Abstract
Background
We assessed multidrug-resistant tuberculosis (MDR-TB) cases and their household contacts (HHCs) to inform the development of an interventional clinical trial.
Methods
We conducted a cross-sectional study of adult MDR-TB cases and their HHCs in 8 countries with high TB burdens. HHCs underwent symptom screenings, chest radiographies, sputum TB bacteriologies, TB infection (TBI) testing (tuberculin skin test [TST] and interferon gamma release assay [IGRA]), and human immunodeficiency virus (HIV) testing.
Results
From October 2015 to April 2016, 1016 HHCs from 284 MDR-TB cases were enrolled. At diagnosis, 69% of MDR-TB cases were positive for acid-fast bacilli sputum smears and 43% had cavitary disease; at study entry, 35% remained smear positive after a median MDR-TB treatment duration of 8.8 weeks. There were 9 HHCs that were diagnosed with TB prior to entry and excluded. Of the remaining 1007 HHCs, 41% were male and the median age was 25 years. There were 121 (12%) HHCs that had new cases of TB identified: 17 (2%) were confirmed, 33 (3%) probable, and 71 (7%) possible TB cases. The TBI prevalence (defined as either TST or IGRA positivity) was 72% and varied by age, test used, and country. Of 1007 HHCs, 775 (77%) were considered high-risk per these mutually exclusive groups: 102 (10%) were aged <5 years; 63 (6%) were aged ≥5 and were infected with HIV; and 610 (61%) were aged ≥5 years, were negative for HIV or had an unknown HIV status, and were TBI positive. Only 21 (2%) HHCs were on preventive therapy.
Conclusions
The majority of HHCs in these high-burden countries were at high risk of TB disease and infection, yet few were receiving routine preventive therapy. Trials of novel, preventive therapies are urgently needed to inform treatment policy and practice.
Keywords: household contacts, multidrug-resistant tuberculosis, TB infection, TB disease, preventive therapy
In a cross-sectional study of multidrug-resistant tuberculosis cases and household contacts in 8 countries, of 1007 contacts screened for tuberculosis, 121 (12%) had new TB identified. Tuberculosis infection prevalence was 72%. Trials of novel preventive therapies are urgently needed.
(See the Major Article by Suryavanshi et al on pages 436–45.)
In 2017, the World Health Organization (WHO) estimated that 558 000 people developed rifampin-resistant (RR) or MDR-TB (Mycobacterium tuberculosis [M.tb] resistant to at least isoniazid [INH] and rifampin). However, only 25% of these cases received appropriate treatment; only 55% had successful treatment outcomes. Patients with extensively drug-resistant (XDR)-TB (MDR-TB with additional resistance to at least 1 fluoroquinolone and 1 injectable) have worse outcomes [1]. These gaps in the diagnosis and treatment of MDR-TB leave close contacts exposed for prolonged periods. With the rollout of rapid molecular diagnostics, coupled with intensified, active case-finding, MDR-TB case notification is growing, with an associated increase in the number of household contacts (HHCs) identified [1–5].
The WHO guidelines acknowledge the lack of quality evidence on how to prevent TB among MDR-TB contacts and specifically recommend clinical trials as a high priority [6–9]. A meta-analysis of 25 studies, which assessed a median of 111 HHCs of MDR-TB cases, found that HHCs are at high risk of acquiring an infection with M.tb (TB infection; TBI) and developing TB disease [10]. HHCs under 5 years old, infected with human immunodeficiency virus (HIV), or with evidence of TBI are at particularly high risk of developing MDR-TB [8, 9, 11, 12]. Since the vast majority of studies included in the meta-analysis were conducted 10 or more years ago, they preceded the rollout of Xpert MTB/RIF (GeneXpert, Cepheid) which rapidly detects RR. These studies also did not use interferon gamma release assay (IGRA) testing for the detection of TBI and were from single countries [10].
We conducted the Protecting Households on Exposure to Newly Diagnosed Index MDR-TB Patients (PHOENIx) feasibility study to prepare for PHOENIx, a large, Phase III, multicountry, interventional trial that aims to evaluate TB preventative therapy among high-risk HHCs of MDR-TB patients. PHOENIx will compare the efficacy and safety of 26 weeks of delamanid versus 26 weeks of isoniazid for preventing TB during 96 weeks of follow-up [13, 14]. The objectives of our feasibility study were to (1) identify, recruit, and characterize adult MDR-TB index cases and their adult and child HHCs; (2) describe the prevalence of TB disease, TBI, and HIV among HHC; and (3) estimate the proportion of HHCs at high risk of TB and, therefore, potentially eligible for our trial.
METHODS
Study Design and Population
We enrolled index cases treated for MDR-TB and their HHCs in a cross-sectional study at 16 sites in 8 countries with high TB burdens (Botswana, Brazil, Haiti, India, Kenya, Peru, South Africa, and Thailand; Supplementary Table 1). These sites were clinical research sites of the National Institutes of Health–funded AIDS Clinical Trials Group (ACTG) and International Maternal Pediatric Adolescent AIDS Trials (IMPAACT) network, selected on the basis of being a non-US site with an anticipated enrollment of at least 10 adult MDR-TB cases within 16 weeks [14, 15]. Our target accrual was 300 cases of patients 18 years or older who were diagnosed with pulmonary MDR-TB (including pre-XDR and XDR-TB), and all of their HHCs. We recruited cases who started MDR-TB treatment within the prior 6 months on regimens following the local standard of care. TB cases had RR-TB, as detected by Xpert MTB/RIF, pending either confirmatory drug-susceptibility testing (DST) or a line probe assay for INH and RR, or had confirmed INH and RR by either genotypic or phenotypic DST. Expanded DST for fluoroquinolones and aminoglycosides to determine XDR and pre-XDR statuses varied per local standards of care.
With the written informed consent of the index cases, HHCs were approached and their written informed consent/assent was also obtained. The study was approved by all local Institutional Review Boards (IRBs)/Ethics Committees and partnering US institutions. There were 2 sites in Brazil and Kenya that did not receive IRB approval to enroll children <18 years.
Study Procedures
Index Cases
Standard data collection forms were used to obtain medical histories, chest images, HIV statuses, and TB diagnostic information from medical records. Sputum samples were also collected at study entry from cases for culture and first- and second-line DST.
Household Contacts
For HHCs, we collected sociodemographic and medical histories, including prior TB diagnoses and HIV statuses. We offered HIV testing for those with unknown statuses or negative results from 1 or more years prior to study entry. All HHCs not already on TB treatment were screened for active TB with a symptom questionnaire, chest radiograph, and sputum sample, as well as a gastric aspirate for children, when needed. HHCs were also tested with a tuberculin skin test (TST) and/or IGRA using QuantiFERON Gold or Gold-in-tube (Qiagen, Venlo, The Netherlands).
Definitions
We defined TST positivity as ≥5 mm [16] and IGRA positivity per the manufacturer instructions [17]. A HHC was defined as a person who shared a dwelling unit and housekeeping arrangement with the index case, and who reported exposure within 6 months prior to the index case starting MDR-TB treatment. We defined high-risk HHCs using the following, mutually exclusive groups: (1) <5 years old, regardless of HIV or TST/IGRA status; (2) ≥5 years old and HIV-infected, regardless of TST/IGRA status; or (3) ≥5 years old, with HIV status negative or unknown and a positive TST and/or IGRA status. We defined any instance of HHC TB as a diagnosis of confirmed, probable, or possible TB, per the below definitions.
Adjudication of Tuberculosis Diagnosis in Household Contacts
Adult and pediatric specialists reviewed the available information from the symptom questionnaires, medical histories, chest radiographies (CXRs), and TB diagnostic testing for HHCs who had any of the following: (1) signs/symptoms suggestive of TB; (2) a chest radiograph with abnormal or uncertain/indeterminate findings; (3) acid fast bacilli (AFB) smear positivity; or (4) M.tb complex, detected through culture or Xpert testing. The review group set definitions according to ACTG/IMPAACT Appendix 100 [8], with study-specific modifications as follows. Confirmed TB was defined as (1) being bacteriologically confirmed using a liquid or solid culture or rapid molecular tests, and (2) either having compatible symptoms of TB or, for children <15 years of age, having a CXR suggestive of TB. Probable TB was defined as (1) having compatible symptoms, and (2) being either AFB positive or having a suggestive CXR. The definition of possible TB was used only for children <15 years old and was defined as either (1) having compatible symptoms, (2) being AFB positive, or (3) having a suggestive CXR. All HHCs diagnosed with TB were referred to local, routine TB services.
Statistical Analyses
Descriptive statistics were performed to assess study population characteristics. Odds ratios (OR) comparing groups are from logistic regression models, fitted using generalized estimating equations, with an exchangeable working correlation structure to take clustering into account. The P values provided are from score tests.
RESULTS
Study Population Characteristics
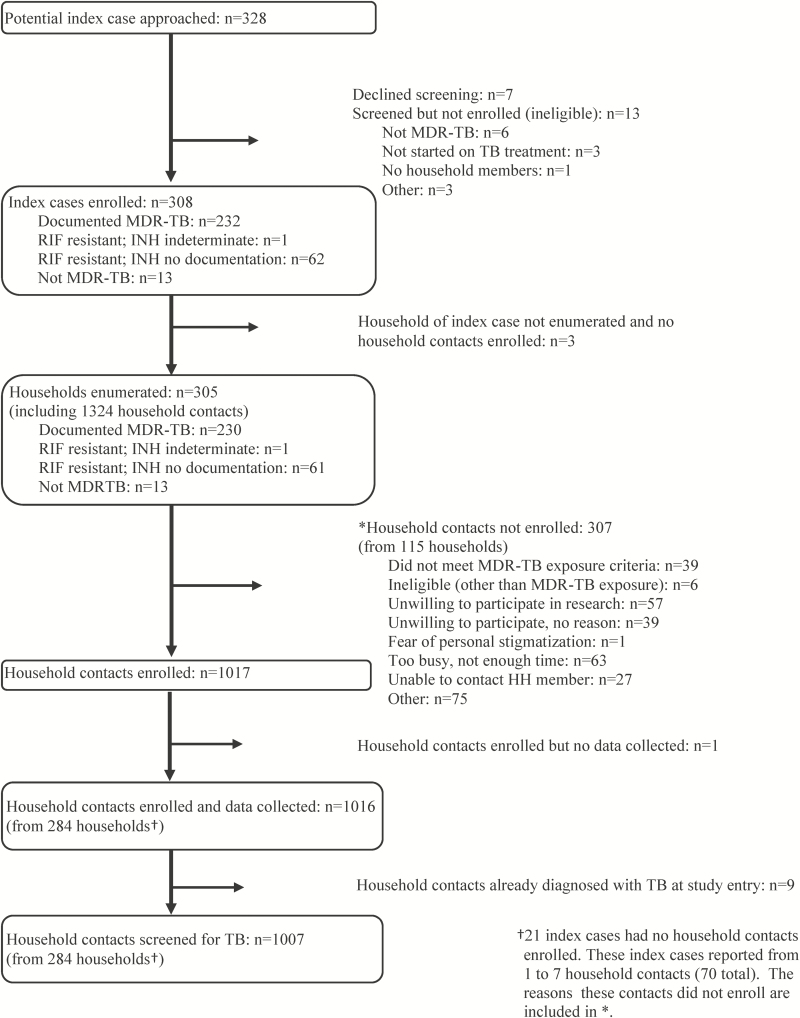
Between October 2015 and April 2016, 308 MDR-TB cases were recruited; 305 had household members, from which 1324 HHCs were enumerated. A total of 1285 met the study criteria as potentially eligible HHCs, of whom 1016 (79%) from 284 households were enrolled (Figure 1). From these households, the median number of HHCs enrolled was 3 (interquartile range 2–4; range 1–19). Of 1016 HHCs, 9 (1%) were already diagnosed with TB at study enrollment (1 child 3 years old and 8 HHCs >15 years of age) and were not further screened for TB.
Figure 1.
Flow of enrollment of index multidrug-resistant TB cases and household contacts in the PHOENIx Feasibility Study. Abbreviations: HH, household; INH, isoniazid; MDR, multidrug-resistant; PHOENIx, Protecting Households on Exposure to Newly Diagnosed Index Multidrug-resistant Tuberculosis Patients; RIF, rifampin; TB, tuberculosis.
Characteristics of the 284 index cases with at least 1 HHC enrolled and their 1007 HHCs who underwent TB screening are shown in Table 1. Among the index cases, 212 (74.6%) had confirmed MDR-TB; 59 (20.8%) had RR, but had no isoniazid resistance documented (majority from Xpert MTB/RIF testing); and 13 (4.6%) had documented RR and isoniazid susceptibility. At enrollment, their median duration of MDR-TB treatment was 8.8 weeks, and 35% remained smear-positive.
Table 1.
Characteristics of the Study Population in the PHOENIx Feasibility Study
| Index Cases | Household Contacts, Overall | Household Contacts | Household Contacts | |
|---|---|---|---|---|
| ≥15 years | <15 years | |||
| Characteristics | (n = 284) | (n = 1007) | (n = 704) | (n = 303) |
| Demographics | n (%) | n (%) | n (%) | n (%) |
| Age, years, median (IQR) | 35.5 (26, 45) | 25 (12, 43) | 34 (24, 49) | 7 (3, 10) |
| <5 | ─ | 102 (10%) | ─ | 102 (34%) |
| 5 to <15 | ─ | 201 (20%) | ─ | 201 (66%) |
| 15 to <50 | 233 (82%) | 530 (53%) | 530 (75%) | ─ |
| ≥50 | 51 (18%) | 174 (17%) | 174 (25%) | ─ |
| Sex | ||||
| Male | 164 (58%) | 414 (41%) | 259 (37%) | 155 (51%) |
| Female | 120 (42%) | 593 (59%) | 445 (63%) | 148 (49%) |
| Pregnant | ─ | 16 (3%) | 16 (4%) | 0 (0%) |
| Countries (# sites) | ||||
| Botswana (1) | 10 (4%) | 38 (4%) | 20 (3%) | 18 (6%) |
| Brazil (1) | 10 (4%) | 17 (2%) | 17 (2%) | 0 (0%)a |
| Haiti (1) | 14 (5%) | 52 (5%) | 37 (5%) | 15 (5%) |
| India (2) | 58 (20%) | 205 (20%) | 169 (24%) | 36 (12%) |
| Kenya (1) | 7 (3%) | 12 (1%) | 12 (2%) | 0 (0%)a |
| Peru (2) | 54 (19%) | 203 (20%) | 130 (18%) | 73 (24%) |
| South Africa (7) | 121 (43%) | 450 (45%) | 294 (42%) | 156 (51%) |
| Thailand (1) | 10 (4%) | 30 (3%) | 25 (4%) | 5 (2%) |
| Race | ||||
| Asian | 69 (24%) | 237 (24%) | 196 (28%) | 41 (14%) |
| Black | 115 (40%) | 383 (38%) | 261 (37%) | 122 (40%) |
| White | 8 (3%) | 9 (1%) | 9 (1%) | 0 (0%) |
| Mixed race/other | 88 (31%) | 378 (38%) | 238 (34%) | 140 (46%) |
| Employed/in school | ─ | 503 (50%) | 306 (43%) | 197 (65%) |
| Highest education attained | ||||
| None | ─ | 245 (24%) | 84 (12%) | 161 (53%) |
| Primary | ─ | 327 (32%) | 205 (29%) | 122 (40%) |
| Secondary | ─ | 351 (35%) | 332 (47%) | 19 (6%) |
| College/university or higher | ─ | 83 (8%) | 83 (12%) | ─ |
| History of incarceration | ─ | 21 (2%) | 20 (3%) | 1 (<1%) |
| Medical history | ||||
| HIV status | ||||
| Negative | 144 (51%) | 695 (69%) | 515 (73%) | 180 (59%) |
| Positive | 102 (36%) | 65 (6%) | 59 (8%) | 6 (2%) |
| Newly diagnosed | ─ | 26 (3%) | 22 (37%) | 4 (67%) |
| Unknownb | 38 (13%) | 247 (25%) | 130 (19%) | 117 (39%) |
| Self-reported diabetes | 20 (7%) | 26 (3%) | 26 (4%) | 0 (0%) |
| Current or former smoker | 125 (44%) | 207 (21%) | 205 (29%) | 2 (1%) |
| Daily alcohol consumption | ─ | 59 (6%) | 56 (8%) | 3 (1%) |
| History of substance use | ─ | 57 (6%) | 56 (8%) | 1 (<1%) |
| Prior history of TB treatment | 150 (53%) | 86 (9%) | 77 (11%) | 9 (3%) |
| TB-related characteristics | ||||
| On TB preventive therapy | ─ | 21 (2%) | 1 (<1%) | 20 (7%) |
| Prevalent TBc | 284 (100%) | 121 (12%) | 37 (5%) | 84 (28%) |
| Chest X-ray cavitation(s) | N = 184 | N = 969 | N = 670 | N = 299 |
| Present | 80 (43%) | 8 (1%) | 7 (1%) | 1 (<1%) |
| AFB smear | N = 257 | N = 276 | N = 183 | N = 93 |
| Negative | 168 (65%) | 261 (96%) | 170 (95%) | 91 (98%) |
| Positive | 89 (35%) | 15 (5%) | 13 (5%) | 2 (2%) |
| Drug-susceptibility resultd | N = 284 | N = 16 | N = 12 | N = 4 |
| XDR/pre-XDR | 9 (2%) | 0 (0%) | 0 (0%) | 0 (0%) |
| MDR | 203 (71%) | 4 (26%) | 3 (25%) | 1 (25%) |
| Rif R, INH S | 13 (4%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Rif R, INH unk, FQ unk, SLID unk | 59 (21%) | 1 (6%) | 0 (0%) | 1 (25%) |
| DS | 0 (0%) | 11 (68%) | 9 (75%) | 2 (50%) |
| MDR-TB treatment duration, weeks median (IQR) | 8.8 (3, 17) | ─ | ─ | ─ |
Data on diabetes diagnoses missing for n = 10 (7 aged ≥15 and 3 aged <15); daily alcohol consumptions for n = 8 (aged ≥15); history of substance use for n = 3 (aged ≥15); highest education attained for n = 1 (aged <15); history of incarceration for n = 1 (aged ≥15); and chest X-ray cavitations for n = 38 (24 aged ≥15 and 4 aged <15).
Abbreviations: AFB, acid-fast bacilli; DS, drug-susceptible; DST, drug-susceptibility testing; FQ, fluoroquinolone; HHC, household contact; HIV, human immunodeficiency virus; INH, isoniazid; IQR, interquartile range; MDR, multidrug-resistant; R, resistant; Rif, rifampin; S, susceptible; SLID, second line injectable drug; TB, tuberculosis; unk, unknown; XDR, extremely drug resistant.
aThese sites had not received Institutional Review Board approval to enroll children.
bIf the last HIV test was not performed within the last 365 days, the HIV status was classified as unknown.
cAll index cases had TB at entry. Of 1016 HHCs, n = 9 (including n = 8 aged ≥15 and n = 1 aged <15 years) with active TB diagnosed prior to study entry had limited data collected and were excluded from this table.
dAmong HHCs, some drug-susceptibility results were available for 16 out of 17 culture-confirmed TB cases. When including the 9 already diagnosed cases prior to study entry and the 17 newly confirmed cases, 11 (42%) were rifampin-resistant or MDR-TB, 14 (54%) were drug-susceptible TB, and 1 (4%) had no DST data available. Since not all samples had DST for fluoroquinolones and injectable drugs, participants classified as having with MDR-TB may have additional resistance.
The majority of the 1007 HHCs were from South Africa (45%), India (20%), and Peru (20%); 593 (59%) were female, 16 (3%) were pregnant, and 303 (30%) were <15 years old, including 102 (10%) <5 years old. There were 39 (4%) with known HIV-infected statuses, 187 (19%) had done HIV testing in the past year and were HIV-negative, and 781 (78%) had unknown HIV statuses. A total of 554 (71%) agreed to HIV testing and, of these, 26 (5%) new infections were identified (reported elsewhere) [18].
Household Contact Screening and Prevalent Tuberculosis Identified
Of the 1007 HHCs screened for TB, 228 (23%) had signs/symptoms of TB; this proportion was similar for children and adolescents/adults (23% of HHCs <15 years old vs 22% of adolescents/adults ≥15 years old; OR 0.83, 95% confidence interval 0.60–1.13; P = .23). A total of 969 (96%) HHCs had CXRs; among the 38 who did not, 16 (42%) were pregnant. A total of 169 (17%) CXRs were abnormal, including 8 (1%) with cavitary lesions. Of 55 children <15 years old with abnormal CXRs, 27 (49%) CXRs were suggestive of or compatible with TB.
Of the 1007 HHCs screened, 121 (12%) were newly diagnosed with TB. There were 31 households (11%) that had more than 1 HHC with a new TB diagnosis. Of the 121 new TB cases, 17 (2% of 1007) were confirmed, 33 (3%) were probable, and 71 (7%) were possible TB (all children <15 years old); 115 (95%) were pulmonary TB. Among the 303 HHCs <15 years old, 84 (28%) had TB diagnosed: 78 of those cases were pulmonary TB (4 confirmed, 9 probable, 65 possible) and 6 were possible extrapulmonary TB. Of the 17 confirmed TB cases, 5 (30%) were RR/MDR-TB, 11 (65%) were drug-susceptible, and 1 (5%) had no DST data available (Table 1). When including the 9 already diagnosed cases prior to study entry and 17 new confirmed cases, 11 (42%) were RR/MDR-TB, 14 (54%) were drug-susceptible TB, and 1 (4%) had no DST data available.
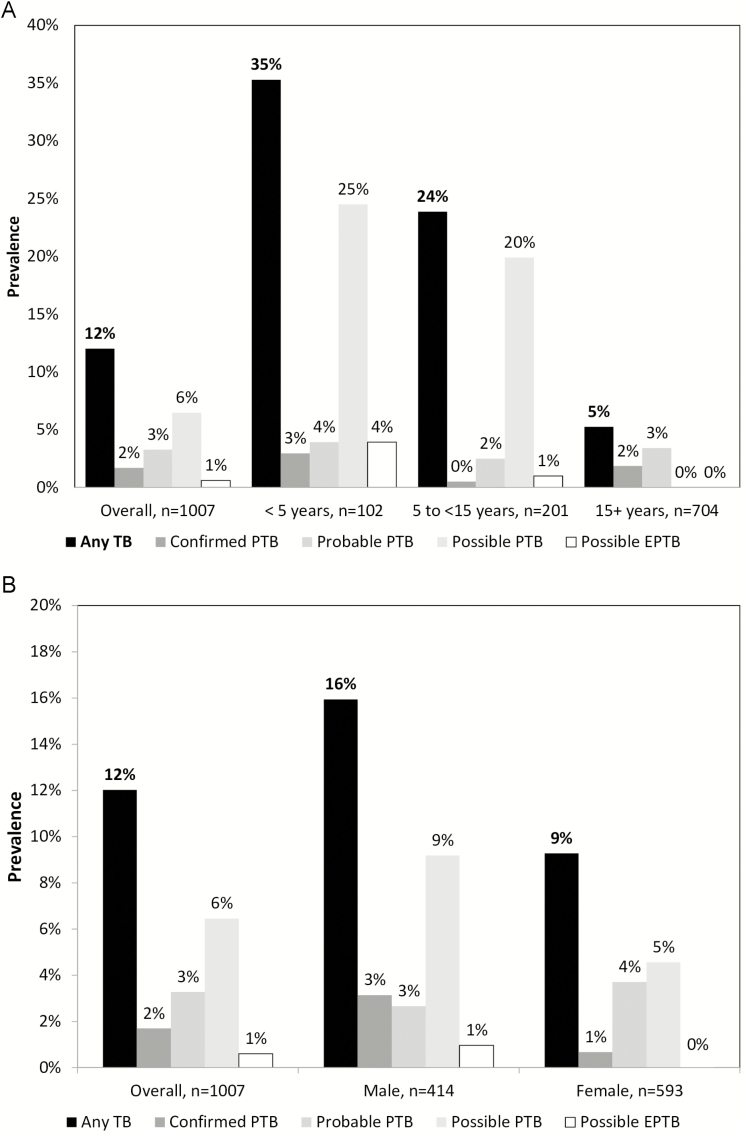
The proportion of new TB cases diagnosed varied by sex, by age, and by country (Figure 2; Tables 2 and 3; Supplementary Table 2). While all possible TB cases were, by definition, in children, there were overall differences in the proportions of confirmed or probable TB cases by age groups (<5 years old, 7%; 5 to <15 years old, 3%; 15 to <50 years old, 4%; and 50+ years old, 9%; P = .027). The proportions of TB cases diagnosed also varied by TBI statuses (98 of 708 [14%] who were TBI positive; 20 of 272 [7%] who were TBI negative; 3 of 27 [11%] whose TBI statuses were unknown; P = .08). Specifically, TB diagnosis rates were higher for those that were TST positive (16% in those TST+, 10% in those TST−, and 9% in those who did not have a TST done, for all TB diagnoses; 9% in those TST+, 2% in those TST−, and 3% in those who did not have a TST done, in confirmed/probable TB cases) and IGRA positive (14% in those IGRA+, 8% in those IGRA−, 38% in those with an indeterminate IGRA result, and 4% in those with an unknown IGRA result, for all TB diagnoses; 7% in those IGRA+, 1% in those IGRA−, 0% in those with an indeterminate IGRA result, and 13% in those with an unknown IGRA result, in confirmed/probable TB cases). Differences were also observed by HIV statuses for all TB diagnoses (4 of 65 [6%] who were HIV positive; 77 of 695 [11%] who were HIV negative; 40 of 247 [16%] whose HIV statuses were unknown; P = .021). However, there were no differences by HIV status when restricting the analysis to only confirmed or probable TB cases (4 of 65 [6%] who were HIV positive; 37 of 695 [5%] who were HIV negative; 9 of 247 [4%] whose HIV statuses were unknown; P = .57), indicating the possibility that the associations with possible TB cases were confounded by age.
Figure 2.
Prevalent TB among household contacts screened for TB, (A) by age group and (B) by sex. Abbreviations: EPTB, extrapulmonary tuberculosis; PTB, pulmonary tuberculosis; TB, tuberculosis.
Table 2.
Tuberculosis Diagnoses Prior to Study Entry and According to Study Outcome Review Group by Age
| Age | TB Diagnosed Prior to Entry | Any New TB | New Confirmed PTB | New Probable PTB | New Possible PTB | New Possible EPTB | No TB | Total Excluding TB Prior to Entry |
|---|---|---|---|---|---|---|---|---|
| n (column %) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (column%) | |
| <5 years | 1 (11.1%) | 36 (36.3%) | 3 (2.9%) | 4 (3.9%) | 25 (24.5%) | 4 (3.9%) | 66 (64.7%) | 102 (10.1%) |
| 5 to <15 years | 0 (0.0%) | 48 (23.9%) | 1 (0.5%) | 5 (2.5%) | 40 (19.9%) | 2 (1.0%) | 153 (76.1%) | 201 (20.0%) |
| 15 to <50 years | 6 (66.7%) | 21 (4.0%) | 10 (1.9%) | 11 (2.1%) | ─ | ─ | 509 (96.0%) | 530 (52.6%) |
| ≥50 years | 2 (22.2%) | 16 (9.2%) | 3 (1.7%) | 13 (7.5%) | ─ | ─ | 158 (90.8%) | 174 (17.3%) |
| Total n (row%) | 9 | 121 (12.0%) | 17 (1.7%) | 33 (3.3%) | 65 (6.5%) | 6 (0.6%) | 886 (88.0%) | 1007 |
Percentages do not always add up to subtotals because of rounding. Numbers shown in the table are row percentages, unless otherwise specified.
The proportion of new TB cases diagnosed varied by age (<5 years 36%, OR [compared to ≥50 years] 4.50, 95% CI 2.35–8.60; 5 to <15 years 24%, OR 2.79, 95% CI 1.61–4.83; and 15 to <50: 4%, OR 0.38, 95% CI 0.22–0.65; ≥50: 9%; overall P < .001).
Abbreviations: CI, confidence interval; EPTB, extrapulmonary tuberculosis; OR, odds ratio; PTB, pulmonary tuberculosis; TB, tuberculosis.
Table 3.
Tuberculosis Diagnoses Prior to Study Entry and According to Study Outcome Review Group by Sex
| Sex | TB Diagnosed Prior to Entry | Any New TB | New Confirmed PTB | New Probable PTB | New Possible PTB | New Possible EPTB | No TB | Total Excluding TB Prior to Entry |
|---|---|---|---|---|---|---|---|---|
| n (column%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (column%) | |
| Male | 4 (41.1%) | 66 (15.9%) | 13 (3.1%) | 11 (2.7%) | 38 (9.2%) | 4 (1.0%) | 348 (84.1%) | 414 (41.1%) |
| Female | 5 (58.9%) | 55 (9.3%) | 4 (0.7%) | 22 (3.7%) | 27 (4.5%) | 2 (0.3%) | 538 (90.7%) | 593 (58.9%) |
| Total n (row%) | 9 | 121 (12.0%) | 17 (1.7%) | 33 (3.3%) | 65 (6.5%) | 6 (0.6%) | 886 (88.0%) | 1007 |
Percentages do not always add up to subtotals because of rounding. Numbers shown in the table are row percentages, unless otherwise specified.
The proportion of new TB cases diagnosed varied by sex (males 66 of 414 [16%] vs. females 55 of 593 [9%], OR 1.88, 95% CI 1.31- 2.71; P = .002).
Abbreviations: CI, confidence interval; EPTB, extrapulmonary tuberculosis; OR, odds ratio; PTB, pulmonary tuberculosis; TB, tuberculosis.
Prevalence of Tuberculosis Infection Among Household Contacts and Proportion of Household Contacts Who Met High-risk Contact Definition
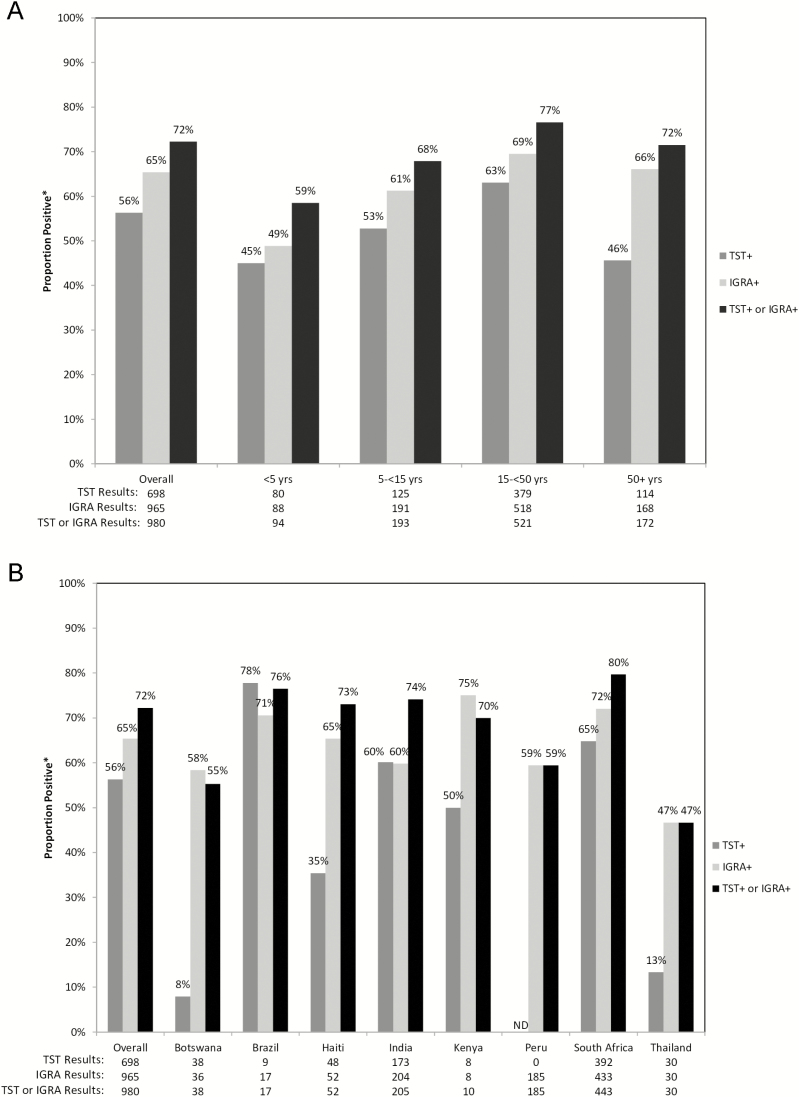
There were variations in the TBI tests used by country and the TBI prevalences by test used, country, and age (Figure 3A and 3B). Overall, the TBI rates were 56% by TST, 65% by IGRA, and 72% by either TST or IGRA. The TBI positivity rate by either test was highest in South Africa (80% positive) and lowest in Thailand (47% positive). The TST positivity rates ranged from 8% in Botswana to 78% in Brazil, while IGRA positivity rates ranged from 47% in Thailand to 75% in Kenya. By age group, TBI was identified in 59% of HHCs <5 years old, 68% of HHCs 5 to <15 years old, 77% of HHCs 15 to <50 years old, and 72% of HCCs ≥50 years old.
Figure 3.
Distribution of TST and IGRA positivity, (A) by age group and (B) by country. Abbreviations: IGRA, interferon gamma release assay; ND, not done; TB, tuberculosis; TST, tuberculin skin test. *Among those with definitive results.
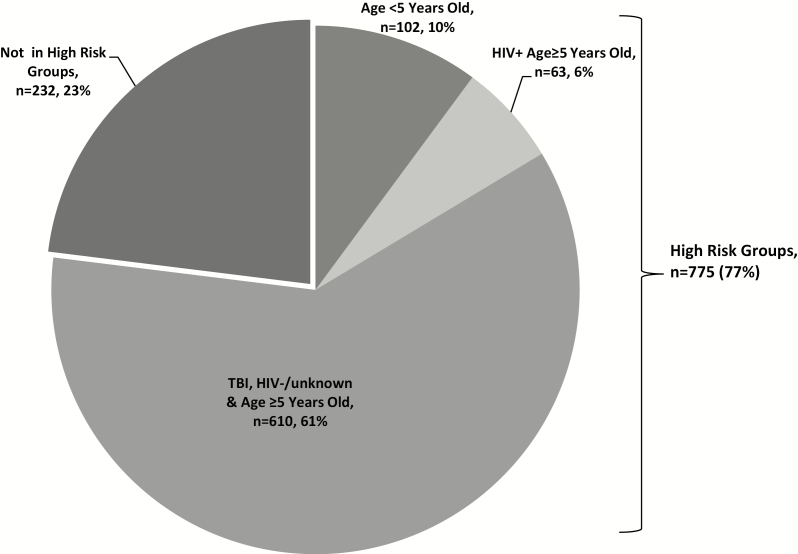
A total of 775 (77%) HHCs met the high-risk criteria (Figure 4): 102 (10%) were <5 years old; 63 (6%) were ≥5 years old and HIV-infected; and 610 (61%) were ≥5 years old, HIV-negative or with an unknown HIV status, and had TBI. A median of 2 high-risk contacts per index case household were identified. Very few individuals were receiving TB preventive therapy at screening (1 [<1%] of those ≥15 years old and 20 [7%] of those <15 years old).
Figure 4.
Proportion of household contacts that were identified as high-risk contacts. Some household contacts met multiple criteria as follows: age <5 years old group: 1 HIV+ & TBI, 1 HIV+ only, 1 TBI only; HIV+ & age ≥5 years old group: 43 TBI. TBI was diagnosed by either interferon gamma release assay or tuberculin skin test. Abbreviations: HIV, human immunodeficiency virus; TBI, tuberculosis infection.
DISCUSSION
Our study is among the largest household TB screening studies of RR/MDR-TB contacts conducted in multiple countries with high TB burdens. First, we observed that active case finding for TB among recently exposed HHCs identified a substantial proportion of individuals with undiagnosed TB, with 12% meeting the study criteria for a new diagnosis of TB. Second, we observed a high prevalence of TBI among the HHCs of MDR-TB cases. Using a definition of TBI positivity by either TST or IGRA, nearly three-quarters of HHCs were infected: a proportion substantially higher than what is reported in the general population in these settings [20]. Lastly, we found that <1% of adults and only 7% of child HHCs were on any TB preventive therapy. Finding that the majority of HHCs of RR/MDR-TB cases either had TB disease or TBI is alarming, highlighting the importance of timely screening, the potential for preventing future TB disease, and the urgent need for novel, effective, preventive TB therapies.
Identifying TB cases is a global priority, and the active screening of recently exposed HHCs is recommended yet remains inconsistent in most settings with high TB burdens. We found that 6% of HHCs had confirmed or probable TB, only 15% of which had been previously diagnosed. This is a high percentage that is consistent with recent meta-analyses, which showed pooled estimates of 3.4% to 6.5% of MDR-TB contacts with TB [10, 13]. The inclusion of possible TB diagnoses among children <15 years old, however, made our estimates higher than earlier estimates, as many programs either do not perform intensive screening (ie, with gastric aspirates and CXRs) and/or do not routinely report screening yields from this age group. We observed that 42% of HHCs with culture-confirmed TB had evidence of at least RR-TB. Studies in Peru found higher proportions (72% to 91%) [21–23] of MDR-TB HHCs with confirmed TB, while a study in South Africa found a similar proportion, of 44% [23], and studies in Asia found lower proportions (9% in Hong Kong and 12.5% in India) [24, 25]. The variability is likely a reflection of the diverse populations, community transmission dynamics, and screening algorithms (Supplementary Table 1). The observation that less than half of contacts with confirmed TB had MDR-TB supports our PHOENIx trial decision to use isoniazid in our control arm, rather than placebo.
We found that young children have a high prevalence of TB (28% of children <15 years old who were screened met our study definition) and should be rapidly screened after an adult in the household is diagnosed with MDR-TB. Meta-analyses have shown that child HHCs <5 years old have the highest risk for progression to active TB, compared to older groups [26]. Pediatric TB diagnoses, however, remain a substantial challenge, even with optimal collection and processing of samples, such as gastric aspirates. The spectrum of radiological abnormalities in children is also broad, further complicating clinical diagnoses [26]. A noninvasive, highly sensitive and specific, rapid, and easy-to-perform diagnostic is urgently needed to advance pediatric TB identification [27].
The 2018 WHO TBI guidelines recommend using TST or IGRA in low-/middle-income countries and strongly recommend using TBI testing for HHCs of MDR-TB cases [7]. Using TST for TBI diagnoses, systematic reviews have found a pooled proportion of TBI between 47% and 52%; when restricted to MDR-TB contacts, the proportion was between 51% and 61% [10, 12]. The proportions were higher in settings with high, compared to low, TB burdens. As expected, age influenced the proportion of HHCs found to be TB positive. Shah et al [10] found a pooled TBI prevalence among children of 27%; among adults, this rose to 52%. Fox et al [12] found that 36% of children <5 years old were TBI positive, 53% of those 5–14 years old were positive, and 65% of those ≥15 years old were positive. Our study is among the first to assess TBI rates using both TST and IGRA in MDR-TB contacts in diverse settings with high TB burdens. We observed differences in the proportions diagnosed as TBI positive by the test used, with notable country and age differences. We observed a higher proportion (72%) of HHCs with TBI than that reported in the systematic reviews. Using either TST or IGRA positivity as our definition of TBI was the main reason, as these are not completely concordant tests for TBI [28]. Even children <5 years old had a high TBI prevalence, of more than 50%. Importantly, the proportion of HHCs with TBI is much higher than estimates for the general population. Houben et al [20] estimated that 23% of the world’s population has TBI, ranging from 11% in the Americas to 31% in Southeast Asia, and that TBI rates among children range from 2% in Europe to 13% in Africa.
We found that a very low proportion of HHCs had been initiated on TB preventive therapy (<1% of adults/adolescents and 7% of children <15 years old). INH alone or INH/ethambutol/fluoroquinolone were used as preventive therapies, per local standards of care. This reflects the fact that most TB control programs lack recommendations for preventive therapy for MDR-TB contacts. In fact, until 2018, the WHO recommended only careful observation for TB symptoms for up to 2 years after exposure, as there was no high-quality evidence to guide specific preventive therapy regimens [7–9]. Small observational studies have suggested benefits of fluoroquinolone or fluoroquinolone-containing regimens [7]. Given the high proportion of HHCs that have diagnosed TB or evidence of TBI, it is urgent that novel, preventive therapies are carefully studied. There are 2 trials now underway to assess fluoroquinolones in adults/adolescents (V-QUIN ACTRN12616000215426/U1111-1177–9052) and in children (TB CHAMP ISRCTN92634082), and our team is initiating a trial assessing the novel agent delamanid (PHOENIx NCT03568383), which has the potential added advantage of treating contacts of fluoroquinolone-resistant TB [1, 29, 30].
Limitations of our study include that we could not perform TST on 20% of the enrolled contacts, due to a shortage of tests in Peru. We also had 2 sites that could not enroll HHCs <18 years old, due to in-country regulatory challenges. Furthermore, TB diagnoses could not be confirmed in half of cases among children <15 years old who had signs/symptoms of TB and/or abnormal CXRs, because no organism could be identified with current standard molecular testing or cultures. We therefore have an incomplete picture of TB disease and TBI prevalence in children. In the context of our proposed trial, rigorous, multiple samples will be examined for bacteriology, central chest radiographic reviews will be completed, and blinded outcome reviews will be completed for children to address these challenges and ensure that more children are diagnosed with confirmed and probable TB.
In summary, the HHCs of MDR-TB cases are at high risk of TBI and disease, so it is critical that HHCs be rapidly screened after a TB case is diagnosed. With the majority of HHCs having evidence of TBI by either TST or IGRA, most would be eligible for TB preventive therapy. Results from the ongoing trials, together with our planned PHOENIx study, will fill the major data gap that exists on how best to prevent TB among those recently exposed to MDR-TB.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the study participants; site community advisory boards; Dan Johnson, Clinical Representative; George K. Siberry, clinical representative; Anne-Marie Demers, consultant microbiologist; Richard E. Chaisson, investigator; Savita Kanade, field representative; Janet Nicotera, field representative; Patricia Anthony, laboratory technologist; Christopher Lane, laboratory technologist; Ujwala A. Kadam, community scientific subcommittee representative; R. Ssenyonga, community scientific subcommittee representative; Akbar Shahkolahi, international site specialist; and Adam Manzella, laboratory data manager, for their contributions.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of any funders.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Institute of Mental Health (NIMH), UM1AI068634, UM1AI068636, UM1AI106701, UM1A1068616, UM1AI068632, UM1AI068616 and UM1AI106716), and the NICHD contract number HHSN275201800001I. A. G. and S. C. are supported by the National Institute of Allergy and Infectious Diseases (grant number UM1AI069465).
Potential conflicts of interest. L. M. has received grants from Janssen Pharmaceutica, Merck Sharp & Dohme Corp, ViiV Healthcare, Johnson and Johnson, Pfizer Pharmaceuticals, and Bristol Myers Squibb and nonfinancial support from Sanofi Aventis and Kowa Pharmaceuticals America, outside the submitted work. S. S. has received grants from Merck and ViiV, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global tuberculosis report 2018 Available at: https://www.who.int/tb/publications/global_report/en/. Accessed 1 March 2019.
- 2. World Health Organization. Monitoring of Xpert MTB/RIF roll-out Available at: http://www.who.int/tb/areas-of-work/laboratory/mtb-rif-rollout/en/. Accessed 1 April 2018.
- 3. Fatima R, Qadeer E, Yaqoob A, et al. . Extending ‘contact tracing’ into the community within a 50-metre radius of an index tuberculosis patient using Xpert MTB/RIF in urban, Pakistan: did it increase case detection? PLOS One 2016; 11:e0165813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Creswell J, Codlin AJ, Andre E, et al. . Results from early programmatic implementation of Xpert MTB/RIF testing in nine countries. BMC Infect Dis 2014; 14:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khanal S, Baral S, Shrestha P, et al. . Yield of intensified tuberculosis case-finding activities using Xpert(®) MTB/RIF among risk groups in Nepal. Public Health Action 2016; 6:136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cain KP, Nelson LJ, Cegielski JP. Global policies and practices for managing persons exposed to multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2010; 14:269–74. [PubMed] [Google Scholar]
- 7. World Health Organization. Latent TB infection: updated and consolidated guidelines for programmatic management 2018. Geneva, Switzerland: World Health Organization, 2018. Available at: http://www.who.int/tb/publications/2018/latent-tuberculosis-infection/en/. Accessed 1 March 2019. [PubMed] [Google Scholar]
- 8. World Health Organization. Recommendations for investigating contacts of persons with infectious tuberculosis in low- and middle-income countries. Geneva, Switzerland: World Health Organization, 2012. Available at: https://www.who.int/tb/publications/2012/contact_investigation2012/en/. Accessed 1 March 2019. [PubMed] [Google Scholar]
- 9. World Health Organization. Guidelines on the management of latent tuberculosis infection. Geneva, Switzerland: World Health Organization, 2015. [PubMed] [Google Scholar]
- 10. Shah NS, Yuen CM, Heo M, Tolman AW, Becerra MC. Yield of contact investigations in households of patients with drug-resistant tuberculosis: systematic review and meta-analysis. Clin Infect Dis 2014; 58:381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morrison J, Pai M, Hopewell PC. Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis 2008; 8:359–68. [DOI] [PubMed] [Google Scholar]
- 12. Fox GJ, Barry SE, Britton WJ, Marks GB. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J 2013; 41:140–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. International Maternal Pediatric Adolescent AIDS Trials. IMPAACT 2003B: (PHOENIx) (DAIDS ID 12041), Protecting households on exposure to newly diagnosed index multidrug-resistant TB patients Available at: http://impaactnetwork.org/studies/IMPAACT2003B.asp. Accessed 1 March 2019.
- 14. International Maternal Pediatric Adolescent AIDS Trials. Network sites Available at: http://impaactnetwork.org/studies/sites.asp. Accessed 1 March 2019.
- 15. AIDS Clinical Trials Group. ACTG sites Available at: https://actgnetwork.org/actg-sites. Accessed 1 March 2019.
- 16. American Thoracic Society. Diagnostic Standards and Classification of Tuberculosis in Adults and Children. Am J Respir Crit Care Med. 2000; 161:1376–95. Available at: https://www.cdc.gov/tb/publications/pdf/1376.pdf [DOI] [PubMed] [Google Scholar]
- 17. Qiagen. QuantiFERON®-TB Gold Plus (QFT®-Plus) [package insert]. Germantown, Maryland: Qiagen, 2017:31–6. [Google Scholar]
- 18. Opollo VS, Wu X, Hughes MD, et al. . HIV testing uptake among the household contacts of multidrug-resistant tuberculosis index cases in eight countries. Int J Tuberc Lung Dis 2018; 22:1443–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Churchyard G, Kim P, Shah NS, et al. . What we know about tuberculosis transmission: an overview. J Infect Dis 2017; 216(Suppl 6):S629–35. Available at: https://www.ncbi.nlm.nih.gov/pubmed/29112747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Houben RM, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLOS Med 2016; 13:e1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Becerra MC, Appleton SC, Franke MF, et al. . Tuberculosis burden in households of patients with multidrug-resistant and extensively drug-resistant tuberculosis: a retrospective cohort study. Lancet 2011; 377:147–52. [DOI] [PubMed] [Google Scholar]
- 22. Becerra MC, Franke MF, Appleton SC, et al. . Tuberculosis in children exposed at home to multidrug-resistant tuberculosis. Pediatr Infect Dis J 2013; 32:115–9. [DOI] [PubMed] [Google Scholar]
- 23. Vella V, Racalbuto V, Guerra R, et al. . Household contact investigation of multidrug-resistant and extensively drug-resistant tuberculosis in a high HIV prevalence setting. Int J Tuberc Lung Dis 2011; 15:1170–5, i. Available at: https://www.ncbi.nlm.nih.gov/pubmed/21943840 [DOI] [PubMed] [Google Scholar]
- 24. Leung EC, Leung CC, Kam KM, et al. . Transmission of multidrug-resistant and extensively drug-resistant tuberculosis in a metropolitan city. Eur Respir J 2013; 41:901–8. [DOI] [PubMed] [Google Scholar]
- 25. Singla N, Singla R, Jain G, Habib L, Behera D. Tuberculosis among household contacts of multidrug-resistant tuberculosis patients in Delhi, India. Int J Tuberc Lung Dis 2011; 15:1326–30. [DOI] [PubMed] [Google Scholar]
- 26. Roya-Pabon CL, Perez-Velez CM. Tuberculosis exposure, infection and disease in children: a systematic diagnostic approach. Pneumonia (Nathan) 2016; 8:1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perez-Velez CM, Roya-Pabon CL, Marais BJ. A systematic approach to diagnosing intra-thoracic tuberculosis in children. J Infect 2017; 74(Supp 1):S74–83. [DOI] [PubMed] [Google Scholar]
- 28. Pai M, Behr M. Latent Mycobacterium tuberculosis infection and interferon-gamma release assays. Microbiol Spectr 2016; 4:1-10. doi: 10.1128/microbiolspec.TBTB2-0023-2016. [DOI] [PubMed] [Google Scholar]
- 29. Chen X, Hashizume H, Tomishige T, et al. . Delamanid kills dormant mycobacteria in vitro and in a guinea pig model of tuberculosis. Antimicrob Agents Chemother 2017; 61:1-11. doi: 10.1128/AAC.02402-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Matsumoto M, Hashizume H, Tomishige T, et al. . OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. PLOS Med 2006; 3:e466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.