Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been spread worldwide. The mortality rate of patients with COVID-19 can be as high as 4.2%.1 Despite optimized medical treatment, some patients with end-stage COVID-19 pneumonia progressed to irreversible loss of lung function, and those critical patients need to be admitted to the intensive care unit (ICU).2–4 Lung transplantation is an effective treatment for end-stage pulmonary chronic diseases.5,6 At present, there is no report of lung transplantation in patients with COVID-19. Here we report 2 lung transplantations of elderly patients with severe COVID-19 pneumonia, hoping to provide an additional choice for those critical patients.
TREATMENT BEFORE LUNG TRANSPLANTATION
Case 1
A 66-year-old woman was admitted to the local hospital for cough (11 days) and fever (1 day) on January 31, 2020. She was diagnosed with COVID-19 pneumonia by abnormal chest computed tomography (CT) findings and positive SARS-CoV-2 nucleic acid test result. She was transferred to our hospital on February 2, 2020 for more comprehensive treatment. A course of Lopinavir/Ritonavir, Arbidol, convalescent plasma, and methylprednisolone did not abate her declining condition. On February 16, life-supporting extracorporeal membrane oxygenation (ECMO) was initiated. The SARS-CoV-2 nucleic acid test results of sputum, bronchoalveolar lavage (BAL), blood, and feces became negative on February 23. Although there was no evidence of continued virus replication, lung function continued to deteriorate. On February 26, her comprehensive assessment read: The x-ray manifestations showed “white lung.” Despite ECMO with mechanical ventilation, oxygenation index was <60 mm Hg. Echocardiography showed the right heart was enlarged, estimated pulmonary artery systolic pressure was 80 mm Hg, and left ventricular ejection fraction (LVEF) was 60%. Kidney, liver, and left heart function were normal. With best practices exhausted and continued decline, lung transplantation was considered and approved by the ethics committee. After her family signed informed consent, we registered the patient into the China Organ Transplant Response System (COTRS).
Case 2
A 70-year-old man was diagnosed with COVID-19 with a fever on February 2. Underlying medical conditions included hypertension, diabetes, and psoriasis for many years. His continued status decline motivated transfer to our hospital after 1 week. Although he received antiviral, hormonal, convalescent plasma, and immune-enhancing supportive treatments, his condition continued to worsen. Tracheal intubation was performed on February 9, and ECMO-assisted support was performed on February 26. The SARS-CoV-2 nucleic acid test results of sputum, BAL, blood, and faeces were negative on February 16. His comprehensive evaluation on March 5: Chest radiographs showed large blurry images of both lungs, and he was relying on ECMO and mechanical ventilation to maintain life. This patient had no obvious transplant contraindications, so we registered the patient into the COTRS.
PROCESS AND OUTCOME OF THE LUNG TRANSPLANTATION
The x-ray of the first patient manifested “white lung” before surgery (Fig. 1A). Lung transplantation occurred on March 1. Donor lungs were acquired by COTRS protocol. During the operation, we found no visible pleural effusion in the thorax cavity, and the right heart of the recipient was enlarged and turned clockwise. The shape of the resected lungs was complete, dark red, tough, without apparent mass, and the surface was uneven. The lungs were consolidated entirely in the cross-section, without noticeable mucus (Fig. 1B). The patient's vital signs were stable after surgery, but 40 hours later, she showed a remarkably decreased oxygenation index, and CT scan presented an extensive consolidation of the donor's lungs. Those manifestations suggested acute rejection. Steroid pulse therapy was used to control rejection (methylprednisolone 500 mg/day for 3 days, then weaning 40 mg daily to a final dose of 40 mg/day). After the first dose, the patient's oxygenation index improved rapidly, and the chest x-ray became clear (Fig. 1C). The ECMO was removed successfully on the fifth day.
FIGURE 1.
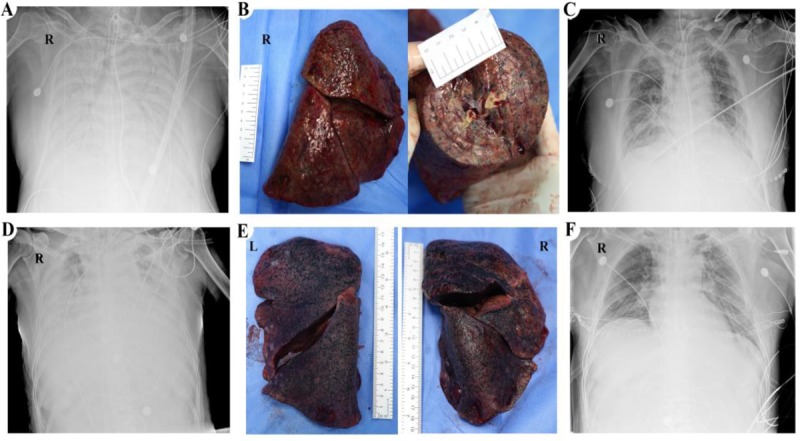
Chest x-rays and gross photos of patients. (A) The first patient's chest x-ray on February 27. (B) Frontal view and cross-sectional appearance of the first patient's resected right lung. (C) The first patient's chest x-ray on four days after lung transplantation. (D) The second patient's chest x-ray on March 7. (E) Resected lungs of the second patient. (F) The second patient's chest x-ray on the next day of lung transplantation. L indicates left; R, right.
On March 8, the second patient underwent bilateral lung transplantation after the nucleic acid test of SARS-CoV-2 turned to negative for 20 days. His lungs gradually lost function as the disease progressed before surgery (Fig. 1D). Similar to the first patient, we found no visible pleural effusion in the thorax, and the recipient's heart was healthy. The resected lungs exhibited extensive consolidation, but they were softer than the first patient's lungs (Fig. 1E). Considering the high proportion of HLA mismatch between donor and recipient, we used high-dose methylprednisolone (methylprednisolone 500 mg/day for 3 days, then weaning 40 mg daily to a final dose of 40 mg/day) to prevent acute rejection after surgery. This patient's condition was stable, and ECMO was withdrawn on the second day after surgery. The chest x-ray showed the transplant lungs were clear (Fig. 1F).
During the operation, we sampled specimens from the recipient's 5 different lung lobes for SARS-CoV-2 nucleic acid test. All specimens were negative. After surgery, the patient's results of SARS-CoV-2 nucleic acid test were still negative in sputum, BAL, and feces. Importantly, live virus in resected lungs was not detected through Vero cell culture in a biological safety protection third-level (P3) laboratory.
DISCUSSION
Lung transplantation is an important therapy for end-stage chronic lung disease,6 but it was rarely used for the treatment of acute infectious pneumonia. Therefore, there is no guideline for lung transplantation for treating patients with COVID-19 pneumonia. We do not recommend lung transplantation in patients with positive viral RNA tests. Systemic virus could damage the donor's lungs after lung transplantation and medical staff would be put at risk to a virus with a high reproductive index.7 It is recommended that the viral RNA test result be negative at least twice (24-hour interval) in transplant candidates. Test specimens should include sputum and BAL.8
The first patient had a significant decrease in oxygenation around 40 hours after surgery, and the patient was diagnosed with early acute rejection, which was confirmed by CT imaging findings and following treatment outcome. The early acute rejection may be related to the following factors: first, blood transfusion before surgery increased the risk of acute postoperative rejection; second, long-term high-dose immunoenhancement (Thymalfasin) before surgery interfered the recipient's immune recognition system.
Unlike chronic lung diseases, COVID19 is an emerging infectious disease, and the profile of the disease is unclear. It is difficult to judge whether the lung injury in COVID-19 patients is irreversible. Therefore, multiple disciplinary teams are necessary to minimize the possibility of misjudgments. This team might include intensive care unit, respiratory, infectious, and radiology departments. In this study, the 2 patients’ lungs were entirely consolidated, and the patients were utterly dependent on ECMO and mechanical ventilation to maintain life. Lung transplantation was the only way to save their lives.
This report, to our knowledge, is the first description of lung transplantation for elderly patients with end-stage COVID-19 pneumonia. We suggest that lung transplantation could be considered when internal medicine treatments, involving medication, mechanical ventilation, and ECMO cannot improve the lung function. Our experience shows that lung transplantation could be an effective choice for end-stage COVID-19 patients.
Acknowledgments
The authors thank the COTRS (China Organ Transplant Response System) to donor organ allocation and also appreciate the donors for their selfless dedication.
Footnotes
Authors’ contributions: T.L. designed the study; W.H. completed the operation; W.H., M.Z., and J.C. collected the date and wrote the manuscript; J.Z., S.Z., and Q.F. contributed to data collection and analysis. G.W. and T.L. criticized the manuscript. All authors approved the final manuscript.
The authors report no conflicts of interest.
REFERENCES
- 1.Coronavirus disease 2019 (COVID-19) Situation Report – 59. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200319-sitrep-59-covid-19.pdf?sfvrsn=c3dcdef9_2.
- 2.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehra MR, Canter CE, Hannan MM, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10-year update. J Heart Lung Transplant 2016; 35:1–23. [DOI] [PubMed] [Google Scholar]
- 6.Yusen RD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-second Official Adult Lung and Heart-Lung Transplantation Report—2015; Focus Theme: Early Graft Failure. J Heart Lung Transplant 2015; 34:1264–1277. [DOI] [PubMed] [Google Scholar]
- 7.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 2020; 382:1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]


