Abstract
The toxicology of mercury (Hg) is of concern since this metal is ubiquitously distributed in the environment, and living organisms are routinely exposed to Hg at low to high levels. The toxic effects of Hg are well studied and it is known that they may differ depending on the Hg chemical species. In this chapter, we emphasize the neurotoxic effects of Hg during brain development. The immature brain is more susceptible to Hg exposure, since all the Hg chemical forms, not only the organic ones, can harm it. The possible consequences of Hg exposure during the early stages of development, the additive effects with other co-occurring neurotoxicants, and the known mechanisms of action and targets will be addressed in this chapter.
Keywords: Mercury, brain, thimerosal, LMM-SH, HMM-SH
Introduction:
Mercury is a metal with a unique chemical characteristic, namely, it is silvery liquid at room temperature. Its physical properties, including low viscosity, high density, excellent electrical conductance, and reflective surface characteristics, drew the attention of researchers and industries to the application of Hg (Clarkson and Magos 2006). In this context, there are records of Hg usage as early as ancient China, where cinnabar (HgS) was used to make a red dye. On the American continent, Hg was brought by the Spanish Galleons to be employed in the process of gold and silver extraction (Clarkson and Magos 2006; Oliveira et al. 2017a). Nowadays, Hg is used as part of some medical devices, as a preservative of vaccines, and as a catalyst in caustic soda and chloride factories. In many parts of the world, Hg is still a constituent of dental amalgams (Horowitz et al. 2014). Of particular environmental concern, in countries, such as Brazil, Venezuela, Ecuador, Indonesia, Tanzania, and Ghana, Hg is still used in artisanal small-scale gold mining (ASGM) (Kristensen et al. 2014), and this activity has apparently increased in the last year (Branco et al. 2017). The environmental levels of Hg increased considerably with anthropogenic intervention (Figure 1). In fact, it is estimated that the atmospheric burden of Hg nowadays is 300–500% higher than that in the precolonial period (AMAP/UNEP 2013; De Simone et al. 2016).
Figure 1:
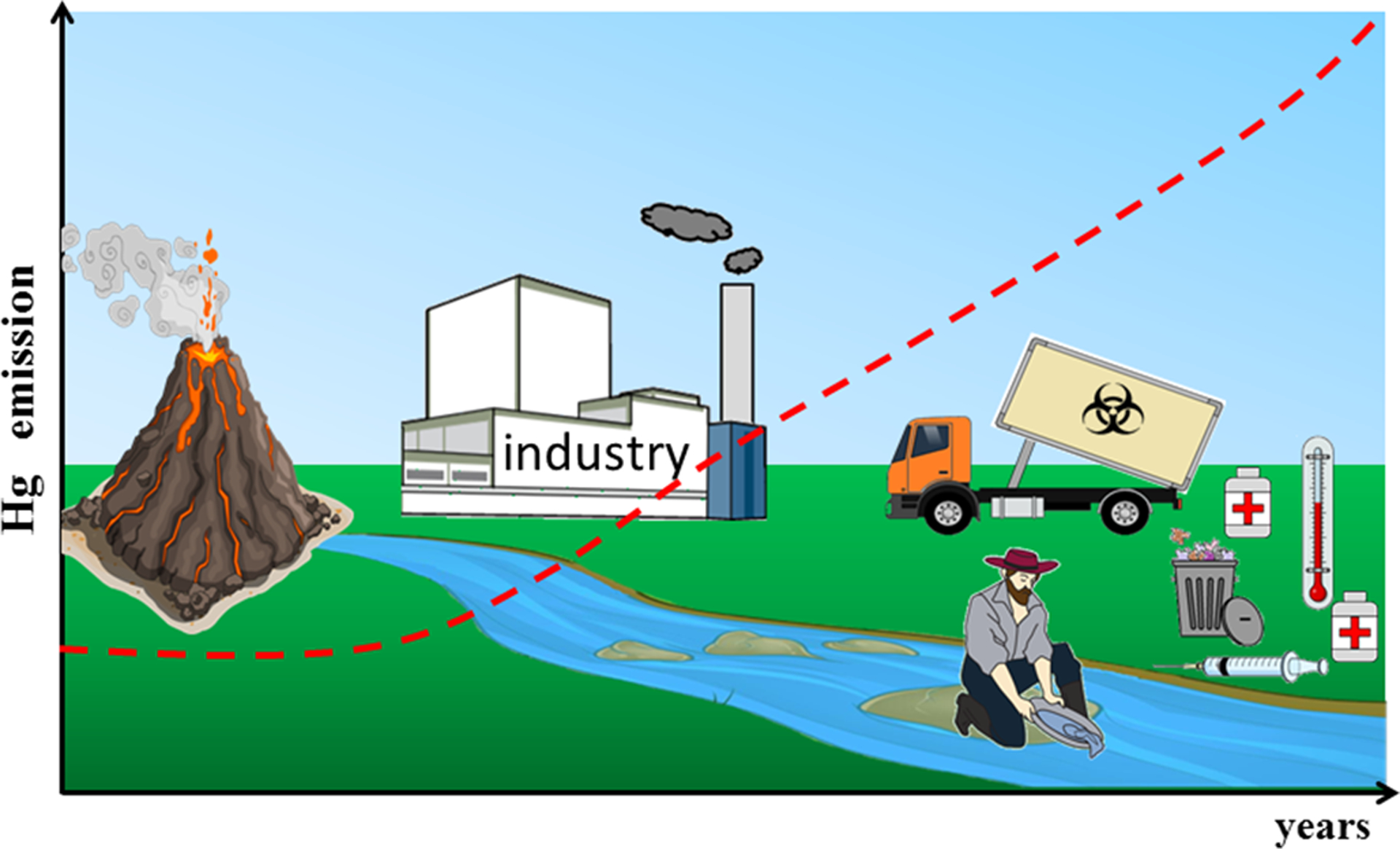
Natural and anthropogenic sources of Hg emission in the environment. The volcanic and erosion processes are natural sources of Hg. The process of gold extraction, the uncontrolled release of waste from industry and medical devices increase the emission/levels of Hg in the environment almost exponentially. The red dotted line represents the emission of Hg.
In this context, the toxicology of Hg has been extensively studied, and it has been established that Hg does not have biochemical and/or physiologic functions in living organisms (WHO 2007). In fact, exposure to even low levels of Hg can be toxic, causing molecular, cellular, developmental and behavioral alterations in different organisms (Chang et al. 2017; Chauhan et al. 2017; Fujimura and Usuki 2017; Mora-Zamorano et al. 2017), including humans (Choi et al. 2017; Junior et al. 2017). The toxic effects are directly influenced by the chemical form of Hg: inorganic elemental Hg (Hg0), ionic inorganic mercury (Hg2+) and organic mercury (Hg atom bound to one carbon atom, for example, CH3Hg+ and C2H5Hg+) (Figure 2).
Figure 2:
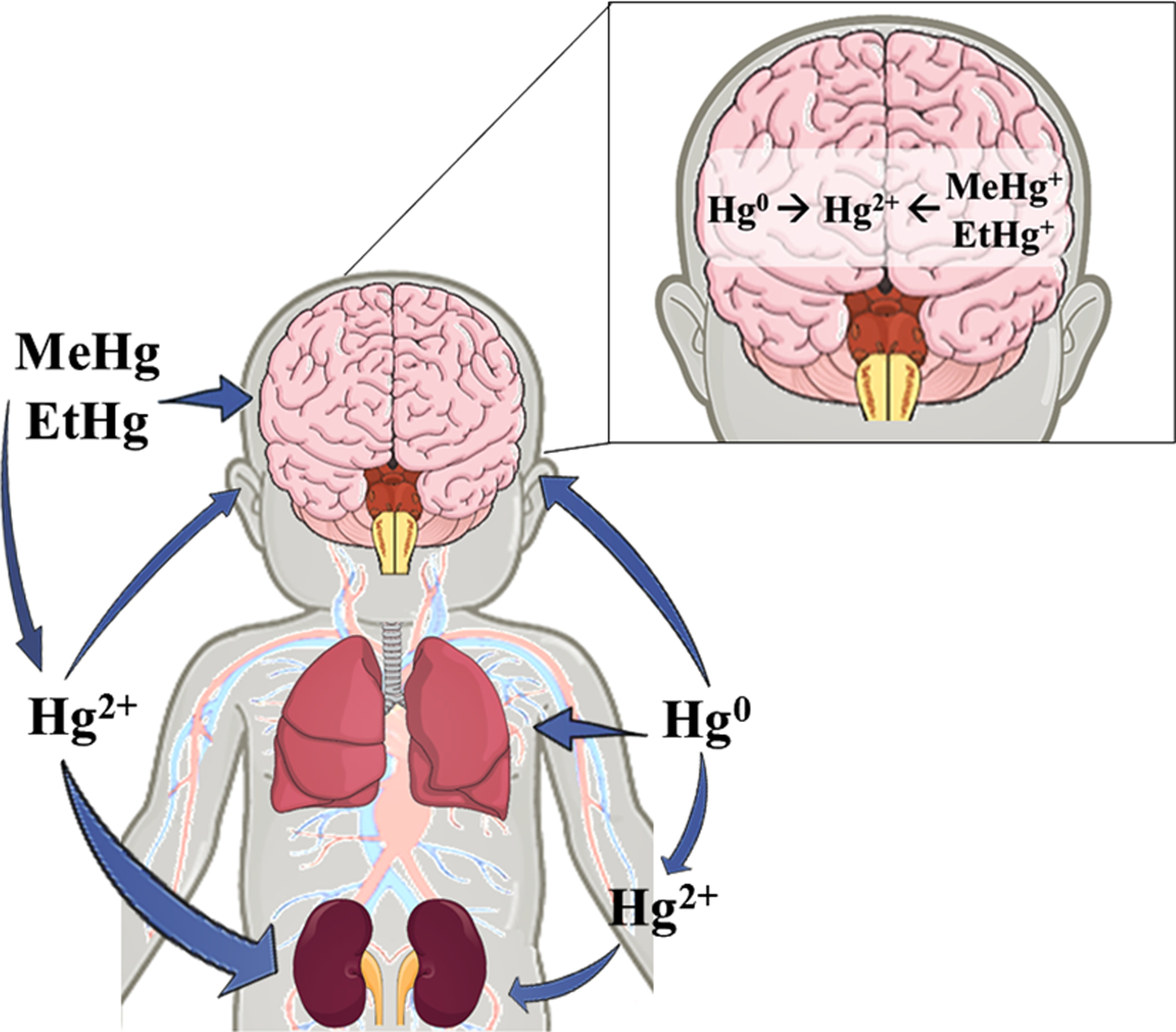
Main targets of Hg different chemical species. The main interconversions among the Hg species are demonstrated. The thickness of the arrows is proportional to the amount of Hg that reaches a given organ.
Although immediately after intoxication, Hg chemical species accumulate in different target organs, multiple studies have demonstrated that they can reach the brain causing the accumulation of Hg2+ (Figure 2). The neurotoxic effects of Hg are usually much more pronounced in the immature nervous system. This review will briefly discuss the neurodevelopmental toxic effects caused by mercury exposure.
1. Inorganic Elemental Mercury
Inorganic elemental Hg (Hg0) is found as a liquid at room temperature, but due to its low vapor pressure (0.2613 Pa at 25 °C), it volatilizes slowly and increasingly with rise in temperature (Risher et al. 2003; Lee et al. 2009; Huber et al. 2006; Gonzalez-Raymat et al. 2017). As a vapor, it can be inhaled and the first target is the respiratory tract (Oz et al. 2012) followed by the nervous and renal systems (Reinhardt 1992; Oliveira et al 2017a). Hg0 can easily cross plasma membranes, reaching the intracellular milieu due to its high diffusibility and lipid solubility (Clarkson 1972). In the blood, Hg0 can be oxidized to Hg2+ in erythrocytes by the enzyme catalase (Halbach and Clarkson 1978). The remaning Hg0 can reach the brain and once inside the brain cells, it can be oxidized to Hg2+ (Eide and Syversen 1983). The oxidized form (Hg2+) has a great affinity for protein thiol (–SH) and selenol (–SeH) groups, causing several alterations that will be further discussed in Section 5 of this chapter.
Hg0 is found in medical devices such as thermometers, barometers, sphygmomanometers (Risher et al. 2003), and in dental amalgams (Reinhardt 1992); it is also commonly used in fluorescent lamps (Hu and Cheng 2012). The main anthropogenic source of Hg0 is ASGM, and data from UNEP (United Nations Environment Programme) estimate that 727 tons of mercury were released by gold extraction in 2010 (AMAP/UNEP 2013). Occupational exposure to elemental Hg is common among workers that inappropriately handled the elemental mercury. Contaminated individuals presented symptoms such as skin rash, pruritus, myalgia, sleep disturbance fatigue, insomnia, nightmares, anxiety disorder and elevated hair, urine and blood Hg levels (Schuurs 1999; Mostafazadeh et al. 2013; Noble et al. 2016; Do et al. 2017). In addition, there are cases of accidental or even intentional contamination with Hg0 (Florentine and Sanfilippo 1991; Gul Oz et al. 2012; Gao et al. 2017).
Intrauterine exposure to Hg0 has been reported to cause Hg accumulation and neurobehavioral changes in metallothionein-null mice (Yoshida et al. 2002, 2005) and in rats (Danielsson et al. 1993; Morgan et al. 2002). Vimy et al (1990), used pregnant sheep as the experimental model and demonstrated that the Hg0 released from dental amalgam fillings crossed the placental barrier and accumulated in the fetus’ organs. Despite these experimental findings, Watson and co-workers (2012, 2013) did not find a correlation between mothers’ dental amalgam and the development of their children at ages 9 and 30 months and 5 years in the Seychelles cohort.
2. Ionic Inorganic Mercury
The ionic inorganic form of Hg, Hg2+, can be formed from the oxidation of elemental mercury or by the cleavage of the carbon-mercury bound in the organic mercury molecules. The process of carbon-mercury bond cleavage is very slow for MeHg+, but it is much faster for EtHg+ (Burbacher et al. 2005; Dórea et al. 2013). These chemical conversions occur in both the environment and in the body (Oliveira et al. 2017a).
Presently, populations are exposed to Hg2+ mainly through the ingestion of contaminated water and food (Park and Zheng 2012). Another reported means of Hg2+ exposure is the addition of Hg2+ to cosmetic creams for skin lightening and acne treatments (Chan 2011; Copan et al. 2015). Although it is known that Hg2+ is poorly absorbed by the intestine and skin cells, the toxic effects of the exposure to inorganic Hg2+ cannot be neglected.
The primary target of Hg2+ is the kidney (Zalups 2000). Some studies have demonstrated that a few hours after intravenous Hg2+ injection, approximately 50% of the injected dose is found in the kidneys (Zalups 1993; Zalups and Barfuss 1995; Oliveira et al. 2017b). Hg cations are not found free in the bloodstream; rather, they are bound to thiol (–SH)-containing molecules such as albumin, cysteine, and glutathione (GSH) (Bridges and Zalups 2017; section 5). The high affinity of −SH-containing molecules for Hg2+ (Fuhr and Rabenstein 1973; Rabenstein and Isab 1982), particularly for cysteine, which is abundant in the blood, will direct Hg-cysteine complexes to the kidneys (section 5). Interestingly, the fate of Hg2+ in the soil is also dictated by the thiol present in the dissolved organic matter (Skyllberg 2008; Leterme et al. 2014). Hg bound to two cysteines (Cys-Hg-Cys) gets entry into the kidney’s proximal tubular cells as a mimic of the amino acid cystine by System b0+, OAT1 and OAT3 transporters (Bridges et al. 2004; Zalups and Ahmad 2004; Bridges and Zalups 2017). Due to its electrical polarity, Hg2+ poorly crosses the plasma membrane or the blood-brain and placental barriers. However, some studies with experimental animals demonstrated that some of Hg2+ crosses the placental barrier and accumulates in the fetus’s organs, which can be harmful to the animal’s development (Danielsson et al. 1984; Bernard et al. 1992; Oliveira et al. 2012, 2015). Moreover, several studies have demonstrated the neurotoxic effects of inorganic mercury in developing mammalian brains (Peixoto et al. 2007; Franciscato et al 2009; Moraes-Silva et al. 2014; Sahin et al. 2017). Although there are studies showing that in small quantities Hg2+ crosses the placental and blood-brain barriers (Alves et al. 2017), the mechanism by which it crosses these barriers is not well understood. Amino acid transporters, similar to those that transport Cys-Hg-Cys complexes in the kidneys, are probably involved.
Of toxicological significance, the access of Hg2+ into the immature mammalian brain is higher to that observed in the adult brain. In early life, the blood-brain barrier (BBB) is not completely developed and is leakier to charged toxic metals (Johansson 1990). Rat pups exposed subcutaneously to Hg2+ (HgCl2) during the second week of post-natal life showed behavioral alterations such as loss of motor coordination and delay in the development of the geotaxis reflex (Peixoto et al. 2007; Moraes-Silva et al. 2014). These alterations were observed even after the cessation of HgCl2 exposure (Franciscato et al. 2009). Huang and colleagues (2014) demonstrated that mice pups exposed to Hg2+ accumulate this metal in the cerebral neuron bodies.
Cases of human Hg2+ direct contamination causing neurotoxic effects do not exist. However, there is evidence that other chemical species of mercury (organic and elemental) are converted to Hg2+ and trapped within the brain (Yamamoto et al. 1986; Burbacher et al. 2005; Ishitobi et al. 2010). Based on a systematic review, Rooney (2014) concluded that the half-life of Hg2+ in the brain varies from years to decades. Of particular neurotoxicological significance, data have been published linking Hg accumulation in the brain with neurodegenerative diseases, namely, Alzheimer’s disease (Mutter et al. 2004; Walach et al. 2014; Chakraborty 2017), amyotrophic lateral sclerosis (Johnson and Atchison 2009; Pamphlett and Jew 2013, 2014) and Autism Spectrum Disorders (Bjørklund et al. 2017a,b), as will be discussed in section 4.
3. Organic Mercury
The organic mercury form is represented by a Hg atom bound to a C atom. The major concern of the human population on the subject of the organic mercury species exposure is related to methylmercury (CH3Hg+; MeHg+) and ethylmercury (C2H5Hg+; EtHg+). MeHg+ and EtHg+ are potent neurotoxic agents. These organic mercury molecules form complexes with low molecular mass thiols (LMM-SH, e.g., cysteine and reduced glutathione, see section 5 for more details) that can easily cross the plasma membrane and the blood-brain barrier as well as the placental barrier (Farina et al. 2011a,b; Dórea et al. 2013).
Exposure to MeHg+ is related to eating habits, i.e. populations consuming a diet based on seafood/fish from contaminated sites are more likely to be exposed to MeHg (Burger et al. 2005; Gochfeld and Burger 2005; Schmidt et al. 2013; Montero-Alvez et al. 2014; Bosch et al. 2016). Another source of dietary MeHg is rice from contaminated areas (Li R. et al. 2016; Brombach et al. 2017; Cui et al. 2017; Wu et al. 2018). Regarding EtHg+, the main source of exposure is Thimerosal, a preservative in vaccines. After the immunization with thimerosal-containing vaccines (TCV), this molecule is metabolized, releasing EtHg+ (Dórea et al. 2013; Dórea 2017a,b). There are several controversies about the use of thimerosal to preserve vaccines. On the one hand, there are scientists from the pharmaceutical industry supporting the use of thimerosal, claiming that the levels used are within safe limits. On the other hand, there are concerns that the cumulative number of shots during the critical periods of brain development may cause moderate to high dose exposure, and indeed these concerns have led rich countries to ban its use for young children. Independently, , studies have questioned the double standard of using Thimerosal in pediatric vaccines for children in less developed countries (Dórea 2017a,b). From a toxicological point of view, the intentional exposure of humans or animals to any form of mercury is illogical.
The neurotoxic effects of the organic forms of mercury became evident after two outbreaks of human contamination. The first case is known as Minamata disease, which took place in Minamata Bay, Japan. For aproximately 20 years (mid 1950s to mid 1970s) a factory released large amounts of Hg2+ into Minamata Bay (Kudo and Turner 1999; Harada 1995; Hachiya 2012). Hg2+ was used as the catalyst in the synthesis of acetaldehyde, but a small amount of MeHg+ (about 1%) was formed as byproduct, which may have increased the extent of human and environmental contamination (Kudo and Turner 1999). Once in the aquatic environment, Hg is methylated by bacteria and incorporated in the food chain, leading to the biomagnification and bioaccumulation processes, i.e., predatory old fish accumulated more MeHg+ than herbivorous or predatory juvenile fish (Oliveira et al. 2017a). As the main source of protein for the Minamata population was fish, they were consequently exposed to high levels of MeHg+. Although the adults showed low to moderate neurologic symptoms, the infants, who were exposed indirectly to MeHg+ (in utero or via breast milk), presented severe learning and locomotor deficits (Harada 1995; Ekino et al. 2007).
The second major case of organic Hg contamination happened in Iraq (1971–1972), where farmers and their families, inadvertently prepared and ate homemade bread made with seeds that had been treated with a fungicide containing organic Hg (MeHg+ and EtHg+). Approximately 6,500 people were poisoned and had severe neurological symptoms, and about 500 died. As in Minamata, infants were much more incapacitated neurologically than adults (Bakir et al. 1973; Shikara and Al Dabbagh 1973).
However, human contamination with EtHg+ (in TCVs) is silent, when compared with the poisonous outbreaks of organic Hg. As mentioned before, infants and children in less developed countries are constantly exposed to EtHg+ after immunization with TCVs during vulnerable periods of brain development (Dórea 2017a,b). Based on this, several epidemiological studies have indicated adverse effects of TCVs in the normal development of children (Geier and Geier 2006; Mrozek-Budzyn et al. 2012; Marques et al. 2014, 2016a).
4. Neurodevelopmental toxicity: Hg versus Autism Spectrum Disorder
The brain is a complex organ that presents non-uniform development, i.e., it occurs in multiple stages. The cortex, hippocampus, and cerebellum are examples of brain structures that show late development (Andersen 2003). Consequently, the vulnerability of different brain areas to neurotoxic insults will be different depending on the developmental period of exposure. In addition, symptoms/effects that are not present during the exposure period may appear later in life. For example, epidemiological evidence has been accumulated supporting the proposal that environmental insults in utero and/or in early postnatal life in association with a genetic predisposition may facilitate the development of neurodegenerative diseases in adult life (Grandjean and Landrigan 2006; Modgil 2014).
Among the environmental insults, metals such as cadmium, lead, manganese, aluminum, and mercury are known to be important neurotoxicants. Neurodevelopmental exposure to mercury, in utero and/or in breast milk or even in vaccines, has been associated with learning/locomotor deficits, low IQ, attention deficit disorder, mental retardation and Autism Spectrum Disorder (Johansson et al. 2007; Drum 2009; Debes et al. 2016; Jeong et al. 2017; Geier et al. 2018a).
Autism Spectrum Disorder (ASD) is a group of heterogeneous dysfunctions in social interaction and communication that lead to the appearance of unusual and repetitive patterns of behavior (American Psychiatric Association 2013). The causes of ASD are usually attributed to complex genetic modifications, but the exact genes involved in ASD development are not fully known. A recent review from Fakhoury (2018) highlighted that mutations in genes involved in the pre- and post-synaptic binding proteins, neuronal cell adhesion, and GABA A receptor, among others, may be involved in the development of ASD. However, to quote Dr. Fakhoury, “the characterization of the neurobiological mechanisms by which common genetic risk variants might operate to give rise to ASD symptomatology has proven to be far more difficult than expected.”
As mentioned above, Hg has emerged as a putative environmental associated factor for ASD development. In fact, several studies have demonstrated that ASD patients presented elevated levels of Hg in blood and hair when compared to the same age control subjects (see Table 1 and the elegant review by Kern et al. 2016). The difference in the mercury values in the studies mentioned in Table 1 can probably be explained by different protocols used to quantify mercury, dietary habits of the subjects, and the occurrence of environmental mercury, among others. If on the one hand there are a great number of studies correlating Hg exposure to ASD development, on the other hand, studies carried out by the pharmaceutical industry or entities with an apparent conflict of interest have most often shown no clear evidence (see the critical review of Kern et al. 2017).
Table 1:
Levels of Hg in ASD patients: examples of case-control studies.
| Hg levels | Sample | Country | Reference |
|---|---|---|---|
| Control: 0.30 μg/g Autism: 4.50 μg/g | Hair | Kuwait | Fido and Al-Saad 2005 |
| Control: 0.37 μg/g Autism: 1.61 μg/g | Hair | India | Priya and Geetha 2011 |
| Control: 2.87 μg/g Autism: 4.02 μg/g | Nail | India | Priya and Geetha 2011 |
| Control: 0.07 μg/g Autism: 0.15 μg/g | Baby teeth | United States | Adams et al. 2007 |
| Control: 0.25 μg/g Autism: 0.39 μg/g | Hair | Egypt | Mohamed et al. 2015 |
| Control: 0.12 μg/g Autism: 0.79 μg/g | Hair | Egypt | El-baz et al. 2010 |
| Control: 2.71 μg/g Autism: 3.66 μg/g | Red Blood Cells | Saudi Arabia | El-Ansary et al. 2017 |
| Control: 13.47 μg/L Autism: 55.59 μg/L | Blood | China | Li et al. 2018 |
| Control: 12.08 μg/L Autism: 32.90 μg/L | Blood | Egypt | Khaled et al. 2016 |
| Control: 0.60 μg/g Autism: 7.00 μg/g | Hair | Oman | Hodgson et al. 2014 |
Hg is a neurotoxic agent, and an immature organism can be exposed in utero and/or via breast milk (if the mother is/was exposed to metal) or via TCVs, food (fish and rice), and from environmental contamination (for example, children that live close to ASGM areas (Ruggieri et al. 2017). Once Hg reaches the brain, it causes several alterations, including oxidative stress, enzyme inhibition, depletion of thiol molecules, DNA and gene expression alteration, excitotoxicity, and cell apoptosis. These alteration can permanently change the normal brain connectivity either by changing the migration/connection between cells or by decreasing the number of cells (Ceccatelli et al 2010; Farina et al. 2011a,b; Garrecht and Austin 2011; Oliveira et al. 2017a). Hg exposure can also activate the immune system (Hultman et al. 1998; Brenden et al. 2001), and several papers have indicated that the maternal and/or fetal activation of the brain immune system can be interconnected with the neurodevelopmental deficit observed in ASD (Estes and McAllister 2016; Fernández de Cossío et al. 2017; Meltzer and Van De Water 2017; Bilbo et al. 2018).
Further support for the potential role Hg in the etiology of ADS is the fact that males are more affected than females (Loomes et al. 2017; Palmer et al. 2017). Several studies have demonstrated that males are more sensitive than females to mercury exposure (Mckeown-Eyssen et al. 1983; Rossi et al. 1997; Gimenez-Llort et al. 2001; Malagutti et al. 2009; Ekstrand et al. 2010; Marques et al. 2015), which corroborates the hypothesis that Hg may be involved in ASD etiology. The differential susceptibility of males to toxic agents, when compared to females, is elegantly discussed by McCarthy and Wright (2017). They hypothesized that during the process of brain masculinization (exposure to high levels of testosterone) the brain is more susceptible to immune activation and/or neurotoxins.
5. Potential molecular target of Hg and mechanism of neurotoxicity
5.1. Thiol (−SH) and Selenol (−SeH) groups
The electrophilic forms of mercury (E+Hg, i.e., Hg2+, MeHg+ and EtHg+) can interact with different ligands (functional groups) found in the cell milieu and in the natural organic matter found in the environment (Table 2). However, the affinity of E+Hg for soft nucleophiles (SNu−) is orders of magnitude higher than that for hard nucleophiles (HNu−) (Table 3). Thus, the fate of Hg0 and E+Hg forms in the environment and inside living cells is greatly influenced by the interaction of Hg0 or E+Hg with thiol/thiolate groups (−SH/S−) ubiquitously found in complex biological systems (Loux 1998; Skyllberg et al. 2006; Farina et al. 2011a,b, 2017; Gu et al. 2011; LoPachin et al. 2012). Another important functional group that can bind E+Hg with high affinity is the selenol/selenolate (−SeH/−Se−) groups found in a small number of selenoproteins (Labunskyy et al. 2014). Although selenol/selenolate groups have a stronger affinity for E+Hg than their sulfur-containing analogs (Farina et al. 2011a,b; Bjørklund et al. 2017c), the number of studies analyzing the constant of formation are limited (Table 2). Despite this limitation, the affinity of −SeH/−Se− for E+Hg is possibly 1 to 4 orders of magnitude higher than that of −SH/−S− analogs (Sugiura et al. 1976; Rabenstein et al. 1986; Khan and Wang 2009; Farina et al. 2011a,b).
Table 2.
Formation constants of MeHg+ or Hg2+ with nucleophilic centers found in biomacromolecules. The nucleophilic centers can be categorized in hard (HNu−) or soft (SNu−) centers. −SeH is the softest of the SNu− centers found in living cells, followed by −SH. The −NH and −COOH-containing molecules are HNu− and the centers containing O are harder than those containing N (Hancock and Martel 1996; Martin 2002).
| Functional group | MeHg+ | Hg2+ |
|---|---|---|
| R-COOH | ≈ 2.5–3.0 | ≈ 4–7 |
| R-(COOH)2 | --- | ≈ 5–9 |
| R-(COOH)3 (citrate) | --- | ≈ 11 |
| R-NH2 | ≈ 7–8 | ≈ 4–5 |
| R-(NH2)2 | --- | ≈ 11 |
| R2-NH | ≈ 6.7 | ≈ 4–5 |
| R-S-R | ≈ 7–8 | ≈ 6–12 |
| R-SH | ≈ 15–26 | ≈ 40–50 |
| R-SeH | ≈ 16–18 | ≈ 50–60 |
The values represent the log of the constant and a high value indicates that a given complex has high thermodynamic stability. The values were obtained from Stricks and Kolthoff 1953; Rabenstein et al. 1974; Rabenstein and Fairhurst 1975; Rabenstein 1978; Jawaid and Ingman 1981; Arnold and Canty 1983; Arnold et al. 1986; Berthon 1995; Seby et al. 2001; Anderegg et al. 2003; Mousavi 2011; Liem-Nguyem et al. 2017.
Table 3:
Examples of human thiol-containing proteins involved in the regulation of brain physiology that are targets of E+Hg forms. The Hg effects were based on animal models or in vitro studies. The proteins affected in rodents or in cultured cells were selected, and then the human protein information was obtained at www.uniprot.org .
| Part A: ENZYMES | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Protein | Gene | N° CXXC | N° CXC | N° CC | N° C | N° aa | Function | Localization | Hg effects | Reference |
| 5’-nucleotidase | NT5E | 1 | 0 | 0 | 10 | 574 | Nucleotide-binding | Plasma membrane | Inhibition | Sood et al. 1995 |
| Acetylcholinesterase | ACHE | 0 | 0 | 0 | 8 | 614 | Neurotransmitter degradation | Plasma membrane | Inhibition | Franciscato et al. 2009; Moraes-Silva et al. 2014 |
| Caspase-8 | CASP8 | 1 | 0 | 1 | 13 | 479 | Apoptosis | Cytoplasm | Activation | Shenker et al. 2002 |
| Complex I (NADH-ubiquinone oxidoreductase 75 kDa subunit) | NDUFS1 | 3 | 0 | 0 | 18 | 727 | Electron transport | Mitochondrion | Inhibition | Glaser et al. 2010a |
| Complex II (Succinate dehydrogenase flavoprotein subunit) | SDHA | 0 | 0 | 2 | 18 | 664 | Electron transport | Mitochondrion | Inhibition | Glaser et al. 2010a |
| Complex III (Cytochrome b-c1 complex subunit 1) | UQCRC1 | 0 | 0 | 0 | 12 | 480 | Electron transport | Mitochondrion | Inhibition | Glaser et al. 2010a |
| Complex IV (Cytochrome C oxidase subunit 1) | MT-CO1 | 0 | 0 | 0 | 1 | 513 | Electron transport | Mitochondrion | Inhibition | Glaser et al. 2010a |
| Cytochrome C | CYCS | 1 | 0 | 0 | 2 | 105 | Apoptosis | Mitochondrion | Release | Shenker et al. 1999; 2002 |
| Creatine kinase B-type | CKB | 0 | 0 | 0 | 5 | 381 | Transferase | Cytoplasm | Inhibition | Glaser et al. 2010b |
| Creatine kinase S-type | CKMT2 | 0 | 0 | 0 | 9 | 419 | Transferase | Mitochondrion | Inhibition | Glaser et al. 2010a |
| Cystathionine gamma-lyase | CTH | 2 | 0 | 0 | 10 | 405 | Cysteine synthesis | Cytoplasm | Inhibition | Bridges et al. 2012 |
| Glutamate-cysteine ligase catalytic subunit | GCLC | 0 | 1 | 1 | 14 | 637 | Glutathione synthesis | Cytoplasm | Induction | Thompson et al. 1999 |
| Glutamine synthetase | GLUL | 0 | 0 | 0 | 11 | 373 | Glutamine and GABA synthesis | Mitochondrion | Inhibition | Known and Park 2003 |
| Glutathione reductase | GSR | 0 | 0 | 0 | 10 | 522 | Glutathione reduction | Mitochondrion | Activation | Stringari et al. 2008; Glaser et al. 2010a; Zemolin et al. 2012 |
| Glutathione S-transferase | GSTA1 | 0 | 0 | 0 | 1 | 222 | Conjugation of reduced glutathione | Cytoplasm | Activation | Zemolin et al. 2012 |
| Phospholipase A2, membrane associated | PLA2G2A | 0 | 2 | 2 | 14 | 144 | Lipid degradation | Plasma membrane | Activation | Mazerik et al. 2007; Verity et al. 1994; |
| Part B: RECEPTORS | ||||||||||
| Protein | Gene | N° CXXC | N° CXC | N° CC | N° C | N° aa | Function | Localization | Hg effects | Reference |
| D(1A) dopamine receptor | DRD1 | 0 | 0 | 1 | 16 | 446 | G-protein coupled receptor | Plasma membrane, Endoplasmic reticulum membrane | Dysfunction | Daré et al. 2003 |
| D(2) dopamine receptor | DRD2 | 0 | 1 | 0 | 12 | 443 | G-protein coupled receptor | Plasma membrane | Dysfunction | Daré et al. 2003 |
| D(3) dopamine receptor | DRD3 | 1 | 0 | 1 | 16 | 400 | G-protein coupled receptor | Plasma membrane | Dysfunction | Daré et al. 2003 |
| Gamma-aminobutyric acid receptor subunit alpha-1 | GABRA1 | 0 | 0 | 0 | 5 | 456 | Ion channel, receptor | Plasma membrane | Inhibition | Bondy and Agrawal 1980 |
| Gamma-aminobutyric acid receptor subunit beta-1 | GABRB1 | 0 | 0 | 1 | 5 | 474 | Ion channel, receptor | Plasma membrane | Inhibition | Bondy and Agrawal 1980 |
| Muscarinic acetylcholine receptor M3 | CHRM3 | 1 | 1 | 0 | 12 | 590 | Inhibition of adenylate cyclase | Plasma membrane | Inhibition | Abd-Elfattah and Shamoo 1981; Abdallah and Shamoo 1984 |
| Muscarinic acetylcholine receptor M5 | CHRM5 | 2 | 0 | 0 | 17 | 532 | Inhibition of adenylate cyclase | Plasma membrane | Inhibition | Abd-Elfattah and Shamoo 1981; Abdallah and Shamoo 1984 |
| Part C: TRANSPORTERS | ||||||||||
| Protein | Gene | N° CXXC | N° CXC | N° CC | N° C | N° aa | Function | Localization | Hg Effect | Reference |
| Calcium-transporting ATPase 1 | ATP2B1 | 0 | 0 | 0 | 17 | 1,258 | Calcium transport | Plasma Membrane | Inhibition | Berg and Miles 1979 |
| Cysteine/glutamate transporter | SLC7A11 | 0 | 0 | 0 | 7 | 501 | Amino-acid transport | Plasma Membrane | Inhibition | Allen et al. 2001; Fonfría et al. 2005 |
| Part D: OTHER | ||||||||||
| Protein | Gene | N° CXXC | N° CXC | N° CC | N° C | N° aa | Function | Localization | Hg Effect | Reference |
| Kelch-like ECH-associated protein 1 | KEAP1 | 0 | 1 | 1 | 27 | 624 | Transcription regulation | Nucleus, Cytoplasm | Modulation | Yoshida et al. 2014 |
| Nuclear factor erythroid 2-related factor 2 | NFE2L2 | 0 | 0 | 0 | 6 | 605 | DNA-binding | Nucleus, Cytoplasm | Up-regulation | Ni et al. 2010 |
| Tubulin beta chain | TUBB | 0 | 1 | 0 | 8 | 444 | Nucleotide-binding, microtubule | Cytoskeleton | Oxidation | Kromidas et al. 1990 |
Exposure to MeHg+ occurs via fish consumption, whereas EtHg+ is intramuscularly injected in the chemical form of thimerosal in vaccines. Exposure to Hg2+ can occur after the absorption of Hg0 or occasionally via ingestion of contaminated food and water. For instance, about 2–10% of the total Hg found in fish meat can be present as inorganic Hg (Montero-Alvarez et al. 2014).
The strong affinity of E+Hg for −SH and −SeH groups implies that nearly all the E+Hg will be bound to one of these groups. Since −SH is much more abundant than −SeH (Rocha et al. 2017), the majority of E+Hg forms will be complexed with −SH groups. In fact, MeHg+ can bind to low molecular mass thiol molecules (LMM-SH, e.g., cysteine, glutathione (GSH)) or to cysteinyl residues in proteins or high molecular mass thiol molecules (HMM-SH). Some important complexes of E+Hg forms with abundant LMM-SH molecules found in the cellular environment are shown in Figure 3.
Figure 3 -.
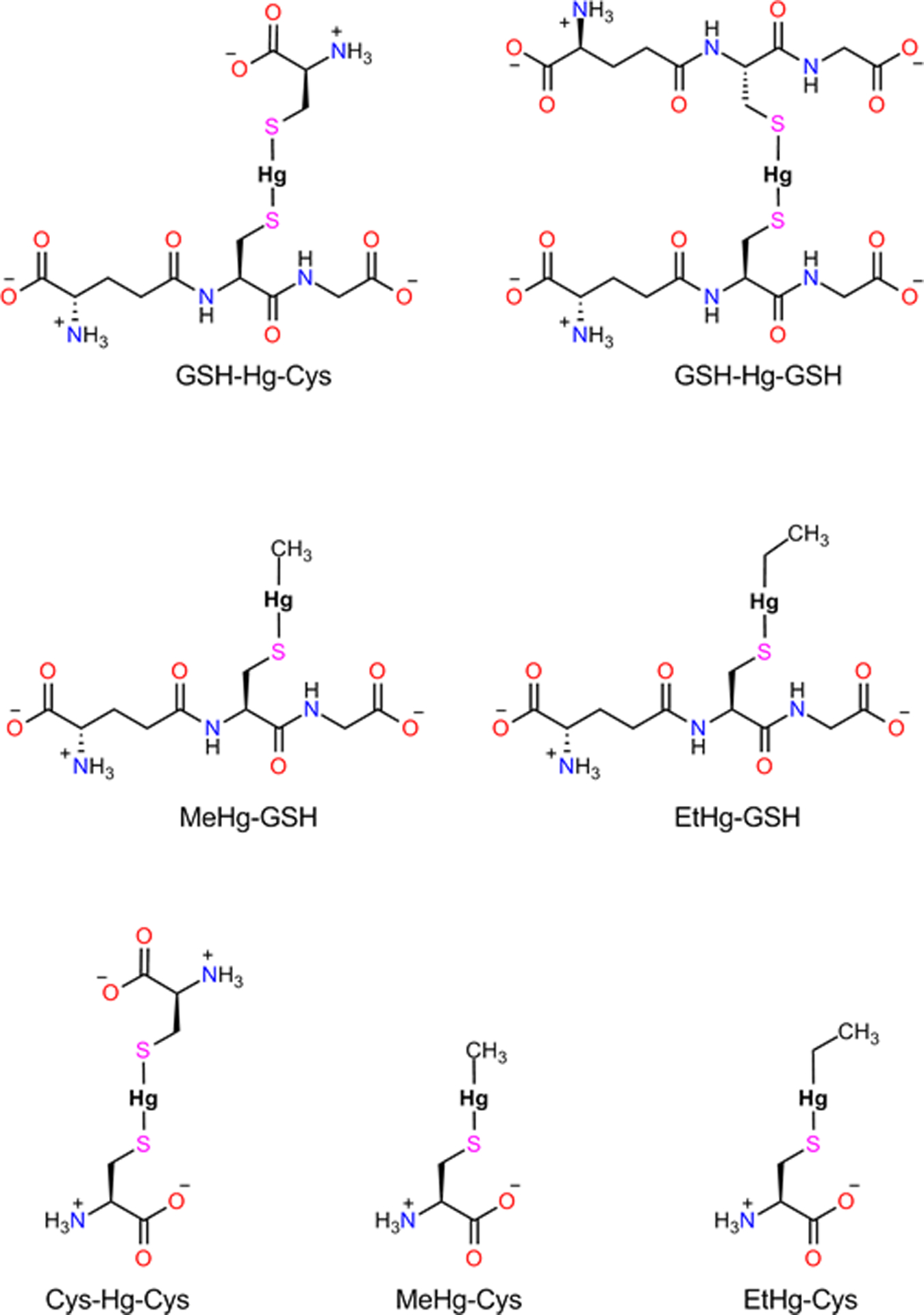
Low Molecular Mass (LMM-SH) complexes of cysteine and/or glutathione with electrophile forms of mercury (E+Hg, i.e., MeHg+; EtHg+ or Hg2+). The formation of the LMM-S-E+Hg complexes depicted here is of crucial importance for the preferential distribution of E+Hg forms to their target organs (Zalups 2000; Bridges and Zalups 2005, 2010, 2017; Farina et al. 2011a,b). The distribution of EtHg+-S-complexes has been rarely studied in the literature (Dórea et al. 2013).
Exposure to MeHg+ usually occurs via fish consumption (Burger et al. 2005; Gochfeld and Burger 2005; Schmidt et al. 2013; Montero-Alvez et al. 2014; Bosch et al. 2016), where the majority of MeHg+ is in the complexed form with cysteine (MeHg+-S-Cys) (Kuwabara et al. 2007). After the digestion of fish proteins containing the MeHg+-S-Cys complex, the mercurial part can be absorbed by distinct mechanisms that are not yet well characterized. The interaction of E+Hg with LMM-SH is critical for its distribution in the body of vertebrates and determined by exchange reactions of the type:
Where, R1-SH and R2-SH can be hypothetically either LMM-SH or HMM-SH (for a detailed discussion see Rabenstein and Fairhurst 1975; Rabenstein 1978; Rabenstein et al. 1982; Rabenstein and Reid 1984; Farina et al. 2011a,b; 2017). A schematic representation of the fate of dietary MeHg+ or intramuscularly administered EtHg+ is depicted in Figure 4. Hg2+’s fate will also depend on the binding to LMM-SH groups, and here the chemistry is a little more complicated because the exchange reactions can involve one or two thiol groups. The binding of Hg2+ to two cysteine molecules is critical for its movement in the body and, particularly, for its renal toxicity (Zalups 2000; Bridges and Zalups 2005, 2010, 2017; Figure 5).
Figure 4 –
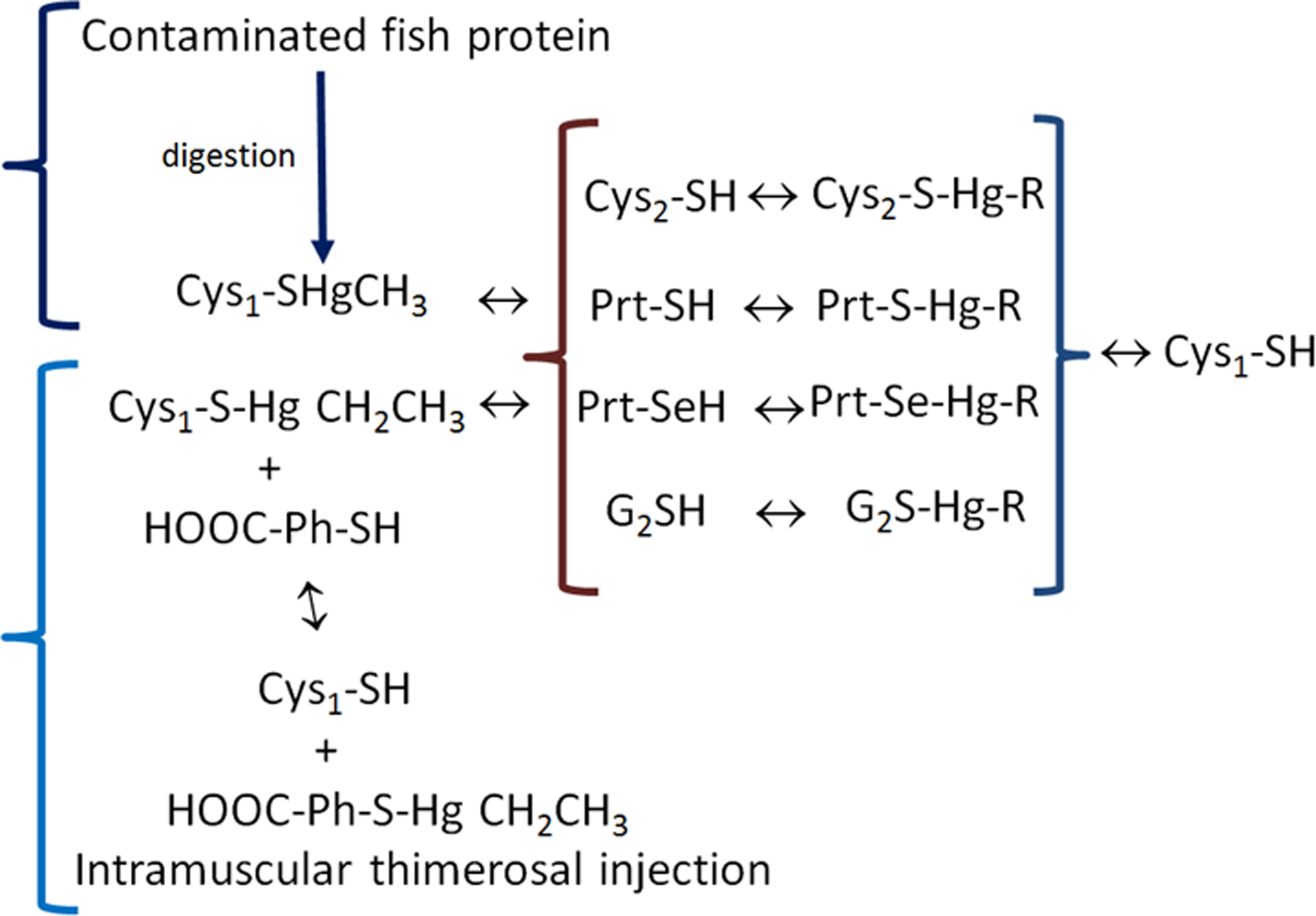
The fate of methylmercury (MeHg+) from the diet and ethylmercury (thimerosal) intramuscularly injected. The methylmercury-cysteine complex is represented by Cys1-SHgCH3 and thimerosal (ethylmercury) is represented by HOOC-Ph-S-Hg-CH2-CH3. In the figure, the first complex of MeHg+ (Cys1-SHgCH3) will enter the body and be exchanged with a second cysteine (Cys2-SH) to form a new complex (Cys2-S-Hg-R). Thimerosal (HOOC-Ph-S-Hg-CH2CH3, which is the complex of Ethylmercury (EtHg+) with thiobenzoic acid (HOOC-Ph-SH) will exchange with a free cysteine or Cys1-SH to release the free thiobenzoic acid (HOOC-Ph-SH) and the complex of ethylmercury with the Cys1-SH (Cys1-S-Hg-CH2CH3). To simplify, both the complexes of ethyl- and methyl-mercury are represented as R-Hg-Cys in the second set of reactions (inside the {}). The reaction of either MeHg-Cys1 or EtHg-Cys1 complexes with different free thiols in LMM-SH (Cys2-SH or G2SH) or HMM-SH molecules (Prt-SH or PrtSeH) will form new complexes of E+Hg with target and non-target molecules. The symbol “↔” indicates that the exchange reactions and the reactions can occur in both directions. Here it is important to emphasize that although the constant of formation of Hg-S bond is extremely high and, consequently, thermodynamically favorable, the Hg-S can rapidly exchange with another similar thiolate group (R-S−).
Figure 5–
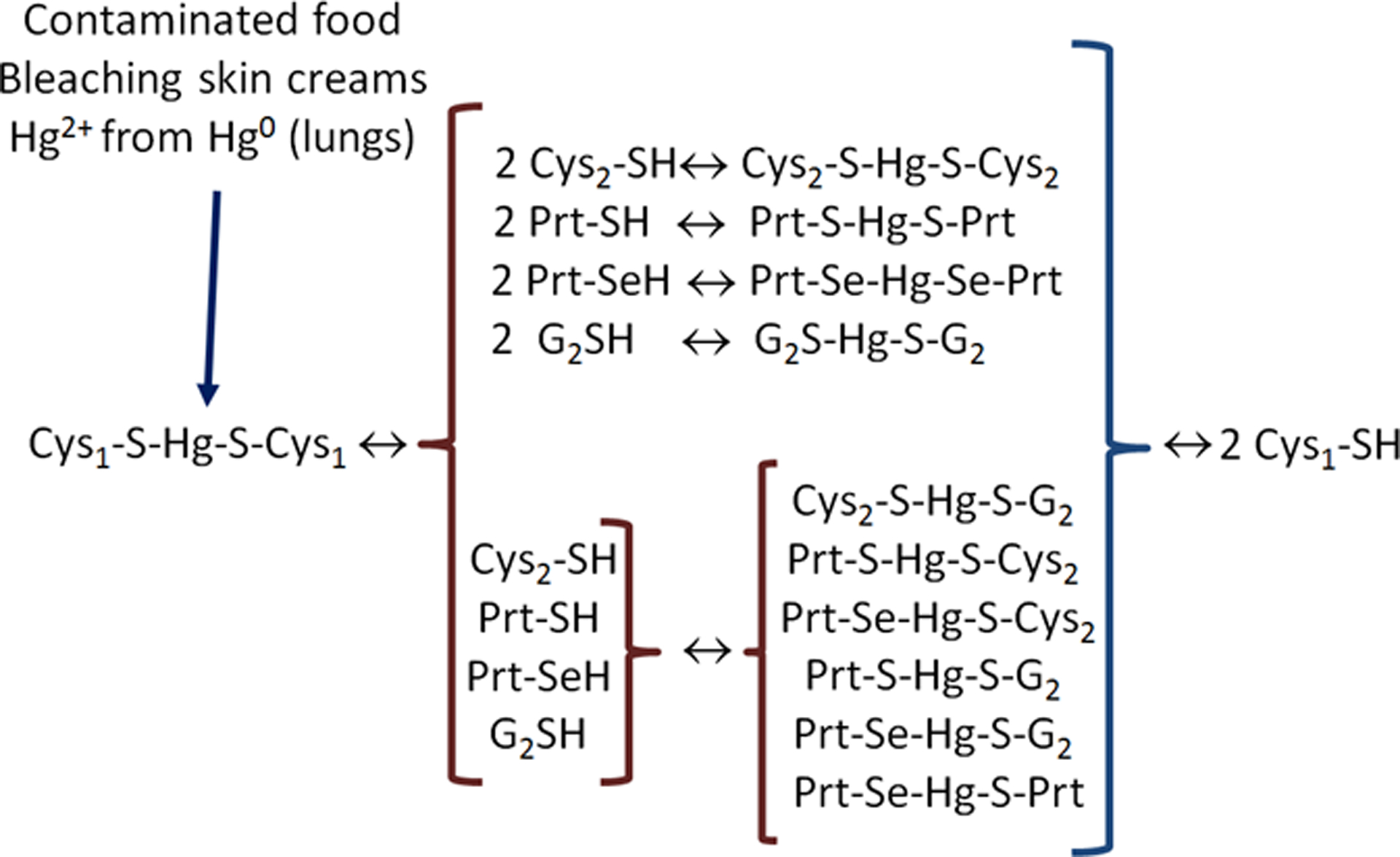
The fate of Hg2+ from the diet, bleaching skin creams and those oxidized from Hg0 absorbed in the lungs. After entering the body, any free Hg2+ will rapidly react with free thiol groups. Here the low molecular mass thiol-containing molecules (LMM-SH molecules) are represented by cysteine (Cys1-SH, Cys2-SH or complexed with Hg2+ and other organic groups represented by G2S−, Prt-S− or Prt-Se−). The complex Cys1-S-Hg-S-Cys1 can be absorbed from the intestinal tract and transported in the blood to the main target tissue (i.e., the kidney). (The molecular basis of why the kidney is the target organ of Hg2+ is elegantly reviewed in Zalups 2000). The reactions represented here can involve only one new thiol group (for instance, only one Cys2-SH can react with the complex Cys1-S-Hg-S-Cys1 forming Cys2-S-Hg-S-Cys1 and Cys1-SH as products and this applies to all the other thiols and complexes depicted in Figure 3.
More recently, MeHg+ and other E+Hg forms have been described in rice (Li R. et al. 2016; Brombach et al. 2017; Cui et al. 2017; Wu et al. 2018), and the exposure to E+Hg forms via rice ingestion can cause neurodevelopmental toxicity in humans (Rothenberg et al. 2016). Little is know about the speciation of E+Hg forms in rice (Wu et al. 2018), but in view of their high affinity for −SH groups, we can presume that they will be found forming stable complexes with both LMM-SH and HMM-SH molecules. Accordingly, the metalloproteomic analysis of rice experimentally exposed to E+Hg forms has indicated that E+Hg can bind to HMM-SH molecules (Li Y. et al. 2016).
Thimerosal is a complex EtHg+ with 2-mercaptobenzoic acid or thiosalicyclic acid (Figure 6) and is usually administered intramuscularly (as part of the TCV). The exposure to free Hg2+ is rare, but the presence of Hg2+ in contaminated food and whitening or bleaching skin creams has been reported frequently (Agrawal and Sharma 2017; Sun et al. 2017). In food, low and intermediate levels of Hg2+ (from picomolar to low micromolar concentrations) will be complexed with low molecular mass containing thiol molecules (LMM-SH, e.g. cysteine and reduced glutathione-GSH as complexes of the type LMM-S-Hg-S-LMM; Figure 3). In the case of contamination via skin creams, absorption will involve inding to either LMM-SH or to thiol-containing proteins (HMM-SH). Hg0 is normally absorbed by the lungs, and in the tissues and in the blood it can be oxidized to Hg2+. The free Hg2+ formed will rapidly react with free thiol groups of LMM-SH and/or HMM-SH molecules found in the blood or tissues (Figure 3).
Figure 6 -.
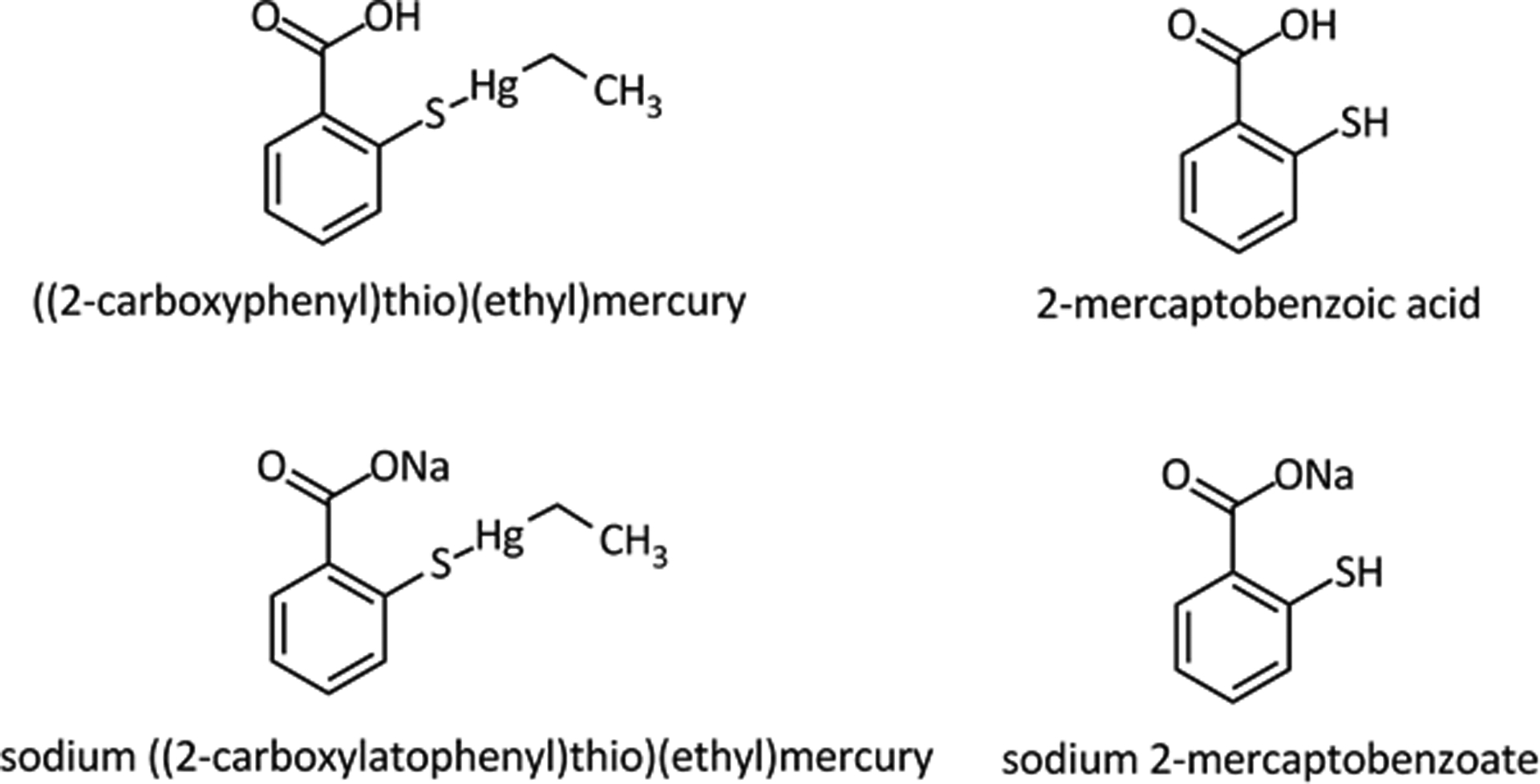
Thimerosal is a complex of Mercaptobezoate with ethylmercury (Et-Hg+) – The structures of thimerosal (either as an acid or a sodium salt) and mercaptobenzoic or salicyclic acid are depicted in the figure.
As described in Figure 4 for organic mercury forms, the basic molecular events involved in the absorption, transport, distribution and toxicity of Hg2+ are determined by exchange reactions of the original complex with one or two free cysteines (Cys2-SH) to form a new complex (Cys1-S-Hg-S-Cys2 or Cys2-S-Hg-S-Cys2; for more details, see the caption of Figure 3). The exchange reactions can occur with any free −SH group, and they are depicted in the scheme by the high molecular mass thiol- or selenol-containing proteins (HMM-SH or HMM-SeH) molecules or by LMM-SH molecules (Cys-SH or G2-SH). The reaction of Cys-Hg complexes with other thiol-containing molecules can generate a diverse number of R-S-Hg-S-R complexes either with different LMM-SH free thiols (Cys-SH or GSH) or HMM-SH/HMM-SeH molecules (Prt-SH or PrtSeH), which can be both target or non-target molecules of E+Hg forms.
In view of the strong affinity of thiol/thiolate and selenol/selenolate groups for electrophilic forms of mercury (E+Hg), the interaction of E+Hg forms with −SH or −SeH groups will be critical for initiating their toxicity. Nevertheless, our knowledge about the preferential targets of E+Hg is still incomplete and here we will discuss some of the caveats when deciphering the primary targets of E+Hg. The first seems to be related to the absorption of E+Hg forms, which will depend greatly on the type and level of exposure. Here we will not discuss the exposure to high levels of E+Hg forms, but only to environmentally or occupationally relevant levels. As a rule, exposure to low (pico- or nanomolar) or moderate levels (low micromolar concentrations) of E+Hg forms does not saturate the E+Hg binding sites (i.e. the thiol groups of non-target and target proteins) because the concentration of −SH groups is in the millimolar range in vertebrate fluids and tissues.
The question of potential saturation of −SeH groups found in a few selenoproteins, which are present at a much lower concentration than the thiol groups, is rather complex because we have practically no information about the distribution and concentration of selenoproteins in different cells and subcellular compartments. Indeed, our knowledge about the physiological function of the roughly two dozen selenoproteins found in vertebrates is incomplete, and only about one-half of them have a defined biochemical role in living cells (Fomenko et al. 2008; Labunskyy et al. 2014). Despite the limited number of studies on the interaction of MeHg+ with selenoproteins, data in the literature have indicated that important selenoenzymes might be targets for both in vitro and in vivo MeHg+ exposures (Wagner et al. 2010; Branco et al. 2011, 2012, 2014, 2017; Dalla Corte et al. 2013; Carvalho et al. 2008, 2011). Studies on the effects of EtHg+ on selenoenzymes are scarce, but analogous to Hg2+ and MeHg+, EtHg+ is also a potent inhibitor of TrxR (Carvalho et al. 2008, 2011; Rodrigues et al. 2015).
There is an large number of studies corroborating the inhibitory effect of E+Hg in thiol-containing proteins (Farina et al. 2017; some examples are listed in Table 3). Among them, are neurotransmitter receptors, various enzymes and cation channels (Farina et al. 2017). However, the question of concentration, distribution and reactivity of cysteinyl residues in thiol-containing proteins is also a critical point and serious limitation in understanding the neurotoxicity of E+Hg forms. As in the case of selenoproteins, we have only limited knowledge on the concentration, reactivity, and distribution of the majority of the thousands of proteins containing −SH groups (Weerapana et al. 2010; Ferrer-Sueta et al. 2011; Reisz et al. 2013; Dröse et al. 2014; Rudyk and Eaton 2014; Pereira et al. 2015; Riemer et al. 2015; Short et al. 2016; Wible and Sutter 2017; Heppner et al. 2018).
As mentioned above, any thiol group found in a protein is a potential target for E+Hg. The total number of cysteinyl residues codified in the human genome is about 550,000, found in about 72,000 proteins recovered from the UniProt database (www.uniprot.org). Considering only the proteins with experimental evidence of expression, which means about 19,000 proteins, one calculates the existence of 260,000 cysetinyl residues.
The human cysteinyl proteome has been estimated to be around 214,000 (Jones and Go 2011; Go et al. 2015), corroborating the number we have calculated above for proteins with experimental evidence. The thiol-containing proteins can have from one to several cysteinyl residues. Cysteine is one of the less abundant amino acids in proteins (Miseta and Csutora 2000; Poole 2015; Wible and Sutter 2017), representing only about 2–3% of amino acids comprising the human proteome. This is possibly due to the reactivity and nucleophilic softness of the cysteinyl group (Fomenko et al. 2008; LoPachin et al. 2012). Indeed, the −SH group is the second most powerful soft nucleophile group found in biomolecules, and the first is the −SeH group (Rocha et al. 2017). Their high reactivity likely restrained their abundance.
In contrast to the thiol groups, which are found in thousands of proteins (Jones and Go 2011; Go et al. 2015), the human genome codifies only about 25 selenoproteins (proteins containing the selenol group found in the selenocysteinyl, U or Sec residues) (Labunskyy et al. 2014), and normally they have 1 to a maximum of about 10 Sec residues in selenoprotein P (Table 4), which is a protein involved in the transport of Se to different tissues in mammals (Labunskyy et 2014; Oliveira et al. 2017a). In fact, the majority of selenoproteins have only one Sec residue and, in some cases, it participates in the analog motif of thiol oxidoreductase −C-X-X-C−, i.e., −C-X-X-U− or −U-X-X-C− (Table 4).
Table 4:
Examples of human selenoproteins involved in the regulation of brain physiology that can be inhibited by E+Hg. The Hg effects were based on animal or in vitro models. The proteins that affected rodents or in cultured cells were selected and then the human protein information was obtained at www.uniprot.org.
| Protein | Gene | CXXC | CXC | CC | C | CXXU | CXU | CU | U | aa | Function | Localization | Hg effects | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutathione peroxidase 1 | GPX1 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 1 | 203 | Oxidoreductase | Cytoplasm | Inhibition | Franco et al. 2009 |
| Glutathione peroxidase 4 | GPX4 | 0 | 0 | 0 | 9 | 0 | 0 | 0 | 1 | 197 | Peroxidase | Mitochondrion | Inhibition | Zemolin et al. 2012 |
| Iodothyronine deiodinase Type I | DIO1 | 0 | 0 | 0 | 4 | 0 | 1 | 0 | 1 | 249 | Thyroid hormone synthesis | Endoplasmic reticulum | Inhibition | Pantaleão et al. 2017 |
| Iodothyronine deiodinase Type II | DIO2 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 2 | 273 | Thyroid hormone biosynthesis, | Plasma Membrane | Inhibition | Mori et al. 2006 |
| Selenoprotein P | SELENOP | 1 | 1 | 1 | 17 | 1 | 4 | 1 | 10 | 381 | Transport of selenium | Extracellular region | Inhibition | Sasakura et al. 1998 |
| Selenoprotein W | SELENOW | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 87 | Antioxidant, redox-related process | Cytoplasm | Down-regulation | Kim et al. 2005 |
| Thioredoxin reductase 1 | TXNRD1 | 1 | 0 | 0 | 17 | 0 | 0 | 1 | 1 | 649 | Oxidoreductase | Cytoplasm | Inhibition | Carvalho et al. 2008 |
In short, there are thousands of targets for EtHg+ forms in the case of HMM-SH molecules, and only a few for HMM-SeH molecules. One important aspect that determines the toxicity of E+Hg forms is the accessibility and the intrinsic reactivity of the functional or catalytic role of a given cysteinyl or selenocysteinyl residue found in HMM-SH or HMM-SeH molecules. In the case of thiol groups, the cysteinyl residues in several HMM-SH molecules have no significant functional or catalytic role. Consequently, the binding of E+Hg to their −SH groups will not modify the protein activity. For instance, keratin has several cysteinyl residues that will form disulfide bonds in the secreted protein found in nails and hair. Keratinocytes can accumulate mercury in hair keratin either by incorporating cysteine-E+Hg complexes in the place of cysteine (Cernichiari et al. 2007), or E+Hg forms can binding to cysteinyl thiol groups in keratin before the formation of the disulfide bonds in the pre-keratin (Sippel 1973). Here we have an example where the binding of mercury to a protein will not significantly change the biological function of the protein (Figure 7). Of possible toxicological significance, the high quantity of mercury incorporated in secreted keratin found in hair and nails can be considered as a route of diversion of E+Hg forms from their target to non-target HMM-SH containing molecules.
Figure 7 –
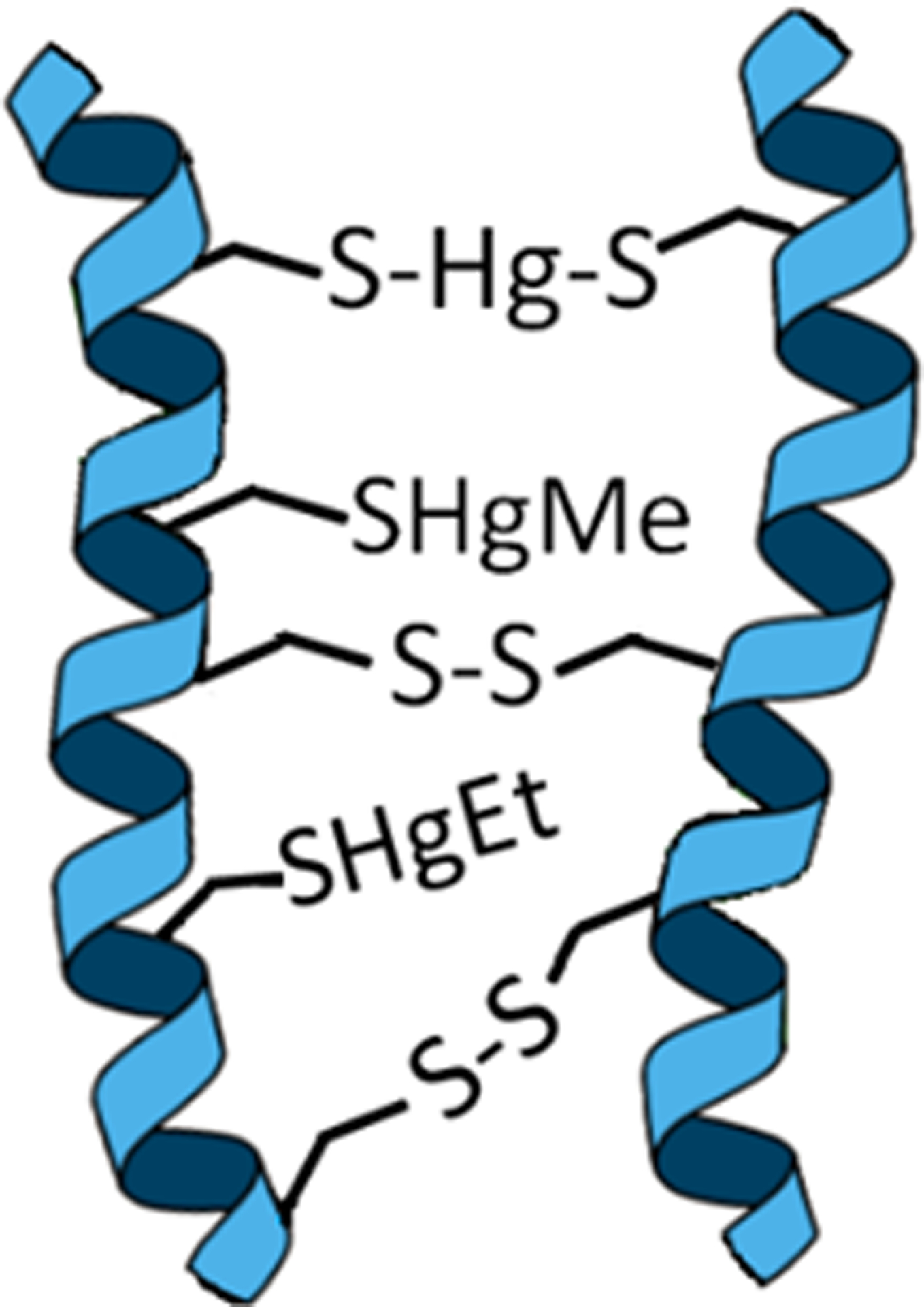
Schematic representation of accumulation of E+Hg forms in the keratin in hair or nails. The MeHg+ and EtHg+ coordinate with one −S− group, whereas Hg2+ coordinates with two −S− groups. The deposition of mercury in hair or nails usually does not change the biological function of the altered protein, and the quantification of Hg in hair is largely used to evaluate the exposure to Hg (Marques et al. 2009; 2011, 2012; Kusanagi et al. 2018)
The cysteinyl residues (Cys) in HMM-SH molecules can play either a direct or an indirect catalytic role in different classes of oxidoreductases, but an essential role in the catalysis or function of other types of proteins (Miseta and Csutora 2000; Fomenko et al. 2008; Poole 2015). Thiol-groups can also participate in the catalysis of non-redox reactions or have a critical structural role in proteins (Fomenko et al. 2008). Here, the binding of E+Hg forms to those cysteinyl residues can inactivate the physiological role of the protein, which can have an important toxicological impact on the exposed organism (Table 3). Some hypothetical types of interaction of E+Hg forms with HMM-SH molecules and the biological consequences of these interactions are depicted in Figure 8.
Figure 8 −.
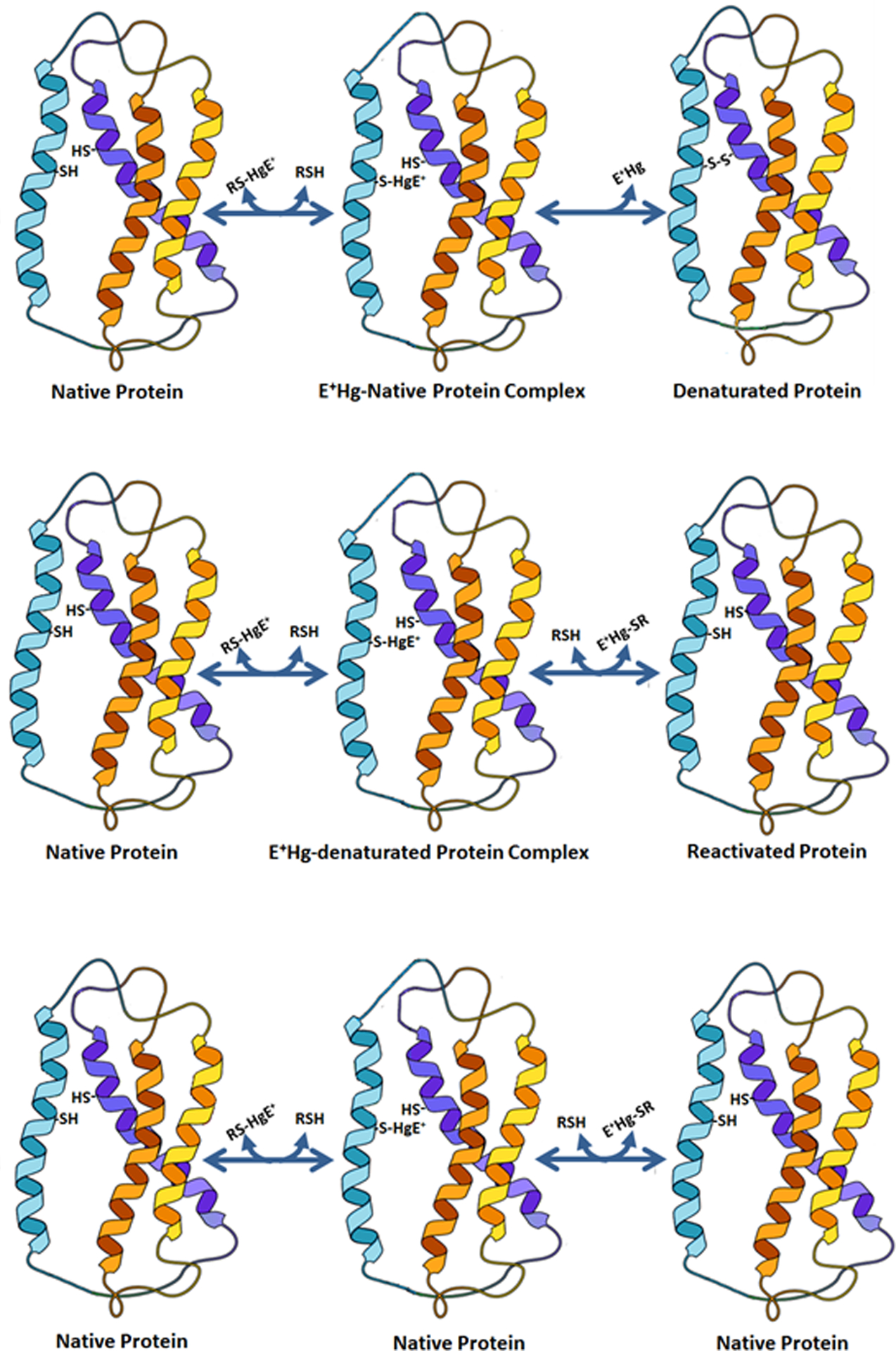
MeHg+ or EtHg+ can interact with thiol-containing proteins (HMM-SH molecules) and inactivate permanently (top) or transiently (middle) the protein function. In the third type of interaction, the binding of E+Hg to the HMM-SH did not modify the protein function (this is somewhat similar to that represented in Figure 7, except that here the E+Hg will only transiently interact with the HMM-SH molecule.
Some important motifs containing cysteinyl and/or selenocysteinyl residues are −C-X-X-C− or −C-X-X-U−, which are found in several oxidoreductases (Fomenko et al. 2008). The number of times that the motif −C-X-X-C− appears in human genes are near 19,000 in about 6,000 proteins (www.uniprot.org), but note that not all of these motifs will appear in native proteins, and not all proteins having the motif −C-X-X-C− will be an oxidoreductase (Table 5). For instance, the motif −C-X-X-C− can also be found in metal-coordinating proteins (Miseta and Csutora 2000; Fomenko et al. 2008), such as in delta-aminolevulinate dehydratase (porphobilinogen synthase; δ-AlaD) (Rocha et al. 2012). This enzyme is an in vitro and in vivo target for E+Hg (Rocha et al. 2012); however, a causal link between the enzyme inhibition and the triggering of toxic effects is difficult to establish. Broadly speaking, this is a critical caveat in the neurotoxicology of E+Hg forms, i.e. the observation that E+Hg forms can inhibit or disrupt a target protein does not establish a causal relationship between the phenomenon and the toxicological outcomes. Some examples of proteins that have been reported to be inhibited after either in vitro or in vivo exposure to MeHg+ are depicted in Table 3 (note that the proteins present in Table 3 are from the human genome, and the experimental studies were usually done in rodents or in vitro). The number of codified cysteinyl residues in these proteins varied from 1 to 27, and some of them have at least one of the motifs −C-X-X-C−, −C-X-C− or −C-C−. Others have only different numbers of “independent” −C− residues (Table 3 and 5).
Table 5.
Thiol groups residues found in the proteome of two mammals, human and mouse. We separated the proteins into two main types: ‘sp’ for UniProtKB/Swiss-Prot and ‘tr’ for UniProtKB/TrEMBL. This is the regular classification used in the UniProt Database.
| Organism | Total Protein | Protein Type | Thiol groups | |||
|---|---|---|---|---|---|---|
| CXC | CXXC | CC | C | |||
| Homo sapiens | 71,444 | sp | 8,573 | 19,038 | 8,486 | 259,182 |
| tr | 9,574 | 17,146 | 9,036 | 278,316 | ||
| Mus musculus | 51,949 | sp | 7,290 | 12,629 | 6,277 | 212,172 |
| tr | 9,407 | 21,372 | 10,107 | 290,020 | ||
An examination of Table 3 provides some examples of the bottlenecks found in the search for targets of E+Hg. MeHg+ can either inhibit or stimulate HMM-SH molecules (see for instance, glutathione-S-transferase and phospholipase A2, Table 3). One additional problem that can be inferred from Table 3 is the fact that the inhibition of E+Hg can be indirect. For instance, acetylcholinesterase has been reported to be inhibited after exposure to MeHg+ or Hg2+; however, the inhibition cannot be mediated by modifications of its thiol groups because none of them are reactive. In fact, the majority of them participate in −S-S− bridge formation.
Another important factor that we have commented on above is the reactivity of thiol groups. The presence of vicinal thiol groups can increase the potential reactivity of these groups (as found in −C-X-X-C− motifs), but some proteins have “independent”, but highly reactive −C− residues, for instance, in human muscle and mitochondrial creatine kinase (CK). CK and mitochondrial CK have one cysteinyl residue with a very low pKa (Wang et al. 2001; Naor and Jensen 2004), which explains their strong reactivity with electrophiles (Stachowiak et al. 1998; Alvarez and Radi 2003; Glaser et al. 2010a, 2014). Thus, it is imperative to develop new in silico tools in the area of predictive toxicology to envisage the structure and the reactivity of thiol groups using the primary structure of a given protein. Another aspect that has to be investigated is the interaction of E+Hg forms with nascent proteins, i.e. prior to post-translational processing (this may be the case of pre-keratin, where E+Hg forms are incorporated in the protein structure before the assembly of −S-S− bonds).
The physiological role of thiol-containing protein can be modulated by transitory S-glutathionylation, nitrosylation or reversible oxidation of cysteinyl residues (Dröse et al. 2014; Pereira et al. 2015; Poole 2015; Comini 2016). The binding of E+Hg to this class of cysteinyl residues can drastically modify the normal dynamics of activating-deactivating redox-regulated thiol-containing proteins. As noted above, the stable binding of E+Hg to cysteinyl residues can permanently inactivate the HMM-SH molecules involved in the regulation of cell physiology via thiol switches. Of particular toxicological significance, those proteins that are modulated by thiolation or nitrosylation can be preferential targets of E+Hg forms, because their cysteinyl residues involved in these reactions are normally highly accessible and reactive towards strong and soft electrophiles, such as E+Hg. However, there are no systematic studies about the interaction of E+Hg with these types of proteins. Thus, it is imperative to determine the effects of E+Hg in proteins regulated by thiolation and other similar mechanisms. Here we posit that E+Hg forms will disrupt the cell metabolism via blockage of cysteinyl residues involved in protein regulation via glutathionylation or nitrosylation. One example of such proteins is complex I in respiratory the chain (Dröse et al. 2014), and data from the literature have indicated that Complex I is inhibited by MeHg+ treatment (Table 3; Glaser et al. 2010a, 2013). The detailed in silico and in vitro systematic studies about the interaction of E+Hg with thiol groups in such proteins can help in the construction of virtual and molecular predictive tools to facilitate the difficult task of determining the primary molecular targets of E+Hg.
In contrast to thiol groups, the selenol group found in selenoproteins is normally involved in the oxidoreductase catalytic activity of selenoenzymes (Fomenko et al. 2007) and possibly in the physiological role of all the selenoproteins. Thus, the binding of E+Hg to the selenocysteinyl residues in selenoproteins is expected to inactivate them. Most characterized selenoproteins are oxidoreductases and catalyze important redox reactions, such as the degradation of peroxides (glutathione peroxidase or GPx isoforms) and the reduction of thioredoxin (thioredoxin reductase or TrxR isoforms). Thus, the inhibition of these antioxidant enzymes is believed to play a role in causing oxidative stress in MeHg+ poisoning (Farina et al. 2011a,b).
In addition, the binding mode of E+Hg species in the respective protein target, depends on the ligand (Cys, GSH or H2O) bound to the Hg atom; i.e., the molecular structure of the Hg molecule will determine which is its target.
For example, the molecular docking simulations demonstrated that MeHg+ and EtHg+ species (H2O-HgMe, Cys-HgMe, GSH-HgMe, H2O-HgEt, Cys-HgEt, GSH-HgEt) (Figure 9) and Hg2+ forms (H2O-Hg-OH, Cys-Hg-H2O, GSH-Hg-H2O, Cys-Hg-Cys, Cys-Hg-GSH, GSH-Hg-GSH) (Figure 10) can access the active site of the δ-AlaD enzyme. These E+Hg species can establish H-bonds, π-π and cation-π interactions, and the Zn⋯O and Hg⋯S coordination that helps to stabilize the Hg species in the active site of δ-AlaD. Specifically, the Hg⋯S coordination from the C124 and/or C132 residues can lead to the formation of an adduct between the enzyme and the Hg species, inhibiting the enzyme due to the denaturation of the active site, as depicted in Figure 11.
Figure 9.
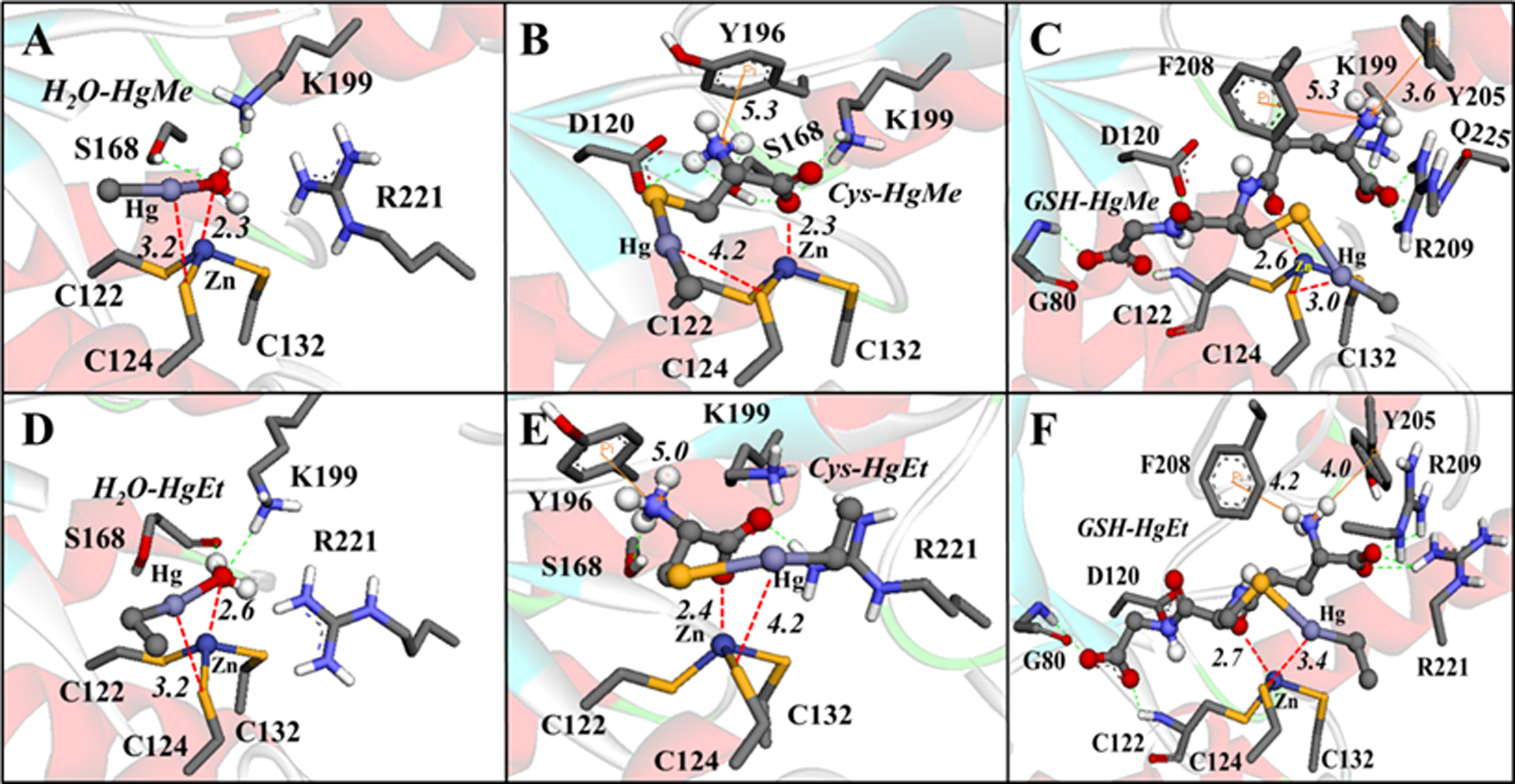
Interactions between the MeHg+ and EtHg+ species with δ-AlaD. H-bonds are shown in green dotted lines, Zn⋯O and Hg⋯S interactions are in red dotted lines, and the cation-π interactions are in orange lines. Only the main residues involved in the interactions are shown. The distances are in Å and the molecular docking simulations were carried out with the Auto Dock Vina 1.1.1 program (Trott and Olson 2010) using the docking configuration according to Nogara and Rocha 2017.
Figure 10.
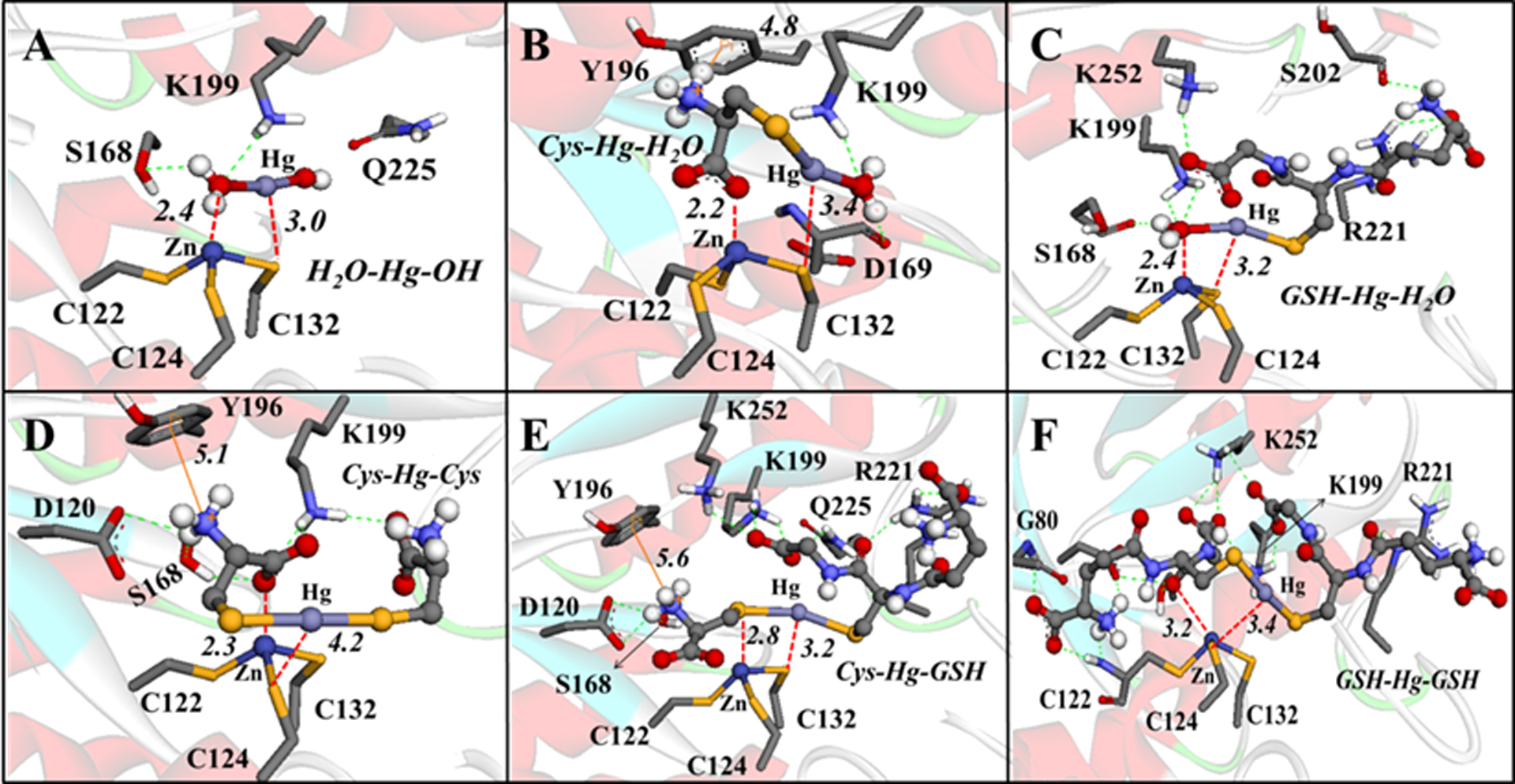
Interactions between the Hg2+ species with δ-AlaD. H-bonds are shown in green dotted lines, Zn⋯O and Hg⋯S interactions are in red dotted lines, and the cation-π interactions are in orange lines. Only the main residues involved in the interactions are shown. The distances are in Å and the molecular docking simulations were carried out with the Auto Dock Vina 1.1.1 program (Trott and Olson 2010) using the docking configuration according to Nogara and Rocha 2017.
Figure 11.
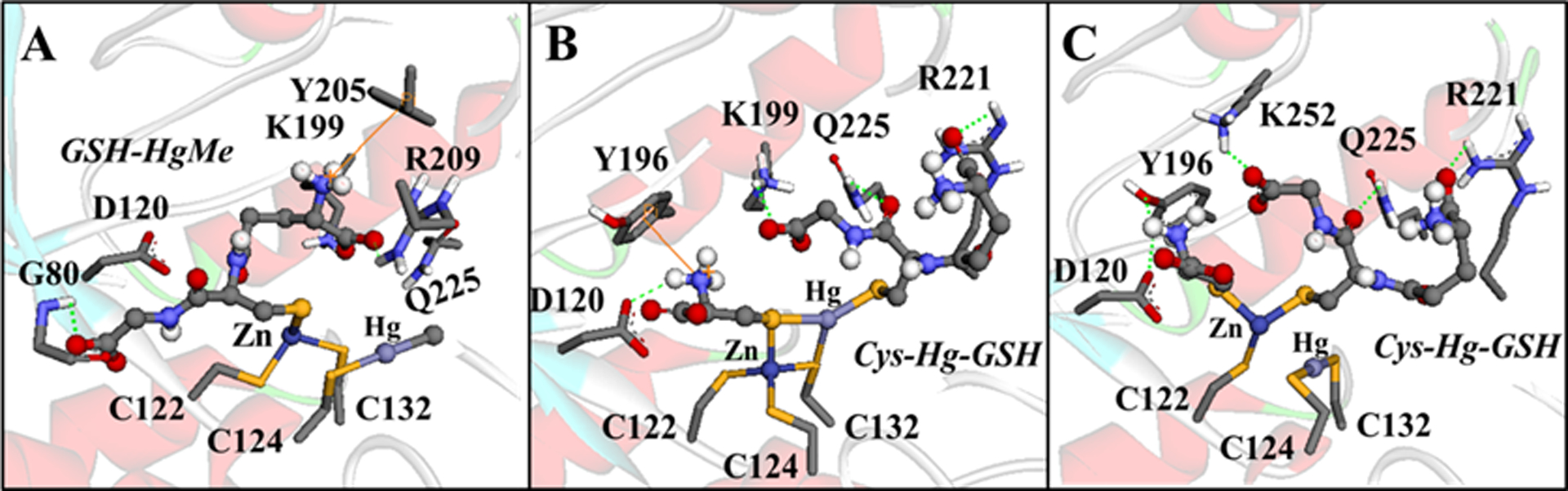
Simulation of the δ-AlaD denaturation caused by MeHg+ (A) and Hg2+ (B-C). H-bonds are showed in green dotted lines and the cation-π interactions are in orange lines. Only the main residues involved in the interactions are shown. The simulation of the enzyme denaturation was done manually in the software Avogadro 1.1.1 (Hanwell et al. 2012) using the amino acid residues at 5Å of the ligands (with the Cα frozen) and geometrically optimized with the universal force field (UFF) (5000 steps).
On the other hand, the docking simulation between the E+Hg species and the CK enzyme showed that only the small molecules (E+Hg species that do not present GSH bound to the Hg atom) can interact in the active site of the enzyme. The C283 residue could be a target for the organic mercury molecules H2O-HgMe, H2O-HgEt (Figure12A), Cys-HgMe and Cys-HgEt (Figure 12B). In addition, the Hg2+ molecule, Cys-Hg-H2O, also showed interactions with C283 (Figure 12C). However, the species H2O-Hg-OH showed S⋯Hg interactions with the C146 residue (Figure 12C), indicating that this residue could be a target as well. Due to the proximity of the C283 residue to the active site of CK, the conjugation between C283 and MeHg (Figure 12D) can lead to the enzyme inhibition. The simulations with the Hg conjugated with GSH do not present S⋯Hg interactions, due to the large distance between the molecule and the cysteinyl residue from the protein (C283(S)....Hg distance > 7Å) (data not shown).
Figure 12.
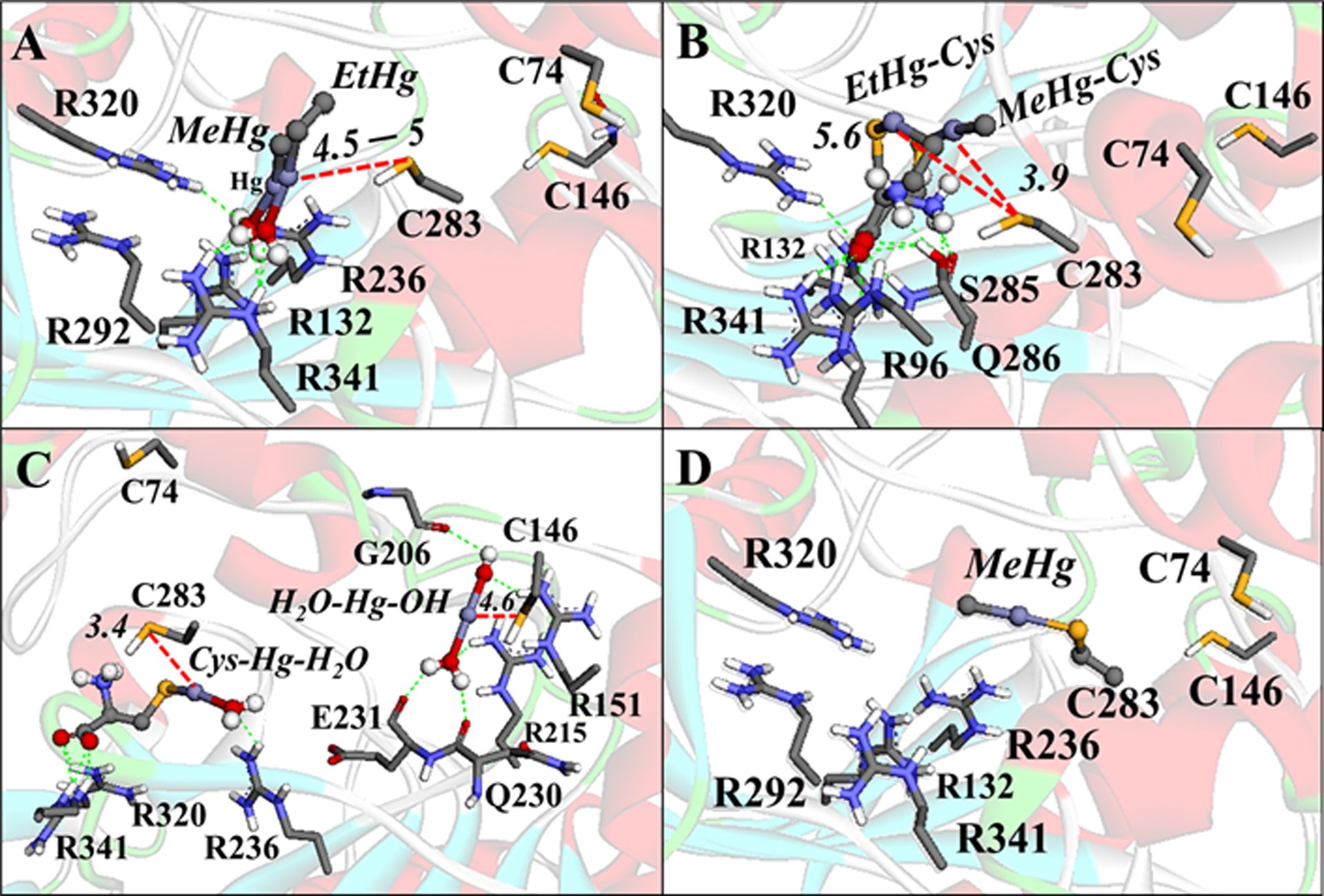
Interactions between the Hg species with CK. (A) MeHg-H2O and EtHg-H2O interactions. (B) Cys-HgMe and Cys-HgEt interactions. (C) Cys-Hg-H2O and [H2O-Hg-OH]+ interactions. (D) Simulation of the Se–Hg bond formation. H-bonds are shown in green dotted lines, Zn⋯O and Hg⋯S interactions are in red dotted lines, and the cation-π interactions are in orange lines. Only the main residues involved in the interactions are shown. The distances are in Å. The molecular blind docking simulations were carried out with the Auto Dock Vina 1.1.1 program (Trott and Olson 2010) using the human brain-type Creatine Kinase (PDB: 3DRB) (Bong et al. 2008), with the coordinates x= −58.518, y=1.229, z=5.558, and the grid box size of 64×68×50Å, and an exhaustiveness = 100. The simulation of the enzyme S−Hg conjugation was done manually in the software Avogadro 1.1.1 (Hanwell et al 2012) using the amino acid residues at 5Å of the ligands (with the Cα frozen) and geometrically optimized with the universal force field (UFF) (5000 steps).
As discussed above, the fate of E+Hg species in the body will depend greatly on the exchange reactions of the type:
Taking into consideration the results of the docking presented in Figures A-D, we can deduce that the neurotoxicity of E+Hg-S- complexes will vary depending on the size of the complexes and the reactivity/accessibility of the cysteinyl or selenocysteinyl residues involved in the reaction. Consequently, the targets of different types of LMM-S-HgE+ complexes must be identified for a better understanding of Hg neurotoxicity.
Another crucial aspect that has yet to be studied in detail in animal models is the simultaneous exposure to E+Hg forms and other potential neurotoxicants (e.g. Pb2+, Al3+, Sn2+, PCBs, etc). This type of study is needed, because humans and animals are normally exposed to a variety of chemical agents (Grandjean and Landrigan 2006). Of particular toxicological significance, synergistic, additive or antagonistic interactions among the toxicants can occur after the simultaneous exposure to E+Hg forms and one or more neurotoxicants. The determination of specific metabolic pathways and/or molecular targets disrupted after the simultaneous exposure to different neurotoxicants is also required, particularly on how the binary, tertiary, quartenary, etc. combinations of toxicants can have additive or synergistic neurotoxic effects. In the next section, some examples of the interaction between mercury and other neurotoxicants in developing humans are exemplified.
4.2. Hg and other neurotoxicants: additive effect?
As reviewed in Palumbo et al (2000), hair Hg in pregnancy has been associated with mild neurological effects from 5 to10 ppm (based on data from the Iraq MeHg+ poisoning), while studies of hair Hg derived from fish-eating (in New Zealand and Canada) have indicated an exposure range between 10 and 20 ppm may provoke adverse effects on the central nervous system (Palumbo et al. 2000).
Nevertheless, important longitudinal studies linking Hg-associated neurological deficits have shown inconsistent outcomes; while adverse associations were shown for Faroese children, no adverse effects have been reported for Seychelles children (Palumbo et al. 2000). Among differences between studies related to neurobehavioral-testing methods, source of exposure (whale meat and fish) and concomitant exposure to neurotoxicants may explain inconsistency between the two cohorts (Palumbo et al. 2000).
Often children-based studies examined neurobehavioral effects of one neurotoxicant at the time, when in real-life scenarios low-dose exposure to Hg occurs simultaneously with multiple co-occurring neurotoxic substances. Association between exposure to mercury and neurobehavioral outcomes requires consideration of co-exposure (to other neurotoxicants) and accompanying confounding factors. Indeed there is a body of literature that addressed combined low-level Hg (MeHg+ and EtHg+) exposures for additive and/or interactions with other environmental toxicants that can contribute to deficits in neurobehavioral test scores. Some of those studies are summarized in Tables 6 and 7.
Table 6.
Summary of studies addressing co-occurring exposures to neurotoxicants and their effects on neurobehavioral tests in infants (adapted and expanded from Dórea 2017a,b).
| Neurotoxic Agents | Aim of study: | Neuro-behavioral Tests (age): Outcomes | Reference (Country) |
|---|---|---|---|
| MeHg; EtHg | To compare the effect of time Hepatitis-B shots (<24h or >24hs after birth) on neurodevelopment. | Gesell Developmental Schedules (6 months): Infants immunized 2–4 days after delivery showed no significant difference in neurodevelopment compared to the group immunized within 24 h. | Marques et al. 2007 (Brazil) |
| MeHg; EtHg | To use Principal Component Analysis as a screening of variables linked to pre- and post-natal Hg exposure. | Gesell Developmental Schedules (6 months): Principal Component Analysis discriminated variability of neurodevelopment outcomes associated with variables that included pre- and post-natal Hg exposure. | Marques et al. 2008 (Brazil) |
| Hg2+; MeHg; EtHg | To evaluate the association between infant Hg and Gesell Developmental Schedules. | Gesell Developmental Schedules (6, 36, 60 months): Length of lactation was positively correlated with GDS scores and hair-Hg concentrations were negatively correlated with Gesell Developmental Schedules scores. | Marques et al. 2009 (Brazil) |
| MeHg; EtHg | To study the neurodevelopment of infants exposed to thimerosal in tetanus-diphtheria vaccines during pregnancy. | Gesell Developmental Schedules (6 months): No difference was observed. | Marques et al. 2010 (Brazil) |
| PCB; MeHg | To study a birth cohort to clarify the effects of PCB and MeHg on infants’ neurodevelopment. | Neonatal Behavioral Assessment Scale (3days): MeHg adversely affects neurobehavioral function. | Suzuki et al. 2010 (Japan) |
| MeHg; EtHg | To compare Hg effects on neurodevelopment of infants from one urban center and two rural villages. | Gesell Developmental Schedules (6 months): A higher score of neuro-development was negatively associated with exposure to additional TCV-EtHg in urban infants. | Dórea et al. 2012 (Brazil) |
| PCB; MeHg; Pb | To examine the effects of prenatal exposure to PCBs, MeHg, and Pb on cognitive development infants. | Fagan Test of Infant Intelligence; A-not-B test; Bayley Scales of Infant Development–2nd Edition (6.5 and 11 months): Outcomes of impairment in specific domains were different for the tested neurotoxicants; Hg was associated with poorer performance on A-not-B test. | Boucher et al. 2014 (Canada) |
| MeHg; EtHg; Pb; Al | To study the neurodevelopment of children living in the vicinity of tin-ore kilns and smelters and of children living in a fishing village. | Bayley Scales of Infant Development–2nd Edition (6 and 24 months): Mental Development Index and Psychomotor Development Index were lower at 24 months of age for children living in the vicinity of tin-ore kilns and smelters. | Marques et al. 2014 (Brazil) |
| MeHg; EtHg | To study Hg exposure and neurodevelopment in children from a tin-ore open-pit mine. | Bayley Scales of Infant Development–2nd Edition (6 and 24 months): There was a significant sex difference with boys showing more sensitivity related to Bayley Scales of Infant Development–2nd Edition delays and Thimerosal-containing vaccines. | Marques et al. 2015 (Brazil) |
| MeHg; EtHg | To study neurodevelopment of children in relation to prolonged breastfeeding and Hg exposure. | Bayley Scales of Infant Development–2nd Edition (6 and 24 months): MeHg and EtHg exposure did not show a significant dose effect (related to the length of breastfeeding) on neurodevelopment among groups. | Marques et al. 2016b (Brazil) |
| MeHg; EtHg | To study neurodevelopment of children exposed to MeHg and EtHg. | Bayley Scales of Infant Development–2nd Edition (6 and 24 months): There was a significant delay in Bayley Scales of Infant Development related to the combined exposure to Hg. | Marques et al. 2016a (Brazil) |
EtHg: ethylmercury; MeHg: methylmercury; PCBs: polychlorinated biphenyls; TCV: Thimerosal-containing vaccines
Table 7.
Summary of studies addressing co-occurring exposures of neurotoxicants and their effects on neurobehavioral tests in preschooler and school-aged children. (adapted and expanded from Dórea 2017a,b).
| Neurotoxic Agents | Aim of study: | Neuro-behavior Tests (age): Outcomes | Reference (Country) |
|---|---|---|---|
| PRESCHOOLERS | |||
| PCB; MeHg | To verify the possible additive and/or interactive effects between PCB and MeHg. | McCarthy Scales of Children’s Abilities (38, 54 months): A significant interaction between cord blood PCBs and maternal hair Hg was found. | Stewart et al. 2003 (United States) |
| PCB; MeHg; Pb | To evaluate the impact of chronic exposure to PCBs and MeHg on visual brain processing. | Visual Evoked Potentials (5–6 years): Exposure to MeHg and PCBs was associated with alterations of visual evoked potential responses, especially for the latency of the N75 and of the P100 components. | Saint-Amour et al. 2006 (Canada) |
| MeHg; EtHg | To assess the dependence on fish consumption of families and its impact on nutritional status and neurodevelopment of pre-school children. | Gesell Developmental Schedules (1–59 months): Fish consumption (as hair Hg) had no impact on Gesell Developmental Schedules. | Marques et al. 2011 (Brazil) |
| PCB; MeHg; Pb | To assess whether PCB, MeHg, and Pb influence infants’ maladaptive behavior problems. | Child Behavior Checklist (30 months): Internalizing behavior was affected by prenatal exposure to low levels of PCBs. No statistically significant association with Hg or Pb. | Tatsuta et al. 2012 (Japan) |
| EtHg; Pb | To examine the relationship between neonatal exposure to Thimerosal-containing vaccines and child development. | Bayley Scales of Infant Development–2nd Edition (12, 24 months): The overall deficit in the psychomotor development index was significantly higher in TCV group. | Mrozek-Budzyn et al. 2012 (Poland) |
| MeHg; EtHg; Al | To assess the effects of fish consumption and undernutrition on neurodevelopment of pre-school children. | Gesell Developmental Schedules (1–59 months): Among others, hair Hg was associated with a delay in language and personal-social development. | Marques et al. 2012 (Brazil) |
| Hg, Mn; As; Pb | To explore the relationship between in utero exposure to environmental neurotoxic metals and neurodevelopment. | Comprehensive Developmental Inventory for Infants and Toddlers (24 months): Significant adverse association with neurodevelopment only for manganese and lead. | Lin et al. 2013 (Taiwan) |
| MeHg; EtHg | To assess milestone achievements and neurodevelopment in toddlers associated with MeHg and EtHg exposure. | Gesell Developmental Schedules (12, 59 months): Milestone achievement was delayed in toddlers from tin-ore mining communities. | Dórea et al. 2014 (Brazil) |
| PCB; MeHg; Pb | To study the relationships between PCB, MeHg and Pb and IQ scores. | Kaufman Assessment Battery for Children (42 months): Scores were negatively affected by prenatal exposure to PCBs. No statistically significant association with Hg or Pb. | Tatsuta et al. 2014 (Japan) |
| PCB; Hg; Pb; Cd | To assess the neurocognitive development of children exposed in utero to environmental neurotoxicants. | Bayley Scales of Infant Development–2nd Edition (24 months): Only PCB118 had a negative impact on neurocognitive development. | Brucker-Davis et al. 2015 (France) |
| Hg; Pb | To study the associations between prenatal and early life exposure to Hg and Pb with toddler temperament. | Toddler Temperament Scale (24 months): No significant association between Pb and Hg exposure was observed in the temperament classification. | Stroustrup et al. 2016 (Mexico) |
| Bisphenol A; PCB; Phtalate; Mn; Hg; Pb | To evaluate the association of prenatal exposure to neurotoxicants and neurodevelopment. | Bayley Scales of Infant Development–2nd Edition (12, 24 months): Maternal exposure to several xenobiotics was associated with adverse neurodevelopmental performances among the children. | Kim et al. 2017 (South Korea) |
| Hg, Pb; Cd | To investigate cognitive development during the first five years of life in relation to heavy metal exposure. | Bayley Scales of Infant Development–2nd Edition and Wechsler Preschool and Primary Scale of Intelligence (6 to 60 m): The stability of cognitive development was unrelated to the umbilical cord level of heavy metals. | Lee et al. 2017 (South Korea) |
| As; Cd; Hg; Mn; Pb | To investigate the effects of prenatal co-exposure to As, Cd, Hg, Mn, and Pb on infants’ cognitive and motor function. | McCarthy Scales of Children’s Abilities (4–5 years): Hg was associated in a dose-response manner with lower scores on the verbal function, and non-linearly related to poorer motor function and gross motor skills. | Freire et al. 2018 (Spain) |
| SCHOOL-AGED CHILDREN | |||
| PCB; MeHg; Pb | To study performance in delayed reinforcement schedules in children exposed to environmental mixtures of PCBs, MeHg, and Pb. | Differential reinforcement of low rates (9.5 years): Children exposed to PCB, MeHg, and Pb demonstrated an impaired performance. | Stewart et al. 2006 (United States) |
| Hg; Pb; Al | Possible contribution of environmental neurotoxicants on attention-deficit hyperactivity disorder. | Test battery for attention performance of children (8–12 years): Evidence confirms that elements of attention-deficit hyperactivity disorder are adversely affected by low levels of Pb, but not by other neurotoxic trace metals. | Nicolescu et al. 2010 (Romenia) |
| MeHg; Pb | To evaluate the effects of MeHg and Pb exposure on infants’ neurodevelopment. | Bayley Scales of Infant Development–2nd Edition (2, 5, 7 years): Tested postnatal MeHg exposure levels had no adverse effects on children’s IQ. | Cao et al. 2010 (United States) |
| MeHg; Pb | To assess the effects of prenatal Pb and MeHg exposure on cognitive deficits. | Wechsler Intelligence Scale for Children (7, 14 years): Some interaction terms between lead and methylmercury suggested that the combined effect of the exposures was less than additive. | Yorifuji et al. 2011 (Faroe Islands) |
| PCB; MeHg | To determine brain function alterations related to neurobehavior in subjects with high prenatal exposure to MeHg and PCBs. | Functional Magnetic Resonance Imaging (15 years): Subjects with high mixed exposure to MeHg and PCBs showed activation in more areas of the brain and different and wider patterns of activation than the low mixed exposure group. | White et al. 2011 (Faroe Islands) |
| PCB; MeHg; Pb | To examine the neurophysiological correlations of response inhibition deficits in children exposed to PCB, MeHg, and Pb. | Go/No-go Performance (11.3 years): Hg concentrations were not related to any outcome on this task but showed significant interactions with other contaminants on certain outcomes. | Boucher et al. 2012a (Canada) |
| PCB; MeHg; Pb | To examine the relationship of developmental exposure to MeHg, PCBs, and Pb to behavioral problems at school age. | Disruptive Behavior Disorders Rating Scale (11.3 years): Hg was not related to impairment in response inhibition but showed significant interactions with Pb and PCB. | Boucher et al. 2012b (Canada) |
| PCB; Hg; Pb | To assess the impact of exposure to contaminants on visual brain development in school-age children. | Visual Evoked Potentials (10.9 years): Cord blood Hg was associated with a reduction of the N75 amplitude at the highest contrast level and with a delay of the N75 latency at the 12% contrast level. | Ethier et al. 2012 (Canada) |
| Cigarette smoke; Hg; Pb | To assess the interactions of prenatal smoking with Pb and Hg. | Teacher Report Form and the Disruptive Behavior Disorders Rating Scale (11.3 years): Co-exposure to Pb and Hg do not appear to exacerbate tobacco effects. | Desrosiers et al. 2013 (Canada) |
| MeHg; Pb | To study the effects of low-level postnatal MeHg and Pb exposure. | Rapid Sequential Movements from the Neurological Examination for Subtle Signs, Full-Scale IQ, Broad Reading, Memory and Learning Slope, Attention/Executive Functions from the Neuropsychological Assessment, Adaptive Skills, and Hit Reaction Time (7 years): No detectable adverse effect on neuropsychological development due to the relatively low MeHg exposure. | Wang et al. 2014 (United States) |
| PCB; Hg; Organochlorine | To evaluate the effects of prenatal neurotoxicant exposures on memory and learning. | Wide Range Assessment of Memory and Learning (8 years): Hg was associated with decrements of Visual Memory, Learning, and Verbal Memory. | Orenstein et al 2014 (United States) |
| PCB; MeHg; Pb | To verify the association of prenatal exposure to neurotoxicants and fine motor function. | Santa Ana Form Board, Finger Tapping Test (11.3 years): High Hg levels were independently associated with poorer Finger Tapping performance. | Boucher et al. 2016 (Canada) |
The effects of multiple exposures during pre- and postnatal life are linked to children’s neuropsychological development, but the weight of Hg as the neurotoxic causative factors will depend on the tested parameter. The results revealed a great variability in Hg exposure related to time (prenatal and postnatal) and route of exposure (injected and breastfeeding), marker of exposure (blood, hair, vaccination records), and co-occurring neurotoxicants (Al, Pb, PCBs, and unidentified sources of pollutants related to mining and smoking). In most of the studies, prenatal Hg exposure did not show a predominant neurodevelopment outcome in the first months of postnatal age.
Infants (less than six months) have the highest combined exposures of neurotoxicants. Most of the exposures originate during pregnancy and likely continue during breastfeeding. The potential for the highest body burden at this early stage is further increased by the EtHg-Al exposure after a full schedule of thimerosal-containing vaccines. Nevertheless, the measured outcomes seem to be limited (Table 6). On the basis of prenatal exposure to neurotoxicants, the additional burden of EtHg-Al (in thimerosal-containing vaccines) is a main cause of concern; the risk of insults that may change neurodevelopmental outcomes has been shown in some studies (Table 6).
Table 7 summarizes the outcomes of neurobehavioral tests in preschoolers and schoolchildren. In preschoolers, most studies did not show significant associations of Hg and deficits in test outcomes; coincidentally, the few studies showing statistically significant Hg interactions were in children living in mining environments (Marques et al. 2012; Dórea et al. 2014), or exposed to a larger suite of neurotoxic metals (Boucher et al. 2014; Freire et al. 2018). It is worth mentioning that in 30 month-old children, internalizing behavior was affected by prenatal levels of PCBs but not by Hg (Tatsuta et al. 2012); neurodevelopment measured at 42 months by the Kaufman Assessment Battery for Children showed similar results (Tatsuta et al. 2014).
Studies in schoolchildren (Table 7) and prenatal Hg exposure used different neurobehavioral tests (assessing sensory processing, speech, hearing, and socialization). Sensitivities of tests regarding motor, cognitive, and mental performance are not discussed here. However, the diversity of constitutional and environmental factors coupled to the different combinations of neurotoxicants makes it challenging to extricate the weight of the Hg toxicity. Nevertheless, the outcomes highlight the importance of the statistical models chosen. Neurophysiological outcomes related to response inhibition deficits were differently affected by cord-blood PCBs and Pb but not by Hg (Boucher et al. 2012a).
Significant association of neurodevelopmental delays with cord-blood-Hg was seen in few studies (Yorifuji et al. 2011; Boucher et al. 2012a, 2016; Tratnik et al. 2017). Prenatal exposure showed distinct domain-specific adverse effects for PCBs (impaired visual recognition memory), Hg (work memory), and Pb (slower speed of information processing) measured by the Fagan Test of Infant Intelligence and the A-not-B tests (Boucher et al. 2014). Altered fine-motor function in school children that were prenatally exposed to these neurotoxicants in traditional diets showed distinct neurological outcomes: higher current PCB levels were associated with poor Santa Ana Form Board and Finger Tapping performances; higher current Hg levels were independently associated with poor Finger Tapping (Boucher et al. 2016). Recently, Kalia et al (2017) reported a beneficial effect on Gesell Development Scores in Chinese children by reducing Policycle Aromatic Hydrocarbon exposure but no significant association with Hg. Indeed, several studies showed neurotoxic effects of Hg in parallel with other pollutants: Boucher et al (2012b) reported no MeHg effect per se, but significant interactions with lead and PCB. White et al (2011) showed that interaction of MeHg and PCB displays more Functional Magnetic Resonance Imaging in wide areas of the brain.
Children on a traditional diet that were prenatally exposed to MeHg, PCBs, and Pb showed significant associations between Attention Deficit Hyperactivity Disorder and neurotoxicants from children’s own dietary sources at the time of tests (Boucher et al. 2012b). Gump et al (2017) showed distinct domain influences of low-level exposures Pb (children with higher Pb levels “exhibit higher levels of hostile distrust and oppositional defiant behaviors, were more dissatisfied and uncertain about their emotions, and had difficulties with communication”) and Hg (positively associated with autism spectrum behaviors). Wang et al (2014) studied low-level MeHg exposure in school children already exposed to Pb and reported no adverse effects on several neurodevelopment tests (Rapid Sequential Movements from the Neurological Examination for Subtle Signs; Full-Scale IQ, Broad Reading, List A Memory and Learning Slope, Attention/Executive Functions from the Neuropsychological Assessment, Adaptive Skills, and Hit Reaction Time).
In order to evaluate cognitive and psychomotor functions in older children, the results of Table 7 are organized as preschool (1–5 years) and school children (> 5 years). In preschoolers, performance tests were assessed by cognitive and psychomotor scales (Gesell Development Scores; Bayley Scales of Infant Development; Comprehensive Developmental Inventory for Infants and Toddlers; McCarthy Scales of Children’s abilities; Fagan Test of Infant Intelligence; Kaufman Assessment Battery for Children), visual function (visual evoked potentials; A-not-B). Personality tests were assessed as behavioral outcomes (Child Behavior Checklist; Toddler Temperament Scale). During this intermediary stage, the weight of mercury was not outstanding; most outcomes with a significant weight of mercury happened with an unmeasured mining pollution (Table 7).
As the child develops and matures other tests measure behavioral problems (Differential Reinforcement of Low Rates; Attention-deficit hyperactivity disorder; Teacher Report Form; Disruptive Behavior Disorders Rating Scale; Neurological Examination for Subtle Signs), learning ability (Wide Range Assessment of Memory and Learning), motor performance (Santa Ana Form Board; Finger Tapping Test) and intelligence (Intelligence Quotient; Wechsler Intelligence Scale for Children-Revised) in school children. Because of the variety and specificity of tests, it is challenging to draw an outcome profile for the exposure combinations. However, it is possible to conclude that unaccounted variables can interfere positively/negatively with neurodevelopment.
Gascon et al (2017) summarized the prenatal and postnatal exposures to neurotoxicants and neuroprotective components co-occurring during childhood; they showed that depending on the study characteristic, for the individual role of Hg there was no effect or a negative effect. In their Spanish cohort, neurodevelopment was seen to be positively impacted by breastfeeding, fish consumption, and nutritional profiles. This overall positive effect of prenatal fish consumption and breastfeeding were also observed in association with students’ achievement scores (Schmiedel et al. 2017).
The variation of a specific time window for exposure and comparable age of testing, along with a defined combination of neurotoxicants, makes neurobehavioral outcomes challenging to interpret. Due to environmental and constitutional confounding factors, the children`s neurologic outcomes related to low-level exposures to either fish-MeHg or EtHg per se remain unpredicted. In most cases of multiple low-level Hg exposures with other neurotoxicants, deficits are in the normal range and are not prodromes of neurological diseases. However, the clinically unperceived or “subtle” individual neurodevelopmental deficit has societal costs that are reflected in public spending on special education resources and/or in diminished social achievements or work productivity. Economic models to estimate these costs were made for exposures to MeHg (Gaskin et al. 2015; Bellinger et al. 2016; Trasande et al. 2016) and EtHg (Geier et al. 2016, 2018b)
Concluding Remarks
Mercury is one of the most neurotoxic elements found in the environment, and exposure to E+Hg forms can have long-lasting behavioral consequences. Consequently, the banning of intentional exposure to E+Hg forms (e.g. thimerosal) should be extended to all young children (not just in rich countries). In addition, unintentional exposure to MeHg should be avoided by adopting preventive attitudes; for instance, by decreasing or eliminating the consumption of piscivorous or predatory fish during gestation and lactation. A link between exposure to low levels of mercury during human brain development and permanent behavioral disabilities remains debatable. However, a massive set of experimental data supports the deleterious neurobehavioral and neurochemical effects of low levels of exposure to Hg, strongly suggesting that E+Hg forms are contributing factors in neurodevelopmental diseases. Indeed, the neurochemical indications that E+Hg forms disrupt normal brain functioning at different subcellular and molecular levels are conclusive. For instance, E+Hg forms have been shown to impair the synaptic biochemistry and physiology at multiple points, which can have a causal role in neurodegenerative diseases (Poirel et al. 2018). Nevertheless, although many targets for E+Hg forms have been described, we know little about their primary molecular targets. As discussed above, co-exposure to mercurial and other neurotoxicants can exacerbate the neurodevelopmental effects of Hg in developing humans, but our knowledge about the molecular events that synergistically or additively exacerbate the neurotoxocity of E+Hg forms is still elusive. Thus, in addition to taking preventative measures to avoid Hg exposure, the development of new experimental and virtual approaches to study the molecular targets of E+Hg forms and other neurotoxicants will be crucial to better understand how neurotoxicants inflict permanent neurodevelopmental disabilities on humans.
Acknowledgements
MA was supported in part by grants from the National Institute of Environmental Health Sciences R01 ES07331, R01 ES020852.
Contributor Information
Michael Aschner, Department of Molecular Pharmacology, Albert Einstein College of Medicine, Bronx, New York, NY, USA.
João B. T. Rocha, Departamento de Bioquímica e Biologia Molecular, Centro de Ciências Naturais e Exatas, Universidade Federal de Santa Maria, Santa Maria, RS, Brazil.
José G. Dórea, Professor Emeritus, Faculdade de Ciências da Saúde, Universidade de Brasília, Brasília, DF, Brazil.
References:
- Abdallah EAM, Shamoo AE. Protective effect of dimercaptosuccinic acid on methylmercury and mercuric chloride inhibition of rat brain muscarinic acetylcholine receptors. Pest Biochem Physiol 21:385–393, 1984. [Google Scholar]
- Abd-Elfattah ASA, Shamoo AE. Regeneration of a functionally active rat brain muscarinic receptor by D-penicillamine after inhibition with methylmercury and mercuric chloride. Evidence for essential sulfhydryl groups in muscarinic receptor binding sites. Mol Pharmacol 20:492–497, 1981. [PubMed] [Google Scholar]
- Adams JB, Romdalvik J, Ramanujam VMS, Legator MS: Mercury, lead, and zinc in baby teeth of children with autism versus controls. J Toxicol Environ Health 70:1046–1051, 2007. [DOI] [PubMed] [Google Scholar]
- Agrawal SS, Sharma P. Current status of mercury level in skin whitening creams. Current Med Res Prac 30:47–50, 2017. [Google Scholar]
- Allen JW, Mutkus LA, Aschner M. Methylmercury-mediated inhibition of 3H-D-aspartate transport in cultured astrocytes is reversed by the antioxidant catalase. Brain Res 902:92–100, 2001. [DOI] [PubMed] [Google Scholar]
- Alvarez B, Radi R. Peroxynitrite reactivity with amino acids and proteins. Amino acids 25:295–311, 2003. [DOI] [PubMed] [Google Scholar]
- Alves AC, Monteiro MS, Machado AL, Oliveira M, Bóia A, Correia A, Oliveira N, Soares AM, Loureiro S. Mercury levels in parturient and newborns from Aveiro region, Portugal. J Toxicol Environ Health Part A 9:1–3, 2017. [DOI] [PubMed] [Google Scholar]
- AMAP/UNEP, 2013. Technical Background Report for the Global Mercury Assessment 2013 Arctic Monitoring and Assessment Programme, Oslo, Norway/UNEP Chemicals Branch, Geneva, Switzerland. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth ed American Psychiatric Association, Arlington: 2013. [Google Scholar]
- Anderegg G, Arnaud-Neu F, Delgado R, Felcman J, Popov K. Critical evaluation of stability constants of metal complexes of complexones for biomedical and environmental applications. Pure Appl Chem 77:1445–1495, 2003. [Google Scholar]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev 27:3–18, 2003. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Canty AJ. Methylmercury(II) sulfhydryl interactions. Potentiometric determination of the formation constants for complexation of methylmercury(II) by sulfhydryl containing amino acids and related molecules, including glutathione. Can J Chem 61:1428–1434, 1983. [Google Scholar]
- Arnold AP, Tan K-S, Rabenstein DL. Nuclear magnetic resonance studies of the solution chemistry of metal complexes. 23. complexation of methylmercury by selenohydryl-containing amino acids and related molecules Inorg Chem 25:2433–2437, 1986. [Google Scholar]
- Bakir F, Damluji SF, Amin-Zaki L, Murtadha M, Khalidi A, Al-Rawi NY, Tikriti S, Dhahir HI, Clarkson TW, Smith JC, Doherty RA. Methylmercury poisoning in Iraq. Science 181:230–241, 1973. [DOI] [PubMed] [Google Scholar]
- Bellinger DC, O’Leary K, Rainis H, Gibb HJ. Country-specific estimates of the incidence of intellectual disability associated with prenatal exposure to methylmercury. Environ Res 147:159–163, 2016. [DOI] [PubMed] [Google Scholar]
- Berg GG, Miles EF. Mechanisms of inhibition of active transport ATPases by mercurial. Chem-Biol Int 27:199–219, 1979. [DOI] [PubMed] [Google Scholar]
- Bernard AM, Collette C, Lauwerys R. Renal effects of in utero exposure to mercuric chloride in rats. Arch Toxicol 66:508–513, 1992. [DOI] [PubMed] [Google Scholar]
- Berthon G Critical evaluation of the stability constants of metal complexes of amino acids with polar side chains. Pure Appl Chem 67:1117–1240, 1995. [Google Scholar]
- Bilbo SD, Block CL, Bolton JL, Hanamsagar R, Tran PK. Beyond infection – Maternal immune activation by environmental factors, microglial development, and relevance for autism spectrum disorders. Expl Neurol 299:241–251, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørklund G, Aaseth J, Ajsuvakova OP, Nikonorov AA, Skalny AV, Skalnaya MG, Tinkov AA. Molecular interaction between mercury and selenium in neurotoxicity. Coord Chem Rev 332:30–37, 2017c. [Google Scholar]
- Bjørklund G, Dadar M, Mutter J, Aaseth J. The toxicology of mercury: current research and emerging trends. Environ Res 159:545–554, 2017a. [DOI] [PubMed] [Google Scholar]
- Bjørklund G, Mutter J, Aaseth J. Metal chelators and neurotoxicity: lead, mercury, and arsenic. Arch Toxicol 91:3787–3797, 2017b. [DOI] [PubMed] [Google Scholar]
- Bondy SC, Agrawal AK. Methylmercury modulates GABAA receptor complex differentially in rat cortical and cerebellar membranes in vitro. Arch Toxicol 46:249–256, 1980.6112975 [Google Scholar]
- Bong SM, Moon JH, Nam KH, Lee KS, Chi YM, Hwang KY. Structural studies of human brain-type creatine kinase complexed with the ADP-Mg2+-NO3− creatine transition-state analogue complex. Febs Let 582:3959–3965, 2008. [DOI] [PubMed] [Google Scholar]
- Bosch AC, O’Neill B, Sigge GO, Kerwath SE, Hoffman LC. Mercury accumulation in Yellowfin tuna (Thunnus albacares) with regards to muscle type, muscle position and fish size. Food Chem 190:351–356, 2016. [DOI] [PubMed] [Google Scholar]
- Boucher O, Burden MJ, Muckle G, Saint-Amour D, Ayotte P, Dewailly É, Nelson CA, Jacobson SW, Jacobson JL. Response inhibition and error monitoring during a visual go/no-go task in Inuit children exposed to lead, polychlorinated biphenyls, and methylmercury. Environ Health Perspect 120:608–615, 2012a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher O, Jacobson SW, Plusquellec P, Dewailly E, Ayotte P, Forget-Dubois N, Jacobson JL, Muckle G. Prenatal methylmercury, postnatal lead exposure, and evidence of attention deficit/hyperactivity disorder among Inuit children in Arctic Québec. Environ Health Perspect 120:1456–1461, 2012b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher O, Muckle G, Ayotte P, Dewailly E, Jacobson SW, Jacobson JL. Altered fine motor function at school age in Inuit children exposed to PCBs, methylmercury, and lead. Environ Int 95:144–151, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher O, Muckle G, Jacobson JL, Carter RC, Kaplan-Estrin M, Ayotte P, Dewailly É, Jacobson SW. Domain-specific effects of prenatal exposure to PCBs, mercury, and lead on infant cognition: results from the Environmental Contaminants and Child Development Study in Nunavik. Environ Health Perspect 122:310–316, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco V, Caito S, Farina M, Rocha JB, Aschner M, Carvalho C. Biomarkers of mercury toxicity: Past, present, and future trends. J Toxicol Environ Health Part B 20:119–154, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco V, Canário J, Holmgren A, Carvalho C. Inhibition of the thioredoxin system in the brain and liver of zebra-seabreams exposed to waterborne methylmercury. Toxicol Appl Pharmacol 251:95–103, 2011. [DOI] [PubMed] [Google Scholar]
- Branco V, Godinho-Santos A, Gonçalves J, Lu J, Holmgren A, Carvalho C. Mitochondrial thioredoxin reductase inhibition, selenium status, and Nrf-2 activation are determinant factors modulating the toxicity of mercury compounds. Free Rad Biol Med 73:95–105, 2014. [DOI] [PubMed] [Google Scholar]
- Branco V, Ramos P, Canário J, Lu J, Holmgren A, Carvalho C. Biomarkers of adverse response to mercury: histopathology versus thioredoxin reductase activity. BioMed Res Int 19: 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenden N, Rabbani H, Abedi-Valugerdi M. Analysis of mercury-induced immune activation in nonobese diabetic (NOD) mice. Clin Exp Immunol 125:202–210, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Bauch C, Verrey F, Zalups RK. Mercuric conjugates of cysteine are transported by the amino acid transporter System b0,+: Implications of molecular mimicry. J Am Soc Nephrol 15:663–673, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Krasnikov BF, Joshee L, Pinto JT, Hallen A, Li J, Zalups RK, Cooper AJL. New insights into the metabolism of organomercury compounds: Mercury-containing cysteine S-conjugates are substrates of human glutamine transaminase K and potent inactivators of cystathionine γ-lyase. Arch Biochem Bioph, 517:20–29, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Zalups RK. Mechanisms involved in the transport of mercuric ions in target tissues. Arch Toxicol 91:63–81, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Zalups RK. Molecular and ionic mimicry and the transport of toxic metals. Toxicol Appl Pharmacol 204:274–308, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Zalups RK. Transport of inorganic mercury and methylmercury in target tissues and organs. J Toxicol Environ Health - Part B 13:385–410, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brombach C-C, Manorut P, Kolambage-Dona PPP, Ezzeldin MF, Chen B, Corns WT, Feldmann J, Krupp EM. Methylmercury varies more than one order of magnitude in commercial European rice. Food Chem 214:360–365, 2017. [DOI] [PubMed] [Google Scholar]
- Brucker-Davis F, Ganier-Chauliac F, Gal J, Panaïa-Ferrari P, Pacini P, Fénichel P, Hiéronimus S. Neurotoxicant exposure during pregnancy is a confounder for assessment of iodine supplementation on neurodevelopment outcome.Neurotoxicol Teratol 51:45–51, 2015. [DOI] [PubMed] [Google Scholar]
- Burbacher TM, Shen DD, Liberato N, Grant KS, Cernichiari E, Clarkson T. Comparison of blood and brain mercury levels in infant monkeys exposed to methylmercury or vaccines containing thimerosal. Environ Health Perspect 113:1015–1021, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J, Stern AH, Gochfeld M. Mercury in commercial fish: optimizing individual choices to reduce risk. Environ Health Perspec 113:266–271, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Chen A, Jones RL, Radcliffe J, Caldwell KL, Dietrich KN, Rogan WJ. Does background postnatal methyl mercury exposure in toddlers affect cognition and behavior? Neurotoxicology 31:1–9, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho CML, Chew E-H, Hashemy SI, Lu J, Holmgren A. Inhibition of the human thioredoxin system: A molecular mechanism of mercury toxicity. J Biol Chem 283:11913–11923, 2008. [DOI] [PubMed] [Google Scholar]
- Carvalho CML, Lu J, Zhang X, Arnér ESJ, Holmgren A. Effects of selenite and chelating agents on mammalian thioredoxin reductase inhibited by mercury: Implications for treatment of mercury poisoning. FASEB J 25:370–381, 2011. [DOI] [PubMed] [Google Scholar]
- Ceccatelli S, Daré E, Moors M. Methylmercury-induced neurotoxicity and apoptosis. Chem-Biol Interac 188:301–308, 2010. [DOI] [PubMed] [Google Scholar]
- Cernichiari E, Myers GJ, Ballatori N, Zareba G, Vyas J, Clarkson T. The biological monitoring of prenatal exposure to methylmercury. Neurotoxicology 28:1015–1022, 2007. [DOI] [PubMed] [Google Scholar]
- Chakraborty P Mercury exposure and Alzheimer’s disease in India - An imminent threat? Sci Total Environ 589:232–235, 2017. [DOI] [PubMed] [Google Scholar]
- Chan TY. Inorganic mercury poisoning associated with skin-lightening cosmetic products. Clin Toxicol 49:886–891, 2011. [DOI] [PubMed] [Google Scholar]
- Chang Y, Lee WY, Lin YJ, Hsu T. Mercury (II) impairs nucleotide excision repair (NER) in zebrafish (Danio rerio) embryos by targeting primarily at the stage of DNA incision. Aquat Toxicol 92: 97–104, 2017. [DOI] [PubMed] [Google Scholar]
- Chauhan V, Srikumar S, Aamer S, Pandareesh MD, Chauhan A. Methylmercury exposure induces sexual dysfunction in male and female Drosophila Melanogaster. Int J Environ Res Public Health 14(10):1108, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Bae S, Lim H, Lim JA, Lee YH, Ha M, Kwon HJ. Mercury exposure in association with decrease of liver function in adults: A longitudinal study. J Prev Med Public Health 50:377–385, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol 36:609–662, 2006. [DOI] [PubMed] [Google Scholar]
- Clarkson TW. The pharmacology of mercury compounds. Annu Rev Pharmacol. 12:375–406, 1972. [DOI] [PubMed] [Google Scholar]
- Comini MA. Measurement and meaning of cellular thiol: Disufhide redox status. Free Rad Res 50:246–271, 2016. [DOI] [PubMed] [Google Scholar]
- Copan L, Fowles J, Barreau T, McGee N. Mercury toxicity and contamination of households from the use of skin creams adulterated with mercurous chloride (calomel). Int J Environ Res Public Health 12:10943–10954, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W, Liu G, Bezerra M, Lagos DA, Li Y, Cai Y. Occurrence of methylmercury in rice-based infant cereals and estimation of daily dietary intake of methylmercury for infants. J Agricultural Food Chem 65:9569–9578, 2017. [DOI] [PubMed] [Google Scholar]
- Dalla Corte CL, Wagner C, Sudati JH, Comparsi B, Leite GO, Busanello A, Soares FAA, Aschner M, Rocha JBT. Effects of diphenyl diselenide on methylmercury toxicity in rats. BioMed Res Int 983821, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsson BRG, Dencker L, Orsen I, Khayat A. Fetotoxicity of inorganic mercury in the mouse: Distribution and effects on nutrient uptake by placenta and fetus. Biol Res Preg Perinatol 5:102–109, 1984. [PubMed] [Google Scholar]
- Danielsson BRG, Fredriksson A, Dahlgren L, Gardlund AT, Olsson L, Dencker L, Archer T. Behavioural effects of prenatal metallic mercury inhalation exposure in rats. Neurotoxicol Teratol 15:391–396, 1993. [DOI] [PubMed] [Google Scholar]
- Daré E, Fetissov S, Hökfelt T, Hall H, Ore Ögren S, Ceccatelli S. Effects of prenatal exposure to methylmercury on dopamine-mediated locomotor activity and dopamine D2 receptor binding. Arch Pharmacol 367:500–508, 2003. [DOI] [PubMed] [Google Scholar]
- De Simone F, Gencarelli CN, Hedgecock IM, Pirrone N. A Modeling comparison of mercury deposition from current anthropogenic mercury emission inventories. Environ Sci Tech 50:5154–5162, 2016. [DOI] [PubMed] [Google Scholar]
- Debes F, Weihe P, Grandjean P. Cognitive deficits at age 22 years associated with prenatal exposure to methylmercury. Cortex 74:358–369, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers C, Boucher O, Forget-Dubois N, Dewailly E, Ayotte P, Jacobson SW, Jacobson JL, Muckle G. Associations between prenatal cigarette smoke exposure and externalized behaviors at school age among Inuit children exposed to environmental contaminants. Neurotoxicol Teratol 39:84–90, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do SY, Lee CG, Kim JY, Moon YH, Kim MS, Bae IH, Song HS. Cases of acute mercury poisoning by mercury vapor exposure during the demolition of a fluorescent lamp factory. Ann Occup Environ Med 29(1):19, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dórea JG, Farina M, Rocha JBT. Toxicity of ethylmercury (and Thimerosal): A comparison with methylmercury. J Appl Toxicol 33:700–711, 2013. [DOI] [PubMed] [Google Scholar]
- Dórea JG, Marques RC, Abreu L. Milestone achievement and neurodevelopment of rural Amazonian toddlers (12 to 24 months) with different methylmercury and ethylmercury exposure. J Toxicol Environ Health A 77:1–13, 2014. [DOI] [PubMed] [Google Scholar]
- Dórea JG, Marques RC, Isejima C. Neurodevelopment of Amazonian infants: antenatal and postnatal exposure to methyl- and ethylmercury. J Biomed Biotechnol. 2012:132876, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dórea JG. Abating mercury exposure in young children should include thimerosal-free vaccines. Neurochem Res 42:2673–2685, 2017b. [DOI] [PubMed] [Google Scholar]
- Dórea JG. Low-dose Thimerosal in pediatric vaccines: Adverse effects in perspective. Environ Res 152:280–293, 2017a. [DOI] [PubMed] [Google Scholar]
- Dröse S, Brandt U, Wittig I. Mitochondrial respiratory chain complexes as sources and targets of thiol-based redox-regulation. Biochim Biophys Acta 1844:1344–1354, 2014. [DOI] [PubMed] [Google Scholar]
- Drum DA. Are toxic biometals destroying your children’s future? Biometals 22:697–700, 2009. [DOI] [PubMed] [Google Scholar]
- Eide I, Syversen TLM. Relationship between catalase activity and uptake of elemental mercury by rat brain. Acta Pharmacol Toxicol 52: 217–223, 1983. [DOI] [PubMed] [Google Scholar]
- Ekino S, Susa M, Ninomiya T, Imamura K, Kitamura T. Minamata disease revisited: an update on the acute and chronic manifestations of methyl mercury poisoning. J Neurol Sci 262:131–144, 2007. [DOI] [PubMed] [Google Scholar]
- Ekstrand J, Nielsen JB, Havarinasab S, Zalups RK, Söderkvist P, Hultman P. Mercury toxicokinetics—dependency on strain and gender. Toxicol Appl Pharmacol 243:283–291, 2010. [DOI] [PubMed] [Google Scholar]
- El-Ansary A, Bjorklund G, Tinkov AA, Skalny A, Dera HA. Relationship between selenium, lead, and mercury in red blood cells of Saudi autistic children. Metab Brain Dis 32:1073–1080, 2017. [DOI] [PubMed] [Google Scholar]
- El-baz F, Elhossiny RM, Elsayed AB, Gaber GM. Hair mercury measurement in Egyptian autistic children. Egyptian J Med Human Gen 11:135–141, 2010. [Google Scholar]
- Estes ML, McAllister AK. Maternal immune activation: Implications for neuropsychiatric disorders. Science 353:772–777, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethier AA, Muckle G, Bastien C, Dewailly É, Ayotte P, Arfken C, Jacobson SW, Jacobson JL, Saint-Amour D. Effects of environmental contaminant exposure on visual brain development: a prospective electrophysiological study in school-aged children. Neurotoxicology 33:1075–1085, 2012. [DOI] [PubMed] [Google Scholar]
- Fakhoury M Imaging genetics in autism spectrum disorders: Linking genetics and brain imaging in the pursuit of the underlying neurobiological mechanisms. Prog Neuro-Psychopharmacol Biol Psychiatry 80:101–114, 2018. [DOI] [PubMed] [Google Scholar]
- Farina M, Aschner M, Rocha JBT. Oxidative stress in MeHg-induced neurotoxicity. Toxicol Applied Pharmacol 256:405–417, 2011a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina M, Aschner M, Rocha JBT. The catecholaminergic neurotransmitter system in methylmercury-induced neurotoxicity Aschner M, Costa LG (Eds) In: Advances in Neurotoxicology, Academic Press, 1: 47–81, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina M, Rocha JBT, Aschner M. Mechanisms of methylmercury-induced neurotoxicity: evidence from experimental studies. Life Sci 89:555–563, 2011b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández de Cossío L, Guzmán A, van der Veldt S, Luheshi GN. Prenatal infection leads to ASD-like behavior and altered synaptic pruning in the mouse offspring. Brain Behav Immun 63:88–98, 2017. [DOI] [PubMed] [Google Scholar]
- Ferrer-Sueta G, Manta B, Botti H, Radi R, Trujillo M, Denicola A. Factors affecting protein thiol reactivity and specificity in peroxide reduction. Chem Res Toxicol 24:434–450, 2011. [DOI] [PubMed] [Google Scholar]
- Fido A, Al-Saad S. Toxic trace elements in the hair of children with autism. Autism 9:290–298, 2005. [DOI] [PubMed] [Google Scholar]
- Florentine MJ, Sanfilippo II DJ. Elemental mercury poisoning. Clin Phar 10: 213–221, 1991. [PubMed] [Google Scholar]
- Fomenko DE, Marino SM, Gladyshev VN. Functional diversity of cysteine residues in proteins and unique features of catalytic redox-active cysteines in thiol oxidoreductases. Mol Cells 26:228–235, 2008. [PMC free article] [PubMed] [Google Scholar]
- Fomenko DE, Xing W, Adair BM, Thomas DJ, Gladyshev VN. High-throughput identification of catalytic redox-active cysteine residues. Science 387–389, 2007. [DOI] [PubMed] [Google Scholar]
- Fonfría E, Vilaró MT, Babot Z, Rodríguez-Farré E, Suñol C. Mercury compounds disrupt neuronal glutamate transport in cultured mouse cerebellar granule cells. J Neurosc Res 79:545–553. [DOI] [PubMed] [Google Scholar]
- Franciscato C, Goulart FR, Lovatto NM, Duarte FA, Flores EMM, Dressler VL, Peixoto NC, Pereira ME. ZnCl2 exposure protects against behavioral and acetylcholinesterase changes induced by HgCl2. Int J Dev Neurosci 27:459–468, 2009. [DOI] [PubMed] [Google Scholar]
- Franco JL, Posser T, Dunkley PR, Dickson PW, Mattos JJ, Martins R, Bainy ACD, Marques MR, Dafre AL, Farina M. Methylmercury neurotoxicity is associated with inhibition of the antioxidant enzyme glutathione peroxidase. Free Radic Biol Med 47:449–457, 2009. [DOI] [PubMed] [Google Scholar]
- Freire C, Amaya E, Gil F, Fernández MF, Murcia M, Llop S, Andiarena A, Aurrekoetxea J, Bustamante M, Guxens M, Ezama E, Fernández-Tardón G, Olea N. Prenatal co-exposure to neurotoxic metals and neurodevelopment in preschool children: The Environment and Childhood (INMA) Project. Sci Total Environ 621:340–351, 2018. [DOI] [PubMed] [Google Scholar]
- Fuhr BJ, Rabenstein DL. Nuclear magnetic resonance studies of the solution chemistry of metal complexes. IX. The binding of cadmium, zinc, lead, and mercury by glutathione. J Amer Chem Soc 95:6944–6950, 1973. [DOI] [PubMed] [Google Scholar]
- Fujimura M, Usuki F. In situ different antioxidative systems contribute to the site-specific methylmercury neurotoxicity in mice. Toxicology 392: 55–63, 2017. [DOI] [PubMed] [Google Scholar]
- Gao Z, Ying X, Yan J, Wang J, Cai S, Yan C. Acute mercury vapor poisoning in a 3-month-old infant: A case report. Clin Chim Acta 465: 119–122, 2017. [DOI] [PubMed] [Google Scholar]
- Garrecht M, Austin DW. The plausibility of a role for mercury in the etiology of autism: A cellular perspective. Toxicol Environ Chem 93:1251–1273, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascon M, Guxens M, Vrijheid M, Torrent M, Ibarluzea J, Fano E, Llop S,Ballester F, Fernández MF, Tardón A, Fernández-Somoano A, Sunyer J. The INMA-INfancia y Medio Ambiente-(Environment and Childhood) project: More than 10 years contributing to environmental and neuropsychological research. Int J Hyg Environ Health 220:647–658, 2017. [DOI] [PubMed] [Google Scholar]
- Gaskin J, Rennie C, Coyle D. Reducing periconceptional methylmercury exposure: cost-utility analysis for a proposed screening program for women planning a pregnancy in Ontario, Canada. Environ Health Perspect 123:1337–1344, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier DA, Geier MR. A meta-analysis epidemiological assessment of neurodevelopmental disorders following vaccines administered from 1994 through 2000 in the United States. Neuroendocrinol Lett 27:401–413, 2006. [PubMed] [Google Scholar]
- Geier DA, Kern JK, Homme KG, Geier MR. A cross-sectional study of the association between infant hepatitis B vaccine exposure in boys and the risk of adverse effects as measured by receipt of special education services. Int J Environ Res Public Health 15(1):123(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier DA, Kern JK, Homme KG, Sykes LK, Geier MR.Thimerosal-containing Hepatitis B Vaccine Exposure is highly associated with childhood obesity: A case-control study using the Vaccine Safety Datalink. N Am J Med Sci 7:297–306, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier DA, Kerna JK, Hommed KG, Geier MR. A cross-sectional study of the relationship between infant Thimerosal containing hepatitis B vaccine exposure and attention-deficit/hyperactivity disorder. J Trace Elem Med Biol 46:1–9, 2018a. [DOI] [PubMed] [Google Scholar]
- Gimenez-Llort L, Ahlbom E, Daré E, Vahter M, Ogren S, Ceccatelli S. Prenatal exposure to methylmercury changes dopamine-modulated motor activity during early ontogeny: age and gender-dependent effects. Environ Toxicol Pharmacol 9:61–70, 2001. [DOI] [PubMed] [Google Scholar]
- Glaser V, de Paula Martins R, Vieira AJ, de Medeiros Oliveira E, Straliotto MR, Mukdsi JH, Torres AI, de Bem AF, Farina M, Rocha JBT, De Paul AL. Diphenyl diselenide administration enhances cortical mitochondrial number and activity by increasing hemeoxygenase type 1 content in a methylmercury-induced neurotoxicity mouse model. Mol Cellular Biochem 390:1–8, 2014. [DOI] [PubMed] [Google Scholar]
- Glaser V, Leipnitz G, Straliotto MR, Oliveira J, dos Santos VV, Wannmacher CMD, de Bem AF, Rocha JBT, Farina M, Latini A. Oxidative stress-mediated inhibition of brain creatine kinase activity by methylmercury. NeuroToxicology 31:454–460, 2010b. [DOI] [PubMed] [Google Scholar]
- Glaser V, Moritz B, Schmitz A, Dafré AL, Nazari EM, Müller YM, Feksa L, Straliottoa MR, de Bem AF, Farina M, Rocha JBT. Protective effects of diphenyl diselenide in a mouse model of brain toxicity. Chem-Biol Interac 206:18–26, 2013. [DOI] [PubMed] [Google Scholar]
- Glaser V, Nazari EM, Müller YMR, Feksa L, Wannmacher CMD, Rocha JBT, Bem AFD, Farina M, Latini A. Effects of inorganic selenium administration in methylmercury-induced neurotoxicity in mouse cerebral cortex. Int J Dev Neurosc 28:631–637, 2010a. [DOI] [PubMed] [Google Scholar]
- Go YM, Chandler JD, Jones DP. The cysteine proteome. Free Radic Biol Med. 84:227–245, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go YM, Jones DP. Cysteine/cystine redox signaling in cardiovascular disease. Free Radic Biol Med. 50:495–509, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gochfeld M, Burger J. Good fish/bad fish: a composite benefit–risk by dose curve. Neurotoxicology 26:511–520, 2005. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Raymat H, Liu G, Liriano C, Li Y, Yin Y, Shi J, Jiang G, Cai Y. Elemental mercury: Its unique properties affect its behavior and fate in the environment. Environ Pollut 229: 69–86, 2017. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet 368:2167–2178, 2006. [DOI] [PubMed] [Google Scholar]
- Gu B, Bian Y, Miller CL, Dong W, Jiang X, Liang L. Mercury reduction and complexation by natural organic matter in anoxic environments. Proc Nat Acad Sci 108:1479–1483, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gul Oz S, Tozlu M, Yalcin SS, Sozen T, Sain Guven G. Mercury vapor inhalation and poisoning of a family. Inhal Toxicol 24: 652–658, 2012. [DOI] [PubMed] [Google Scholar]
- Gump BB, Dykas MJ, MacKenzie JA, Dumas AK, Hruska B, Ewart CK, Parsons PJ, Palmer CD, Bendinskas K. Background lead and mercury exposures: Psychological and behavioral problems in children. Environ Res 158:576–582, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachiya N Epidemiological Update of Methylmercury and Minamata Disease In: Ceccatelli S, Aschner M (eds) Methylmercury and Neurotoxicity. Current Topics in Neurotoxicity, 2012, vol 2 Springer, Boston, MA. [Google Scholar]
- Halbach S, Clarkson TW. Enzymatic oxidation of mercury vapor by erythrocytes. Biochim Biophys Acta 523: 522–531, 1978. [DOI] [PubMed] [Google Scholar]
- Hancock RD, Martell AE. Hard and soft acid-base behavior in aqueous solution: Steric effects make some metal ions hard: A quantitative scale of hardness-softness for acids and bases. J Chem Educ 73: 654–661, 1996. [Google Scholar]
- Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J Cheminform 4:1–17, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada M Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol 25:1–24, 1995. [DOI] [PubMed] [Google Scholar]
- Heppner DE, Hristova M, Ida T, Mijuskovic A, Dustin CM, Bogdándi V, Fukuto JM, Dick TP, Nagy P, Li J, Akaike T, van der Vliet A. Cysteine perthiosulfenic acid (Cys-SSOH): A novel intermediate in thiol-based redox signaling? Redox Biol 14:379–385, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson NW, Waly MI, Al-Farsi YM, Al-Sharbati MM, Al-Farsi O, Ali A, Ouhtit A, Zang T, Zhou ZS, Deth RC. Decreased glutathione and elevated hair mercury levels are associated with nutritional deficiency-based autism in Oman. Experim Biol Med 239:697–706, 2014. [DOI] [PubMed] [Google Scholar]
- Horowitz HM, Jacob DJ, Amos HM, Streets DG, Sunderland EM. Historical mercury releases from commercial products: global environmental implications. Environ Sci Technol 48: 10242–10250, 2014. [DOI] [PubMed] [Google Scholar]
- Hu Y, Cheng H. Mercury risk from fluorescent lamps in China: Current status and future perspective. Environ Int 44: 141–150, 2012. [DOI] [PubMed] [Google Scholar]
- Huang X, Wang C, Li S, Liu Y, Zhang Z. Highly sensitive visualization of inorganic mercury in mouse neurons using a fluorescent probe. J Fluoresc 24:1313–1317, 2014. [DOI] [PubMed] [Google Scholar]
- Huber ML, Laesecke A, Friend DG. Correlation for the vapor pressure of mercury. Ind Eng Chem Res 45:7351–7361, 2006. [Google Scholar]
- Hultman P, Lindh U, Horsted-Bindslev P. Activation of the immune system and systemic immune-complex deposits in Brown Norway rats with dental amalgam restorations. J Dental Res 77:1415–1425, 1998. [DOI] [PubMed] [Google Scholar]
- Ishitobi H, Stern S, Thurston SW, Zareba G, Langdon M, Gelein R, Weiss B. Organic and inorganic mercury in neonatal rat brain after prenatal exposure to methylmercury and mercury vapor. Environ Health Perspec 118:242–248, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawaid M, Ingman F. Potentiometric studies on the complex formation between methylmercury(II) and some keto- and amino- carboxylic acids. Talanta 28:137–143, 1981. [DOI] [PubMed] [Google Scholar]
- Jeong KS, Park H, Ha E, Shin J, Hong Y-C, Ha M, Park H, Kim B-N, Lee B, Lee S-J, Lee KY, Kim JH, Kim Y. High maternal blood mercury level is associated with low verbal IQ in children. J Korean Med Sci 32:1097–1104, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson BB. The physiology of the blood-brain barrier In: Porter JC, Ježová D (eds) Circulating Regulatory Factors and Neuroendocrine Function. Advances in Experimental Medicine and Biology, vol 274 Springer, Boston, MA. [Google Scholar]
- Johansson C, Castoldi AF, Onishchenko N, Manzo L, Vahter M, Ceccatelli S. Neurobehavioural and molecular changes induced by methylmercury exposure during development. Neurotox Res 11:241–260, 2007. [DOI] [PubMed] [Google Scholar]
- Johnson FO, Atchison WD. The role of environmental mercury, lead and pesticide exposure in development of amyotrophic lateral sclerosis. NeuroToxicology 30:761–765, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DP, Go YM. Mapping the cysteine proteome: analysis of redox-sensing thiols. Curr Opin Chem Biol. 15:103–112, 2011. [DOI] [PubMed] [Google Scholar]
- Junior JMFC, Lima AADS, Junior DR, Khoury EDT, Souza GDS, Silveira LCL, Pinheiro MDCN. Emotional and motor symptoms in riverside dwellers exposed to mercury in the Amazon. Rev Bras Epidemiol 20: 212–224, 2017. [DOI] [PubMed] [Google Scholar]
- Kalia V, Perera F, Tang D. Environmental pollutants and neurodevelopment: Review of benefits from closure of a coal-burning power plant in Tongliang, China. Glob Pediatr Health. 2017. DOI: 4:2333794X17721609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern JK, Geier DA, Deth RC, Sykes LK, Hooker BS, Love JM, Bjørklund G, Chaigneau CG, Haley BE, Geier MR. Systematic assessment of research on Autism Spectrum Disorder (ASD) and mercury reveals conflicts of interest and the need for transparency in autism research. Sci Eng Ethics 23:1691–1718, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kern JK, Geier DA, Sykes LK, Haley BE, Geier MR. The relationship between mercury and autism: A comprehensive review and discussion. J Trace Elem Med Biol 37: 8–24, 2016. [DOI] [PubMed] [Google Scholar]
- Khaled EM, Meguid NA, Bjorklund G, Gouda A, Bahary MH, Hashish A, Sallam NM, Chirumbolo S, El-Bana MA. Altered urinary porphyrins and mercury exposure as biomarkers for autism severity in Egyptian children with autism spectrum disorder. Metab Brain Dis 31: 1419–1426, 2016. [DOI] [PubMed] [Google Scholar]
- Khan MAK, Wang F. Mercury-selenium compounds and their toxicological signifcance: toward a molecular understanding of the mercury-selenium antagonism. Environ Toxicol Chem 28:1567–1577, 2009. [DOI] [PubMed] [Google Scholar]
- Kim S, Eom S, Kim HJ, Lee JJ, Choi G, Choi S, Kim S, Kim SY, Cho G, Kim YD, Suh E, Kim SK, Kim S, Kim GH, Moon HB, Park J, Kim S, Choi K, Eun SH. Association between maternal exposure to major phthalates, heavy metals, and persistent organic pollutants, and the neurodevelopmental performances of their children at 1 to 2years of age- CHECK cohort study. Sci Total Environ 624:377–384, 2017. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Chai YG, Ryu JC. Selenoprotein W as molecular target of methylmercury in human neuronal cells is down-regulated by GSH depletion. Biochem Biophys Res Commun 20:1095–10200, 2005. [DOI] [PubMed] [Google Scholar]
- Kristensen AKB, Thomsen JF, Mikkelsen S. A review of mercury exposure among artisanal small-scale gold miners in developing countries. Int Arch Occup Environ Health 87: 579–590, 2014. [DOI] [PubMed] [Google Scholar]
- Kromidas L, Trombetta LD, Jamall IS. The protective effects of glutathione against methylmercury cytotoxicity. Toxicology Letters, 51 (1), pp. 67–80, 1990. [DOI] [PubMed] [Google Scholar]
- Kudo A, Turner RR. Mercury contamination of Minamata Bay: historical overview and progress towards recovery In Mercury Contaminated Sites 1999. (pp. 143–158). Springer, Berlin, Heidelberg. [Google Scholar]
- Kusanagi E, Takamura H, Chen SJ, Adachi M, Hoshi N. Children’s hair mercury concentrations and seafood consumption in five regions of Japan. Arch Environ Contam Toxicol 2018. DOI: 10.1007/s00244-017-0502-x. [DOI] [PubMed] [Google Scholar]
- Kuwabara JS, Arai Y, Topping BR, Pickering IJ, George GN. Mercury speciation in piscivorous fish from mining-impacted reservoirs. Environ Sci Technol 41:2745–2749, 2007. [DOI] [PubMed] [Google Scholar]
- Kwon O-S, Park Y-J. In vitro and in vivo dose-dependent inhibition of methylmercury on glutamine synthetase in the brain of different species. Environ Toxicol Pharmacol 14:17–24, 2003. [DOI] [PubMed] [Google Scholar]
- Labunskyy VM, Hatfield DL, Gladyshev VN. Selenoproteins: molecular pathways and physiological roles. Physiol Rev 94:739–777, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Park H, Ha E, Hong YC, Ha M, Park H, Kim BN, Lee SJ, Lee KY, Kim JH, Jeong KS, Kim Y. Stability of cognitive development during the first five years of life in relation to heavy metal concentrations in umbilical cord blood: Mothers’ and Children’s Environmental Health (MOCEH) birth cohort study. Sci Total Environ 609:153–159, 2017. [DOI] [PubMed] [Google Scholar]
- Lee R, Middleton D, Caldwell K, Dearwent S, Jones S, Lewis B, Monteilh C, Mortensen ME, Nickle R, Orloff K, Reger M, Risher J, Rogers HS, Watters M. A review of events that expose children to elemental mercury in the United States. Cien Saude Colet 15:585–598, 2009. [DOI] [PubMed] [Google Scholar]
- Leterme B, Blanc P, Jacques D. A reactive transport model for mercury fate in soil—application to different anthropogenic pollution sources Environ Sci Pollut Res 21:12279–12293, 2014. [DOI] [PubMed] [Google Scholar]
- Li H, Li H, Li Y, Liu Y, Zhao Z. Blood Mercury, arsenic, cadmium, and lead in children with autism spectrum disorder. Biol Trace Elem Res 181:31–37, 2018. [DOI] [PubMed] [Google Scholar]
- Li R, Xu X, Xu Z, Ao M, Shang L, Qiu G, Tang S. Total mercury and methylmercury concentrations and risk assessments in rice plants collected from a river watershed in a typical mercury mining area of Guizhou. Res Environ Sci 29:1829–1839, 2016. [Google Scholar]
- Li Y, Zhao J, Li Y-F, Xu X, Zhang B, Liu Y, Cui L, Li B, Gao Y, Chai Z. Comparative metalloproteomic approaches for the investigation proteins involved in the toxicity of inorganic and organic forms of mercury in rice (Oryza sativa L.) roots. Metallomics 8:663–671, 2016. [DOI] [PubMed] [Google Scholar]
- Liem-Nguyen V, Skyllberg U, Nam K, Björn E. Thermodynamic stability of mercury(II) complexes formed with environmentally relevant low-molecular-mass thiols studied by competing ligand exchange and density functional theory. Environ Chem 14:243–253, 2017. [Google Scholar]
- Lin CC, Chen YC, Su FC, Lin CM, Liao HF, Hwang YH, Hsieh WS, Jeng SF, Su YN, Chen PC. In utero exposure to environmental lead and manganese and neurodevelopment at 2 years of age. Environ Res 123:52–57, 2013. [DOI] [PubMed] [Google Scholar]
- Loomes R, Hull L, Mandy WPL. What is the male-to-female ratio in Autism Spectrum Disorder? A Systematic review and meta-analysis. J Am Acad Child Adolesc Psych 56:466–474, 2017. [DOI] [PubMed] [Google Scholar]
- LoPachin RM, Gavin T, DeCaprio A, Baarber DS. Application of the hard and soft, acids and bases (HSAB) theory to toxicants-target interactions. Chem Res Toxicol 25:239–251, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loux NT. An assessment of mercury-species-dependent binding with natural organic carbon. Chem Spec Bioavail 10:127–136, 1998. [Google Scholar]
- Malagutti KS, Silva AP, Braga HC, Mitozo PA, Santos ARS, Dafre AL, de Bem AF, Farina M. 17β-estradiol decreases methylmercury-induced neurotoxicity in male mice. Environ Toxicol Pharmacol 27:293–297, 2009. [DOI] [PubMed] [Google Scholar]
- Marques R, Dórea JG, Bernardi JVE, Bastos WR, Malm O. Prenatal and postnatal mercury exposure, breastfeeding and neurodevelopment during the first 5 years. Cogn Behav Neurol 22:134–141, 2009. [DOI] [PubMed] [Google Scholar]
- Marques RC, Abreu L, Bernardi JV, Dórea JG. Neurodevelopment of Amazonian children exposed to ethylmercury (from Thimerosal in vaccines) and methylmercury (from fish). Environ Res 149:259–265, 2016a. [DOI] [PubMed] [Google Scholar]
- Marques RC, Abreu L, Bernardi JV, Dórea JG. Traditional living in the Amazon: extended breastfeeding, fish consumption, mercury exposure and neurodevelopment. Ann Hum Biol 43:360–370, 2016b. [DOI] [PubMed] [Google Scholar]
- Marques RC, Bernardi JV, Abreu L, Dórea JG. Neurodevelopment outcomes in children exposed to organic mercury from multiple sources in a tin-ore mine environment in Brazil. Arch Environ Contam Toxicol 68:432–441, 2015. [DOI] [PubMed] [Google Scholar]
- Marques RC, Bernardi JV, Dórea JG, Bastos WR, Malm O. Principal component analysis and discrimination of variables associated with pre- and post-natal exposure to mercury. Int J Hyg Environ Health 211:606–614, 2008. [DOI] [PubMed] [Google Scholar]
- Marques RC, Bernardi JV, Dórea JG, de Fatima R, Moreira M, Malm O. Perinatal multiple exposure to neurotoxic (lead, methylmercury, ethylmercury, and aluminum) substances and neurodevelopment at six and 24 months of age. Environ Pollut 187:130–135, 2014. [DOI] [PubMed] [Google Scholar]
- Marques RC, Dórea JG, Bernardi JVE. Thimerosal exposure (from tetanus-diphtheria vaccine) during pregnancy and neurodevelopment of breastfed infants at six months. Acta Paediatr 99:1–6, 2010. [DOI] [PubMed] [Google Scholar]
- Marques RC, Dórea JG, Leão RS, Dos Santos VG, Bueno L, Marques RC, Brandão KG, Palermo EF, Guimarães JR. Role of methylmercury exposure (from fish consumption) on growth and neurodevelopment of children under 5 years of age living in a transitioning (tin-mining) area of the western Amazon, Brazil. Arch Environ Contam Toxicol 62:341–350, 2012. [DOI] [PubMed] [Google Scholar]
- Marques RC, Dórea JG, Manzatto AG Bastos WR, Bernardi JV, Malm O. Time of perinatal immunization, thimerosal exposure and neurodevelopment at 6 months in breastfed infants. Acta Paediatr 96:864–868, 2007. [DOI] [PubMed] [Google Scholar]
- Marques RC, Dórea JG, McManus C, Leão RS, Brandão KG, Marques RC, Vieira IH, Guimarães JR, Malm O. Hydroelectric reservoir inundation (Rio Madeira Basin, Amazon) and changes in traditional lifestyle: impact on growth and neurodevelopment of pre-school children. Public Health Nutr 14:661–669, 2011. [DOI] [PubMed] [Google Scholar]
- Martin RB. Practical hardness scales for metal ion complexes. Inorganica Chim Acta 339:27–33, 2002. [Google Scholar]
- Mazerik JN, Hagele T, Sherwani S, Ciapala V, Butler S, Kuppusamy ML, Hunter M, Kuppusamy P, Marsh CB, Parinandi NL. Phospholipase A2 activation regulates cytotoxicity of methylmercury in vascular endothelial cells. Int J Toxicol 26:553–569, 2007. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Wright CL. Convergence of sex differences and the neuroimmune system in Autism Spectrum Disorder. Biol Psy 81:402–410, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckeown-Eyssen GE, Ruedy J, Neims A. Methyl mercury exposure in northern Quebec. II. Neurologic findings in children. Am J Epidemiol 118:470–479, 1983. [DOI] [PubMed] [Google Scholar]
- Meltzer A, Van De Water J. The role of the immune system in Autism Spectrum Disorder. Neuropsychopharmacology 42:284–298, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miseta A, Csutora P. Relationship between the occurrence of cysteine in proteins and the complexity of organisms. Mol Biol Evol 17:1232–1239, 2000. [DOI] [PubMed] [Google Scholar]
- Modgil S, Lahiri DK, Sharma VL, Anand A. Role of early life exposure and environment on neurodegeneration: implications on brain disorders. Transl Neurodegener. 3(1):9, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed FEB, Zaky EA, El-Sayed AB, Elhossieny RM, Zahra SS, Eldin WS, Youssef WY, Khaled RA, Youssef AM. Assessment of hair aluminum, lead, and mercury in a sample of autistic Egyptian children: environmental risk factors of heavy metals in autism. Behavioural Neurol 2015:545674, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Alvarez A, Fernández de la Campa MD, Sanz-Medel A. Mercury speciation in Cuban commercial edible fish by HPLC-ICP-MS using the double spike isotope dilution analysis strategy. Int J Environ Anal Chem 2:36–47, 2014. [Google Scholar]
- Moraes-Silva L, Siqueira LF, Oliveira VA, Oliveira CS, Ineu RP, Pedroso TF, Fonseca MM, Pereira ME. Preventive effect of CuCl2 on behavioral alterations and mercury accumulation in central nervous system induced by HgCl2 in newborn rats. J Biochem Mol Toxicol 28:328–35, 2014. [DOI] [PubMed] [Google Scholar]
- Mora-Zamorano FX, Klingler R, Basu N, Head J, Murphy CA, Binkowski FP, Larson JK, Carvan MJ. Developmental methylmercury exposure affects swimming behavior and foraging efficiency of yellow perch (Perca flavescens) Larvae. ACS Omega 31: 4870–4877, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DL, Chanda SM, Price HC, Fernando R, Liu J, Brambila E, O’Connor RW, Beliles RP, Barone S Jr. Disposition of inhaled mercury vapor in pregnant rats: Maternal toxicity and effects on developmental outcome. Toxicol Sci 66:261–273, 2002. [DOI] [PubMed] [Google Scholar]
- Mori K, Yoshida K, Tani J, Hoshikawa S, Ito S, Watanabe C. Ethylmercury inhibits type II 5′-deiodinase activity in NB41A3 neuroblastoma cells. Toxicol Lett 161:96–101, 2006. [DOI] [PubMed] [Google Scholar]
- Mostafazadeh B, Kiani A, Mohamadi E, Shaki F, Shirazi FH. Mercury exposure of gold mining workers in the northwest of Iran. Pakistan J Pharm Sci 26: 1267–1270, 2013. [PubMed] [Google Scholar]
- Mousavi A Predicting mercury(II) binding by organic ligands: A chemical model of therapeutic and environmental interests. Environ Forensics 12:327–332, 2011. [Google Scholar]
- Mrozek-Budzyn D, Majewska R, Kieltyka A, Augustyniak M. Neonatal exposure to Thimerosal from vaccines and child development in the first 3 years of life. Neurotoxicol Teratol 34:592–597, 2012. [DOI] [PubMed] [Google Scholar]
- Mutter J, Naumann J, Sadaghiani C, Schneider R, Walach H. Alzheimer Disease: Mercury as pathogenetic factor and apolipoprotein E as a moderator. Neuroendocrinol Lett 25:331–339, 2004. [PubMed] [Google Scholar]
- Naor MM, Jensen JH. Determinants of cysteine pKa values in creatine kinase and α1‐antitrypsin. PROT: Struc Func Bioinfor 57:799–803, 2004. [DOI] [PubMed] [Google Scholar]
- Ni M, Li X, Yin Z, Jiang H, Sidoryk-Wegrzynowicz M, Milatovic D, Cai J, Aschner M. Methylmercury induces acute oxidative stress, altering Nrf2 protein level in primary microglial cells. Toxicol Sci 116:590–603, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolescu R, Petcu C, Cordeanu A, Fabritius K, Schlumpf M, Krebs R, Krämer U, Winneke G. Environmental exposure to lead, but not other neurotoxic metals, relates to core elements of ADHD in Romanian children: performance and questionnaire data. Environ Res 110:476–483, 2010. [DOI] [PubMed] [Google Scholar]
- Noble MJ, Decker SL, Horowitz BZ. Inhalational mercury toxicity from artisanal gold extraction reported to the Oregon poison center, 2002–2015. Clin Toxicol 54: 847–851, 2016. [DOI] [PubMed] [Google Scholar]
- Nogara PA and Rocha JBT. In Silico Studies of Mammaliand-ALAD Interactions with Selenides and Selenoxides. Mol Inf 36:1700091, 2017. [DOI] [PubMed] [Google Scholar]
- Oliveira CS, Joshee L, Bridges CC. MRP2 and the transport kinetics of cysteine conjugates of inorganic mercury. Biol Trace Elem Res DOI: 10.1007/s12011-017-1163-3, 2017b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira CS, Joshee L, Zalups RK, Pereira ME, Bridges CC. Disposition of inorganic mercury in pregnant rats and their offspring. Toxicology 335:62–71, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira CS, Oliveira VA, Ineu RP, Moraes-Silva L, Pereira ME. Biochemical parameters of pregnant rats and their offspring exposed to different doses of inorganic mercury in drinking water. Food Chem Toxicol 50:2382–2387, 2012. [DOI] [PubMed] [Google Scholar]
- Oliveira CS, Piccoli BC, Aschner M, Rocha JBT. Chemical speciation of selenium and mercury as determinant of their neurotoxicity In: Aschner M, Costa LG (eds.), Neurotoxicity of Metals, Advances in Neurobiology, 2017a. DOI 10.1007/978-3-319-60189-2_4. [DOI] [PubMed] [Google Scholar]
- Orenstein ST, Thurston SW, Bellinger DC, Schwartz JD, Amarasiriwardena CJ, Altshul LM, Korrick SA. Prenatal organochlorine and methylmercury exposure and memory and learning in school-age children in communities near the New Bedford Harbor Superfund site, Massachusetts. Environ Health Perspect 122:1253–1259, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz SG, Tozlu M, Yalcin SS, Sozen T, Guven GS. Mercury vapor inhalation and poisoning of a family. Inhal Toxicol 24:652–658, 2012. [DOI] [PubMed] [Google Scholar]
- Palmer N, Beam A, Agniel D, Eran A, Manrai A, Spettell C, Steinberg G, Mandl K, Fox K, Nelson SF, Kohane I. Association of sex with recurrence of autism spectrum disorder among siblings. JAMA Pediatrics 171:1107–1112, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamphlett R, Jew SK. Heavy metals in locus ceruleus and motor neurons in motor neuron disease. Acta Neuropathol Comm 1(1):81, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamphlett R, Jew SK. Uptake of inorganic mercury by human locus ceruleus and corticomotor neurons: Implications for amyotrophic lateral sclerosis. Acta Neuropathol Comm 1(1):13, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleão TU, Ferreira ACF, Santos MCS, Figueiredo ASP, Louzada RAN, Rosenthal D, Carvalho DP, Costa VMC. Effect of thimerosal on thyroid hormones metabolism in rats. Endocr Connect 6:741–747, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J-D, Zheng W. Human exposure and health effects of inorganic and elemental mercury. J. Prev Med Public Health 45:344–352, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto NC, Roza T, Morsch VM, Pereira ME. Behavioral alterations induced by HgCl2 depend on the postnatal period of exposure. Int J Dev Neurosci 25:39–46, 2007. [DOI] [PubMed] [Google Scholar]
- Pereira CD, Minamino N, Takao T. Free Thiol of transthyretin in human plasma most accessible to modification/oxidation. Anal Chem 87:10785–10791, 2015. [DOI] [PubMed] [Google Scholar]
- Poirel O, Mella S, Videau C, Ramet L, Davoli MA, Herzog E, Katsel P, Mechawar N, Haroutunian V, Epelbaum J, Daumas S, Mestikawy SE. Moderate decline in select synaptic markers in the prefrontal cortex (BA9) of patients with Alzheimer’s disease at various cognitive stages. Sci Rep 8(1):938, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole LB. The basics of thiols and cysteines in redox biology and chemistry. Free Rad Biol Med 80:148–157, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priya MDL, Geetha A. Level of trace elements (copper, zinc, magnesium and selenium) and toxic elements (lead and mercury) in the hair and nail of children with autism. Biol Trace Elem Res 142:148–158, 2011. [DOI] [PubMed] [Google Scholar]
- Rabenstein DL, Fairhurst MT. Nuclear magnetic resonance studies of the solution chemistry of metal complexes. XI. The binding of methylmercury by sulfhydryl-containing amino acids and by glutathione. J Am Chem Soc 97:2086–2092, 1975. [DOI] [PubMed] [Google Scholar]
- Rabenstein DL, Isab AA. A proton nuclear magnetic resonance study of the interaction of mercury with intact human erythrocytes. Biochim Bioph Acta 721:374–384, 1982. [DOI] [PubMed] [Google Scholar]
- Rabenstein DL, Ozubko R, Libich S, Evans CA, Fairhurst MT, Suvanprakorn C. Nuclear magnetic resonance studies of the solution chemistry of metal complexes. X. Determination of formation constants of the methylmercury complexes of selected amines and aminocarboxylic acids. J Coord Chem 3:263–271, 1974. [Google Scholar]
- Rabenstein DL, Reid RS, Yamashita G, Tan KS, Arnold AP. Determination of thiols and selenols by titration with methylmercury with end point detection by nuclear magnetic resonance spectrometry. Anal Chem 58:1266–1269, 1986. [Google Scholar]
- Rabenstein DL, Reid RS. Nuclear magnetic resonance studies of the solution chemistry of metal complexes. 20. Ligand-exchange kinetics of methylmercury(II)-thiol complexes. Inor Chem 23:1246–1250, 1984. [Google Scholar]
- Rabenstein DL. The chemistry of methylmercury toxicology J Chem Educ 54:292–296, 1978. [Google Scholar]
- Reinhardt JW. Side-effects: mercury contribution to body burden from dental amalgam. Ad Dent Res 6: 110–113, 1992. [DOI] [PubMed] [Google Scholar]
- Reisz JA, Bechtold E, King SB, Poole LB, Furdui CM. Thiol-blocking electrophiles interfere with labeling and detection of protein sulfenic acids. FEBS J 280:6150–6161, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemer J, Schwarzländer M, Conrad M, Herrmann JM. Thiol switches in mitochondria: Operation and physiological relevance. Biol Chem 396:465–482, 2015. [DOI] [PubMed] [Google Scholar]
- Risher JF, Nickle RA, Amler SN. Elemental mercury poisoning in occupational and residential settings. Int J Hyg Environ Health 206: 371–379, 2003. [DOI] [PubMed] [Google Scholar]
- Rocha JBT, Piccoli BC, Oliveira CS. Biological and chemical interest in selenium: a brief historical account. ARKIVOC. 2017. DOI: 10.3998/ark.5550190.p009.784. [DOI] [Google Scholar]
- Rocha JBT, Saraiva RA, Garcia SC, Gravina FS, Nogueira CW. Aminolevulinate dehydratase (δ-ALA-D) as marker protein of intoxication with metals and other pro-oxidant situations. Toxicol Res 1:85–102, 2012. [Google Scholar]
- Rodrigues J, Branco V, Lu J, Holmgren A, Carvalho C. Toxicological effects of thiomersal and ethylmercury: Inhibition of the thioredoxin system and NADP+-dependent dehydrogenases of the pentose phosphate pathway. Toxicol Appl Pharmacol 286:216–223, 2015. [DOI] [PubMed] [Google Scholar]
- Rooney JPK The retention time of inorganic mercury in the brain - A systematic review of the evidence. Toxicol Appl Pharmacol 274:425–435, 2014. [DOI] [PubMed] [Google Scholar]
- Rossi AD, Ahlbom E, Ogren SO, Nicotera P, Ceccatelli S. Prenatal exposure to methylmercury alters locomotor activity of male but not female rats. Exp Brain Res 117:428–436, 1997. [DOI] [PubMed] [Google Scholar]
- Rothenberg SE, Yu X, Liu J, Biasini FJ, Hong C, Jiang X, Nong Y, Cheng Y, Korrick SA. Maternal methylmercury exposure through rice ingestion and offspring neurodevelopment: A prospective cohort study. Int J Hyg Environ Health 219:832–842, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudyk O and Eaton P. Biochemical methods for monitoring protein thiol redox states in biological systems. Redox Biol 2:803–813, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggieri F, Majorani C, Domanico F, Alimonti A. Mercury in children: Current state on exposure through human biomonitoring studies. Int J Environ Res Public Health 14(5), 519, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin D, Erdolu CO, Karadenizli S, Kara A, Bayrak G, Beyaz S, Demir B, Ateş N. Effects of gestational and lactational exposure to low dose mercury chloride (HgCl2) on behaviour, learning and hearing thresholds in WAG/Rij rats. EXCLI J 15:391–402, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Amour D, Roy MS, Bastien C, Ayotte P, Dewailly E, Després C, Gingras S, Muckle G. Alterations of visual evoked potentials in preschool Inuit children exposed to methylmercury and polychlorinated biphenyls from a marine diet. Neurotoxicology 27:567–578; 2006. [DOI] [PubMed] [Google Scholar]
- Sasakura C, Suzuki KT. Biological interaction between transition metals (Ag, Cd and Hg), selenide/sulfide and selenoprotein P. J Inorg Biochem 71:159–162, 1998. [DOI] [PubMed] [Google Scholar]
- Schmidt L, Bizzi CA, Duarte FA, Dressler VL, Flores EM. Evaluation of drying conditions of fish tissues for inorganic mercury and methylmercury speciation analysis. Microchem J 108:53–59, 2013. [Google Scholar]
- Schmiedel V, Vogt H, Walach H. Are pupils’ ‘Programme for International Student Assessment (PISA)’ scores associated with a nation’s fish consumption? Scand J Public Health 2017. DOI: 10.1177/1403494817717834. [DOI] [PubMed] [Google Scholar]
- Schuurs AHB. 1999. Reproductive toxicity of occupational mercury. A review of the literature. J Dent 27: 249–256, 1999. [DOI] [PubMed] [Google Scholar]
- Seby F, Potin-Gautier M, Giffaut E, Borge G, Donard OFX. A critical review of thermodynamic data for selenium species at 25 oC. Chem Geol 171:173–194, 2001. [Google Scholar]
- Shenker BJ, Guo TL, Insug O, Shapiro IM. Induction of apoptosis in human T-cells by methyl mercury: Temporal relationship between mitochondrial dysfunction and loss of reductive reserve. Toxicol Appl Pharmacol 157:23–35, 1999. [DOI] [PubMed] [Google Scholar]
- Shenker BJ, Pankoski L, Zekavat A, Shapiro IM. Mercury-induced apoptosis in human lymphocytes: Caspase activation is linked to redox status. Antiox Redox Signal 4:379–389, 2002. [DOI] [PubMed] [Google Scholar]
- Shikara IA, Al Dabbagh TQ. Neurological manifestations in organic mercury poisoning. Ann Coll Med Mosul 4:33–42, 1973. [Google Scholar]
- Short JD, Downs K, Tavakoli S, Asmis R. Protein thiol redox signaling in monocytes and macrophages. Antioxid Redox Signal 25:816–835, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippel TO. The histochemistry of thiols and disulphides. I. The use of N-(4-aminophenyl) maleimide for demonstrating thiol groups. Histochem J 5:413–423, 1973. [DOI] [PubMed] [Google Scholar]
- Skyllberg U, Bloom PR, Qian J, Lin CM, Bleam WF. Complexation of mercury (II) in soil organic matter: EXAFS evidence for linear two-coordination with reduced sulfur groups. Environ Sci Technol 40:4174–4180, 2006. [DOI] [PubMed] [Google Scholar]
- Skyllberg U Competition among thiols and inorganic sulfides and polysulfides for Hg and MeHg in wetland soils and sediments under suboxic conditions: illumination of controversies and implications for MeHg net production. J Geophys Res 113, 2008 DOI: 10.1029/2008jg000745. [DOI] [Google Scholar]
- Sood PP, Bapu C, Vijayalakshmi K. Vitamins and monothiols efficacy in the restoration of adenosine nucleotide degradation enzymes altered during methylmercury intoxication. J Environ Pathol Toxicol Oncol 14:101–105, 1995. [PubMed] [Google Scholar]
- Stachowiak O, Dolder M, Wallimann T, Richter C. Mitochondrial creatine kinase is a prime target of peroxynitrite-induced modificationand inactivation. J Biol Chem 273:16694–16699, 1998. [DOI] [PubMed] [Google Scholar]
- Stewart PW, Reihman J, Lonky EI, Darvill TJ, Pagano J. Cognitive development in preschool children prenatally exposed to PCBs and MeHg. Neurotoxicol Teratol 25:11–22, 2003. [DOI] [PubMed] [Google Scholar]
- Stewart PW, Sargent DM, Reihman J, Gump BB, Lonky E, Darvill T, Hicks H, Pagano J. Response inhibition during Differential Reinforcement of Low Rates (DRL) schedules may be sensitive to low-level polychlorinated biphenyl, methylmercury, and lead exposure in children. Environ Health Perspect 114:1923–1929, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricks W, Kolthoff IM. Reactions between mercuric mercury and cysteine and glutathione. Apparent dissociation constants, heats and entropies of formation of various forms of mercuric mercaptocysteine and -glutathione. J Am Chem Soc 75:5673–5681, 1953. [Google Scholar]
- Stringari J, Nunes AKC, Franco JL, Bohrer D, Garcia SC, Dafre AL, Milatovic D, Souza DO, Rocha JBT, Aschner M, Farina M. Prenatal methylmercury exposure hampers glutathione antioxidant system ontogenesis and causes long-lasting oxidative stress in the mouse brain. Toxicol Appl Pharmacol 227:147–154, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroustrup A, Hsu HH, Svensson K, Schnaas L, Cantoral A, Solano González M, Torres-Calapiz M, Amarasiriwardena C, Bellinger DC, Coull BA, Téllez-Rojo MM, Wright RO, Wright RJ. Toddler temperament and prenatal exposure to lead and maternal depression. Environ Health 15(1):71, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura Y, Hojo Y, Tamai Y, Tanaka H. Selenium protection against mercury toxicity. Binding of methylmercury by the selenohydryl-containing ligand. J Am Chem Soc 98:2339–2341, 1976. [DOI] [PubMed] [Google Scholar]
- Sun GF, Hu WT, Yuan ZH, Zhang BA, Lu H. Characteristics of mercury intoxication induced by skin-lightening products. Chinese Med J 1:130, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Nakai K, Sugawara T, Nakamura T, Ohba T, Shimada M, Hosokawa T, Okamura K, Sakai T, Kurokawa N, Murata K, Satoh C, Satoh H. Neurobehavioral effects of prenatal exposure to methylmercury and PCBs, and seafood intake: neonatal behavioral assessment scale results of Tohoku study of child development. Environ Res 110, 699–704, 2010. [DOI] [PubMed] [Google Scholar]
- Tatsuta N, Nakai K, Murata K, Suzuki K, Iwai-Shimada M, Kurokawa N, Hosokawa T, Satoh H. Impacts of prenatal exposures to polychlorinated biphenyls, methylmercury, and lead on intellectual ability of 42-month-old children in Japan. Environ Res 133:321–326, 2014. [DOI] [PubMed] [Google Scholar]
- Tatsuta N, Nakai K, Murata K, Suzuki K, Iwai-Shimada M, Yaginuma-Sakurai K, Nakamura T, Hosokawa T, Satoh H. Prenatal exposures to environmental chemicals and birth order as risk factors for child behavior problems. Environ Res 114:47–52, 2012. [DOI] [PubMed] [Google Scholar]
- Thompson SA, White CC, Krejsa CM, Diaz D, Woods JS, Eaton DL, Kavanagh TJ. Induction of glutamate-cysteine ligase (γ-glutamylcysteine synthetase) in the brains of adult female mice subchronically exposed to methylmercury. Toxicol Lett 110:1–9, 1999. [DOI] [PubMed] [Google Scholar]
- Trasande L, DiGangi J, Evers DC, Petrlik J, Buck DG, Šamánek J, Beeler B, Turnquist MA, Regan K. Economic implications of mercury exposure in the context of the global mercury treaty: Hair mercury levels and estimated lost economic productivity in selected developing countries. J Environ Manage 183:229–235, 2016. [DOI] [PubMed] [Google Scholar]
- Tratnik JS, Falnoga I, Trdin A, Mazej D, Fajon V, Miklavčič A, Kobal AB, Osredkar J, Sešek Briški A, Krsnik M, Neubauer D, Kodrič J, Stropnik S, Gosar D, Lešnik Musek P, Marc J, Jurkovič Mlakar S, Petrović O, Vlašić-Cicvarić I, Prpić I, Milardović A, Radić Nišević J, Vuković D, Fišić E, Špirić Z, Horvat M. Prenatal mercury exposure, neurodevelopment and apolipoprotein E genetic polymorphism. Environ Res. 2017;152:375–385. [DOI] [PubMed] [Google Scholar]
- Trott O and Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem 31:455–461, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verity MA, Sarafian T, Pacifici EHK, Sevanian A. Phospholipase A2 stimulation by methyl mercury in neuron culture. J Neurochem 62:705–714, 1994. [DOI] [PubMed] [Google Scholar]
- Vimy MJ, Takahashi Y, Lorscheider FL. Maternal-fetal distribution of mercury (203Hg) released from dental amalgam fillings. Am J Physiol 258:939–945, 1990. [DOI] [PubMed] [Google Scholar]
- Wagner C, Sudati JH, Nogueira CW, Rocha JBT. In vivo and in vitro inhibition of mice thioredoxin reductase by methylmercury. Biometals 23:1171–1177, 2010. [DOI] [PubMed] [Google Scholar]
- Walach H, Mutter J, Deth R. Inorganic mercury and Alzheimer’s Disease-Results of a review and a molecular mechanism. Diet and Nutrition in Dementia and Cognitive Decline. Chapter 55, page: 593–601, 2014. [Google Scholar]
- Wang PF, McLeish MJ, Kneen MM, Lee G, Kenyon GL. An unusually low pKa for Cys282 in the active site of human muscle creatine kinase. Biochemistry 40:11698–11705, 2001. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chen A, Dietrich KN, Radcliffe J, Caldwell KL, Rogan WJ. Postnatal exposure to methyl mercury and neuropsychological development in 7-year-old urban inner-city children exposed to lead in the United States. Child Neuropsychol 20:527–538, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson GE, Evans K, Thurston SW, van Wijngaarden E, Wallace JMW, McSorley EM, Bonham MP, Mulhern MS, McAfee AJ, Davidson PW, Shamlaye CF, Strain JJ, Love T, Zareba G, Myers GJ. Prenatal exposure to dental amalgam in the Seychelles Child Development Nutrition Study: Associations with neurodevelopmental outcomes at 9 and 30 months. NeuroToxicology 33:1511–1517, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson GE, van Wijngaarden E, Love TMT, McSorley EM, Bonham MP, Mulhern MS, Yeates AJ, Davidson PW, Shamlaye CF, Strain JJ, Thurston SW, Harrington D, Zareba G, Wallace JMW, Myers GJ. Neurodevelopmental outcomes at 5 years in children exposed prenatally to maternal dental amalgam: The seychelles child development nutrition study. Neurotoxicol Teratol 39:57–62, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerapana E, Wang C, Simon GM, Richter F, Khare S, Dillon MBD, Bachovchi DA, Mowen K, Baker D, Cravatt BF. Quantitative reactivity profiling predicts functional cysteines in proteomes. Nature 468:790–797, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RF, Palumbo CL, Yurgelun-Todd DA, Heaton KJ, Weihe P, Debes F, Grandjean P. Functional MRI approach to developmental methylmercury and polychlorinated biphenyl neurotoxicity. Neurotoxicology 32:975–980, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization) (2007) Exposure to mercury: a major public health concern Environmental Health Criteria. Geneva: World Health Organization. [Google Scholar]
- Wible RS, Sutter TR. Soft cysteine cignaling network: The functional significance of cysteine in protein function and the soft acids/bases thiol chemistry that facilitates cysteine modification. Chem Res Toxicol 30:729–762, 2017. [DOI] [PubMed] [Google Scholar]
- Wu C, Wu G, Wang Z, Zhang Z, Qian Y, Ju L. Soil mercury speciation and accumulation in rice (Oryza sativa L.) grown in wastewater-irrigated farms. Appl Geochem 89:202–209, 2018. [Google Scholar]
- Yamamoto R, Suzuki T, Satoh H, Kawais K. Generation and dose as modifying factors of inorganic mercury accumulation in brain, liver, and kidneys of rats fed methylmercury. Environ Res 41:309–318, 1986. [DOI] [PubMed] [Google Scholar]
- Yorifuji T, Debes F, Weihe P, Grandjean P. Prenatal exposure to lead and cognitive deficit in 7- and 14-year-old children in the presence of concomitant exposure to similar molar concentration of methylmercury. Neurotoxicol Teratol 33:205–211, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida E, Abiko Y, Kumagai Y. Glutathione adduct of methylmercury activates the Keap1–Nrf2 pathway in SH-SY5Y cells. Chem Res Toxicol 27:1780–1786, 2014. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Satoh M, Shimada A, Yamamoto E, Yasutake A, Tohyama C. Maternal-to-fetus transfer of mercury in metallothionein-null pregnant mice after exposure to mercury vapor. Toxicology 175:215–222, 2002. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Watanabe C, Horie K, Satoh M, Sawada M, Shimada A. Neurobehavioral changes in metallothionein-null mice prenatally exposed to mercury vapor. Toxicol Lett 155:361–368, 2005. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Ahmad S. Homocysteine and the renal epithelial transport and toxicity of inorganic mercury: role of basolateral transporter organic anion transporter 1. J Am Soc Nephrol 15:2023–2031, 2004. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Barfuss DW. Pretreatment with p-aminohippurate inhibits the renal uptake and accumulation of injected inorganic mercury in the rat. Toxicology 103: 23–35, 1995. [DOI] [PubMed] [Google Scholar]
- Zalups RK. Early aspects of the intrarenal distribution of mercury after the intravenous administration of mercuric chloride. Toxicology 79: 215–228, 1993. [DOI] [PubMed] [Google Scholar]
- Zalups RK. Molecular interactions with mercury in the kidney. Pharmacol Rev 52:113–143, 2000. [PubMed] [Google Scholar]
- Zemolin APP, Meinerz DF, de Paula MT, Mariano DOC, Rocha JBT, Pereira AB, Posser T, Franco JL. Evidences for a role of glutathione peroxidase 4 (GPx4) in Methylmercury induced neurotoxicity in vivo. Toxicology 302:60–67, 2012. [DOI] [PubMed] [Google Scholar]


