INTRODUCTION
The rapidly expanding coronavirus disease 2019 (COVID-19) acute respiratory infection has changed many aspects of daily life. The outbreak was declared a public health emergency of international concern on 30 January 2020 by the World Health Organization. The dramatically raising number of general population and health care professionals infected in Western countries is profoundly changing daily clinical practice. Resources of health care systems across Europe and globally have been temporarily reallocated to cope with the pandemic. As no vaccine is effective to date, social distancing has been promoted in order to decrease the diffusion and reduce the preventable deaths from overloading the public health system. All nonurgent procedures such as elective surgery and diagnostic testing have been markedly impacted. In this particular environment, the lack of availability of operating theaters, endoscopic suites and infusion sessions, radiation planning, or planned hospital admissions has forced the entire oncologic community to carefully consider how to deliver best care to cancer patients during this crisis.
Additional risk among oncologic patients in the COVID-19 emergency
It is well-known that elderly patients and those with comorbidity have been the most victims to the most serious respiratory effects of COVID-19. Cancer patients are vulnerable, and the balance between fear of immunosuppression, with the threat of COVID-19, and a compromised cancer outcome due to either delays in treatment or the use of suboptimal alternatives is a matter of great concern taking place daily in MDTs throughout the world. The Chinese experience to date highlights these concerns, in particular the added risk of Infection due to cancer-associated or treatment-induced therapy, as well as poorer outcomes from infection itself with higher risk of severe events. Patients who underwent chemotherapy (CT) or surgery in the prior month had a clinically severe form in 75% of cases, with an odds ratio of 5.34 (95% confidence interval 1·80–16·18; P = 0·0026).1 Therefore, the risk of acquiring COVID-19, and its implications with respect to mortality in particular, is highly relevant at this time in treatment planning and informed consent.2
Esophageal tumors
Surgery of esophageal cancer in particular is associated with a higher risk of mortality compared with most other cancer surgeries, up to 5% even in the best high-volume centers. Moreover, the rate of postoperative major respiratory complications is high, at approximately 25%; hence, a severe acute respiratory syndrome, from COVID-19, would present a major risk to life, particularly if respiratory and intensive care resources were unavailable or suboptimal.3–8
A key question consequently, uncertain at this time, is the timing of progression of an esophageal cancer that can be treated with curative intent from the initial clinical presentation and staging. We acknowledge the insufficiency of data on this topic and suggest that doubling time and metastatic potential is likely to be highly variable across the spectrum of tumor biology seen in esophageal cancers, and most estimates in this context are speculative.
As the pandemic is going on, with no clear signs of abating, and no vaccine likely for approximately a year, difficult decisions will have to be made, in part depending on patient factors and preferences, with the access to safe treatments, including CT, radiation therapy, and surgery being paramount within local and regional structures, as well as population prevalence and position on the curve of COVID-19 infections, and the key element of critical care facilities.
EMERGENCY CASES
This includes bleeding and perforations, as well as obstruction.
For bleeding, consider the endoscopic and/or interventional radiology options first, including embolization or hemostatic radiotherapy in selected cases.
In cases of perforation, if the patient shows relevant comorbidity (cardiovascular disease, previous respiratory condition, end-stage renal disease, moderate–severe liver disease, diabetes, obesity), low performance status (PS) (American Society of Anesthesiologists score > 3, Eastern Cooperative Oncology Group (ECOG) PS ≥ 2, or Karnofsky ≤ 60%) and/or organ dysfunction is present, and critical beds are limited due to a high volume of COVID-19 positive patients, always try conservative measures first.
Esophageal obstruction can be managed endoscopically in line with local endoscopic protocols.
ELECTIVE CASES
Given the implications of serious respiratory distress in response to COVID-19 in the postoperative course, every patient scheduled for elective upper gastrointestinal cancer surgery should be triaged and investigated with proper testing.
COVID-19 positive or suspected patients
For COVID-19 positive patients or those with symptoms every intervention should be delayed. Every case should be discussed in a multidisciplinary setting assessing the timing of future intervention, and patients should be made aware of the additional surgical risk posed by COVID-19. In addition, more intensive surveillance or treatment should be considered when patients with cancer are infected, especially in the elderly or those with other comorbidities. In asymptomatic positive patients, the same principle should apply, and surgery delayed until the patient test is negative.
COVID-19 confirmed negative patients
Due to the high respiratory risk of esophageal surgery, and the implications of COVID-19 infection, together with the higher chance of abnormal presentations of the infection due to tumor or treatment-related immunosuppression, there is a compelling case for accurate testing of these patients before progressing to surgery. Accurate triage is advisable 1 day before accessing the hospital for elective procedures. A phone call assessing if the patient present any of high-risk symptoms (fever > 37.5°C, new-onset cough, dyspnea, contact with patient infected or high prevalence areas) is mandatory to stratify the risks for the patient, the health care provider, and other patients hospitalized.
Ai et al.,9 reporting on 1,014 patients from the city of Wuhan, revealed that a chest computed tomography demonstrated a higher sensitivity for diagnosis of COVID-19 in comparison with reverse transcription polymerase chain reaction (RT-PCR) assay from swab samples, with respective rates of 88% (888/1,014) and 59% (601/1,014). Using RT-PCR as a reference, the sensitivity of chest computed tomography for COVID-19 was 97% (580/601). Interestingly, 60 to 93% of patients had an initial positive chest computed tomography before a positive RT-PCR result, and the mean interval time between the initial negative to positive RT-PCR results was 5.1 ± 1.5 days. These data, combined with the advice of Italian expert’s,10 provide strong support and recommendation to combine a chest computed tomography with an RT-PCR assay from swab samples in every patient in COVID-19 pandemic areas who require oncologic surgery, most particularly for esophageal and lung surgery where respiratory complications are well-reported.
The second issue is eligibility for elective surgery, and we propose that patients should be stratified according to tumor and patient factors (Fig. 1).
Fig. 1.
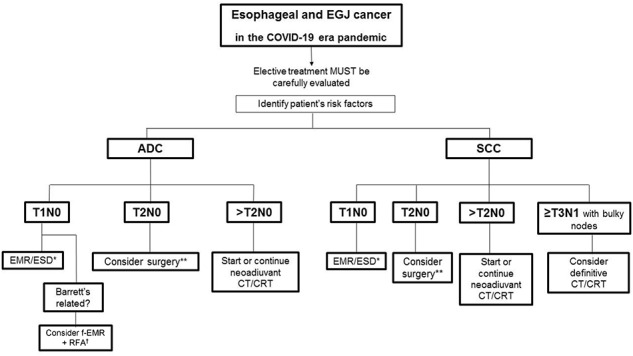
Proposed flowchart for esophageal and gastroesophageal junction cancer treatment during COVID-19 pandemics.20 If pT1bN0 is confirmed, consider adding CRT adjuvancy and/or closer follow-up and/or elective surgery after the COVID19 emergency has passed, according to MDT decision. †Consider the patient’s personal factors, ASA, nutritional status; if compromised, try to improve with preassessment and reconsider the case collectively. ADC, adenocarcinoma; ASA, American Society of Anesthesiologists; CT, chemotherapy; CRT, chemoradiotherapy; EMR, endoscopic mucosal resection; ESD, endoscopic submucosal dissection; f-EMR + RFA, focal endoscopic mucosal resection + radiofrequency ablation; GEJ, gastroesophageal junction; SCC, squamous cell carcinoma.
Establishing patient’s risk factors
Age > 7511
ECOG PS ≥ 2 or Karnofsky ≤ 60%
Preexisting comorbidities (cardiovascular disease, previous respiratory condition, end-stage renal disease, moderate–severe liver disease, diabetes, obesity)
Risk of postoperative complications and the need for intensive care unit
Due to high risk of postoperative complications, elective esophagectomy should be delayed when possible, especially in patients with comorbidity or where an extensive lymphadenectomy is advocated. Radical chemoradiotherapy (CRT) represents an alternative for squamous cell carcinoma (SCC) patients, which is supported by international guidelines.1,2 In patients who have received neoadjuvant CRT, a limited longer period before surgery, as per the NeoRes II trial, is justified.12 In patients who have a complete clinical response based on computed tomography–positron emission tomography, bite-on-bite biopsies of an endoscopically normal esophagus, then a watch and wait policy can be considered at this time, notwithstanding lack of Level I evidence, this particularly applies to esophageal SCC.13
Establishing cancer priority
Symptoms related to the tumor
Local compressive symptoms: consider stents or interventional radiology options first
cTNM: locoregional (I) versus advanced stage (II–III).
Consider endoscopic mucosal resection or endoscopic submucosal dissection, already standard for cT1a disease, for all cT1N0 disease. In patients presenting with T2N0 tumors, with no or few comorbidities, surgery should be proposed; postneoadjuvant stage II and III disease should, if possible, continue to be treated surgically but priority can be determined based on likely postneoadjuvant nodal involvement and poorly differentiated histology.14 Based on the predicted prognosis and the need for extensive surgical lymphadenectomy, for poorly differentiated tumors and SCC, a switch from neoadjuvant to a definitive CT or CRT can be considered (Fig. 1).
With respect to operative approach, there is no evidence yet to avoid minimally invasive approaches to reduce infection diffusion, but, even in cases negative, we strongly recommend the use of all the disposable personal protection equipment including masks (level 2 or 3 filtering face piece depending on the aerosol-generating risk level), eye protection, double nonsterile gloves, gowns, suites, caps, and socks.
SYSTEMIC ANTICANCER TREATMENTS
Currently, there is no strong evidence about how esophagogastric specific anticancer treatments should be adapted in the COVID-19 emergency setting. The Thésaurus National de Cancérologie Digestive have proposed alternative schemes for every digestive cancer, including esophagogastric tumors, based on the consensus of experts (Fig. 2)15 Clinicians will also need to consider the level of immunosuppression associated with an individual therapy and the condition itself, and patients’ other risk factors. Neoadjuvant therapy that requires clinic visits and clinician–patient contact must also be considered, and potentially be modified or protracted.16
Fig. 2.
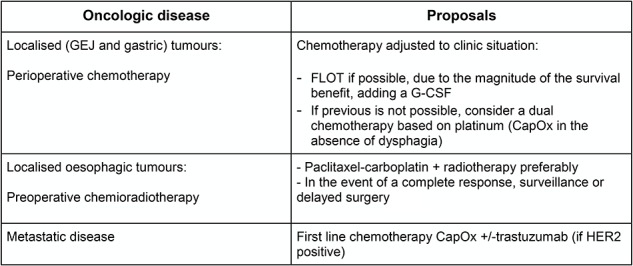
Alternative anticancer treatment scheme proposed by the French Intergroup Thésaurus National de Cancérologie Digestive for esophagogastric tumors during COVID-19 crisis (expert’s agreement).15 CapOx, capecitabine + oxaliplatin; G-CSF, granulocyte colony-stimulating factor; GEJ, gastroesophageal junction.
The American Society of Radiation Oncology17 advises that new patient consults and new patient starts may be triaged on a case-by-case basis according to the urgency of the situation following discussion with the MDT. The European Society for Radiotherapy and Oncology (ESTRO) adds that therapies that have been considered standard of care should be reconsidered. ESTRO also gives some guidance about the modification of radiotherapy treatments under a tumor-type basis in their recent publication. In respect of esophagogastric tumors, while gastric tumors should be treated with CT only, esophageal tumors in the view of the ESTRO should be treated by ‘resection or CRT rather than trimodality therapy.’18
To apply these recommendations, it would appear that starting or extending CRT for localized esophageal tumors (including cT2N0 stages) should be a valid option to consider for these patients, this is also consistent with the French proposal.15 If services are disrupted, patients should be prioritized for treatment accordingly, and the National Institute for Health and Care Excellence (NICE) guideline’s prioritization system is a useful resource in this scenario.19
NUTRITIONAL STATUS
During waiting time for a postponed surgery, in order to maintain or even gain optimal PS prior to surgery, it is highly recommended to closely monitor nutritional status by usual nutritional scores (e.g. Nutrition Risk Screening 2002 (NRS-2002) or Malnutrition Universal Screening Tool (MUST) scales). Dietary input through virtual clinics is also recommended. It is also advised to correct anemic condition by normally used methods in your institution.
PREOPERATIVE REHABILITATION
If possible, consider to prepare patient-adjusted rehabilitation programs to do at home, to maintain patients fitness and musculoskeletal tone prior to surgery, limit sarcopenia and its consequences. These programs should be sent virtually or by ordinary post to minimize face-to-face contact.
PSYCHOLOGIC SUPPORT
Communicate with patients and support their mental well-being to help alleviate any anxiety and fear they may have about COVID-19. Discuss the risks and benefits of changing treatment regimens or having treatment breaks with patients, their families, and caregivers.
Implementation of nontraditional care delivery strategies through information technology platforms and telehealth options can implement managing cancer-related issues.
FOLLOW-UP
Consider postponing long-term follow-up patients until the crisis has passed, or use preferably telehealth medicine if it cannot be postponed. Consider that computed tomography scanning and other imaging may be limited as radiology departments divert resources toward the coronavirus pandemic. In cases when CT or CRT is indicated by the multidisciplinary team, this must not be delayed and the access of the patient should follow the same route of triaging as for surgery intervention with phone call and oropharyngeal and nasopharyngeal swabs prior to hospitalization.
CONCLUSIONS
The sanitary systems were not prepared to face such a pandemic: thousands of people everywhere in the world are infected and need support to fight the respiratory distress. Many people died for this reason. We should take into account our previous daily battle trying to help our patients deal with a particularly difficult cancer, and this battle within a global war on a potentially devastating virus must be maintained as best we can, and these recommendations, not formal guidelines, are a proposed guide that hopefully will be of value at a time where traditional paradigms are upended.
Conflict of Interest
None of the named authors have any conflict of interest to disclose.
Funding information
No funding was received for this article.
IMPORTANT NOTE: This document is not intended as guideline, but has been drawn up to help cope with the temporary emergency and manage the surgical priority. These suggestions are subjected to change based on the pandemic evolution and must be adapted to the local situation in terms of resources and incidence of infection. In ALL centers, decisions should be made through an MDT process. Ensure each patient is considered on an individual basis by the MDT and record the reasoning behind each decision.
References
- 1. Liang W, Guan W, Chen R et al. . Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 2020; 21(3): 335–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tuech J J, Gangloff A, Di Fiore F et al. . Strategy for the practice of digestive and oncological surgery during the Covid-19 epidemic. J Visc Surg 2020. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mariette C, Markar S, Dabakuyo-Yonli T S et al. . Health-related quality of life following hybrid minimally invasive versus open esophagectomy for patients with esophageal cancer, analysis of a Multicenter, open-label, randomized phase III controlled trial: the MIRO trial. Ann Surg 2019. In press. [DOI] [PubMed] [Google Scholar]
- 4. Rutegård M, Lagergren P, Rouvelas I et al. . Surgical complications and long-term survival after esophagectomy for cancer in a nationwide Swedish cohort study. Eur J Surg Oncol 2012; 38: 555. [DOI] [PubMed] [Google Scholar]
- 5. Raymond DP. Complications of esophageal resection In: Post TW, (ed.) UpToDate. Waltham, MA: UpToDate Inc. [Cited on 27 March 2020] Available from URL: https://www.uptodate.com [Google Scholar]
- 6. Biere S S, Maas K W, Cuesta M A, Peet D L. Cervical or thoracic anastomosis after esophagectomy for cancer: a systematic review and meta-analysis. Dig Surg 2011; 28: 29. [DOI] [PubMed] [Google Scholar]
- 7. Inoue J, Ono R, Makiura D et al. . Prevention of postoperative pulmonary complications through intensive preoperative respiratory rehabilitation in patients with esophageal cancer. Dis Esophagus 2013; 26: 68. [DOI] [PubMed] [Google Scholar]
- 8. Schieman C, Wigle D A, Deschamps C et al. . Patterns of operative mortality following esophagectomy. Dis Esophagus 2012; 25: 645. [DOI] [PubMed] [Google Scholar]
- 9. Ai T, Yang Z, Hou H, Zhan C et al. . Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology 2020; 26: 200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pansurg Webinar Surgery during the Covid-19 pandemic. Italian experience. Available from URL: https://www.pansurg.org/archive.
- 11. McLoughlin J M1, Lewis J M, Meredith K L. The impact of age on morbidity and mortality following esophagectomy for esophageal cancer. Cancer Control 2013; 20(2): 144–50. [DOI] [PubMed] [Google Scholar]
- 12. Klevebro F, Alexandersson von Döbeln G, Wang N et al. . A randomized clinical trial of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the oesophagus or gastro-oesophageal junction. Ann Oncol 2016; 27(4): 660–7. [DOI] [PubMed] [Google Scholar]
- 13. Noordman B J, Spaander M C W, Valkema R et al. . Detection of residual disease after neoadjuvant chemoradiotherapy for oesophageal cancer (preSANO): a prospective multicentre, diagnostic cohort study. Lancet Oncol 2018l; 19(7): 965–74. [DOI] [PubMed] [Google Scholar]
- 14. Association of Upper Gastrointestinal Surgery of Great Britain and Ireland (AUGIS) Surgical priority in oesophageal and gastric cancer. March 2020. Available from URL: https://www.augis.org/wp-content/uploads/2020/03/Surgical-Priority-in-Oesophageal-and-Gastric-Cancer.pdf.
- 15. Di Fiore F, Bouché O, Lepage C et al. . Propositions of alternatives in digestive cancers management during the COVID-19 epidemic period: a French intergroup clinical point of view (TNCD). sous presse. Dig Liver Dis Thésaurus National de Cancérologie DigestiveMarch 2020, en ligne. Available from URL http://www.tncd.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. American Society of Clinical Oncology (ASCO) COVID-19 patient care information guideline. https://www.asco.org/asco-coronavirus-information/care-individuals-cancer-during-covid-19 (Accessed on 29 March 2020).
- 17. American Society of Radiation Oncology (ASTRO) Should we be delaying new consult/starts of patients who can be triaged for two to three (e.g., prostate cancers on ADT) when significant community spread of COVID-19 is detectable in our area? Should we delay new starts of more indolent cancers (e.g., skin cancers, new adjuvant breast radiation, new prostate radiation, etc.)?. [Cited 29 March 2020.] Available from URL: https://www.astro.org/Daily-Practice/COVID-19-Recommendations-and-Information/COVID-19-FAQs#q8.
- 18. Simcock R, Thomas T V, Mercy C E et al. . COVID-19: global radiation Oncology’s targeted response for pandemic preparedness. Clin Transl Radiat Oncol 2020. doi: 10.1016/j.ctro.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. NICE guidelines COVID-19 rapid guideline: delivery of systemic anticancer treatments. NICE guideline [NG161]. [Published March 2020.] Available from URL: https://www.nice.org.uk/guidance/ng161/chapter/6-Prioritising-patients-for-treatment. [PubMed]
- 20. Kitagawa Y, Uno T, Oyama T, Kato K et al. . Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 1. Esophagus 2019 Jan; 16(1): 1–24. doi: 10.1007/s10388-018-0641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Desai M, Saligram S, Gupta N et al. . Efficacy and safety outcomes of multimodal endoscopic eradication therapy in Barrett's esophagus-related neoplasia: a systematic review and pooled analysis. Gastrointest Endosc 2017; 85(3): 482–95 e4. doi: 10.1016/j.gie.2016.09.022 Epub 23 Sep 2016. [DOI] [PubMed] [Google Scholar]


