Abstract
The role of advanced glycation end products (AGEs) in promoting and/or exacerbating metabolic dysregulation is being increasingly recognized. AGEs are formed when reducing sugars nonenzymatically bind to proteins or lipids, a process that is enhanced by hyperglycemic and hyperlipidemic environments characteristic of numerous metabolic disorders including obesity, diabetes, and its complications. In this mini-review, we put forth the notion that AGEs span the spectrum from cause to consequence of insulin resistance and diabetes, and represent a “common soil” underlying the pathophysiology of these metabolic disorders. Collectively, the surveyed literature suggests that AGEs, both those that form endogenously as well as exogenous AGEs derived from environmental factors such as pollution, smoking, and “Western”-style diets, contribute to the pathogenesis of obesity and diabetes. Specifically, AGE accumulation in key metabolically relevant organs induces insulin resistance, inflammation, and oxidative stress, which in turn provide substrates for excess AGE formation, thus creating a feed-forward–fueled pathological loop mediating metabolic dysfunction.
Keywords: advanced glycation end products (AGEs), insulin resistance (IR), receptor for advanced glycation end products (RAGE), soluble receptor for advanced glycation end products (sRAGE), obesity, diabetes
Michael Stern’s 1995 “common soil” hypothesis proposed that macrovascular disorders, specifically atherosclerosis, may not just be a complication of diabetes but rather that these disorders share common genetic and environmental pathogenic factors, or a common soil. The author proposed central obesity and what was referred to as insulin resistance (IR) syndrome, including hypertension, glucose intolerance, hyperinsulinemia, and hyperlipidemia, as common factors (1). Recent and burgeoning evidence suggests that in the milieus of these metabolic perturbations, the formation of advanced glycation end products (AGEs) is enhanced, thereby implicating AGEs as major contributors to this common soil because AGEs are connected to the pathophysiology of obesity, diabetes, and diabetic complications through the induction of systemic IR.
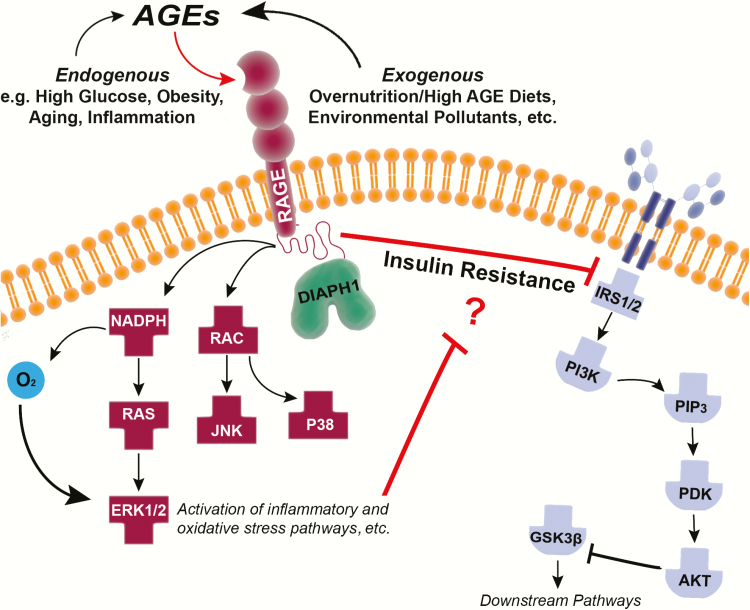
AGEs are the resulting irreversible molecular adducts formed from the nonenzymatic reaction between reducing sugars such as fructose and glucose with proteins or lipids, through a series of complex reactions accelerated by heat and first described in 1912 by Louis-Camille Maillard (2, 3). One way to classify AGEs is through their modes of formation. The endogenous AGEs form as products of Schiff bases/Amadori products and from the polyol pathway (Table 1) (4). Endogenous AGEs include species such as pyrraline, pentosidine, Nε-carboxyethyl lysine (CEL), and the prototypical in vivo AGE, Nε-carboxymethyl lysine (CML) (3, 5, 6). Several receptors with affinity for AGEs have been described, including scavenger receptors (SRs) (7) such as CD36 (8) and some that are preferentially expressed by macrophages (8): galectin-3 (9), OST-48 (10), AGE receptor 1 (AGER-1) (11), the lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) (12), and the best characterized and most widely studied receptor for AGEs, RAGE (13). Multiple isoforms of RAGE have been identified but the 2 most prominent are the forms that lack the transmembrane and cytoplasmic domains, known as truncated or soluble (sRAGE), and an endogenous secretory (esRAGE), which forms via alternative RNA splicing (14, 15). Because of the lack of transmembrane and cytoplasmic domains of RAGE, these soluble receptors are believed to act as circulating decoy receptors that suppress the interaction between membrane-bound (full-length) RAGE and its ligands, including AGEs. Full-length RAGE is expressed at low levels in multiple tissues, including endothelial cells, smooth muscle cells, neurons, cardiomyocytes (16), renal cells (17), and in adipose tissue (18, 19). On ligand binding, RAGE induces inflammatory cascades (20), including the activation of the Ras–mitogen-activated protein kinase and nuclear factor–κB pathways, and the production of reactive oxygen species (21), processes that are exacerbated in high-glucose environments (22) and that may also contribute to the pathogenesis of IR (Fig. 1). A distinct means of AGE formation is via exogenous mechanisms. In the sections to follow, we consider how exogenous AGEs may be imbibed and in turn exacerbate endogenous AGE formation.
Table 1.
| Polyol Pathway | Schiff Base/Amadori Products | |||||
|---|---|---|---|---|---|---|
| Source | Lipid Peroxidation | Triosine/Ketone Derived | Fructose 3-Phosphate Derived | Oxidative | Nonoxidative | Isomerization |
| Precursor | Glyoxal | Methylglyoxal | 3-deoxyglucosone | |||
| AGEs | Carboxymethyl- lysine | Carboxyethyl- lysine | Deoxyglucosone- lysine dimer | Carboxymethyl- lysine | Pyrraline | Argpyrimidine |
| Glyoxal-lysine dimer | Methylglyoxal- lysine dimer | Pentosidine |
Figure 1.
Depiction of endogenous and exogenous advanced glycation end product (AGE) sources and their relative contribution to the well-established signaling pathway activated by receptor for advanced glycation end products (RAGE)-ligand binding (left) along with a proposed link between AGE-RAGE and impaired insulin signaling (right).
Advanced Glycation End Products and Obesity
Whereas AGEs form endogenously at physiological levels, exogenous AGEs are derived from environmental factors such as dietary intake; particularly, highly processed foods characteristic of “Western” diets (23), air pollutants (24), and tobacco smoke (25) are major contributors to pathological accumulation, all of which are intricately associated with the development of obesity (26, 27). The incidence of obesity worldwide has paralleled the increased use of Western-style diets, which in addition to the well-established macronutrient differences (eg, higher fat content), also contain high levels of exogenous AGEs (23). Remarkably, increased dietary AGE intake results in significant elevation of circulating AGEs (28), including CEL, methylglyoxal (MGO), and CML (29), providing evidence that orally ingested AGEs are absorbed and circulated throughout the body, a process that is enhanced in foods with high fat content (30) and especially in those rich in saturated fatty acids (31). This likely results from lipoxidation providing excess substrates for AGE formation and induction of the myeloperoxidase inflammatory pathway (32), which contributes to the formation of AGEs (33), thereby creating a feed-forward–fueled pathological loop. The following section reviews evidence assessing the link between exogenous AGEs and obesity.
Exposing rodent diets to extreme heat or supplementing diets with AGE precursors (eg, MGO) have been commonly used techniques to increase dietary AGE content (32, 34–37). A caveat with this approach is that these manipulations alter macronutrients and micronutrients including fats, niacin, and vitamin A content, which may confound the interpretation of the results of these studies because the reported changes vary even when similar foods and processing protocols are employed (32, 35, 36). Hofmann et al demonstrated that high dietary AGE intake increases circulating AGEs, which is exacerbated in a genetic (db/db) mouse model of obesity likely because of the associated hyperglycemia and hyperlipidemia characteristic of this model, which provide extra substrates for AGE formation. The high-AGE diet promoted weight gain in the obesity-prone female db/db mouse but not in female wild-type mice and resulted in impaired glucose tolerance and insulin sensitivity, as well as impaired glucose uptake in fat, liver, (35) and muscle (36). Importantly, AGEs accumulate in key metabolic organs such as adipose tissue, a process that requires RAGE (18) and likely contributes to obesity and organ dysfunction.
Notably, the AGE-RAGE system is implicated in obesity as overexpression of RAGE in vitro via induced adipocyte hypertrophy (38) and compared to mice fed a high-fat, low-AGE diet, those fed a high-fat, high-AGE diet for 6 weeks exhibited greater weight gain and more visceral adiposity (32). Findings from our laboratory further support this notion by demonstrating that mice globally devoid of RAGE (39) with adipose tissue-specific RAGE deletion or wild-type mice surgically transplanted with RAGE-devoid adipocytes of brown or white subcutaneous adipose tissue depots are protected from diet-induced obesity by enhancing adipocyte lipid breakdown, increasing whole-body energy expenditure and promoting white adipose tissue browning, which collectively are processes linked to improved metabolic health (19). Importantly, systemic administration of sRAGE, the RAGE ligand decoy receptor, prevented high-fat diet–induced weight gain, providing further support for a role of circulating AGEs in promoting and/or exacerbating obesity.
Studies in humans report that obesity is generally associated with low levels of circulating AGEs, which appears paradoxical but may be explained by the “tissue AGE trapping” hypothesis. Sebeková et al provided some of the first evidence for this paradox when the authors reported circulating CML levels to be significantly lower in obese male and female children and adolescents when compared to lean controls (40). In apparent opposition, Uribarri and colleagues reported that obese adults (average body mass index [BMI] 33.2 kg/m2) exhibited significantly higher circulating AGEs (CML and MGO) compared to overweight participants (average BMI 26.3 kg/m2). However, when considering only individuals not at risk for developing metabolic syndrome, there was no association between the circulating AGE precursor MGO and percentage of body fat (41). Analysis from 1270 patients enrolled in the Cohort on Diabetes and Atherosclerosis Maastricht and Hoorn studies reported by Gaens et al revealed that circulating CML levels are inversely associated with BMI and central obesity, which was determined by waist circumference. Specifically, morbidly obese and severely obese patients had the lowest levels of serum CML compared to normal-weight controls (42). In addition to BMI and central obesity, de Courten et al found that neither circulating CML nor MGO were associated with percentage of body fat in overweight and obese individuals (43). This inverse association between fat mass and circulating CML had also been reported by Semba and colleagues, who raised the possibility that lower serum CML in obesity may be due to AGEs being preferentially deposited or “trapped” in adipose tissue (44). In fact, compared to lean individuals, obese patients exhibit significant accumulation of CML and concurrent overexpression of RAGE in subcutaneous and visceral fat depots, with greater accumulation in visceral fat. Adipose tissue CML and RAGE expression colocalized with macrophage markers, highlighting the role of AGE-RAGE in adipose tissue inflammation and likely, metabolic dysfunction (18).
Weight loss studies provide further support for the involvement of the AGE-RAGE system in obesity, demonstrating that circulating levels of sRAGE are increased after bariatric surgery, which is associated with improved homeostatic assessment of insulin resistance (HOMA-IR), fasting insulin, and glucose tolerance (45). Further, a report by Parikh et al suggested that high baseline serum sRAGE levels are a strong predictor for successful surgical weight loss 6 months postsurgery (46). Collectively, these studies suggest that AGEs, specifically exogenous AGEs derived from Western-like diets, may be important contributors to the pathogenesis of obesity by promoting adipocyte hypertrophy and to metabolic dysregulation by accumulating in metabolically relevant organs, such as adipose tissue, where they induce inflammation and overall organ dysfunction.
Advanced Glycation End Products and Insulin Resistance
Obesity is often accompanied by high levels of glucose and lipids, and it is therefore closely associated with and is considered a major risk factor for the development of IR and diabetes (47). AGE formation is bolstered in pathological states characterized by hyperlipidemia and/or hyperglycemia. As highlighted by the “common soil” hypothesis, among other factors, IR is a hallmark of obesity and diabetes. From the in vitro to the in vivo setting, studies have linked AGEs mechanistically to the pathogenesis of IR by altering insulin signaling cascades. For example, Riboulet-Chavey et al used rat-derived L6 skeletal myoblast cells to assess the effect of MGO on glucose uptake. The authors demonstrated that about 3% of labeled MGO is taken up by these cells within 30 minutes and is sufficient to dose-dependently impair insulin-stimulated glucose uptake. Although insulin receptor phosphorylation was not affected over that time course, activation of downstream proteins involved in insulin signaling, including insulin receptor substrate 1 and Akt (473), was inhibited by MGO treatment (48). A similar observation was made by Afridi and colleagues using Hep-G2 hepatocytes (49) and Wu et al in 3T3-L1–differentiated adipocytes (50), in which longer exposure also resulted in increased reactive oxygen species and inflammation (51). Fiory et al found that 4-hour MGO treatment of INS-1E pancreatic beta cells impaired glucose-stimulated insulin secretion (52), suggesting that AGEs affect not only cell sensitivity to insulin, but also the ability for insulin to be secreted. The caveat with these studies is that the majority relied on the use of α-oxoaldehyde AGE precursors such as glyoxal and MGO (48, 49, 52, 53), and it is unclear whether the relatively short-term exposure is sufficient to allow for significant AGE formation and to truly mimic the in vivo conditions. Furthermore, the specific pathophysiological mechanism(s) by which AGEs alter insulin signaling still remain to be fully elucidated. In vivo rodent studies using dietary manipulations to increase AGE content provide further support for the notion that AGEs impair insulin action as evidenced by impaired glucose tolerance, insulin sensitivity, and glucose uptake in fat, liver (35), and muscle (36) of animals exposed to exogenous dietary AGEs. Using daily injections as an alternative approach to increase AGEs, Pinto-Junior et al recently confirmed that AGEs alter insulin sensitivity, at least in part, by suppressing the expression of the glucose transporter 4 in muscle (54). Taken together, experimental models support the idea that AGEs can alter whole-body insulin sensitivity, thereby implicating these compounds in the pathogenesis of IR.
In human studies, elevated levels of circulating AGEs are associated with IR. In a cohort of 270 nonobese, nondiabetic male and female Chinese individuals, IR assessed via HOMA-IR was associated with high serum AGE levels (55). This finding was confirmed in a comparable report studying Japanese individuals (56), supporting the hypothesis that AGEs are key contributors to human IR per se, irrespective of obesity. However, a report by Sebeková et al from a study of 437 healthy people from Slovakia found that people with more risk factors for metabolic syndrome had lower levels of circulating CML (57). This finding is consistently observed in studies involving children and adolescents who are obese and/or at risk for metabolic disorders, raising the possibility that AGE-induced metabolic dysregulation may start from an early age. The authors found circulating AGEs (including CML) to be lower in obese children and adolescents than in lean controls despite the presence of IR, inflammation, and greater oxidative stress (40). Similarly, Accacha and colleagues analyzed a larger sample of preteens and, although no significant association between CML and BMI was reported, children with a higher percentage of body fat tended to have lower circulating CML levels (P = .07) and exhibited greater IR (58). One possible explanation for the observation that in some individuals (mostly obese) circulating AGEs are lower than in controls is the “tissue-trapping hypothesis” (18, 44). However, one must reconcile the fact that obese patients with established diabetes appear to exhibit the opposite pattern, namely, higher circulating AGE levels. One possibility is that lower levels of AGEs in strict nondiabetes-associated obesity may reflect the body’s defense mechanism to clear excess AGEs, which, on trapping and accumulation in tissues, instigate IR and overall metabolic dysfunction. Consequently, the hyperlipidemia and hyperglycemia characteristic of IR or diabetes results in excess substrate availability for further AGE formation, which in an already overloaded system translates ultimately to the inability of AGEs to further accumulate in tissues and thus leads to elevated levels in the circulation. Although this hypothesis is conceivable and plausible, it is a research area that should be addressed in future studies.
Because the apparent discrepancy in establishing the directionality of the relationship between circulating AGEs and metabolic impairment has been suggested to be attributed to the trapping of AGEs in tissues, assessment of the AGE detoxification mechanisms may provide an alternative approach to address this question. Some of the mechanisms by which AGEs are cleared include SRs (7) as well as AGER-1 receptor-mediated endocytosis (11), some antioxidants, and specifically the nitric oxide synthase inhibitor aminoguanidine, which has potent antiglycation properties that suppress AGE formation. sRAGE is protective against AGE-RAGE–implicated pathologies, including vascular dysfunction (59) and atherosclerosis (60) as well as obesity (39). Thus, it is purported to act as an endogenous decoy receptor that binds AGEs (and other RAGE ligands) to reduce AGE activity and signaling via the effector transmembrane RAGE. Serum sRAGE levels are negatively correlated with BMI in obese (61), severely obese (62), and recently diagnosed type 2 diabetes (T2D) patients (63), as well as in individuals with impaired glucose tolerance (61), high hemoglobin A1c, and HOMA-IR (62, 64). In patients the greater the number of risk factors, including fasting hyperglycemia, the greater the association with lower levels of circulating sRAGE (57, 65). Several enzyme systems have been identified with the ability to suppress the formation of AGEs, including aldehyde reductases and dehydrogenases, amadoriase, fructosamine 3-phosphokinase, and glyoxalase I (GLO1) (66). The glutathione-dependent GLO1 prevents glycation by using reduced glucothione to convert AGE precursors such as glyoxal and MGO into D-lactate (67). In diabetic rats, overexpression of GLO1 suppressed AGE levels and oxidative stress (68), whereas in obese humans with metabolic syndrome, GLO1 gene expression is significantly suppressed in peripheral mononuclear cells (41) and is inversely associated with levels of circulating AGEs (31). Of note, dietary manipulations to enhance GLO1 activity in humans is a promising therapeutic venue for vascular and glycemic regulation in obesity (69), and the commonly used antihyperglycemic drug metformin has been shown to also scavenge MGO, forming an imidazolinone metabolite that promotes endothelial function (70). In summary, obesity and IR are closely associated with impaired AGE detoxification, especially by sRAGE, which likely results from AGE and other RAGE ligand overload.
Advanced Glycation End Products and Diabetes
Moving along the spectrum of AGE biology within the common soil, the effect of AGEs on the development of IR may ultimately lead to the development of diabetes. In the context of T2D and metabolic syndrome, reports from multiple independent research groups from geographically and ethnically different populations further support the hypothesis that elevated circulating AGE levels are linked to IR and metabolic impairment (71–73). In the United Arab Emirates Healthy Futures Study, we observed that AGE levels were significantly associated with diabetes status, and levels of sRAGE and esRAGE were associated with BMI (74). Haddad et al studied 80 controls and 86 patients with metabolic syndrome (~86% with T2D) and found that patients exhibited a 4-fold increase in circulating pentosidine along with more than doubled levels of CML (72). Okura and colleagues, studying a smaller cohort of 20 nondiabetic and 12 T2D patients, reported higher serum levels of CEL and CML, which were negatively associated with HOMA-IR (73). Notably, it appears that circulating AGEs may serve as a predictor of future risk of developing diabetes. In a 7-year prospective study conducted in a cohort of 781 people, the authors observed that normoglycemic individuals had an average of 246 µU/mL of circulating AGEs, whereas those with more than 450 µU/mL had a 10-fold increase in the risk for developing diabetes (75). Similarly, a study by Miranda et al found that low levels of circulating sRAGE were associated with increased odds of developing T2D (61).
The association between AGE-RAGE and T2D is strengthened by the observation that some drugs commonly used to treat diabetes have direct effects on AGE levels regardless of controlled dietary changes, specifically, pioglitazone. Oz Gul et al reported that 12-week treatment of Turkish diabetic patients with pioglitazone but not rosiglitazone increased circulating sRAGE levels, which was associated with improved hemoglobin A1c and HOMA-IR (76). Similar observations were made by Koyama and colleagues, who compared pioglitazone to glimepiride in Japanese patients (77), and Mirmiranpour et al, who compared pioglitazone to metformin and a “no-medication” control group (71). Taken together, these findings suggest a potential role for high-circulating AGEs and impaired AGE detoxification on the pathophysiology of IR and diabetes, but do not allow for a causal relationship to be established.
Advanced Glycation End Products and Other Endocrine Disorders: Polycystic Ovary Syndrome
Interestingly, beyond metabolic syndrome and diabetes mellitus, distinct endocrine disorders may be associated with metabolic dysfunction and IR. Polycystic ovary syndrome (PCOS) is a disorder among women of reproductive age characterized by excess androgens, which is associated with increased susceptibility to obesity, IR, and/or diabetes (78, 79). A study by Diamanti-Kandarakis et al including 293 age- and BMI-matched women, 100 of whom were diagnosed with PCOS, revealed that PCOS is associated with significantly higher levels of circulating AGEs. Among these women, serum AGEs correlated positively with testosterone levels (80). In a separate article, the authors reported that treatment with the lipase inhibitor orlistat for 24 weeks significantly lowered circulating AGE levels (81). Because the authors selected for insulin-sensitive patients, how AGE levels compare in IR PCOS women and/or the pathophysiological role of AGEs in this disorder could not be assessed and, therefore, further research in this area is warranted.
Whether AGEs are causally linked to hyperandrogenism remains to be assessed. However, a study in nondiabetic Japanese men found that serum AGEs are negatively correlated with testosterone levels, thus highlighting potential sex-specific effects of AGEs on this hormone system. PCOS patients are reported to have decreased levels of sRAGE in follicular fluid (82), and in a study of 148 normal-weight and obese PCOS patients, Liao et al demonstrated significantly higher levels of circulating AGEs and lower sRAGE levels in obese individuals. Furthermore, serum sRAGE levels were negatively associated with HOMA-IR (83), thereby implicating the AGE-RAGE system in PCOS-related obesity and IR.
Advanced Glycation End Products and Diabetic Complications
Although beyond the scope of this mini-review, a plethora of studies have linked AGE accumulation to the development of diabetic complications. In diabetes, the key driver to AGE formation is likely to be the higher levels of endogenous glucose in affected individuals, because it is well established that patients with type 1 diabetes (84, 85) and T2D are characterized by increased serum and tissue AGE levels. Elevated AGEs have also been linked to the pathophysiology of diabetic retinopathy, neuropathy, and nephropathy, which has been comprehensively reviewed elsewhere (86–88). Excessive dietary AGE intake is sufficient to cause their accumulation in the heart (89), where they are key players in the pathophysiology of cardiomyopathy. AGEs and RAGE also accumulate in the heart in a rat model of ischemia/reperfusion (90), after myocardial infarction (91), and more so during diabetes (92), where they stimulate dendritic cell differentiation and promote cytokine production (93). In addition, AGE-induced cardiac calcium leaking promotes contractile dysfunction, thereby exacerbating diabetic cardiomyopathy (94), which is prevented by inhibiting AGE formation with aminoguanidine (95). AGEs also accumulate in bone with normal aging, negatively affecting bone mass density (96), a process that is exacerbated in people with T2D, leading to osteoporosis and increased fracture risk (97, 98). Pathological effects of AGEs in bone include impaired adhesion of osteoblastic cells (99) as well as impaired differentiation, proliferation, and induction of apoptosis in osteoblasts (100, 101). Taken together, these observations suggest that although exogenous AGEs may play a key role in the pathogenesis of these disorders, hyperglycemia and hyperlipidemia resulting from established pathology likely further fuel the AGE-RAGE system, resulting in severe complications.
Concluding Remarks
Although the process by which AGEs are formed was first described in the early 1910s, it was not until the 1990s that research on AGEs and pathology was sparked, which coincides with the first isolation and description of the AGE receptors. Since then, AGE-RAGE interactions have been implicated in multiple diseases, including obesity, IR, PCOS, and diabetes and its complications. There are apparently conflicting reports with regards to whether circulating AGEs are elevated in obesity, and if not, whether this can be explained by the tissue-trapping hypothesis still needs to be elucidated either by prospective human studies or by the use of appropriate animal models. Furthermore, these discrepancies may also be explained by the multiple distinct methods used to measure AGEs, ranging from skin fluorescence (a property of some AGEs), immunohistochemistry, enzyme-linked immunosorbent assays, and mass spectrometry, with the latter considered the most sensitive technique for AGE quantification and one that allows assessment of the relative contribution by the different AGE species to pathology. Undoubtedly, each of these detection methods spans a range of the type and quantity of AGEs that can be detected, and thus great care is warranted when attempting comparisons across studies in which distinct AGE measurement strategies were employed. Where there seems to be consensus, however, is that the AGE-RAGE system is tightly implicated in the pathophysiological mechanisms underlying the metabolic disorders discussed in this mini-review. Here, we propose that AGEs are important members of the common soil that affects vascular disease and metabolic diseases such as diabetes. The AGE-induced common factor between these diseases appears to be the induction of IR that consequently instigates a cascade of pathologies, including hyperlipidemia, hyperglycemia, inflammation, and oxidative stress, which may explain why some diabetic complications are present even before a formal diagnosis of diabetes is made (102).
Although AGEs form physiologically in our bodies and the formation is exacerbated in pathologies characterized by hyperlipidemia, hyperglycemia, and hyperinsulinemia, a major source of pathological levels of AGEs is likely environmental. Importantly, diabetes and cardiovascular disorders are aggravated by tobacco smoking, which is another source of exogenous AGEs (25). Highly processed foods are a major and more widely studied source of AGEs, and regulation of dietary AGE intake is beneficial in improving some disease parameters. Because compliance with dietary restriction has been and will likely continue to be a major obstacle in disease management, a comprehensive understanding of the role of AGEs in the pathophysiology of these disorders is paramount. To this end, significant efforts have been made using experimental models that are generally in agreement with the results of research in humans and highlight a potential direct effect of AGEs on the pathogenesis of obesity, IR, and diabetes. The authors hope that this mini-review ignites further interest in this important and promising line of research, which is an active area of investigation in our laboratories.
Literature search
In preparing this manuscript, the authors performed a comprehensive survey of the available literature using the key terms alone or in combination, including the following: advanced glycation end products, obesity, diabetes, insulin resistance, soluble RAGE, and receptors for advanced glycation end products. Literature searches were completed using common scientific databases for peer-reviewed journals. including PubMed and Scopus. Because this is a mini-review, the authors selected what was considered most relevant for the scope of this work.
Glossary
Abbreviations
- AGER-1
AGE receptor 1
- AGEs
advanced glycation end products
- CEL
carboxyethyl lysine
- CML
N ε-carboxymethyl lysine
- esRAGE
endogenous secretory receptor for advanced glycation end products
- Glut4
glucose transporter 4;
- HOMA-IR
homeostatic assessment of insulin resistance
- IR
insulin resistance
- MGO
methylglyoxal
- RAGE
receptor for advanced glycation end products
- sRAGE
soluble receptor for advanced glycation end products
- SRs
scavenger receptors
Acknowledgments
The authors thank Dr Lahortiga and Dr Cox for access to the Library of Science & Medical Illustrations.
Financial Support: This work was supported by grants from the National Institute of Health (5T32HL098129-10 to H.H.R.) and the American Heart Association (17SFRN33520045 to A.M.S. and H.H.R.).
Additional Information
Disclosure Summary: The authors do not have any disclosures to report.
Data Availability. Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Stern MP. Diabetes and cardiovascular disease. The “common soil” hypothesis. Diabetes. 1995;44(4):369–374. [DOI] [PubMed] [Google Scholar]
- 2. Gkogkolou P, Böhm M. Advanced glycation end products: key players in skin aging? Dermatoendocrinol. 2012;4(3):259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44(2):129–146. [DOI] [PubMed] [Google Scholar]
- 4. Henning C, Glomb MA. Pathways of the Maillard reaction under physiological conditions. Glycoconj J. 2016;33(4):499–512. [DOI] [PubMed] [Google Scholar]
- 5. Ahmed MU, Thorpe SR, Baynes JW. Identification of N epsilon-carboxymethyllysine as a degradation product of fructoselysine in glycated protein. J Biol Chem. 1986;261(11):4889–4894. [PubMed] [Google Scholar]
- 6. Reddy S, Bichler J, Wells-Knecht KJ, Thorpe SR, Baynes JW. N epsilon-(carboxymethyl)lysine is a dominant advanced glycation end product (AGE) antigen in tissue proteins. Biochemistry. 1995;34(34):10872–10878. [DOI] [PubMed] [Google Scholar]
- 7. Smedsrød B, Melkko J, Araki N, Sano H, Horiuchi S. Advanced glycation end products are eliminated by scavenger-receptor-mediated endocytosis in hepatic sinusoidal Kupffer and endothelial cells. Biochem J. 1997;322 (Pt 2):567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Horiuchi S, Higashi T, Ikeda K, et al. Advanced glycation end products and their recognition by macrophage and macrophage-derived cells. Diabetes. 1996;45(Suppl 3):S73–S76. [DOI] [PubMed] [Google Scholar]
- 9. Pricci F, Leto G, Amadio L, et al. Role of galectin-3 as a receptor for advanced glycosylation end products. Kidney Int Suppl. 2000;77:S31–S39. [DOI] [PubMed] [Google Scholar]
- 10. Li YM, Mitsuhashi T, Wojciechowicz D, et al. Molecular identity and cellular distribution of advanced glycation endproduct receptors: relationship of p60 to OST-48 and p90 to 80K-H membrane proteins. Proc Natl Acad Sci U S A. 1996;93(20):11047–11052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cai W, He JC, Zhu L, Lu C, Vlassara H. Advanced glycation end product (AGE) receptor 1 suppresses cell oxidant stress and activation signaling via EGF receptor. Proc Natl Acad Sci U S A. 2006;103(37):13801–13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen X, Zhang T, Du G. Advanced glycation end products serve as ligands for lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1): biochemical and binding characterizations assay. Cell Biochem Funct. 2008;26(7):760–770. [DOI] [PubMed] [Google Scholar]
- 13. Schmidt AM, Vianna M, Gerlach M, et al. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J Biol Chem. 1992;267(21):14987–14997. [PubMed] [Google Scholar]
- 14. Yonekura H, Yamamoto Y, Sakurai S, et al. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem J. 2003;370(Pt 3):1097–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park IH, Yeon SI, Youn JH, et al. Expression of a novel secreted splice variant of the receptor for advanced glycation end products (RAGE) in human brain astrocytes and peripheral blood mononuclear cells. Mol Immunol. 2004;40(16):1203–1211. [DOI] [PubMed] [Google Scholar]
- 16. Brett J, Schmidt AM, Yan SD, et al. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am J Pathol. 1993;143(6):1699–1712. [PMC free article] [PubMed] [Google Scholar]
- 17. Tanji N, Markowitz GS, Fu C, et al. Expression of advanced glycation end products and their cellular receptor RAGE in diabetic nephropathy and nondiabetic renal disease. J Am Soc Nephrol. 2000;11(9):1656–1666. [DOI] [PubMed] [Google Scholar]
- 18. Gaens KH, Goossens GH, Niessen PM, et al. Nε-(carboxymethyl)lysine-receptor for advanced glycation end product axis is a key modulator of obesity-induced dysregulation of adipokine expression and insulin resistance. Arterioscler Thromb Vasc Biol. 2014;34(6):1199–1208. [DOI] [PubMed] [Google Scholar]
- 19. Hurtado Del Pozo C, Ruiz HH, Arivazhagan L, et al. A receptor of the immunoglobulin superfamily regulates adaptive thermogenesis. Cell Rep. 2019;28(3):773–791.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108(7):949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab. 2001;280(5):E685–E694. [DOI] [PubMed] [Google Scholar]
- 22. Coughlan MT, Thorburn DR, Penfold SA, et al. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J Am Soc Nephrol. 2009;20(4):742–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Uribarri J, Woodruff S, Goodman S, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2010;110(6):911–916.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gursinsky T, Ruhs S, Friess U, et al. Air pollution-associated fly ash particles induce fibrotic mechanisms in primary fibroblasts. Biol Chem. 2006;387(10–11):1411–1420. [DOI] [PubMed] [Google Scholar]
- 25. Cerami C, Founds H, Nicholl I, et al. Tobacco smoke is a source of toxic reactive glycation products. Proc Natl Acad Sci U S A. 1997;94(25):13915–13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. An R, Ji M, Yan H, Guan C. Impact of ambient air pollution on obesity: a systematic review. Int J Obes (Lond). 2018;42(6):1112–1126. [DOI] [PubMed] [Google Scholar]
- 27. Dare S, Mackay DF, Pell JP. Relationship between smoking and obesity: a cross-sectional study of 499,504 middle-aged adults in the UK general population. PLoS One. 2015;10(4): e0123579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cai W, He JC, Zhu L, et al. Oral glycotoxins determine the effects of calorie restriction on oxidant stress, age-related diseases, and lifespan. Am J Pathol. 2008;173(2):327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scheijen JLJM, Hanssen NMJ, van Greevenbroek MM, et al. Dietary intake of advanced glycation endproducts is associated with higher levels of advanced glycation endproducts in plasma and urine: the CODAM study. Clin Nutr. 2018;37(3): 919–925. [DOI] [PubMed] [Google Scholar]
- 30. Davis KE, Prasad C, Vijayagopal P, Juma S, Adams-Huet B, Imrhan V. Contribution of dietary advanced glycation end products (AGE) to circulating AGE: role of dietary fat. Br J Nutr. 2015;114(11):1797–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lopez-Moreno J, Quintana-Navarro GM, Camargo A, et al. Dietary fat quantity and quality modifies advanced glycation end products metabolism in patients with metabolic syndrome. Mol Nutr Food Res. 2017;61(8). [DOI] [PubMed] [Google Scholar]
- 32. Sayej WN, Knight Iii PR, Guo WA, et al. Advanced glycation end products induce obesity and hepatosteatosis in CD-1 wild-type mice. Biomed Res Int. 2016;2016:7867852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Anderson MM, Requena JR, Crowley JR, Thorpe SR, Heinecke JW. The myeloperoxidase system of human phagocytes generates Nepsilon-(carboxymethyl)lysine on proteins: a mechanism for producing advanced glycation end products at sites of inflammation. J Clin Invest. 1999;104(1):103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sandu O, Song K, Cai W, Zheng F, Uribarri J, Vlassara H. Insulin resistance and type 2 diabetes in high-fat-fed mice are linked to high glycotoxin intake. Diabetes. 2005;54(8):2314–2319. [DOI] [PubMed] [Google Scholar]
- 35. Hofmann SM, Dong HJ, Li Z, et al. Improved insulin sensitivity is associated with restricted intake of dietary glycoxidation products in the db/db mouse. Diabetes. 2002;51(7):2082–2089. [DOI] [PubMed] [Google Scholar]
- 36. Cassese A, Esposito I, Fiory F, et al. In skeletal muscle advanced glycation end products (AGEs) inhibit insulin action and induce the formation of multimolecular complexes including the receptor for AGEs. J Biol Chem. 2008;283(52):36088–36099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cai W, Ramdas M, Zhu L, Chen X, Striker GE, Vlassara H. Oral advanced glycation endproducts (AGEs) promote insulin resistance and diabetes by depleting the antioxidant defenses AGE receptor-1 and sirtuin 1. Proc Natl Acad Sci U S A. 2012;109(39):15888–15893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Monden M, Koyama H, Otsuka Y, et al. Receptor for advanced glycation end products regulates adipocyte hypertrophy and insulin sensitivity in mice: involvement of Toll-like receptor 2. Diabetes. 2013;62(2):478–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Song F, Hurtado del Pozo C, Rosario R, et al. RAGE regulates the metabolic and inflammatory response to high-fat feeding in mice. Diabetes. 2014;63(6):1948–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sebeková K, Somoza V, Jarcusková M, Heidland A, Podracká L. Plasma advanced glycation end products are decreased in obese children compared with lean controls. Int J Pediatr Obes. 2009;4(2):112–118. [DOI] [PubMed] [Google Scholar]
- 41. Uribarri J, Cai W, Woodward M, et al. Elevated serum advanced glycation endproducts in obese indicate risk for the metabolic syndrome: a link between healthy and unhealthy obesity? J Clin Endocrinol Metab. 2015;100(5):1957–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gaens KH, Ferreira I, van de Waarenburg MP, et al. Protein-bound plasma Nε-(carboxymethyl) lysine is inversely associated with central obesity and inflammation and significantly explain a part of the central obesity-related increase in inflammation: the Hoorn and CODAM studies. Arterioscler Thromb Vasc Biol. 2015;35(12):2707–2713. [DOI] [PubMed] [Google Scholar]
- 43. de Courten B, de Courten MP, Soldatos G, et al. Diet low in advanced glycation end products increases insulin sensitivity in healthy overweight individuals: a double-blind, randomized, crossover trial. Am J Clin Nutr. 2016;103(6):1426–1433. [DOI] [PubMed] [Google Scholar]
- 44. Semba RD, Arab L, Sun K, Nicklett EJ, Ferrucci L. Fat mass is inversely associated with serum carboxymethyl-lysine, an advanced glycation end product, in adults. J Nutr. 2011;141(9):1726–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brix JM, Höllerl F, Kopp HP, Schernthaner GH, Schernthaner G. The soluble form of the receptor of advanced glycation endproducts increases after bariatric surgery in morbid obesity. Int J Obes (Lond). 2012;36(11):1412–1417. [DOI] [PubMed] [Google Scholar]
- 46. Parikh M, Chung M, Sheth S, et al. Randomized pilot trial of bariatric surgery versus intensive medical weight management on diabetes remission in type 2 diabetic patients who do NOT meet NIH criteria for surgery and the role of soluble RAGE as a novel biomarker of success. Ann Surg. 2014;260(4):617–622; discussion 622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ye J. Mechanisms of insulin resistance in obesity. Front Med. 2013;7(1):14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Riboulet-Chavey A, Pierron A, Durand I, Murdaca J, Giudicelli J, Van Obberghen E. Methylglyoxal impairs the insulin signaling pathways independently of the formation of intracellular reactive oxygen species. Diabetes. 2006;55(5):1289–1299. [DOI] [PubMed] [Google Scholar]
- 49. Afridi SK, Aftab MF, Murtaza M, et al. A new glycotoxins inhibitor attenuates insulin resistance in liver and fat cells. Biochem Biophys Res Commun. 2016;476(4):188–195. [DOI] [PubMed] [Google Scholar]
- 50. Wu CH, Huang HW, Huang SM, Lin JA, Yeh CT, Yen GC. AGE-induced interference of glucose uptake and transport as a possible cause of insulin resistance in adipocytes. J Agric Food Chem. 2011;59(14):7978–7984. [DOI] [PubMed] [Google Scholar]
- 51. Unoki H, Bujo H, Yamagishi S, Takeuchi M, Imaizumi T, Saito Y. Advanced glycation end products attenuate cellular insulin sensitivity by increasing the generation of intracellular reactive oxygen species in adipocytes. Diabetes Res Clin Pract. 2007;76(2):236–244. [DOI] [PubMed] [Google Scholar]
- 52. Fiory F, Lombardi A, Miele C, Giudicelli J, Beguinot F, Van Obberghen E. Methylglyoxal impairs insulin signalling and insulin action on glucose-induced insulin secretion in the pancreatic beta cell line INS-1E. Diabetologia. 2011;54(11):2941–2952. [DOI] [PubMed] [Google Scholar]
- 53. Passarelli M, Tang C, McDonald TO, et al. Advanced glycation end product precursors impair ABCA1-dependent cholesterol removal from cells. Diabetes. 2005;54(7):2198–2205. [DOI] [PubMed] [Google Scholar]
- 54. Pinto-Junior DC, Silva KS, Michalani ML, et al. Advanced glycation end products-induced insulin resistance involves repression of skeletal muscle GLUT4 expression. Sci Rep. 2018;8(1):8109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tan KC, Shiu SW, Wong Y, Tam X. Serum advanced glycation end products (AGEs) are associated with insulin resistance. Diabetes Metab Res Rev. 2011;27(5):488–492. [DOI] [PubMed] [Google Scholar]
- 56. Tahara N, Yamagishi S, Matsui T, et al. Serum levels of advanced glycation end products (AGEs) are independent correlates of insulin resistance in nondiabetic subjects. Cardiovasc Ther. 2012;30(1):42–48. [DOI] [PubMed] [Google Scholar]
- 57. Sebeková K, Krivošíková Z, Gajdoš M. Total plasma Nε-(carboxymethyl)lysine and sRAGE levels are inversely associated with a number of metabolic syndrome risk factors in non-diabetic young-to-middle-aged medication-free subjects. Clin Chem Lab Med. 2014;52(1):139–149. [DOI] [PubMed] [Google Scholar]
- 58. Accacha S, Rosenfeld W, Jacobson A, et al. Plasma advanced glycation end products (AGEs), receptors for AGEs and their correlation with inflammatory markers in middle school-age children. Horm Res Paediatr. 2013;80(5):318–327. [DOI] [PubMed] [Google Scholar]
- 59. Shoji T, Koyama H, Morioka T, et al. Receptor for advanced glycation end products is involved in impaired angiogenic response in diabetes. Diabetes. 2006;55(8):2245–2255. [DOI] [PubMed] [Google Scholar]
- 60. Park L, Raman KG, Lee KJ, et al. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med. 1998;4(9):1025–1031. [DOI] [PubMed] [Google Scholar]
- 61. Miranda ER, Somal VS, Mey JT, et al. Circulating soluble RAGE isoforms are attenuated in obese, impaired–glucose-tolerant individuals and are associated with the development of type 2 diabetes. Am J Physiol Endocrinol Metab. 2017;313(6):E631–E640.28811295 [Google Scholar]
- 62. Hagen I, Schulte DM, Müller N, et al. Soluble receptor for advanced glycation end products as a potential biomarker to predict weight loss and improvement of insulin sensitivity by a very low calorie diet of obese human subjects. Cytokine. 2015;73(2):265–269. [DOI] [PubMed] [Google Scholar]
- 63. Su XD, Li SS, Tian YQ, Zhang ZY, Zhang GZ, Wang LX. Elevated serum levels of advanced glycation end products and their monocyte receptors in patients with type 2 diabetes. Arch Med Res. 2011;42(7):596–601. [DOI] [PubMed] [Google Scholar]
- 64. Basta G, Sironi AM, Lazzerini G, et al. Circulating soluble receptor for advanced glycation end products is inversely associated with glycemic control and S100A12 protein. J Clin Endocrinol Metab. 2006;91(11):4628–4634. [DOI] [PubMed] [Google Scholar]
- 65. Hudson BI, Dong C, Gardener H, et al. Serum levels of soluble receptor for advanced glycation end-products and metabolic syndrome: the Northern Manhattan Study. Metabolism. 2014;63(9):1125–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Thornalley PJ. The enzymatic defence against glycation in health, disease and therapeutics: a symposium to examine the concept. Biochem Soc Trans. 2003;31(Pt 6):1341–1342. [DOI] [PubMed] [Google Scholar]
- 67. Xue M, Rabbani N, Thornalley PJ. Glyoxalase in ageing. Semin Cell Dev Biol. 2011;22(3):293–301. [DOI] [PubMed] [Google Scholar]
- 68. Brouwers O, Niessen PM, Ferreira I, et al. Overexpression of glyoxalase-I reduces hyperglycemia-induced levels of advanced glycation end products and oxidative stress in diabetic rats. J Biol Chem. 2011;286(2):1374–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Xue M, Weickert MO, Qureshi S, et al. Improved glycemic control and vascular function in overweight and obese subjects by glyoxalase 1 inducer formulation. Diabetes. 2016;65(8):2282–2294. [DOI] [PubMed] [Google Scholar]
- 70. Kinsky OR, Hargraves TL, Anumol T, et al. Metformin scavenges methylglyoxal to form a novel imidazolinone metabolite in humans. Chem Res Toxicol. 2016;29(2):227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mirmiranpour H, Mousavizadeh M, Noshad S, et al. Comparative effects of pioglitazone and metformin on oxidative stress markers in newly diagnosed type 2 diabetes patients: a randomized clinical trial. J Diabetes Complications. 2013;27(5): 501–507. [DOI] [PubMed] [Google Scholar]
- 72. Haddad M, Knani I, Bouzidi H, Berriche O, Hammami M, Kerkeni M. Plasma levels of pentosidine, carboxymethyl-lysine, soluble receptor for advanced glycation end products, and metabolic syndrome: the metformin effect. Dis Markers. 2016;2016:6248264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Okura T, Ueta E, Nakamura R, et al. High serum advanced glycation end products are associated with decreased insulin secretion in patients with type 2 diabetes: a brief report. J Diabetes Res. 2017;2017:5139750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Inman CK, Aljunaibi A, Koh H, et al. The AGE-RAGE axis in an Arab population: the United Arab Emirates Healthy Futures (UAEHFS) pilot study. J Clin Transl Endocrinol. 2017;10:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jiménez IU, Díaz-Díaz E, Castro JS, et al. Circulating concentrations of advanced glycation end products, its association with the development of diabetes mellitus. Arch Med Res. 2017;48(4):360–369. [DOI] [PubMed] [Google Scholar]
- 76. Oz Gul O, Tuncel E, Yilmaz Y, et al. Comparative effects of pioglitazone and rosiglitazone on plasma levels of soluble receptor for advanced glycation end products in type 2 diabetes mellitus patients. Metabolism. 2010;59(1):64–69. [DOI] [PubMed] [Google Scholar]
- 77. Koyama H, Tanaka S, Monden M, et al. Comparison of effects of pioglitazone and glimepiride on plasma soluble RAGE and RAGE expression in peripheral mononuclear cells in type 2 diabetes: randomized controlled trial (PioRAGE). Atherosclerosis. 2014;234(2):329–334. [DOI] [PubMed] [Google Scholar]
- 78. Moghetti P. Insulin resistance and polycystic ovary syndrome. Curr Pharm Des. 2016;22(36):5526–5534. [DOI] [PubMed] [Google Scholar]
- 79. Polak K, Czyzyk A, Simoncini T, Meczekalski B. New markers of insulin resistance in polycystic ovary syndrome. J Endocrinol Invest. 2017;40(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Diamanti-Kandarakis E, Katsikis I, Piperi C, et al. Increased serum advanced glycation end-products is a distinct finding in lean women with polycystic ovary syndrome (PCOS). Clin Endocrinol (Oxf). 2008;69(4):634–641. [DOI] [PubMed] [Google Scholar]
- 81. Diamanti-Kandarakis E, Katsikis I, Piperi C, Alexandraki K, Panidis D. Effect of long-term orlistat treatment on serum levels of advanced glycation end-products in women with polycystic ovary syndrome. Clin Endocrinol (Oxf). 2007;66(1): 103–109. [DOI] [PubMed] [Google Scholar]
- 82. Wang B, Li J, Yang Q, Zhang F, Hao M, Guo Y. Decreased levels of sRAGE in follicular fluid from patients with PCOS. Reproduction. 2017;153(3):285–292. [DOI] [PubMed] [Google Scholar]
- 83. Liao Y, Huang R, Sun Y, et al. An inverse association between serum soluble receptor of advanced glycation end products and hyperandrogenism and potential implication in polycystic ovary syndrome patients. Reprod Biol Endocrinol. 2017;15(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Barat P, Cammas B, Lacoste A, et al. Advanced glycation end products in children with type 1 diabetes: family matters? Diabetes Care. 2012;35(1):e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Brunvand L, Heier M, Brunborg C, et al. Advanced glycation end products in children with type 1 diabetes and early reduced diastolic heart function. BMC Cardiovasc Disord. 2017;17(1):133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yan SF, Ramasamy R, Schmidt AM. Mechanisms of disease: advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat Clin Pract Endocrinol Metab. 2008;4(5):285–293. [DOI] [PubMed] [Google Scholar]
- 87. Goh SY, Cooper ME. Clinical review: the role of advanced glycation end products in progression and complications of diabetes. J Clin Endocrinol Metab. 2008;93(4):1143–1152. [DOI] [PubMed] [Google Scholar]
- 88. Singh VP, Bali A, Singh N, Jaggi AS. Advanced glycation end products and diabetic complications. Korean J Physiol Pharmacol. 2014;18(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Roncero-Ramos I, Niquet-Léridon C, Strauch C, et al. An advanced glycation end product (AGE)-rich diet promotes Nε-carboxymethyl-lysine accumulation in the cardiac tissue and tendons of rats. J Agric Food Chem. 2014;62(25):6001–6006. [DOI] [PubMed] [Google Scholar]
- 90. Bucciarelli LG, Kaneko M, Ananthakrishnan R, et al. Receptor for advanced-glycation end products: key modulator of myocardial ischemic injury. Circulation. 2006;113(9):1226–1234. [DOI] [PubMed] [Google Scholar]
- 91. Blackburn NJR, Vulesevic B, McNeill B, et al. Methylglyoxal-derived advanced glycation end products contribute to negative cardiac remodeling and dysfunction post-myocardial infarction. Basic Res Cardiol. 2017;112(5):57. [DOI] [PubMed] [Google Scholar]
- 92. Nożyński J, Zakliczyński M, Konecka-Mrowka D, et al. Advanced glycation end product accumulation in the cardiomyocytes of heart failure patients with and without diabetes. Ann Transplant. 2012;17(2):53–61. [DOI] [PubMed] [Google Scholar]
- 93. Cao W, Chen J, Chen Y, et al. Advanced glycation end products induced immune maturation of dendritic cells controls heart failure through NF-κB signaling pathway. Arch Biochem Biophys. 2015;580:112–120. [DOI] [PubMed] [Google Scholar]
- 94. Yan D, Luo X, Li Y, et al. Effects of advanced glycation end products on calcium handling in cardiomyocytes. Cardiology. 2014;129(2):75–83. [DOI] [PubMed] [Google Scholar]
- 95. Pei Z, Deng Q, Babcock SA, He EY, Ren J, Zhang Y. Inhibition of advanced glycation endproduct (AGE) rescues against streptozotocin-induced diabetic cardiomyopathy: role of autophagy and ER stress. Toxicol Lett. 2018;284:10–20. [DOI] [PubMed] [Google Scholar]
- 96. Odetti P, Rossi S, Monacelli F, et al. Advanced glycation end products and bone loss during aging. Ann N Y Acad Sci. 2005;1043:710–717. [DOI] [PubMed] [Google Scholar]
- 97. Furst JR, Bandeira LC, Fan WW, et al. Advanced glycation endproducts and bone material strength in type 2 diabetes. J Clin Endocrinol Metab. 2016;101(6):2502–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Momma H, Niu K, Kobayashi Y, et al. Skin advanced glycation end-product accumulation is negatively associated with calcaneal osteo-sono assessment index among non-diabetic adult Japanese men. Osteoporos Int. 2012;23(6):1673–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. McCarthy AD, Uemura T, Etcheverry SB, Cortizo AM. Advanced glycation endproducts interefere with integrin-mediated osteoblastic attachment to a type-I collagen matrix. Int J Biochem Cell Biol. 2004;36(5):840–848. [DOI] [PubMed] [Google Scholar]
- 100. Cheng YZ, Yang SL, Wang JY, et al. Irbesartan attenuates advanced glycation end products-mediated damage in diabetes-associated osteoporosis through the AGEs/RAGE pathway. Life Sci. 2018;205:184–192. [DOI] [PubMed] [Google Scholar]
- 101. Chen H, Liu W, Wu X, Gou M, Shen J, Wang H. Advanced glycation end products induced IL-6 and VEGF-A production and apoptosis in osteocyte-like MLO-Y4 cells by activating RAGE and ERK1/2, P38 and STAT3 signalling pathways. Int Immunopharmacol. 2017;52:143–149. [DOI] [PubMed] [Google Scholar]
- 102. Spijkerman AM, Dekker JM, Nijpels G, et al. Microvascular complications at time of diagnosis of type 2 diabetes are similar among diabetic patients detected by targeted screening and patients newly diagnosed in general practice: the Hoorn Screening Study. Diabetes Care. 2003;26(9):2604–2608. [DOI] [PubMed] [Google Scholar]