Abstract
Fatty acids (FAs) are stored safely in the form of triacylglycerol (TAG) in lipid droplet (LD) organelles by professional storage cells called adipocytes. These lipids are mobilized during adipocyte lipolysis, the fundamental process of hydrolyzing TAG to FAs for internal or systemic energy use. Our understanding of adipocyte lipolysis has greatly increased over the past 50 years from a basic enzymatic process to a dynamic regulatory one, involving the assembly and disassembly of protein complexes on the surface of LDs. These dynamic interactions are regulated by hormonal signals such as catecholamines and insulin which have opposing effects on lipolysis. Upon stimulation, patatin-like phospholipase domain containing 2 (PNPLA2)/adipocyte triglyceride lipase (ATGL), the rate limiting enzyme for TAG hydrolysis, is activated by the interaction with its co-activator, alpha/beta hydrolase domain-containing protein 5 (ABHD5), which is normally bound to perilipin 1 (PLIN1). Recently identified negative regulators of lipolysis include G0/G1 switch gene 2 (G0S2) and PNPLA3 which interact with PNPLA2 and ABHD5, respectively. This review focuses on the dynamic protein-protein interactions involved in lipolysis and discusses some of the emerging concepts in the control of lipolysis that include allosteric regulation and protein turnover. Furthermore, recent research demonstrates that many of the proteins involved in adipocyte lipolysis are multifunctional enzymes and that lipolysis can mediate homeostatic metabolic signals at both the cellular and whole-body level to promote inter-organ communication. Finally, adipocyte lipolysis is involved in various diseases such as cancer, type 2 diabetes and fatty liver disease, and targeting adipocyte lipolysis is of therapeutic interest.
Introduction
The main function of the adipocyte is to act as a buffer for the storage of excess fatty acids (FAs) as inert triacylglycerols (TAGs) in organelles termed lipid droplets (LDs) (1). When energy demand is increased, TAGs are subsequently broken down into their constituent FAs and glycerol through the highly active and dynamic biochemical process of lipolysis. Once released, FAs have a variety of fates including oxidation for energy in the form of ATP, re-esterification back into TAGs (2), and also functioning as signaling molecules (3-5). In brown adipose tissue (BAT), a thermogenic organ, FAs can function as both fuel and allosteric activators of non-shivering thermogenesis (6, 7). While FAs are essential metabolic substrates used for cellular energy, in excess, FAs can have deleterious effects through the buildup of toxic lipid metabolites (8) in a condition known as lipotoxicity (9). This excessive spillover of FAs from adipocytes into non-adipose tissues results in numerous abhorrent processes such as inflammation (10), insulin resistance (11), and apoptosis (9). As a result, adipocyte lipolysis requires exquisite regulation at the physiological level by hormones that have opposing effects. On the surface of the LD, dynamic protein-protein interactions control the trafficking, activation, and inhibition of the lipases involved in lipolysis (12-16).
The canonical pathway of adipocyte lipolysis is catalyzed by three main lipases: patatin-like phospholipase domain containing-2 (PNPLA2)/adipocyte triglyceride lipase (ATGL) (17), hormone sensitive lipase (HSL) (18), and monoacylglycerol lipase (MGL) (19). ATGL will be referred to by its official gene symbol PNPLA2 throughout the rest of this review to note its similarity with other PNPLA paralogs. These three lipases work in concert to degrade TAGs sequentially, resulting in the release of three FAs and glycerol backbone. However, recent work suggests that lipolysis may not be linear, as many of the protein players involved in adipocyte lipolysis are multifunctional enzymes able to catalyze reactions in both directions (20-22), resulting in the re-esterification and recycling of FAs (23), thereby challenging the current model.
Our understanding of adipose tissue has greatly increased in the past 20 years, from an inert depot that stores excess energy to a dynamic organ involved in regulating whole-body energy homeostasis (24), and an active area of interest has been to understand the mechanisms that regulate adipocyte lipolysis. Recent advances in the understanding of adipocyte lipolysis regulation include allosteric control by FA metabolites (16) and regulation of protein levels by active mechanisms that mark proteins for degradation (13). Under normal physiology, these mechanisms of regulation coordinate to control FA efflux from the adipocyte.
Dysregulated lipolysis has been implicated in a wide variety of diseases such as obesity, type 2 diabetes (T2D), fatty liver disease (FLD), and cancer (1, 25). Furthermore, human and rodent genetic studies corroborate the concept that pathways of lipid mobilization in adipocytes are of therapeutic interest (26, 27). This review will focus on the regulation of adipocyte lipolysis by dynamic protein-protein interactions, hormones, allosteric mechanisms and protein turnover. In addition, the concept that lipolytic proteins are multifunctional enzymes and a signaling function for lipolysis will be reviewed. Finally, implications for the dysregulation of lipolysis and how this process is involved in various metabolic disorders and whether activation or inhibition of lipolysis is suitable for treatment of metabolic diseases and will be discussed.
Molecular players and dynamic protein-protein interactions
PNPLA2 is activated by co-regulator alpha/beta hydrolase domain-containing protein 5
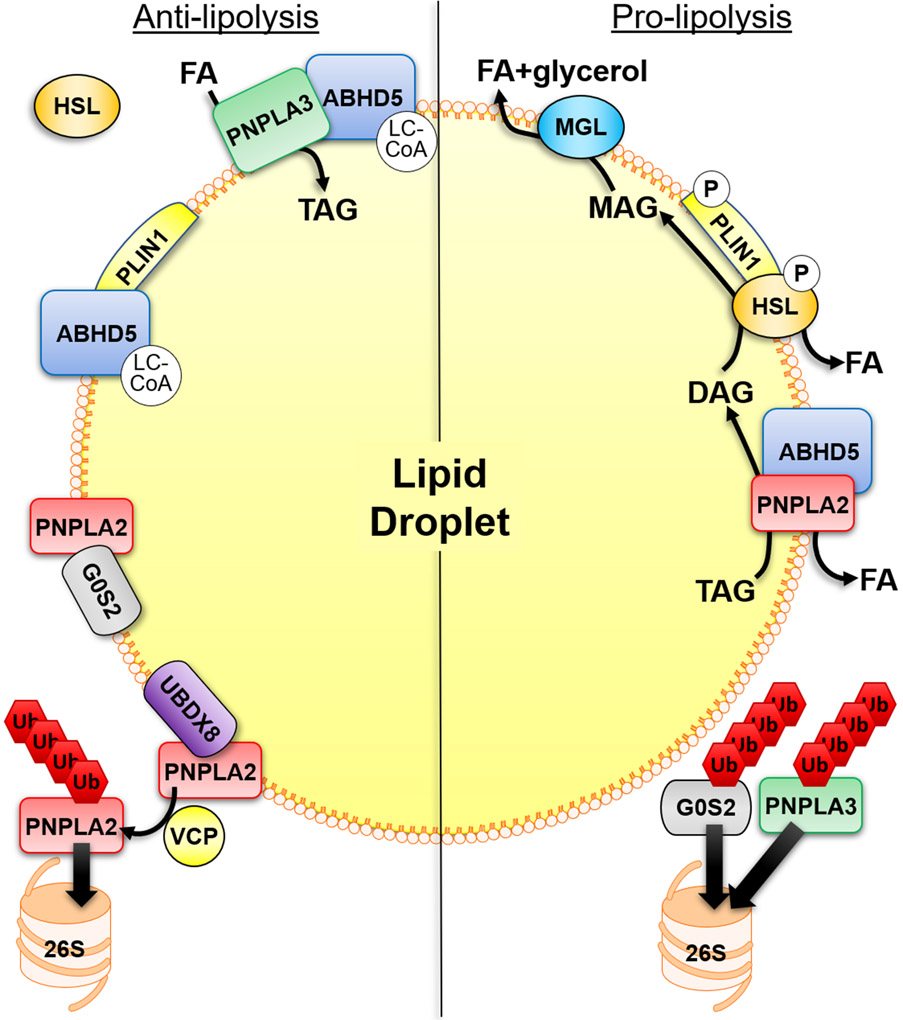
PNPLA2, the rate limiting enzyme for TAG hydrolysis was initially identified independently in 2004 by the groups of Gross (21), Zechner (17) and Sul (28), with subsequent work from the Zechner group demonstrating PNPLA2 as the rate limiting step in TAG hydrolysis in vivo (29). In the basal state PNPLA2 has weak hydrolytic activity which is greatly increased by the co-lipase alpha/beta hydrolase domain-containing protein 5 (ABHD5, also known as CGI-58) by greater than 20-fold (30). Indeed, knockdown of ABHD5 reduces both basal and stimulate lipolysis in adipocytes (31) (Figure 1). ABHD5 was first described as the causative gene in Chanarin-Dorfman syndrome (CDS), a disease marked by neutral lipid storage and ichthyosis (32, 33). Interestingly, ABHD5 mutations causative in CDS do not co-localize to LDs and are unable to stimulate PNPLA2 lipolytic activity (30), demonstrating an important function for the interaction in the etiology of CDS and lipid storage. The exact mechanism by which ABHD5 increases PNPLA2 activity is not completely understood; however, ABHD5 lacks an active serine found in other ABHD family members (33), in which possibilities might include promoting access to the TAG core through remodeling of the phospholipid monolayer, or removal of reaction products. Recent comparative evolutionary analysis of ABHD5 and closely related paralog ABHD4 identified critical surface residues of R299 and G328 that are required for lipase activation of PNPLA2, which can be dissociated from translocation of PNPLA2 (34).
Figure 1. Mechanisms that regulate adipocyte lipolysis on the surface of lipid droplets.
Anti-lipolytic mechanisms of regulation include the binding of ABHD5 to PLIN1 which is regulated allosterically by ABHD5-sensing of long-chain acyl-CoA (LC-CoA). PNPLA2 hydrolytic activity can be inhibited by the interaction with G0S2 which is independent of its interaction with ABHD5. In addition, PNPLA3 can compete with PNPLA2 for binding to ABHD5, thereby sequestering ABHD5 away from PNPLA2. PNPLA3 can also function to sequester fatty acids (FAs) through lysophosphatidic acid acyltransferase or transacylase activity. LC-CoAs also allosterically regulate the interaction between PNPLA3 and ABHD5. UBDX8 can bind PNPLA2 to promote its segregation from the lipid droplet (LD) by VCP, followed by ubiquitination (Ub) and elimination by the proteasome (26S). In the basal state HSL is found mostly in the cytosol. Stimulatory signals lead to the phosphorylation (P) of PLIN1 to release ABHD5 which can bind PNPLA2, and phosphorylation of HSL promotes its trafficking to LDs and interaction with PLIN1. This leads to the sequential hydrolysis of TAG by PNPLA2, hydrolysis of DAG by HSL and hydrolysis of MAG by MGL to three FAs and glycerol. The elimination of G0S2 and PNPLA3 by the ubiquitin-proteasome system (UPS), through currently unknown mechanisms, would be pro-lipolytic.
Perilipin 1, a lipid droplet scaffold protein that coordinates interactions with ABHD5 and HSL
Perilipin 1 (PLIN1), initially discovered by the group of Constantine Londos, is the major adipocyte perilipin/ADRP/TIP47 (PAT) protein (35) that functions as a scaffold to organized protein-protein interactions on the surface of LDs (36). In the basal state, ABHD5 is bound to PLIN1 (14, 37), which upon hormonal stimulation (described in detail below) releases ABHD5 to interact with PNPLA2 (Figure 1). HSL, first cloned by Holm in 1988 (38), is the main diacylglycerol (DAG) lipase in adipocytes. Mice that lack HSL show only a minor defect in lipolysis, are cold tolerant, and demonstrate a dramatic accumulation of DAGs in adipose tissue (39-41). HSL translocates from the cytosol to LDs (42) in response to hormonal stimulation which requires PLIN1 (15) through direct interaction (43, 44) (Figure 1). However, rather than functioning as a barrier to HSL in the hydrolysis of TAG (45), PLIN1 seems to mark a domain of lipolytic attack for HSL (46); a model recently confirmed by super-resolution microscopy (47). The recent discovery of pharmacological ABHD5 ligands that disrupt the interaction between ABHD5 and PLIN1 (48) demonstrate that the dissociation of ABHD5 is sufficient to stimulate adipocyte lipolysis and that these protein interactions can be targeted pharmacologically (34, 48, 49).
G0/G1 switch gene 2 a PNPLA2 inhibitor
G0/G1 switch gene 2 (G0S2) was first identified based on its differential expression from the transition of G0 to G1 phase (50), however a definitive function in cell cycle has not been demonstrated. The initial indication that G0S2 was involved in lipolysis was from studies showing that G0S2 mRNA expression is highest in WAT and BAT, upregulated during adipocyte differentiation (51), and a peroxisome proliferator-activated receptor (PPAR) target gene. Subsequent work from the group of Jun Lui in 2010 identified G0S2 as a bona-fide binding partner of PNPLA2, capable of inhibiting its TAG hydrolase activity (52) (Figure 1). This interaction was mapped at the hydrophobic domain of G0S2 and the patatin-like domain of PNPLA2, whereby overexpression of G0S2 diminished adipocyte lipolysis, and deletion increased both basal and stimulated lipolysis (52). Interestingly, the mechanism by which G0S2 inhibits lipolysis is independent of ABHD5 and occurs in a non-competitive manner (52, 53). The domain of G0S2 which inhibits PNPLA2 comprises amino acids K20-A52 and does not inhibit the function of other PNPLA family members PNPLA6 and 7, or HSL, MGL, and lipoprotein lipase (54); however, more closely related paralogs of PNPLA2 such as PNPLA3 and PNPLA5 were not examined. Hypoxia-inducible lipid droplet-associated protein (HILPDA)/Hypoxia inducible protein 2 (HID2), a hypoxia-inducible factor-1 target (55) and a structurally similar protein to G0S2, also inhibits PNPLA2 activity (56, 57). However, in contrast to its effect on suppressing hepatocyte lipolysis (58), HILPDA does not regulate lipolysis in adipose tissue (59, 60).
PNPLA3 a novel ABHD5 interacting partner
PNPLA3 is an additional member of the PNPLA family of lipases, shares the greatest homology with PNPLA2, and arose in vertebrates by a gene duplication (61). Interestingly, the expression of PNPLA2 and PNPLA3 oppose one another, with expression of PNPLA2 highest during conditions that promote TAG hydrolysis such as fasting, while the expression of PNPLA3 is highest during conditions that promote TAG synthesis such as feeding (62, 63). This regulation of expression strongly suggests that PNPLA2 and PNPLA3 have opposing functions in TAG metabolism. Importantly, a mutation in PNPLA3 (rs738409), I148M, is the single greatest genetic risk factor for the development of FLD (64-67), however its precise function in neutral lipid metabolism is controversial (68, 69). Interestingly, the expression of rodent PNPLA3 is highest in BAT (62, 63), and recent work demonstrates a role in regulating adipocyte lipolysis. Our group demonstrated that PNPLA3 is a novel binding partner of ABHD5 in BAT, functioning to compete with PNPLA2 for binding to ABHD5, thereby inhibiting adipocyte lipolysis (70). Additionally, the mechanism by which PNPLA3 inhibited lipolysis involved the re-esterification of mobilized FAs (Figure 1). Of note, ABHD5 can bind multiple PNPLA family members (37, 70-72), suggesting that it functions as scaffold for forming various enzymatic assemblies, or metabolons, to mediate a wide array of metabolic processes. The opposing expression of PNPLA3 and PNPLA2 (73) likely explains how efficient lipolysis can occur during fasting; however, the I148M variant evades degradation and accumulates on LDs (74). Importantly, the disease causing I148M variant was a gain of function for the interaction with ABHD5 and inhibition of lipolysis, and could promote TAG accumulation in an ABHD5-dependent manner (70). Similarly, PNPLA3 I148M promotes hepatosteatosis through ABHD5-dependent inhibition of PNPLA2 (75), suggesting that small molecules that target the PNPLA3 I148M/ABHD5 interaction could be potential therapeutics for FLD and diabetes.
The molecular players of lipolysis are multifunctional enzymes
Many of the protein players in lipolysis possess additional enzymatic functions which imparts added complexity to the regulation of lipolysis. HSL can catalyze multiple substrates, with studies showing that it can function as a TAG, DAG, retinyl, and cholesterol esterase (39, 76, 77). PNPLA2 has retinyl esterase activity, which is activated by ABHD5 in hepatic stellate cells (78); however, this function has not been examined in adipocytes. Both purified PNPLA2 and PNPLA3 have transacylase activity, able to transfer an acyl donor group to monoacylglycerol (MAG) or DAG (21). Work from Grant Mitchell and Jiang Wei Wu demonstrated a physiological significance to the transacylase function of PNPLA2, where PNPLA2 could synthesize TAGs from DAGs (23). In the genetic absence of HSL, this transacylase activity of PNPLA2 promoted more efficient lipolysis by acylating DAG to generate MAG substrate for MGL (23). PNPLA3 can remodel the phospholipid monolayer in LDs (79) by transferring polyunsaturated FAs from TAG to phospholipids via transacylation, or possibly through TAG hydrolase activity (80) and can promote FA re-esterification during lipolysis possibly through lysophosphatidic acid acyltransferase (LPAAT) or transacylase activity (70). LPAAT activity of ABHD5 has been reported (22, 81); however, whether this is due to intrinsic activity or through a binding partner remains to be verified (82). The Lui group identified an intrinsic LPAAT function in G0S2 which could promote TAG accumulation independent of PNPLA2 inhibition in hepatocytes (20); however, further work will be required to determine a similar role in adipose tissue. Overall, the molecular players involved in adipocyte lipolysis have multiple activities that are likely to be determined by subcellular location, availability of substrates and proximity to binding partners and coupled enzymes.
Hormonal regulation of adipocyte lipolysis
Catecholamines
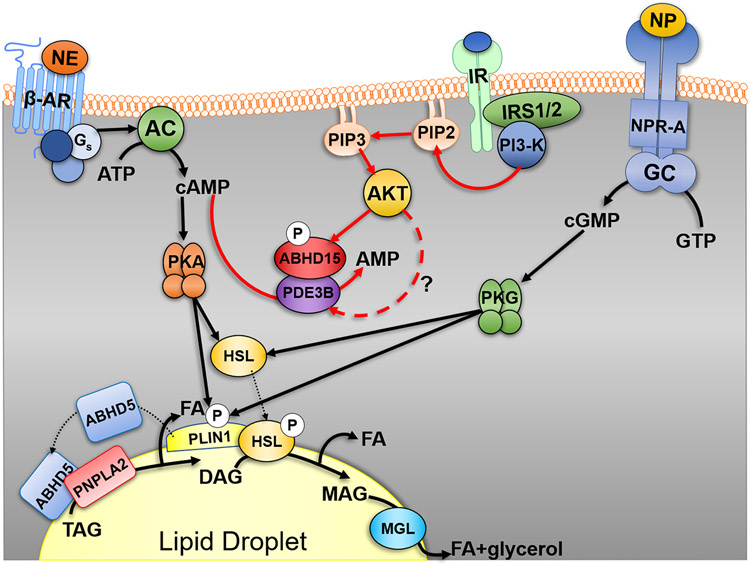
The regulation of TAG stores is exquisitely regulated at the hormonal level by circulating factors that have opposing effects on adipocyte lipolysis (Figure 2). Nor-epinephrine, a major catecholamine that promotes adipocyte lipolysis (83, 84), is released in response to cold stimulation from terminal neurons that innervate fat cells (83) to mediate non-shivering thermogenesis (85-87) through the activation of β-adrenergic receptors (β-ARs). β1, β2, and β3-ARs, which are coupled to Gs, lead to activation of adenylyl cyclase, increasing levels of the second messenger cyclic AMP (cAMP) to promote Protein kinase A (PKA) activation (83) (Figure 2). PKA phosphorylation of PLIN1 releases ABHD5 to fully activate PNPLA2 (12, 16) and phosphorylation of HSL promotes HSL translocation from the cytosol to LDs (88, 89) to interact with PLIN1(15, 88, 90, 91) and increases its hydrolase activity for TAGs and DAGs (92) (Figure 2). Indeed, PLIN1 phosphorylation is required for PKA-dependent lipolysis (44, 93) by promoting the release of ABHD5 and interaction with PNPLA2 in response to PKA (12, 16) and for proper LD recruitment and activation of HSL (44). However, the requirement for HSL phosphorylation has recently been challenged as studies by the Granneman group elegantly show that direct activation of PNPLA2 in the absence of PKA stimulation is sensitive to HSL inhibition, suggesting that PNPLA2-mediated generation of DAG is sufficient to promote HSL activity (49). In addition, PKA can also phosphorylate PLIN5 on S155 to also promote release of ABHD5 and activation of PNPLA2 (94). The role of protein phosphorylation and other kinases in the regulation of lipolysis has been reviewed in depth elsewhere (36, 95).
Figure 2. Hormonal control of adipocyte lipolysis.
Activation of the seven transmembrane β-adrenergic receptors (β-AR) on adipocytes by norepinephrine (NE), leads to Gs-dependent activation of adenylyl cyclase (AC) to increase intracellular levels of cyclic-AMP (cAMP). cAMP activates protein kinase A (PKA) to phosphorylate (P) PLIN1, which release ABHD5 to fully activate PNPLA2 TAG hydrolase activity and release a fatty acid (FA). PKA also phosphorylates HSL to promote its translocation to PLIN1 and increase its DAG hydrolase activity to release a FA and produce MAG. MAG is hydrolyzed by MGL to release a final FA and glycerol. Lipolysis can also be stimulated by natriuretic peptide (NP) which signals through the NP receptor-A (NPR-A) to increase guanylyl cyclase (GC)/cyclic-GMP (cGMP) and activation of protein kinase G (PKG). PKG can also phosphorylate HSL and PLIN1. Insulin, the main anti-lipolytic hormone (red lines), signals through the insulin receptor (IR) and insulin receptor substrate 1 and 2 (IRS1/2) to increase activity of phosphoinositide 3-kinase (PI3K) and levels of PIP3. PIP3 activates the kinase AKT, which through currently unknown mechanisms (?), activates phosphodiesterase 3B (PDE3B) to hydrolyze cAMP to AMP. ABHD15, an AKT phosphorylation substrate, is required for the anti-lipolytic action of insulin and can bind to and stabilize PDE3B. Dotted line indicates movement of proteins, while the dashed red line notes currently unclear pathway of regulation.
Physiological activation of brown fat β3-AR through cold stress, (96-98) or direct pharmacological activation (99) rapidly activates non-shivering thermogenesis. This occurs through the action of mobilized FAs that function as allosteric activators of uncoupling protein 1 (UCP1), the molecular mechanism for heat generation in brown fat (6, 100). Activation of UCP1, a proton channel in the inner mitochondrial membrane of brown adipocytes, produces a leak in the mitochondrial membrane and uncouples ATP synthesis from respiration, thereby dissipating energy as heat (6, 100). However, the role of BAT PNPLA2 in maintaining thermogenesis has been recently challenged with tissue-specific gene knockout (KO) models. Work from the group of Renate Schreiber and Rudy Zechner demonstrated that PNPLA2 in BAT is not required for cold-induced thermogenesis, suggesting that other BAT lipases such as HSL or Carboxylesterase 3 (Ces3) (101) may suffice. However, upon deletion of PNPLA2 from WAT and BAT, mice were cold sensitive under fasting conditions, suggesting that WAT-derived FAs additionally function to support BAT thermogenesis (102). Furthermore, PNPLA2 in the heart is required to maintain the supply of FAs for cardiac function during thermogenesis, as PNPLA2 cardiac-specific deficient mice are cold intolerant (102). Similarly, mice that lack ABHD5 in BAT have normal cold-induced thermogenesis and are not cold-sensitive, but deletion of ABHD5 in both WAT and BAT imparts cold sensitivity in the fasted state (103). Recent evidence also suggests that in response to cold-exposure, WAT derived FAs are metabolized in the liver to acyl-carnitines which then circulate to fuel BAT-mediated thermogenesis (104). Overall, these data suggest that additional lipases may support BAT function, and that a whole-body response involving WAT, liver, and heart is required to mediate thermogenesis, especially under conditions such as fasting. Although, fatty acid oxidation (FAO) in BAT is necessary for maintaining thermogenesis (105, 106). Further evidence for the importance of systemic lipolysis is the fact that PNPLA2 in adipocytes is required to maintain acute exercise performance, however PNPLA2 in skeletal muscle is dispensable (107).
Natriuretic Peptides
Atrial Natriuretic Peptide (ANP) and B-type natriuretic peptide (BNP), the main natriuresis hormones, are synthesized in cardiac tissue in response to stretching that occurs with increases in blood volume and function to decrease extracellular fluid volume and increase renal sodium excretion (108). As part of this response, ANP stimulates lipolysis in adipocytes through the natriuretic peptide receptor-A (NPR-A) (109) and cyclic GMP (cGMP)/Protein kinase G (PKG) pathway to phosphorylate HSL and PLIN1 (110) (Figure 2). ANP-mediated lipolysis may be important in fueling cardiac tissue during increased function required during pressure overload and heart failure; however, in excess could predispose the heart to further complications (111). The effects of ANP on lipolysis are more relevant to primates than rodents, as rodents express over 100 times the level of the clearance receptor NP clearance receptor (NPCR), which mitigates the effect of ANP on lipolysis (112, 113). In humans, direct ANP infusion at maximal dose increases lipolysis greater than 10-fold (114) and can decreases blood pressure, and increases post-prandial lipid oxidation and energy expenditure (111), suggesting therapeutic benefits of ANP treatment are possible in man. Similar to β3-AR activation, ANP treatment promotes BAT-thermogenesis, browning of white fat and reduces body fat in rodents, while increasing respiration in human fat cell cultures (113). These beneficial effects of ANP require action on adipose tissue but not muscle (115).
Insulin
Insulin, a major negative regulator of adipocyte lipolysis, binds the insulin receptor (IR) and through the docking protein insulin receptor substrate 1 (IRS1) and IRS2 (116), activates the lipid kinase phosphatidylinositol 3-kinase (PI3-K), increasing production of PIP3 which stimulates the serine threonine kinase AKT/Protein kinase B (117) (Figure 2). Once active, AKT is thought to phosphorylate numerous proteins including phosphodiesterase 3 B (PDE3B), which leads to the hydrolysis of cAMP, and dampening of catabolic signals that promote adipocyte lipolysis (118, 119). Although, this model has recently been challenged. Mouse brown adipocyte that lack PDE3B lose the ability of insulin to suppress lipolysis; however, this does not require phosphorylation of PDE3B on S273 by AKT2 (117). Surprisingly, deletion of AKT2 only partly impairs the ability of insulin to suppress lipolysis (120). These data suggest the existence of additional mechanisms by which insulin suppresses lipolysis (117). In contrast, insulin-stimulated glucose uptake in adipocytes require AKT2, the predominant isoform in key insulin sensitive tissues (120). These differential effects of insulin on glucose uptake vs. lipolysis can be explained by selective insulin signaling/resistance that occurs in adipocytes (121). More recently, ABHD15, initially identified as a 47 KDa AKT substrate, has been shown to mediate the effects of insulin on lipolysis (122, 123) by stabilizing PDE3B (124, 125). Mice deficient in ABHD15 are unresponsive to the effects of insulin on lipolysis (122, 123). Moreover, the effects of ABHD15 on glucose uptake are somewhat unclear as Xia et al. found a defect in glucose uptake in WAT (122) in the absence of ABHD15, but Stockli et al. failed to find the same effect (123), discrepancies which may be explained by technical differences between the studies. In all, the exact mechanisms by which insulin suppresses adipocyte lipolysis are currently not completely understood.
Adipocyte lipolysis is controlled by intracellular intermediary lipid metabolites
In addition to the regulation of lipolysis by hormonal extracellular signals such as insulin and catecholamines, lipolysis is also controlled by intracellular mechanisms that involve intermediary lipid metabolites. Jepson et al. showed that oleoyl-CoA and oleic acid could inhibit HSL activity at 0.1 μM and 0.5 μM, respectively (126). Similarly, PNPLA2 is also inhibited by long-chain acyl-CoAs in a non-competitive manner with an IC50 for oleoyl-CoA of 33 μM (127). This inhibitory effect was directly through the N-terminal patatin-like domain and did not affect the interaction with ABHD5. ABHD5 ligands that release ABHD5 from PLIN1 or PLIN5 independent of PKA activation, led to the discovery of long-chain acyl-CoA (LC-CoA) as an endogenous allosteric regulator of ABHD5 that promotes the interaction with PLIN1/5 (48). These data suggest that ABHD5 senses LC-CoA to impart a negative feedback mechanism on lipolysis by its sequestration with PLIN1, demonstrating that ABHD5 integrates both extracellular (i.e. hormonal) and intracellular signals (48). Similarly, the interaction between ABHD5 and PNPLA3 is nutritionally regulated by oleoyl-CoA (70). Overall, these studies demonstrate that lipolysis is regulated by intermediary metabolites of FAs and that ABHD5 is an intracellular receptor and transducer of metabolic signals (Figure 1).
Protein turnover in the regulation of adipocyte lipolysis
The degradation of proteins is an essential cellular function necessary for protein quality control and maintaining protein composition of organelles. The two major pathways for the degradation of proteins involve the selective elimination through the ubiquitin-proteasome system (UPS) and removal through chaperone-mediated autophagy (CMA) (128). In the UPS, proteins are selectively marked by ubiquitination, the covalent addition of ubiquitin (Ub) molecules to lysine residues, which signals and targets the protein for proteolytic degradation by the 26S proteasome. The role of the UPS in the regulation of LD proteins will be reviewed, (Figure 1) and a more in-depth review on the regulation of the LD proteome can be found by Bersuker et al. (129).
The evasion of proteasomal degradation by LD proteins likely represents an active process in regulating expression levels. For example, many lipolytic proteins such as PLIN1 (13, 130), PNPLA2 (131, 132) and G0S2 (133) are stabilized by condition that promote LD biogenesis, such as oleic acid supplementation, where association with the phospholipid monolayer is thought to mask motifs that signal degradation. While oleic acid supplementation stabilizes PNPLA3, this occurs independent of LD formation (134). Interestingly, PNPLA3 I148M is not properly targeted by the UPS which likely promotes the disease-causing variant to accumulate on LDs (135) where it can disrupt protein-protein interactions critical for TAG hydrolysis (70, 75). It is currently unclear how the I148M variant evades ubiquitination, however targeting the UPS pathway (74, 136), or preventing LD accumulation of PNPLA3 could be potential therapeutics for FLD.
Protein-protein interactions are an additional mechanism that promote LD protein stability. Work from David Savage’s group showed that PLIN1 mutations from patients with lipodystrophies, which lack the C-terminus, fail to stabilize ABHD5 on LDs, resulting in greater basal lipolysis (137) and likely explains why these patients are unable to sequester FAs into TAGs. Furthermore, the binding of ABHD5 to PLIN1 (138) and binding of G0S2 to PNPLA2 prevents their ubiquitination and degradation by the proteasome (133). Mice that lack ABHD5 fail to localize PNPLA3 to LDs, suggesting that this interaction is important for stabilizing and targeting PNPLA3 to LDs (75). The exact mechanisms by which LD proteins are targeted for proteasomal degradation remain unclear. However, UBXD8, a protein in the ER-associated degradation (ERAD) pathway, can bind PNPLA2, preventing the interaction with ABHD5, thereby segregating PNPLA2 to valosin-containing protein (VCP)/p97 segragrase that promotes extraction and subsequent Ub-dependent degradation (132) (Figure 1). Additionally, Mysterin, an AAA+ ATPase/ubiquitin ligase was recently identified as a resident LD protein (139). Interestingly, the expression of Mysterin was shown to promote LD growth, an effect which was attributed to its ability to eliminate PNPLA2 from LDs (139). It will be important to know the exact mechanism by which Mysterin eliminates PNPLA2 and if it targets other PNPLA family members.
Adipocyte Lipolysis in metabolic signaling
Adipocyte Lipolysis in cellular signaling
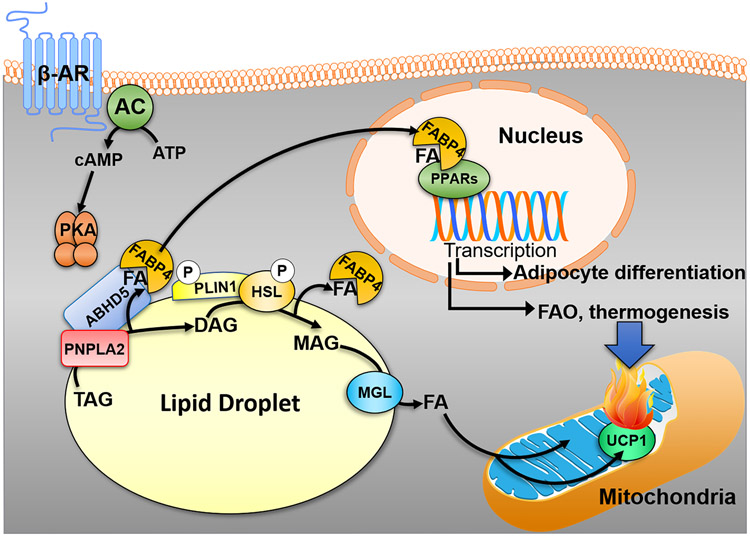
In addition to its metabolic role in providing substrates, lipolysis can also function in signaling to regulate cellular metabolism (Figure 3). Work from Hei Sook Sul’s lab demonstrated that PNPLA2 in adipocytes was required to maintain a BAT phenotype through the regulation of PPARα, a nuclear transcription factor (140). The deletion of PNPLA2 in adipocytes resulted in reduced PPARα activity and expression of target genes, including lower UCP1 protein levels and when exposed to cold, mice were cold sensitive (140). Similarly, pharmacological inhibition of HSL and knockdown of PNPLA2 reduced the expression of known PPAR target genes in which LDs were shown to be a source of PPARα and PPARδ ligands that could be generated within minutes of stimulating lipolysis (3). Additionally, our recent work demonstrates in real-time imaging assays that FAs can traffic from LDs to the nucleus to modulate gene expression in brown adipocytes (141). Furthermore, HSL seems to be important in generating PPARγ ligands (76), a fact which may explain why mice with deletion of adipocyte HSL and patients that lack HSL are lipodystrophic (142). These FAs mobilized by lipolysis are thought to be delivered by fatty acid binding proteins (FABPs), molecular lipid shuttles, which interact with ABHD5 (143), HSL (144) and PPARs (145, 146). The hydrolysis of retinyl esters by HSL in WAT may also generate signals such as ligands for Retinoid X receptors (77). Overall, these studies demonstrate that lipolysis signals a homeostatic response by providing ligands to nuclear receptors to regulate gene expression and thereby modulate cellular metabolism (Figure 3).
Figure 3. Adipocyte lipolysis produces intracellular signals in the homeostatic regulation of lipid metabolism.
Fatty acids (FA) released by the sequential action of adipocyte lipases and regulators (see text and Figure 2) in response to activation of β-adrenergic receptors (β-ARs) can function as ligands for the nuclear receptor peroxisome proliferator-activated receptors (PPARs). These FAs are thought to traffic to the nucleus by fatty acid binding protein 4 (FABP4) which can interact with ABHD5, sequester FAs, and delivery them to PPARs in the nucleus. In brown adipocytes, the mobilization of FAs can produce ligands for PPARα which traffic to the nucleus within minutes of stimulating lipolysis. This leads to a transcriptional program which upregulates the machinery involved in fatty acid oxidation (FAO) and thermogenesis (UCP1) to match supply with oxidation. In brown adipocytes, FAs are also allosteric regulator of UCP1, the molecular mechanism for heat production. In addition, lipolysis can produce PPARγ ligands which are important for adipocyte differentiation and maintenance of an adipocyte phenotype necessary for sequestration of FAs and preventing lipotoxicity.
Adipocyte lipolysis in inter-organ crosstalk
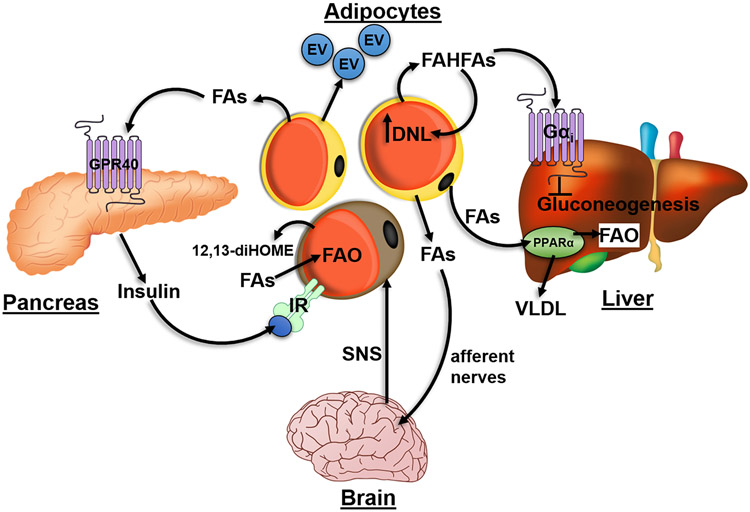
In addition to serving a role in signaling at the cellular level, adipose tissue lipolysis can also communicate metabolic and nutritional status with other tissues to regulate whole body energy homeostasis (Figure 4). Adipose tissue derived FAs modulate PPARα and cyclic AMP-responsive element-binding protein H (CREBH) transcriptional activity in the liver, two important transcription factors that regulate genes in FAO and endoplasmic reticulum (ER) stress, respectively (147-149). These adipocyte-derived FAs promote the expression of G0S2, the hormone fibroblast growth factor 21 (FGF21) (149) and genes involved in FAO (150), and regulate liver FAO and very low-density lipoprotein (VLDL) secretion (150). In addition, in adipose tissue, lipolysis and lipogenesis are coupled as chronic stimulation of lipolysis activates lipogenesis to match adipose tissue catabolism with anabolism (151). PNPLA2-mediated WAT lipolysis triggers insulin secretion to promote delivery of TAG-rich lipoproteins to BAT to replenish fuel for thermogenesis (152). The mechanisms by which released FAs promote insulin secretion is likely through the sensing of FAs by GPR40 receptor found on the surface of beta cells (153). Lipolysis can be locally sensed in white fat by afferent nerves to trigger a neural circuit from WAT to BAT that acutely induces BAT thermogenesis, mediated by representative FAs eicosopentanoic acid and arachidonic acid (154). Fatty Acid ester of Hydroxyl Fatty Acid (FAHFAs) are adipocyte derived lipokines that can improve insulin sensitivity by suppressing endogenous glucose production in liver through Gαi protein coupled receptors and enhance insulin action in adipocytes (155). These FAHFAs can be stored as TAGs (156) and mobilized during cold exposure by PNPLA2 to promote de-novo lipogenesis and FA-esterification in an insulin-independent manner (157), possibly explaining the coupling of lipolysis to lipogenesis in adipocytes (151). 12,13-diHOME, was identified as a lipolysis-derived BAT lipokine that could act in an autocrine–paracrine manner to promote FA transport into brown adipocytes through translocation of the FA transporters FATP1 and CD36 (158).
Figure 4. Adipocyte lipolysis promotes inter-organ crosstalk to regulate whole-body energy homeostasis.
Fatty acids (FAs) released by adipocyte lipolysis can function as PPARα ligands in the liver to upregulate a transcriptional program involved in increasing fatty acid oxidation (FAO) and VLDL secretion. Furthermore, fatty acid ester of hydroxyl fatty acids (FAHFAs) released by adipocytes can act through a Gαi-coupled receptor to suppress hepatic gluconeogenesis. These FAHFAs can also have autocrine effects by upregulating of de novo lipogenesis (DNL) and FA-esterification in adipocytes. FAs released by white adipocytes can activate GRP40 on β-cells in the pancreas to elicit insulin secretion which acts on brown adipose tissue (BAT) to promote greater FA uptake required for FAO. 12,13-diHOME, secreted from brown adipocytes can function in an autocrine manner to similarly promote further uptake and utilization of FAs. FAs released by white adipose tissue can be sensed by afferent nerves which feedback to the brain to increase sympathetic outflow to BAT through the sympathetic nervous system (SNS). Adipocytes can also secrete extracellular vesicles (EVs) in response to lipolysis that can further mediate tissue-tissue communication.
Extracellular vesicles (EVs) are secreted by a variety of cells, including adipocytes (159), and increasing evidence suggests that they may mediate paracrine/endocrine effects (160, 161). EVs can be secreted in response to adipocyte lipolysis where they could mediate signaling events. FABP4 secretion through EVs was increased in response to lipolytic stimulation which could be blocked with pharmacological inhibition of PNPLA2 and HSL (162). The secretion of FABP4 involved unconventional pathways of EV secretion that might involve exosome-like vesicles (163), autophagic vesicles (164) and endosomal/secretory lysosomes (165). Once secreted, FABP4 can mediate various metabolic responses (163, 166). Further work is required to understand additional EV-derived signals that are released by adipose tissue in response to lipolysis and the mechanisms of release. Overall, these studies highlight the role of adipocyte lipolysis in inter-organ crosstalk through the release of lipid signals and EVs to regulate whole body tissue homeostasis (Figure 4).
Adipocyte Lipolysis in Obesity and Insulin Resistance
Adipose tissue buffers the deleterious effects of FAs by storing them as TAGs in LDs (167). However, when the limit for storage of TAGs is reached or adipocyte lipolysis is dysregulated, FAs can accumulate in ectopic tissues such as skeletal muscle and the liver leading to insulin insensitivity and T2D (11). This is best illustrated in the two clinical conditions of lipodystrophy and obesity. In lipodystrophy, patients lack adipose tissue due to genetic mutations in the ability to properly form functional adipocytes or to store FAs as TAGs (168). In obesity, the capacity for adipocytes to store TAGs is reached (1). In both conditions, FA spillover leads to ectopic lipid accumulation in peripheral tissues and overt T2D (11, 168). The concept of adipose tissue as a buffer is also illustrated in genetic (169) and pharmacological models (170) that enhance TAG storage in adipocytes which improves adipocyte function, thereby limiting lipotoxicity and improving metabolic disorders.
In obesity, increased plasma FAs are attributed to a higher basal rate of adipocyte lipolysis compared to lean subjects (171, 172). However, this difference is abolished when tissue mass is taken into consideration (173), and no difference in basal lipolysis is observed when adjusted for adipocyte size in vitro (174). Obesity is characterized by a chronic low-grade inflammatory state with increased secretion of inflammatory cytokines including TNFα (175). The majority of TNFα is secreted by resident macrophages in adipose tissue (176) and affects adipocyte lipolysis through multiple mechanisms. TNFα can promote insulin resistance in adipocytes (177), and FAs can activate JNK to promote the additional release of pro-inflammatory cytokines from macrophages (178) and adipocytes (179), inciting a vicious cycle. TNFα increases phosphorylation of PLIN1 and decreases expression of both PLIN1 and G0S2 (180-182) thereby promoting greater basal lipolysis and release of FAs into plasma. IL-6, which is also increased in obesity (176, 183) can stimulate lipolysis (184) and cause hyperglycemia and hepatic insulin resistance in rats (183) and requires intact JNK signaling in macrophages (183) to promote diet-induced insulin resistance (185).
In T2D patients, insulin-mediated inhibition of lipolysis is thought to be impaired, thereby increasing plasma FA levels and ectopic accumulation of TAGs in key insulin sensitive tissues leading to insulin resistance (186). This concept of adipocyte lipolysis driving insulin resistance is supported by adipocyte specific ATGL KO (187) and HSL KO mice (150), in which plasma FAs are reduced and whole body insulin sensitivity is increased. Nonetheless, the interpretation of these studies is complicated by the fact that these models are constitutive genetic deletions, and due to the lack of FA mobilization from adipocytes, these mice are completely reliant on glucose as a fuel source. Interestingly, in chronically insulin treated 3T3-L1 cells and fat explants from insulin resistant mice, only insulin-stimulated glucose uptake is decreased while insulin suppression of lipolysis is intact (121), effects which are explained by selective insulin resistance in adipocytes. Isolated adipocytes from T2D patients have greater lipolysis (188); however, this is controversial in vivo (189). In a recent study, adipocytes isolated from T2D patients have no deficits in the signaling cascades of lipolysis. Instead, re-esterification of FAs is reduced by 25% in T2D adipocytes leading to increased FA levels (190), suggesting the primary defect may be in sequestration of FAs and not lipolysis per se.
The mechanisms of FA-induced insulin resistance have been intensively studied and have been attributed to two metabolites of FAs - DAGs and ceramides. Both accumulate in insulin-resistant animals and humans (191-193). DAGs are generated de novo from fatty acyl-CoA while ceramides are generated de novo from palmitate (8). DAG-mediated activation of protein kinase C theta (PKC θ) leads to phosphorylation of IRS residue S307. Subsequently, insulin-stimulated tyrosine phosphorylation is decreased leading to impaired glucose transport and resistance (194). Knockout of mitochondrial acyl-CoA:glycerol-sn-3-phosphate acyltransferase 1 (mtGPAT1), a major hepatic glycerol-3-phosphate acyltransferase, decreased de novo DAG generation protecting mice from hepatic insulin resistance (195). Furthermore, DAG generated by PNPLA2 preferentially produces 1,3- DAG which cannot activate PKC (196), suggesting that the de novo generation of DAGs leads to insulin resistance. Like DAG, ceramides can also impact insulin signaling through PKC, specifically PKC zeta (ζ). Ceramide activation of PKCζ leads to phosphorylation of PKB/AKT preventing PKB/AKT trafficking to the cellular membrane and insulin stimulation (197). Specifically, the generation of ceramides by dihydroceramide desaturase 1 (DES1) in adipocytes seems to impart some of the lipotoxic effects such as impaired glucose utilization, insulin resistance and hepatic steatosis (198). Contrary to the spillover hypothesis, ceramides initially decrease adipose tissue lipolysis, where de-novo ceramide synthesis may be a counter-regulatory response to limit FA spillover (198). It remains to be understood the exact mechanisms by which adipocyte-generated ceramides promote lipotoxicity; however, one possibility is that adipocyte derived ceramides might circulate to impair insulin resistance in peripheral tissues.
In addition to impairing insulin signaling, adipocyte derived FAs can also impact gluconeogenesis in the liver, where a hallmark of T2D is the loss of the inverse relationship between gluconeogenesis and lipogenesis. Insulin normally suppresses gluconeogenesis while promoting lipogenesis, however, under insulin resistant states such as T2D, hepatic lipogenesis remains insulin sensitive and suppression of gluconeogenesis is insulin resistant, leading to a metabolic state called “selective hepatic insulin resistance” (199). Recent studies have shown that insulin-mediated suppression of hepatic gluconeogenesis occurs indirectly through the ability of insulin to suppress adipocyte lipolysis and the flux into liver pyruvate carboxylase, the rate-limiting enzyme in gluconeogenesis (183, 200). In T2D, FA spillover from adipocytes increases hepatic acetyl-CoA, an allosteric regulator of pyruvate carboxylase activity, resulting in greater gluconeogenesis in the liver (183, 200). This effect was alleviated with both genetic and pharmacological inhibition of adipocyte lipolysis, which decreased hepatic acetyl-CoA levels and gluconeogenesis, thereby normalizing plasma glucose in mice fed with high fat diet (HFD) (183). Overall, multiple mechanisms including inflammation and reduced FA re-esterification likely explain the impaired buffering capacity in adipocytes during obesity that leads to the spillover of FAs, buildup of toxic lipid metabolites and allosteric regulators that suppress insulin action and increased hepatic glucose output, respectively (Figure 5).
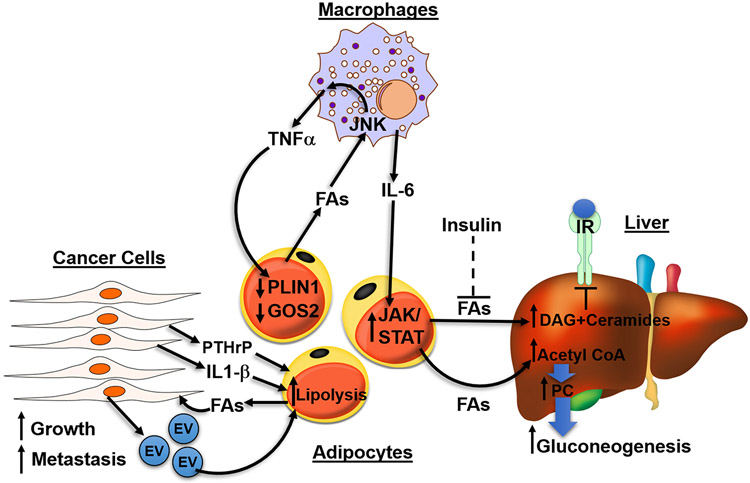
Figure 5. Adipocyte lipolysis is implicated in disease such as diabetes, fatty liver disease and cancer.
In diseased states, excessive fatty acids (FAs) activate signaling in surrounding cells to further increase lipolysis in adipocytes, forming a vicious, positive feedback loop. In obesity, adipocytes lose their ability to safely buffer FAs as TAGs, leading to the spillover of FAs which act upon macrophages through JNK signaling to release inflammatory cytokines TNFα and IL-6. Both cytokines function on adipocytes to further increase lipolysis. TNFα decreases PLIN1 and G0S2, inhibitors of PNPLA2, and IL-6 acts through JAK/STAT pathway to increase lipolysis. Under normal physiology, insulin functions to suppress lipolysis, however, in type 2 diabetes (T2D), the failure of insulin to suppress FA release from adipocytes (dashed line) results in excessive FA flux to the liver. FAs in the liver can form DAG and ceramides which act on PKC to decrease insulin sensitivity. Hepatic acetyl-CoA, generated from adipocyte FAs, allosterically upregulates pyruvate carboxylase (PC) to increase gluconeogenesis in the liver. Lastly, cancer cells secrete EVs and cytokine IL-1β increasing lipolysis of adipocytes in the tumor environment. Tumor-derived parathyroid-hormone-related protein (PTHrP) can promote the upregulation of lipolytic enzymes to promote cancer associated cachexia. Cancer cells use FAs generated from lipolysis as fuel to increase growth and invasiveness.
Adipocyte Lipolysis in Fatty Liver Disease
FLD is a full spectrum disease ranging from excessive accumulation of TAGs in the liver to an advanced stage of disease that additionally includes inflammation known as non-alcoholic steatohepatitis (NASH) and is associated with both obesity and insulin resistance (201). In the liver, there is a delicate balance between FA uptake from peripheral tissues, storage into TAGs, and efflux of TAGs through VLDL particles. Disruption of any of these processes can lead to perturbation of TAG homeostasis and the development of FLD (reviewed in (202). Metabolic tracer studies in humans demonstrate that adipose tissue derived FAs account for the majority of hepatic TAGs in FLD patients, suggesting that dysregulation of adipocyte lipolysis is a major mechanism of diet-induced FLD (203) (Figure 5). Adipocyte specific KO of ABHD5 decreased lipolysis, resulting in reduced FA flux to the liver, and protection against HFD induced hepatic steatosis (149), suggesting that suppressing adipocyte lipolysis could be protective against FLD. Nevertheless, as discussed above, adipocyte derived FAs can reprogram liver metabolism through counterregulatory responses that activate PPARα and CREBH (149).
Inhibiting adipocyte lipolysis may seem an attractive therapeutic option for FLD; however, loss of function mutations in HSL cause FLD in humans (142) and partial lipodystrophy with age (204, 205). To study the mechanism by which deletion of HSL leads to the development of FLD and lipodystrophy, Xia et al. developed tissue specific KOs of HSL in adipocytes and liver. Interestingly, adipocyte deletion of HSL causes FLD by 8 months of age, while hepatocyte KO of HSL does not (150). In addition, the absence of HSL in adipocytes also causes lipodystrophy with decreased expression of lipogenic genes in adipocytes and reduced expression of PPARα target genes and FAO in the liver (150). Taken together, these data demonstrate that HSL-mediated adipocyte lipolysis is required to maintain proper functioning adipose tissue and to activate PPARα in the liver, thereby preventing the accumulation of hepatic TAGs and the development of FLD.
Adipocyte Lipolysis in Cancer
Crosstalk between adipocytes and cancer cells in the tumor microenvironment can create a metabolic symbiosis driving cancer growth and metastasis. Coupled with glucose, FAs are also vital for cancer cells for synthesis of membrane lipids (206), energy through FAO (207), and produce pro-tumorigenic lipid signaling molecules such as LPA (208). As a result, cancer cells upregulate de novo synthesis of FAs, and elevated levels of fatty acid synthase (FAS) correlate negatively with cancer prognosis (209). Besides synthesizing FAs, it has become clear that cancer cells can also use exogenous FAs for fuel as well.
Emerging studies have shown that intracellular lipolysis is critical for fueling cancer cells and promoting their survival. When co-cultured with adipocytes, breast and ovarian cancer cells increase TAG storage, suggesting lipid transfer from adipocytes to cancer cells promote growth and aggressiveness (25, 210). PNPLA2 is upregulated in breast cancer cells co-cultured with adipocytes, enhancing intracellular lipolytic activity of cancer cells (210, 211). In vivo, PNPLA2 is expressed in breast cancer cells and not in normal breast epithelial cells, and PNPLA2 expression is correlated with poor prognosis (211). Moreover, silencing of PNPLA2 dramatically reduces breast cancer cell invasiveness (210, 211). Prostate cancer cells co-cultured with adipocytes increase expression of IL1-β to stimulate adipocyte lipolysis in a vicious positive feedback loop that decreases prostate cancer cell sensitivity to docetaxel and upregulates prostaglandin E2 (PGE2) synthesis in adipocytes, a prostate cancer pro-survival signal (212). Taken together, PNPLA2-mediated intracellular lipolysis promotes crosstalk between cancer cells and adipocytes and subsequently promotes tumor progression and aggressiveness.
The exact mechanisms of communication between adipocytes and tumor cells are not completely understood. FABPs, have high affinity for FAs, bile, and retinoids (213) and have been shown to play a role in transporting FAs between adipocytes and cancer cells in which pharmacological inhibition of FABP4 reduces TAG content in ovarian cells and aggressiveness. Furthermore, FABP4 KO mice have decreased tumor burden and metastasis compared to WT mice when injected with ovarian cancer cells (25), suggesting FABP4 may be an attractive target for cancer.
Besides FABPs, secreted EVs have been implicated in tumor-fat cell communication. Tumors have long been known to promote an energy wasting state known at cachexia, which involves muscle and fat loss. Injection of Lewis lung carcinoma (LLC) or B16 melanoma cells promoted tumor growth and loss of WAT and muscle (214). Cancer-associated cachexia (CAC) was blocked in PNPLA2-deficient mice and to a lesser extent in HSL-deficient mice, demonstrating that lipolysis is essential for CAC (214). The exact signals that induce CAC are not entirely known, however, co-culture of lung cancer-derived EVs can promote adipocyte lipolysis through the delivery of IL-6 (215). Similarly, EVs derived from cancer patients with pancreatic cancer and immortalized pancreatic cancer cell lines can promote adipocyte lipolysis. These effects were inhibited by adrenomedullin receptor (ADMR) blockade, suggesting EV-derived adrenomedullin mediates these effects (216). In addition, coculture of breast cancer cells with adipocytes can promote EV-mediated lipolysis (217). Adipocyte EVs can transfer both FAs and proteins involved in FAO to melanoma cells, thereby increasing aggressiveness (218) and promoting metastasis in melanoma and prostate cancer cells (219). In concert with increased lipolysis, increased FAO has also been shown to drive cancer growth and aggressiveness in breast cancer cells co-cultured with adipocytes (211). Blocking EV production in vivo can prevent CAC in mice bearing LLC tumors (220). In a model of LLC CAC, tumor-derived parathyroid-hormone-related protein (PTHrP) could promote an energy wasting state by reprogramming a thermogenic state in adipose tissue, which involved the upregulation of lipolytic enzymes (221).
Therapeutic interventions that target adipocyte lipolysis
Targeting adipocyte lipolysis for reducing obesity and improving insulin resistance has been of therapeutic interest for decades now (222); however, treatments that directly target this pathway are currently not in clinical use. While seemingly straightforward, the argument for stimulating or inhibiting lipolysis can be made (Table 1). One potential means of targeting adipocyte lipolysis is through the pharmacological activation of β3-ARs. In mice, β3-AR receptors mediate cold-induced thermogenesis (223) and pharmacological activation of β3-ARs acutely stimulate lipolysis leading to two-fold increase in energy expenditure, a dramatic ten-fold increase in insulin levels, and 40–50% reduction in food intake (98). Similarly, chronic treatment with β3-AR agonists have been shown to decrease adiposity and improve insulin resistance in obese rodents (224, 225).
Table 1.
Summary of Therapeutic Targeting of Lipolysis in Diseased States
| Disease | Net Change on Lipolysis |
Therapeutic Targeting |
Strategy | References |
|---|---|---|---|---|
| Obesity | Increased | Stimulate | Increase BAT lipolysis and FA oxidation | 98,222-225,235 |
| Inhibit | Promote storage of FAs into TAGs thereby preventing spillover | 11,27,169,170-172 | ||
| Fatty Liver Disease | Increased | Inhibit | Prevent FA spillover into liver | 27,149,203,241 |
| Type 2 Diabetes | Increased/Unaffected | Stimulate | Increase energy expenditure and insulin levels | 98, 224, 225 |
| Inhibit | Prevent buildup of DAGs and ceramides | 27,183,187,200,239 | ||
| Prevent adipocyte derived FAs from activating gluconeogenesis | 183,200 | |||
| Improve adipose tissue inflammation | 175, 177-183, 185 | |||
| Re-esterification | Consume ATP and increase futile cycling of FAs and sequestration in adipocytes | 151, 190 | ||
| Cancer | Increased | Inhibit | Prevent cancer cells from using FAs as fuel | 27,233-235 |
With such immense success in rodents, β3-AR agonists have not yet translated into treatments for human obesity and diabetes. In humans, β3 receptors are mostly expressed in the bladder, with minimal expression in other tissues (226). Clinical trials show no effect of β3 agonists on activating lipolysis and increasing energy expenditure (227, 228). The failure of these agonists for approval of obesity and diabetes was attributed to poor bioavailability and cross reactivity with the other β receptors (229). More recently, a newer generation β3-AR agonist, Mirabegron, which is approved for incontinence, showed increased BAT activity in healthy patients; however, the dose required was four times that recommended and was associated with adverse cardiac side effects (230). A potential drawback of such a therapy is that the beneficial effects of β3-AR require BAT (96-98), which may be a paucity in humans (231). Indeed, much of the cold-induced thermogenic response is humans is mediated by muscle (232). A potential alleviation to the lack of β3-AR action in humans would be to stimulate lipolysis downstream of receptor activation. Work from the Granneman group identified ABHD5 ligands that could stimulate lipolysis by bypassing receptor activation through the dissociation of ABHD5 from PLIN1 (48). However, such a treatment would require coupling to FAO (106) and metabolic homeostatic mechanisms such as FA signaling (3) to prove successful. Alternatively, Adenosine, A2A and A2B receptors stimulate lipolysis in human brown adipocytes which may represent a novel mechanism of BAT regulation in humans (233). Importantly, adenosine increased blood flow to BAT in healthy lean human subjects (234); however the levels of energy consumption attained in BAT are likely to have minimal effects on whole-body energy expenditure (231).
By taking advantage of the increased 14C atmospheric levels during the Cold War and 14C incorporation into lipid, the group of Peter Arner and Kristy Spalding were able to calculate lipid age and turnover, demonstrating that lipolysis is defective during aging (235). Over 13 years, average lipid age, that is the average amount of time lipids spent in WAT, increased by 0.6 years irrespective of weight change and age, suggesting decreased lipid turnover (235). In individuals that underwent bariatric surgery, lipid age increased in patients with weight regain, while lipid age decreased in individuals with stable weight (235). The mechanism of decreased lipid turnover with aging may be due to decreased sympathetic tone (236). Alternatively, in a cohort of 87 obese women, resistance to both catecholamine and ANP-mediated lipolysis was observed (237). Overall these data suggest increasing lipolysis is a viable therapeutic option in maintaining weight-loss following bariatric surgery.
Adipocyte overexpression of PNPLA2 protected mice from weight gain on chow and HFD, decreased adipocyte size and increased FAO and turnover of TAG (238), supporting the cause for stimulating lipolysis therapeutically. One of the caveats of stimulating lipolysis is the fate of FAs that are released. If excess FAs are deposited in ectopic tissues, this could lead to lipotoxic effects of FAs. Interestingly, overexpression of PNPLA2 does not increase serum FAs or promote the ectopic deposition of TAGs. Instead, this increased UCP1 expression, energy expenditure and thermogenesis (238). This suggests that pharmacological agents that can stimulate PNPLA2-mediated lipolysis may be beneficial for obesity and insulin resistance.
On the other hand, genetic deletion of PNPLA2 has been shown to improve metabolic disease as well. Mice which lack PNPLA2 are highly insulin sensitive and resistant to HFD-induced insulin resistance (239). Surprisingly, deletion of PNPLA2 does not result in obesity, likely due to counter-regulatory mechanisms such as reductions in food intake and reduced PPARγ signaling in adipose tissue (239). Adipocyte specific deletion of PNPLA2 also decreases lipids in serum and improves hepatic insulin sensitivity through decreased TAG accumulation, immune cell infiltration and inflammation in the liver (187). These mice are also protected against HFD-induced increase in hepatic glucose production (183). However, a potential negative finding due to the lack of PNPLA2 was greater inflammation and immune cell infiltration in adipose tissue which could impair adipocyte function (187). Complementary studies demonstrate that pharmacological inhibition of PNPLA2, with ATGListatin (240), prevents HFD-induced weight gain due to a decrease in food consumption (27). A concern would be the development of FLD, since liver specific PNPLA2 KO mice develop FLD; however (241), mice treated with ATGListatin had 50% decrease in hepatic TAG content attributed to decrease FA flux to the liver and increased insulin sensitivity (27). These data suggest that ATGListatin can selectively inhibit adipocyte lipolysis without altering liver TAG levels, however due to poor efficacy of ATGListatin on human PNPLA2 (27), this has not been tested in the clinic.
In summary, these findings support both the stimulation and inhibition of adipocyte lipolysis for therapeutic benefit, which will need to be specifically tailored to the disease state in question (Table 1). Stimulating lipolysis would prevent stagnant lipid pools due to reduced lipid turnover observed in aging (235). Indeed, promoting lipid turnover is an energy consuming process where lipolysis is coupled to de novo synthesis (151), while blocking turnover impairs adipocyte function (187). However, excess FAs in circulation would likely result in the development of FLD and T2D, therefore stimulated lipolysis would need to be coupled to FAO and ATP demand or energy wasting processes (6). Alternatively, stimulating FA re-esterification would be beneficial by preventing ER stress, mitochondrial damage and the lipotoxic effects of FAs (242, 243). Additionally, FA re-esterification consumes ATP to activate AMPK (244), which could improve adipocyte function (245). Furthermore, treatments that target the interaction between PNPLA3 and ABHD5 (70) or the degradation of PNPLA3 (74) could be of therapeutic benefit to patients that carry the PNPLA3 I148M variant. In conclusion, our understanding of adipocyte lipolysis has greatly increased over the past 50 years, and further work should uncover additional layers of regulation that can be targeted.
Acknowledgements
We thank Drs. James Granneman and Otto Muzik for insightful comments regarding human BAT positron emission tomography studies. We thank Patrick Lee for images of adipocytes.
Funding
This work was supported by the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) grants R00DK114471 (E.P.M) and F30-DK116529 (A.Y).
Abbreviations
- FA
Fatty Acid
- TAG
Triacylglycerol
- LD
Lipid Droplet
- BAT
Brown Adipose Tissue
- PNPLA
Phospholipase domain containing
- ATGL
Adipocyte triglyceride lipase
- HSL
Hormone Sensitive Lipase
- MGL
Monoacylglycerol Lipase
- T2D
Type 2 Diabetes
- FLD
Fatty Liver Disease
- ABHD5
alpha/beta hydrolase domain-containing protein 5
- CDS
Chanarin-Dorfman Syndrome
- PLIN1
Perilipin 1
- PAT
perilipin/ADRP/TIP47
- DAG
Diacylglycerol
- G0S2
G0/G1 switch gene 2
- PPAR
peroxisome proliferator-activated receptor
- HILPDA
Hypoxia-inducible lipid droplet-associated protein
- HID2
Hypoxia inducible protein 2
- MAG
Monoacylglycerol
- LPAAT
lysophosphatidic acid acyltransferase
- β-ARs
β- adrenergic receptors
- cAMP
cyclic AMP
- UCP1
uncoupling protein 1
- KO
knockout
- Ces3
Carboxylesterase 3
- FAO
fatty acid oxidation
- ANP
Atrial Natriuretic Peptide
- BNP
B-type Natriuretic Peptide
- PKG
Protein Kinase G
- NPCR
NP Clearance Receptor
- NPR-A
natriuretic peptide receptor-A
- cGMP
cyclic GMP
- WAT
white adipose tissue
- IRS
insulin receptor substrate
- PI3-K
phosphatidylinositol 3-kinase
- PDE3B
phosphodiesterase 3 B
- LC-CoA
long-chain acyl-CoA
- UPS
ubiquitin- proteasome system
- CMA
chaperone-mediated autophagy
- ERAD
ER-associated degradation
- VCP
valosin-containing protein
- LPA
lysophosphatidic acid
- SIRT1
Sirtuin1
- FABP
Fatty acid binding protein
- CREBH
cAMP responsive element binding protein H
- ER
Endoplasmic Reticulum
- VLDL
Very low-density lipoprotein
- FGF21
Fibroblast growth factor 21
- FAHFA
Fatty acid ester of hydroxyl fatty acid
- EVs
extracellular vesicles
- PKC
protein kinase C
- HFD
high fat diet
- DES1
dihydroceramide desaturase 1
- NASH
Non-alcoholic steatohepatitis
- FAS
Fatty acid synthase
- PGE2
prostaglandin E2
- LLC
Lewis lung carcinoma
- CAC
cancer-associated cachexia
- ADMR
adrenomedullin receptor
- PTHrP
parathyroid hormone-related protein
Footnotes
Competing Interests
The authors declare that there are no competing interests.
References
- 1.Unger RH, Scherer PE. Gluttony, sloth and the metabolic syndrome: a roadmap to lipotoxicity. Trends in Endocrinology & Metabolism. 2010;21(6):345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edens NK, Leibel RL, Hirsch J. Mechanism of free fatty acid re-esterification in human adipocytes in vitro. J Lipid Res. 1990;31(8):1423–31. [PubMed] [Google Scholar]
- 3.Mottillo EP, Bloch AE, Leff T, Granneman JG. Lipolytic Products Activate Peroxisome Proliferator-activated Receptor (PPAR) and in Brown Adipocytes to Match Fatty Acid Oxidation with Supply. Journal of Biological Chemistry. 2012;287(30):25038–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ong KT, Mashek MT, Bu SY, Greenberg AS, Mashek DG. Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatology. 2011;53(1):116–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zechner R, Zimmermann R, Eichmann Thomas O, Kohlwein Sepp D, Haemmerle G, Lass A, et al. FAT SIGNALS - Lipases and Lipolysis in Lipid Metabolism and Signaling. Cell Metabolism. 2012;15(3):279–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicholls DG. The hunt for the molecular mechanism of brown fat thermogenesis. Biochimie. 2017;134:9–18. [DOI] [PubMed] [Google Scholar]
- 7.Nicholls DG, Locke RM. Thermogenic mechanisms in brown fat. Physiol Rev. 1984;64(1):1–64. [DOI] [PubMed] [Google Scholar]
- 8.Bikman BT, Summers SA. Ceramides as modulators of cellular and whole-body metabolism. J Clin Invest. 2011;121(11):4222–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Unger RH, Zhou YT. Lipotoxicity of beta-cells in obesity and in other causes of fatty acid spillover. Diabetes. 2001;50 Suppl 1:S118–21. [DOI] [PubMed] [Google Scholar]
- 10.Tripathy D, Mohanty P, Dhindsa S, Syed T, Ghanim H, Aljada A, et al. Elevation of Free Fatty Acids Induces Inflammation and Impairs Vascular Reactivity in Healthy Subjects. Diabetes. 2003;52(12):2882–7. [DOI] [PubMed] [Google Scholar]
- 11.Samuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest. 2016;126(1):12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Granneman JG, Moore H-PH, Granneman RL, Greenberg AS, Obin MS, Zhu Z. Analysis of lipolytic protein trafficking and interactions in adipocytes. The Journal of biological chemistry. 2007;282(8):5726–35. [DOI] [PubMed] [Google Scholar]
- 13.Xu G, Sztalryd C, Londos C. Degradation of perilipin is mediated through ubiquitination-proteasome pathway. Biochim Biophys Acta. 2006;1761(1):83–90. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi T, Omatsu N, Matsushita S, Osumi T. CGI-58 Interacts with Perilipin and Is Localized to Lipid Droplets: POSSIBLE INVOLVEMENT OF CGI-58 MISLOCALIZATION IN CHANARIN-DORFMAN SYNDROME. Journal of Biological Chemistry. 2004;279(29):30490–7. [DOI] [PubMed] [Google Scholar]
- 15.Sztalryd C, Xu G, Dorward H, Tansey JT, Contreras JA, Kimmel AR, et al. Perilipin A is essential for the translocation of hormone-sensitive lipase during lipolytic activation. J Cell Biol. 2003;161(6):1093–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Granneman JG, Moore HP, Krishnamoorthy R, Rathod M. Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 (Abhd5) and adipose triglyceride lipase (Atgl). J Biol Chem. 2009;284(50):34538–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306(5700):1383–6. [DOI] [PubMed] [Google Scholar]
- 18.Haemmerle G, Zimmermann R, Strauss JG, Kratky D, Riederer M, Knipping G, et al. Hormone-sensitive lipase deficiency in mice changes the plasma lipid profile by affecting the tissue-specific expression pattern of lipoprotein lipase in adipose tissue and muscle. J Biol Chem. 2002;277(15):12946–52. [DOI] [PubMed] [Google Scholar]
- 19.Taschler U, Radner FP, Heier C, Schreiber R, Schweiger M, Schoiswohl G, et al. Monoglyceride lipase deficiency in mice impairs lipolysis and attenuates diet-induced insulin resistance. J Biol Chem. 2011;286(20):17467–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Xie X, Heckmann BL, Saarinen AM, Gu H, Zechner R, et al. Identification of an intrinsic lysophosphatidic acid acyltransferase activity in the lipolytic inhibitor G0/G1 switch gene 2 (G0S2). Faseb j. 2019;33(5):6655–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenkins CM, Mancuso DJ, Yan W, Sims HF, Gibson B, Gross RW. Identification, Cloning, Expression, and Purification of Three Novel Human Calcium-independent Phospholipase A2 Family Members Possessing Triacylglycerol Lipase and Acylglycerol Transacylase Activities. Journal of Biological Chemistry. 2004;279(47):48968–75. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh AK, Ramakrishnan G, Chandramohan C, Rajasekharan R. CGI-58, the Causative Gene for Chanarin-Dorfman Syndrome, Mediates Acylation of Lysophosphatidic Acid. Journal of Biological Chemistry. 2008;283(36):24525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Zhang CC, Yang H, Soni KG, Wang SP, Mitchell GA, et al. An Epistatic Interaction between Pnpla2 and Lipe Reveals New Pathways of Adipose Tissue Lipolysis. Cells. 2019;8(5):395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kusminski CM, Bickel PE, Scherer PE. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nature reviews Drug discovery. 2016;15(9):639–60. [DOI] [PubMed] [Google Scholar]
- 25.Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17(11):1498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schweiger M, Lass A, Zimmermann R, Eichmann TO, Zechner R. Neutral lipid storage disease: genetic disorders caused by mutations in adipose triglyceride lipase/PNPLA2 or CGI-58/ABHD5. American Journal of Physiology - Endocrinology And Metabolism. 2009;297(2):E289. [DOI] [PubMed] [Google Scholar]
- 27.Schweiger M, Romauch M, Schreiber R, Grabner GF, Hutter S, Kotzbeck P, et al. Pharmacological inhibition of adipose triglyceride lipase corrects high-fat diet-induced insulin resistance and hepatosteatosis in mice. Nat Commun. 2017;8:14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villena JA, Roy S, Sarkadi-Nagy E, Kim K-H, Sul HS. Desnutrin, an Adipocyte Gene Encoding a Novel Patatin Domain-containing Protein, Is Induced by Fasting and Glucocorticoids: ECTOPIC EXPRESSION OF DESNUTRIN INCREASES TRIGLYCERIDE HYDROLYSIS. Journal of Biological Chemistry. 2004;279(45):47066–75. [DOI] [PubMed] [Google Scholar]
- 29.Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science (New York, NY). 2006;312(5774):734–7. [DOI] [PubMed] [Google Scholar]
- 30.Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, et al. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metabolism. 2006;3(5):309–19. [DOI] [PubMed] [Google Scholar]
- 31.Yamaguchi T, Omatsu N, Morimoto E, Nakashima H, Ueno K, Tanaka T, et al. CGI-58 facilitates lipolysis on lipid droplets but is not involved in the vesiculation of lipid droplets caused by hormonal stimulation. Journal of lipid research. 2007;48(5):1078–89. [DOI] [PubMed] [Google Scholar]
- 32.Akiyama M, Sawamura D, Nomura Y, Sugawara M, Shimizu H. Truncation of CGI-58 protein causes malformation of lamellar granules resulting in ichthyosis in Dorfman-Chanarin syndrome. The Journal of investigative dermatology. 2003;121(5):1029–34. [DOI] [PubMed] [Google Scholar]
- 33.Lefevre C, Jobard F, Caux F, Bouadjar B, Karaduman A, Heilig R, et al. Mutations in CGI-58, the gene encoding a new protein of the esterase/lipase/thioesterase subfamily, in Chanarin-Dorfman syndrome. American journal of human genetics. 2001;69(5):1002–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanders MA, Zhang H, Mladenovic L, Tseng YY, Granneman JG. Molecular Basis of ABHD5 Lipolysis Activation. Scientific reports. 2017;7:42589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenberg AS, Egan JJ, Wek SA, Moos MC Jr., Londos C, Kimmel AR. Isolation of cDNAs for perilipins A and B: sequence and expression of lipid droplet-associated proteins of adipocytes. Proc Natl Acad Sci U S A. 1993;90(24):12035–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sztalryd C, Brasaemle DL. The perilipin family of lipid droplet proteins: Gatekeepers of intracellular lipolysis. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862(10 Pt B):1221–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Subramanian V, Rothenberg A, Gomez C, Cohen AW, Garcia A, Bhattacharyya S, et al. Perilipin A Mediates the Reversible Binding of CGI-58 to Lipid Droplets in 3T3-L1 Adipocytes. Journal of Biological Chemistry. 2004;279(40):42062–71. [DOI] [PubMed] [Google Scholar]
- 38.Holm C, Kirchgessner TG, Svenson KL, Fredrikson G, Nilsson S, Miller CG, et al. Hormone-sensitive lipase: sequence, expression, and chromosomal localization to 19 cent-q13.3. Science. 1988;241(4872):1503–6. [DOI] [PubMed] [Google Scholar]
- 39.Haemmerle G, Zimmermann R, Hayn M, Theussl C, Waeg G, Wagner E, et al. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J Biol Chem. 2002;277(7):4806–15. [DOI] [PubMed] [Google Scholar]
- 40.Wang SP, Laurin N, Himms-Hagen J, Rudnicki MA, Levy E, Robert MF, et al. The adipose tissue phenotype of hormone-sensitive lipase deficiency in mice. Obesity research. 2001;9(2):119–28. [DOI] [PubMed] [Google Scholar]
- 41.Osuga J, Ishibashi S, Oka T, Yagyu H, Tozawa R, Fujimoto A, et al. Targeted disruption of hormone-sensitive lipase results in male sterility and adipocyte hypertrophy, but not in obesity. Proc Natl Acad Sci U S A. 2000;97(2):787–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Egan JJ, Greenberg AS, Chang MK, Wek SA, Moos MC, Londos C. Mechanism of hormone-stimulated lipolysis in adipocytes: translocation of hormone-sensitive lipase to the lipid storage droplet. Proc Natl Acad Sci U S A. 1992;89(18):8537–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen WJ, Patel S, Miyoshi H, Greenberg AS, Kraemer FB. Functional interaction of hormone-sensitive lipase and perilipin in lipolysis. J Lipid Res. 2009;50(11):2306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyoshi H, Souza SC, Zhang HH, Strissel KJ, Christoffolete MA, Kovsan J, et al. Perilipin promotes hormone-sensitive lipase-mediated adipocyte lipolysis via phosphorylation-dependent and -independent mechanisms. J Biol Chem. 2006;281(23):15837–44. [DOI] [PubMed] [Google Scholar]
- 45.Brasaemle DL, Rubin B, Harten IA, Gruia-Gray J, Kimmel AR, Londos C. Perilipin A increases triacylglycerol storage by decreasing the rate of triacylglycerol hydrolysis. J Biol Chem. 2000;275(49):38486–93. [DOI] [PubMed] [Google Scholar]
- 46.Moore H-PH, Silver RB, Mottillo EP, Bernlohr DA, Granneman JG. Perilipin targets a novel pool of lipid droplets for lipolytic attack by hormone-sensitive lipase. The Journal of biological chemistry. 2005;280(52):43109–20. [DOI] [PubMed] [Google Scholar]
- 47.Hansen JS, de Maré S, Jones HA, Göransson O, Lindkvist-Petersson K. Visualization of lipid directed dynamics of perilipin 1 in human primary adipocytes. Scientific Reports. 2017;7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanders Matthew A, Madoux F, Mladenovic L, Zhang H, Ye X, Angrish M, et al. Endogenous and Synthetic ABHD5 Ligands Regulate ABHD5-Perilipin Interactions and Lipolysis in Fat and Muscle. Cell Metabolism. 2015;22(5):851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rondini EA, Mladenovic-Lucas L, Roush WR, Halvorsen GT, Green AE, Granneman JG. Novel Pharmacological Probes Reveal ABHD5 as a Locus of Lipolysis Control in White and Brown Adipocytes. The Journal of pharmacology and experimental therapeutics. 2017;363(3):367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Russell L, Forsdyke DR. A human putative lymphocyte G0/G1 switch gene containing a CpG-rich island encodes a small basic protein with the potential to be phosphorylated. DNA and cell biology. 1991;10(8):581–91. [DOI] [PubMed] [Google Scholar]
- 51.Zandbergen F, Mandard S, Escher P, Tan NS, Patsouris D, Jatkoe T, et al. The G0/G1 switch gene 2 is a novel PPAR target gene. Biochem J. 2005;392(Pt 2):313–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang X, Lu X, Lombes M, Rha GB, Chi YI, Guerin TM, et al. The G(0)/G(1) switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab. 2010;11(3):194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cornaciu I, Boeszoermenyi A, Lindermuth H, Nagy HM, Cerk IK, Ebner C, et al. The Minimal Domain of Adipose Triglyceride Lipase (ATGL) Ranges until Leucine 254 and Can Be Activated and Inhibited by CGI-58 and G0S2, Respectively. PLoS ONE. 2011;6(10):e26349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cerk IK, Salzburger B, Boeszoermenyi A, Heier C, Pillip C, Romauch M, et al. A peptide derived from G0/G1 switch gene 2 acts as noncompetitive inhibitor of adipose triglyceride lipase. J Biol Chem. 2014;289(47):32559–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gimm T, Wiese M, Teschemacher B, Deggerich A, Schodel J, Knaup KX, et al. Hypoxia-inducible protein 2 is a novel lipid droplet protein and a specific target gene of hypoxia-inducible factor-1. Faseb j. 2010;24(11):4443–58. [DOI] [PubMed] [Google Scholar]
- 56.Padmanabha Das KM, Wechselberger L, Liziczai M, De la Rosa Rodriguez M, Grabner GF, Heier C, et al. Hypoxia-inducible lipid droplet-associated protein inhibits adipose triglyceride lipase. J Lipid Res. 2018;59(3):531–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang X, Saarinen AM, Hitosugi T, Wang Z, Wang L, Ho TH, et al. Inhibition of intracellular lipolysis promotes human cancer cell adaptation to hypoxia. Elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DiStefano MT, Danai LV, Roth Flach RJ, Chawla A, Pedersen DJ, Guilherme A, et al. The Lipid Droplet Protein Hypoxia-inducible Gene 2 Promotes Hepatic Triglyceride Deposition by Inhibiting Lipolysis. J Biol Chem. 2015;290(24):15175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dijk W, Mattijssen F, de la Rosa Rodriguez M, Loza Valdes A, Loft A, Mandrup S, et al. Hypoxia-Inducible Lipid Droplet-Associated Is Not a Direct Physiological Regulator of Lipolysis in Adipose Tissue. Endocrinology. 2017;158(5):1231–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DiStefano MT, Roth Flach RJ, Senol-Cosar O, Danai LV, Virbasius JV, Nicoloro SM, et al. Adipocyte-specific Hypoxia-inducible gene 2 promotes fat deposition and diet-induced insulin resistance. Mol Metab. 2016;5(12):1149–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holmes R Comparative studies of adipose triglyceride lipase genes and proteins: an ancient gene in vertebrate evolution. Open Access Bioinformatics. 2012:4, 15–29. [Google Scholar]
- 62.Baulande S, Lasnier F, Lucas M, Pairault J. Adiponutrin, a Transmembrane Protein Corresponding to a Novel Dietary- and Obesity-linked mRNA Specifically Expressed in the Adipose Lineage. Journal of Biological Chemistry. 2001;276(36):33336–44. [DOI] [PubMed] [Google Scholar]
- 63.Kershaw EE, Hamm JK, Verhagen LAW, Peroni O, Katic M, Flier JS. Adipose triglyceride lipase: function, regulation by insulin, and comparison with adiponutrin. Diabetes. 2006;55(1):148–57. [PMC free article] [PubMed] [Google Scholar]
- 64.Dongiovanni P, Donati B, Fares R, Lombardi R, Mancina RM, Romeo S, et al. PNPLA3 I148M polymorphism and progressive liver disease. World journal of gastroenterology. 2013;19(41):6969–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nature genetics. 2008;40(12):1461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Romeo S, Sentinelli F, Cambuli VM, Incani M, Congiu T, Matta V, et al. The 148M allele of the PNPLA3 gene is associated with indices of liver damage early in life. Journal of hepatology. 2010;53(2):335–8. [DOI] [PubMed] [Google Scholar]
- 67.Valenti L, Rumi M, Galmozzi E, Aghemo A, Del Menico B, De Nicola S, et al. Patatin-like phospholipase domain-containing 3 I148M polymorphism, steatosis, and liver damage in chronic hepatitis C. Hepatology (Baltimore, Md). 2011;53(3):791–9. [DOI] [PubMed] [Google Scholar]
- 68.Huang Y, Cohen JC, Hobbs HH. Expression and Characterization of a PNPLA3 Protein Isoform (I148M) Associated with Nonalcoholic Fatty Liver Disease. Journal of Biological Chemistry. 2011;286(43):37085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kumari M, Schoiswohl G, Chitraju C, Paar M, Cornaciu I, Rangrez Ashraf Y, et al. Adiponutrin Functions as a Nutritionally Regulated Lysophosphatidic Acid Acyltransferase. Cell Metabolism. 2012;15(5):691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang A, Mottillo EP, Mladenovic-Lucas L, Zhou L, Granneman JG. Dynamic interactions of ABHD5 with PNPLA3 regulate triacylglycerol metabolism in brown adipocytes. Nature Metabolism. 2019:1 560–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ohno Y, Nara A, Nakamichi S, Kihara A. Molecular mechanism of the ichthyosis pathology of Chanarin–Dorfman syndrome: Stimulation of PNPLA1-catalyzed ω-O-acylceramide production by ABHD5. Journal of Dermatological Science. 2018;92(3):245–53. [DOI] [PubMed] [Google Scholar]
- 72.Kien B, Grond S, Haemmerle G, Lass A, Eichmann TO, Radner FPW. ABHD5 stimulates PNPLA1-mediated ω- O -acylceramide biosynthesis essential for a functional skin permeability barrier. Journal of Lipid Research. 2018;59(12):2360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qiao A, Liang J, Ke Y, Li C, Cui Y, Shen L, et al. Mouse patatin-like phospholipase domain-containing 3 influences systemic lipid and glucose homeostasis. Hepatology (Baltimore, Md). 2011;54(2):509–21. [DOI] [PubMed] [Google Scholar]
- 74.BasuRay S, Wang Y, Smagris E, Cohen JC, Hobbs HH. Accumulation of PNPLA3 on lipid droplets is the basis of associated hepatic steatosis. Proc Natl Acad Sci U S A. 2019;116(19):9521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang Y, Kory N, BasuRay S, Cohen JC, Hobbs HH. PNPLA3, CGI‐58, and Inhibition of Hepatic Triglyceride Hydrolysis in Mice. Hepatology. 2019:hep.30583 69, 2427–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shen W-J, Yu Z, Patel S, Jue D, Liu L-F, Kraemer FB. Hormone-sensitive lipase modulates adipose metabolism through PPARgamma. Biochimica et biophysica acta. 2011;1811(1):9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Strom K, Gundersen TE, Hansson O, Lucas S, Fernandez C, Blomhoff R, et al. Hormone-sensitive lipase (HSL) is also a retinyl ester hydrolase: evidence from mice lacking HSL. Faseb j. 2009;23(7):2307–16. [DOI] [PubMed] [Google Scholar]
- 78.Taschler U, Schreiber R, Chitraju C, Grabner GF, Romauch M, Wolinski H, et al. Adipose triglyceride lipase is involved in the mobilization of triglyceride and retinoid stores of hepatic stellate cells. Biochim Biophys Acta. 2015;1851(7):937–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ruhanen H, Perttila J, Holtta-Vuori M, Zhou Y, Yki-Jarvinen H, Ikonen E, et al. PNPLA3 mediates hepatocyte triacylglycerol remodeling. The Journal of Lipid Research. 2014;55(4):739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mitsche MA, Hobbs HH, Cohen JC. Patatin-like phospholipase domain–containing protein 3 promotes transfers of essential fatty acids from triglycerides to phospholipids in hepatic lipid droplets. Journal of Biological Chemistry. 2018;293(18):6958–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Montero-Moran G, Caviglia JM, McMahon D, Rothenberg A, Subramanian V, Xu Z, et al. CGI-58/ABHD5 is a coenzyme A-dependent lysophosphatidic acid acyltransferase. Journal of lipid research. 2010;51(4):709–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McMahon D, Dinh A, Kurz D, Shah D, Han G-S, Carman GM, et al. Comparative gene identification 58/alpha/beta hydrolase domain 5 lacks lysophosphatidic acid acyltransferase activity. Journal of lipid research. 2014;55(8):1750–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carpene C, Bousquet-Melou A, Galitzky J, Berlan M, Lafontan M. Lipolytic effects of beta 1-, beta 2-, and beta 3-adrenergic agonists in white adipose tissue of mammals. Annals of the New York Academy of Sciences. 1998;839:186–9. [DOI] [PubMed] [Google Scholar]
- 84.Shi F, Collins S. Second messenger signaling mechanisms of the brown adipocyte thermogenic program: an integrative perspective. Hormone molecular biology and clinical investigation. 2017;31(2). [DOI] [PubMed] [Google Scholar]
- 85.Schonbaum E, Johnson GE, Sellers EA, Gill MJ. Adrenergic beta-receptors and non-shivering thermogenesis. Nature. 1966;210(5034):426. [DOI] [PubMed] [Google Scholar]
- 86.Dawkins MJ, Scopes JW. Non-shivering thermogenesis and brown adipose tissue in the human new-born infant. Nature. 1965;206(980):201–2. [DOI] [PubMed] [Google Scholar]
- 87.Davis TR, Johnston DR, Bell FC, Cremer BJ. Regulation of shivering and non-shivering heat production during acclimation of rats. Am J Physiol. 1960;198:471–5. [DOI] [PubMed] [Google Scholar]
- 88.Clifford GM, Londos C, Kraemer FB, Vernon RG, Yeaman SJ. Translocation of hormone-sensitive lipase and perilipin upon lipolytic stimulation of rat adipocytes. J Biol Chem. 2000;275(7):5011–5. [DOI] [PubMed] [Google Scholar]
- 89.Clifford GM, McCormick DK, Vernon RG, Yeaman SJ. Translocation of perilipin and hormone-sensitive lipase in response to lipolytic hormones. Biochem Soc Trans. 1997;25(4):S672. [DOI] [PubMed] [Google Scholar]
- 90.Souza SC, Muliro KV, Liscum L, Lien P, Yamamoto MT, Schaffer JE, et al. Modulation of hormone-sensitive lipase and protein kinase A-mediated lipolysis by perilipin A in an adenoviral reconstituted system. J Biol Chem. 2002;277(10):8267–72. [DOI] [PubMed] [Google Scholar]
- 91.Su CL, Sztalryd C, Contreras JA, Holm C, Kimmel AR, Londos C. Mutational analysis of the hormone-sensitive lipase translocation reaction in adipocytes. J Biol Chem. 2003;278(44):43615–9. [DOI] [PubMed] [Google Scholar]
- 92.Holm C Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem Soc Trans. 2003;31(Pt 6):1120–4. [DOI] [PubMed] [Google Scholar]
- 93.Miyoshi H, Perfield JW 2nd, Souza SC, Shen WJ, Zhang HH, Stancheva ZS, et al. Control of adipose triglyceride lipase action by serine 517 of perilipin A globally regulates protein kinase A-stimulated lipolysis in adipocytes. J Biol Chem. 2007;282(2):996–1002. [DOI] [PubMed] [Google Scholar]
- 94.Pollak NM, Jaeger D, Kolleritsch S, Zimmermann R, Zechner R, Lass A, et al. The Interplay of Protein Kinase A and Perilipin 5 Regulates Cardiac Lipolysis. Journal of Biological Chemistry. 2015;290(3):1295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fruhbeck G, Mendez-Gimenez L, Fernandez-Formoso JA, Fernandez S, Rodriguez A. Regulation of adipocyte lipolysis. Nutrition research reviews. 2014;27(1):63–93. [DOI] [PubMed] [Google Scholar]
- 96.Lowell BB, S-Susulic V, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB, et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993;366(6457):740–2. [DOI] [PubMed] [Google Scholar]
- 97.Hamann A, Benecke H, Le Marchand-Brustel Y, Susulic VS, Lowell BB, Flier JS. Characterization of insulin resistance and NIDDM in transgenic mice with reduced brown fat. Diabetes. 1995;44(11):1266–73. [DOI] [PubMed] [Google Scholar]
- 98.Grujic D, Susulic VS, Harper ME, Himms-Hagen J, Cunningham BA, Corkey BE, et al. Beta3-adrenergic receptors on white and brown adipocytes mediate beta3-selective agonist-induced effects on energy expenditure, insulin secretion, and food intake. A study using transgenic and gene knockout mice. J Biol Chem. 1997;272(28):17686–93. [DOI] [PubMed] [Google Scholar]
- 99.Yoshida T, Sakane N, Wakabayashi Y, Umekawa T, Kondo M. Anti-obesity and anti-diabetic effects of CL 316,243, a highly specific beta 3-adrenoceptor agonist, in yellow KK mice. Life sciences. 1994;54(7):491–8. [DOI] [PubMed] [Google Scholar]
- 100.Fedorenko A, Lishko Polina V, Kirichok Y. Mechanism of Fatty-Acid-Dependent UCP1 Uncoupling in Brown Fat Mitochondria. Cell. 2012;151(2):400–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang L, Li X, Tang H, Gao Z, Zhang K, Sun K. A Unique Role of Carboxylesterase 3 (Ces3) in beta-Adrenergic Signaling-Stimulated Thermogenesis. Diabetes. 2019;68(6):1178–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schreiber R, Diwoky C, Schoiswohl G, Feiler U, Wongsiriroj N, Abdellatif M, et al. Cold-Induced Thermogenesis Depends on ATGL-Mediated Lipolysis in Cardiac Muscle, but Not Brown Adipose Tissue. Cell Metabolism. 2017. 26, 753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shin H, Ma Y, Chanturiya T, Cao Q, Wang Y, Kadegowda AKG, et al. Lipolysis in Brown Adipocytes Is Not Essential for Cold-Induced Thermogenesis in Mice. Cell Metabolism. 2017. 26, 764–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Simcox J, Geoghegan G, Maschek JA, Bensard CL, Pasquali M, Miao R, et al. Global Analysis of Plasma Lipids Identifies Liver-Derived Acylcarnitines as a Fuel Source for Brown Fat Thermogenesis. Cell Metabolism. 2017;26(3):509–22.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gonzalez-Hurtado E, Lee J, Choi J, Wolfgang MJ. Fatty acid oxidation is required for active and quiescent brown adipose tissue maintenance and thermogenic programing. Molecular Metabolism. 2017. 7, 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee J, Ellis JM, Wolfgang MJ. Adipose Fatty Acid Oxidation Is Required for Thermogenesis and Potentiates Oxidative Stress-Induced Inflammation. Cell Reports. 2015;10(2):266–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dubé JJ, Sitnick MT, Schoiswohl G, Wills RC, Basantani MK, Cai L, et al. Adipose triglyceride lipase deletion from adipocytes, but not skeletal myocytes, impairs acute exercise performance in mice. American Journal of Physiology - Endocrinology And Metabolism. 2015;308(10):E879–E90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cannone V, Cabassi A, Volpi R, Burnett JC. Atrial Natriuretic Peptide: A Molecular Target of Novel Therapeutic Approaches to Cardio-Metabolic Disease. Int J Mol Sci. 2019;20(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moro C, Crampes F, Sengenes C, De Glisezinski I, Galitzky J, Thalamas C, et al. Atrial natriuretic peptide contributes to physiological control of lipid mobilization in humans. Faseb j. 2004;18(7):908–10. [DOI] [PubMed] [Google Scholar]
- 110.Sengenes C, Bouloumie A, Hauner H, Berlan M, Busse R, Lafontan M, et al. Involvement of a cGMP-dependent pathway in the natriuretic peptide-mediated hormone-sensitive lipase phosphorylation in human adipocytes. J Biol Chem. 2003;278(49):48617–26. [DOI] [PubMed] [Google Scholar]
- 111.Birkenfeld AL, Budziarek P, Boschmann M, Moro C, Adams F, Franke G, et al. Atrial Natriuretic Peptide Induces Postprandial Lipid Oxidation in Humans. Diabetes. 2008;57(12):3199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sengenes C, Zakaroff-Girard A, Moulin A, Berlan M, Bouloumie A, Lafontan M, et al. Natriuretic peptide-dependent lipolysis in fat cells is a primate specificity. American journal of physiology Regulatory, integrative and comparative physiology. 2002;283(1):R257–65. [DOI] [PubMed] [Google Scholar]
- 113.Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessi-Fulgheri P, Zhang C, et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122(3):1022–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bartels ED, Guo S, Kousholt BS, Larsen JR, Hasenkam JM, Burnett J, Jr., et al. High doses of ANP and BNP exacerbate lipolysis in humans and the lipolytic effect of BNP is associated with cardiac triglyceride content in pigs. Peptides. 2019;112:43–7. [DOI] [PubMed] [Google Scholar]
- 115.Wu W, Shi F, Liu D, Ceddia RP, Gaffin R, Wei W, et al. Enhancing natriuretic peptide signaling in adipose tissue, but not in muscle, protects against diet-induced obesity and insulin resistance. Sci Signal. 2017;10(489). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Miki H, Yamauchi T, Suzuki R, Komeda K, Tsuchida A, Kubota N, et al. Essential role of insulin receptor substrate 1 (IRS-1) and IRS-2 in adipocyte differentiation. Mol Cell Biol. 2001;21(7):2521–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.DiPilato LM, Ahmad F, Harms M, Seale P, Manganiello V, Birnbaum MJ. The Role of PDE3B Phosphorylation in the Inhibition of Lipolysis by Insulin. Mol Cell Biol. 2015;35(16):2752–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wijkander J, Landstrom TR, Manganiello V, Belfrage P, Degerman E. Insulin-induced phosphorylation and activation of phosphodiesterase 3B in rat adipocytes: possible role for protein kinase B but not mitogen-activated protein kinase or p70 S6 kinase. Endocrinology. 1998;139(1):219–27. [DOI] [PubMed] [Google Scholar]
- 119.Kitamura T, Kitamura Y, Kuroda S, Hino Y, Ando M, Kotani K, et al. Insulin-induced phosphorylation and activation of cyclic nucleotide phosphodiesterase 3B by the serine-threonine kinase Akt. Mol Cell Biol. 1999;19(9):6286–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Koren S, DiPilato LM, Emmett MJ, Shearin AL, Chu Q, Monks B, et al. The role of mouse Akt2 in insulin-dependent suppression of adipocyte lipolysis in vivo. Diabetologia. 2015;58(5):1063–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tan S-X, Fisher-Wellman KH, Fazakerley DJ, Ng Y, Pant H, Li J, et al. Selective Insulin Resistance in Adipocytes. Journal of Biological Chemistry. 2015;290(18):11337–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xia W, Pessentheiner AR, Hofer DC, Amor M, Schreiber R, Schoiswohl G, et al. Loss of ABHD15 Impairs the Anti-lipolytic Action of Insulin by Altering PDE3B Stability and Contributes to Insulin Resistance. Cell Rep. 2018;23(7):1948–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stockli J, Zadoorian A, Cooke KC, Deshpande V, Yau B, Herrmann G, et al. ABHD15 regulates adipose tissue lipolysis and hepatic lipid accumulation. Mol Metab. 2019;25:83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gridley S, Lane WS, Garner CW, Lienhard GE. Novel insulin-elicited phosphoproteins in adipocytes. Cell Signal. 2005;17(1):59–66. [DOI] [PubMed] [Google Scholar]
- 125.Chavez JA, Gridley S, Sano H, Lane WS, Lienhard GE. The 47kDa Akt substrate associates with phosphodiesterase 3B and regulates its level in adipocytes. Biochem Biophys Res Commun. 2006;342(4):1218–22. [DOI] [PubMed] [Google Scholar]
- 126.Jepson CA, Yeaman SJ. Inhibition of hormone-sensitive lipase by intermediary lipid metabolites. FEBS Lett. 1992;310(2):197–200. [DOI] [PubMed] [Google Scholar]
- 127.Nagy HM, Paar M, Heier C, Moustafa T, Hofer P, Haemmerle G, et al. Adipose triglyceride lipase activity is inhibited by long-chain acyl-coenzyme A. Biochimica et biophysica acta. 2014;1841(4):588–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pohl C, Dikic I. Cellular quality control by the ubiquitin-proteasome system and autophagy. Science. 2019;366(6467):818–22. [DOI] [PubMed] [Google Scholar]
- 129.Bersuker K, Olzmann JA. Establishing the lipid droplet proteome: Mechanisms of lipid droplet protein targeting and degradation. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862(10 Pt B):1166–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Brasaemle DL, Barber T, Kimmel AR, Londos C. Post-translational regulation of perilipin expression. Stabilization by stored intracellular neutral lipids. J Biol Chem. 1997;272(14):9378–87. [DOI] [PubMed] [Google Scholar]
- 131.Ghosh M, Niyogi S, Bhattacharyya M, Adak M, Nayak DK, Chakrabarti S, et al. Ubiquitin Ligase COP1 Controls Hepatic Fat Metabolism by Targeting ATGL for Degradation. Diabetes. 2016;65(12):3561–72. [DOI] [PubMed] [Google Scholar]
- 132.Olzmann JA, Richter CM, Kopito RR. Spatial regulation of UBXD8 and p97/VCP controls ATGL-mediated lipid droplet turnover. Proceedings of the National Academy of Sciences. 2013;110(4):1345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Heckmann BL, Zhang X, Saarinen AM, Liu J. Regulation of G0/G1 Switch Gene 2 (G0S2) Protein Ubiquitination and Stability by Triglyceride Accumulation and ATGL Interaction. PLoS One. 2016;11(6):e0156742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Huang Y, He S, Li JZ, Seo Y-K, Osborne TF, Cohen JC, et al. A feed-forward loop amplifies nutritional regulation of PNPLA3. Proceedings of the National Academy of Sciences. 2010;107(17):7892–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.BasuRay S, Smagris E, Cohen JC, Hobbs HH. The PNPLA3 variant associated with fatty liver disease (I148M) accumulates on lipid droplets by evading ubiquitylation: Basuray et al. Hepatology. 2017;66(4):1111–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schapira M, Calabrese MF, Bullock AN, Crews CM. Targeted protein degradation: expanding the toolbox. Nature reviews Drug discovery. 2019;18(12):949–63. [DOI] [PubMed] [Google Scholar]
- 137.Gandotra S, Lim K, Girousse A, Saudek V, O’Rahilly S, Savage DB. Human Frame Shift Mutations Affecting the Carboxyl Terminus of Perilipin Increase Lipolysis by Failing to Sequester the Adipose Triglyceride Lipase (ATGL) Coactivator AB-hydrolase-containing 5 (ABHD5). Journal of Biological Chemistry. 2011;286(40):34998–5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Patel S, Yang W, Kozusko K, Saudek V, Savage DB. Perilipins 2 and 3 lack a carboxy-terminal domain present in perilipin 1 involved in sequestering ABHD5 and suppressing basal lipolysis. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(25):9163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sugihara M, Morito D, Ainuki S, Hirano Y, Ogino K, Kitamura A, et al. The AAA+ ATPase/ubiquitin ligase mysterin stabilizes cytoplasmic lipid droplets. The Journal of Cell Biology. 2019;218(3):949.1–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ahmadian M, Abbott Marcia J, Tang T, Hudak Carolyn SS, Kim Y, Bruss M, et al. Desnutrin/ATGL Is Regulated by AMPK and Is Required for a Brown Adipose Phenotype. Cell Metabolism. 2011;13(6):739–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mottillo EP, Zhang H, Yang A, Zhou L, Granneman JG. Genetically-encoded sensors to detect fatty acid production and trafficking. Mol Metab. 2019;29:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Albert JS, Yerges-Armstrong LM, Horenstein RB, Pollin TI, Sreenivasan UT, Chai S, et al. Null Mutation in Hormone-Sensitive Lipase Gene and Risk of Type 2 Diabetes. New England Journal of Medicine. 2014;370(24):2307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hofer P, Boeszoermenyi A, Jaeger D, Feiler U, Arthanari H, Mayer N, et al. Fatty Acid-binding Proteins Interact with Comparative Gene Identification-58 Linking Lipolysis with Lipid Ligand Shuttling. Journal of Biological Chemistry. 2015;290(30):18438–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jenkins-Kruchten AE, Bennaars-Eiden A, Ross JR, Shen WJ, Kraemer FB, Bernlohr DA. Fatty acid-binding protein-hormone-sensitive lipase interaction. Fatty acid dependence on binding. J Biol Chem. 2003;278(48):47636–43. [DOI] [PubMed] [Google Scholar]
- 145.Tan NS, Shaw NS, Vinckenbosch N, Liu P, Yasmin R, Desvergne B, et al. Selective cooperation between fatty acid binding proteins and peroxisome proliferator-activated receptors in regulating transcription. Mol Cell Biol. 2002;22(14):5114–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wolfrum C, Borrmann CM, Borchers T, Spener F. Fatty acids and hypolipidemic drugs regulate peroxisome proliferator-activated receptors alpha - and gamma-mediated gene expression via liver fatty acid binding protein: a signaling path to the nucleus. Proc Natl Acad Sci U S A. 2001;98(5):2323–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Minnich A, Tian N, Byan L, Bilder G. A potent PPARalpha agonist stimulates mitochondrial fatty acid beta-oxidation in liver and skeletal muscle. Am J Physiol Endocrinol Metab. 2001;280(2):E270–9. [DOI] [PubMed] [Google Scholar]
- 148.Zhang K, Shen X, Wu J, Sakaki K, Saunders T, Rutkowski DT, et al. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124(3):587–99. [DOI] [PubMed] [Google Scholar]
- 149.Jaeger D, Schoiswohl G, Hofer P, Schreiber R, Schweiger M, Eichmann TO, et al. Fasting-induced G0/G1 switch gene 2 and FGF21 expression in the liver are under regulation of adipose tissue derived fatty acids. J Hepatol. 2015;63(2):437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xia B, Cai GH, Yang H, Wang SP, Mitchell GA, Wu JW. Adipose tissue deficiency of hormone-sensitive lipase causes fatty liver in mice. PLoS Genet. 2017;13(12):e1007110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mottillo EP, Balasubramanian P, Lee YH, Weng C, Kershaw EE, Granneman JG. Coupling of lipolysis and de novo lipogenesis in brown, beige, and white adipose tissues during chronic beta3-adrenergic receptor activation. J Lipid Res. 2014;55(11):2276–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Heine M, Fischer AW, Schlein C, Jung C, Straub LG, Gottschling K, et al. Lipolysis Triggers a Systemic Insulin Response Essential for Efficient Energy Replenishment of Activated Brown Adipose Tissue in Mice. Cell Metab. 2018;28(4):644–55 e4. [DOI] [PubMed] [Google Scholar]
- 153.Pang Z, Wu N, Zhang X, Avallone R, Croci T, Dressler H, et al. GPR40 is partially required for insulin secretion following activation of beta3-adrenergic receptors. Mol Cell Endocrinol. 2010;325(1–2):18–25. [DOI] [PubMed] [Google Scholar]
- 154.Garretson JT, Szymanski LA, Schwartz GJ, Xue B, Ryu V, Bartness TJ. Lipolysis sensation by white fat afferent nerves triggers brown fat thermogenesis. Molecular Metabolism. 2016;5(8):626–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhou P, Santoro A, Peroni OD, Nelson AT, Saghatelian A, Siegel D, et al. PAHSAs enhance hepatic and systemic insulin sensitivity through direct and indirect mechanisms. J Clin Invest. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tan D, Ertunc ME, Konduri S, Zhang J, Pinto AM, Chu Q, et al. Discovery of FAHFA-Containing Triacylglycerols and Their Metabolic Regulation. J Am Chem Soc. 2019;141(22):8798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Paluchova V, Oseeva M, Brezinova M, Cajka T, Bardova K, Adamcova K, et al. Lipokine 5-PAHSA is Regulated by Adipose Triglyceride Lipase and Primes Adipocytes for de novo Lipogenesis in Mice. Diabetes. 2019:db190494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lynes MD, Leiria LO, Lundh M, Bartelt A, Shamsi F, Huang TL, et al. The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nature Medicine. 2017;23(5):631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Muller G, Jung C, Straub J, Wied S, Kramer W. Induced release of membrane vesicles from rat adipocytes containing glycosylphosphatidylinositol-anchored microdomain and lipid droplet signalling proteins. Cell Signal. 2009;21(2):324–38. [DOI] [PubMed] [Google Scholar]
- 160.Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, Wolfrum C, et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017;542(7642):450–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Crewe C, Joffin N, Rutkowski JM, Kim M, Zhang F, Towler DA, et al. An Endothelial-to-Adipocyte Extracellular Vesicle Axis Governed by Metabolic State. Cell. 2018;175(3):695–708.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mita T, Furuhashi M, Hiramitsu S, Ishii J, Hoshina K, Ishimura S, et al. FABP4 is secreted from adipocytes by adenyl cyclase-PKA- and guanylyl cyclase-PKG-dependent lipolytic mechanisms. Obesity (Silver Spring, Md). 2015;23(2):359–67. [DOI] [PubMed] [Google Scholar]
- 163.Ertunc ME, Sikkeland J, Fenaroli F, Griffiths G, Daniels MP, Cao H, et al. Secretion of fatty acid binding protein aP2 from adipocytes through a nonclassical pathway in response to adipocyte lipase activity. Journal of Lipid Research. 2015;56(2):423–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Josephrajan A, Hertzel AV, Bohm EK, McBurney MW, Imai SI, Mashek DG, et al. Unconventional Secretion of Adipocyte Fatty Acid Binding Protein 4 Is Mediated By Autophagic Proteins in a Sirtuin-1-Dependent Manner. Diabetes. 2019;68(9):1767–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Villeneuve J, Bassaganyas L, Lepreux S, Chiritoiu M, Costet P, Ripoche J, et al. Unconventional secretion of FABP4 by endosomes and secretory lysosomes. J Cell Biol. 2018;217(2):649–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Prentice KJ, Saksi J, Hotamisligil GS. Adipokine FABP4 integrates energy stores and counterregulatory metabolic responses. J Lipid Res. 2019;60(4):734–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Frayn KN. Adipose tissue as a buffer for daily lipid flux. Diabetologia. 2002;45(9):1201–10. [DOI] [PubMed] [Google Scholar]
- 168.Robbins AL, Savage DB. The genetics of lipid storage and human lipodystrophies. Trends Mol Med. 2015;21(7):433–8. [DOI] [PubMed] [Google Scholar]
- 169.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117(9):2621–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. [DOI] [PubMed] [Google Scholar]
- 171.Coppack SW, Jensen MD, Miles JM. In vivo regulation of lipolysis in humans. J Lipid Res. 1994;35(2):177–93. [PubMed] [Google Scholar]
- 172.Bickerton AS, Roberts R, Fielding BA, Tornqvist H, Blaak EE, Wagenmakers AJ, et al. Adipose tissue fatty acid metabolism in insulin-resistant men. Diabetologia. 2008;51(8):1466–74. [DOI] [PubMed] [Google Scholar]
- 173.Jansson PA, Larsson A, Smith U, Lonnroth P. Glycerol production in subcutaneous adipose tissue in lean and obese humans. J Clin Invest. 1992;89(5):1610–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tchernof A, Belanger C, Morisset AS, Richard C, Mailloux J, Laberge P, et al. Regional differences in adipose tissue metabolism in women: minor effect of obesity and body fat distribution. Diabetes. 2006;55(5):1353–60. [DOI] [PubMed] [Google Scholar]
- 175.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145(5):2273–82. [DOI] [PubMed] [Google Scholar]
- 176.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW, Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nguyen MT, Satoh H, Favelyukis S, Babendure JL, Imamura T, Sbodio JI, et al. JNK and tumor necrosis factor-alpha mediate free fatty acid-induced insulin resistance in 3T3-L1 adipocytes. J Biol Chem. 2005;280(42):35361–71. [DOI] [PubMed] [Google Scholar]
- 178.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282(48):35279–92. [DOI] [PubMed] [Google Scholar]
- 179.Mottillo EP, Shen XJ, Granneman JG. beta3-adrenergic receptor induction of adipocyte inflammation requires lipolytic activation of stress kinases p38 and JNK. Biochimica et biophysica acta. 2010;1801(9):1048–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yang X, Zhang X, Heckmann BL, Lu X, Liu J. Relative contribution of adipose triglyceride lipase and hormone-sensitive lipase to tumor necrosis factor-alpha (TNF-alpha)-induced lipolysis in adipocytes. J Biol Chem. 2011;286(47):40477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Langin D, Arner P. Importance of TNFalpha and neutral lipases in human adipose tissue lipolysis. Trends Endocrinol Metab. 2006;17(8):314–20. [DOI] [PubMed] [Google Scholar]
- 182.Ryden M, Arner P. Tumour necrosis factor-alpha in human adipose tissue -- from signalling mechanisms to clinical implications. J Intern Med. 2007;262(4):431–8. [DOI] [PubMed] [Google Scholar]
- 183.Perry RJ, Camporez JG, Kursawe R, Titchenell PM, Zhang D, Perry CJ, et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell. 2015;160(4):745–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.van Hall G, Steensberg A, Sacchetti M, Fischer C, Keller C, Schjerling P, et al. Interleukin-6 stimulates lipolysis and fat oxidation in humans. The Journal of clinical endocrinology and metabolism. 2003;88(7):3005–10. [DOI] [PubMed] [Google Scholar]
- 185.Han MS, Jung DY, Morel C, Lakhani SA, Kim JK, Flavell RA, et al. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013;339(6116):218–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hickner RC, Racette SB, Binder EF, Fisher JS, Kohrt WM. Suppression of whole body and regional lipolysis by insulin: effects of obesity and exercise. The Journal of clinical endocrinology and metabolism. 1999;84(11):3886–95. [DOI] [PubMed] [Google Scholar]
- 187.Schoiswohl G, Stefanovic-Racic M, Menke MN, Wills RC, Surlow BA, Basantani MK, et al. Impact of Reduced ATGL-Mediated Adipocyte Lipolysis on Obesity-Associated Insulin Resistance and Inflammation in Male Mice. Endocrinology. 2015;156(10):3610–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yki-Jarvinen H, Kubo K, Zawadzki J, Lillioja S, Young A, Abbott W, et al. Dissociation of in vitro sensitivities of glucose transport and antilipolysis to insulin in NIDDM. Am J Physiol. 1987;253(3 Pt 1):E300–4. [DOI] [PubMed] [Google Scholar]
- 189.Pereira MJ, Skrtic S, Katsogiannos P, Abrahamsson N, Sidibeh CO, Dahgam S, et al. Impaired adipose tissue lipid storage, but not altered lipolysis, contributes to elevated levels of NEFA in type 2 diabetes. Degree of hyperglycemia and adiposity are important factors. Metabolism. 2016;65(12):1768–80. [DOI] [PubMed] [Google Scholar]
- 190.Jonsson C, Castor Batista AP, Kjolhede P, Stralfors P. Insulin and beta-adrenergic receptors mediate lipolytic and anti-lipolytic signalling that is not altered by type 2 diabetes in human adipocytes. Biochem J. 2019;476(19):2883–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Turinsky J, O’Sullivan DM, Bayly BP. 1,2-Diacylglycerol and ceramide levels in insulin-resistant tissues of the rat in vivo. J Biol Chem. 1990;265(28):16880–5. [PubMed] [Google Scholar]
- 192.Haus JM, Kashyap SR, Kasumov T, Zhang R, Kelly KR, Defronzo RA, et al. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes. 2009;58(2):337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cantley JL, Yoshimura T, Camporez JP, Zhang D, Jornayvaz FR, Kumashiro N, et al. CGI-58 knockdown sequesters diacylglycerols in lipid droplets/ER-preventing diacylglycerol-mediated hepatic insulin resistance. Proc Natl Acad Sci U S A. 2013;110(5):1869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Szendroedi J, Yoshimura T, Phielix E, Koliaki C, Marcucci M, Zhang D, et al. Role of diacylglycerol activation of PKCtheta in lipid-induced muscle insulin resistance in humans. Proc Natl Acad Sci U S A. 2014;111(26):9597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Neschen S, Morino K, Hammond LE, Zhang D, Liu ZX, Romanelli AJ, et al. Prevention of hepatic steatosis and hepatic insulin resistance in mitochondrial acyl-CoA:glycerol-sn-3-phosphate acyltransferase 1 knockout mice. Cell Metab. 2005;2(1):55–65. [DOI] [PubMed] [Google Scholar]
- 196.Eichmann TO, Kumari M, Haas JT, Farese RV, Zimmermann R, Lass A, et al. Studies on the Substrate and Stereo/Regioselectivity of Adipose Triglyceride Lipase, Hormone-sensitive Lipase, and Diacylglycerol- O -acyltransferases. Journal of Biological Chemistry. 2012;287(49):41446–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Fox TE, Houck KL, O’Neill SM, Nagarajan M, Stover TC, Pomianowski PT, et al. Ceramide recruits and activates protein kinase C zeta (PKC zeta) within structured membrane microdomains. J Biol Chem. 2007;282(17):12450–7. [DOI] [PubMed] [Google Scholar]
- 198.Chaurasia B, Tippetts TS, Mayoral Monibas R, Liu J, Li Y, Wang L, et al. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science. 2019;365(6451):386–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7(2):95–6. [DOI] [PubMed] [Google Scholar]
- 200.Titchenell PM, Quinn WJ, Lu M, Chu Q, Lu W, Li C, et al. Direct Hepatocyte Insulin Signaling Is Required for Lipogenesis but Is Dispensable for the Suppression of Glucose Production. Cell Metab. 2016;23(6):1154–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.McCullough AJ. The clinical features, diagnosis and natural history of nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8(3):521–33, viii. [DOI] [PubMed] [Google Scholar]
- 202.Kawano Y, Cohen DE. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. Journal of gastroenterology. 2013;48(4):434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115(5):1343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zolotov S, Xing C, Mahamid R, Shalata A, Sheikh-Ahmad M, Garg A. Homozygous LIPE mutation in siblings with multiple symmetric lipomatosis, partial lipodystrophy, and myopathy. American journal of medical genetics Part A. 2017;173(1):190–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Farhan SM, Robinson JF, McIntyre AD, Marrosu MG, Ticca AF, Loddo S, et al. A novel LIPE nonsense mutation found using exome sequencing in siblings with late-onset familial partial lipodystrophy. The Canadian journal of cardiology. 2014;30(12):1649–54. [DOI] [PubMed] [Google Scholar]
- 206.Rysman E, Brusselmans K, Scheys K, Timmermans L, Derua R, Munck S, et al. De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res. 2010;70(20):8117–26. [DOI] [PubMed] [Google Scholar]
- 207.Zha S, Ferdinandusse S, Hicks JL, Denis S, Dunn TA, Wanders RJ, et al. Peroxisomal branched chain fatty acid beta-oxidation pathway is upregulated in prostate cancer. Prostate. 2005;63(4):316–23. [DOI] [PubMed] [Google Scholar]
- 208.Ren J, Xiao YJ, Singh LS, Zhao X, Zhao Z, Feng L, et al. Lysophosphatidic acid is constitutively produced by human peritoneal mesothelial cells and enhances adhesion, migration, and invasion of ovarian cancer cells. Cancer Res. 2006;66(6):3006–14. [DOI] [PubMed] [Google Scholar]
- 209.Kuhajda FP. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition. 2000;16(3):202–8. [DOI] [PubMed] [Google Scholar]
- 210.Yang D, Li Y, Xing L, Tan Y, Sun J, Zeng B, et al. Utilization of adipocyte-derived lipids and enhanced intracellular trafficking of fatty acids contribute to breast cancer progression. Cell Commun Signal. 2018;16(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wang YY, Attane C, Milhas D, Dirat B, Dauvillier S, Guerard A, et al. Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI Insight. 2017;2(4):e87489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Herroon MK, Diedrich JD, Rajagurubandara E, Martin C, Maddipati KR, Kim S, et al. Prostate Tumor Cell-Derived IL1beta Induces an Inflammatory Phenotype in Bone Marrow Adipocytes and Reduces Sensitivity to Docetaxel via Lipolysis-Dependent Mechanisms. Mol Cancer Res. 2019. 17, 2508–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Besnard P, Niot I, Poirier H, Clement L, Bernard A. New insights into the fatty acid-binding protein (FABP) family in the small intestine. Mol Cell Biochem. 2002;239(1–2):139–47. [PubMed] [Google Scholar]
- 214.Das SK, Eder S, Schauer S, Diwoky C, Temmel H, Guertl B, et al. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science. 2011;333(6039):233–8. [DOI] [PubMed] [Google Scholar]
- 215.Hu W, Ru Z, Zhou Y, Xiao W, Sun R, Zhang S, et al. Lung cancer-derived extracellular vesicles induced myotube atrophy and adipocyte lipolysis via the extracellular IL-6-mediated STAT3 pathway. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864(8):1091–102. [DOI] [PubMed] [Google Scholar]
- 216.Sagar G, Sah RP, Javeed N, Dutta SK, Smyrk TC, Lau JS, et al. Pathogenesis of pancreatic cancer exosome-induced lipolysis in adipose tissue. Gut. 2016;65(7):1165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wu Q, Sun S, Li Z, Yang Q, Li B, Zhu S, et al. Tumour-originated exosomal miR-155 triggers cancer-associated cachexia to promote tumour progression. Molecular cancer. 2018;17(1):155. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 218.Clement E, Lazar I, Attane C, Carrie L, Dauvillier S, Ducoux-Petit M, et al. Adipocyte extracellular vesicles carry enzymes and fatty acids that stimulate mitochondrial metabolism and remodeling in tumor cells. Embo j. 2020;39(3):e102525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lazar I, Clement E, Dauvillier S, Milhas D, Ducoux-Petit M, LeGonidec S, et al. Adipocyte Exosomes Promote Melanoma Aggressiveness through Fatty Acid Oxidation: A Novel Mechanism Linking Obesity and Cancer. Cancer Res. 2016;76(14):4051–7. [DOI] [PubMed] [Google Scholar]
- 220.Hu W, Ru Z, Xiao W, Xiong Z, Wang C, Yuan C, et al. Adipose tissue browning in cancer-associated cachexia can be attenuated by inhibition of exosome generation. Biochem Biophys Res Commun. 2018;506(1):122–9. [DOI] [PubMed] [Google Scholar]
- 221.Kir S, White JP, Kleiner S, Kazak L, Cohen P, Baracos VE, et al. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature. 2014;513(7516):100–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Arch JR. beta(3)-Adrenoceptor agonists: potential, pitfalls and progress. Eur J Pharmacol. 2002;440(2–3):99–107. [DOI] [PubMed] [Google Scholar]
- 223.Granneman JG, Lahners KN, Chaudhry A. Molecular cloning and expression of the rat beta 3-adrenergic receptor. Mol Pharmacol. 1991;40(6):895–9. [PubMed] [Google Scholar]
- 224.Umekawa T, Yoshida T, Sakane N, Kondo M. Effect of CL316,243, a highly specific beta(3)-adrenoceptor agonist, on lipolysis of epididymal, mesenteric and subcutaneous adipocytes in rats. Endocr J. 1997;44(1):181–5. [DOI] [PubMed] [Google Scholar]
- 225.Cawthorne MA, Sennitt MV, Arch JR, Smith SA. BRL 35135, a potent and selective atypical beta-adrenoceptor agonist. Am J Clin Nutr. 1992;55(1 Suppl):252S–7S. [DOI] [PubMed] [Google Scholar]
- 226.Andersson KE, Martin N, Nitti V. Selective beta(3)-adrenoceptor agonists for the treatment of overactive bladder. The Journal of urology. 2013;190(4):1173–80. [DOI] [PubMed] [Google Scholar]
- 227.Redman LM, de Jonge L, Fang X, Gamlin B, Recker D, Greenway FL, et al. Lack of an effect of a novel beta3-adrenoceptor agonist, TAK-677, on energy metabolism in obese individuals: a double-blind, placebo-controlled randomized study. The Journal of clinical endocrinology and metabolism. 2007;92(2):527–31. [DOI] [PubMed] [Google Scholar]
- 228.Larsen TM, Toubro S, van Baak MA, Gottesdiener KM, Larson P, Saris WH, et al. Effect of a 28-d treatment with L-796568, a novel beta(3)-adrenergic receptor agonist, on energy expenditure and body composition in obese men. Am J Clin Nutr. 2002;76(4):780–8. [DOI] [PubMed] [Google Scholar]
- 229.Arch JR. Challenges in beta(3)-Adrenoceptor Agonist Drug Development. Ther Adv Endocrinol Metab. 2011;2(2):59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Cypess Aaron M, Weiner Lauren S, Roberts-Toler C, Elía Elisa F, Kessler Skyler H, Kahn Peter A, et al. Activation of Human Brown Adipose Tissue by a β3-Adrenergic Receptor Agonist. Cell Metabolism. 2015;21(1):33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Muzik O, Mangner TJ, Leonard WR, Kumar A, Janisse J, Granneman JG. 15O PET Measurement of Blood Flow and Oxygen Consumption in Cold-Activated Human Brown Fat. Journal of Nuclear Medicine. 2013;54(4):523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Blondin DP, Labbé SM, Phoenix S, Guérin B, Turcotte ÉE, Richard D, et al. Contributions of white and brown adipose tissues and skeletal muscles to acute cold-induced metabolic responses in healthy men: Cold-induced energy metabolism of WAT, BAT and skeletal muscle. The Journal of Physiology. 2015;593(3):701–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Gnad T, Scheibler S, von Kugelgen I, Scheele C, Kilic A, Glode A, et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature. 2014;516(7531):395–9. [DOI] [PubMed] [Google Scholar]
- 234.Lahesmaa M, Oikonen V, Helin S, Luoto P, M UD, Pfeifer A, et al. Regulation of human brown adipose tissue by adenosine and A2A receptors - studies with [(15)O]H2O and [(11)C]TMSX PET/CT. Eur J Nucl Med Mol Imaging. 2019;46(3):743–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Arner P, Bernard S, Appelsved L, Fu KY, Andersson DP, Salehpour M, et al. Adipose lipid turnover and long-term changes in body weight. Nat Med. 2019;25(9):1385–9. [DOI] [PubMed] [Google Scholar]
- 236.Camell CD, Sander J, Spadaro O, Lee A, Nguyen KY, Wing A, et al. Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature. 2017;550(7674):119–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ryden M, Backdahl J, Petrus P, Thorell A, Gao H, Coue M, et al. Impaired atrial natriuretic peptide-mediated lipolysis in obesity. International journal of obesity (2005). 2016;40(4):714–20. [DOI] [PubMed] [Google Scholar]
- 238.Ahmadian M, Duncan RE, Varady KA, Frasson D, Hellerstein MK, Birkenfeld AL, et al. Adipose Overexpression of Desnutrin Promotes Fatty Acid Use and Attenuates Diet-Induced Obesity. Diabetes. 2009;58(4):855–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Schreiber R, Hofer P, Taschler U, Voshol PJ, Rechberger GN, Kotzbeck P, et al. Hypophagia and metabolic adaptations in mice with defective ATGL-mediated lipolysis cause resistance to HFD-induced obesity. Proceedings of the National Academy of Sciences. 2015:201516004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Mayer N, Schweiger M, Romauch M, Grabner GF, Eichmann TO, Fuchs E, et al. Development of small-molecule inhibitors targeting adipose triglyceride lipase. Nat Chem Biol. 2013;9(12):785–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wu JW, Wang SP, Alvarez F, Casavant S, Gauthier N, Abed L, et al. Deficiency of liver adipose triglyceride lipase in mice causes progressive hepatic steatosis. Hepatology. 2011;54(1):122–32. [DOI] [PubMed] [Google Scholar]
- 242.Chitraju C, Mejhert N, Haas JT, Diaz-Ramirez LG, Grueter CA, Imbriglio JE, et al. Triglyceride Synthesis by DGAT1 Protects Adipocytes from Lipid-Induced ER Stress during Lipolysis. Cell Metabolism. 2017;26(2):407–18.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Nguyen TB, Louie SM, Daniele JR, Tran Q, Dillin A, Zoncu R, et al. DGAT1-Dependent Lipid Droplet Biogenesis Protects Mitochondrial Function during Starvation-Induced Autophagy. Developmental Cell. 2017;42(1):9–21.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Gauthier MS, Miyoshi H, Souza SC, Cacicedo JM, Saha AK, Greenberg AS, et al. AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte: potential mechanism and physiological relevance. J Biol Chem. 2008;283(24):16514–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Townsend LK, Knuth CM, Wright DC. Cycling our way to fit fat. Physiol Rep. 2017;5(7). [DOI] [PMC free article] [PubMed] [Google Scholar]