations, separately. Female stress-induced cort repeatability was not measured due to a lack of repeated individual samples. We used a gaussian distribution and grouped by individual. Parametric bootstrapping was used to obtain 95% confidence intervals for our population repeatability estimates (nboot=1500).
2.3.2. Profile repeatability: Intra-individual
We measured PR scores for 18 individual male MWCSs following methods created by Reed et al. (2019; Appendix S1). We were able to collect a stress series within each life-history stage (territory establishment, mate-pairing, and egg/chick rearing; Sup. Fig. 2) for a total of 3 stress series per individual for 13 males. We also included 5 males with only 2 stress series collected from 2 of the 3 life-history stages. Reed et al. (2019) found that removing one stress series from an individual had little effect on that individual’s PR score. To test if this finding was true for a free-living species, we experimentally removed from the 13 males with 3 stress series 1) the first stress series (collected during territory establishment), 2) the second stress series (collected during mate-pairing), 3) the third stress series (collected during egg/chick rearing), and 4) randomly removing one stress series, and re-calculated PR scores.
2.3.3. Reaction norm repeatability: Inter- and intra-individual
To measure the level of repeatability of the slope and intercept of the stress response during three different life-history stages, we used a reaction norm approach following Araya-Ajoy et al. (2015). Only males with 3 full stress series were included in this analysis (n=13, series=39). We used a multi-level random regression mixed-effect model to quantify variation in reaction norm intercepts and slopes within and among individual males (see supplementary materials for model structure). Cort levels were modelled as a function of the stress response over time (0, 15, 30 mins after capture) in interaction with life-history stage, which was fitted as an environmental covariate with three levels (territory establishment, mate-pairing, and egg/chick rearing). Random intercepts were included for individual and series; random slopes with respect to time were also included at two hierarchical levels. We fitted the random regression model using a Bayesian framework implemented with the package “MCMCglmm” (Hadfield, 2010). We ran 3,003,000 iterations per model, from which we discarded the initial 3000 (burn in period). Each chain was sampled at an interval of 3000 iterations. Repeatabilities of slope and intercept were calculated using equation 3 from Araya-Ajoy et al. (2015). Posterior means and 95% credible intervals were estimated across the thinned samples for the mean effects (fixed effects), (co)variances, and repeatabilites.
2.3.4. Corticosterone and testosterone
We used a linear mixed model to test for a relationship between T and baseline cort levels. T was used as the dependent variable and baseline cort and body condition were included as covariates. Individual ID was included as a random effect to account for repeated measures. We only used baseline cort levels in this model as T samples were collected from baseline cort blood samples.
2.3.5. Hormones and home-range
We used GPS points to estimate male home-range size (m2; n=17). Home-ranges were calculated as the 95% kernel utilization distribution (UD), a method which summarizes an individual’s locations in a 2-dimensional probability space (Worton, 1989). Kernel UD was estimated using the R package “adehabitatHR” v0.4.15 (Calenge, 2006). We used a linear model to test whether territory size was related to male pre-breeding cort (baseline or stress-induced) or T concentrations. We focused on male pre-breeding hormone levels as those are the most relevant during territory establishment. Body condition collected during territory establishment was used as a covariate. To test for a pattern of cort (baseline or stress-induced) and T distribution across home-ranges, we used Moran’s I test (R package “spdep” v1.0– 2; Bivand and Wong, 2018). Moran’s I is a measure of spatial autocorrelation (Cliff and Ord, 1973), which compares hormone levels of each territorial male with adjacent males within a specified distance. In this analysis, we interactively ran Moran’s I tests by specifying different distances (from 140m and 400m) to progressively include more neighboring males in the comparison.
2.3.6. Hormones and nesting success
To test for a difference in the stress response between male and females during the pre-breeding season, we used separate Welch’s t-tests for samples collected at 0, 15, and 30 minutes after capture. To measure reproductive success, we used nesting success (proportion of young fledged over total young hatched) as our metric as there was low variation in the number of fledglings (>75% had 4 young). We ran separate models for each sex, as we collected fewer hormone measures for females than males to minimize nest desertion and home-range size was measured for territory-establishing males. We ran generalized linear mixed models (GLMMs) with a binomial distribution to test if male nesting success was related to absolute baseline and stress-induced cort and T concentrations with an interaction of life- history stage, feather cort, PR scores, and home-range size with body condition as a covariate. We ran GLMMs with a binomial distribution to test if female nesting success was related to absolute baseline and stress-induced cort concentrations with an interaction of life-history stage and feather cort with body condition as a covariate. Individual ID was included as a random effect in all models to account for repeated measures. Our set of candidate models included all variables described above, and we evaluated combinations of all parameters using the corrected Akaike information criterion (AICc) to avoid overparameterization (Burnham and Anderson, 2002). We found no issues of collinearity between all parameters, which we checked by creating a correlation matrix (r<0.5 for all pairwise comparisons).
3. Results
3.1. Repeatability: Inter-individual
Stress-induced cort was repeatable for male MWCSs (R=0.43, p=0.002; Table 2). Male and female baseline cort and male T concentrations were not repeatable (Table 2).
Table 2. Repeatability of absolute values of hormone levels in mountain white crowned sparrows, Zonotrichia leucophrys oriantha.
Repeatability estimates were calculated using intra-class correlation coefficients with parametric bootstrapping to obtain 95% confidence intervals. Significant p-values are in bold.
| Hormone | Sex | R (CI) | P-value |
|---|---|---|---|
| Baseline corticosterone | Male | 0.06 (0, 0.33) | 0.90 |
| Female | 0.13 (0, 0.34) | 0.20 | |
| Stress-induced corticosterone | Male | 0.43 (0.08, 0.68) | 0.002 |
| Testosterone | Male | 0.12 (0, 0.49) | 0.27 |
3.2. Profile Repeatability: Intra-individual
PR scores range between 0 (no consistency) and 1 (high consistency). There was high variation in the 18 PR scores (PR range: 0.0001 – 0.99; Sup. Table 1). Average PR score was higher for males with only 2 stress series (Sup. Table 1). We removed one stress series from each male that had 3 stress series at different life-history stages to see how PR scores and rank would change. Average PR scores increased slightly from the original average PR score (0.18 ± 0.34) in all cases (see section 2.3.2); 1.) 0.25 ± 0.20, 2.) 0.36 ± 0.26, 3.) 0.21 ± 0.17, and 4.) 0.28 ± 0.22. However, rank order significantly changed (Sup. Table 2).
Due to the contrasting results from the group with 3 stress series compared to the group with 2 stress series, we separated the groups for all further analyses. Out of the 13 individuals measured with 3 stress series, 3 individuals showed high PR scores (>0.56; high consistency), while the remaining 10 scores showed low consistency (<0.06). For example, the individual with the highest intra-individual consistency in their stress response had a PR score of 0.86 whereas the individual with the lowest consistency had a PR score of <0.0001 (Fig. 1). For the individuals in the 2 stress series group, 4 out of 5 males showed high PR scores (>0.61).
3.3. Reaction norm repeatability: Inter- and intra-individual
Cort levels positively correlated with time since capture (Table 3). The interaction of time and life-history stage was also significant: the reaction norm of territory establishment and mate-pairing in relation to time since capture had a steeper slope than egg/chick rearing (Table 3). This indicates that males had a lower stress response to capture and restraint during egg/chick rearing than during territory establishment and mate-pairing. We found large credible intervals for repeatability in both slope (R=0.50) and intercept (R=0.35) of the stress response over different life-history stages (Table 3), suggesting low confidence in repeatability.
Table 3. Variation in individual corticosterone concentration as a function of life-history stage and time.
We used a MCMCglmm with random intercepts and slopes (with respect to life-history stage*time) at the level of the individual and series within individual. Variance in intercepts are printed with subscript ‘0’, variances in slopes with subscript ‘1’ for the among-individual (‘ind’), among-series (‘series’) levels; intercept-slope covariances (‘cov’) are presented at each level. Reference level for life-history stage is chick rearing. All values are reported as mean with 95% credible intervals.
| Model Output | β (95% CI) | P-Value | ||
| Fixed effects | Intercept | 36.99 (27.32, 47.30) | < 0.001 | |
| Time | 38.72 (26.34, 50.17) | < 0.001 | ||
| Life-history stage (Mate-pairing) | 6.55 (−5.17, 18.46) | 0.26 | ||
| Life-history stage (Territory estab.) | 4.33 (−7.20, 15.46) | 0.45 | ||
| Time × Life-history stage (Mate-pairing) | 21.72 (7.38, 37.92) | 0.01 | ||
| Time × Life-history stage (Territory estab.) | 16.89 (7.38, 37.92) | 0.02 | ||
| Random effects | σ2 (95% CI) | |||
| Among individuals | Vind0 | 103.84 (0.001, 272.6) | ||
| Vind1 | 86.11 (0.001, 254.3) | |||
| Cov ind0, ind1 | 55.75 (−18.17, 191.3) | |||
| Within-individuals among-series | Vseries0 | 177.30 (58.9, 329.9) | ||
| Vseries1 | 86.2 (0.001, 250.3) | |||
| Cov series0, series1 | 57.1 (−31.0, 188.9) | |||
| Residuals | 153.1 (87.09, 205.4) | |||
| Repeatability | Intercept | 0.35 (0.001, 0.69) | ||
| Slope | 0.50 (0.001, 0.99) | |||
3.4. Corticosterone and testosterone
We found significant intra-individual correlations between T and cort: when individual males had higher levels of baseline cort, their T concentration was significantly lower (coef: −0.66, SE: 0.17, p=0.0005; Sup. Fig. 3).
3.5. Hormones and home-range
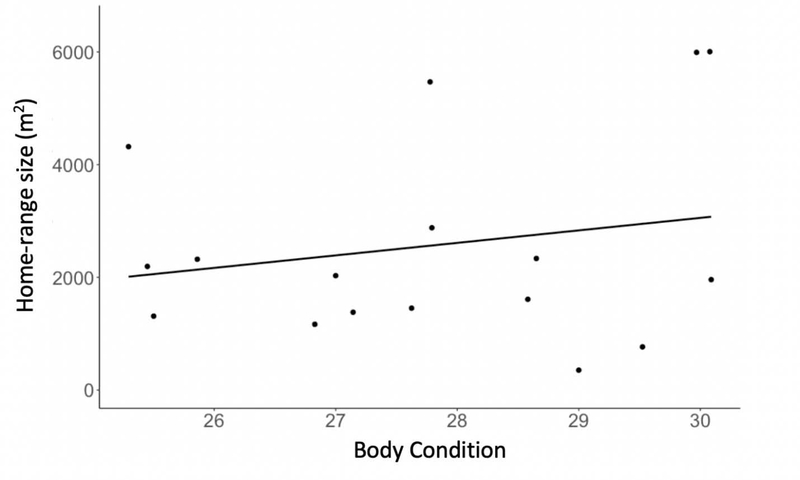
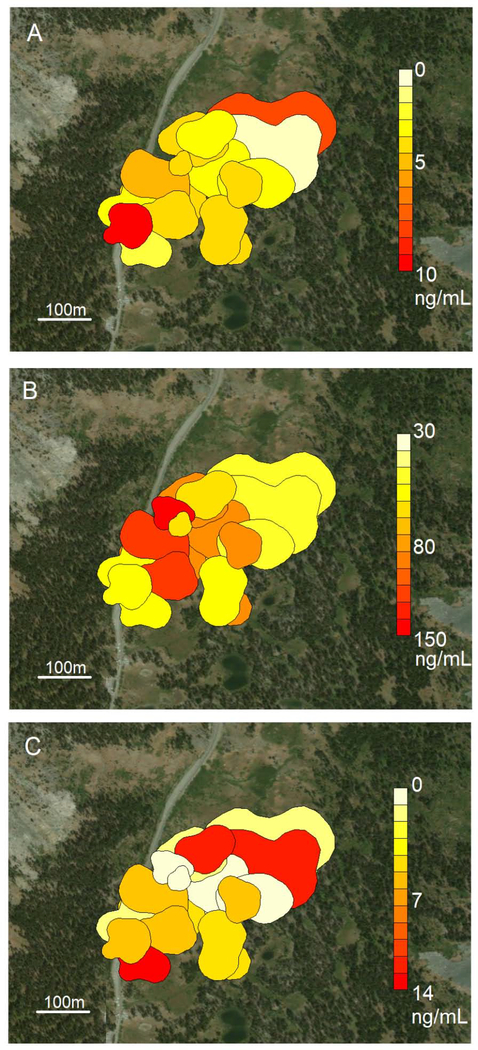
We did not find a significant relationship between male cort or T concentrations and home-range size (baseline: F2, 19=2.54, p=0.51; stress-induced: F2, 19=3.37, p=0.20; T: F2, 34=1.36, p=0.27). We found that males with larger home-ranges had better body condition (F1, 20=4.76, p=0.04; Fig. 2). With respect to baseline cort levels and T, male home-ranges were randomly distributed across the breeding grounds (Fig. 3A and C), as we found non-significant results at all distances from Moran’s I test (Sup. Fig. 4). For stress-induced cort, we found that male home-ranges were nonrandomly distributed within 225–250m of each other, with males showing higher levels of stress-induced cort clustered together in more central home-ranges (Fig. 3B). On average, each male had 10.35 neighbors within 240m (Fig. 3). As we measured home-range size, which are larger and more flexible than territories, there is a higher rate of overlap between neighbors.
Figure 2. Home-range size and body condition.
Male mountain white-crowned sparrows in better pre-breeding body condition had larger home-ranges (p=0.041).
Figure 3. Mountain white-crowned sparrow hormone home-range maps.
Spatial distribution of male mountain white-crowned sparrow home-ranges with baseline corticosterone (A), stress-induced corticosterone (B), and testosterone levels (C).
3.6. Hormones and nesting success
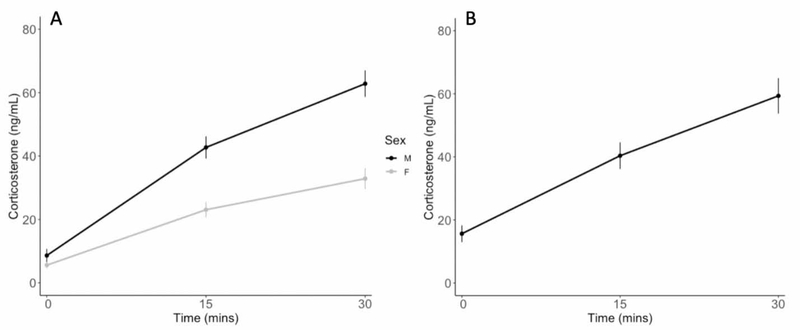
We found a significant difference between average male and females stress responses during the pre-breeding life-history stage in that females had a lower stress response compared to males (Welch’s T- test: 0 mins: t(49.735)=−1.373, p=0.176; 15 mins: t(48.693)=−4.7232, p=<0.0001; 30 mins: t(163.88)=6.007, p=<0.0001; Fig. 4). Of the 18 nests we found, 8 were successful and 10 failed (due to desertion or depredation). Male and female nesting success was not explained by any predictor variables. The top model for both males and females was an intercept only model (Sup. Table 3; Sup. Table 4).
Figure 4. Mountain white-crowned sparrow average corticosterone levels across life-history stages.
Average male and female mountain white-crowned sparrow stress response during the pre-breeding life- history stage (A) and average male stress response during the breeding life-history stage (B). Plotted are means ± 95% confidence intervals.
4. Discussion
This study uses a novel dataset with repeated within-individual hormone samples and home-range size to predict nesting success in a free-living songbird. Although absolute concentrations of male stress- induced cort levels were repeatable at the population level, PR scores and reaction norm analyses revealed low intra-individual consistency and low repeatability in the shape of the stress response, respectively. Males were non-randomly distributed across territories with individual males having higher concentrations of stress-induced cort clustered in central territories. We found no evidence that cort (baseline, stress-induced, PR scores, or feather) or T concentrations were related to an individual’s nesting success in males or females.
4.1. Repeatability
The lack of repeatability in baseline cort and T levels is in accordance with other studies (e.g., Baugh et al., 2014; Pavitt et al., 2015). One reason for the lack of repeatability in baseline cort levels is the relationship between baseline cort and metabolic functions (Sapolsky et al., 2000), which have strong temporal variations, as well as variations within life-history stage (Cockrem et al., 2017). Both internal and external conditions impact an individual’s level of baseline cort, such as individual quality (Husak and Moore, 2008), adding natural variation at any given time (Rensel and Schoech, 2011). Natural variation in T due to social factors (dominance and aggressive interactions during territory establishment) likely also account for the low repeatability in T concentrations (While et al., 2010; Pavitt et al., 2015; Fanson and Biro, 2019). Stress-induced cort, however, is less variable, as it is a response to a standardized stressor. In line with current literature (Rensel and Schoech, 2011; Holtmann et al., 2017; Schoenemann and Bonier, 2018; Taff et al., 2018), we found repeatability in male stress-induced cort levels. Given that stress-induced cort is a proxy for the maximum level of cort that an individual secretes in response to a stressor (Rensel and Schoech, 2011), finding repeatability among individuals is more common compared to baseline concentrations.
PR scores may be able to address the uncertainties of measuring the stress response, i.e., subtracting baseline from stress-induced or calculating the area under the curve, as PR scores encompass cross-over events and the shape of the response. Although we found absolute concentrations of stress- induced cort in males to be repeatable, we found a wide range of PR scores with most individuals (10 of 13) with 3 stress series showing a low level of intra-individual consistency and most individuals (4 of 5) with 2 stress series showing a high level of intra-individual consistency (Sup. Table 1). These results indicate that a single measurement of a stress response in this population will not accurately represent the cort profile of an individual across different life-history stages. Our results indicate that either the PR metric cannot holistically assess intra-individual variance or the relatedness among an individual’s stress responses are highly variable.
This is the first study to use the PR metric on a free-living organism. Reed et al. (2019) tested the metric on captive European starlings (Sturnus vulgaris) and found high intra-individual consistency with an average PR score of 0.84. In their dataset, removing one stress series did not change PR scores; however, in our population, removing one stress series from each individual male significantly changed the PR score ranks for individuals (Sup. Table 2). This finding, combined with the large credible intervals for repeatability in the reaction norm intercept and slope, suggest that individual stress response profiles are not consistent. The population of MWCSs used for this study live in a highly variable environment, experiencing a wide range of temperatures across seasons (Sup. Fig. 1). Additionally, as this population borders Yosemite National Park, human traffic is high, which has been shown to impact GC levels (Thiel et al., 2008). In a study by Addis et al. (2011), high elevation white-crowned sparrows had a different stress response compared to low elevation white-crowned sparrows, which they propose may be due to the increase in phenotypic variation in response to novel environments (Holway and Suarez, 1999). Therefore, the variability in the environment of the MWCS population in Tioga Pass is likely a significant factor in their overall low levels of intra-individual consistency in the stress response.
4.2. Corticosterone and testosterone
Studies relating baseline cort and T concentrations show negative, positive, and no relationships (Ketterson et al., 1991; Wikelski et al., 1999; Hau et al., 2010; Li et al., 2017; Zito et al., 2017). The inconsistent correlations between these hormones could be due to differences across life-history stages (Deviche et al., 2014). We had multiple samples of the same individual across different life-history stages and our results indicate that within individuals, there is a negative relationship between T and baseline cort across time. This finding indicates that the relationship between these two hormones may only be evident when taking within-individual differences into account and why there is not a clear relationship among individuals with one-time sampling. We did not assess T after the standard capture and restraint, so the relationship between these hormones will likely differ after exposure to a stressor (Deviche et al., 2014). Deviche et al. note that because not all stressors elicit an identical relationship between cort and T, this is not a direct relationship but one mediated by a suite of physiological mechanisms (2014).
4.3. Spatial relationships
To our knowledge, this is the first time MWCS home-range size and distributions have been measured and mapped; however, male MWCSs generally return to the same or similar territory locations between years (Morton, 2002). Our results suggest that there is a non-random pattern in the spatial distribution of individuals and their cort phenotypes in territorial male MWCSs. As this is a correlative study, we were unable to test if cort levels determined home-range location or, rather, home-range location affected an individual’s cort phenotype. Habitat composition and nest site availability likely create competition for better nesting areas (Merilä and Wiggins, 1995; Strubbe and Matthysen, 2009) and may select for spatial patterning of individuals with different physiological profiles. Viblanc et al. (2014) found that king penguins with peripheral territories (few neighbors) had lower baseline cort levels than individuals with central territories (surrounded by neighbors). In MWCSs, males with higher stress- induced cort levels inhabited centralized home-ranges. Higher stress-induced cort concentrations in central home-ranges may be due to a higher rate of intraspecific interactions (Côté, 2000). Although, assumedly this would increase concentrations of T as well (Wingfield and Wada, 1989; Wingfield et al., 1990), but individual males were randomly distributed in space in regard to T. One reason for this difference may be due to T levels decreasing after a conspecific interaction too quickly for our study to have detected a significant spatial distribution of individuals and their respective T concentrations. We also point out that a colonial nesting seabird may have different territory establishment rules and different social interactions between breeding pairs than territorial temperate songbirds. Measuring territory quality in addition to size and location may give a more accurate representation of environmental factors affecting hormone levels and fitness (Both and Visser, 2000; Meadows, 2001; Steury and Murray, 2003; Machín et al., 2018).
4.4. Hormones and nesting success
In line with previous research (Wingfield et al., 1982; Wingfield et al., 1994), we found that female MWCSs had a suppressed stress response compared to males during the pre-breeding life-history stage (Fig. 4). Since female MWCSs usually provide more parental care than males (Morton, 2002), our finding corroborates with other studies indicating that the parent that gives the most parental care tends to have a more dampened stress response (Wingfield et al., 1995; O’Reilly and Wingfield, 2001, Bókony et al., 2009). Moreover, males had a lower stress response during chick-rearing than other life-history stages, which also supports the hypothesis that the stress response is dampened during breeding to minimize desertion and increase parental care (O’Reilly and Wingfield, 2001).
We found no relationship between hormone levels (baseline cort, stress-induced cort, feather cort, or T) and nesting success, even with repeated within-individual hormone measurements. This suggests that nesting success is either unrelated to an individual’s hormone phenotype or this relationship is highly complex, with many physiological and environmental components playing species-specific and context- dependent roles. Our data may also lack the statistical power needed to detect relationships among these variables, or female stress responses during breeding may be more important for nesting success than those of males (we did not collect multiple stress series from females to minimize nest desertion). After collecting three stress series per individual across different life-history stages, we found that male PR scores did not predict nesting success. The natural variability in baseline cort (e.g., Breuner et al., 1999) and the wide range of PR scores we found are likely why there is inconsistency between GC levels and fitness (Bonier et al., 2009a). Although studies have found that high GC plasticity is related to increased reproductive success (Bonier et al., 2009b; Ouyang et al., 2013), our study cautions that more data should be collected across seasons (Hau and Goymann, 2015). As for feather cort, the environmental conditions an individual experiences during pre-breeding and breeding likely have a greater impact on the success of a nest compared to cort levels during the time of feather growth (Boves et al., 2016). Nesting success in male MWCSs was also not related to T concentrations. In other studies of T and fitness, reproductive success was measured with respect to extra-pair paternity (Reed et al., 2006), and it is possible that MWCSs nesting success is affected by extra-pair possibilities (Sherman and Morton, 1988; Morton, 2002).
It is important to note a critical difference between individuals that experience nest depredation compared to nest abandonment. Out of the 10 failed nests we recorded, 5 were abandoned and 5 were depredated and due to low sample size, we could not separate them in the analyses. Nests are abandoned primarily due to perceived risk of immediate survival, e.g., extreme weather conditions (Wingfield et al., 1983) or increased predation risk (Beckmann and Martin, 2016). A breeding individual that is unable to or does not suppress their stress response, and therefore has a more elevated response to stressors, is more likely to abandon a nesting attempt (Wingfield and Sapolsky, 2003; Ouyang et al., 2012; Thierry et al., 2013). Nest abandonment can be detrimental to fitness; however, it can be advantageous if an individual is able to perceive a failed nesting attempt and re-nest (Morton, 2002). Nest depredation does not directly interfere with the survival of a parent unlike inclement weather or a predator, leading to a difference in the way physiological responses are mediated (Wingfield, 2003).
4.5. Conclusion
Although absolute concentrations of male stress-induced cort is repeatable at the population level, we provide evidence that the stress response is highly plastic in a population of MWCSs. We encourage further studies with repeated measures of the cort phenotype in free-living species to test the generality of this pattern and the use of PR as a metric for intra-individual variation of the stress response. Spatial distribution of individuals and their physiological traits can reveal non-random patterns of home- range/territory establishment and is an interesting avenue of research linking spatial and temporal variation of individuals and their hormone levels. Longer-term studies (i.e., the same individual breeding in different years with varying environmental factors, such as habitat quality) may disentangle the relationship between hormones and fitness. By gaining a better understanding of these relationships, we can identify how selection is acting on hormone expression, when and how the stress response promotes individual survival, and how habitat alterations can affect fitness in free-living organisms.
Supplementary Material
Highlights.
Male stress-induced corticosterone was repeatable at the population level
Male stress-response profiles were highly variable
Males with higher body condition had larger home-ranges
Males with higher levels of testosterone had lower levels of baseline corticosterone
Hormones and home-range size did not relate to nesting success
Acknowledgments
We thank three anonymous reviewers and the associate editor for helpful comments on the manuscript. We thank E. Johnson, A. Chrisler, D. Capasso, M. Clapp, and B. Malisch for help in the field. Thank you to UNR’s Evol Doers lab group, particularly J. Jahner, for advice on analyses and K. Nussear for his help on the spatial analysis. Thanks to the Mono Lake Committee for logistic support and to The Roberts Environmental Center of Claremont McKenna College for the use of their Eastern Sierra Research Station. JLM is funded by the American Association of University Women. MJK received support from the Flores Award administered by St. Mary’s College of Maryland’s Department of Biology. JQO is funded by the National Institute of Health (P20 GM103650).
Footnotes
Declaration of Interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addis EA, Davis JE, Miner EM, Wingfield JC 2011. Variation in circulating corticosterone levels is associated with altitudinal range expansion in a passerine bird. Oecologia. 167, 369–378. 10.1007/s00442-011-2001-5. [DOI] [PubMed] [Google Scholar]
- Adkins-Regan E 2005. Hormones and Animal Social Behavior. Princeton University Press. [Google Scholar]
- Apfelbeck B, Flinks H, Goymann W 2016. Variation in circulating testosterone during mating predicts reproductive success in a wild songbird. Front. Ecol. Evol 4,107 10.3389/fevo.2016.00107. [DOI] [Google Scholar]
- Apfelbeck B, Mortega KG, Flinks H, Illera JC, Helm B 2017. Testosterone, territorial response, and song in seasonally breeding tropical and temperate stonechats. BMC Evol. Biol 17, 101 10.1186/s12862-017-0944-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya-Ajoy YG, Mathot KJ, Dingemanse NJ 2015. An approach to estimate short-term, long-term and reaction norm repeatability. Methods Ecol. Evol 6(12), 1462–1473. 10.1111/2041-210X.12430 [DOI] [Google Scholar]
- Baird TA, Hews DK 2007. Hormone levels in territorial and non-territorial male collared lizards. Phys. Behav 92(4), 755–763. 10.1016/j.physbeh.2007.05.069 [DOI] [PubMed] [Google Scholar]
- Baugh AT, van Oers K, Dingemanse NJ, Hau M 2014. Baseline and stress-induced glucocorticoid concentrations are not repeatable but covary within individual great tits (Parus major). Gen. Comp. Endocrinol 208, 154–163. 10.1016/J.YGCEN.2014.08.014. [DOI] [PubMed] [Google Scholar]
- Beckmann C, Martin K 2016. Testing hypotheses about the function of repeated nest abandonment as a life history strategy in a passerine bird. Ibis. 158, 335–342. 10.1111/ibi.12361. [DOI] [Google Scholar]
- Bivand RS, Wong DWS 2018. Comparing implementations of global and local indicators of spatial association. TEST. 27, 716–748. 10.1007/s11749-018-0599-x. [DOI] [Google Scholar]
- Bókony V, Lendvai Z, Liker A, Angelier F, Wingfield JC, Chastel O 2009. Stress response and the value of reproduction: Are birds prudent parents? Am. Nat 173, 589–598. 10.1086/597610. [DOI] [PubMed] [Google Scholar]
- Bonier F, Quigley H, Austad S 2004. A Technique for Non-Invasively Detecting Stress Response in Cougars. Wildlife Society Bulletin (1973–2006), 32(3), 711–717 [Google Scholar]
- Bonier F, Martin PR 2016. How can we estimate natural selection on endocrine traits? Lessons from evolutionary biology. Proc. R. Soc. B 283, 20161887 10.1098/rspb.2016.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonier F, Martin PR, Moore IT, Wingfield JC 2009a. Do baseline glucocorticoids predict fitness? Trends Ecol. Evol 24, 634–642. 10.1016/j.tree.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Bonier F, Moore IT, Martin PR, Robertson RJ 2009b. The relationship between fitness and baseline glucocorticoids in a passerine bird. Gen. Comp. Endocrinol 163, 208–213. 10.1016/J.YGCEN.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Bortolotti GR, Marchant T, Blas J, Cabezas S 2009. Tracking stress: localisation, deposition and stability of corticosterone in feathers. J. Exp. Biol 212, 1477–1482. 10.1242/jeb.022152 [DOI] [PubMed] [Google Scholar]
- Bortolotti GR, Marchant TA, Blas J, German T 2008. Corticosterone in feathers is a long-term, integrated measure of avian stress physiology. Funct. Ecol 22, 494–500. 10.1111/j.1365-2435.2008.01387.x. [DOI] [Google Scholar]
- Both C, Visser ME 2000. Breeding territory size affects fitness: An experimental study on competition at the individual level. J. Anim. Ecol 69, 1021–1030. 10.1111/j.1365-2656.2000.00458.x. [DOI] [Google Scholar]
- Boves TJ, Fairhurst GD, Rushing CS, Buehler DA 2016. Feather corticosterone levels are related to age and future body condition, but not to subsequent fitness, in a declining migratory songbird. Conserv. Physiol 4, cow041 10.1093/conphys/cow041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuner CW, Greenberg AL, Wingfield JC 1998. Noninvasive corticosterone treatment rapidly increases activity in gambel’s white-crowned sparrows (Zonotrichia leucophrys gambelii). Gen. Comp. Endocrinol 111, 386–394. 10.1006/gcen.1998.7128. [DOI] [PubMed] [Google Scholar]
- Breuner CW, Wingfield JC, Romero ML 1999. Diel rhythms of basal and stress-induced corticosterone in a wild, seasonal vertebrate, Gambel’s white-crowned sparrow. J. Exp. Zool 284, 334–342. . [DOI] [PubMed] [Google Scholar]
- Brischoux F, Lourdais O, Boissinot A, Angelier F 2018. Influence of temperature, size and confinement on testosterone and corticosterone levels in breeding male spined toads (Bufo spinosus). Gen. Comp. Endocrinol 269, 75–80. 10.1016/j.ygcen.2018.08.017. [DOI] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR 2002. Model Selection and Inference: A Practical Information-Theoretic Approach. 2nd Ed. Springer-Verlag, New York. [Google Scholar]
- Calenge C 2006. The package adehabitat for the R software: a tool for the analysis of space and habitat use by animals. Ecol. Modell 197, 516–519. 10.1016/j.ecolmodel.2006.03.017. [DOI] [Google Scholar]
- Casao A, Cebrián I, Asumpçâo ME, Pérez-Pé R, Abecia JA, Forcada F, et al. 2010. Seasonal variations of melatonin in ram seminal plasma are correlated to those of testosterone and antioxidant enzymes. Reprod Biol Endocrinol. 8, 59. doi: 10.1186/1477-7827-8-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler CR, Ketterson ED, Nolan V, Ziegenfus C 1994. Effects of testosterone on spatial activity in free-ranging male dark-eyed juncos, Junco hyemalis. Anim. Behav 47, 1445–1455. 10.1006/ANBE.1994.1191. [DOI] [Google Scholar]
- Cliff AD, Ord JK 1973. Spatial autocorrelation. London: Pion [Google Scholar]
- Cockrem JF, Silverin B 2002. Variation within and between Birds in Corticosterone Responses of Great Tits (Parus major). Gen. Comp. Endocrinol 125(2): 197–206. 10.1006/GCEN.2001.7750 [DOI] [PubMed] [Google Scholar]
- Cockrem JF, Barrett DP, Candy EJ, Potter MA 2009. Corticosterone responses in birds: Individual variation and repeatability in Adelie penguins (Pygoscelis adeliae) and other species, and the use of power analysis to determine sample sizes. Gen. Comp. Endocrinol 163(1–2): 158–168. 10.1016/j.ygcen.2009.03.029 [DOI] [PubMed] [Google Scholar]
- Cockrem JF, Candy EJ, Barrett DP, Agnew P, Potter MA 2017. Individual variation and repeatability of corticosterone responses of little penguins (Eudyptula minor) sampled in two successive years at Oamaru, New Zealand. Gen. Comp. Endocrinol 244, 86–92. 10.1016/j.ygcen.2016.01.010. [DOI] [PubMed] [Google Scholar]
- Côté SD 2000. Aggressiveness in king penguins in relation to reproductive status and territory location. Anim. Behav 59, 813–821. 10.1006/anbe.1999.1384. [DOI] [PubMed] [Google Scholar]
- Crino OL, Klaassen Van Oorschot B, Johnson EE, Malisch JL, Breuner CW 2011. Proximity to a high traffic road: Glucocorticoid and life history consequences for nestling white-crowned sparrows. Gen. Comp. Endocrinol 173, 323–332. 10.1016/j.ygcen.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Cyr NE, Romero LM 2007. Chronic stress in free-living European starlings reduces corticosterone concentrations and reproductive success. Gen. Comp. Endocrinol 151, 82–89. 10.1016/j.ygcen.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Dekkers TJ, van Rentergem JAA, Meijer B, Popma A, Wagemaker E, Huizenga HM 2019. A meta-analytical evaluation of the dual-hormone hypothesis: Does cortisol moderate the relationship between testosterone and status, dominance, risk taking, aggression, and psychopathy? Neurosci. Biobehav. Rev 96, 250–271. [DOI] [PubMed] [Google Scholar]
- DeNardo DF, Sinervo B 1994. Effects of Corticosterone on Activity and Home-Range Size of Free- Ranging Male Lizards. Horm. Behav 28, 53–65. 10.1006/hbeh.1994.1005. [DOI] [PubMed] [Google Scholar]
- Deviche P, Beouche-Helias B, Davies S, Gao S, Lane S, Valle S 2014. Regulation of plasma testosterone, corticosterone, and metabolites in response to stress, reproductive stage, and social challenges in a desert male songbird. Gen. Comp. Endocrinol 203, 120–131. 10.1016/j.ygcen.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Diemer KM, Nocera JJ 2014. Associations of bobolink territory size with habitat quality. Ann. Zool. Fenn 5, 515–525. 10.5735/086.051.0607. [DOI] [Google Scholar]
- Dohm MR 2002. Repeatability estimates do not always set an upper limit to heritability. Func. Ecol 16(2): 273–280. 10.1046/j.1365-2435.2002.00621.x [DOI] [Google Scholar]
- Edwards KL, Shultz S, Pilgrim M, Walker SL 2015. Male reproductive success is correlated with testosterone in the eastern black rhinoceros (Diceros bicornis michaeli). Gen. Comp. Endocrinol 213, 40–49. [DOI] [PubMed] [Google Scholar]
- Enstrom DA, Ketterson ED, Nolan V 1997. Testosterone and mate choice in the dark-eyed junco. Anim. Behav. 54, 1135–1146. 10.1006/anbe.1997.0555. [DOI] [Google Scholar]
- Fanson KV, Biro PA 2019. Meta-analytic insights into factors influencing the repeatability of hormone levels in agricultural, ecological, and medical fields. Am. J. Physiol. Regul. Integr. Comp. Physiol 316(2): R101–R109. 10.1152/ajpregu.00006.2018 [DOI] [PubMed] [Google Scholar]
- Fanson KV, Wielebnowski NC, Shenk TM, Lucas JR 2012. Comparative patterns of adrenal activity in captive and wild canada lynx (lynx canadensis). J Comp Physiol.B, Biochem Syst Environ Physiol. 182(1), 157–65. doi: 10.1007/s00360-011-0597-8 [DOI] [PubMed] [Google Scholar]
- Fusani L 2008. Testosterone control of male courtship in birds. Horm. Behav 54, 227–233. 10.1016/J.YHBEH.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Gangloff EJ, Sparkman AM, Holden KG, Corwin CJ, Topf M, Bronikowski AM 2017. Geographic variation and within-individual correlations of physiological stress markers in a widespread reptile, the common garter snake (Thamnophis sirtalis). Comp. Biochem. Physiol. A 205, 68–76. 10.1016/j.cbpa.2016.12.019. [DOI] [PubMed] [Google Scholar]
- Garamszegi LZ, Hirschenhauser K, Bó V, Eens M, Hurtrez-Boussè S, Møller AP, Oliveira RF, Wingfield JC 2008. Latitudinal distribution, migration, and testosterone levels in birds. Am. Nat 172, 533–546. 10.1086/590955. [DOI] [PubMed] [Google Scholar]
- Gerlach NM, Ketterson ED 2013. Experimental elevation of testosterone lowers fitness in female dark-eyed juncos. Horm. Behav 63, 782–790. 10.1016/j.yhbeh.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Gesquiere LR, Khan M, Shek L, Wango TL, Wango EO, Alberts SC, Altmann J 2008. Coping with a challenging environment: Effects of seasonal variability and reproductive status on glucocorticoid concentrations of female baboons (Papio cynocephalus). Horn. Behav 54(3), 410–416. 10.1016/j.yhbeh.2008.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace JK, Anderson DJ 2014. Corticosterone stress response shows long-term repeatability and links to personality in free-living Nazca boobies. Gen. Comp. Endocrinol 208: 39–48. 10.1016/J.YGCEN.2014.08.020 [DOI] [PubMed] [Google Scholar]
- Grebe NM, Del Giudice M, Thompson ME, Nickels N, Ponzi D, Zilioli S, et al. 2019. Testosterone, cortisol, and status-striving personality features: A review and empirical evaluation of the Dual Hormone hypothesis. Horm. Behav 109, 25–37. 10.1016/J.YHBEH.2019.01.006 [DOI] [PubMed] [Google Scholar]
- Guimont FS, Wynne-Edwards KE 2006. Individual variation in cortisol responses to acute “on-back” restraint in an outbred hamster. Horm. Behav 50, 252–260. 10.1016/j.yhbeh.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Hadfield JD 2010. MCMC Methods for Multi-Response Generalized Linear Mixed Models: The MCMCglmm R Package. J Stat Softw 33(2), 1–22.20808728 [Google Scholar]
- Harris CM, Madliger CL, Love OP 2017. An evaluation of feather corticosterone as a biomarker of fitness and an ecologically relevant stressor during breeding in the wild. Oecologia. 183, 987996 10.1007/s00442-017-3836-1. [DOI] [PubMed] [Google Scholar]
- Hau M, Casagrande S, Ouyang JQ, Baugh AT 2016. Glucocorticoid-mediated phenotypes in vertebrates: Multilevel variation and evolution. Adv. Study Behav 48, 41–115. 10.1016/bs.asb.2016.01.002. [DOI] [Google Scholar]
- Hau M, Goymann W 2015. Endocrine mechanisms, behavioral phenotypes and plasticity: known relationships and open questions. Front. Zool 12, S7 10.1186/1742-9994-12-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hau M, Ricklefs RE, Wikelski M, Lee KA, Brawn JD 2010. Corticosterone, testosterone and life- history strategies of birds. Proc. R. Soc. B 277, 3203–3212. 10.1098/rspb.2010.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann AA 2000. Laboratory and field heritability; some lessons from Drosophila Adaptive Genetic Variation in the Wild (eds. Mousseau TA, Sinervo B & Endler JA), pp. 200–218. Oxford University Press, New York. [Google Scholar]
- Holtmann B, Lagisz M, Nakagawa S 2017. Metabolic rates, and not hormone levels, are a likely mediator of between-individual differences in behaviour: a meta-analysis. Func. Ecol 31, 685–696. 10.1111/1365-2435.12779. [DOI] [Google Scholar]
- Holway DA, Suarez AV 1999. Animal behavior: an essential component of invasion biology. Trends Ecol. Evol 14, 328–330. 10.1016/S0169-5347(99)01636-5. [DOI] [PubMed] [Google Scholar]
- Hunt G, Hunt M 1976. Gull Chick Survival: The Significance of Growth Rates, Timing of Breeding and Territory Size. Ecology. 57(1), 62–75. 10.2307/1936398 [DOI] [Google Scholar]
- Husak JF, Irschick DJ, Meyers JJ, Lailvaux SP, Moore IT 2007. Hormones, sexual signals, and performance of green anole lizards (Anolis carolinensis). Horm. Behav 52(3), 360–367. 10.1016/j.yhbeh.2007.05.014 [DOI] [PubMed] [Google Scholar]
- Husak J, Moore IT 2008. Stress hormones and mate choice. Trends Ecol. Evol 23, 532–534. 10.1016/j.tree.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Ippi S, Cerón G, Alvarez LM, Aráoz R, Blendinger PG 2018. Relationships among territory size, body size, and food availability in a specialist river duck. Emu 118, 293–303. 10.1080/01584197.2018.1438848. [DOI] [Google Scholar]
- Jaatinen K, Seltmann MW, Hollmén T, Atkinson S, Mashburn K, Öst M 2013. Context dependency of baseline glucocorticoids as indicators of individual quality in a capital breeder. Gen. Comp. Endocrinol 191, 231–238. 10.1016/j.ygcen.2013.06.022. [DOI] [PubMed] [Google Scholar]
- Jachowski DS, Washburn BE, Millspaugh JJ 2015. Revisiting the Importance of Accounting for Seasonal and Diel Rhythms in Fecal Stress Hormone Studies. Wildlife Society Bulletin. 39(4), 738–745. 10.1002/wsb.592. [DOI] [Google Scholar]
- Janati A, Talbi R, Klosen P, Mikkelsen JD, Magoul R, Simonneaux V, El Ouezzani S 2013. Distribution and seasonal variation in hypotha-lamic RF-amide peptides in a semi-desert rodent, the jerboa. J Neuroendocrinol 25(4), 402–411. 10.1111/jne.12015. [DOI] [PubMed] [Google Scholar]
- Johnson MD 2007. Measuring habitat quality: A review. Condor. 109, 489–504. 10.1650/8347.1. [DOI] [Google Scholar]
- Ketterson ED, Nolan V, Wolf L, Ziegenfus C, Dufty AM, Ball GF, Johnsen TS 1991. Testosterone and avian life histories: The effect of experimentally elevated testosterone on corticosterone and body mass in dark-eyed juncos. Horm. Behav 25, 489–503. 10.1016/0018-506X(91)90016-B. [DOI] [PubMed] [Google Scholar]
- Ketterson ED, Nolan V Jr, Wolf L, Ziegenfus C 1992. Testosterone and avian life histories: Effects of experimentally elevated testosterone on behavior and correlates of fitness in the dark-eyed junco (Junco hyemalis). Am. Nat 140, 980–999. 10.1086/285451. [DOI] [Google Scholar]
- Klukowski M 2011. Effects of breeding season, testosterone and ACTH on the corticosterone response of free-ranging male fence lizards (Sceloporus undulatus). Gen. Com. Endocrinol 173, 295–302. 10.1016/j.ygcen.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Kralj-Fiser S, Scheiber IBR, Blejec A, Moestl E, Kotrschal K 2007. Individualities in a flock of free-roaming greylag geese: behavioral and physiological consistency over time and across situations. Horm. Behav 5: 239–248. 10.1016/j.yhbeh.2006.10.006 [DOI] [PubMed] [Google Scholar]
- Lattin CR, Reed JM, Desrochers DW, Romero LM 2011. Elevated corticosterone in feathers correlates with corticosterone-induced decreased feather quality: A validation study. J. Avian Biol 42, 247–252. 10.1111/j.1600-048X.2010.05310.x. [DOI] [Google Scholar]
- Lessells CM, Boag PT 1987. Unrepeatable repeatabilities: A common mistake. Auk. 104, 116–121. 10.2307/4087240. [DOI] [Google Scholar]
- Li Y, Sun Y, Krause JS, Li M, Liu X, Zhu W, Yao Y, Wu Y, Li D 2017. Dynamic interactions between corticosterone, corticosteroid binding globulin and testosterone in response to capture stress in male breeding Eurasian tree sparrows. Comp. Biochem. Physiol., Part A Mol. Integ. Physiol 205, 41–47. 10.1016/LCBPA.2016.12.016. [DOI] [PubMed] [Google Scholar]
- Love OP, Shutt LJ, Silfies JS, Bird DM 2003. Repeated Restraint and Sampling Results in Reduced Corticosterone Levels in Developing and Adult Captive American Kestrels (Falco sparverius). Physiol. Biochem. Zool 76(5): 753–761. 10.1086/376431 [DOI] [PubMed] [Google Scholar]
- Lynn SE, Porter AJ 2008. Trapping initiates stress response in breeding and non-breeding house sparrows Passer domesticus: implications for using unmonitored traps in field studies. J Avian Biol 39, 87–94. 10.1111/j.0908-8857.2008.04204.x [DOI] [Google Scholar]
- Machín P, Fernández-Elipe J, Klaassen RHG 2018. The relative importance of food abundance and weather on the growth of a sub-arctic shorebird chick. Behav. Ecol. Sociobiol 72, 1–12. 10.1007/s00265-018-2457-y. [DOI] [Google Scholar]
- Maestripieri D, Hoffman CL, Anderson GM, Carter CS, Higley JD 2009. Mother-infant interactions in free-ranging rhesus macaques: Relationships between physiological and behavioral variables. Physiol. Behav 96, 613–619. 10.1016/j.physbeh.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm KD, McShea WJ, Van Deelen TR, Bacon HJ, Liu F, Putman S, Zhu X, Brown JL 2013. Analyses of fecal and hair glucocorticoids to evaluate short- and long-term stress and recovery of Asiatic black bears (Ursus thibetanus) removed from bile farms in China. Gen. Comp. Endocrinol 185, 97–106. 10.1016/j.ygcen.2013.01.014 [DOI] [PubMed] [Google Scholar]
- Malisch JL, Bennett DA, Davidson BA, Wenker E, Suzich R, Johnson EE. 2018. Stress hyperglycemia in white-throated and white-crowned sparrows: A new technique for rapid glucose measurement in the field. Physiol. Biochem. Zool 91, 943–949. 10.1086/698536. [DOI] [PubMed] [Google Scholar]
- Meadows D 2001. Centre-Edge Differences in Behaviour, Territory Size and Fitness in Clusters of Territorial Damselfish: Patterns, Causes, and Consequences. Behaviour. 138(9), 1085–1116. 10.1163/156853901753287154 [DOI] [Google Scholar]
- McGlothlin JW, Jawor JM, Ketterson ED 2007. Natural variation in a testosterone-mediated trade-off between mating effort and parental effort. Am. Nat 170, 864–875. 10.1086/522838. [DOI] [PubMed] [Google Scholar]
- Mehta PH, Prasad S 2015. The dual-hormone hypothesis: a brief review and future re-search agenda. Curr. Opin. Behav. Sci 3, 163–168. [Google Scholar]
- Merilä J, Wiggins DA 1995. Interspecific competition for nest holes causes adult mortality in the collared flycatcher. Condor. 97, 445–450. 10.2307/1369030. [DOI] [Google Scholar]
- Mokkonen M, Koskela E, Mappes T, Mills SC 2012. Sexual antagonism for testosterone maintains multiple mating behaviour. J. Anim. Ecol 81, 277–283. 10.1111/jT365-2656.2011.01903.x [DOI] [PubMed] [Google Scholar]
- Morton ML 2002. Mountain white-crowned sparrow: migration and reproduction at high altitude. Cooper Ornithological Society. [Google Scholar]
- Navarro-Castilla Á, Barja I 2019. Stressful living in lower-quality habitats? Body mass, feeding behavior and physiological stress levels in wild wood mouse populations. Integrative Zoology. 14, 114–126. 10.1111/1749-4877.12351. [DOI] [PubMed] [Google Scholar]
- Newmark WD, Stanley TR 2016. The influence of food abundance, food dispersion and habitat structure on territory selection and size of an Afrotropical terrestrial insectivore. Ostrich. 87, 199–207. 10.2989/00306525.2016.1216903. [DOI] [Google Scholar]
- O’Reilly KM, Wingfield JC 2001. Ecological factors underlying the adrenocortical response to capture stress in arctic-breeding shorebirds. Gen. Comp. Endocrinol 124, 1–11. 10.1006/GCEN.2001.7676. [DOI] [PubMed] [Google Scholar]
- Ouyang JQ, Hau M, Bonier F 2011. Within seasons and among years: When are corticosterone levels repeatable? Horm. Behav 60(5): 559–564. 10.1016/J.YHBEH.201L08.004 [DOI] [PubMed] [Google Scholar]
- Ouyang JQ, Lendvai ÁZ, Moore IT, Bonier F, Haussmann MF 2016. Do hormones, telomere lengths, and oxidative stress form an integrated phenotype? A case study in free-living tree swallows. Integr. Comp. Biol 56, 138–145. 10.1093/icb/icw044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang JQ, Quetting M, Hau M 2012. Corticosterone and brood abandonment in a passerine bird. Anim. Behav. 84, 261–268. 10.1016/J.ANBEHAV.2012.05.006. [DOI] [Google Scholar]
- Ouyang JQ, Sharp P, Quetting M, Hau M 2013. Endocrine phenotype, reproductive success and survival in the great tit, Parus major. J. Evol. Biol 26, 1988–1998. 10.1111/jeb.12202. [DOI] [PubMed] [Google Scholar]
- Patterson SH, Hahn TP, Cornelius JM, Breuner CW 2014. Natural selection and glucocorticoid physiology. J. Evol. Biol 27, 259–274. 10.1111/jeb.12286. [DOI] [PubMed] [Google Scholar]
- Pavitt AT, Walling CA, Möstl E, Pemberton JM, Kruuk LEB 2015. Cortisol but not testosterone is repeatable and varies with reproductive effort in wild red deer stags. Gen. Comp. Endocrinol 222, 62–68. 10.1016/j.ygcen.2015.07.009. [DOI] [PubMed] [Google Scholar]
- Peig J, Green AJ 2009. New perspectives for estimating body condition from mass/length data: The scaled mass index as an alternative method. Oikos. 118, 1883–1891. 10.1111/j.1600-0706.2009.17643.x. [DOI] [Google Scholar]
- R Core Team, 2018. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: https://www.R-project.org/. (accessed August 2018). [Google Scholar]
- Raynaud J, Müller K, Schradin C 2012. Experimental increase of testosterone levels in free-ranging juvenile male African striped mice (Rhabdomys pumilio) induces physiological, morphological, and behavioral changes. Gen. Comp. Endocrinol 178(1), 108–115. [DOI] [PubMed] [Google Scholar]
- Reed WL, Clark ME, Parker PG, Raouf SA, Arguedas N, Monk DS, Snajdr E, Nolan V Jr., Ketterson ED 2006. Physiological effects on demography: A long-term experimental study of testosterone’s effects of fitness. Am. Nat 167, 667–683. 10.1086/503054. [DOI] [PubMed] [Google Scholar]
- Reed JM, Harris DR, Romero LM 2019. Profile repeatability: A new method for evaluating repeatability of individual hormone response profiles. Gen. Comp. Endocrinol 270, 1–9. 10.1016/J.YGCEN.2018.09.015. [DOI] [PubMed] [Google Scholar]
- Rensel MA, Schoech SJ 2011. Repeatability of baseline and stress-induced corticosterone levels across early life stages in the Florida scrub-jay (Aphelocoma coerulescens). Horm. Behav 59, 497–502. 10.1016/j.yhbeh.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Romero LM, Reed JM 2005. Collecting baseline corticosterone samples in the field: is under 3 min good enough? Comp. Biochem. Physiol., Part A Mol. Integr. Physiol 140, 73–79. 10.1016/J.CBPB.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Romero LM, Reed JM 2008. Repeatability of baseline corticosterone concentrations. Gen. Comp. Endocrinol 156, 27–33. 10.1016/i.ygcen.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Romero LM, Romero RC 2002. Corticosterone Responses in Wild Birds: The Importance of Rapid Initial Sampling. Condor 104(1), 129 10.1650/0010-5422(2002)104[0129:criwbt]2.0.co;2 [DOI] [Google Scholar]
- Romero LM, Wikelski M 2001. Corticosterone levels predict survival probabilities of Galápagos marine iguanas during El Niño events. Proc. Natl. Acad. Sci. U.S.A 98, 7366–7370. https://doi.org/10.1073pnas.131091498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev 21, 55–89. 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schoenemann KL, Bonier F 2018. Repeatability of glucocorticoid hormones in vertebrates: a meta-analysis. PeerJ. 6, e4398 10.7717/peeri.4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman PW, Morton ML 1988. Extra-pair fertilizations in mountain white-crowned sparrows. Behav. Ecol. Sociobiol 22(6), 413–420. 10.1007/BF00294979 [DOI] [Google Scholar]
- Skorupski J, Jankowiak Ł, Kiriaka B, Rek T, Wysocki D 2017. Beech forest structure and territory size of four songbird species in Puszcza Bukowa, NW Poland: implications for bird-friendly silvicultural practices in a temperate forest. Ethol. Ecol. Evol 30, 128–140. 10.1080/03949370.2017.1329232. [DOI] [Google Scholar]
- Sorenson GH, Dey CJ, Madliger CL, Love OP 2017. Effectiveness of baseline corticosterone as a monitoring tool for fitness: a meta-analysis in seabirds. Oecologia. 183, 353–365. 10.1007/s00442-016-3774-3. [DOI] [PubMed] [Google Scholar]
- Steingrímsson SÓ, Grant JWA 1999. Allometry of territory size and metabolic rate as predictors of self-thinning in young-of-the-year Atlantic salmon. J. Animal Ecol 68(1), 17–26. 10.1046/j.1365-2656.1999.0026Lx [DOI] [Google Scholar]
- Steury T, Murray D. 2003. Causes and Consequences of Individual Variation in Territory Size in the American Red Squirrel. Oikos. 101(1), 147–156. 10.1034/j.1600-0706.2003.12278.x [DOI] [Google Scholar]
- Stoffel MA, Nakagawa S, Schielzeth H 2017. rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol. Evol 8, 1639–1644. 10.1111/2041-210X.12797. [DOI] [Google Scholar]
- Strubbe D, Matthysen E. 2009. Experimental evidence for nest-site competition between invasive ringnecked parakeets (Psittacula krameri) and native nuthatches (Sitta europaea). Biol. Conserv 142, 1588–1594. 10.1016/j.biocon.2009.02.026. [DOI] [Google Scholar]
- Taff CC, Schoenle LA, Vitousek MN 2018. The repeatability of glucocorticoids: A review and meta-analysis. Gen. Comp. Endocrinol 260, 136–145. 10.1016/j.ygcen.2018.01.011. [DOI] [PubMed] [Google Scholar]
- Thiel D, Jenni-Eiermann S, Braunisch V, Palme R, Jenni L 2008. Ski tourism affects habitat use and evokes a physiological stress response in capercaillie Tetrao urogallus: a new methodological approach. J. Appl. Ecol 45, 845–853. 10.1111/j.1365-2664.2008.01465.x. [DOI] [Google Scholar]
- Thierry AM, Massemin S, Handrich Y, Raclot T 2013. Elevated corticosterone levels and severe weather conditions decrease parental investment of incubating Adélie penguins. Horm. Behav 63, 475–483. 10.1016/J.YHBEH.2012.12.01L [DOI] [PubMed] [Google Scholar]
- Uller T, Astheimer L, Olsson M 2007. Consequences of Maternal Yolk Testosterone for Offspring Development and Survival: Experimental Test in a Lizard. Func. Ecol 21(3), 544–551. 10.1111/i.1365-2435.2007.01264.x [DOI] [Google Scholar]
- Veiga JP, Polo V 2008. Fitness consequences of increased testosterone levels in female spotless starlings. Am. Nat 172, 42–53. 10.1086/587850. [DOI] [PubMed] [Google Scholar]
- Viblanc VA, Gineste B, Stier A, Robin JP, Groscolas R 2014. Stress hormones in relation to breeding status and territory location in colonial king penguin: a role for social density? Oecologia. 1, 763–772. 10.1007/s00442-014-2942-6. [DOI] [PubMed] [Google Scholar]
- Wada H, Moore IT, Breuner CW, Wingfield JC 2006. Stress responses in tropical sparrows: Comparing tropical and temperate Zonotrichia. Physiol. Biochem. Zool 79, 784–792. 10.1086/505509. [DOI] [PubMed] [Google Scholar]
- Washburn BE, Millspaugh JJ, Morris DL, Schulz JH, Faaborg J 2007. Using a commercially available enzyme immunoassay to quantify testosterone in avian plasma. Condor. 109, 181–186. 10.1650/0010-5422(2007)109[181:UACAEI]2.0.C0;2. [DOI] [Google Scholar]
- Watson A, Parr R 1981. Hormone implants affecting territory size and aggressive and sexual behaviour in red grouse. Ornis Scandinavica (Scandinavian J. Ornithol). 12, 55–61. 10.2307/3675905. [DOI] [Google Scholar]
- While GM, Isaksson C, McEvoy J, Sinn DL, Komdeur J, Wapstra E, Groothuis TGG 2010. Repeatable intra-individual variation in plasma testosterone concentration and its sex-specific link to aggression in a social lizard. Horm. Behav, 58, 208–213. 10.1016/J.YHBEH.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Wikelski M, Lynn S, Breuner C, Wingfield JC, Kenagy GJ 1999. Energy metabolism, testosterone and corticosterone in white-crowned sparrows. J. Comp. Phys. A 185, 463–470. 10.1007/s003590050407. [DOI] [Google Scholar]
- Williams TD 2008. Individual variation in endocrine systems: Moving beyond the “tyranny of the Golden Mean.” Philos. Trans. R. Soc. Lond., B, Biol. Sci 363, 1687–1698. 10.1098/rstb.2007.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield JC 1984. Androgens and mating systems: Testosterone-induced polygyny in normally monogamous birds. Auk. 101, 665–671. 10.2307/4086893. [DOI] [Google Scholar]
- Wingfield JC 2003. Control of behavioural strategies for capricious environments. Anim Behav. 66, 807–816. 10.1006/ANBE.2003.2298. [DOI] [Google Scholar]
- Wingfield JC 2013. Ecological processes and the ecology of stress: the impacts of abiotic environmental factors. Funct. Ecol 27, 37–44. 10.1111/1365-2435.12039. [DOI] [Google Scholar]
- Wingfield JC, Deviche P, Sharbaugh S, Astheimer LB, Holberton R, Suydam R, Hunt K. 1994. Seasonal changes of the adrenocortical responses to stress in redpolls, Acanthis flammea, in Alaska. J. Exp. Zool 270, 372–380. 10.1002/jez.1402700406. [DOI] [Google Scholar]
- Wingfield JC, Hegner RE, Dufty AM, Ball GF 1990. The “Challenge Hypothesis”: Theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am. Nat 136, 829–846. 10.1086/285134. [DOI] [Google Scholar]
- Wingfield JC, Moore MC, Farner DS 1983. Endocrine responses to inclement weather in naturally breeding populations of white-crowned sparrows (Zonotrichia leucophrys pugetensis). Auk. 100, 56–62. 10.1093/auk/100.L56. [DOI] [Google Scholar]
- Wingfield JC, O’Reilly KM, Astheimer LB 1995. Modulation of the adrenocorticol responses to acute stress in arctic birds: A possible ecological basis. Am. Zool 35, 285–294. 10.1093/icb/35.3.285. [DOI] [Google Scholar]
- Wingfield JC, Sapolsky RM 2003. Reproduction and resistance to stress: When and how. J. Neuroendocrinol. 15, 711–724. 10.1046/j.1365-2826.2003.01033.x. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Smith JP, Farner DS 1982. Endocrine responses of white-crowned sparrows to environmental stress. Condor. 84, 399–409. 10.2307/1367443. [DOI] [Google Scholar]
- Wingfield JC, Wada M 1989. Changes in plasma levels of testosterone during male-male interactions in the song sparrow, Melospiza melodia: time course and specificity of response. J. Comp. Physiol. A 166, 189–194. 10.1007/BF00193463 [DOI] [Google Scholar]
- Worton BJ 1989. Kernel methods for estimating the utilization distribution in home-range studies. Ecology. 70, 164–168. 10.2307/1938423. [DOI] [Google Scholar]
- Zito JB, Hanna A, Kadoo N, Tomaszycki ML 2017. Early life stress increases testosterone and corticosterone and alters stress physiology in zebra finches. Horm. Behav 95, 57–64. 10.1016/j.yhbeh.2017.08.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.