Abstract
To limit excessive glucocorticoid secretion following hypothalamic-pituitary-adrenal (HPA) axis stimulation, circulating glucocorticoids inhibit corticotropin-releasing hormone (CRH) expression in paraventricular nucleus (PVN) neurons. As HPA function differs between sexes and depends on circulating estradiol (E2) levels in females, we investigated sex/estrous stage-dependent glucocorticoid regulation of PVN Crh. Using NanoString nCounter technology, we first demonstrated that adrenalectomized (ADX’d) diestrous female (low E2), but not male or proestrous female (high E2), mice exhibited a robust decrease in PVN CRH mRNA following 2-day treatment with the glucocorticoid receptor (GR) agonist RU28362. Immunohistochemical analysis of PVN CRH neurons in Crh-IRES-Cre;Ai14 mice, where TdTomato fluorescence permanently tags CRH-expressing neurons, showed similarly abundant co-expression of GR-immunoreactivity in males, diestrous females, and proestrous females. However, we identified sex/estrous stage-related glucocorticoid regulation or expression of GR transcriptional coregulators. Out of 17 coregulator genes examined using nCounter multiplex analysis, mRNAs that were decreased by RU28362 in ADX’d mice in a sex/estrous stage-dependent fashion included: GR (males = diestrous females > proestrous females), signal transducer and activator of transcription 3 (STAT3) (males < diestrous = proestrous), and HDAC1 (males < diestrous > proestrous). Steroid receptor coactivator 3 (SRC-3), nuclear corepressor 1 (NCoR1), heterogeneous nuclear ribonucleoprotein U (hnrnpu), CREB binding protein (CBP) and CREB-regulated transcription coactivator 2 (CRTC2) mRNAs were lower in ADX’d diestrous and proestrous females versus males. Additionally, most PVN CRH neurons co-expressed methylated CpG binding protein 2 (MeCP2)-immunoreactivity in diestrous female and male Crh-IRES-Cre;Ai14 mice. Our findings collectively suggest that GR’s sex-dependent regulation of PVN Crh may depend upon differences in the GR transcriptional machinery and an underlying influence of E2 levels in females.
Keywords: corticotropin-releasing hormone (CRH), paraventricular nucleus (PVN), glucocorticoid receptor (GR), adrenalectomy, sex differences
The neuropeptide corticotropin-releasing hormone (CRH) is a critical regulator of the body-wide adaptation to homeostatic challenges. In response to stressors, CRH synthesis dramatically increases in neuroendocrine neurons of the hypothalamic paraventricular nucleus (PVN) that direct the activity of the hypothalamic-pituitary-adrenal (HPA) axis (1, 2). The HPA axis orchestrates physiological and psychological reactions to stress by increasing production of adrenal glucocorticoids (GCs). Acute, stress-related rises in GCs are beneficial, coordinating numerous physiological responses while negatively regulating PVN Crh to reduce their own production (3, 4). However, chronic, unrestrained rises in GCs increase risk for numerous stress-related disorders, partly due to disrupted negative feedback on the HPA axis (4–7).
To reduce Crh expression in PVN neurons and inhibit acute stress responses, GCs can act through either the mineralocorticoid receptor (MR) or the GC receptor (GR) (8). However, the GR is thought to play a more dominant role in limiting PVN Crh expression in times of stress (8). The GR classically behaves as a nuclear receptor and, following ligand binding, translocates from the cytoplasm to the nucleus where it interacts with hormone response elements on DNA to directly decrease Crh transcription in the presence of high GC levels (9, 10). The mechanism underlying the direct downregulation of PVN Crh transcription by the GR is not fully understood. However, one possibility proposed by Sharma et al is that GR activation recruits DNA methyl-transferase 3B (DnMT3b) to increase methylation of the Crh promoter and create binding sites for methylated CpG binding protein 2 (MeCP2) (11). MeCP2 is thought to be a key player in the regulation of Crh, as CRH mRNA is increased in MeCP2-null mice (12) and MeCP2 interacts with the transcriptional repressor histone deacetylase 1 (HDAC1) (11), which also participates in the GC repression of Crh (13). Other work has demonstrated an essential role for steroid receptor coactivator-1 (SRC-1) (14). Moreover, GR signaling has been shown to limit Crh expression by opposing the excitatory influence of brain-derived neurotrophic factor (BDNF), which involves tropomyosin receptor kinase B (TrkB)-cAMP response element binding protein (CREB) signaling (15). Ultimately, GR’s repression of PVN Crh likely occurs through extremely complex interactions among these proposed players, and potentially other transcriptional regulators commonly associated with the GR but uninvestigated in the context of PVN Crh regulation.
Unfortunately, most studies examining the repression of PVN Crh by the GR have been performed in vitro, while in vivo studies have concentrated on male rodents. This is particularly problematic given the known sex differences at all levels of HPA axis activation (16), as well as emerging evidence for sex differences in GC-negative feedback (17, 18). In support of the latter, one study recently reported that GR expression by neurons of the PVN is necessary for negative feedback in male, but not female, mice (17). In addition, we recently demonstrated a more rapid response of PVN Crh expression to the removal of GC negative feedback by adrenalectomy (ADX) in male versus female mice (18). As psychopathologies are often related to altered GC negative feedback on the HPA axis and exhibit a striking sex difference in prevalence, continued investigations of sex differences in the GC regulation of PVN Crh are essential (19).
In the present studies, we explored potential sex differences in the mechanisms of direct GC negative feedback on PVN Crh in adult mice. As fluctuations in circulating estradiol (E2) that occur across the female rodent estrous cycle can influence HPA axis activity (20), we also examined females with low circulating E2 (diestrous) versus females with peak E2 levels (proestrous). We first demonstrated a pronounced sex difference in the involvement of the GR in the downregulation of PVN Crh. Adrenalectomized (ADX’d) diestrous female, but not male, mice exhibited a robust decrease in CRH mRNA following short term GR agonist treatment. However, proestrous ADX’d females did not have decreased CRH mRNA after the same treatment. Co-expression of GRs in PVN CRH neurons was similarly abundant in male, diestrous female, and proestrous female Crh-IRES-Cre;Ai14 mice, where the fluorescent TdTomato protein permanently tags CRH neurons. Thus, we sought to determine whether sex/estrous stage-dependent differences in the GR repression of PVN Crh are accompanied by differences in the gene expression of the GR and/or its known transcriptional coregulators, including DnMT3b, MeCP2, HDAC1, SRC-1, and players along the TrkB-CREB signaling cascade. We also examined other transcriptional regulators commonly associated with the GR but underinvestigated in the context of PVN Crh regulation. Taken together, our findings demonstrate sex differences in the GR transcriptional machinery that may depend on circulating E2 levels in females and help explain the presence of sex differences in GC negative feedback on Crh and HPA activity as a whole.
Methods
Animals
Animals in these studies were housed in the laboratory animal research facility at Colorado State University. They were maintained in a 12-hour light/12-hour dark cycle with lights on starting at 0600 h, and they were provided ad libitum access to food and water. All animal protocols were approved by the Institutional Animal Care and Use Committee at Colorado State University and were performed within National Institutes of Health and Association for Assessment and Accreditation of Laboratory Animal Care International guidelines.
C57BL/6N mice
Adult (2–4 months of age) male and female C57BL/6N mice were obtained from Charles River Laboratories (Wilmington, MA) and allowed to acclimate to the laboratory animal research facility for at least 1 week prior to use in experiments. At the start of experiments, intact C57BL/6N male (n = 5–8/group) and female (n = 5–7/group) mice were bilaterally ADX’d under isoflurane anesthesia and given 0.9% saline as drinking water to maintain osmolarity. Beginning 2 days after surgery, ADX’d animals received 1 subcutaneous (s.c.) injection of the GR agonist RU28362 (0.4 mg/kg) or vehicle (27% hydroxypropyl-β-cyclodextrin; Cyclodextrin Technologies Development, Inc., Alacua, FL) per day between 0900 h and 1000 h for 2 days. This dose of RU28362 is based on prior publications and was selected due to its ability to suppress HPA axis activity (21, 22). RU28362 is also highly selective for the GR, has little or no affinity for the MR (8, 23), and like other synthetic glucocorticoids, likely has a relatively short half-life in plasma (approximately 2–4 hours), but longer-lasting biological effects due to the long-lasting (12–36 hours) interaction of the ligand-receptor complex and ensuing transcriptional changes (24). Two days following the start of treatment, animals were injected with a third and final dose of RU28362 between 0900 and 1000 h and, 4 hours later, were anesthetized with isoflurane and decapitated in less than 2 minutes following the first cage disturbance. This occurred no later than 1400 h (lights on at 0600 h, off at 1800 h). Brains were removed, fresh frozen and stored at −80°C until they were sectioned for PVN microdissection and eventually droplet digital PCR (ddPCR) or N-Counter multiplexing to examine PVN gene expression.
Crh-IRES-Cre;Ai14 mice
Adult (2–4 months of age) male and female Crh-IRES-Cre;Ai14 mice were also used in these studies to enable identification of CRH-expressing neurons without the use of immunohistochemical approaches, which are subject to limitations in peptide detection based upon GC-mediated downregulation of Crh expression. Crh-IRES-Cre;Ai14 mice were generated from our colonies by crossing the B6(Cg)-Crhtml(cre)Zjh/J (Crh-IRES-Cre) (Jackson Laboratories, Bar Harbor, ME; stock #012704, RRID:IMSR_JAX:012704) (25) and B6.Cg-Gt(ROSA)26Sortml4(CAG-TdTomato)Hze/J (Ai14) (Jackson Laboratories; stock #007914, RRID:IMSR_JAX:007914) (26) strains. These original stocks were generated as described previously (27, 28). Genotyping was performed at weaning using an ear punch to identify Crh-IRES-Cre and Ai14 mutants for use in the generation of heterozygous Crh-IRES-Cre;Ai14 offspring as previously reported (29). Adult male and female Crh-IRES-Cre;Ai14 offspring were used to examine co-expression of GR and MeCP2 immunoreactivity (ir) in PVN CRH neurons. These animals have previously been validated for the examination of CRH (29–31) and the expression of CRH-ir matches that of Cre-driven reporter gene expression (29).
Vaginal cytology
For studies comparing females versus males, female C57BL/6N and Crh-IRES-Cre;Ai14 mice were killed on a day of diestrus when circulating levels of E2 are low. A separate cohort of ADX’d vehicle- and RU28362-treated C57BL/6N females were also killed on proestrus to examine the influence of peak E2 levels on PVN gene expression. Cycle day was determined using vaginal cytology according to previously established methods (32). Briefly, each female underwent vaginal lavage daily using a saline (0.9% NaCl) solution, then samples were dried on glass slides and dipped in methylene blue (0.05%) for visualization using light microscopy. For C57BL/6N female mice, estrous cycle stage was monitored daily for roughly 2 weeks prior to ADX to allow for habituation to handling and to enable prediction of estrous stage at time of death.
PVN microdissection and RNA isolation
Frozen brains were sectioned at −16°C into 300-μm-thick sections containing the PVN using a CM3050 S Cryostat (Leica, Wetzlar, Germany). PVN punches were obtained from 2 atlas-matched thick sections per animal using a micropunch fashioned from sharpened heavy wall stainless steel type 304 tubing with an internal diameter of 0.991 ± 0.0381 mm (Small Parts Inc., Miami Lakes, FL). Tissue punches were kept frozen at all times and stored at −80°C until RNA extraction. Total RNA was isolated from PVN punches using the RNeasy mini kit (Qiagen, Germantown, MD) according to the manufacturer’s instructions.
NanoString nCounter technology
RNA concentrations were measured using a Qubit 4 Fluorometer (Invitrogen, Carlsbad, CA). Multiplexed mRNA quantification was performed by the University of Arizona Genetics Core using a NanoString nCounter Custom CodeSet (Seattle, WA), which contained bar-coded hybridization probes against mRNAs for CRH, arginine vasopressin (AVP), period circadian regulator 1 (Per1), GR, and GR coregulators, including DnMT3b, HDAC1, MeCP2, SRC-1, steroid receptor coactivator 2 (SRC-2), steroid receptor coactivator 3 (SRC-3), nuclear corepressor 1 (NCoR1), CREB binding protein (CBP), death domain associated protein (DAXX), heterogeneous nuclear ribonucleoprotein U (hnrnpu), SIN3 transcription regulator family member A (Sin3a), signal transducer and activator of transcription 3 (STAT3), BDNF, CREB, TrkB, and CREB-regulated transcription coactivator 2 (CRTC2). nSolver software was used for gene expression analysis, and, for each gene, transcript count was normalized to the geometric mean expression of 4 housekeeping genes (glyceraldehyde 3-phosphate dehydrogenase, eukaryotic translation elongation factor 2, TATA-box binding protein, and beta-actin). Background expression level in each sample was calculated as the mean plus standard deviation of raw counts for 8 synthetic negative control RNA probes for that sample. Only DnMT3b values were limited by this threshold. Specifically, 2 vehicle-treated males, 2 RU28362-treated males, 3 vehicle-treated diestrous females, 4 RU28362-treated diestrous females, 2 vehicle-treated proestrous females, and 5 RU28362-treated proestrous females had normalized DnMT3b transcript numbers at or below the background threshold. The threshold values in these cases were included only for calculation of approximate means but were not used for further statistical analyses.
Droplet digital (dd)PCR
Total RNA was quantitated using an Epoch Microplate Spectrophotometer and Gen5 v. 1.11 data analysis software (BioTek, Winooski, VT). Complementary DNA (cDNA) was generated using an iScript cDNA synthesis kit (Bio-Rad, Munich, Germany). The ddPCR was then used to measure target cDNA copies as a number of molecules. The ddPCR reaction mix was prepared by adding cDNA to an EvaGreen supermix (10 μl; Bio-Rad, Hercules, CA) combined with forward (1 μl) and reverse (0.4 μl) primers and nuclease-free water up to a total volume of 20 μl. Primers used for CRH were: forward = 5′-ATGCTGCTGGTGGCTCTGTC-3′ and reverse- 5′-GGATCAGAACCGGCTGAGGT-3′. All systems and reagents used for ddPCR were obtained from Bio-Rad (Hercules, CA). Droplet generation, PCR amplification of template molecules in each individual droplet, reading, and quantification of absolute template expression in copies/μl using QuantaSoft software were all performed as detailed previously (18). All template expression values were normalized to the amount of cDNA loaded in each reaction, which was calculated based on cDNA concentrations quantified using the Quant-iT OliGreen ssDNA Assay Kit (Thermo Scientific, Waltham, MA) according to manufacturer’s instructions.
Immunohistochemistry (IHC)
Adult male, diestrous female, and proestrous female Crh-IRES-Cre;Ai14 mice (n = 3–4) were intracardially perfused with ice-cold phosphate-buffered saline (PBS; 0.01M; pH 7.4), followed by 4% paraformaldehyde (PFA) in phosphate buffer (PB, 4°C). Brains were removed from the skull, placed in 4% buffered PFA for 24 hours, and then infiltrated with 30% sucrose as a cryoprotectant. Four series of 35-μm-thick coronal sections were obtained using a Leica CM3050 S cryostat. IHC was performed on free-floating sections. Five 10-minute washes were performed in PBS (0.01M; pH 7.4). Sections were then incubated in blocking solution (4% normal goat serum in PBS) for 1 hour prior to incubation overnight in primary antibody solution: PBS with 0.1% Triton-X 100 (PBST; pH 7.4) (plus 4% normal goat serum for MeCP2 IHC). Primary antibodies used were: previously validated rabbit anti-GR (33) (1:500 dilution; Thermo Fisher Scientific, Waltham, MA, Cat# PA1-511A, RRID:AB_2236340) (34) and rabbit anti-MeCP2 (1:1600; Cell Signaling Tech, Danvers, MA, Cat# 3456, RRID:AB_2143849 (35)). Sections were then washed 3 × 10 minutes in PBST before secondary antibody incubation in the serum blocking solution. For MeCP2 IHC, Alexa Flour-488-conjugated goat anti-rabbit (1:1000; Thermo Fisher Scientific, Waltham, MA, Cat# A11008, RRID:AB_143165 (36)) was used as the secondary antibody and incubation occurred for 2 hours. Following 3 × 10-minute washes in PBS, MeCP2 IHC-treated sections were mounted and coverslipped using Vectashield H-1000 mounting medium for fluorescence (Vector Laboratories, Burlingame, CA). For GR IHC, biotinylated goat anti-rabbit (Vector Laboratories, Burlingame, CA, Cat# BA-1000, RRID:AB_2313606) (37) was used as the secondary antibody and incubation occurred for 1 hour. Sections were washed 3 × 10 minutes in PBST then incubated for 1 hour in a tertiary antibody solution, containing Alexa Fluor-488-conjugated streptavidin (1:200; Thermo Fisher Scientific, Waltham, MA, Cat#S32354, RRID:AB_2315383) (38) and 4% normal goat serum in PBS. Following 6 × 10-minute washes in PBS, sections were mounted and coverslipped using Vectashield. All wash and antibody incubation steps were performed on a shaker table at room temperature.
We also performed a preadsorption control for the anti-MeCP2 antibody in which the primary antibody was incubated with a 50× molar excess of immunizing peptide for 3 hours at room temperature prior to application to the tissue. The blocking peptide used was human MeCP2 (Prospec, Rehovot, Israel). All other staining steps were completed as described above. Preadsorption of anti-MeCP2 resulted in the total loss of MeCP2-ir. Additionally, the widespread MeCP2 labeling throughout the brain in our studies matches the MeCP2 mRNA expression reported in the Allen in situ hybridization data set (https://mouse.brain-map.org/experiment/show/79904518) (39).
Confocal imaging and colocalization quantification
IHC-treated brain sections containing the PVN were identified using Paxinos and Franklin’s The Mouse Brain in Stereotaxic Coordinates (40), and images for CRH:TdTomato neurons with GR or MeCP2-ir were obtained using a Zeiss 880 laser scanning confocal microscope and a 20× (W Plan-Apochromat 20X/1.0 DIC Vis-ir ∞/0.17) objective. Confocal images were taken of the rostral (Bregma −0.83 mm), middle (Bregma −0.95 mm), or caudal (Bregma −1.07 mm) PVN bilaterally. Z stacks composed of 0.64-μm-thick optical sections, spanning approximately 29μm were created for each image.
Three-dimensional images were rendered from confocal Z-stacks with Imaris v9.1 software (Bitplane Inc, Zurich, Switzerland). CRH:TdTomato positive neurons and GR-ir– or MeCP2-ir–positive neurons were automatically counted using Imaris software (version 9.1) and manually checked. CRH:TdTomato and GR-ir or MeCP2-ir were considered to be colocalized when automatically determined cell centers were within 4.5 μm of each other. Colocalization was confirmed visually, in 3 dimensions. Percentages of CRH:TdTomato neurons containing GR-ir or MeCP2-ir were determined as the number of colocalized CRH:TdTomato neurons divided by the total number of these neurons multiplied by 100.
Statistical methods
All data shown are mean values, and all error bars represent standard error of the mean (SEM). For comparison of CRH:TdTomato and GR-ir or MeCP2-ir colocalization percentages, as well as CRH:TdTomato cells numbers, across the rostral to caudal extent of the PVN in male versus female mice, 2-way repeated measures ANOVAs were performed. All other statistical comparisons were made using 2-way ANOVAs. Fisher’s Least Significant Difference post hoc test was used where appropriate. All statistics were done using the Prism statistical program (version 8.0.1, GraphPad Software, La Jolla, CA) and results were considered statistically significant when P < 0.05. Additionally, all data were analyzed using the Extreme Studentized Deviate method (GraphPad) to detect significant outliers. Only 6 outliers were detected and excluded from further analyses.
Results
Sex- and estrous stage-dependent effects of GR agonist treatment on PVN Crh expression
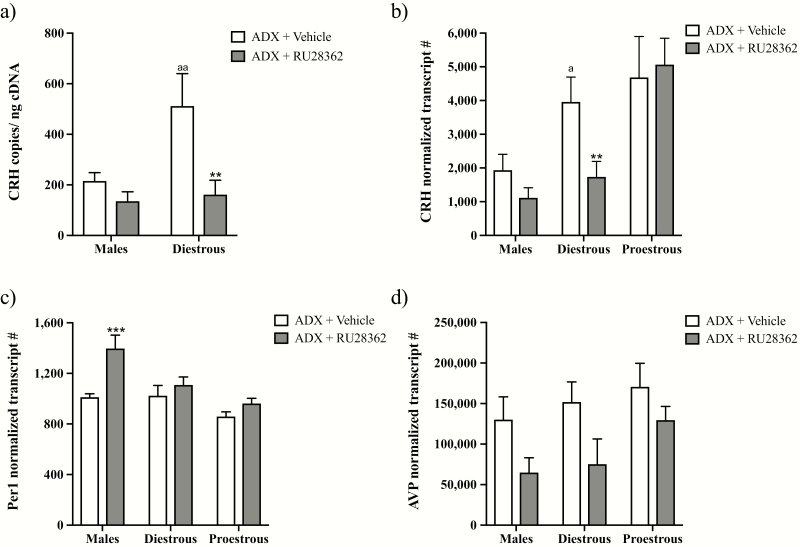
To determine if the GR similarly regulates PVN Crh expression in male and diestrous female mice, we examined the effect of treatment with the GR agonist RU28362 on PVN CRH mRNA levels in ADX’d mice of both sexes using ddPCR (Fig. 1a). Two-way ANOVA showed significant main effects of RU28362 (F(1,23) = 12.27; P < 0.01), sex (F(1,23) = 6.87; P < 0.05) and a sex × RU28362 interaction (F(1,23) = 4.83; P < 0.05) on PVN CRH mRNA. Post hoc analyses revealed that RU28362 treatment did not significantly alter CRH mRNA levels in ADX’d males. However, it significantly decreased diestrous female CRH mRNA levels (P < 0.01), largely due to significantly higher baseline CRH mRNA levels present in ADX’d females versus males (P < 0.01).
Figure 1.
Effect of glucocorticoid receptor (GR) agonist treatment on expression of glucocorticoid-regulated genes within the hypothalamic paraventricular nucleus (PVN). Changes in PVN gene expression in adrenalectomized (ADX’d) male, diestrous female, or proestrous female mice treated with RU28362 (0.4 mg/kg s.c.) or vehicle are shown. Panels (a) and (b) show CRH mRNA levels in males and females measured using (a) droplet digital (dd)PCR or (b) nCounter technology. Panel (c) shows period circadian regulator 1 (Per1) mRNA levels, and panel (d) shows arginine vasopressin (AVP) mRNA levels measured using nCounter technology. For the nCounter technology, mRNA levels were normalized to the geometric mean expression of 4 housekeeping genes. For ddPCR, mRNA levels were normalized to the amount of input cDNA. Each bar represents the mean normalized gene expression ± SEM of n = 4–8 mice. Two-way ANOVAs revealed main effects of RU28362 for CRH (a); (P < 0.01), Per1 (P < 0.001), and AVP (P < 0.01) mRNAs. Main effects of sex/estrous stage were found for CRH (a): (P < 0.05); (b): (P < 0.01) and Per1 (P < 0.001), and interaction effects were found for CRH (a); (P < 0.05) and Per 1 (P = 0.0531). Error bar symbols: a = P < 0.05 and aa = P < 0.01 for diestrous females versus males of the same experimental group. ** = P < 0.01 for RU28362- versus vehicle-treated diestrous female mice. *** = P < 0.001 for RU28362- versus vehicle-treated male mice.
Using nCounter technology, we not only validated our ddPCR findings, but we also examined females on proestrus, as well as diestrus, to determine whether estrous cycle stage influences GR-mediated negative feedback on PVN Crh (Fig. 1b). Two-way ANOVA revealed a significant effect of sex/estrous stage (F(2,29) = 8.5; P < 0.01), but not of RU28362 treatment or interaction. Because we originally hypothesized that the Crh response to GR agonist treatment would depend on sex and estrous stage, we also performed post hoc analyses to examine the effects of RU28362 in ADX’d males versus diestrous females, as well as in proestrous females. A 2-way ANOVA for males versus diestrous females exposed significant effects of sex (F(1,17) = 6.27; P < 0.05) and RU28362 (F(1,17) = 8.31; P < 0.05); and a priori comparisons showed significantly higher CRH mRNA levels in vehicle-treated ADX’d diestrous females versus males (P < 0.05), as well as significantly lower (P < 0.01) CRH mRNA following RU28362 exclusively in diestrous females. In ADX’d proestrous females, alternatively, there was no significant decrease in PVN CRH mRNA following RU28362 treatment.
To determine if the absence of a significant effect of RU28362 in ADX’d male and ADX’d proestrous female mice was unique to PVN CRH mRNA, we examined changes in the expression of other GR-regulated genes. Two-way ANOVA revealed a significant effect of RU28362 (F(1,31) = 13.26; P < 0.001) to increase PVN Per1 mRNA in ADX’d mice, as well as a significant effect of sex/estrous stage (F(2,31) = 10.80; P < 0.001; Fig. 1c). There was also a strong trend toward a significant RU28362 by sex/estrous stage interaction (F(2,31) = 3.232; P = 0.0531); therefore, we performed post hoc comparisons to examine the effects of RU28362 within male and female groups. RU28362 administration significantly increased PVN Per1 mRNA in ADX’d males (P < 0.001, Fig. 1c), but not females in either estrous stage. Furthermore, RU28362 decreased AVP mRNA levels in male and female ADX’d subjects (Fig. 1d). Accordingly, a 2-way ANOVA showed a significant effect of RU28362 (F(1,31) = 8.319; P < 0.01), but no significant effects of sex or interaction were found.
GR co-expression in PVN CRH neurons of both sexes
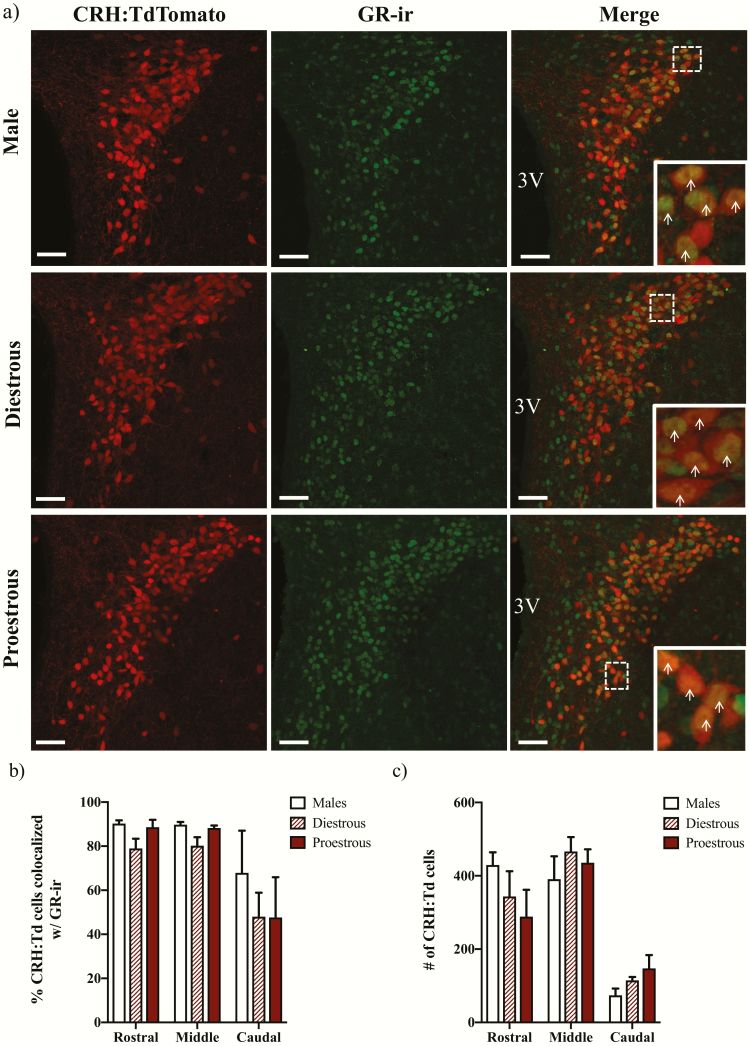
To determine whether differences in GR expression may differentially regulate CRH mRNA in male versus diestrous or proestrous female mice, we next examined the expression of GR-ir in PVN CRH neurons that were marked by the stable expression of a TdTomato fluorophore (CRH:TdTomato; Fig. 2a). Two-way repeated measures ANOVAs (PVN level by sex/estrous stage) revealed significant effects of PVN level, but not of sex or interaction, on both the percentage of CRH:TdTomato neurons colocalized with GR-ir (F(1.132,7.921) = 11.34; P < 0.01) (Fig. 2b) as well as the number of CRH:TdTomato neurons (F(1.172,8.202) = 26.28; P < 0.001) (Fig. 2c).
Figure 2.
Glucocorticoid receptor (GR) expression in corticotropin-releasing hormone (CRH) neurons. Representative photomicrographs of brain sections taken from CRH-IRES-Cre;Ai14 male, diestrous female, and proestrous female mice containing the rostral paraventricular nucleus (PVN; Bregma −0.83 mm) and immunolabeled for GR are shown in (a). The images on the left show CRH:TdTomato neurons in the PVN, the middle panels show GR immunoreactivity (ir), and the right panels show merged images at 26× magnification. Magnified insets highlight co-expression of CRH:TdTomato and GR-ir in the outlined boxes. Arrows indicate CRH:TdTomato neurons that co-express GR-ir. Scale bars: 40 μm. 3V: third ventricle. Panels (b) and (c) show the mean ± SEM percentage of CRH:TdTomato neurons that co-express GR-ir (b) or number of CRH:TdTomato neurons (c) in the PVN of n = 3–4 mice. Repeated measures 2-way ANOVAs showed main effects of PVN level on colocalization percentages (P < 0.01) and on the number of CRH:TdTomato cells (P < 0.001), but not of sex/estrous stage or interaction.
Sex- and estrous stage-dependent effects of GR agonist treatment on GR and coregulator mRNAs
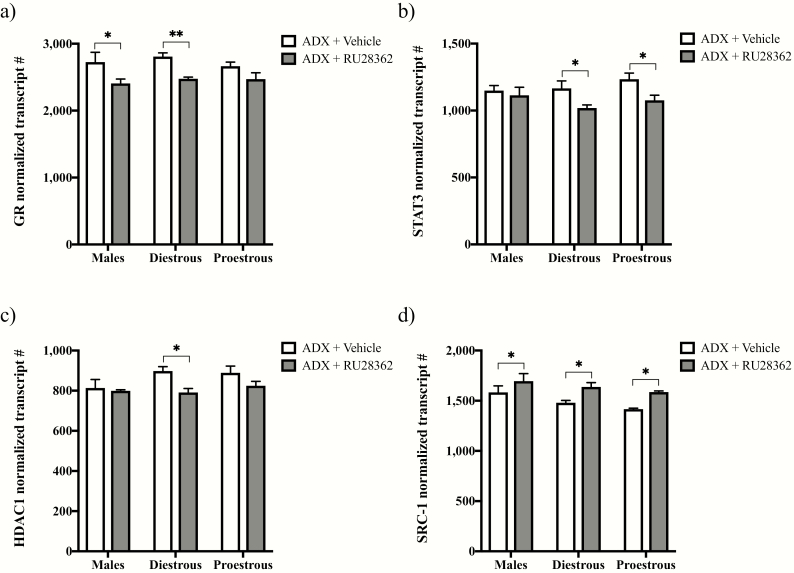
We next examined gene expression changes for the GR and its known coregulators following vehicle or RU28362 treatment of male and diestrous or proestrous female ADX’d mice using nCounter technology (Fig. 3). Two-way ANOVAs revealed significant effects of RU28362, to decrease GR mRNA (F(1,31) = 16.85; P < 0.0001; Fig. 3a), as well as that of its co-repressors STAT3 (F(1,31) = 8.836; P < 0.01; Fig. 3b) and HDAC1 (F(1,30) = 7.203; P < 0.05; Fig. 3c). Although no significant effects of sex/estrous stage or interaction were detected for GR, HDAC1, or STAT3, we performed post hoc analyses based upon our original hypothesis that sex- and/or estrous stage dependent differences exist in the regulation of the GR transcriptional machinery by RU28362. The RU28362 treatment significantly decreased GR mRNA in ADX’d males (P < 0.05) and diestrous females (P < 0.01), but not ADX’d proestrous females (P = 0.0893). STAT3 mRNA levels, alternatively, were decreased following RU28362 treatment in ADX’d diestrous (P < 0.05) and proestrous females (P < 0.05), but not ADX’d males. HDAC1 mRNA levels were significantly decreased after RU28362 exclusively in ADX’d diestrous females (P < 0.05). In contrast to the GR agonist-induced decreases in GR, STAT3, and HDAC1 mRNAs, the RU28362 treatment significantly increased mRNA levels for the GR co-activator SRC-1, regardless of sex or estrous stage (Fig. 3d). Accordingly, a 2-way ANOVA showed significant effects of RU28362 (F(1,30) = 14.63; P < 0.001) and of sex/estrous stage (F(2,30) = 4.364; P < 0.05), but not an interaction effect. Planned comparisons showed significant increases in SRC-1 mRNA levels following RU28362 in all groups (P < 0.05).
Figure 3.
Effect of glucocorticoid receptor (GR) agonist treatment on GR and coregulator gene expression within the paraventricular nucleus (PVN). Changes in PVN mRNAs in adrenalectomized (ADX’d) male, diestrous female, and proestrous female mice treated with RU28362 (0.4 mg/kg s.c.) or vehicle are shown. mRNA levels for GR (a), signal transducer and activator of transcription 3 (STAT3) (b), histone deacetylase 1 (HDAC1) (c), and steroid receptor coactivator 1 (SRC-1) (d) were measured using nCounter technology and normalized to the geometric mean expression of 4 housekeeping genes. Each bar represents mean normalized gene expression ± SEM of n = 5–7 mice. Main effects of RU28362 were found for GR (P < 0.001), STAT3 (P < 0.01), HDAC1 (P < 0.05), and SRC-1 (P < 0.001) mRNA levels by 2-way ANOVAs. A main effect of sex/estrous stage was also found for SRC-1 mRNA (P < 0.05). Planned comparisons: * = P < 0.05 and ** = P < 0.01 for RU28362- versus vehicle-treated ADX’d male, diestrous female, or proestrous female mice.
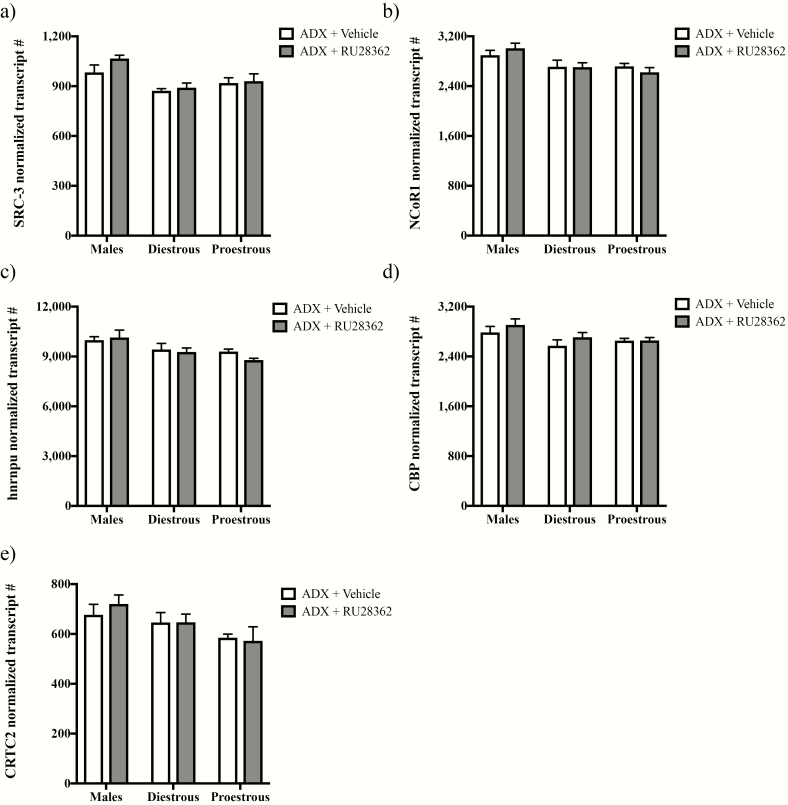
Even though no effects of RU28362 treatment or RU28362 by sex/estrous stage interaction were observed for other GR coregulatory genes examined, we found main effects of sex/estrous stage for SRC-3 (F(2,30) = 7.325; P < 0.01; Fig. 4a), NCoR1 (F(2,31) = 6.806; P < 0.01; Fig. 4b) and hnrnpu (F(2,31) = 6.670; P < 0.01; Fig. 4c) by 2-way ANOVAs. Specifically, SRC-3, NCoR1, and hnrnpu mRNA levels appeared lower in ADX’d females versus males. Table 1 shows other genes associated with GR coregulation that were unaltered by sex/estrous stage, GR agonist treatment, or sex/estrous stage by RU28362 interaction.
Figure 4.
Sex/estrous stage-dependent gene expression of glucocorticoid receptor (GR) coregulators within the paraventricular nucleus (PVN). Changes in PVN mRNAs in adrenalectomized (ADX’d) male, diestrous female, and proestrous female mice treated with RU28362 (0.4 mg/kg s.c.) or vehicle are shown. mRNA levels for steroid receptor coactivator 3 (SRC-3) (a), nuclear corepressor 1 (NCoR1 (b), heterogeneous nuclear ribonucleoprotein U (hnrnpu) (c), cAMP response element binding protein (CREB) binding protein (CBP (d), and CREB regulated transcription coactivator 2 (CRTC2 (e) were measured using nCounter technology and normalized to the geometric mean expression of 4 housekeeping genes. Each bar represents mean normalized gene expression +/- SEM of n = 4–7 mice. (a-e) Only main effects of sex/estrous stage were found by 2-way ANOVAs for SRC-3 (P < 0.01), NCoR1 (P < 0.01), hnrnpu (P < 0.01), CBP (P < 0.05), and CRTC2 (P < 0.05) mRNA levels.
Table 1.
Mean ± SEM Normalized Transcript Numbers in the Paraventricular Nucleus (PVN) of ADX’d Male, Diestrous Female and Proestrous Female Mice Measured Using nCounter Technology.
| Males | Diestrous Females | Proestrous Females | ||||
|---|---|---|---|---|---|---|
| ADX + Vehicle (n = 5) | ADX + RU28362 (n = 6) | ADX + Vehicle (n = 5) | ADX + RU28362 (n = 7) | ADX + Vehicle (n = 7) | ADX + RU28362 (n = 7) | |
| GR repression | ||||||
| DAXX | 192.6 ± 3.9 | 195.8 ± 8.6 | 208.0 ± 7.8 | 198.9 ± 7.1 | 200.1 ± 5.5 | 198.8 ± 5.3 |
| DnMT3b | 38.4 ± 3.0a(2) | 33.6 ± 1.6a(2) | 33.9 ± 1.7a(3) | 32.5 ± 1.6a(4) | 33.4 ± 1.5a(2) | 31.4 ± 1.2a(5) |
| MeCP2 | 1172.7 ± 39.4 | 1244.3 ± 29.2 | 1130.1 ± 14.3 | 1208.2 ± 65.7 | 1114.0 ± 15.7 | 1197.9 ± 81.8 |
| Sin3a | 1131.0 ± 41.4 | 1119.7 ± 76.6 | 1145.0 ± 39.4 | 1112.8 ± 24.0 | 1161.1 ± 46.6 | 1046.8 ± 45.1 |
| SRC-2 | 3217.8 ± 142.8 | 3689.9 ± 108.8 | 3530.3 ± 91.6 | 3497.9 ± 139.0 | 916.9 ± 33.7 | 928.0 ± 46.0 |
| BDNF activation | ||||||
| BDNF | 125.5 ± 2.9 | 133.4 ± 10.0 | 119.1 ± 8.2 | 124.5 ± 9.8 | 123.2 ± 11.6 | 115.1 ± 6.3 |
| CREB | 987.7 ± 31.7 | 967.8 ± 21.5 | 930.1 ± 30.9 | 950.7 ± 15.7 | 923.4 ± 21.6 | 939.9 ± 31.8 |
| TrkB | 12938.9 ± 246.2 | 13389.8 ± 652.7 | 13090.2 ± 387.9 | 13131.0 ± 293.7 | 11734.9 ± 344.8 | 12896.7 ± 254.8 |
Abbreviations: ADX, adrenalectomy; BDNF, brain-derived neurotrophic factor; CREB, cAMP response element binding protein; DAXX, death domain associated protein; DnMT3b, DNA methyl-transferase 3B; GR, glucocorticoid receptor; MeCP2, methylated CpG binding protein 2; Sin3a, SIN3 transcription regulator family member A; SRC-2, steroid receptor coactivator 2; TrkB, tropomyosin receptor kinase B.
a(#) Indicates that mean values represent the specified number of background threshold values.
Because GR can downregulate PVN Crh expression by inhibiting Crh induction by BDNF (15), we also included genes associated with the BDNF regulation of Crh in the nCounter multiplex analysis study. No significant effects of RU28362 or RU28362 by sex/estrous stage interaction were identified for any of the genes examined (Table 1). However, we observed significant main effects of sex/estrous stage for CBP mRNA (F(2,31) = 3.993; P < 0.05; Fig. 4d) and CRTC2 mRNA (F(2,31) = 4.458; P < 0.05; Fig. 4e), where levels appeared lower in ADX’d female versus male mice.
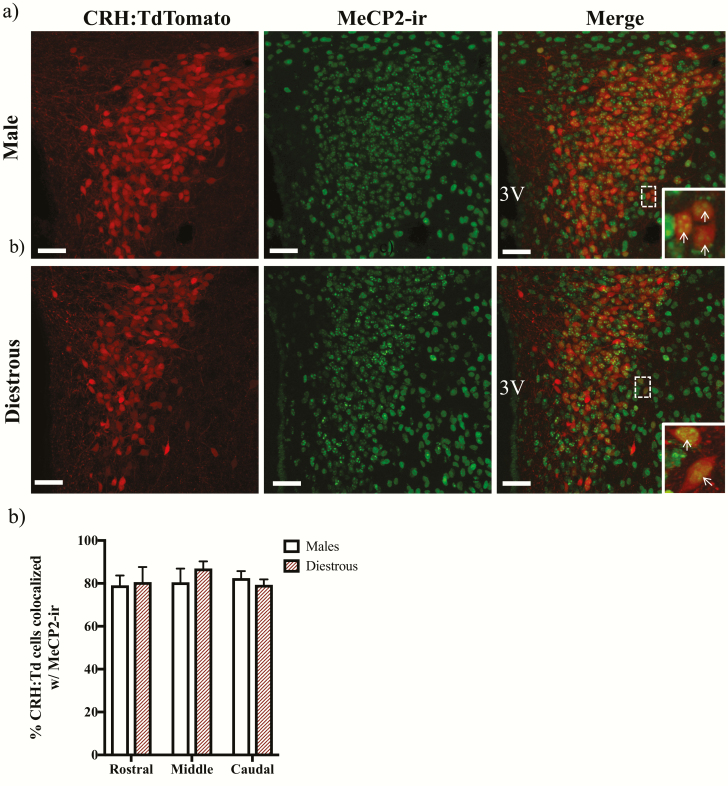
MeCP2 co-expression in PVN CRH neurons of both sexes
We identified abundant co-expression of MeCP2-ir in CRH neurons throughout the rostral-caudal extent of the PVN of male (79.9% ± 5.5%) and diestrous female (84.8% ± 4.3%) mice that stably express the TdTomato fluorophore in CRH neurons (CRH:TdTomato) (Fig. 5). No significant effects of sex, PVN level, or interaction were found by a 2-way ANOVA with repeated measures across PVN level (Fig. 5b).
Figure 5.
Methylated CpG binding protein 2 (MeCP2) expression in corticotropin-releasing hormone (CRH) neurons. Panel (a) shows representative photomicrographs of brain sections taken from Crh-IRES-Cre;Ai14 male and diestrous female mice containing the middle paraventricular nucleus (PVN; Bregma −0.95 mm) and immunolabeled for MeCP2. The images on the left show CRH:TdTomato neurons in the PVN, the middle panels show MeCP2 immunoreactivity (ir), and the right panels show merged images at 26X magnification. Magnified insets highlight co-expression of CRH:TdTomato and MeCP2-ir in the outlined boxes. Arrows indicate CRH:TdTomato neurons that co-express MeCP2-ir. Scale bars: 40 um. 3V: third ventricle. Panel (b) shows the mean ± SEM percent of CRH:TdTomato neurons that co-express MeCP2-ir throughout the PVN of n = 3 mice.
Discussion
Despite the sex-related risks for psychopathologies and their correlation with sex differences in HPA axis activity (19), few studies have examined GC regulation of the HPA axis in both sexes. In the present studies, we demonstrated a striking sex difference in GR-mediated negative feedback on PVN Crh expression. Whereas ADX’d male mice exhibited a small, nonstatistically significant decrease in CRH mRNA after 2 days of GR agonist treatment, ADX’d diestrous females showed a more robust and highly significant decrease. When examined on proestrus, however, ADX’d females showed an absence of a response of CRH mRNA. Interestingly, this sex/estrous stage-dependent difference was unique to Crh expression, as GR agonist administration decreased AVP mRNA regardless of sex or estrous stage. Although PVN CRH neurons co-expressed GRs abundantly in males, diestrous females, and proestrous females, the mRNAs for GR, STAT3, and HDAC1 exhibited sex/estrous stage-dependent regulation by GR agonist treatment. SRC-3, NCoR1, hnrnpu, CBP, and CRTC2 mRNAs also all were lower in diestrous and proestrous females than in males. Conversely, diestrous females and males had similar PVN MeCP2 mRNA levels, as well as similar percentages of MeCP2 co-expressing PVN CRH neurons. Thus, taken together, our findings suggest that sex differences in GR-mediated negative feedback on PVN Crh may depend on sex differences in the GR transcriptional machinery and an underlying influence of circulating E2 levels in females.
Previous studies have only begun to examine sex differences in GR-mediated negative feedback on PVN Crh, even though sex differences have been found at all levels of the HPA axis (16). In a recent study, Solomon et al demonstrated that PVN-selective GR knockdown results in increased adrenocorticotropic hormone (ACTH) and corticosterone responses to acute stress in male mice, but not females, suggesting that PVN GR may only be necessary for feedback inhibition of the stress-activated HPA axis in males (17). However, Solomon et al used randomly-cycling female mice, which leaves open the possibility that fluctuating E2 levels alter the GR involvement (17). Our finding that ADX’d females exhibited a robust decrease in PVN CRH mRNA on diestrus that was absent on proestrus supports this possibility. Notably, Solomon et al also did not observe effects of PVN GR depletion on basal or stress-induced CRH and AVP mRNA in either sex (17). As RU28362 has previously been shown to decrease PVN CRH mRNA in ADX’d male rats (41), corresponding to our present findings in mice, this discrepancy likely reflects differences in experimental approaches. The GR knockdown technique employed by Solomon et al involved crossing transgenic mice such that GR was depleted in the PVN throughout development and into adulthood (17). Thus, consequences of GR depletion during key developmental windows that greatly influence the activity of the HPA axis in adulthood (42, 43) could help explain inconsistencies between our findings and those of Solomon et al (17).
Nonetheless, we observed a robust decrease in PVN CRH mRNA in ADX’d diestrous female mice following GR agonist treatment that was absent in males. The response in female mice was largely driven by greater expression of CRH mRNA in vehicle-treated ADX’d diestrous female versus male mice, suggesting that the response to ADX may be sex-dependent. In a recent study, we demonstrated that male mice exhibit a more rapid response to ADX than do diestrous female mice, as increased CRH mRNA levels measured by in situ hybridization were found 2 days after ADX in males, but not diestrous females (18). Although CRH mRNA was significantly increased 4 days after ADX in both sexes, no statistical comparisons between sexes could be made, as males and females were run in separate in situ hybridization assays (18). Consequently, the possibility remains that females have a greater increase in Crh expression after ADX than do males, which enables their robust Crh response to GR agonist treatment. While ADX’d male mice showed a small decrease in CRH mRNA following short-term RU28362 administration in the present studies, it did not reach statistical significance, unlike the findings of a previous study in male rats (41). Such discrepancies between our findings and those of previous studies (41) could be related to the use of mice versus rats or to insufficiencies in the timing and/or dose of RU28362 in our studies. However, we observed a significant effect of RU28362 that led to increased expression of Per1, a gene previously shown to be stimulated by GCs, in males (44, 45). We also observed a reduction in AVP mRNA following GR agonist treatment of ADX’d males and females. Thus, the dose of RU28362 was sufficient for regulation of Avp, but not Crh, in both sexes, suggesting that males may ultimately rely more on GR regulation of PVN Avp versus Crh to limit neuroendocrine stress responses. Accordingly, in male rats, PVN AVP mRNA levels have been suggested to be more sensitive to GC negative feedback than are levels of CRH mRNA (46).
Interestingly, the sex difference in GR regulation of PVN Crh may only hold true when females have low E2 levels. As in males, no significant effect of GR agonist treatment on PVN CRH mRNA was observed in ADX’d proestrous females. That females can have varying sensitivity to Crh regulation by the GR depending on their estrous cycle stage is well supported by evidence that the estrous cycle alters the activity of the HPA axis (16). Increasing E2 concentrations throughout the estrous cycle have been shown to be directly proportional to the basal and stress-induced activity of the HPA axis. Accordingly, female rodents on diestrus (low E2) have low basal GC secretion and a relatively quick on-off response to stressors, whereas females on proestrus (high E2) have elevated basal GC levels and a prolonged GC response to stressors (20, 47, 48). Importantly, elevations in HPA activity are greatest on proestrous morning, when E2 levels are peaking, but elevations in progesterone have not yet occurred, as progesterone appears to inhibit E2’s effects on HPA output (20). Because we examined female mice no later than 1400 h on proestrus, it is likely that the changes in the Crh response to RU28362 we observed on proestrus versus diestrus are due to E2 rather than progesterone. Supporting this possibility, E2 has been shown to inhibit GC negative feedback on PVN CRH in female rats (49).
Notably, androgens may also influence GR-mediated negative feedback on PVN Crh to drive sex differences, as we previously demonstrated an effect for the potent androgen, dihydrotestosterone, to facilitate the CRH mRNA response 2 days after ADX in males (18). Although it does not appear that gonadal hormones influence the response of PVN CRH mRNA 4 days after ADX, as both male and female mice exhibited increased CRH mRNA following ADX alone or ADX paired with gonadectomy (18), the possibility remains that androgens influence the effects of the GR on Crh expression. The upregulation of Per1 mRNA in the PVN of ADX’d males, but not diestrous or proestrous females, we observed after RU28362 treatment supports the possibility that the presence of androgens in males amplifies some of the actions of the GR. Moreover, this effect of androgens, which may contribute to sex differences in GR sensitivity, also appears to be gene-specific, since there was no sex effect on the ability of RU28362 to decrease AVP mRNA expression.
Despite the sex and estrous stage-related response of Crh to GR regulation, male, diestrous female, and proestrous female mice all exhibited co-expression of the GR in almost all CRH neurons within the rostral and middle regions of the PVN. In the mouse, the anterior two-thirds of the PVN contains the vast majority of neuroendocrine neurons that regulate the HPA axis, whereas the posterior one-third mostly contains preautonomic neurons that coordinate autonomic outflow (50). The greater percentage of CRH:TdTomato and GR-ir colocalization we observed in the rostral/ middle versus caudal PVN may, therefore, be related to the predominant role the rostral/ middle PVN plays in regulating the HPA axis. However, the lesser colocalization in the caudal PVN may also simply reflect the presence of significantly fewer CRH:TdTomato neurons within the region.
Although abundant GR co-expression in PVN CRH neurons has previously been reported in male rats (51), subjects were ADX’d in that study to overcome the technical hurdle of capturing the entire population of CRH neurons using immunohistochemical approaches. CRH is readily downregulated by endogenous GCs, making this difficult without such manipulation (52). In the present studies, we utilized CRH-IRES-Cre;Ai14 mice, which provide the unique advantage of permanently labeling all CRH expressing neurons without the need for nonphysiological manipulations, such as ADX or colchicine for complete visualization. In these mice, the Crh promoter drives expression of Cre recombinase, which in turn, induces the expression of a TdTomato reporter through the removal of a loxp-STOP-loxp site upstream of the TdTomato reporter. TdTomato expression is then driven by a CAG promoter and permanently marks CRH neurons. Importantly, TdTomato fluorescence highly overlaps with CRH protein (29, 30) in the PVN but not with other neuropeptide neurons characteristic of the region (29). TdTomato also reliably targets stress-responsive neuronal populations in the PVN (29). Thus, our GR and CRH colocalization findings are novel in the use of the CRH-IRES-Cre;Ai14 mouse model, as well as in their incorporation of both male and female subjects.
While the localization of GRs in CRH neurons supports their involvement in a direct mechanism of Crh regulation in both sexes, further studies are necessary to determine if ADX and/or GR agonist treatment influence GR/ CRH colocalization in a sex and/or estrous stage-dependent fashion, as GRs are also subject to downregulation by GCs (46, 53). Then again, GR gene expression was similar in the PVN of vehicle-treated ADX’d male, diestrous female, and proestrous female mice. The lack of sex/estrous stage-related differences in both CRH/ GR colocalization and GR mRNA levels of vehicle-treated mice in the present studies is perhaps surprising, given evidence of lower GC binding in the hypothalamus of randomly-cycling female versus male rats (54) and that E2 treatment can downregulate GR in female rat hypothalamus (55). These previous studies suggest that females have fewer hypothalamic corticosteroid receptors. However, they used randomly cycling females (54) or ovariectomized females treated with E2 (55) and did not measure GR in the PVN selectively. While it is possible that sex/estrous stage-related changes in GR gene expression exist within PVN CRH neurons, a more quantitative and anatomically selective approach is necessary to assess this. An alternative possibility is that sex differences are present in GR function, rather than numbers, reflecting other sex/estrous stage-reliant molecular mechanisms downstream of receptor binding.
In this regard, we also examined sex/estrous-dependent effects of RU28362 treatment on the expression of a putative GR-DnMT3b-MeCP2-HDAC1 repressor complex, as well as SRC-1, both of which have been proposed to negatively regulate Crh (11, 14). Specifically, whereas SRC-1 mRNA levels were significantly increased following RU28362 treatment in ADX’d mice, irrespective of sex and estrous stage, GR and HDAC1 mRNAs exhibited sex/estrous stage- dependent regulation by RU28362. GR mRNA was decreased in males and diestrous females, but not proestrous females, supporting the possibility that increasing E2 levels inhibit GR’s autologous regulation, as previously reported in the hypothalamus (55), to influence sex differences in GR-mediated negative feedback on PVN Crh. HDAC1 mRNA, alternatively, was decreased by RU28362 exclusively in diestrous females, again supporting a role for increasing E2 to inhibit the function of an HDAC1-containing repressor complex and thereby influence sex-based Crh expression. Further studies are necessary to determine if and/or how decreases in GR and HDAC1 expression correspond to changes in Crh expression. Nonetheless, such changes support the possibility of sex/estrous stage-dependent involvement of a GR-DnMT3b-MeCP2-HDAC1 repressor complex in GR-mediated negative feedback on PVN Crh. Our findings similarly support the sex-dependent involvement of STAT3, a coregulator previously associated with GR regulation outside the context of PVN Crh (56), as STAT3 mRNA levels were decreased by RU28362 in both ADX’d diestrous and proestrous females, but not in males. However, an important caveat to consider here and throughout this discussion is that we cannot restrict our gene expression findings to only CRH neurons in the PVN. Hence, any of the changes in the gene expression of GR-associated transcriptional regulators could ultimately alter the expression of other GR-regulated genes, or other genes outside of GR regulation altogether.
Although we did not observe an effect of RU28362 on PVN MeCP2 mRNA in ADX’d subjects, we also chose to examine expression of MeCP2 in CRH neurons of the male and female mouse PVN, under the assumption that MeCP2 can be found in many neuropeptide neurons of the PVN. In addition to its role in a putative GR-DnMT3b-MeCP2-HDAC1 repressor complex (11), MeCP2 has been implicated in Crh transcriptional repression in vivo (12). Accordingly, increased PVN CRH mRNA and decreased MeCP2 binding to the Crh promoter have been demonstrated in mice expressing a mutated MeCP2 protein (12). Thus, MeCP2 is thought to be central to Crh downregulation, and further assessment of its sex-dependent involvement in our studies was warranted. We found that most PVN CRH:TdTomato neurons co-expressed MeCP2-ir in male and diestrous female Crh-IRES-Cre;Ai14 mice, supporting its involvement in Crh regulation of mice of both sexes. Future studies using single cell analysis to quantify MeCP2 expression in PVN CRH neurons and examine sex/estrous stage-dependent differences will further enhance our understanding of its potentially sex-dependent involvement in the GR’s regulation of Crh.
Sex-dependent expression of GR transcriptional coregulators, including SRC-3, NCoR1, hnrnpu, CBP, and CRTC2 mRNAs, was also found in ADX’d subjects, supporting the possibility of their sex-dependent involvement in GR-mediated negative feedback on PVN Crh. Although roles for SRC-3 and hnrnpu in the GR regulation of PVN Crh have not yet been identified, both proteins have been associated with GR’s regulation of other genes (57, 58). A role for CBP and CRTC2, on the other hand, has been better established. Jeanneteau et al proposed a mechanism of negative feedback in which GR signaling opposes the excitatory influence of BDNF-TrkB-CREB signaling on PVN Crh (15). Thus, CBP and CRTC2, as components of the excitatory TrkB-CREB signaling cascade, may be decreased in ADX’d females to shift the balance in Crh regulation toward the GC influence. This may contribute to the greater GR inhibition of Crh evident in diestrous females versus males. A role for NCoR1 has also been identified in the repression of CREB-mediated Crh induction in a cell model system for Crh repression by GCs (59), and it may be decreased in ADX’d females to further alter the balance between CREB and GC signaling at the Crh promoter.
Taken together, our findings have demonstrated a pronounced sex difference in GR-mediated negative feedback on PVN Crh that may rely on sex differences in the GR transcriptional machinery, as well as an underlying influence of estrous cycle stage in female mice. Using a novel NanoString nCounter technology, we observed significant changes in mRNAs for the GR and its transcriptional coregulators that might have otherwise been undetectable. This technology offers the unique advantage of quantifying expression of multiple mRNAs with great accuracy and sensitivity directly from total RNA without the need for reverse transcription and amplification, steps which can add variance (60). Although we cannot definitively say that the sex-, estrous cycle- and GC- dependent changes in the transcriptional regulator mRNAs we identified influence GR’s repression of Crh, these findings will help guide future investigations of the sex-dependent mechanisms of GC negative feedback on the HPA axis.
Acknowledgments
Financial Support: This research was supported by the National Institutes of Health (Grant R01-DK105826 to R.J.H. and R01-MH082900 to R.M.U.)
Glossary
Abbreviations
- ADX
adrenalectomy
- ADX’d
adrenalectomized
- AVP
arginine vasopressin
- BDNF
brain-derived neurotrophic factor
- CRB
CREB binding protein
- CREB
cAMP response element binding protein
- CRH
corticotropin-releasing hormone
- CRTC2
CREB-regulated transcription coactivator 2
- DAXX
death domain associated protein
- DnMT3b
DNA methyl-transferase 3B
- E2
estradiol
- GC
glucocorticoid
- GR
glucocorticoid receptor
- HDAC1
histone deacetylase 1
- hnrnpu
heterogeneous nuclear ribonucleoprotein U
- HPA
hypothalamic-pituitary-adrenal
- IHC
immunohistochemistry
- ir
immunoreactivity
- MeCP2
methylated CpG binding protein 2
- MR
mineralocorticoid receptor
- NCoR1
nuclear corepressor 1
- PBS
phosphate-buffered saline
- PBST
PBS with 0.1% Triton-X 100
- Per1
period circadian regulator 1
- PVN
paraventricular nucleus
- s.c.
subcutaneous
- SEM
standard error of the mean
- Sin3a
SIN3 transcription regulator family member A
- SRC-1
steroid receptor coactivator 1
- SRC-2
steroid receptor coactivator 2
- SRC-3
steroid receptor coactivator 3
- STAT3
signal transducer and activator of transcription 3
- TrkB
tropomyosin receptor kinase B
Additional Information
Disclosure Summary: The authors have nothing to disclose. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Whitnall MH. Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog Neurobiol. 1993;40(5):573–629. [DOI] [PubMed] [Google Scholar]
- 2. Herman JP, Figueiredo H, Mueller NK, et al. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24(3):151–180. [DOI] [PubMed] [Google Scholar]
- 3. Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984;5(1):25–44. [DOI] [PubMed] [Google Scholar]
- 4. Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55–89. [DOI] [PubMed] [Google Scholar]
- 5. Holsboer F. Stress, hypercortisolism and corticosteroid receptors in depression: implications for therapy. J Affect Disord. 2001;62(1–2):77–91. [DOI] [PubMed] [Google Scholar]
- 6. de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6(6):463–475. [DOI] [PubMed] [Google Scholar]
- 7. Dallman MF, Akana SF, Scribner KA, et al. Stress, feedback and facilitation in the hypothalamo-pituitary-adrenal axis. J Neuroendocrinol. 1992;4(5):517–526. [DOI] [PubMed] [Google Scholar]
- 8. Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117(6):2505–2511. [DOI] [PubMed] [Google Scholar]
- 9. Brink M, Humbel BM, De Kloet ER, Van Driel R. The unliganded glucocorticoid receptor is localized in the nucleus, not in the cytoplasm. Endocrinology. 1992;130(6):3575–3581. [DOI] [PubMed] [Google Scholar]
- 10. De Kloet ER, Vreugdenhil E, Oitzl MS, Joëls M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19(3):269–301. [DOI] [PubMed] [Google Scholar]
- 11. Sharma D, Bhave S, Gregg E, Uht R. Dexamethasone induces a putative repressor complex and chromatin modifications in the CRH promoter. Mol Endocrinol. 2013;27(7):1142–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McGill BE, Bundle SF, Yaylaoglu MB, Carson JP, Thaller C, Zoghbi HY. Enhanced anxiety and stress-induced corticosterone release are associated with increased Crh expression in a mouse model of Rett syndrome. Proc Natl Acad Sci USA. 2006;103(48):18267–18272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miller L, Foradori CD, Lalmansingh AS, Sharma D, Handa RJ, Uht RM. Histone deacetylase 1 (HDAC1) participates in the down-regulation of corticotropin releasing hormone gene (crh) expression. Physiol Behav. 2011;104(2):312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lachize S, Apostolakis EM, van der Laan S, et al. Steroid receptor coactivator-1 is necessary for regulation of corticotropin-releasing hormone by chronic stress and glucocorticoids. Proc Natl Acad Sci USA. 2009;106(19):8038–8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jeanneteau FD, Lambert WM, Ismaili N, et al. BDNF and glucocorticoids regulate corticotrophin-releasing hormone (CRH) homeostasis in the hypothalamus. Proc Natl Acad Sci USA. 2012;109(4):1305–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heck AL, Handa RJ. Sex differences in the hypothalamic-pituitary-adrenal axis’ response to stress: an important role for gonadal hormones. Neuropsychopharmacology. 2019;44(1):45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Solomon MB, Loftspring M, de Kloet AD, et al. Neuroendocrine function after hypothalamic depletion of glucocorticoid receptors in male and female mice. Endocrinology. 2015;156(8):2843–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heck AL, Handa RJ. Androgens drive sex biases in hypothalamic corticotropin-releasing hormone gene expression after adrenalectomy of mice. Endocrinology. 2019;160(7):1757–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bangasser DA, Valentino RJ. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol. 2014;35(3):303–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129(5):2503–2511. [DOI] [PubMed] [Google Scholar]
- 21. Burgess LH, Handa RJ. Chronic estrogen-induced alterations in adrenocorticotropin and corticosterone secretion, and glucocorticoid receptor-mediated functions in female rats. Endocrinology. 1992;131(3):1261–1269. [DOI] [PubMed] [Google Scholar]
- 22. Francis AB, Pace TW, Ginsberg AB, Rubin BA, Spencer RL. Limited brain diffusion of the glucocorticoid receptor agonist RU28362 following i.c.v. administration: implications for i.c.v. drug delivery and glucocorticoid negative feedback in the hypothalamic-pituitary-adrenal axis. Neuroscience. 2006;141(3):1503–1515. [DOI] [PubMed] [Google Scholar]
- 23. De Kloet ER. Brain corticosteroid receptor balance and homeostatic control. Front. Neuroendocrinol. 1990;12:94–165 [DOI] [PubMed] [Google Scholar]
- 24. De Kloet ER, Ortiz Zacarias NV, Meijer OC. Manipulating the brain corticosteroid receptor balance: focus on ligands and modulators. In: Fink G, ed. Stress: Neuroendocrinology and Neurobiology. Cambridge, MA: Academic Press; 2017. [Google Scholar]
- 25. RRID:IMSR_JAX:012704 https://scicrunch.org/resolver/RRID:IMSR_JAX:012704.
- 26. RRID:IMSR_JAX:007914 https://scicrunch.org/resolver/IMSR_JAX:007914.
- 27. Taniguchi H, He M, Wu P, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71(6):995–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Madisen L, Zwingman TA, Sunkin SM, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13(1):133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wamsteeker Cusulin JI, Füzesi T, Watts AG, Bains JS. Characterization of corticotropin-releasing hormone neurons in the paraventricular nucleus of the hypothalamus of Crh-IRES-Cre mutant mice. Plos One. 2013;8(5):e64943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smith JA, Wang L, Hiller H, Taylor CT, de Kloet AD, Krause EG. Acute hypernatremia promotes anxiolysis and attenuates stress-induced activation of the hypothalamic-pituitary-adrenal axis in male mice. Physiol Behav. 2014;136:91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Walker LC, Cornish LC, Lawrence AJ, Campbell EJ. The effect of acute or repeated stress on the corticotropin releasing factor system in the CRH-IRES-Cre mouse: a validation study. Neuropharmacology. 2019;154:96–106. [DOI] [PubMed] [Google Scholar]
- 32. Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80(2):84–97. [DOI] [PubMed] [Google Scholar]
- 33. Weiser MJ, Foradori CD, Handa RJ. Estrogen receptor beta activation prevents glucocorticoid receptor-dependent effects of the central nucleus of the amygdala on behavior and neuroendocrine function. Brain Res. 2010;1336:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. RRID:AB_2236340 https://scicrunch.org/resolver/AB_2236340.
- 35. RRID:AB_2143849 https://scicrunch.org/resolver/AB_2143849.
- 36. RRID:AB_143165 https://scicrunch.org/resolver/AB_143165.
- 37. RRID:AB_2313606 https://scicrunch.org/resolver/AB_2313606.
- 38. RRID:AB_2315383 https://scicrunch.org/resolver/AB_2315383.
- 39. Shen EH, Overly CC, Jones AR. The Allen Human Brain Atlas: comprehensive gene expression mapping of the human brain. Trends Neurosci. 2012;35(12):711–714. [DOI] [PubMed] [Google Scholar]
- 40. Paxinos G, Franklin K.. Paxinos and Franklin’s The Mouse Brain in Stereotaxic Coordinates. Waltham, MA: Academic Press; 2013. [Google Scholar]
- 41. Albeck DS, Hastings NB, McEwen BS. Effects of adrenalectomy and type I or type II glucocorticoid receptor activation on AVP and CRH mRNA in the rat hypothalamus. Brain Res Mol Brain Res. 1994;26(1-2):129–134. [DOI] [PubMed] [Google Scholar]
- 42. Romeo RD. The metamorphosis of adolescent hormonal stress reactivity: a focus on animal models. Front Neuroendocrinol. 2018;49:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Green MR, McCormick CM. Sex and stress steroids in adolescence: gonadal regulation of the hypothalamic-pituitary-adrenal axis in the rat. Gen Comp Endocrinol. 2016;234:110–116. [DOI] [PubMed] [Google Scholar]
- 44. Balsalobre A, Brown SA, Marcacci L, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289(5488):2344–2347. [DOI] [PubMed] [Google Scholar]
- 45. So AY, Bernal TU, Pillsbury ML, Yamamoto KR, Feldman BJ. Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proc Natl Acad Sci USA. 2009;106(41):17582–17587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Makino S, Smith MA, Gold PW. Increased expression of corticotropin-releasing hormone and vasopressin messenger ribonucleic acid (mRNA) in the hypothalamic paraventricular nucleus during repeated stress: association with reduction in glucocorticoid receptor mRNA levels. Endocrinology. 1995;136(8):3299–3309. [DOI] [PubMed] [Google Scholar]
- 47. Herman JP, McKlveen JM, Ghosal S, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016;6(2):603–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Carey MP, Deterd CH, de Koning J, Helmerhorst F, de Kloet ER. The influence of ovarian steroids on hypothalamic-pituitary-adrenal regulation in the female rat. J Endocrinol. 1995;144(2):311–321. [DOI] [PubMed] [Google Scholar]
- 49. Weiser MJ, Handa RJ. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience. 2009;159(2):883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Biag J, Huang Y, Gou L, et al. Cyto- and chemoarchitecture of the hypothalamic paraventricular nucleus in the C57BL/6J male mouse: a study of immunostaining and multiple fluorescent tract tracing. J Comp Neurol. 2012;520(1):6–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Uht RM, McKelvy JF, Harrison RW, Bohn MC. Demonstration of glucocorticoid receptor-like immunoreactivity in glucocorticoid-sensitive vasopressin and corticotropin-releasing factor neurons in the hypothalamic paraventricular nucleus. J Neurosci Res. 1988;19(4):405–11, 468. [DOI] [PubMed] [Google Scholar]
- 52. Kovács KJ. CRH: the link between hormonal-, metabolic- and behavioral responses to stress. J Chem Neuroanat. 2013;54:25–33. [DOI] [PubMed] [Google Scholar]
- 53. Patchev VK, Brady LS, Karl M, Chrousos GP. Regulation of HSP90 and corticosteroid receptor mRNA by corticosterone levels in vivo. Mol Cell Endocrinol. 1994;103(1-2):57–64. [DOI] [PubMed] [Google Scholar]
- 54. Turner BB, Weaver DA. Sexual dimorphism of glucocorticoid binding in rat brain. Brain Res. 1985;343(1):16–23. [DOI] [PubMed] [Google Scholar]
- 55. Burgess LH, Handa RJ. Estrogen-induced alterations in the regulation of mineralocorticoid and glucocorticoid receptor messenger RNA expression in the female rat anterior pituitary gland and brain. Mol Cell Neurosci. 1993;4(2):191–198. [DOI] [PubMed] [Google Scholar]
- 56. Petta I, Dejager L, Ballegeer M, et al. The interactome of the glucocorticoid receptor and its influence on the actions of glucocorticoids in combatting inflammatory and infectious diseases. Microbiol Mol Biol Rev. 2016;80(2):495–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rollins DA, Coppo M, Rogatsky I. Minireview: nuclear receptor coregulators of the p160 family: insights into inflammation and metabolism. Mol Endocrinol. 2015;29(4):502–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Eggert M, Michel J, Schneider S, et al. The glucocorticoid receptor is associated with the RNA-binding nuclear matrix protein hnRNP U. J Biol Chem. 1997;272(45):28471–28478. [DOI] [PubMed] [Google Scholar]
- 59. van der Laan S, Lachize SB, Vreugdenhil E, de Kloet ER, Meijer OC. Nuclear receptor coregulators differentially modulate induction and glucocorticoid receptor-mediated repression of the corticotropin-releasing hormone gene. Endocrinology. 2008;149(2):725–732. [DOI] [PubMed] [Google Scholar]
- 60. Veldman-Jones MH, Brant R, Rooney C, et al. Evaluating robustness and sensitivity of the nanostring technologies nCounter platform to enable multiplexed gene expression analysis of clinical samples. Cancer Res. 2015;75(13):2587–2593. [DOI] [PubMed] [Google Scholar]