Abstract
Purpose of review:
Autologous hematopoietic stem cell transplantation (HSCT) is a promising therapeutic modality for severe autoimmune diseases. In this review, we will outline the immunological mechanisms and the clinical evidence and experiences for therapeutic HSCT in autoimmune diseases, with particular focus on systemic sclerosis (SSc) and multiple sclerosis (MS).
Recent findings:
Approximately 3000 patients with autoimmune diseases worldwide have been treated with HSCT. HSCT in SSc has been shown in three randomized controlled trials to be associated with significant long-term event-free survival despite some transplant-related mortality in the first year. A recent controlled trial in MS has also show benefit with transplant.
Summary:
The aim of HSCT is to ‘reset’ one’s immune system into a naïve and self-tolerant state through immune depletion and regulation. HSCT requires careful patient selection, close collaboration between physicians and expertise of transplant team to ensure optimal outcome.
Keywords: Stem cell transplantation, HSCT, autoimmune diseases
Introduction
Hematopoietic stem cell transplantation (HSCT) has emerged as a promising treatment for severe and therapy-refractory autoimmune diseases. The pathogenesis of autoimmune diseases is currently attributed to T and B cells inappropriately recognizing self-antigens and initiating a cell-mediated or humoral reaction, or both, resulting in inflammatory tissue and vascular damage (1). The rationale for using HSCT in autoimmune diseases is to eradicate one’s autoreactive immune cells and to regenerate a naïve, self-tolerant immune system (2).
Autologous HSCT process
The process of HSCT involves: 1) hematopoietic cell mobilization, harvesting, selection and cryopreservation, 2) preparative conditioning with chemotherapy with or without irradiation, 3) infusion of stem cell graft, and 4) supportive care after transplantation (3–5). CD34+ hematopoietic stem cells are mobilized from the marrow to the bloodstream by the administration of granulocyte colony-stimulating factor, with or without cyclophosphamide (Cyc) priming. Peripheral blood stem cells are then collected by leukapheresis. The conditioning regimen used in autologous HSCT for patients with autoimmune disease is usually non-myeloablative and consist of the combination of antithymocyte globulin (ATG) with either high dose Cyc or other chemotherapeutic agents (6). The conditioning is followed by infusion of autologous (CD34+) stem cells. Patients can be discharged from hospital once their neutrophil counts have recovered, which occurs typically within 1–3 weeks after stem cell infusion. Most patients remain severely lymphopenic for several months after HSCT while their immune system fully reconstitutes.
Mechanistic aspects of autologous HSCT
The rationale in using HSCT in autoimmune diseases is to ‘reset’ one’s immune system by purging the existing immune system and regenerating a new and healthy repertoire of immune cells (7). Several factors may contribute to the resetting and regulation of the immunological clock. Lymphotoxic chemotherapy leads to a profound and persistent lymphopenia and reduced levels of putative pathogenic antibodies (8). There is growing evidence that autologous HSCT can re-establish immunological tolerance by: (i) activating thymopoiesis and establishing a diversified T cell receptor repertoire (9, 10); (ii) increasing the number of regulatory, FoxP3-positive T cells which are important in the preservation of tolerance (11); (iii) ATG targeting long-living, antibody-producing plasma cells by complement-mediated lysis and apoptosis (12). This ‘reset’ of the immunological clock could underlie the prolonged clinical remissions in some patients. However, relapses are possible which may be due to persistence of autoreactive memory cells or incomplete immunologic renewal and regulation.
Preclinical models
The experimental basis for the treatment of autoimmune diseases with HSCT derives from the pioneering translational research in murine models of autoimmunity of Ikehara and Good (13), who first evidenced that the origin of autoimmune diseases is in the bone marrow and that bone marrow can restore tolerance. Similar results were demonstrated in animal experiments by van Bekkum showing that conditioning followed by transplantation of syngeneic and autologous bone marrow transplant resulted in cure of induced models of autoimmunity. In particular, collagen-induced arthritis (as model for rheumatoid arthritis) and experimental autoimmune encephalomyelitis (as model for multiple sclerosis, MS), suggesting that tolerance induced by HSCT can prevent autoimmunity even after antigenic re-encounter (14, 15).
Initial clinical experiences
The potential for cure of autoimmune diseases with HSCT stemmed from patients with coincident autoimmune disease and hematologic malignancy or aplastic anaemia who remained in long-term remission of both diseases after allogeneic transplantation (16). By 1997, several teams had published clinical designs for HSCT for autoimmune diseases (17, 18). The first case of successful treatment of a 45-year-old female with untreatable pulmonary hypertension and systemic sclerosis (SSc) with autologous HSCT was reported in 1997 (19). Retrospective analyses from the European Bone Marrow Transplantation (EBMT) autoimmune disease registry and the Center for International Bone Marrow Transplant Registry (CIBMTR) in the USA, together with small prospective phase I and II trials, supported the feasibility and efficacy of HSCT in several severe, therapy-resistant autoimmune diseases and led to large-scale phase II and III HSCT trials (6, 20). The EBMT Autoimmune Diseases Working Party database has accumulated over 2000 patients with HSCT procedures between January 1994 and December 2015, as reported by 247 centers in 40 countries (21). The trends in HSCT activity in autoimmune diseases are shown in Figure 1. From registries reports, the two most frequent refractory autoimmune diseases in patients undergoing autologous HSCT are that of MS and SSc (6, 22).
Figure 1:
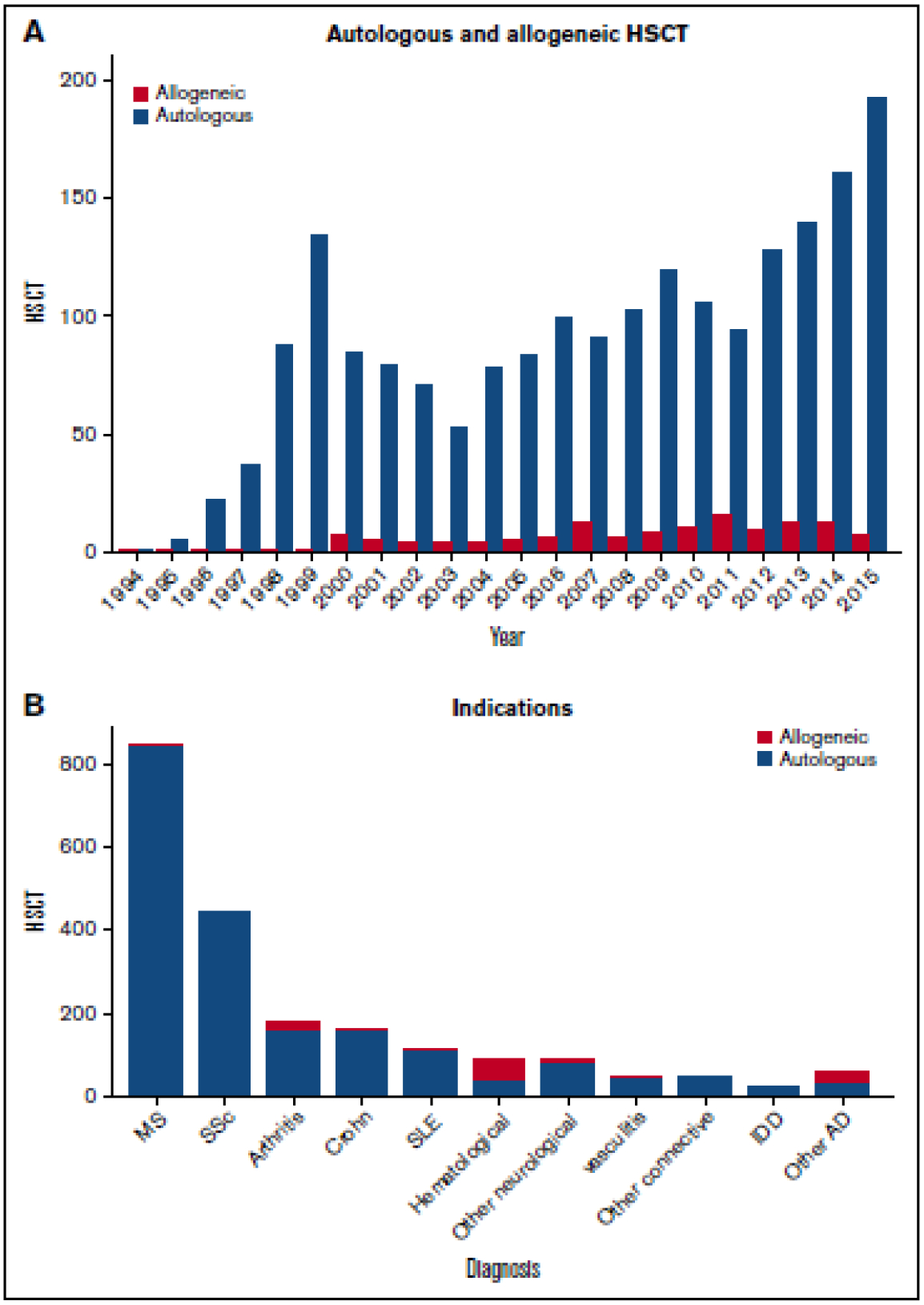
Trends in activity for hematopoetic stem cell transplantation (HSCT) in autoimmune diseases. A. By autologous and allogeneic HSCT. B. By disease indication.
Reproduced with permission of Blood advances, 2017;1(27):2742–55.
HSCT for MS
MS is a chronic, autoimmune, demyelinating and degenerative disease of the central nervous system (CNS) which is mediated by T cells triggered against structural components of myelin in the CNS. Most patients present at age 20–40 years with a relapsing-remitting (RR) course. After 10–15 years, most individuals with RR MS transition to a secondary progressive course characterized by neurodegeneration and neurologic worsening (23). About 10–15% of patients present with primary progressive MS, characterized by gradual neurologic decline (23). No evidence of disease activity (NEDA)--- defined by absence of relapses, disability worsening on the Expanded Disability Status Scale, or MRI lesion activity (new or enlarging T2 lesions or Gadolinium-enhancing lesions) has been proposed as a goal for MS disease-modifying therapy (24). Currently, despite MS disease-modifying therapies, a high proportion of patients fail to achieve NEDA (25). HSCT has been investigated as treatment of various MS phenotypes in both retrospective studies and clinical trials and beneficial results have been observed in a subset of patients with highly active relapsing forms of MS (26).
Retrospective studies have consistently supported the efficacy of HSCT in patients with relapsing forms of MS based on relapse reduction, progression-free survival, improvement in disability and reduction of MRI lesion activity (26–30). Single-arm phase I and II clinical trials, using varying regimens for mobilization and conditioning have also demonstrated efficacy of HSCT for RRMS based on MS disease activity-free survival, disability worsening and improvement (26, 31–37).
The randomized phase II ASTIMS trial showed superior efficacy of HSCT on the development of MRI lesions and lower annualized relapse rate compared to mitoxantrone (38). The MIST phase III trial randomized patients to either HSCT using non-myeloablative conditioning regimen or standard MS disease-modifying therapy and demonstrated efficacy of HSCT on disability worsening, relapse reduction and decrease in MRI lesions (39).
Recent meta-analyses of the efficacy and safety of HSCT for the treatment of MS have importantly demonstrated the decrease in treatment-related mortality over time (40–42). It has been reported that in patients with MS, HSCT resulted in NEDA rates of 78–83% at 2 years and 60–68% at 5 years; in contrast, studies of conventional disease-modifying therapies, including those considered to be highly efficacious, reported NEDA rates of only 13–46% at 2 years (41). Sormani et al also reported the proportion of patients with MS who remained NEDA over time under different treatments (Figure 2) and demonstrated that the proportion of NEDA patients in the autologous HSCT treated subjects was considerably and persistently higher than in those treated with all the other drugs (41).
Figure 2:
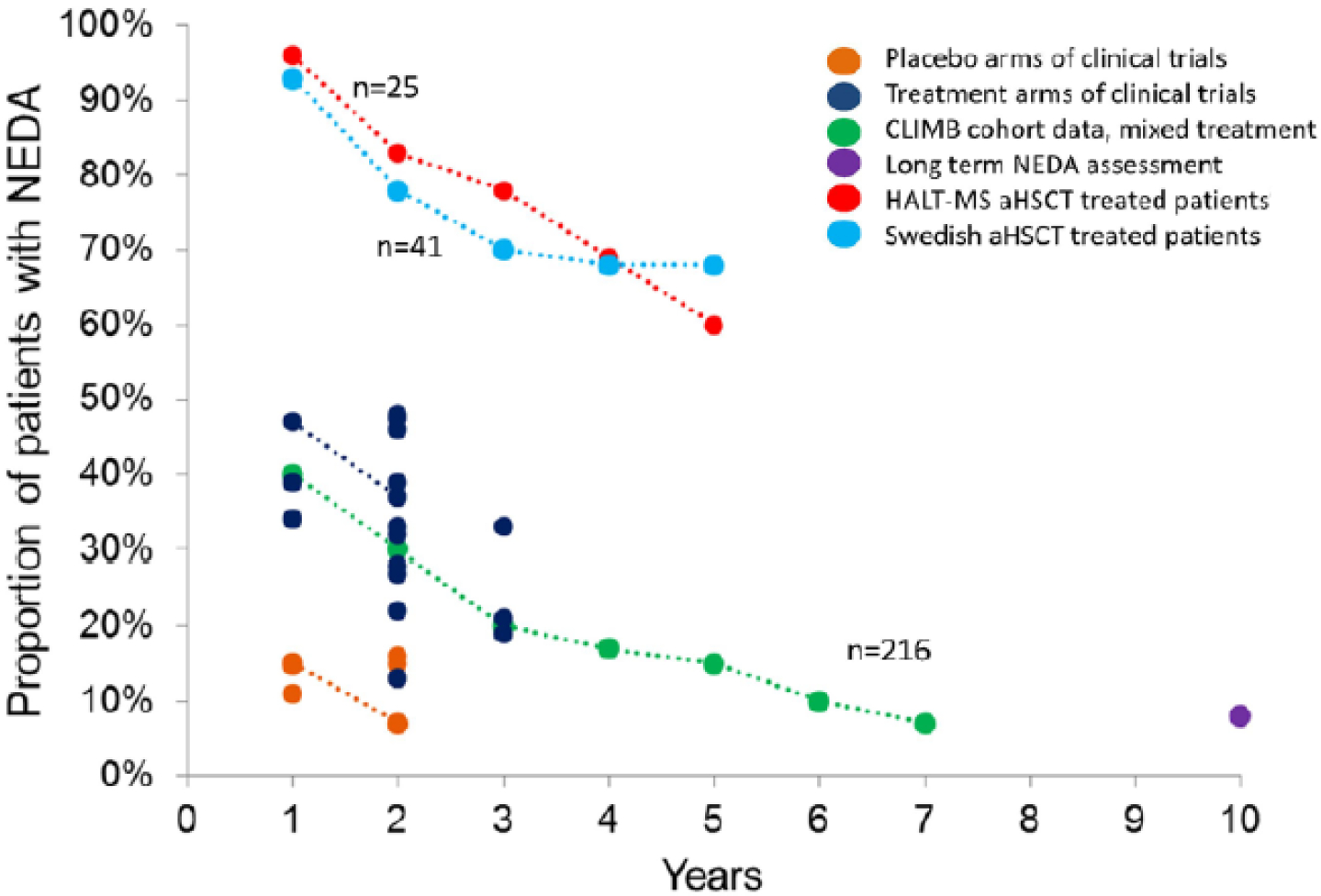
Proportion of patients maintaining no evidence of disease activity (NEDA) status over time under different treatment strategies. Points connected by a line represent longitudinal observations in the same study.
Reproduced with permission of Multiple sclerosis, 2017;23(2):201–4.
In the position statement from the American Society for Blood and Marrow Transplantation (ASBMT), HSCT is endorsed as a “standard of care, clinical evidence available” for patients with treatment-refractory relapsing MS (26).
HSCT for SSc
SSc is a chronic immune-mediated disease characterized by immune dysregulation, vascular damage and diffuse fibrosis. Immunosuppressive and biologic agents commonly used to treat patients with severe skin or organ involvement offer only modest benefit in delaying disease progression but do not reverse the natural and often fatal course of this disease (43, 44).
Several observational studies, registry-based studies and phase I/II trials have demonstrated the efficacy of HSCT in inducing major regression of both skin and lung fibrosis in SSc patients (45–52). The positive results from these studies form the basis for three randomized trials that compared HSCT and standard care (Cyc only) in the treatment of severe SSc: the American Scleroderma Stem Cell versus Immune Suppression Trial (ASSIST) (53), the Autologous Stem Cell Transplantation International Scleroderma (ASTIS) trial (54), and the Scleroderma: Cyclophosphamide or Transplantation (SCOT) trial (55). Table 1 detailed these 3 trials that confirmed the efficacy of HSCT in SSc.
Table 1:
Outline of ASSIST, ASTIS, and SCOT trials
| ASSIST | ASTIS | SCOT | ||||
|---|---|---|---|---|---|---|
| Trial design | Randomized phase II | Randomized phase III | Randomized phase III | |||
| No. of centers | 1 (USA) | 29 (Europe) | 26 (USA) | |||
| Recruitment period | 2006–2009 | 2001–2009 | 2006–2011 | |||
| No. of patients randomized | 19 | 156 | 75 | |||
| Age range, yr | <60 | 18–65 | 18–69 | |||
| Primary outcome | Improvement at 12 mo defined as a decrease in mRSS or an increase in FVC | Event failure-free survival at 24 mo | Change in global rank composite score at 54 months* | |||
| Treatment | Cyc | HSCT | Cyc | HSCT | Cyc | HSCT |
| Cyc 1g/m2/mo × 6 doses | Cyc 200mg/kg + rabbit ATG 6.5mg/kg + MP 5g | Cyc 750mg/m2/mo × 12 doses | Cyc 200mg/kg + rabbit ATG 7.5mg/kg | Cyc 750mg/m2/mo × 12 doses | Cyc 120mg/kg + horse ATG 90mg/kg + TBI 800 cGy | |
| Stem cell mobilization | - | Cyc 2g/m2 & GCSF | - | Cyc 4g/m2 & GCSF | - | GCSF & prednisolone |
| Autologous cells | - | Unselected | - | CD34+ selected | - | CD34+ selected |
| Patients randomized | 9 | 10 | 77 | 79 | 39 | 36 |
| Primary endpoint | Clinical improvement in 0/9 patients on Cyc arm vs 10/10 patients on HSCT arm (OR, 110; p=.0001) | HR for death or major organ failure at 2y follow-up, .35(95% CI, .16–.74; p=.006) favouring HSCT arm | At 54 mo, median global rank composite score −6.0 in Cyc arm vs. 17.0 in HSCT arm (p=.01) | |||
| Overall survival | 100% at 12 mo for both arms | HR at 10y follow-up, .29(95% CI, .13–.64; p=.002) favouring HSCT arm | At 72 mo, 51% in Cyc arm vs 86% in HSCT arm (p=.02) | |||
| TRM | 0% at 12 mo for both arms | 0% in Cyc arm vs 10.1% in HSCT arm (p=.007) | At 54 mo, 0% in Cyc arm vs 3% in HSCT arm (p=.48) | |||
| Recurrent disease | Disease progression in 8/9 patients in Cyc arm vs. 0/10 patients in HSCT arm (p=.0001) | Between 12–24 mo, 43.8% in Cyc arm received immunosuppresants vs. 22.4% in HSCT arm (p=.02) | At 54 mo, 44% in Cyc arm restarted DMARDs vs. 9% in HSCT arm (p=.001) | |||
Abbreviations: ATG, anti-thymocyte globulin; Cyc, cyclophosphamide; DMARDS, disease modifying anti-rheumatic drugs; FVC, forced vital capacity; HR, hazard ratio; HSCT, hematopoietic stem cell transplant; MP, methylprednisone; mRSS, modified Rodnan skin score; TBI, total body irradiation.
Global rank composite score is based on hierarchy of following disease outcomes: death, event-free survival (survival without respiratory, renal, or cardiac failure), FVC, Disability Index of Health Assessment Questionnaire score, and mRSS.
The ASSIST trial which included 19 patients randomized to receive either autologous HSCT after non-myeloablative conditioning with Cyc and rabbit ATG or monthly Cyc demonstrated that autologous HSCT resulted in significant improvements in both respiratory and skin manifestations at 12 months (53). This study did not show any treatment-related mortality. In the open-label phase III ASTIS study which recruited 156 patients with early diffuse SSc and compared CD34+ selected HSCT after conditioning with Cyc (200 mg/kg) and ATG versus 12 monthly intravenous pulse of Cyc, and demonstrated that HSCT confers better long-term survival than Cyc. Despite increased treatment-related mortality (TRM) of 10% during the first year, treatment responses in SSc clinical outcomes 2 years after HSCT were better than controls, allowing superior event-free and overall survival rates during the 10 years following HSCT (54).
The SCOT trial investigated CD34+ selected autologous HSCT after a myeloablative conditioning with total body irradiation (TBI, 800 cGy with lung and renal shielding to 200 cGy) and reduced dose Cyc (120mg/kg) versus 12 monthly infusions of Cyc and demonstrated a change in the primary end-point which was the global rank composite score (GRCS). The GRCS compares participants with each other on the basis of a hierarchy of disease features assessed at 54 months: death, event-free survival (survival without respiratory, renal, or cardiac failure), forced vital capacity, the score on the Disability Index of the Health Assessment Questionnaire, and the modified Rodnan skin score (55). At 54 months, GRCS was significantly improved after HSCT compared to monthly Cyc infusions.
Systematic reviews and meta-analysis concluded that HSCT is beneficial in some patients with SSc (56, 57). Compared with the control, HSCT reduced all-cause mortality (risk ratio, 0.50 [95% confidence interval, 0.33 to 0.75; p = 0 .0007) and improved skin thickness, forced vital capacity, total lung capacity, and quality of life (57). Based on the results of these controlled studies, the European League Against Rheumatism has issued evidence-based guidelines for the treatment of SSc that recommend HSCT for the treatment of selected patients with rapidly progressive disease at risk of organ failure (58). In the position statement from the ASBMT, HSCT is recommended as “standard of care” for patients with severe SSc (59).
Adverse effects of HSCT
The immune system of patients with autoimmune disease can be substantially weakened by both the disease and chronic use of immunosuppressants (20). The process of stem cells mobilization, conditioning regimen and T‑cell depletion of the autologous graft is also associated with an increased risk of infections (6). Due to the loss of regulatory mechanisms within the immune system, autoimmunity can develop de novo during immune reconstitution after HSCT. The cumulative incidence of a secondary autoimmune disease was reported to be 9.8% at 5 years after HSCT (60).
Initially associated with increased TRM, HSCT was considered as salvage therapy in patients with severe refractory disease with poor outcomes. Over the past years however, TRM has significantly improved due to greater center experience, better patient selection and supportive care (21).
Relapse of disease after HSCT
Disease relapse after HSCT is possible and remains a challenge. As illustrated in Figure 3, analysis of the EBMT registry-based data showed that disease relapse after HSCT has remained a complication over the different years of transplant (21). In this survey, almost all individuals received non-myeloablative transplants. Therein lies the question whether there is a preparative conditioning regimen that has lower relapse rates. Based on current evidence, there appears to be lower relapse rates in myeloablative in comparison to non-myeloablative regimens. In the SCOT trial where myeloablative regimen was utilised, risk of relapse as defined by disease modifying anti-rheumatic drugs (DMARDs) initiation was reported to be lower than those in previous reports of non-myeloablative transplantation (55). Relapses may be due to the persistence of autoreactive memory cells. Myeloablation with TBI is used to maximally deplete T cells before progenitor-cell immune reconstitution and may result in better long-term outcomes and potentially cure. The unique property of TBI to ablate dividing and resting autoreactive clones probably contributed to the durable remissions observed in the SCOT trial (55), mirroring the findings of preclinical transplantation studies in autoimmune diseases (61).
Figure 3:
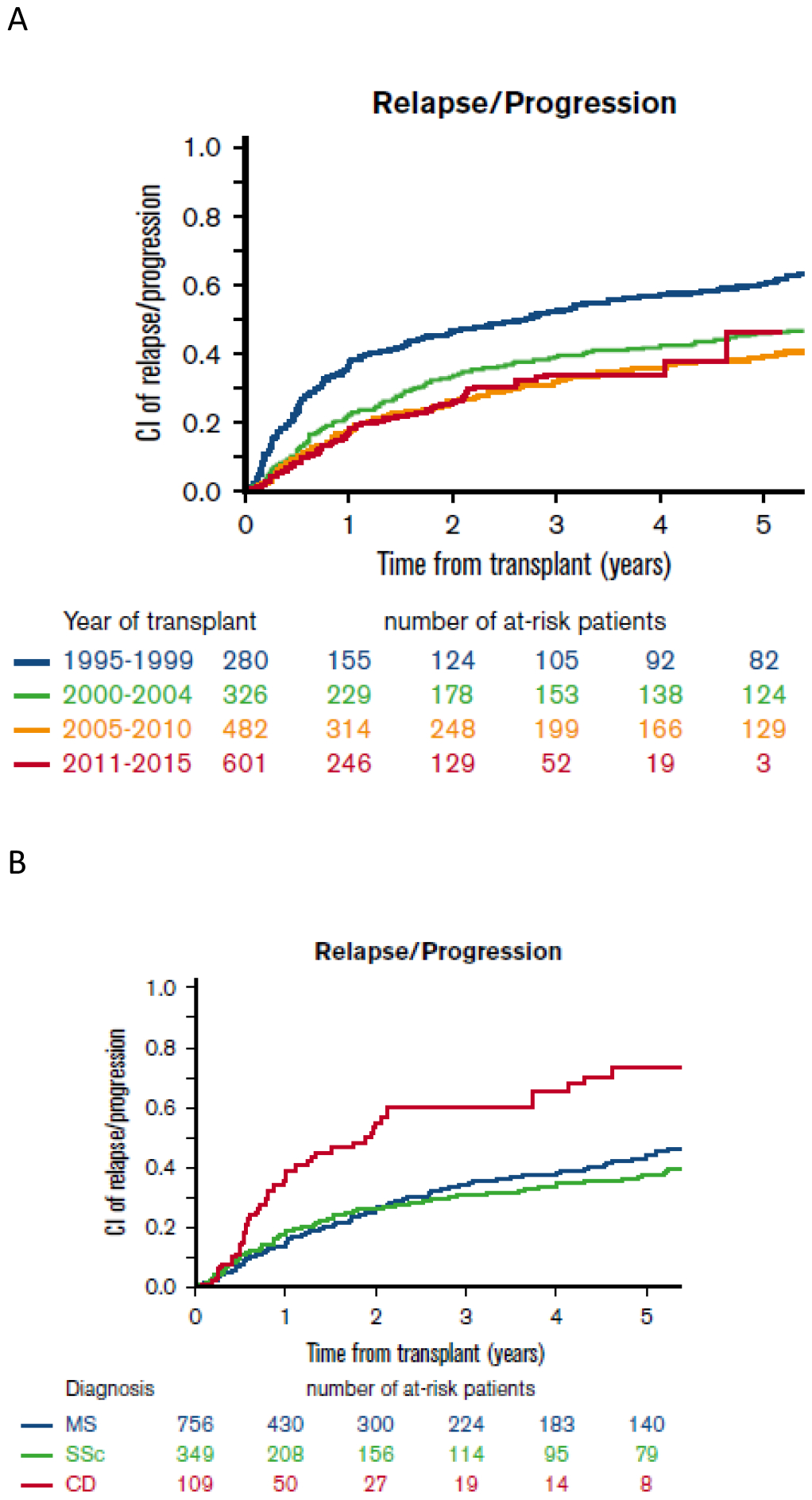
Relapse of autoimmune disease. A. Trends in incidence of relapse over the different years of transplant. B. Cumulative relapse incidence following first autologous HSCT in multiple sclerosis (MS), systemic sclerosis (SSc) and Crohn disease (CD).
Reproduced with permission of Blood advances, 2017;1(27):2742–55.
Conclusion
Considerable advances have deepened our understanding of the role of HSCT in autoimmune diseases. Autologous HSCT may be the treatment that is able to induce long-term, symptom-free remission and potential cure in autoimmune diseases that are refractory to conventional medication therapy. Close collaboration between physicians with expertise in treating autoimmune diseases and transplant physicians is critical to identify patients who are candidates for HSCT and to ensure optimal outcomes. Future trials to address patient selection, transplantation timing, optimal preparative regimens, maintenance therapy post-transplantation and longer term prognosis will be helpful to optimise the efficacy of HSCT in autoimmune diseases.
Key points:
The rationale of haematopoietic stem cell transplantation (HSCT) is to ‘reset’ one’s immune system into a naïve and self-tolerant state through immune depletion and regulation.
HSCT has emerged as a promising treatment for severe and therapy-refractory autoimmune diseases.
Safety of HSCT has generally improved with careful patient selection and greater transplant expertise.
Acknowledgements
Dr Andrea Low for her expertise on systemic sclerosis.
Financial support and sponsorship
The SCOT trial was supported by NIH grants N01-AI05419 and HHSN 272201100025C.
Footnotes
Conflict of interest
None
References
Papers of particular interest, published within the annual period of review, have been highlighted as:
*of special interest
**of outstanding interest
- 1.Shlomchik MJ, Craft JE, Mamula MJ. From T to B and back again: positive feedback in systemic autoimmune disease. Nature reviews Immunology. 2001;1(2):147–53. [DOI] [PubMed] [Google Scholar]
- 2.Alexander T, Bondanza A, Muraro PA, Greco R, Saccardi R, Daikeler T, et al. SCT for severe autoimmune diseases: consensus guidelines of the European Society for Blood and Marrow Transplantation for immune monitoring and biobanking. Bone marrow transplantation. 2015;50(2):173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas E, Storb R, Clift RA, Fefer A, Johnson FL, Neiman PE, et al. Bone-marrow transplantation (first of two parts). The New England journal of medicine. 1975;292(16):832–43. [DOI] [PubMed] [Google Scholar]
- 4.Thomas ED, Storb R, Clift RA, Fefer A, Johnson L, Neiman PE, et al. Bone-marrow transplantation (second of two parts). The New England journal of medicine. 1975;292(17):895–902. [DOI] [PubMed] [Google Scholar]
- 5.Blume KG, Thomas ED. A review of autologous hematopoietic cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2000;6(1):1–12. [DOI] [PubMed] [Google Scholar]
- 6.Snowden JA, Saccardi R, Allez M, Ardizzone S, Arnold R, Cervera R, et al. Haematopoietic SCT in severe autoimmune diseases: updated guidelines of the European Group for Blood and Marrow Transplantation. Bone marrow transplantation. 2012;47(6):770–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sullivan KM, Muraro P, Tyndall A. Hematopoietic cell transplantation for autoimmune disease: updates from Europe and the United States. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010;16(1 Suppl):S48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hügle T, Daikeler T. Stem cell transplantation for autoimmune diseases. Haematologica. 2010;95(2):185–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexander T, Thiel A, Rosen O, Massenkeil G, Sattler A, Kohler S, et al. Depletion of autoreactive immunologic memory followed by autologous hematopoietic stem cell transplantation in patients with refractory SLE induces long-term remission through de novo generation of a juvenile and tolerant immune system. Blood. 2009;113(1):214–23. [DOI] [PubMed] [Google Scholar]
- 10.Muraro PA, Douek DC, Packer A, Chung K, Guenaga FJ, Cassiani-Ingoni R, et al. Thymic output generates a new and diverse TCR repertoire after autologous stem cell transplantation in multiple sclerosis patients. The Journal of experimental medicine. 2005;201(5):805–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roord ST, de Jager W, Boon L, Wulffraat N, Martens A, Prakken B, et al. Autologous bone marrow transplantation in autoimmune arthritis restores immune homeostasis through CD4+CD25+Foxp3+ regulatory T cells. Blood. 2008;111(10):5233–41. [DOI] [PubMed] [Google Scholar]
- 12.Zand MS, Vo T, Pellegrin T, Felgar R, Liesveld JL, Ifthikharuddin JJ, et al. Apoptosis and complement-mediated lysis of myeloma cells by polyclonal rabbit antithymocyte globulin. Blood. 2006;107(7):2895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikehara S, Good RA, Nakamura T, Sekita K, Inoue S, Oo MM, et al. Rationale for bone marrow transplantation in the treatment of autoimmune diseases. Proceedings of the National Academy of Sciences of the United States of America. 1985;82(8):2483–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Bekkum DW, Bohre EP, Houben PF, Knaan-Shanzer S. Regression of adjuvant-induced arthritis in rats following bone marrow transplantation. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(24):10090–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Bekkum DW. Stem cell transplantation in experimental models of autoimmune disease. Journal of clinical immunology. 2000;20(1):10–6. [DOI] [PubMed] [Google Scholar]
- 16.Nelson JL, Torrez R, Louie FM, Choe OS, Storb R, Sullivan KM. Pre-existing autoimmune disease in patients with long-term survival after allogeneic bone marrow transplantation. The Journal of rheumatology Supplement. 1997;48:23–9. [PubMed] [Google Scholar]
- 17.Tyndall A, Gratwohl A. Blood and marrow stem cell transplants in auto-immune disease: a consensus report written on behalf of the European League against Rheumatism (EULAR) and the European Group for Blood and Marrow Transplantation (EBMT). Bone marrow transplantation. 1997;19(7):643–5. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan KM, Furst DE. The evolving role of blood and marrow transplantation for the treatment of autoimmune diseases. The Journal of rheumatology Supplement. 1997;48:1–4. [PubMed] [Google Scholar]
- 19.Tamm M, Gratwohl A, Tichelli A, Perruchoud AP, Tyndall A. Autologous haemopoietic stem cell transplantation in a patient with severe pulmonary hypertension complicating connective tissue disease. Annals of the rheumatic diseases. 1996;55(10):779–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swart JF, Delemarre EM, van Wijk F, Boelens JJ, Kuball J, van Laar JM, et al. Haematopoietic stem cell transplantation for autoimmune diseases. Nature reviews Rheumatology. 2017;13(4):244–56. [DOI] [PubMed] [Google Scholar]
- 21.Snowden JA, Badoglio M, Labopin M, Giebel S, McGrath E, Marjanovic Z, et al. Evolution, trends, outcomes, and economics of hematopoietic stem cell transplantation in severe autoimmune diseases. Blood advances. 2017;1(27):2742–55. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This publication summarizes the trends, factors and outcomes in patients with autoimmune diseases who underwent hematopoietic stem cell transplantation using the European Society for Blood and Marrow Transplantation registry data.
- 22.Pasquini MC, Voltarelli J, Atkins HL, Hamerschlak N, Zhong X, Ahn KW, et al. Transplantation for autoimmune diseases in north and South America: a report of the Center for International Blood and Marrow Transplant Research. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18(10):1471–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sorensen PS, Thompson AJ, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giovannoni G, Turner B, Gnanapavan S, Offiah C, Schmierer K, Marta M. Is it time to target no evident disease activity (NEDA) in multiple sclerosis? Multiple sclerosis and related disorders. 2015;4(4):329–33. [DOI] [PubMed] [Google Scholar]
- 25.Rotstein DL, Healy BC, Malik MT, Chitnis T, Weiner HL. Evaluation of no evidence of disease activity in a 7-year longitudinal multiple sclerosis cohort. JAMA neurology. 2015;72(2):152–8. [DOI] [PubMed] [Google Scholar]
- 26.Cohen JA, Baldassari LE, Atkins HL, Bowen JD, Bredeson C, Carpenter PA, et al. Autologous Hematopoietic Cell Transplantation for Treatment-Refractory Relapsing Multiple Sclerosis: Position Statement from the American Society for Blood and Marrow Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2019. [DOI] [PubMed] [Google Scholar]
- 27.Mancardi GL, Sormani MP, Di Gioia M, Vuolo L, Gualandi F, Amato MP, et al. Autologous haematopoietic stem cell transplantation with an intermediate intensity conditioning regimen in multiple sclerosis: the Italian multi-centre experience. Multiple sclerosis (Houndmills, Basingstoke, England). 2012;18(6):835–42. [DOI] [PubMed] [Google Scholar]
- 28.Burman J, Iacobaeus E, Svenningsson A, Lycke J, Gunnarsson M, Nilsson P, et al. Autologous haematopoietic stem cell transplantation for aggressive multiple sclerosis: the Swedish experience. Journal of neurology, neurosurgery, and psychiatry. 2014;85(10):1116–21. [DOI] [PubMed] [Google Scholar]
- 29.Burt RK, Balabanov R, Han X, Sharrack B, Morgan A, Quigley K, et al. Association of nonmyeloablative hematopoietic stem cell transplantation with neurological disability in patients with relapsing-remitting multiple sclerosis. Jama. 2015;313(3):275–84. [DOI] [PubMed] [Google Scholar]
- 30.Muraro PA, Pasquini M, Atkins HL, Bowen JD, Farge D, Fassas A, et al. Long-term Outcomes After Autologous Hematopoietic Stem Cell Transplantation for Multiple Sclerosis. JAMA neurology. 2017;74(4):459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shevchenko YL, Novik AA, Kuznetsov AN, Afanasiev BV, Lisukov IA, Kozlov VA, et al. High-dose immunosuppressive therapy with autologous hematopoietic stem cell transplantation as a treatment option in multiple sclerosis. Experimental hematology. 2008;36(8):922–8. [DOI] [PubMed] [Google Scholar]
- 32.Shevchenko JL, Kuznetsov AN, Ionova TI, Melnichenko VY, Fedorenko DA, Kartashov AV, et al. Autologous hematopoietic stem cell transplantation with reduced-intensity conditioning in multiple sclerosis. Experimental hematology. 2012;40(11):892–8. [DOI] [PubMed] [Google Scholar]
- 33.Burt RK, Loh Y, Cohen B, Stefoski D, Balabanov R, Katsamakis G, et al. Autologous non-myeloablative haemopoietic stem cell transplantation in relapsing-remitting multiple sclerosis: a phase I/II study. The Lancet Neurology. 2009;8(3):244–53. [DOI] [PubMed] [Google Scholar]
- 34.Nash RA, Hutton GJ, Racke MK, Popat U, Devine SM, Griffith LM, et al. High-dose immunosuppressive therapy and autologous hematopoietic cell transplantation for relapsing-remitting multiple sclerosis (HALT-MS): a 3-year interim report. JAMA neurology. 2015;72(2):159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nash RA, Hutton GJ, Racke MK, Popat U, Devine SM, Steinmiller KC, et al. High-dose immunosuppressive therapy and autologous HCT for relapsing-remitting MS. Neurology. 2017;88(9):842–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atkins HL, Bowman M, Allan D, Anstee G, Arnold DL, Bar-Or A, et al. Immunoablation and autologous haemopoietic stem-cell transplantation for aggressive multiple sclerosis: a multicentre single-group phase 2 trial. Lancet (London, England). 2016;388(10044):576–85. [DOI] [PubMed] [Google Scholar]
- 37.Moore JJ, Massey JC, Ford CD, Khoo ML, Zaunders JJ, Hendrawan K, et al. Prospective phase II clinical trial of autologous haematopoietic stem cell transplant for treatment refractory multiple sclerosis. Journal of neurology, neurosurgery, and psychiatry. 2018. [DOI] [PubMed] [Google Scholar]
- 38.Mancardi GL, Sormani MP, Gualandi F, Saiz A, Carreras E, Merelli E, et al. Autologous hematopoietic stem cell transplantation in multiple sclerosis: a phase II trial. Neurology. 2015;84(10):981–8. [DOI] [PubMed] [Google Scholar]
- 39.Burt RK, Balabanov R, Burman J, Sharrack B, Snowden JA, Oliveira MC, et al. Effect of Nonmyeloablative Hematopoietic Stem Cell Transplantation vs Continued Disease-Modifying Therapy on Disease Progression in Patients With Relapsing-Remitting Multiple Sclerosis: A Randomized Clinical Trial. Jama. 2019;321(2):165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study demonstrated the efficacy of hematopoietic stem cell transplantation on disability worsening, relapse reduction and decrease in MRI lesions in patients with multiple sclerosis compared to standard disease-modifying therapy.
- 40.Sormani MP, Muraro PA, Schiavetti I, Signori A, Laroni A, Saccardi R, et al. Autologous hematopoietic stem cell transplantation in multiple sclerosis: A meta-analysis. Neurology. 2017;88(22):2115–22. [DOI] [PubMed] [Google Scholar]; **This publication demonstrated that the proportion of patients with multiple sclerosis with no evidence of disease activity (NEDA) was higher in the autologous hematopoetic stem cell transplant treated subjects than in those treated with all the other drugs.
- 41.Sormani MP, Muraro PA, Saccardi R, Mancardi G. NEDA status in highly active MS can be more easily obtained with autologous hematopoietic stem cell transplantation than other drugs. Multiple sclerosis (Houndmills, Basingstoke, England). 2017;23(2):201–4. [DOI] [PubMed] [Google Scholar]
- 42.Muraro PA, Martin R, Mancardi GL, Nicholas R, Sormani MP, Saccardi R. Autologous haematopoietic stem cell transplantation for treatment of multiple sclerosis. Nature reviews Neurology. 2017;13(7):391–405. [DOI] [PubMed] [Google Scholar]
- 43.Elhai M, Meune C, Avouac J, Kahan A, Allanore Y. Trends in mortality in patients with systemic sclerosis over 40 years: a systematic review and meta-analysis of cohort studies. Rheumatology (Oxford, England). 2012;51(6):1017–26. [DOI] [PubMed] [Google Scholar]
- 44.Tashkin DP, Elashoff R, Clements PJ, Roth MD, Furst DE, Silver RM, et al. Effects of 1-year treatment with cyclophosphamide on outcomes at 2 years in scleroderma lung disease. American journal of respiratory and critical care medicine. 2007;176(10):1026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vonk MC, Marjanovic Z, van den Hoogen FH, Zohar S, Schattenberg AV, Fibbe WE, et al. Long-term follow-up results after autologous haematopoietic stem cell transplantation for severe systemic sclerosis. Annals of the rheumatic diseases. 2008;67(1):98–104. [DOI] [PubMed] [Google Scholar]
- 46.Nash RA, McSweeney PA, Crofford LJ, Abidi M, Chen CS, Godwin JD, et al. High-dose immunosuppressive therapy and autologous hematopoietic cell transplantation for severe systemic sclerosis: long-term follow-up of the US multicenter pilot study. Blood. 2007;110(4):1388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burt RK, Oliveira MC, Shah SJ, Moraes DA, Simoes B, Gheorghiade M, et al. Cardiac involvement and treatment-related mortality after non-myeloablative haemopoietic stem-cell transplantation with unselected autologous peripheral blood for patients with systemic sclerosis: a retrospective analysis. Lancet (London, England). 2013;381(9872):1116–24. [DOI] [PubMed] [Google Scholar]
- 48.Henes JC, Schmalzing M, Vogel W, Riemekasten G, Fend F, Kanz L, et al. Optimization of autologous stem cell transplantation for systemic sclerosis -- a single-center longterm experience in 26 patients with severe organ manifestations. The Journal of rheumatology. 2012;39(2):269–75. [DOI] [PubMed] [Google Scholar]
- 49.Farge D, Labopin M, Tyndall A, Fassas A, Mancardi GL, Van Laar J, et al. Autologous hematopoietic stem cell transplantation for autoimmune diseases: an observational study on 12 years’ experience from the European Group for Blood and Marrow Transplantation Working Party on Autoimmune Diseases. Haematologica. 2010;95(2):284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oyama Y, Barr WG, Statkute L, Corbridge T, Gonda EA, Jovanovic B, et al. Autologous non-myeloablative hematopoietic stem cell transplantation in patients with systemic sclerosis. Bone marrow transplantation. 2007;40(6):549–55. [DOI] [PubMed] [Google Scholar]
- 51.Del Papa N, Onida F, Zaccara E, Saporiti G, Maglione W, Tagliaferri E, et al. Autologous hematopoietic stem cell transplantation has better outcomes than conventional therapies in patients with rapidly progressive systemic sclerosis. Bone marrow transplantation. 2017;52(1):53–8. [DOI] [PubMed] [Google Scholar]
- 52.Binks M, Passweg JR, Furst D, McSweeney P, Sullivan K, Besenthal C, et al. Phase I/II trial of autologous stem cell transplantation in systemic sclerosis: procedure related mortality and impact on skin disease. Annals of the rheumatic diseases. 2001;60(6):577–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burt RK, Shah SJ, Dill K, Grant T, Gheorghiade M, Schroeder J, et al. Autologous non-myeloablative haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for systemic sclerosis (ASSIST): an open-label, randomised phase 2 trial. Lancet (London, England). 2011;378(9790):498–506. [DOI] [PubMed] [Google Scholar]
- 54.van Laar JM, Farge D, Sont JK, Naraghi K, Marjanovic Z, Larghero J, et al. Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: a randomized clinical trial. Jama. 2014;311(24):2490–8. [DOI] [PubMed] [Google Scholar]
- 55.Sullivan KM, Goldmuntz EA, Keyes-Elstein L, McSweeney PA, Pinckney A, Welch B, et al. Myeloablative Autologous Stem-Cell Transplantation for Severe Scleroderma. The New England journal of medicine. 2018;378(1):35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]; **The SCOT trial showed that myeloablative autologous hematopoietic stem-cell transplantation achieved long-term benefits in patients with scleroderma, with significant improvement in the global rank composite score at 54 months.
- 56.Host L, Nikpour M, Calderone A, Cannell P, Roddy J. Autologous stem cell transplantation in systemic sclerosis: a systematic review. Clinical and experimental rheumatology. 2017;35 Suppl 106(4):198–207. [PubMed] [Google Scholar]
- 57.Shouval R, Furie N, Raanani P, Nagler A, Gafter-Gvili A. Autologous Hematopoietic Stem Cell Transplantation for Systemic Sclerosis: A Systematic Review and Meta-Analysis. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2018;24(5):937–44. [DOI] [PubMed] [Google Scholar]
- 58.Kowal-Bielecka O, Fransen J, Avouac J, Becker M, Kulak A, Allanore Y, et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Annals of the rheumatic diseases. 2017;76(8):1327–39. [DOI] [PubMed] [Google Scholar]
- 59.Sullivan KM, Majhail NS, Bredeson C, Carpenter PA, Chatterjee S, Crofford LJ, et al. Systemic Sclerosis as an Indication for Autologous Hematopoietic Cell Transplantation: Position Statement from the American Society for Blood and Marrow Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2018;24(10):1961–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Daikeler T, Labopin M, Di Gioia M, Abinun M, Alexander T, Miniati I, et al. Secondary autoimmune diseases occurring after HSCT for an autoimmune disease: a retrospective study of the EBMT Autoimmune Disease Working Party. Blood. 2011;118(6):1693–8. [DOI] [PubMed] [Google Scholar]
- 61.van Bekkum DW. Conditioning regimens for the treatment of experimental arthritis with autologous bone marrow transplantation. Bone marrow transplantation. 2000;25(4):357–64. [DOI] [PubMed] [Google Scholar]


