Abstract
Background
The severity of the 2017–2018 influenza season in the United States was high, with influenza A(H3N2) viruses predominating. Here, we report influenza vaccine effectiveness (VE) and estimate the number of vaccine-prevented influenza-associated illnesses, medical visits, hospitalizations, and deaths for the 2017–2018 influenza season.
Methods
We used national age-specific estimates of 2017–2018 influenza vaccine coverage and disease burden. We estimated VE against medically attended reverse-transcription polymerase chain reaction–confirmed influenza virus infection in the ambulatory setting using a test-negative design. We used a compartmental model to estimate numbers of influenza-associated outcomes prevented by vaccination.
Results
The VE against outpatient, medically attended, laboratory-confirmed influenza was 38% (95% confidence interval [CI], 31%–43%), including 22% (95% CI, 12%–31%) against influenza A(H3N2), 62% (95% CI, 50%–71%) against influenza A(H1N1)pdm09, and 50% (95% CI, 41%–57%) against influenza B. We estimated that influenza vaccination prevented 7.1 million (95% CrI, 5.4 million–9.3 million) illnesses, 3.7 million (95% CrI, 2.8 million–4.9 million) medical visits, 109 000 (95% CrI, 39 000–231 000) hospitalizations, and 8000 (95% credible interval [CrI], 1100–21 000) deaths. Vaccination prevented 10% of expected hospitalizations overall and 41% among young children (6 months–4 years).
Conclusions
Despite 38% VE, influenza vaccination reduced a substantial burden of influenza-associated illness, medical visits, hospitalizations, and deaths in the United States during the 2017–2018 season. Our results demonstrate the benefit of current influenza vaccination and the need for improved vaccines.
Keywords: influenza, vaccination, prevented illnesses, burden
During the 2017–2018 influenza season, we estimate that influenza vaccination reduced the risk of medically attended influenza by 38% and prevented 7 million illnesses, 4 million medical visits, 109 000 hospitalizations, and 8000 deaths in the United States.
(See the Editorial Commentary by Neuzil and Fitzpatrick on pages 1854–5.)
The 2017–2018 influenza season in the United States was a high severity season [1, 2]. Circulation of influenza viruses was widespread for an extended period throughout the country. Influenza A(H3N2) viruses predominated, but influenza A(H1N1)pdm09 and B viruses also circulated [2]. The Centers for Disease Control and Prevention (CDC) has estimated that there were 48.8 million influenza illnesses, 959 000 hospitalizations, and 79 400 influenza-associated deaths during 2017–2018, the highest morbidity and mortality since the 2009 pandemic [3].
Influenza vaccination is the primary strategy to prevent influenza illness and its complications. Recent reports estimate that 42% of the US population was vaccinated against influenza during the 2017–2018 season [4, 5]; the mid-season estimates of the effectiveness of influenza vaccine were 36% against all influenza A and B virus infections and 25% against A(H3N2) virus infections [6]. Here, we report end-of-season vaccine effectiveness (VE) and apply it with vaccine coverage to estimate the number of influenza-associated illnesses, medical visits, hospitalizations, and deaths prevented by influenza vaccination.
METHODS
Influenza Vaccine Composition
The recommended composition of the 2017–2018 Northern Hemisphere trivalent influenza vaccine included an A/Michigan/45/2015 (H1N1)–like virus, an A/Hong Kong/4801/2014 (H3N2)–like virus, and a B/Brisbane/60/2008–like virus (Victoria lineage). In addition, quadrivalent vaccines included a B/Phuket/3073/2013–like virus (Yamagata lineage) [7].
Influenza Vaccine Effectiveness
Effectiveness of 2017–2018 influenza vaccination for the prevention of outpatient medically attended influenza illness was determined through the US Influenza Vaccine Effectiveness (Flu VE) Network, which has been described in detail previously [8–11]. Briefly, study staff recruited, consented, and enrolled patients aged ≥6 months who sought outpatient care for acute respiratory illness (including cough) within 7 days of symptom onset at 52 participating healthcare facilities in 5 research sites in Michigan, Pennsylvania, Texas, Washington, and Wisconsin. Patients who received an antiviral medication in the 7 days before enrollment or who were enrolled in the prior 14 days were not eligible. Study staff collected a combined nasal and throat swab from patients aged ≥2 years or a nasal swab only from children aged <2 years. Reverse-transcription polymerase chain reaction (RT-PCR) was used to detect influenza viruses, including subtype and lineage. All diagnostic laboratories used primers and probes from the CDC and passed proficiency testing. Staff interviewed patients for demographic data, current health status, symptoms, and reported receipt of 2017–2018 influenza vaccine. We looked for International Classification of Diseases codes assigned to medical encounters in the year prior to enrollment to determine whether participants had a preexisting health condition associated with increased risk of severe influenza [12, 13].
For all US Flu VE Network sites, a participant’s vaccination status was based on documented receipt of 2017–2018 influenza vaccine in electronic immunization records (medical records, state immunization systems, and employee health records). In addition, at 4 sites (excluding Wisconsin), we considered adults aged ≥18 years vaccinated if they reported timing and place of vaccination without documented receipt. We excluded children (aged 6 month–8 years) who were partially vaccinated. We used a test-negative design to estimate VE, contrasting the odds of influenza vaccination among participants with RT-PCR–positive influenza (cases) to the odds of vaccination among participants who were negative for influenza (controls) using a logistic regression model [14]. We estimated VE and 95% confidence intervals (CIs) against any influenza and by influenza virus type or subtype in separate models and stratified models by participant age (6 months–4 years, 5–17 years, 18–49 years, 50–64 years, and ≥65 years). We adjusted all logistic regression models, a priori, for network site, calendar time (in bi-week increments), participant age, and high-risk status.
The Flu VE Network study was approved by institutional review boards at each participating site and the CDC.
Estimates of Influenza-associated Outcomes
The methods for estimating age-specific influenza burden have been detailed elsewhere, and estimates from the 2017–2018 season are available from the CDC [3, 15]. This method uses mathematical multipliers to calculate illnesses, medical visits, and deaths from data on hospitalized cases reported through the Influenza Hospitalization Surveillance Network (FluSurv-NET), as illustrated in Supplementary Figure 1. For this analysis, we restricted burden estimates to those aged ≥6 months. We further estimated the burden by influenza virus type and subtype using virologic distributions observed in the US Flu VE Network patients for illnesses and medical visits and the distributions observed in FluSurv-NET to estimate hospitalizations and deaths for each (sub)type [16]. As data on influenza A subtype were missing for 60% of FluSurv-NET patients with influenza A virus infection, we used multiple imputation (70 imputations) to estimate the rate of hospitalization for each subtype, including patient age, surveillance site, and admission time period (October–December, January, February, or March–May) in the imputation model.
Influenza Vaccine Coverage
We obtained annual estimates of influenza vaccination coverage in the United States by month, from August 2017 through April 2018, which were reported by the CDC (Supplementary Figure 2) [4, 5].
Influenza-associated Outcomes Prevented by Vaccination
We estimated the effect of seasonal influenza vaccination on disease burden using a mathematical compartmental model, stratified by age group [17]. We began the model with all members of the US population unvaccinated and susceptible to influenza. Each month the susceptible population was divided, based on observed data, into those who became infected (using data on estimated illness), those who were vaccinated and protected against influenza (using data on vaccine coverage and effectiveness), and those who remained susceptible to infection. Each month we estimated age-specific rates of illness (and medical visits, hospitalizations, and deaths) by dividing the monthly illnesses by the prior month’s susceptible population. Using these rates among susceptible persons, we estimated the number of outcomes that would have occurred in the same population without influenza vaccination. We calculated the prevented outcomes as the difference between outcomes in the absence of vaccination and those estimated under current levels of vaccination [15, 18, 19].
Estimates of VE in adult outpatients and inpatients during 2017–2018 were similar in the United States, thus we assumed that VE estimates from the US Flu VE Network applied to all influenza outcomes and were also constant across the season [20]. We applied (sub)type-specific VE estimates to the (sub)type-specific models.
We estimated the number needed to vaccinate (NNV) to prevent 1 influenza-associated hospitalization by dividing the number of vaccinated individuals by hospitalizations prevented by vaccination. When VE 95% CIs included the null, the undefined value of NNV was indicated as >999 999. Our estimates of NNV were stratified by age group.
We used a Monte Carlo algorithm to estimate a 95% credible interval (CrI) around the estimates of prevented outcomes, incorporating uncertainty in each data input. Briefly, we chose a value at random from the assumed distribution for each of the model inputs (Supplementary Table 1) and calculated the estimated prevented outcome and repeated the process 5000 times. Distributions for VE and vaccine coverage were truncated at 0.
Sensitivity Analysis for Vaccine Coverage
Missing responses to the influenza vaccination question were more common in the telephone survey in 2017–2018 compared with 2016–2017. We conducted sensitivity analyses to assess the effect of differences in vaccine coverage on estimates of prevented hospitalizations [4]. We explored the following scenarios for age group–specific coverage: as observed in 2016–2017; 2017–2018 coverage assuming individuals with missing responses were vaccinated; 2017–2018 coverage assuming individuals with missing responses were unvaccinated; and reducing coverage by 3%–17% to account for overestimation by self-report [21–25].
RESULTS
Among the population eligible for influenza vaccination and aged ≥6 months, we estimated there were 47.9 million illnesses, 22.1 million medical visits, 953 000 hospitalizations, and 79 400 deaths associated with influenza in 2017–2018. Adults aged ≥65 years accounted for 15% of illnesses but 70% and 90% of all hospitalizations and deaths, respectively.
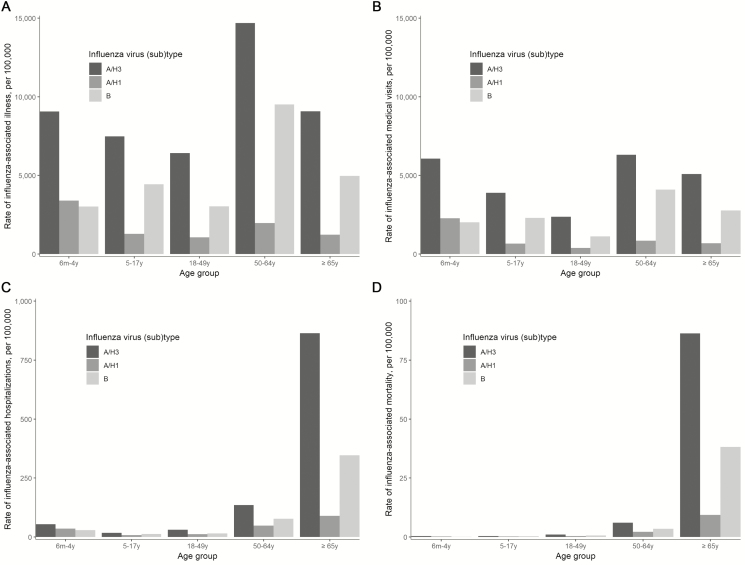
Influenza A(H3N2) was associated with the highest rates of illness, affecting 9% of children aged 6 months–4 years and 15% of adults aged 50–64 years (Figure 1 and Supplementary Table 2). After applying these rates to the US population, influenza A(H3N2) was associated with an estimated 28.4 million illnesses, 13.0 million medical visits, 587 000 hospitalizations, and 49 000 deaths overall (Supplementary Table 3). Influenza A(H1N1)pdm09 virus infections were less common, with 4.6 million illnesses. Influenza B virus infections accounted for 15.7 million illnesses, 32% of all influenza illnesses.
Figure 1.
Adjusted rates of influenza-associated (A) illnesses, (B) medical visits, (C) hospitalizations, and (D) deaths, by age group and influenza (sub)type—United States, 2017–2018 influenza season.
Vaccine Effectiveness
From the US Flu VE Network, 8900 people were enrolled and 8436 were included in analysis for the 2017–2018 influenza season, including 3050 case-patients with RT-PCR–confirmed influenza and 5386 controls with noninfluenza acute respiratory illness (Table 1; Supplementary Table 4). Influenza A virus infections were identified from November 2017 through February 2018 (Supplementary Figure 3). Influenza A(H3N2) viruses accounted for 84% of influenza A virus infections; and influenza B virus infections occurred later in the season with a peak in mid-March.
Table 1.
Demographic and Clinical Characteristics of Participants Enrolled in the US Influenza Vaccine Effectiveness Network—United States, 2017–2018 Influenza Season
| Test Result Status | Vaccination Status | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Influenza Positive | Influenza Negative | Vaccinated | |||||||
| Characteristic | No. | (%) | No. | (%) | P Valuea | Total | No. | (%) | P Valueb |
| Overall | 3050 | … | 5386 | … | 8436 | 4113 | … | ||
| Study site | … | … | … | … | <.001 | … | … | … | <.001 |
| Michigan | 532 | (39) | 836 | (61) | 1368 | 750 | (55) | ||
| Pennsylvania | 501 | (38) | 804 | (62) | 1305 | 599 | (46) | ||
| Texas | 725 | (37) | 1260 | (63) | 1985 | 753 | (38) | ||
| Washington | 501 | (29) | 1224 | (71) | 1725 | 1022 | (59) | ||
| Wisconsin | 791 | (39) | 1262 | (61) | 2053 | 989 | (48) | ||
| Male sex | 1322 | (38) | 2131 | (62) | .001 | 3453 | 1553 | (45) | <.001 |
| Age group (y) | … | … | … | … | <.001 | … | … | … | <.001 |
| <5 | 262 | (24) | 847 | (76) | 1109 | 551 | (50) | ||
| 5–17 | 837 | (46) | 965 | (54) | 1802 | 632 | (35) | ||
| 18–49 | 965 | (34) | 1894 | (66) | 2859 | 1128 | (39) | ||
| 50–64 | 571 | (38) | 937 | (62) | 1508 | 891 | (59) | ||
| ≥65 | 415 | (36) | 743 | (64) | 1158 | 911 | (79) | ||
| Race/ethnicity | … | … | … | … | .02 | … | … | … | <.001 |
| White, non-Hispanic | 2171 | (36) | 3888 | (64) | 6059 | 3117 | (51) | ||
| Black, non-Hispanic | 266 | (40) | 392 | (60) | 658 | 226 | (34) | ||
| Other, non-Hispanic | 269 | (33) | 543 | (67) | 812 | 418 | (51) | ||
| Hispanic | 331 | (38) | 550 | (62) | 881 | 339 | (38) | ||
| Unknown | 13 | (50) | 13 | (50) | 26 | 13 | (50) | ||
| Any high-risk conditionc | 1370 | (34) | 2633 | (66) | .001 | 4003 | 2445 | (61) | <.001 |
| Asthma/pulmonary high-risk condition | 537 | (32) | 1125 | (68) | <.001 | 1662 | 994 | (60) | <.001 |
| Cardiovascular high-risk condition | 274 | (34) | 540 | (66) | .12 | 814 | 587 | (72) | <.001 |
| Diabetes high-risk condition | 232 | (34) | 449 | (66) | .24 | 681 | 480 | (70) | <.001 |
| Body mass index ≥40d | 179 | (32) | 381 | (68) | .03 | 560 | 360 | (64) | <.001 |
| Other high-risk condition | 922 | (35) | 1704 | (65) | .18 | 2626 | 1702 | (65) | <.001 |
| Interval from onset to enrollment (days) | … | … | … | … | <.001 | … | … | … | <.001 |
| <3 | 1444 | (45) | 1759 | (55) | 3203 | 1472 | (46) | ||
| 3–4 | 1066 | (35) | 2008 | (65) | 3074 | 1501 | (49) | ||
| 5–7 | 540 | (25) | 1619 | (75) | 2159 | 1140 | (53) | ||
| Influenza test resulte | |||||||||
| Negative | … | … | 5386 | … | 5386 | 2842 | (53) | ||
| Influenza B positive | 958 | …. | … | … | 958 | 377 | (39) | ||
| B/Victoria | 39 | … | … | … | 39 | 8 | (21) | ||
| B/Yamagata | 908 | … | … | … | 908 | 369 | (41) | ||
| Influenza A positive | 2103 | … | … | … | 2103 | 899 | (43) | ||
| A (H1N1)pdm09 | 318 | … | … | … | 318 | 93 | (29) | ||
| A (H3N2) | 1761 | … | … | … | 1761 | 795 | (45) |
a P value calculated using χ2 comparing frequency of participants testing influenza positive vs negative by characteristic.
b P value calculated using χ2 test that compares the frequency of vaccination by participant characteristic.
cPresence of a high-risk health condition is defined as the presence of ≥1 medical record–documented International Classification of Disease, 10 Edition, high risk code from 1 October 2016 to enrollment, as defined by the Advisory Committee on Immunization Practices guidance for conditions that increase risk for complications from influenza [26].
dBody mass index was calculated as kg/m2 from height and weight recorded in the electronic medical record. Calculated for adults aged ≥18 years only.
eFourteen influenza B viruses were of unknown lineage; 34 influenza A viruses were of unknown subtype. There were 25 coinfections that are each counted twice in the table above: 11 A(H3N2) and A(H1N1)pdm09, 9 B/Yamagata and A(H3N2), 3 B/Victoria and B/Yamagata, 1 A(H1N1)pdm09 and B/Yamagata, and 1 B/Yamagata and A of unknown subtype.
Among those enrolled in the US Flu VE Network, 42% of influenza-positive case-patients and 53% of influenza-negative controls were vaccinated against influenza (Supplementary Table 5). Of the vaccinated participants aged <65 years with known vaccine type, 97% received quadrivalent inactivated influenza vaccine (IIV4) and 3% received trivalent inactivated influenza vaccine (IIV3). Of vaccinated adults aged ≥65 years with known vaccine type, 51% received high-dose IIV3, 47% received standard-dose IIV4 or IIV3, and 2% received adjuvanted IIV3.
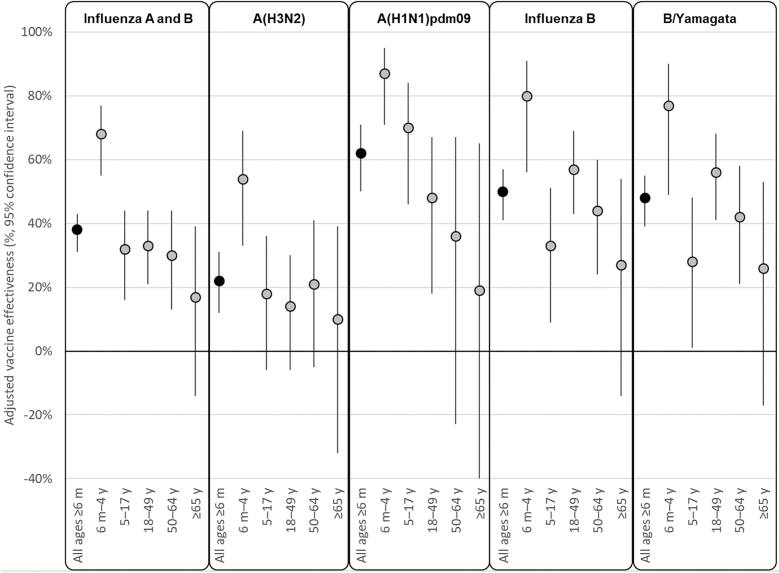
VE against any influenza A or B virus infection was 38% (95% CI, 31%–43%) after adjustment for study site, age, high-risk condition, and calendar time (Figure 2; Supplementary Table 5). The VE estimates against any influenza virus infection varied by age group and were statistically significant in all age groups except for people aged ≥65 years (Figure 2). The adjusted VE against A(H3N2) was 22% (95% CI, 12%–31%) overall but also varied by age and was only statistically significant in children aged 6 months–4 years. The adjusted VE against A(H1N1)pdm09 was 62% (95% CI, 50%–71%) and VE against influenza B was 50% (95% CI, 41%–57%).
Figure 2.
Adjusted vaccine effectiveness (VE) against outpatient, medically attended influenza-associated illness, US Flu VE Network—2017–2018 influenza season. The y-axis scale has been truncated for simplicity; however, for adults aged ≥65 years, the 95% confidence interval around the adjusted VE estimate against influenza A(H1N1)pdm09 extends beyond the lower limit of the y-axis (adjusted VE = 0.19, 95% confidence interval, –.91, .65).
Vaccine-prevented Burden
We estimated that influenza vaccination prevented 7.1 million (95% CrI, 5.4 million–9.3 million) illnesses and 3.7 million (95% CrI, 2.8 million–4.9 million) medical visits (Table 2). Prevented illnesses included 2.3 million illnesses due to A(H3N2) viruses and 1.4 million illnesses due to A(H1N1)pdm09 viruses; 48% and 70% of which, respectively, were prevented among children (Supplementary Table 6). Additionally, more than 3 million illnesses from influenza B viruses were prevented with vaccination.
Table 2.
Estimates of Influenza A- and B-Associated Illness, Medical Visits, Hospitalizations, and Deaths Prevented by Influenza Vaccination—United States, 2017–2018 Influenza Season
| Illnesses | Medical Visits | Hospitalization | Death | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age Group | Number Prevented | 95% CrIa | Number Prevented | 95% CrI | Number Prevented | 95% CrI | % Prevented | 95% CrI | Number Prevented | 95% CrI |
| 6 months–4 years | 2 121 511 | (1 445 133, 2 928 929) | 1 421 413 | (971 080, 1 966 976) | 14 790 | (10 075, 20 419) | 41 | (33, 47) | 74 | (0, 189) |
| 5–17 years | 1 366 965 | (613 310, 2 178 412) | 710 822 | (319 168, 1 143 256) | 3748 | (1682, 5973) | 15 | (7, 22) | 89 | (28, 197) |
| 18–49 years | 1 138 407 | (663 181, 1 610 481) | 421 211 | (243 149, 603 887) | 6390 | (3722, 9040) | 7 | (4, 10) | 228 | (119, 403) |
| 50–64 years | 1 792 530 | (673 687, 2 937 768) | 770 788 | (292 197, 1 263 230) | 19 009 | (7144, 31 154) | 10 | (4, 15) | 868 | (330, 1591) |
| ≥65 years | 715 073 | (0, 2 033 756) | 400 441 | (0, 1 145 616) | 65 007 | (0, 184 887) | 9 | (0, 21) | 6796 | (0, 19 844) |
| All ages | 7 134 487 | (5 393 925, 9 310 339) | 3 724 674 | (2 819 761, 4 877 688) | 108 944 | (38 854, 230 943) | 10 | (4, 19) | 8054 | (1059, 21 320) |
Abbreviation: CrI, credible interval.
aThe 95% CrI is from 5000 Monte Carlo simulations.
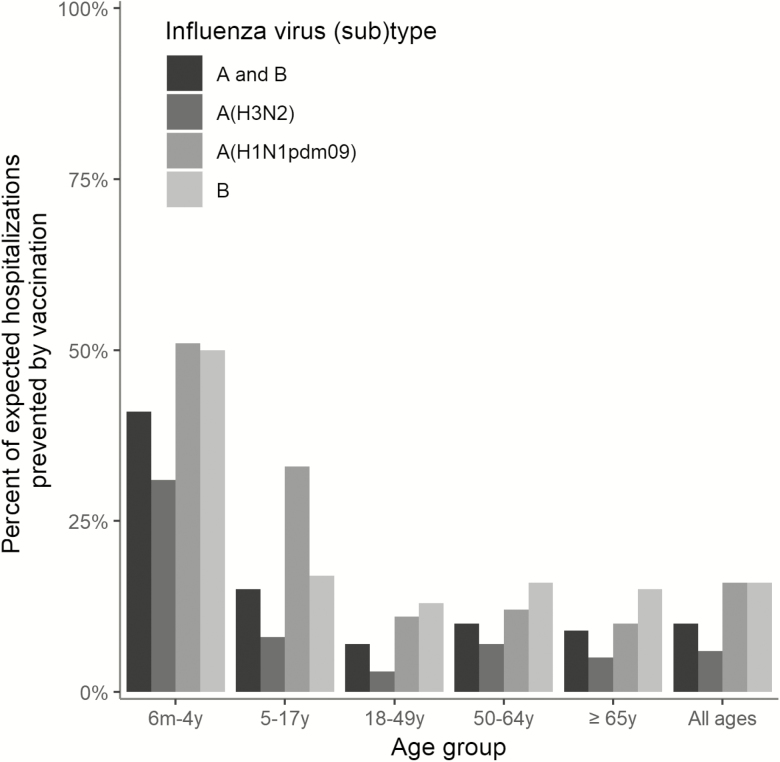
Overall, an estimated 109 000 (95% CrI, 38 900–231 000) hospitalizations were prevented by vaccination, or 10% (95% CrI, 4%–19%) of expected hospitalizations (Table 2). However, the percent of expected hospitalizations prevented by vaccination varied by age group, from a low of 7% (95% CrI, 4%–10%) in adults aged 18–49 years, who had the lowest vaccine coverage, to a high of 41% (95% CrI, 33%–47%) in children aged 6 months–4 years, who had high vaccine coverage and the highest VE (Figure 3).
Figure 3.
Estimated percent of expected influenza-associated hospitalizations prevented by vaccination—United States, 2017–2018 influenza season.
The burden of influenza-associated hospitalizations was greatest in adults aged ≥65 years, and our model estimated that influenza vaccination prevented approximately 65 000 influenza-associated hospitalizations (95% CrI, 0–185 000; 9% of expected, 95% CrI, 0%–21%) in this age group despite lower VE compared with other age groups. Using the estimated vaccine coverage and the overall prevented hospitalizations, we estimate that 462 people (95% CrI, 162–>999 999) aged ≥65 years needed to be vaccinated for each influenza-associated hospitalization prevented (Table 3).
Table 3.
Number Needed to Vaccinate to Prevent 1 Influenza A- and B-Associated Hospitalization—United States, 2017–2018 Influenza Season
| Age Group | Number Needed to Vaccinate | 95% Credible Intervala |
|---|---|---|
| 6 mo–4 y | 821 | (606, 1190) |
| 5–17 y | 7811 | (4925, 17 494) |
| 18–49 y | 5758 | (4105, 9849) |
| 50–64 y | 1311 | (808, 3502) |
| 65+ y | 462 | (162, >999 999) |
| All ages | 1223 | (578, 3438) |
aThe 95% credible interval is from 5000 Monte Carlo simulations.
Finally, an estimated 8000 (95% CrI, 1100–21 000) influenza-associated deaths were prevented by vaccination (9% of expected deaths, overall; 95% CrI, 1%–20%). Influenza vaccination prevented an estimated 39% (95% CrI, 30%–45%) of influenza-related mortality in children aged 6 months–4 years.
In sensitivity analysis, all CrIs for estimates of prevented hospitalizations using various vaccine coverage scenarios overlapped with the CrIs using the reported 2017–2018 coverage (Supplementary Table 7).
DISCUSSION
During the 2017–2018 season, currently available influenza vaccines reduced the risk of any influenza-associated medically attended illness by 38% and A(H3N2)-associated illness by 22%. When modeled with burden and vaccine coverage, we estimated that influenza vaccination prevented 7.1 million illnesses, 109 000 hospitalizations, and 8000 deaths related to influenza. In children aged 6 months–4 years, the benefits of vaccination were greatest, with 41% of all expected hospitalizations prevented by vaccination. VE against A(H1N1)pdm09 and B viruses was greater in all age groups than for A(H3N2); accordingly, the benefit of vaccination against these viruses was greater than against A(H3N2) viruses. Nevertheless, our results suggest that currently available vaccines provided substantial benefit during a season with high rates of influenza-associated medical visits, hospitalizations, and deaths.
The population benefit of influenza vaccination in our model depends on burden, VE, and vaccine coverage. During 2017–2018, the benefit of influenza vaccination was substantial mainly because of the high burden of influenza-associated disease. Vaccination prevented 109 000 hospitalizations, but this number represents only 10% of expected hospitalizations overall. Thus, while vaccination is an important strategy to mitigate some of the burden and severity of the influenza season, improvements in both VE and vaccine coverage are needed and would result in a greater reduction in burden, enhancing both the public health and economic benefits of annual influenza vaccination. Our model of prevented illness may underestimate the population benefit of vaccination as it only accounts for direct effects of vaccination. Various studies suggest that influenza vaccination, particularly of school-aged children, may also provide indirect protection (ie, herd immunity) against influenza virus infection, largely by reducing the probability of contact with an infected person [27–32]. The magnitude of indirect protection is inconsistent between studies [33]; however, the population benefit of seasonal influenza vaccination would be greater if indirect effects were present and considered in the model [34, 35].
VE against circulating A(H3N2) viruses and prevented fraction of A(H3N2) disease were lower than with influenza A(H1N1)pdm09 and B viruses. Reduced vaccine protection against A(H3N2) viruses is likely multifactorial and was also observed during the 2016–2017 influenza season with the same A(H3N2) vaccine reference virus (A/Hong Kong/4801/2014) [36]. Antigenic characterization indicated that most circulating A(H3N2) viruses in 2017–2018 remained antigenically similar to the cell-propagated A/Hong Kong/4801/2014 reference virus, suggesting limited antigenic drift between the seasons [2]. However, A(H3N2) viruses continued to evolve, and several viral genetic groups circulated. Further, many circulating A(H3N2) viruses were poorly inhibited by antisera raised against egg-adapted viruses used for production of the majority of influenza vaccines in the United States [2]. The higher VE against A(H3N2) viruses that we observed in young children may suggest that the immune response to the current A(H3N2) vaccine virus differs by age. This deserves more attention as young children had higher VE despite being vaccinated with egg-based vaccines. Among older adults, egg adaptation of A(H3N2) vaccine viruses may have contributed to reduced effectiveness despite increasing use of high-dose vaccine, which was shown previously to be more effective than standard-dose influenza vaccines in previous A(H3N2) predominant seasons [37]. Even with reduced VE among older adults, vaccination still prevented 1 influenza-related hospitalization for every 462 people vaccinated. More broadly, we need to better understand the factors that contribute to differences in VE in order to improve influenza vaccines.
Our estimates of the effect of vaccination rely on large, multistate research and surveillance platforms, but there are limitations to the available data. First, multipliers are used to scale surveillance data to national burden estimates. Data to calculate the multipliers often lag by 2 years; thus, we use multipliers measured during previous influenza seasons. Any changes in testing practices, care-seeking behavior, or disease severity patterns that occurred during 2017–2018 would not be reflected in the multipliers. Our estimates of the effect of vaccination will be revised on CDC websites as data are updated. Second, we imputed subtype-specific hospitalization rates because subtyping was not performed systematically in FluSurv-Net. Third, our model does not currently account for possible waning effectiveness of influenza vaccination over the season [38–44]. The current literature is inconsistent about the amount of waning that occurs; however, including any amount of waning effectiveness in the model would have reduced our estimated population benefit. Fourth, vaccination coverage estimates from self-report and telephone surveys have limitations, including lower response rates and possible inaccuracy of vaccination status [21–25, 45, 46]. All results of our sensitivity analysis fell within the CrIs using reported coverage. Fifth, as we assumed that influenza vaccination would not increase the risk of infection, our CrIs are truncated at zero and thus skewed in favor of a population benefit. Finally, the role of genetic and antigenic diversity on the VE and estimated population benefit deserves further investigation; full antigenic and genetic characterization of specimens from the US Flu VE Network is ongoing toward this effort.
Our results highlight the large burden of influenza-associated illnesses, medical visits, hospitalizations, and deaths during 2017–2018 and the value of current vaccines to reduce the burden of disease, even with a VE of 38% against influenza A and B viruses and 22% against A(H3N2) viruses. Given the substantial burden of influenza-associated illness, efforts to improve influenza vaccines are imperative. An A(H3N2) vaccine component with improved effectiveness could substantially reduce the number of influenza-associated hospitalizations among older adults [47]. Several studies have suggested that vaccines with a higher dose of antigen may offer protective advantages over standard-dose inactivated influenza vaccines in older adults [37, 48, 49]. Also, it is possible that vaccine viruses not propagated in eggs could be advantageous, especially for the A(H3N2) vaccine component. There were 2 licensed vaccines (cell culture–derived inactivated vaccine and recombinant vaccine) that did not include egg-propagated A(H3N2) viruses in 2017–2018 [50]. Efforts to determine the advantages of nonegg-based and enhanced vaccines are ongoing. At this time, vaccination remains an important component of influenza prevention; and our results indicate that current vaccines prevented a substantial burden of illness during the 2017–2018 influenza season.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
US Flu VE Network. Huong Q. McLean, Jennifer P. King, Mary Patricia Nowalk, G.K. Balasubramani, Todd M. Bear, Robert Hickey, John V. Williams, Evelyn C. Reis, Krissy K. Moehling, Heather Eng, Lisa A. Jackson, Michael Smith, Chandni Raiyani, Lydia Clipper, Kempapura Murthy, Wencong Chen, Michael Reis, Joshua G. Petrie, Ryan E. Malosh, E.J. McSpadden, Hannah E. Segaloff, Caroline K. Cheng, Rachel Truscon, Emileigh Johnson, and Lois E. Lamerato.
Acknowledgments. The authors acknowledge the great work and support of the following: Bret Rosenblum, Samantha Ford, Monika Johnson, Jonathan M. Raviotta, Terrie Sax, Jonathan Steele, Michael Susick, Rina Chabra, Edward Garofolo, Philip Iozzi, Barbara Kevish, Donald B. Middleton, Leonard Urbanski, from University of Pittsburgh Schools of the Health Sciences and University of Pittsburgh Medical Center; Teresa Ponder, Todd Crumbaker, Iosefo Iosefo, Patricia Sleeth, Virginia Gandy, Kelsey Bounds, Mary Kylberg, Arundhati Rao, Robert Fader, Kimberley Walker, Marcus Volz, Jeremy Ray, Deborah Price, Jennifer Thomas, Hania Wehbe-Janek, Madhava Beeram, John Boyd, Jamie Walkowiak, Robert Probe, Glen Couchman, Shahin Motakef and Alejandro Arroliga from Baylor Scott and White Health; Anne Kaniclides, Emerson Bouldin, Christoph Baker, Kimberly Berke, Mackenzie Smith, and Niharika Rajesh from University of Michigan School of Public Health; Elizabeth Alleman, Sarah Bauer, Michelle Groesbeck, Kristyn Brundidge, Neha Hafeez, Jayla Jackson, Ian Anastasia, and Gabriel Kadoo from Henry Ford Health System; Sarah Petnic and Alison Ryan from the California Emerging Infections Program; Amber Maslar, James Meek, and Rona Chen from the Connecticut Emerging Infections Program; Samantha Stephens from the Colorado Emerging Infections Program Stepy Thomas, Suzanne Segler, Kyle Openo, Emily Fawcett, Monica Farley, and Andrew Martin from Georgia Emerging Infections Program; Patricia Ryan, Robert Sunkel, Taylor Lutich, Rebecca Perlmutter, Brittany Grace, Timothy Blood, and Cindy Zerrlaut from the Maryland Department of Health; Melissa McMahon, Anna Strain, and Jamie Christensen from the Minnesota Department of Health; Kathy Angeles, Lisa Butler, Sarah Khanlian, Robert Mansmann from the University of Mexico Emerging Infectious Program and Chelsea McMullen from the New Mexico Department of Health; Eva Pradhan and Katarina Manzi from the New York–Albany Emerging Infections Program; Christina Felsen and Maria Gaitan from the University of Rochester (New York) School of Medicine and Dentistry; Krista Long, Nicholas Fisher, Emily Hawley, and Rory O’Shaughnessy from the Ohio Influenza Hospitalization Surveillance Project; Magdalena Scott and Courtney Crawford from the Oregon Emerging Infections Program; and William Schaffner, Tiffanie Markus, Karen Leib, and Katie Dyer from Vanderbilt University Medical Center and Tennessee Emerging Infections Program. The authors also acknowledge the work of Tammy Santibanez, Yusheng Zhai (Leidos, Inc.), Pengjun Lu, Anup Srivastav (Leidos, Inc.), and Mei-Chuan Hung (Leidos, Inc.) in the Immunization Services Division and Charisse Cummings in the Influenza Division at the Centers for Disease Control and Prevention (CDC).
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Financial support. This work was supported by CDC through the following cooperative agreements: Emerging Infections Programs (CDC-RFA-CK17-1701), the Influenza Hospital Surveillance Project (5U38OT000143), the University of Michigan (1U01 IP001034), Kaiser Permanente Washington Research Institute (1U01 IP001037), Marshfield Clinic Research Institute (1U01 IP001038), University of Pittsburgh (1U01 IP001035), and Baylor Scott and White Healthcare (1U01 IP001039). At the University of Pittsburgh, the project was also supported by the National Institutes of Health through grant UL1TR001857.
Potential conflicts of interest.A. S. M. reports consultancy fees from Sanofi and Seqirus outside the submitted work. E. A. reports grants from Sanofi Pasteur, Merck, Pfizer, Micron, MedImmune, and PaxVax and has received consultancy fees from AbbVie outside the submitted work. E. B. reports grants from Seqirus outside the submitted work. H. K. T. reports grants from Sanofi Pasteur and personal fees from Seqirus during the conduct of the study. M. J. reports grants from Sanofi Pasteur outside the submitted work. M. G. reports grants from the CDC outside the submitted work. R. L. received payment to coedit a book on infectious disease surveillance; royalties are donated to the Minnesota Department of Health. R. Z. reports grants from Sanofi Pasteur, Merck & Co., and Pfizer Inc outside the submitted work. All other authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
US Influenza Vaccine Effectiveness (Flu VE) Network, the Influenza Hospitalization Surveillance Network, and the Assessment Branch, Immunization Services Division, Centers for Disease Control and Prevention:
Huong Q McLean, Jennifer P King, Mary Patricia Nowalk, G K Balasubramani, Todd M Bear, Robert Hickey, John V Williams, Evelyn C Reis, Krissy K Moehling, Heather Eng, Lisa A Jackson, Michael Smith, Chandni Raiyani, Lydia Clipper, Kempapura Murthy, Wencong Chen, Michael Reis, Joshua G Petrie, Ryan E Malosh, E J McSpadden, Hannah E Segaloff, Caroline K Cheng, Rachel Truscon, Emileigh Johnson, Lois E Lamerato, Bret Rosenblum, Samantha Ford, Monika Johnson, Jonathan M Raviotta, Terrie Sax, Jonathan Steele, Michael Susick, Rina Chabra, Edward Garofolo, Philip Iozzi, Barbara Kevish, Donald B Middleton, Leonard Urbanski, Teresa Ponder, Todd Crumbaker, Iosefo Iosefo, Patricia Sleeth, Virginia Gandy, Kelsey Bounds, Mary Kylberg, Arundhati Rao, Robert Fader, Kimberley Walker, Marcus Volz, Jeremy Ray, Deborah Price, Jennifer Thomas, Hania Wehbe-Janek, Madhava Beeram, John Boyd, Jamie Walkowiak, Robert Probe, Glen Couchman, Shahin Motakef, Alejandro Arroliga, Anne Kaniclides, Emerson Bouldin, Christoph Baker, Kimberly Berke, Mackenzie Smith, Niharika Rajesh, Elizabeth Alleman, Sarah Bauer, Michelle Groesbeck, Kristyn Brundidge, Neha Hafeez, Jayla Jackson, Ian Anastasia, Gabriel Kadoo, Sarah Petnic, Alison Ryan, Amber Maslar, James Meek, Rona Chen, Samantha Stephens, Stepy Thomas, Suzanne Segler, Kyle Openo, Emily Fawcett, Monica Farley, Andrew Martin, Patricia Ryan, Robert Sunkel, Taylor Lutich, Rebecca Perlmutter, Brittany Grace, Timothy Blood, Cindy Zerrlaut, Melissa McMahon, Anna Strain, Jamie Christensen, Kathy Angeles, Lisa Butler, Sarah Khanlian, Robert Mansmann, Chelsea McMullen, Eva Pradhan, Katarina Manzi, Christina Felsen, Maria Gaitan, Krista Long, Nicholas Fisher, Emily Hawley, Rory O’Shaughnessy, Magdalena Scott, Courtney Crawford, William Schaffner, Tiffanie Markus, Karen Leib, Katie Dyer, Tammy Santibanez, Yusheng Zhai, Pengjun Lu, Anup Srivastav, and Mei-Chuan Hung
References
- 1. Biggerstaff M, Kniss K, Jernigan DB, et al. Systematic assessment of multiple routine and near-real time indicators to classify the severity of influenza seasons and pandemics in the United States, 2003–04 through 2015–2016. Am J Epidemiol 2018; 187:1040–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garten R, Blanton L, Elal AIA, et al. Update: influenza activity in the United states during the 2017–18 season and composition of the 2018–19 influenza Vaccine. MMWR Morb Mortal Wkly Rep 2018; 67:634–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. Estimated influenza illnesses, medical visits, hospitalizations, and deaths in the United States—2017–2018 influenza season Available at: https://www.cdc.gov/flu/about/burden/estimates.htm. Accessed 29 October 2018.
- 4. Centers for Disease Control and Prevention. Estimates of influenza vaccination coverage and adults—United States, 2017–18 flu season Available at: https://www.cdc.gov/flu/fluvaxview/coverage-1718estimates.htm. Accessed 29 October 2018.
- 5. Centers for Disease Control and Prevention. Estimates of flu vaccination coverage among children—United States, 2017–18 flu season Available at: https://www.cdc.gov/flu/fluvaxview/coverage-1718estimates-children.htm. Accessed 29 October 2018.
- 6. Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb Mortal Wkly Rep 2018; 67:180–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices—United States, 2017–18 influenza season. MMWR Recomm Rep 2017; 66:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gaglani M, Pruszynski J, Murthy K, et al. Influenza vaccine effectiveness against 2009 pandemic influenza A(H1N1) virus differed by vaccine type during 2013–2014 in the United States. J Infect Dis 2016; 213:1546–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McLean HQ, Thompson MG, Sundaram ME, et al. Influenza vaccine effectiveness in the United States during 2012–2013: variable protection by age and virus type. J Infect Dis 2015; 211:1529–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ohmit SE, Petrie JG, Malosh RE, et al. Influenza vaccine effectiveness in the community and the household. Clin Infect Dis 2013; 56:1363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zimmerman RK, Nowalk MP, Chung J, et al. 2014–2015 influenza vaccine effectiveness in the United States by vaccine type. Clin Infect Dis 2016; 63:1564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jackson ML, Chung JR, Jackson LA, et al. Influenza vaccine effectiveness in the United States during the 2015–2016 season. N Engl J Med 2017; 377:534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines. MMWR Recomm Rep 2016; 65:1–54. [DOI] [PubMed] [Google Scholar]
- 14. Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine 2013; 31:2165–8. [DOI] [PubMed] [Google Scholar]
- 15. Reed C, Chaves SS, Daily Kirley P, et al. Estimating influenza disease burden from population-based surveillance data in the United States. PLoS One 2015; 10:e0118369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chaves SS, Lynfield R, Lindegren ML, Bresee J, Finelli L. The US influenza hospitalization surveillance network. Emerg Infect Dis 2015; 21:1543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tokars JI, Rolfes MA, Foppa IM, Reed C. An evaluation and update of methods for estimating the number of influenza cases averted by vaccination in the United States. Vaccine 2018; 36:7331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reed C, Angulo FJ, Swerdlow DL, et al. Estimates of the prevalence of pandemic (H1N1) 2009, United States, April–July 2009. Emerg Infect Dis 2009; 15:2004–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rolfes MA, Foppa IM, Garg S, et al. Annual estimates of the burden of seasonal influenza in the United States: a tool for strengthening influenza surveillance and preparedness. Influenza Other Respir Viruses 2018; 12:132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Flannery B, Chung J, Ferdinands J. Preliminary estimates of 2017–18 seasonal influenza vaccine effectiveness against laboratory-confirmed influenza from the US Flu VE and HAIVEN Networks. In: Advisory Committee on Immunization Practices; Atlanta, GA, 2018. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-06/flu-02-Flannery-508.pdf.
- 21. Brown C, Clayton-Boswell H, Chaves SS, et al. ; New Vaccine Surveillance Network Validity of parental report of influenza vaccination in young children seeking medical care. Vaccine 2011; 29:9488–92. [DOI] [PubMed] [Google Scholar]
- 22. Mangtani P, Shah A, Roberts JA. Validation of influenza and pneumococcal vaccine status in adults based on self-report. Epidemiol Infect 2007; 135:139–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Poehling KA, Vannoy L, Light LS, et al. Assessment of parental report for 2009–2010 seasonal and monovalent H1N1 influenza vaccines among children in the emergency department or hospital. Acad Pediatr 2012; 12:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Irving SA, Donahue JG, Shay DK, Ellis-Coyle TL, Belongia EA. Evaluation of self-reported and registry-based influenza vaccination status in a Wisconsin cohort. Vaccine 2009; 27:6546–9. [DOI] [PubMed] [Google Scholar]
- 25. King JP, McLean HQ, Belongia EA. Validation of self-reported influenza vaccination in the current and prior season. Influenza Other Respir Viruses 2018; 12:808–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2017–18 influenza season. Am J Transplant 2017; 17:2970–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hurwitz ES, Haber M, Chang A, et al. Effectiveness of influenza vaccination of day care children in reducing influenza-related morbidity among household contacts. JAMA 2000; 284:1677–82. [DOI] [PubMed] [Google Scholar]
- 28. Monto AS, Davenport FM, Napier JA, Francis T Jr. Modification of an outbreak of influenza in Tecumseh, Michigan by vaccination of schoolchildren. J Infect Dis 1970; 122:16–25. [DOI] [PubMed] [Google Scholar]
- 29. Pebody RG, Sinnathamby MA, Warburton F, et al. Uptake and impact of vaccinating primary school-age children against influenza: experiences of a live attenuated influenza vaccine programme, England, 2015/16. Euro Surveill 2018; 23: pii=1700496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Piedra PA, Gaglani MJ, Kozinetz CA, et al. Herd immunity in adults against influenza-related illnesses with use of the trivalent-live attenuated influenza vaccine (CAIV-T) in children. Vaccine 2005; 23:1540–8. [DOI] [PubMed] [Google Scholar]
- 31. Rudenko LG, Slepushkin AN, Monto AS, et al. Efficacy of live attenuated and inactivated influenza vaccines in schoolchildren and their unvaccinated contacts in Novgorod, Russia. J Infect Dis 1993; 168:881–7. [DOI] [PubMed] [Google Scholar]
- 32. Loeb M, Russell ML, Moss L, et al. Effect of influenza vaccination of children on infection rates in Hutterite communities: a randomized trial. JAMA 2010; 303:943–50. [DOI] [PubMed] [Google Scholar]
- 33. Mertz D, Fadel SA, Lam PP, et al. Herd effect from influenza vaccination in non-healthcare settings: a systematic review of randomised controlled trials and observational studies. Euro Surveill 2016; 21: pii=30378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Arinaminpathy N, Kim IK, Gargiullo P, et al. Estimating direct and indirect protective effect of influenza vaccination in the United States. Am J Epidemiol 2017; 186:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sah P, Medlock J, Fitzpatrick MC, Singer BH, Galvani AP. Optimizing the impact of low-efficacy influenza vaccines. Proc Natl Acad Sci U S A 2018; 115:5151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Flannery B, Chung JR, Monto AS, et al. Influenza vaccine effectiveness in the United States during the 2016–2017 season. Clin Infect Dis 2018; 64:736–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med 2014; 371:635–45. [DOI] [PubMed] [Google Scholar]
- 38. Belongia EA, Sundaram ME, McClure DL, Meece JK, Ferdinands J, VanWormer JJ. Waning vaccine protection against influenza A (H3N2) illness in children and older adults during a single season. Vaccine 2015; 33:246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jimenez-Jorge S, de Mateo S, Delgado-Sanz C, et al. Effectiveness of influenza vaccine against laboratory-confirmed influenza, in the late 2011–2012 season in Spain, among population targeted for vaccination. BMC Infect Dis 2013; 13:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kissling E, Valenciano M, Larrauri A, et al. Low and decreasing vaccine effectiveness against influenza A(H3) in 2011/12 among vaccination target groups in Europe: results from the I-MOVE multicentre case-control study. Euro Surveill 2013; 18: pii=20390. [DOI] [PubMed] [Google Scholar]
- 41. Pebody R, Andrews N, McMenamin J, et al. Vaccine effectiveness of 2011/12 trivalent seasonal influenza vaccine in preventing laboratory-confirmed influenza in primary care in the United Kingdom: evidence of waning intra-seasonal protection. Euro Surveill 2013; 18: pii=20389. [DOI] [PubMed] [Google Scholar]
- 42. Petrie JG, Ohmit SE, Truscon R, et al. Modest waning of influenza vaccine efficacy and antibody titers during the 2007–2008 influenza season. J Infect Dis 2016; 214:1142–9. [DOI] [PubMed] [Google Scholar]
- 43. Sullivan SG, Komadina N, Grant K, Jelley L, Papadakis G, Kelly H. Influenza vaccine effectiveness during the 2012 influenza season in Victoria, Australia: influences of waning immunity and vaccine match. J Med Virol 2014; 86:1017–25. [DOI] [PubMed] [Google Scholar]
- 44. Ferdinands JM, Fry AM, Reynolds S, et al. Intraseason waning of influenza vaccine protection: evidence from the US Influenza Vaccine Effectiveness Network, 2011–12 through 2014–15. Clin Infect Dis 2017; 64:544–50. [DOI] [PubMed] [Google Scholar]
- 45. Centers for Disease Control and Prevention. Interim results: state-specific seasonal influenza vaccination coverage—United States, August 2009–January 2010. MMWR Morb Mortal Wkly Rep 2010; 59:477–84. [PubMed] [Google Scholar]
- 46. Zimmerman RK, Raymund M, Janosky JE, Nowalk MP, Fine MJ. Sensitivity and specificity of patient self-report of influenza and pneumococcal polysaccharide vaccinations among elderly outpatients in diverse patient care strata. Vaccine 2003; 21:1486–91. [DOI] [PubMed] [Google Scholar]
- 47. Fry AM, Kim IK, Reed C, et al. Modeling the effect of different vaccine effectiveness estimates on the number of vaccine-prevented influenza-associated hospitalizations in older adults. Clin Infect Dis 2014; 59:406–9. [DOI] [PubMed] [Google Scholar]
- 48. Dunkle LM, Izikson R, Patriarca P, et al. ; PSC12 Study Team Efficacy of recombinant influenza vaccine in adults 50 years of age or older. N Engl J Med 2017; 376:2427–36. [DOI] [PubMed] [Google Scholar]
- 49. Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 influenza season. MMWR Recomm Rep 2018; 67:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Barr IG, Donis RO, Katz JM, et al. Cell culture-derived influenza vaccines in the severe 2017–2018 epidemic season: a step towards improved influenza vaccine effectiveness. NPJ Vaccines 2018; 3:44; doi:10.1038/s41541-018-0079-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.