Abstract
Glucagon (GCG) is an essential regulator of glucose and lipid metabolism that also promotes weight loss. We have shown that glucagon-receptor (GCGR) signaling increases fatty acid oxidation (FAOx) in primary hepatocytes and reduces liver triglycerides in diet-induced obese (DIO) mice; however, the mechanisms underlying this aspect of GCG biology remains unclear. Investigation of hepatic GCGR targets elucidated a potent and previously unknown induction of leptin receptor (Lepr) expression. Liver leptin signaling is known to increase FAOx and decrease liver triglycerides, similar to glucagon action. Therefore, we hypothesized that glucagon increases hepatic LEPR, which is necessary for glucagon-mediated reversal of hepatic steatosis. Eight-week-old control and liver-specific LEPR-deficient mice (LeprΔliver) were placed on a high-fat diet for 12 weeks and then treated with a selective GCGR agonist (IUB288) for 14 days. Liver triglycerides and gene expression were assessed in liver tissue homogenates. Administration of IUB288 in both lean and DIO mice increased hepatic Lepr isoforms a-e in acute (4 hours) and chronic (72 hours,16 days) (P < 0.05) settings. LeprΔliver mice displayed increased hepatic triglycerides on a chow diet alone (P < 0.05), which persisted in a DIO state (P < 0.001), with no differences in body weight or composition. Surprisingly, chronic administration of IUB288 in DIO control and LeprΔliver mice reduced liver triglycerides regardless of genotype (P < 0.05). Together, these data suggest that GCGR activation induces hepatic Lepr expression and, although hepatic glucagon and leptin signaling have similar liver lipid targets, these appear to be 2 distinct pathways.
Keywords: Glucagon, Leptin, Hepatic Steatosis, NAFLD
Nonalcoholic fatty liver disease (NAFLD) is the most common liver disease in the United States and is present in 80% to 90% of obese individuals (1). NAFLD is a result of hepatic steatosis, an ectopic accumulation of triglycerides within hepatocytes of the liver. Hepatic steatosis increases the risk of systemic metabolic abnormalities, including impaired glucose homeostasis and insulin resistance. These, in turn, lead to an increased risk for type 2 diabetes and cardiovascular disease (2), both of which contribute to a substantial burden for patients and the healthcare system. Although lifestyle interventions such as diet and exercise can prevent liver damage and reverse early stages, there are no approved medical treatments for NAFLD.
The fasting hormone glucagon is well characterized for its role in glycemic homeostasis. More recently, glucagon has been appreciated for its regulation of lipid metabolism and energy expenditure (3). Chronic glucagon receptor (GCGR) activation reduces body weight and reverses hepatic steatosis in diet-induced obese (DIO) mice (4). Consistent with these observations, both fasting and GCGR agonism stimulate hepatic fatty acid oxidation (FAOx). Moreover, this regulation occurs in a GCGR-dependent manner (5). Conversely, mice lacking GCGR are not only resistant to fasting-stimulated FAOx, but are also characterized by elevated plasma free-fatty acids and triglycerides (5). Although an integral role for hepatic GCGR in the regulation of hepatic and whole-body lipid homeostasis is clear, the intracellular mechanisms by which glucagon regulates this metabolism has yet to be fully elucidated.
The prototypical adipokine, leptin, has been at the forefront of obesity research since first described in 1994 (6). Although produced and secreted from adipocytes, leptin acts upon its receptor, LEPR, to regulate a wide array of physiologies, including energy balance, glucose and lipid homeostasis, reproduction, exercise response, and immune function (7). Many of these actions are centrally regulated; therefore, research in the field has primarily focused on LEPR signaling in the hypothalamus (8). However, peripheral actions of LEPR signaling have also been described, including hepatic lipid accumulation in mice lacking hepatic LEPR (9,10). This accumulation occurs even when mice are maintained on a standard chow diet, implicating the hepatic LEPR as a critical regulator of liver lipid metabolism (9). In support of this role, acute peripheral, but not intracerebroventricular, infusion of leptin decreases liver and plasma triglycerides concomitant with increased liver FAOx and ketogenesis in mice with sucrose-induced hepatic steatosis (11). Obesity and NAFLD are associated with a rise in circulating leptin levels and a state of hepatic leptin resistance, including reduced hepatic leptin receptor (Lepr) expression in multiple tissues (12). Importantly, selective reexpression of hepatic Lepr ameliorates hepatic steatosis in Zucker fatty rats (13) and acute (90 minute) infusion of leptin in isolated liver reduces liver triglycerides (14), suggesting that LEPR signaling is sufficient to treat NAFLD. Our recent investigation into GCGR regulation of energy balance uncovered potent regulation of Lepr in mice with chronic GCGR activation (4). This upregulation of Lepr expression may result in increased leptin sensitivity; therefore, we hypothesized that GCGR-signaling increases the hepatic Lepr, which is necessary for glucagon-mediated reversal of hepatic steatosis.
Materials and Methods
Animal models. All studies were approved by and performed according to the guidelines of the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham. Mice were single or group-housed on a 12:12-hour light-dark cycle (light on from 6:00 am to 6:00 pm) at 22°C and constant humidity with free access to food and water, except as noted. Lepr-floxed and Alb-Cre mice obtained from Jackson Labs (strain nos. 008327 and 003574, Bar Harbor, ME). Liver-deficient Gcgr, Fgf21, and Fxr mice were generated as previously described (4,15). All mice were maintained in our facilities and fed a standard chow (Teklad LM-485, 5.6% fat) or high-fat diet (HFD, 58.0 kcal% fat; D12331 Research Diets, New Brunswick NJ). Knockout of hepatic Lepr confirmed using primers amplifying floxed exon 1 (Mm01262072_m1, Applied Biosystems), as previously validated (8,16).
Peptides. GCGR agonist (IUB288) was synthesized as previously described (17). Native glucagon and insulin (Humulin R) were obtained from American Peptide Co. (Sunnyvale, CA) and Eli Lilly and Co. (Indianapolis, IN), respectively.
Glucose and insulin tolerance tests. Glucose and insulin tolerance tests were performed in 5- to 6-hour fasted 8- to 10-week chow-fed, or 24-week diet-induced obese male mice by IP injection of glucose (1.5-2 g/kg, 25% wt/vol D-glucose [Sigma, St. Louis, MO] in 0.9% wt/vol saline) or insulin (0.5-0.75 unit/kg in 0.9% wt/vol saline). Blood glucose was determined by Ascensia Contour Glucometer (Parsippany, NJ). Glucose disappearance rate was defined as (Δ blood glucose/minute).
Oral lipid tolerance test. Oral, lipid tolerance tests were performed in overnight fasted 8- to 10-week-old chow-fed, or 24-week old diet-induced obese male mice by oral gavage of 400 µL corn oil. Blood was collected at t = 0, 60, 120, and 180 minutes via tail bleed.
Plasma and tissue analyses. Lipids in plasma and tissue samples from 2-hour fasted mice were determined using Infinity Triglycerides (Thermo Fisher Scientific, Waltham, MA; #TR22421) and Infinity Cholesterol (Thermo Fisher Scientific; #TR13421). Hepatic lipid measurements were conducted following extraction as previously described (18). Briefly, liver (40-80 mg) was homogenized for 2 minutes at 30 seconds-1 (x2) using a TissueLyser II (Qiagen, Valencia, CA) and lipid was extracted using chloroform/methanol (2:1, vol/vol) and ultrapure water. The organic phase was then separated via centrifugation, dried, and reconstituted in chloroform and vortexed. Values are represented as milligram of triglyceride per gram of liver.
Quantitative real-time PCR. Liver RNA was isolated from 2-hour fasted mice using the RNeasy Lipid Mini-Kit (Qiagen) and cDNA was synthesized by reverse transcription PCR using SuperScriptIII, DNase treatment, and anti-RNase treatment according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). Single gene quantitative PCR was performed as previously described (15,19), whereas TaqMan Gene Expression Assay was used for identification of Cre-mediated Lepr recombination as previously described (8) with the following primer/probe sets: Lepr (Mm01262072_m1, Applied Biosystems) and Hprt1 (Mm03024075_m1, Applied Biosystems). Data were normalized to housekeeping genes Rps18, β-actin, or HPrt1 as noted using the ΔΔct calculation. See Table 1 for list of primer sets.
Table 1.
Primer Sequences
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| Lepr-a | ACA CTG TTA ATT TCA CAC CAG AG | AGT CAT TCA AAC CAT AGT TTA GG |
| Lepr-b | TGG TCC CAG CAG CTA TGG T | ACC CAG AGA AGT TAG CAC TGT |
| Lepr-c | CAAGCAGCAGAATGACGCAG | GTGACCTTTTGGAAATTCAGTCCT |
| Lepr-d | ACGCAGGGCTGTATGTCATT | TCCTTTTGGAAATTCAGTCCTTG |
| Lepr-e | TGT TAT ATC TGG TTA TTG AAT GG | CAT TAA ATG ATT TAT TAT CAG AAT TGC |
| Lepr | GAC CGC CGA ACA CAA CCG ATG AC | ACA CCT AGC TGG CGA AAA ACT GAA G |
| Leprot | GGGCTGACTTTTCTTATGCTG | CCCAGTGGTGAAGAAATACGC |
| Leptin | CAG CCT GCC TTC CCA AAA | CAT CCA GGC TCT CTG GCT TCT |
| Rps18 | TTC TGG CCA ACG GTC TAG ACA AC | CCA GTG GTC TTG GTG TGC TGA |
| β-actin | GGC TGT ATT CCC CTC CAT CG | CCA GTT GGT AAC AAT GCC ATG T |
Statistics. All data are represented as mean and SEM. Statistical significance was determined using unpaired Student t tests or, where appropriate, 1- and 2-way ANOVA with multiple comparisons Tukey and Sidak posttest, respectively. Statistics were completed using GraphPad Prism, version 6.0, for Macintosh (GraphPad Software, San Diego, CA) and significance assigned when P < 0.05.
Results
GCGR Agonism Increases Hepatic Leptin Receptor Expression
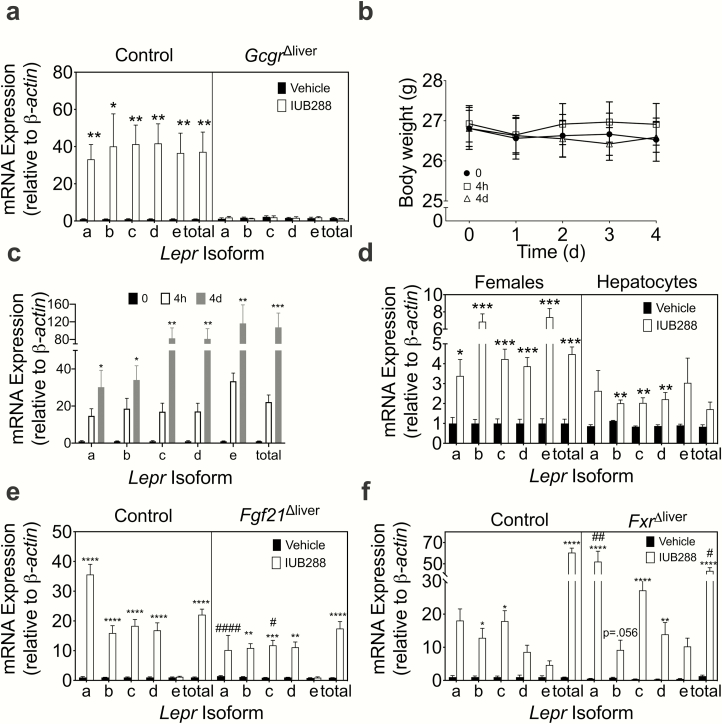
We have previously observed that IUB288, a long-acting GCGR agonist, is suitable for once-daily subcutaneous administration in mice to study the pharmacological actions of GCGR signaling (17). Prior RNA-sequencing in liver tissue from DIO mice treated chronically with IUB288 (GSE135881) highlighted a robust increase in the hepatic Lepr expression as well as a clear upregulation of genes involved in hepatic FAOx (4). In confirmation with this initial screen, hepatic Lepr expression increased in DIO animals treated with IUB288 (P < 0.05, Fig. 1a, left). Moreover, this induction was absent in animals deficient for hepatic Gcgr (Gcgr∆liver, Fig. 1a, right), suggesting hepatic glucagon signaling is necessary for direct induction of hepatic Lepr expression. The leptin receptor is alternatively spliced into multiple isoforms (Lepr-a-e) (20). All isoforms were similarly induced following GCGR agonism (P < 0.05, Fig. 1a). Gcgr∆liver mice are resistant to the antiobesity effects of IUB288 (4), thus it is unclear whether Lepr induction is a direct effect of GCGR activation or a consequence of weight loss. Therefore, we tested GCGR-stimulated induction of hepatic Lepr mRNA expression in lean animals where we observe a defense of body weight (Fig. 1b and (17)). Lepr expression increases in lean, chow-fed males after an acute IUB288 injection (4 hours) or chronic (4 days) treatment (P < 0.05, Fig. 1c), with no changes in body weight (Fig. 1b). This suggests GCGR signaling induces Lepr mRNA expression independent of weight loss. Likewise, Lepr was induced in lean, IUB288-treated females (5-day treatment, P < 0.001, Fig. 1d, left), suggesting this is also a sex-independent effect. Furthermore, IUB288 increased Lepr expression in isolated primary hepatocytes (P < 0.05, Fig. 1d, right), suggesting cell-autonomous regulation. We have previously shown that the antiobesity properties of GCGR agonism are mediated through 2 downstream pathways: farnesoid X receptor (FXR) and fibroblast growth factor 21 (FGF21) (4,17). Robust GCGR stimulation of hepatic Lepr mRNA expression is maintained in both of these models (Fig. 1e-f), although some isoforms exhibit slightly, but significantly altered patterns. Lepr-a and Lepr-c were slightly dampened in Fgf21∆liver IUB288-treated mice (P < 0.05, Fig. 1e), whereas Lepr-a was upregulated in Fxr∆liver IUB288-treated mice (P < 0.01, Fig. 1f). Together, these data suggest that GCGR agonism increases Lepr expression, independent of body weight and sex in vivo and in vitro.
Figure 1.
Glucagon receptor (GCGR) agonism increases liver leptin receptor mRNA expression. (a) Liver Lepr expression in diet-induced obese (DIO) male control and GcgrΔliver mice (n = 4-7). (b) Body weight and (c) liver Lepr isoform expression in lean 9- to 10-week-old male mice (n = 4) following an acute (4-hour) or chronic (4-day) IUB288 treatment. Liver Lepr isoform expression in (d, left) females (n = 5) and (d, right) primary hepatocytes (n = 3). Liver Lepr isoform expression in (e) DIO male control and (f) Fgf21Δliver (n = 5-7) or FxrΔliver (n =3-8) mice. Data are represented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared with respective vehicle controls. #P <0.05 compared between genotypes.
Hepatic LEPR alters liver triglyceride homeostasis
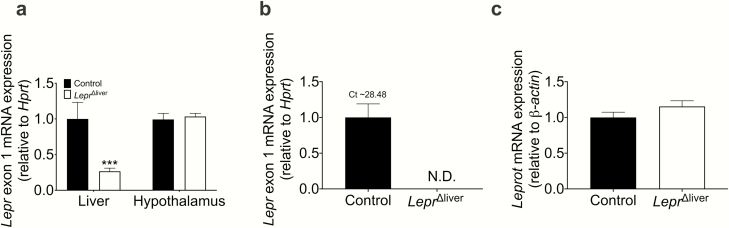
LEPR and GCGR are both potent regulators of hepatic triglyceride homeostasis (4,9,13,17,21). To evaluate the role of hepatic LEPR downstream of GCGR signaling in the regulation of hepatic triglyceride homeostasis, we used mice deficient for hepatic leptin receptors (LeprΔliver). Lepr gene recombination averaged 80% efficiency in liver and did not alter Lepr mRNA expression in hypothalamus (Fig. 2a). Importantly, Lepr mRNA expression was completely ablated in primary hepatocytes isolated from LeprΔliver mice (Fig. 2b). Thus, the remaining 20% expression is likely the result of amplification of Lepr in other liver cell types (e.g., Kupffer cells or sinusoidal endothelial cells (22)). Lepr also shares the promoter region and the first 2 5′ untranslated exons with leptin receptor overlapping transcript (Leprot) (23). Cre-mediated recombination of exon 1 in our model did not alter hepatic Leprot expression (Fig. 2c), suggesting this knockout is specific to Lepr alone.
Figure 2.
Lepr knockout validation. (a) Lepr exon 1 mRNA analysis in liver and hypothalamus in male chow-fed control and LeprΔliver mice (n = 4-9). (b) Lepr exon 1 and (c) Leptin receptor overlapping transcript (Leprot) mRNA expression in primary hepatocytes from male control and LeprΔliver mice (n = 3).
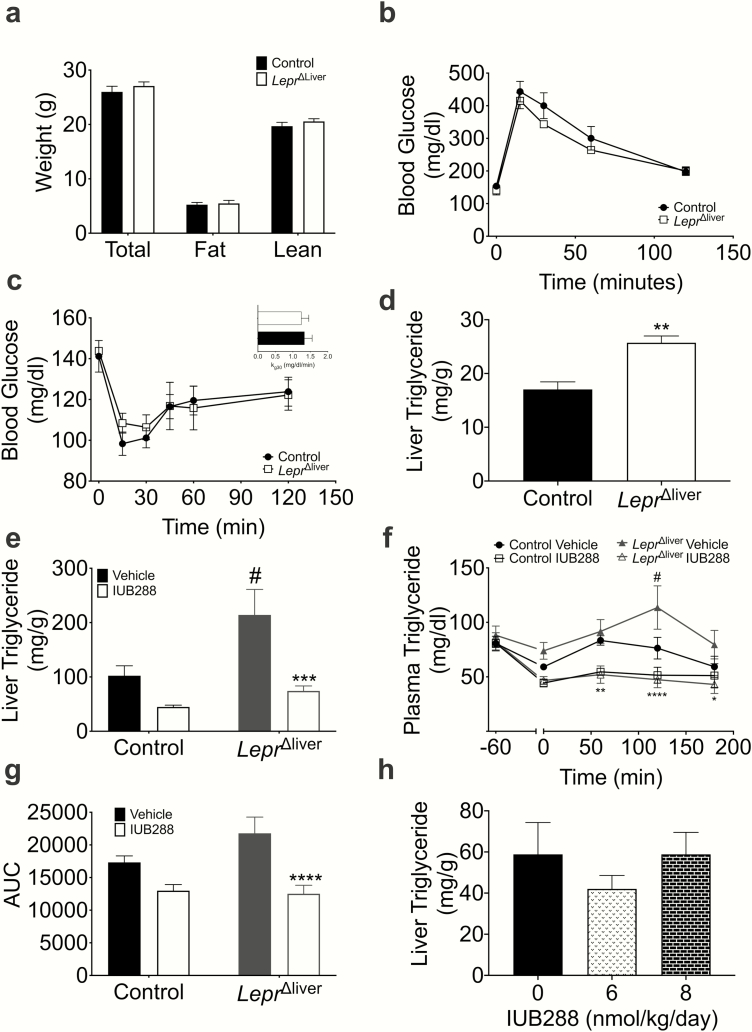
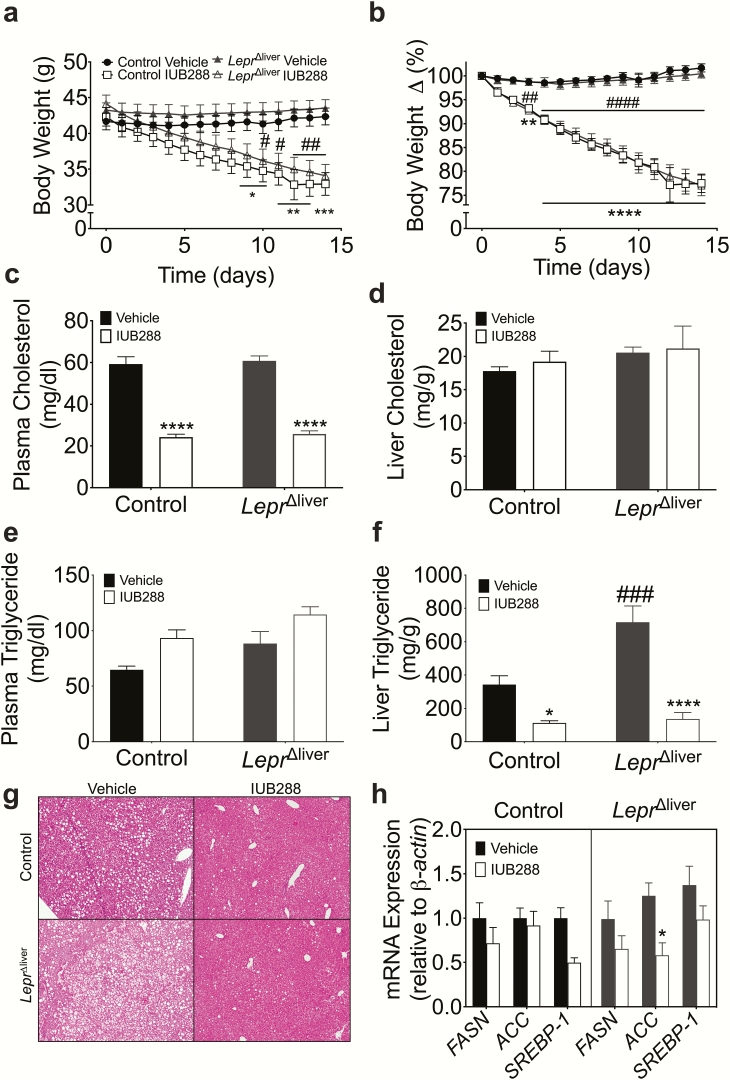
Chow-fed, LeprΔliver mice displayed similar total body weight and composition compared with littermate controls (Fig. 3a). Likewise, we observed no genotypic differences in glucose or insulin tolerance (Fig. 3b-c). Consistent with previous reports, chow-fed, LeprΔliver mice exhibit an increase in liver triglycerides compared with littermate controls in both early (8- to 10-week-old mice, Fig. 3d) and later (24- to 32-weeks old, Fig. 3e, bars 1 and 3) adulthood. Alterations in liver lipid homeostasis were also associated with mild defects in whole body lipid homeostasis. LeprΔliver mice displayed no significant difference in fasting plasma triglycerides (TG) (Fig. 3f), but exhibited a slight intolerance to an oral lipid challenge in comparison to control mice, specifically at the 120-minute time point (P < 0.05) (Fig. 3f). This intolerance, however, did not significantly alter total triglyceride excursion, analyzed via area under the curve (Fig. 3g). Conversely, IUB288 improves whole-body lipid tolerance following a 60-minute pretreatment and decreases liver triglycerides after chronic (14-day) treatment (4). Thus, we expected that loss of hepatic leptin signaling would result in a blunted effect of IUB288 on lipid metabolism. However, we found that IUB288 treatment improved liver TG (10 nmol/kg/d 4-day treatment) and lipid tolerance (Fig. 3e-f) in both LeprΔliver and control mice compared with vehicle-treated mice. IUB288 did not decrease liver TG at lower doses of IUB288 (6 or 8nmol/kg/d 4-day treatment; Fig. 3h), suggesting that 10 nmol/kg/d IUB288 is the minimal dose necessary to decrease liver TG. These findings demonstrate that although pharmacological GCGR signaling potently drives Lepr expression, this induction, and even hepatic LEPR itself, is not necessary for GCGR agonism-mediated improvements in TG homeostasis during normal chow-feeding.
Figure 3.
Hepatic LEPR alters liver triglyceride homeostasis. (a) Body composition, (b) glucose (6-hour fast, 2 g/kg glucose), and (c) insulin (5-hour fast, 0.5 U/kg insulin) tolerance tests, and (c, inset) kg30 in 8- to 10-week-old male mice (n = 6). (d) Liver triglycerides in chow-fed male 8- to 10-week-old mice (n = 3-4) and (e) 24- to 32-week-old mice following 4 days of vehicle or glucagon receptor agonism (10 nmol/kg/d IUB288) (n = 11-15). (f) Oral lipid tolerance test and (g) area under the curve with IUB288. Pretreatment of IUB288 at t = -60 and corn oil oral gavage t = 0 (n = 8-9). (h) Liver triglycerides with 4-day dose response (6 nmol and 8 nmol/kg/d) IUB288 in chow-fed 21- to 28-week-old male mice (n = 5-6). Data are represented as mean ± SEM. *P < 0.05, **P < 0.01, ****P < 0.0001 compared with respective genotypic control. #P < 0.05 compared with control vehicle animals. ND, no data.
Hepatic Lepr contributions to diet-induced obesity and lipid homeostasis
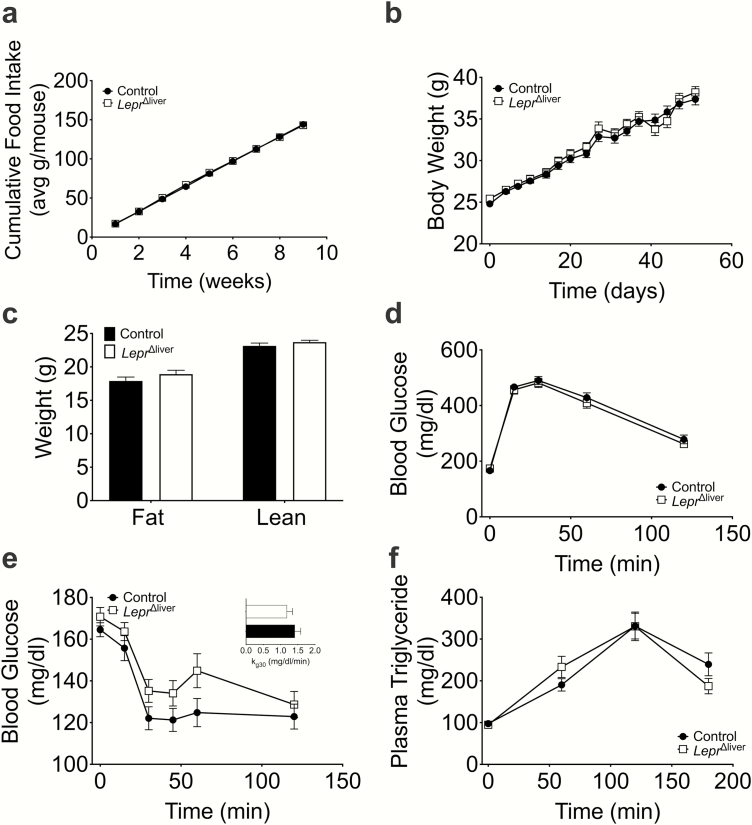
The metabolic insult of high-fat feeding exerts profound dysregulation of whole-body metabolism, including defects in energy balance, glucose, and lipid homeostasis. To assess the role of hepatic LEPR in the adaptation to this insult, we exposed control and LeprΔliver mice to an HFD known to induce obesity and hepatic steatosis (4). Similar to our observations in chow-fed mice, LeprΔliver mice were characterized by a similar level of food intake, body weight, and body composition compared with high-fat fed control mice (Fig. 4a-c). Consistent with the proportionate obesity levels, control and LeprΔliver mice displayed comparable glucose, insulin, and lipid tolerance (Fig. 4d-f). Together, these data suggest that the hepatic leptin receptor does not contribute to HFD-induced metabolic intolerance.
Figure 4.
Diet-induced obesity in LeprΔliver mice. (a) Food intake, (b) body weight, and (c) body composition in high fat-fed mice. (d) Glucose (6-hour fast, 1.5 g/kg glucose), (e) insulin (5-hour fast, 0.75 U/kg insulin), (e, inset) kg30, and (f) oral lipid tolerance tests in diet-induced obese control and LeprΔliver male mice (n = 22-27). Data are represented as mean ± SEM.
Hepatic Lepr is dispensable for glucagon-mediated reversal of hepatic steatosis
Following 12 weeks of HFD, mice were weight matched to receive vehicle or IUB288 treatment for 14 days. Consistent with our previous observations (4,17,24,25), this chronic administration of IUB288 in DIO control mice results in considerable body weight reduction (Fig. 5a-b). As expected, LeprΔliver mice also lost a significant amount of body weight in comparison to their respective vehicle controls (Fig. 5a-b); however, the weight loss was genotype-independent. This potent antiobesity effect was associated with a considerable suppression of plasma cholesterol in both control and LeprΔliver mice (Fig. 5c), with no differences in liver cholesterol (Fig. 5d). Conversely, we observed a mild increase in plasma TG resulting from both genotype (2-way ANOVA; P < 0.01) and treatment (2-way ANOVA; P < 0.01), but no post hoc differences within genotype/treatment groups (Fig. 5e). Intriguingly, although high-fat feeding induced steatosis in both genotypes compared with chow-feeding, DIO LeprΔliver mice displayed considerably more liver triglycerides than control animals (P < 0.001, Fig. 5f-g). However, this steatosis was accompanied by a profound reduction in liver triglycerides in IUB288-treated control and LeprΔliver mice compared with vehicle-treated mice (Fig. 5f-g). Consistent with a reduction in liver triglycerides, chronic IUB288 treatment decreases fatty acid synthesis gene expression (Fig. 5h).
Figure 5.
GCGR agonism in LeprΔliver mice. (a) Body weight and (b) body percent change in control and LeprΔliver male mice treated for 14 days with IUB288 (10 nmol/kg/d) (n = 9-13). (c) Plasma cholesterol, (d) liver cholesterol, (e) plasma triglycerides, (f) liver triglycerides, (g) hematoxylin and eosin liver slices, and (h) fatty acid synthesis genes following treatment. Data are represented as mean ± SEM. *P < 0.05, **P < 0.01, ****P < 0.0001 compared with respective genotypic controls. ###P < 0.001 compared between genotypes.
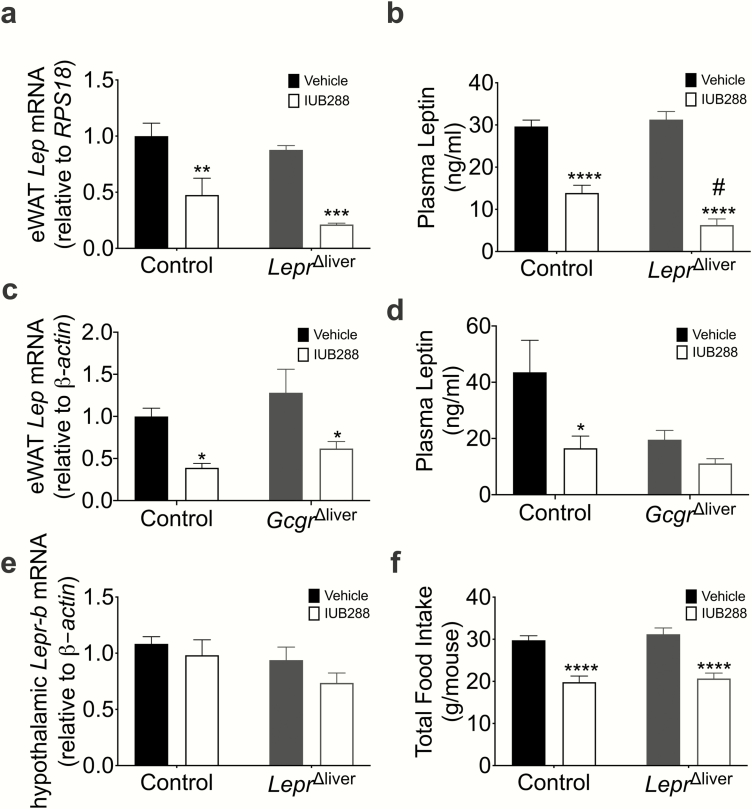
Although we were focused on liver LEPR effects, we acknowledge the potential compensatory upregulation of the ligand (i.e., leptin) or the receptor in other tissues (e.g., hypothalamus) that this genetic ablation may induce. To interrogate potential compensation via leptin levels, we assessed epididymal white adipose tissue (eWAT) leptin expression and plasma leptin levels. IUB288 treatment decreased eWAT leptin expression (P < 0.01) (Fig. 6a), and this suppression remained consistent in IUB288 treated LeprΔliver mice (P < 0.001). Plasma leptin was similarly reduced in IUB288-treated mice (Fig. 6b); however, plasma leptin in IUB288-treated LeprΔliver mice was significantly lower than control, IUB288-treated mice (P < 0.05) (Fig. 6b). This down-regulation appeared to be a direct effect of IUB288, rather than a reduction in body weight because leptin expression remained consistently suppressed in Gcgr∆liver mice, which are resistant to IUB288’s antiobesity properties (4) (Fig. 6c-d). In addition, hypothalamic Lepr-b expression was unchanged (Fig. 6e) and food intake was similarly reduced in control and LeprΔliver-treated mice (Fig. 6f), suggesting compensatory hypothalamic leptin signaling is unlikely. Together, these data demonstrate that although both glucagon and leptin regulate liver lipid metabolism, the beneficial effects of GCGR agonism on liver triglycerides are independent of the hepatic LEPR.
Figure 6.
Leptin profile in LeprΔliver mice. Epididymal white adipose leptin expression and (a-b) plasma leptin in LeprΔliver (n = 4-13) and (c-d) GcgrΔliver (n = 4-5) diet-induced obese mice following 14 days of IUB288 treatment. (e) Hypothalamic Lepr-b expression (n = 8-11) and (f) total food intake (n = 8-13). Data are represented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared with respective genotypic control. #P < 0.05 compared between genotypes. Abbreviation: eWAT, epididymal white adipose tissue.
Discussion
Emerging evidence has elucidated novel roles for GCGR signaling as a crucial regulator of energy balance, glucose, and lipid metabolism (3,4,15,17,24,25). Initial attempts to reverse hyperglycemia by antagonizing this pathway resulted in an unexpected dyslipidemia, which slowed enthusiasm for attenuating glucagon action as a therapeutic approach (26,27). Conversely, we have shown that pharmacological GCGR agonism enhances insulin action (15), stimulates energy expenditure, suppresses food intake, and reduces dyslipidemia (4,17). Moreover, we observed a potent antisteatotic effect following pharmacological GCGR stimulation in mice (4). Thus, an important and emerging question revolves around the identification of downstream mechanisms mediating GCGR regulation of liver lipids. In this study, we investigated the unexpected regulation of hepatic Lepr expression downstream of GCGR agonism.
GCGR signaling regulates hepatic Lepr mRNA expression
We previously identified FGF21 and FXR as downstream targets of hepatic GCGR signaling (4). Here we have identified another target: hepatic Lepr expression. Previous reports of Lepr regulation are limited to its ligand, leptin, and food deprivation (28). We found that pharmacological GCGR activation via the long-acting glucagon analog, IUB288, stimulates liver Lepr expression independent of body weight, sex, or diet. Lepr is alternatively spliced into multiple isoforms (20), yet we observed similar up-regulation of all 5 isoforms suggesting that GCGR-specific induction is driven by a common promoter element. Importantly, pharmacological GCGR-stimulated induction of Lepr mRNA was lost in the hepatic GCGR-depleted (Gcgr∆liver) mice and this stimulation remained consistent in isolated primary hepatocytes, suggesting this occurs in a cell-autonomous manner. Converse to our observations in mice, glucagon was recently reported to upregulate hepatic Leptin transcripts AI and AII in goldfish, while simultaneously downregulating hepatic Lepr expression (29). With regard to previously characterized GCGR transcription targets, IUB288-stimulated Lepr expression of isoforms a and c are altered in mice deficient for hepatic FXR or FGF21, suggesting that hepatic FXR and FGF21 may play a minor role in pharmacological GCGR-stimulated Lepr expression. However, Lepr stimulation in Fxr∆liver and Fgf21∆liver mice are still quite robust, and thus the biological relevance of these alterations is likely to be subtle.
Leptin is expressed in a circadian manner (30) and it is likely that Lepr expression is also time-of-day dependent. With this in mind, our studies in lean mice were consistently conducted with injection at Zeitgeber time (ZT) 6 (hours after lights on) and expression assessed at ZT 10. Using this paradigm, we identified increased Lepr expression 4 hours after a single IUB288 injection. Although a focus of future studies, we have yet to examine if this regulation remains consistent at other times of day. Our chronic, pharmacological GCGR-stimulation studies assessed gene expression at ZT 5 and suggest that at least in a chronic state, Lepr induction remains high, despite possible time-of-day variations.
LEPR regulation of liver and whole-body lipid homeostasis
Since the discovery of the obese mutant (ob/ob) mouse in 1950 (31), the study of leptin and LEPR have been a predominant area of research to uncover regulatory aspects of energy balance. Mice deficient for leptin (ob/ob) or leptin receptor signaling (db/db) exhibit severe obesity, characterized by reduced energy expenditure, hyperphagia, and dyslipidemia (32). This dyslipidemia is marked by elevated cholesterol (33) and fatty acids (34). Further, loss of leptin or leptin receptors results in ectopic fat accumulation in the liver, driven by an increase in de novo lipogenesis and a decrease in mitochondrial fatty acid oxidation (35).
Central LEPRs have been implicated in leptin’s effects on food intake and energy expenditure; however, the roles of peripherally expressed LEPR are less defined. Contrary to the systemic energy imbalances seen with the ob/ob or db/db mice, hepatocyte-specific deletion of leptin receptors results in an inconspicuous phenotype. With the first publication of this model, Cohen et al. (8) reported no alterations in body weight, body composition, or liver triglycerides with hepatic-specific deletion of all LEPR isoforms. Thus, they concluded that the hepatic steatosis phenotype seen in db/db mice was independent of hepatic LEPR. Distinct from the Cohen study (8), Huynh et al. (10,36), using hepatocyte-specific removal of the leptin signaling domain, reported elevated liver TG and cholesterol. These data suggest that the Lepr signaling isoform (b) could be important for upregulating lipid metabolism, whereas other isoform(s) may counteract this effect. Furthermore, additional studies support liver-specific leptin modulation of FAOx and decreases in liver and plasma triglycerides (11). Together, some of these data suggest a role for hepatic LEPR-signaling in lipid homeostasis.
To test the hypothesis that hepatic LEPRs are necessary for glucagon’s reversal of hepatic steatosis, we used Cre lox recombination to generate mice lacking all hepatic Lepr isoforms. This was accomplished via excision of exon 1, which encodes the signal sequence necessary for proper translocation of LEPR to the cellular membrane. Similar to the work of Cohen et al. (37) and Huynh et al. (9), we found the loss of all hepatic Lepr isoforms does not influence body weight or body composition, whether on chow or challenged with an HFD. However, despite using the same floxed model as Cohen et al. (37), we observed increases in liver triglycerides on chow diet alone, which was exacerbated with age or HFD. These observed differences may arise from differences in the albumin-Cre strain. Intriguingly these data are consistent with those of Huynh et al. (9), who also observed Lepr-dependent hepatic steatosis. Together, these data suggest that hepatic LEPR signaling is a crucial regulator of liver triglyceride homeostasis and warrants further investigation.
Hypothalamic regulation of energy homeostasis is altered in obesity and emerging therapies aim to target central regulation of energy balance, including lipid metabolism. Specifically, the melanocortin system has received much attention because this signaling pathway regulates feeding behavior, energy expenditure, glucose homeostasis, and autonomic flow (38). Leptin is an endogenous ligand activating the melanocortin pathway (39) and thus can regulate peripheral lipid metabolism through a central mechanism. Studies have uncovered that the level of melanocortin activity regulates peripheral lipid metabolism independent of changes in food intake and body weight (40). Manipulation of this pathway, via pharmacological melanocortin receptor blockade increases triglyceride content in eWAT and liver (40,41), highlighting the importance of this central pathway in peripheral lipid homeostasis.
Central leptin signaling can also induce liver FAOx in an AMPK-dependent manner (42). However, in our studies, removal of hepatic Lepr did not alter hypothalamic Lepr mRNA expression, suggesting that the phenotype is not a result of compensatory central leptin signaling. Conversely, IUB288-treated LeprΔliver mice displayed lower plasma leptin levels compared with IUB288-treated controls. Although the mechanism of this decrease is likely associated with direct eWAT GCGR activation, it may also suggest an increase in central leptin sensitivity. Central leptin signaling suppresses food intake (43), which we used as an indirect measure of leptin sensitivity. However, we observed no differences in food intake between control and LeprΔliver IUB288-treated mice, suggesting that hypothalamic leptin sensitivity is unaltered. Because food intake is an indirect endpoint, future studies will aim to address the direct effects of centrally regulated decreases in liver triglycerides in this Lepr model (via vagotomy).
GCGR regulation of liver and whole-body lipid metabolism
In addition to its well-characterized role in glucose homeostasis, glucagon is also a key regulator of whole-body and liver lipid metabolism. Starting in the 1960s, studies have implicated glucagon in decreasing plasma cholesterol (44,45), plasma triglycerides (5,45,46), and total esterified fatty acids (47). Studies using exogenous glucagon treatments and GCGR-deficient rodent models have uncovered glucagon’s role in decreasing liver triglyceride production (5,48) and decreasing hepatic lipoprotein synthesis, while concomitantly increasing hepatic fatty acid oxidation (4,5,49). Pharmacological GCGR activation increases hepatic Lepr expression, and we hypothesized that this regulation was an important mediator for the antisteatotic properties of IUB288. Although we have provided data to suggest that hepatic LEPR is not necessary for GCGR-mediated reductions in liver triglycerides following pharmacological activation, the physiological relevance of this regulation remains unclear. Leptin also regulates inflammation (50) and glucose homeostasis via crosstalk with the insulin-signaling pathway (51). Although beyond of the scope of this paper, it is possible that GCGR-stimulated Lepr expression may be important for either of these endpoints. A final caveat to these studies is the use of the GCGR agonist, IUB288, rather than the native glucagon peptide. To this point, we have published 3 independent studies confirming that the biological actions of glucagon are faithfully replicated by IUB288, including increasing hepatic FAOx (4,15,17). Therefore, although this remains an important caveat to our studies, we expect that there is little potential for divergence of biological effects between our agonist and the native peptide.
Conclusion
We report that hepatic GCGR activation increases hepatic leptin receptor expression, acutely and chronically, and is independent of sex or body weight. Although both glucagon and leptin have been implicated in regulating liver fatty acid oxidation to decrease liver triglycerides, LEPR are not required for GCGR regulation of liver triglycerides following pharmacological activation.
Glossary
Abbreviations
- DIO
diet-induced obese
- eWAT
epididymal white adipose tissue
- FAOx
fatty acid oxidation
- FGF
fibroblast growth factor
- FXR
farnesoid X receptor
- GCGR
glucagon receptor
- HFD
high-fat diet
- NAFLD
nonalcoholic fatty liver disease
- TG
triglyceride
- ZT
Zeitgeber time
Acknowledgments
Financial Support: The project described was supported by Award Number P30DK079626 from the National Institute of Diabetes and Digestive and Kidney Diseases, as well as National Institutes of Health grants 1R01DK112934 (to K.M.H.), R01DK111483 (to C.S.H.), and American Diabetes Association 1-16-JDF-044 (to C.S.H.). Trainee support was provided by the National Heart, Lung, and Blood Institute training grant (T32HL105349).
Author Contributions: S.N. and K.M.H. were responsible for study conception and design, data analyses and interpretation, and drafting the article; T.K., S.N., and J.P.A. generated experimental data; B.F., R.D., and C.S.H. advised study concept and critical revision of the article. K.M.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
References
- 1. Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28(1):155–161. [DOI] [PubMed] [Google Scholar]
- 2. Rasouli N MB, Elbein SC, Kern PA. Ectopic fat accumulation and metabolic syndrome. Diabetes Obes Metab. 2007;9(1):1–10. [DOI] [PubMed] [Google Scholar]
- 3. Habegger KM, Heppner KM, Geary N, Bartness TJ, DiMarchi R, Tschöp MH. The metabolic actions of glucagon revisited. Nat Rev Endocrinol. 2010;6(12):689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim T, Nason S, Holleman C, Pepin M, Wilson L, Berryhill TF, Wende AR, Steele C, Young ME, Barnes S, Drucker DJ, Finan B, DiMarchi R, Perez-Tilve D, Tschöp M, Habegger KM. Glucagon receptor signaling regulates energy metabolism via hepatic farnesoid X receptor and fibroblast growth factor 21. Diabetes. 2018;67(9):1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Longuet C, Sinclair EM, Maida A, Baggio LL, Maziarz M, Charron MJ, Drucker DJ. The glucagon receptor is required for the adaptive metabolic response to fasting. Cell Metab. 2008;8(5):359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. [DOI] [PubMed] [Google Scholar]
- 7. Park HK, Ahima RS. Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metabolism. 2015;64(1):24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, Mombaerts P, Friedman JM. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest. 2001;108(8):1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huynh FK, Neumann UH, Wang Y, Rodrigues B, Kieffer TJ, Covey SD. A role for hepatic leptin signaling in lipid metabolism via altered very low density lipoprotein composition and liver lipase activity in mice. Hepatology. 2013;57(2):543–554. [DOI] [PubMed] [Google Scholar]
- 10. Huynh FK, Levi J, Denroche HC, Gray SL, Voshol PJ, Neumann UH, Speck M, Chua SC, Covey SD, Kieffer TJ. Disruption of hepatic leptin signaling protects mice from age- and diet-related glucose intolerance. Diabetes. 2010;59(12):3032–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang W, Dedousis N, O’Doherty RM. Hepatic steatosis and plasma dyslipidemia induced by a high-sucrose diet are corrected by an acute leptin infusion. J Appl Physiol (1985). 2007;102(6):2260–2265. [DOI] [PubMed] [Google Scholar]
- 12. Brabant G, Müller G, Horn R, Anderwald C, Roden M, Nave H. Hepatic leptin signaling in obesity. Faseb J. 2005;19(8):1048–1050. [DOI] [PubMed] [Google Scholar]
- 13. Lee Y, Wang MY, Kakuma T, Wang ZW, Babcock E, McCorkle K, Higa M, Zhou YT, Unger RH. Liporegulation in diet-induced obesity. The antisteatotic role of hyperleptinemia. J Biol Chem. 2001;276(8):5629–5635. [DOI] [PubMed] [Google Scholar]
- 14. Huang W, Dedousis N, Bhatt BA, O’Doherty RM. Impaired activation of phosphatidylinositol 3-kinase by leptin is a novel mechanism of hepatic leptin resistance in diet-induced obesity. J Biol Chem. 2004;279(21):21695–21700. [DOI] [PubMed] [Google Scholar]
- 15. Kim T, Holleman CL, Nason S, Arble DM, Ottaway N, Chabenne J, Loyd C, Kim JA, Sandoval D, Drucker DJ, DiMarchi R, Perez-Tilve D, Habegger KM. Hepatic Glucagon receptor signaling enhances insulin-stimulated glucose disposal in rodents. Diabetes. 2018;67(11):2157–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Lartigue G, Ronveaux CC, Raybould HE. Deletion of leptin signaling in vagal afferent neurons results in hyperphagia and obesity. Mol Metab. 2014;3(6):595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Habegger KM, Stemmer K, Cheng C, Müller TD, Heppner KM, Ottaway N, Holland J, Hembree JL, Smiley D, Gelfanov V, Krishna R, Arafat AM, Konkar A, Belli S, Kapps M, Woods SC, Hofmann SM, D’Alessio D, Pfluger PT, Perez-Tilve D, Seeley RJ, Konishi M, Itoh N, Kharitonenkov A, Spranger J, DiMarchi RD, Tschöp MH. Fibroblast growth factor 21 mediates specific glucagon actions. Diabetes. 2013;62(5):1453–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37(8):911–917. [DOI] [PubMed] [Google Scholar]
- 19. Loyd C, Magrisso IJ, Haas M, Balusu S, Krishna R, Itoh N, Sandoval DA, Perez-Tilve D, Obici S, Habegger KM. Fibroblast growth factor 21 is required for beneficial effects of exercise during chronic high-fat feeding. J Appl Physiol (1985). 2016;121(3):687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379(6566):632–635. [DOI] [PubMed] [Google Scholar]
- 21. Uotani S, Abe T, Yamaguchi Y. Leptin activates AMP-activated protein kinase in hepatic cells via a JAK2-dependent pathway. Biochem Biophys Res Commun. 2006;351(1):171–175. [DOI] [PubMed] [Google Scholar]
- 22. Ikejima K, Lang T, Zhang YJ, Yamashina S, Honda H, Yoshikawa M, Hirose M, Enomoto N, Kitamura T, Takei Y, Sato N. Expression of leptin receptors in hepatic sinusoidal cells. Comp Hepatol. 2004;3 Suppl 1:S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bailleul B, Akerblom I, Strosberg AD. The leptin receptor promoter controls expression of a second distinct protein. Nucleic Acids Res. 1997;25(14):2752–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Finan B, Yang B, Ottaway N, Smiley DL, Ma T, Clemmensen C, Chabenne J, Zhang L, Habegger KM, Fischer K, Campbell JE, Sandoval D, Seeley RJ, Bleicher K, Uhles S, Riboulet W, Funk J, Hertel C, Belli S, Sebokova E, Conde-Knape K, Konkar A, Drucker DJ, Gelfanov V, Pfluger PT, Müller TD, Perez-Tilve D, DiMarchi RD, Tschöp MH. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat Med. 2015;21(1):27–36. [DOI] [PubMed] [Google Scholar]
- 25. Finan B, Clemmensen C, Zhu Z, Stemmer K, Gauthier K, Müller L, De Angelis M, Moreth K, Neff F, Perez-Tilve D, Fischer K, Lutter D, Sánchez-Garrido MA, Liu P, Tuckermann J, Malehmir M, Healy ME, Weber A, Heikenwalder M, Jastroch M, Kleinert M, Jall S, Brandt S, Flamant F, Schramm KW, Biebermann H, Döring Y, Weber C, Habegger KM, Keuper M, Gelfanov V, Liu F, Köhrle J, Rozman J, Fuchs H, Gailus-Durner V, Hrabě de Angelis M, Hofmann SM, Yang B, Tschöp MH, DiMarchi R, Müller TD. Chemical hybridization of glucagon and thyroid hormone optimizes therapeutic impact for metabolic disease. Cell. 2016;167(3):843–857.e14. [DOI] [PubMed] [Google Scholar]
- 26. Campbell JE, Drucker DJ. Islet α cells and glucagon–critical regulators of energy homeostasis. Nat Rev Endocrinol. 2015;11(6):329–338. [DOI] [PubMed] [Google Scholar]
- 27. Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest. 2012;122(1):4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cohen P, Yang G, Yu X, Soukas AA, Wolfish CS, Friedman JM, Li C. Induction of leptin receptor expression in the liver by leptin and food deprivation. J Biol Chem. 2005;280(11):10034–10039. [DOI] [PubMed] [Google Scholar]
- 29. Yan AF, Chen T, Chen S, Tang DS, Liu F, Jiang X, Huang W, Ren CH, Hu CQ. Signal transduction mechanism for glucagon-induced leptin gene expression in goldfish liver. Int J Biol Sci. 2016;12(12):1544–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kettner NM, Mayo SA, Hua J, Lee C, Moore DD, Fu L. Circadian dysfunction induces leptin resistance in mice. Cell Metab. 2015;22(3):448–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered. 1950;41(12):317–318. [DOI] [PubMed] [Google Scholar]
- 32. Fève B, Bastard JP. From the conceptual basis to the discovery of leptin. Biochimie. 2012;94(10):2065–2068. [DOI] [PubMed] [Google Scholar]
- 33. Kennedy AJ, Ellacott KL, King VL, Hasty AH. Mouse models of the metabolic syndrome. Dis Model Mech. 2010;3(3-4):156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Giesbertz P, Padberg I, Rein D, Ecker J, Höfle AS, Spanier B, Daniel H. Metabolite profiling in plasma and tissues of ob/ob and db/db mice identifies novel markers of obesity and type 2 diabetes. Diabetologia. 2015;58(9):2133–2143. [DOI] [PubMed] [Google Scholar]
- 35. Perfield JW 2nd, Ortinau LC, Pickering RT, Ruebel ML, Meers GM, Rector RS. Altered hepatic lipid metabolism contributes to nonalcoholic fatty liver disease in leptin-deficient Ob/Ob mice. J Obes. 2013;2013:296537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huynh FK, Neumann UH, Wang Y, Rodrigues B, Kieffer TJ, Covey SD. A role for hepatic leptin signaling in lipid metabolism via altered very low density lipoprotein composition and liver lipase activity in mice. Hepatology. 2013;57(2):543–554. [DOI] [PubMed] [Google Scholar]
- 37. Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, Mombaerts P, Friedman JM. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest. 2001;108(8):1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Krashes MJ, Lowell BB, Garfield AS. Melanocortin-4 receptor-regulated energy homeostasis. Nat Neurosci. 2016;19(2):206–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oswal A, Yeo GS. The leptin melanocortin pathway and the control of body weight: lessons from human and murine genetics. Obes Rev. 2007;8(4):293–306. [DOI] [PubMed] [Google Scholar]
- 40. Nogueiras R, Wiedmer P, Perez-Tilve D, Veyrat-Durebex C, Keogh JM, Sutton GM, Pfluger PT, Castaneda TR, Neschen S, Hofmann SM, Howles PN, Morgan DA, Benoit SC, Szanto I, Schrott B, Schürmann A, Joost HG, Hammond C, Hui DY, Woods SC, Rahmouni K, Butler AA, Farooqi IS, O’Rahilly S, Rohner-Jeanrenaud F, Tschöp MH. The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest. 2007;117(11):3475–3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wiedmer P, Chaudhary N, Rath M, Yi CX, Ananthakrishnan G, Nogueiras R, Wirth EK, Kirchner H, Schweizer U, Jonas W, Veyrat-Durebex C, Rohner-Jeanrenaud F, Schürmann A, Joost HG, Tschöp MH, Perez-Tilve D. The HPA axis modulates the CNS melanocortin control of liver triacylglyceride metabolism. Physiol Behav. 2012;105(3):791–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miyamoto L, Ebihara K, Kusakabe T, Aotani D, Yamamoto-Kataoka S, Sakai T, Aizawa-Abe M, Yamamoto Y, Fujikura J, Hayashi T, Hosoda K, Nakao K. Leptin activates hepatic 5’-AMP-activated protein kinase through sympathetic nervous system and α1-adrenergic receptor: a potential mechanism for improvement of fatty liver in lipodystrophy by leptin. J Biol Chem. 2012;287(48):40441–40447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest. 1996;98(5):1101–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Caren R, Corbo L. Glucagon and cholesterol metabolism. Metabolism. 1960;9:938–945. [PubMed] [Google Scholar]
- 45. Guettet C, Rostaqui N, Mathé D, Lecuyer B, Navarro N, Jacotot B. Effect of chronic glucagon administration on lipoprotein composition in normally fed, fasted and cholesterol-fed rats. Lipids. 1991;26(6):451–458. [DOI] [PubMed] [Google Scholar]
- 46. Guettet C, Rostaqui N, Navarro N, Lecuyer B, Mathe D. Effect of chronic glucagon administration on the metabolism of triacylglycerol-rich lipoproteins in rats fed a high sucrose diet. J Nutr. 1991;121(1):24–30. [DOI] [PubMed] [Google Scholar]
- 47. Caren R, Corbo L. Transfer of plasma lipid to platelets by action of glucagon. Metabolism. 1970;19(8):598–607. [DOI] [PubMed] [Google Scholar]
- 48. Penhos JC, Wu CH, Daunas J, Reitman M, Levine R. Effect of glucagon on the metabolism of lipids and on urea formation by the perfused rat liver. Diabetes. 1966;15(10):740–748. [DOI] [PubMed] [Google Scholar]
- 49. Keller U, Shulman G. Effect of glucagon on hepatic fatty acid oxidation and ketogenesis in conscious dogs. Am J Physiol. 1979;237(2):E121–E129. [DOI] [PubMed] [Google Scholar]
- 50. Iikuni N, Lam QL, Lu L, Matarese G, La Cava A. Leptin and Inflammation. Curr Immunol Rev. 2008;4(2):70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brabant G, Müller G, Horn R, Anderwald C, Roden M, Nave H. Hepatic leptin signaling in obesity. Faseb J. 2005;19(8):1048–1050. [DOI] [PubMed] [Google Scholar]