Abstract
Objective
To investigate the natural history of fibrotic lung disease in recipients of a single lung transplant for scleroderma-associated interstitial lung disease (ILD).
Methods
Global ILD (including ground glass, nodular opacities and fibrosis) was categorized into severity quintiles on first and last post-transplant CT scans, and percent fibrosis by manual contouring was also determined, in nine single lung transplant recipients. Quantitative mean lung densities and volumes for the native and allograft lungs were also acquired.
Results
In the native lung, global ILD severity quintile worsened in two cases and percent fibrosis worsened in four cases (range 5–28%). In the lung allograft, one case each developed mild, moderate and severe ILD; of these, new fibrotic ILD (involving <10% of lung) occurred in two cases and acute cellular rejection occurred in one. The average change in native lung density over time was +2.2 Hounsfield Units per year and lung volume +1.4 ml per year, whereas the allograft lung density changed by –5.5 Hounsfield Units per year and total volume +27 ml per year (P = 0.011 and P = 0.039 for native vs allograft density and volume comparisons, respectively).
Conclusions
While the course of ILD in the native and transplanted lungs varied in this series, these cases illustrate that disease progression is common in the native lung, suggesting that either the immune process continues to target autoantigens or ongoing fibrotic pathways are active in the native lung. Mild lung disease may occur in the allograft after several years due to either allograft rejection or recurrent mild ILD.
Keywords: scleroderma, systemic sclerosis, lung transplant, interstitial lung disease, pulmonary fibrosis, longitudinal, CT imaging
Rheumatology key messages
Interstitial lung disease commonly worsens in the native lung of single lung transplant recipients with scleroderma.
A lung allograft has long-term survival in patients with scleroderma.
Mild lung fibrosis can occur after years of follow-up in the lung allograft.
Introduction
Interstitial lung disease (ILD) affects up to 90% of patients with scleroderma when evaluated by high-resolution CT [1]. The progression of ILD in scleroderma is highly variable, ranging from limited non-progressive bibasilar fibrosis with normal pulmonary function testing to progressive lung involvement causing hypoxaemic respiratory failure. Approximately 10–15% of patients with scleroderma-associated ILD will progress to severe end-stage lung disease [2]. For these patients, lung transplantation is a life-saving option. Despite initial concerns that patients with scleroderma undergoing lung transplantation may have poor survival due to extra-pulmonary disease, studies show similar survival as compared with patients undergoing lung transplant for idiopathic pulmonary fibrosis or pulmonary hypertension not related to connective tissue disease [3–10].
Both single lung transplant (SLT) and bilateral lung transplant procedures are performed in patients with scleroderma. Bilateral lung transplant is now preferred due to a lower incidence of chronic lung allograft dysfunction compared with SLT [11]. In patients with high lung allocation scores at time of transplant, bilateral lung transplant is also associated with improved survival [11]. While recurrence of ILD in the lung allograft in patients with scleroderma-associated ILD has not been reported, it has also not been rigorously defined and studied. However, in studies assessing outcomes in lung transplant recipients with scleroderma, recurrent ILD has not been reported to be the cause of death [3–10]. It is ultimately unknown whether scleroderma disease activity or chronic allograft rejection will cause the development of progressive ILD in the lung allograft. Furthermore, the long-term outcome of the native lung has not been studied in cases of SLT.
Patients with scleroderma who have undergone SLT provide a unique opportunity to evaluate the course and progression (or not) of ILD in the single lung allograft and the native diseased lung. In this case series, we report the clinical history and detailed CT imaging findings of nine subjects from two institutions who underwent SLT for advanced scleroderma-associated ILD. In addition to blinded radiology reviews of CT images, we applied reader-independent quantitative methodology to determine the density of lung parenchyma and lung volume on longitudinal CT scans [12]. Density determination by this methodology has been validated as an objective measurement of the extent of ILD in idiopathic pulmonary fibrosis [13]. Quantitative CT has also been used to evaluate ILD progression in scleroderma [14]. We hypothesized that the ILD in the native lung would worsen over time, as evidenced by qualitative increase in global ILD severity quintile, semi-quantitative increase in percent fibrosis and quantitative increase in density over time, whereas the transplanted lung would remain largely unchanged.
Methods
Study cohort
Demographic, clinical, serological and radiographic information has been collected on >3500 patients with scleroderma in the Johns Hopkins Scleroderma Cohort and >300 patients in the University of California San Francisco Scleroderma Cohort. A total of 16 cases from these two cohorts were identified as having undergone a SLT. Cases were included in the study if they had undergone an SLT for scleroderma-associated ILD and had at least two CT scans at least 18 months apart, as it was felt that this would be the minimum time required to identify a significant change in ILD. A total of nine cases (seven from Johns Hopkins Scleroderma Cohort and two from University of California San Francisco Scleroderma Cohort) met the inclusion criteria. Of the seven SLT recipients that did not meet inclusion criteria, two died within the immediate post-operative period—one from acute respiratory distress syndrome and one from multisystem ischaemic reperfusion injury. A third recipient died 14 months post-transplant from resistant CMV pneumonitis. A fourth patient died 7 years post-transplant from post-transplant lymphoproliferative disorder; we were unable acquire serial CT images for this patient. The remaining three recipients were lost to follow-up; attempts at contacting them using last known contact information were unsuccessful. All nine included cases fulfilled the 2013 ACR classification criteria for scleroderma [15]. The presence of scleroderma organ involvement was prospectively collected during routine clinical visits occurring every 6 months and documented in a database. Informed consent forms and other recruitment materials were approved by the Institutional Review Board at each institution before study initiation. The study was conducted in compliance with the International Conference on Harmonization for Good Clinical Practice Guidelines and the Declaration of Helsinki.
The diagnosis and grading of acute cellular rejection were carried out on the basis of the results of transbronchial biopsies and in accordance with the International Society for Heart and Lung Transplantation [16]. The diagnosis and grading of bronchiolitis obliterans syndrome (BOS), a subtype of chronic lung allograft dysfunction, was made on the basis of percent decline in post-transplant FEV1 in accordance with the International Society for Heart and Lung Transplantation, American Thoracic Society and European Respiratory Society clinical guidelines [17]. Formal criteria for the diagnosis of restrictive allograft syndrome, a subtype of chronic lung allograft dysfunction, does not yet exist. Restrictive allograft syndrome may be suggested by restrictive lung physiology on pulmonary function tests in combination with new ILD; however, restrictive lung physiology by pulmonary function testing is confounded by progression of ILD in the native lung in SLT, thus making restrictive allograft syndrome particularly challenging to diagnose with certainty in the SLT population [18].
Imaging data
All known CT images of the chest that participants had undergone post-transplantation were acquired for analysis. Routine post-transplant monitoring was the most common clinical indication for the scans.
Digital Imaging and Communications in Medicine (DICOM) datasets were anonymized and assigned random study identification numbers prior to review, and radiologists were blinded to all clinical data including dates of transplants and scans. A board-certified thoracic radiologist (C.T.L.) and board-certified diagnostic radiologist (A.F.H.) reviewed the earliest and latest post-transplant CT scans for each participant in a blinded fashion, and global ILD severity was visually estimated. Prior to reading, both radiologists had trained and agreed on the scoring method. Global ILD severity was categorized as follows: none, trace (1–10%), mild (11–25%), moderate (26–50%), severe (51–75%) and very severe (>75%) [19]. In any cases in which there was a disagreement between radiologists, scans were reviewed together and consensus reached. Qualitative assessment of CT images was conducted on OsiriX (version 7.0; Pixmeo, Geneva, Switzerland). ILD patterns were characterized as cellular non-specific interstitial pneumonia (NSIP), fibrotic NSIP or usual interstitial pneumonia as described previously [20, 21]. Fibrotic NSIP on CT was defined as the presence of any of the following: reticulations, traction bronchiectasis, honeycombing and architectural distortion. Cellular NSIP on CT was defined as the presence of ground-glass and/or nodular opacities. Semi-quantitative analysis of fibrosis was performed by certified thoracic radiologist on a free, open-source imaging software (3D Slicer, www.slicer.org) with the Chest Imaging Platform extension. Percent fibrosis was quantified on axial CT images by manual contouring of the areas that demonstrated fibrotic ILD, using methods described previously [22]. The percent fibrosis was determined by dividing the area of fibrotic lung by the total lung area. All imaging data were collected using a standardized form with separate entries for each native and transplanted lung.
For assessment of density and volume, only non-contrasted CT scans were analysed, as contrast could variably affect density measurements. A total of 69 scans were utilized for parenchymal density and lung volume measurements. These scans were obtained at irregular intervals over time beginning post-transplant, such that patients in general were not followed for the same amount of time. All 69 CT images were analysed using the Pulmonary Workstation 2 software (VIDA Diagnostics, Coralville, IA, USA), applying CT quantitative methodology [23]. Automated segmentation of the right and left lungs from the chest wall and mediastinum allowed for separate measurements of right and left lung density and volume. Lung density was expressed in Hounsfield Units (HU), where 0 HU represents the density of water and −1000 HU represents the density of air. Normal lung density at total lung capacity ranges from −750 to −950 HU [24]. Air volume and tissue volume were also obtained, and expressed in millilitres (ml).
For exploratory analysis of the longitudinal trends in lung densities and volumes, a linear model with random patient effect for each native and transplanted lung was estimated for the nine subjects.
Results
Characteristics of study participants
A total of nine subjects underwent SLT for scleroderma-related ILD (Table 1). Median disease duration at the time of transplant, from onset of RP, was 13 years (interquartile range 9–14) and from onset of first non-Raynaud’s symptom was 9 years (interquartile range 8–12). The median time of follow-up post-transplantation was 7 years (interquartile range 6–14), and seven subjects were alive at the end of follow-up. Scleroderma subtype, autoantibody status, organ involvement and immunosuppression regimens for each of the nine cases are summarized in Table 1.
Table 1.
Clinical characteristics and immunosuppression regimen(s) of nine SLT recipients with scleroderma
| Case | Age (years) at SLT | Sex | Race | Disease duration (years) at SLT (from RP/first non-RP symptom) | Years followed post- transplant | Status at end of follow- up | Subtype | Serology | Scleroderma organ involvement | Immunosuppression regimen(s)a | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ANA pattern | Scl70 | ACA | RNA Pol III | Raynaud's | PAH | Cardiac | Upper GI | Renal Crisis | Articular | Telangiectasia | Calcinosis | Prednisone | MMF | Azathioprine | Tacrolimus | Cyclosporine | Everolimus | ||||||||
| 1 | 46 | F | C | 14/7 | 17 | Dead | Limited | Nucleolar | − | − | NT | + | − | − | + | − | − | + | − | + | − | + | + | − | − |
| 2 | 37 | F | C | 17/8 | 18 | Alive | Limited | Speckled | − | − | NT | + | − | − | + | − | − | + | − | + | − | + | − | + | − |
| + | + | − | + | − | − | ||||||||||||||||||||
| 3 | 45 | M | Other | 9/9 | 7 | Alive | Diffuse | Speckled | − | − | + | + | − | + | + | − | + | + | + | + | + | − | + | − | − |
| 4 | 48 | F | C | 1/3 | 7 | Dead | Sine | Speckled | + | − | NT | + | − | − | + | − | − | − | − | + | + | − | + | − | − |
| 5 | 32 | F | C | 9/9 | 6 | Alive | Diffuse | Homogenous | + | − | − | + | − | − | + | − | + | + | − | + | + | − | + | − | − |
| 6 | 38 | F | C | 13/13 | 17 | Alive | Limited | Speckled | − | + | − | + | + | + | + | − | − | + | − | + | − | + | + | − | − |
| + | − | − | + | − | + | ||||||||||||||||||||
| + | − | − | + | − | − | ||||||||||||||||||||
| 7 | 47 | F | C | 28/13 | 2 | Alive | Limited | Nucleolar | − | − | − | + | − | − | + | − | − | + | + | + | + | − | + | − | − |
| 8 | 61 | M | Asian | 10/8 | 14 | Alive | Limited | Speckled | − | − | − | + | + | − | + | − | − | + | − | + | + | − | + | − | − |
| 9 | 53 | F | C | 13/12 | 2.5 | Alive | Diffuse | Negative | − | − | NT | + | + | − | + | − | − | + | − | + | + | − | + | − | − |
Cases with more than one regimen listed had change in regimen during clinical course. Scl70: anti-topoisomerase I antibody; RNA Pol III: anti-RNA Polymerase III antibody. PAH: pulmonary arterial hypertension as defined by right heart catheterization with pulmonary artery systolic pressure ≥25 mmHg with pulmonary capillary wedge pressure <15 mmHg; cardiac: presence of arrhythmia requiring treatment, ECG evidence of conduction defect, left ventricular systolic function <50%, or clinical signs of left or right heart failure; upper GI: gastroesophageal reflux disease; articular: presence of synovitis or tendon friction rubs; SLT: single lung transplant; F: female; M: male; C: Caucasian; NT: not tested.
Two subjects were deceased at time of study closure. One subject died of respiratory failure secondary to BOS and one subject died of shock, presumed septic although no organism was identified. Five subjects (cases 1, 3, 5, 6 and 8) developed BOS between 5–12 years post-transplantation. Three subjects (cases 2, 5 and 9) developed acute cellular rejection of the lung allograft between 1 and 3 years post-transplant. Two subjects recovered with steroid treatment; immunosuppression regimen was changed in one subject (case 2). The third subject (case 9) with acute cellular rejection had failure of the single lung allograft and subsequently underwent bilateral lung transplantation at 2.5 years post-transplant.
Pulmonary function testing at the time of the first and last post-transplant CT scan are presented in supplementary Table S1, available at Rheumatology online.
ILD and fibrosis evaluation
Three methods were employed to evaluate the presence and progression of ILD and fibrosis in the native and transplanted lungs.
First, the overall global severity quintile of ILD (including ground glass, nodular opacities and fibrotic changes) for each lung on the first and last post-transplant CT scans was determined (Table 2). On assessment of the native lung, the ILD global severity worsened in two subjects, improved in three subjects and remained unchanged in four subjects. Of the four subjects with no change in global ILD severity for the native lung at the two time points, one subject (case 1) already had ‘very severe’ lung involvement on the first scan (the highest quintile at >75% involvement). On assessment of the transplanted lung, three cases had evidence of new global ILD on the last post-transplant CT scan compared with the first post-transplant CT scan, which was classified as ‘mild severity’ (11–25% involvement) in one case, ‘moderate severity’ (26–50% involvement) in one case and ‘severe’ in one case (51–75% involvement). The pattern of ILD in the subject (case 9) that developed severe ILD changes in the transplant lung was cellular NSIP; this subject also had biopsy changes consistent with acute cellular rejection and ultimately underwent re-transplantation. The three subjects with new ILD in the transplanted lung also had either worsening in global severity of the native lung (two subjects) or unchanged severity (one subject; already at ‘very severe’ global severity).
Table 2.
Qualitative and semi-quantitative ILD findings on first and last post-transplant CT scans
| Case | Years post- transplant first scan last scan | Native or transplanted lung | ILD global severitya | ILD pattern | Fibrosis % | Ground glass opacities | Tree-in-bud | Consolidation | Mosaic pattern | Non-traction bronchiectasis | Traction bronchiectasis | Reticular abnormalities | Honeycombing |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 8 | Native | Very severe | NSIP-fibrotic | 100 | + | − | − | − | BWT | + | + | − |
| Transplant | None | None | 0 | + | − | − | − | − | − | − | − | ||
| 17 | Native | Very severe | NSIP-fibrotic | 100 | − | − | − | − | Sig | + | + | + | |
| Transplant | Moderate | NSIP-fibrotic | 7 | + | − | − | − | Min | + | − | − | ||
| 2 | 12 | Native | Severe | UIP | 55 | − | − | − | − | BWT | + | + | + |
| Transplant | None | None | 0 | − | − | − | + | − | − | − | − | ||
| 18 | Native | Severe | UIP | 60 | − | − | − | − | BWT | + | + | + | |
| Transplant | None | None | 0 | − | − | − | + | − | − | − | − | ||
| 3 | 1 | Native | Very severe | NSIP-fibrotic | 48 | + | − | − | − | Sig | + | + | − |
| Transplant | None | None | 0 | − | − | − | − | − | − | − | − | ||
| 6 | Native | Severe | NSIP-fibrotic | 47 | − | − | − | − | + | + | + | − | |
| Transplant | None | None | 0 | − | − | − | − | − | − | − | − | ||
| 4 | 2 | Native | Very severe | NSIP-cellular | 0 | − | − | − | − | BWT | + | − | − |
| Transplant | None | None | 0 | − | − | − | − | − | − | − | − | ||
| 7 | Native | Severe | NSIP-cellular | 0 | + | + | + | − | BWT | + | − | − | |
| Transplant | None | None | 0 | − | − | + | − | Min | − | − | − | ||
| 5 | 0 | Native | Moderate | NSIP-fibrotic | 15 | − | − | − | − | BWT | + | + | − |
| Transplant | None | None | 0 | − | − | − | − | − | − | − | − | ||
| 5 | Native | Severe | NSIP-fibrotic | 43 | + | − | − | − | Sig | + | + | − | |
| Transplant | Mild | NSIP-fibrotic | 9 | + | + | + | − | Min | + | − | − | ||
| 6 | 5 | Native | Mild | NSIP-fibrotic | 2 | − | − | − | − | BWT | − | + | − |
| Transplant | None | None | 0 | − | + | + | − | Min | − | − | − | ||
| 15 | Native | Mild | NSIP-fibrotic | 2 | − | − | − | − | BWT | + | − | − | |
| Transplant | None | None | 0 | − | − | − | − | − | − | − | − | ||
| 7 | 0 | Native | Moderate | UIP | 16 | + | − | − | − | − | + | + | − |
| Transplant | Mild | NSIP-cellular | 0 | + | − | − | − | − | − | − | − | ||
| 6 | Native | Moderate | UIP | 14 | − | − | − | − | BWT | + | + | − | |
| Transplant | None | None | 0 | − | − | + | − | − | − | − | − | ||
| 8 | 8 | Native | Very severe | NSIP-fibrotic | 28 | + | − | − | − | BWT | + | + | − |
| Transplant | None | None | 0 | − | − | − | − | − | − | − | − | ||
| 14 | Native | Severe | NSIP-fibrotic | 50 | + | − | − | − | BWT | + | − | + | |
| Transplant | None | None | 0 | − | − | − | − | BWT | − | − | − | ||
| 9 | 0 | Native | Severe | UIP | 10 | + | − | − | − | − | + | + | + |
| Transplant | None | None | 0 | − | − | − | − | − | − | − | − | ||
| 2 | Native | Very severe | UIP | 35 | + | − | + | − | BWT | + | + | + | |
| Transplant | Severe | NSIP-cellular | 0 | + | − | + | + | − | − | + | − | ||
Severity: trace (1–10%), mild (11–25%), moderate (26–50%), severe (51–75%) and very severe (>75%). BWT: bronchial wall thickening; ILD: interstitial lung disease; Min: minimal; NSIP: non-specific interstitial pneumonia; Sig: significant; UIP: usual interstitial pneumonia.
The second assessment included determination of percent fibrosis for each lung on the first and last post-transplant CT scan (Table 2). On assessment of the native lung, the percent fibrosis worsened in four subjects (increase in area fibrosis range: 5–28%), improved minimally in two subjects (decrease in area fibrosis range: 1–2%, essentially unchanged) and remained unchanged in three subjects (of which one subject, case 1, already had 100% fibrosis) (Table 2). Regarding the transplanted lung, there was no fibrosis present on the first post-transplant CT scan for any of the nine subjects. Two subjects (cases 1 and 5) showed peripheral and basal fibrosis in the transplanted lung (both <10%) on the last post-transplant scan. Longitudinal review of available scans revealed that fibrosis was first seen 12 years post-transplantation in case 1, and over 4 years post-transplantation in case 5. Of interest, case 1 had 100% fibrosis in the native lung on first and last post-transplant scan (time interval between scans 11 years), and case 5 had worsening fibrosis in the native lung (time interval between scans 5 years). The remaining seven subjects did not develop evidence of fibrosis in the transplanted lung, despite worsening of fibrosis in the native lung in three of those subjects. Representative axial CT images from case 3 (Fig. 1) and case 5 (Fig. 2) are presented.
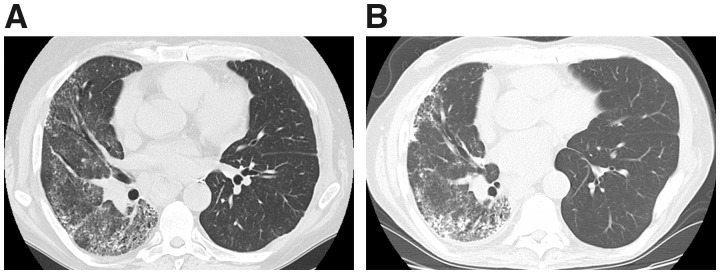
Fig. 1.
Representative axial CT images from case 3
Severe NSIP pattern of fibrosis in the native right lung is shown, unchanged when comparing the most recent post-transplant scan (B) with a scan obtained 5 years prior (A). The transplanted left lung also remained stable without de novo ILD or CT abnormality. NSIP: non-specific interstitial pneumonia; ILD: interstitial lung disease.
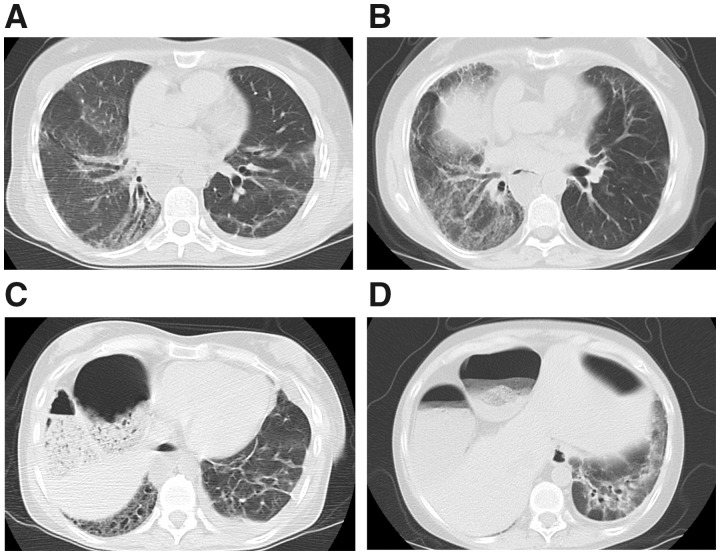
Fig. 2.
Representative axial CT images from case 5
Progression of fibrotic NSIP pattern in the native right lung on the most recent post-transplant scan (B) is shown, compared with a scan obtained 5 years prior (A). The transplanted left lung developed peribronchovascular fibrosis with traction bronchiectasis at the lower lung zone on the most recent scan (D). In comparison, linear atelectasis and a small pleural effusion without evidence of ILD was seen in the transplanted left lung on the scan obtained 5 years prior (C). NSIP: non-specific interstitial pneumonia; ILD: interstitial lung disease.
Quantitative lung density, total lung volume, tissue volume and air volume were obtained on 69 longitudinal CT images. Subjects received from 3 to 16 CT scans each, with a median of 6 scans per subject. Actual follow-up time covered by lung scans ranged from 2 to 18 years (median 7). Given that the number of overlapping intervals among cases was small, statistical analysis of changes in density and volume over time was considered exploratory. This analysis of the average change in lung density over time (in years) revealed that the native lung density increased by 2.2 HU per year, whereas the transplanted lung density decreased by 5.5 HU per year, a significant difference when the native and transplanted lung densities were compared (P = 0.011) (Table 3). Total lung volume increased, on average, 1.4 ml per year in the native lung, whereas the total volume increased by 27 ml per year in the transplanted lung, a statistically significant difference when native and transplanted lungs were compared (P = 0.039). The increase in volume was largely due to an increase in air volume and not tissue volume.
Table 3.
Average change in lung densities and volumes over time, by native and transplanted lungs
| Average change over time (per year) | |||||
|---|---|---|---|---|---|
| Native lung | Transplanted lung | Test for difference (P-value) | |||
| Measure | Estimate | 95% CI | Estimate | 95% CI | |
| Mean density (HU) | 2.2 | (−3.8, 8.18) | −5.5 | (−11.5, 0.48) | 0.011 |
| Total volume (ml) | 1.4 | (−27.85, 30.73) | 27.0 | (−2.34, 56.24) | 0.039 |
| Tissue volume (ml) | −1.9 | (−6.88, 3.04) | 0.0 | (−4.96, 4.95) | 0.330 |
| Air volume (ml) | 2.8 | (−25.42, 30.95) | 26.4 | (−1.82, 54.54) | 0.049 |
HU: Hounsfield Units.
Discussion
This case series employs three different methodologies for assessing ILD, and to our knowledge, represents the most detailed longitudinal assessment of ILD in SLT recipients with scleroderma. Our results show that four subjects had worsening of global ILD severity and/or percent fibrosis in the native lung. There was also a trend towards increasing density in the native lung, consistent with worsening fibrosis. These data suggest that either the immune process continues to target autoantigens or ongoing fibrotic pathways are active in the native lung leading to progression of ILD. Two cases of de novo fibrotic ILD in the transplanted lung were observed. The de novo ILD occurred in a peripheral and basal distribution in patients who also had concurrent evidence of BOS. The de novo fibrotic ILD in the transplanted lungs involved <10% of the lung area, despite the fibrotic area tripling in the native lung in one case (with the other case having 100% fibrosis on baseline study). Despite progression of ILD in the native lung in four cases, indicating continued disease activity, only one of the lung allografts developed de novo ILD. While we cannot distinguish the cause for the interstitial changes in the allografts, both cases did have BOS, indicating the presence of chronic allograft rejection; thus, the ‘restrictive allograft syndrome’, a subtype of chronic allograft rejection characterized by ILD and restrictive lung physiology, is certainly plausible. While an apical appearance of fibrosis is more typical of restrictive allograft syndrome, a peripheral and basal pattern has been described in restrictive allograft syndrome [18, 25]. However, the pleural parenchymal fibroelastosis pattern characteristically associated with restrictive allograft syndrome was not observed. While we suspect allograft rejection, we recognize that the basilar distribution of fibrotic changes may represent scleroderma disease occurrence in the allograft.
It is important to note that if fibrosis reoccurred in the transplant, progression appeared to be slow and mild. Specifically, in the case observed for the longest duration of 17 years, the first appearance of ILD occurred 12 years post-transplantation and only mildly increased over the next 5 years. Of interest, the new areas of fibrosis in the lung allograft only occurred in the setting of end-stage fibrotic lung disease or progressive fibrotic lung disease in the native lung. However, there were three other cases in which progression of fibrosis in the native lung was seen without any evidence of de novo fibrosis in the allograft.
Recurrence of the primary lung disease in the lung allograft has been reported in patients undergoing lung transplantation for other indications, including sarcoidosis [26–29], Langerhans cell histiocytosis [30] and desquamative interstitial pneumonitis [31]. Recurrence of usual interstitial pneumonia, to the best of our knowledge, has not been reported in lung transplant recipients with idiopathic pulmonary fibrosis. We now report on the radiographic presence of fibrosis of the lung allograft in two out of nine cases with scleroderma-related ILD. As both cases already had evidence of chronic allograft rejection with the diagnosis of BOS, we speculate that in our cases the de novo fibrosis in the lung allograft was secondary to a restrictive allograft syndrome, a subtype of chronic lung allograft dysfunction that can present with fibrotic lung disease. While we suspect allograft rejection accounts for these changes, we recognize that the pattern of ILD is consistent with that seen in scleroderma-associated lung disease.
In addition to blinded reads by two board-certified radiologists, we also evaluated longitudinal change in lung density, which has been shown to associate with extent of interstitial lung involvement in idiopathic pulmonary fibrosis [13]. Congruent with our findings of worsening global ILD severity and fibrosis in the native lung in almost half of cases, we found a trend towards worsening density in the native lung over time. Density actually decreased in the lung allograft, which is likely a function of the increase in air volume that was also found in these cases. The increase in air volume over time in the lung allograft may reflect mild hyperinflation from BOS, as four out of nine patients met International Society for Heart and Lung Transplantation/American Thoracic Society/European Respiratory Society criteria for BOS [17]. Quantitative CT methodology has been used to assess BOS in lung transplant recipients, and predicts forced expiratory volume in this population [32–34]. Recently, quantitative density measurement from inspiratory CT scans has been shown to define risk of mortality in transplant recipients and may be useful not only for assessment of the detection and progression of ILD, but also detection of BOS [34]. As lungs can be analysed individually, this methodology may be particularly useful to monitor complications in SLT recipients.
This study is limited by its small sample size of nine cases and retrospective design. The CT scans were obtained for clinical indications, and while many scans were obtained for routine transplant monitoring, some scans were obtained for other indications such as dyspnoea, concern for infection or in the setting of acute cellular rejection. This likely helps explain improvements in ILD that were seen over time in some cases. Additionally, blinded radiologist review of the first and last post-transplant CT images allowed for identification of features that could potentially be associated with infection such as focal consolidation vs features associated with fibrotic ILD such as reticulations and honeycombing. Furthermore, semi-quantitative determination of percent fibrotic area identified only non-inflammatory changes (e.g. reticulations, traction bronchiectasis, honeycombing and architectural distortion). Interpretation of the trends in quantitative measurements of density and volume on longitudinal CT images was limited by the sample size and few overlapping post-transplant time intervals at which the CT scans were obtained. However, we primarily sought to utilize this method as an exploratory analysis of trends in changes in density and volume over time. The trend towards increasing density in the native lung was consistent with our qualitative findings of increased ILD in the native lung, as we hypothesized. Furthermore, the increase in air volume in the transplanted lungs is consistent with the air trapping that occurs in BOS, which was present in five of our cases. Finally, as the CT scans were obtained for clinical indications at different institutions, variations in volume of air inspired during each scan may differ, leading to variations in densities and volumes measured over time. However, as the thorax is a fixed cavity, the variation in volume of air inspired during different scans would be expected to affect both the native and transplanted lung equally. Further studies with larger sample sizes and standardized inspiratory and expiratory CT methodology will be needed to determine the utility of reader-independent quantitative CT measurements to monitor ILD progression and BOS in SLT recipients with scleroderma.
Overall, this case series demonstrated that while the global ILD severity and fibrosis worsens in nearly half of the native lungs in SLT recipients, involvement of the allograft with de novo ILD and/or fibrosis occurs less frequently. In the two cases of de novo fibrosis in the lung allograft, involvement was mild and occurred in cases with severe baseline native lung ILD or severely worsening native lung ILD. We speculate that the fibrosis was secondary to restrictive allograft dysfunction, as these cases also had declines in forced expiratory volumes by pulmonary function testing, consistent with chronic lung allograft dysfunction. However, we recognize that the distribution of fibrosis occurred in a peripheral and basal distribution, rather than the apical distribution more typical of restrictive allograft dysfunction, thus recurrence of scleroderma ILD was possible.
Supplementary Material
Acknowledgements
The authors would like to thank Ms Alli Brooker for her excellent technical assistance with the quantitative CT data acquisition. F.M.W., A.M.H. and R.A.W. contributed to the conception and design of the study. F.M.W., A.M.H., C.T.L., A.F.H., A.V., J.A.G., F.B. and R.H.B. contributed to the acquisition of data. F.M.W., A.M.H., C.T.L., J.P., R.H.B. and R.A.W. contributed to the analysis and interpretation of data. A.M.H. was responsible for drafting the initial manuscript. All authors were involved in critically revising the manuscript for important intellectual content, approved the final draft to be published, and agree be accountable for all aspects of the work and in ensuring that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved.
Funding: Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number T32AR048522 (A.M.H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Research reported in this publication was also supported by the Scleroderma Research Foundation (F.M.W.) and the Martha McCrory Professorship (F.M.W.).
Disclosure statement: R.A.W. reports grants and personal fees from AstraZeneca/Medimmune, grants and personal fees from Boehringer Ingelheim, personal fees from Contrafect, personal fees from Pulmonx, personal fees from Roche/Genentech, personal fees from Spiration, personal fees from Sunovion, grants from Pearl Therapeutics, personal fees from Merck, personal fees from Circassia, personal fees from Pneuma, personal fees from Verona, personal fees from Bonti, personal fees from Denali, personal fees from Aradigm, personal fees from Mylan, personal fees from Theravance, personal fees from Propeller Health, personal fees from Kiniksa, personal fees from Syneos, grants and personal fees from Sanofi/Regeneron, and grants and personal fees from GlaxoSmithKline, outside the submitted work. The other authors have declared no conflicts of interest.
References
- 1. Schurawitzki H, Stiglbauer R, Graninger W. et al. Interstitial lung disease in progressive systemic sclerosis: high-resolution CT versus radiography. Radiology 1990;176:755–9. [DOI] [PubMed] [Google Scholar]
- 2. Steen VD, Conte C, Owens GR, Medsger TA Jr.. Severe restrictive lung disease in systemic sclerosis. Arthritis Rheum 1994;37:1283–9. [DOI] [PubMed] [Google Scholar]
- 3. Schachna L, Medsger TA Jr, Dauber JH. et al. Lung transplantation in scleroderma compared with idiopathic pulmonary fibrosis and idiopathic pulmonary arterial hypertension. Arthritis Rheum 2006;54:3954–61. [DOI] [PubMed] [Google Scholar]
- 4. Massad MG, Powell CR, Kpodonu J. et al. Outcomes of lung transplantation in patients with scleroderma. World J Surg 2005;29:1510–5. [DOI] [PubMed] [Google Scholar]
- 5. Crespo MM, Bermudez CA, Dew MA. et al. Lung transplant in patients with scleroderma compared with pulmonary fibrosis. short- and long-term outcomes. Ann Am Thorac Soc 2016;13:784–92. [DOI] [PubMed] [Google Scholar]
- 6. Shitrit D, Amital A, Peled N. et al. Lung transplantation in patients with scleroderma: case series, review of the literature, and criteria for transplantation. Clin Transplant 2009;23:178–83. [DOI] [PubMed] [Google Scholar]
- 7. Sottile PD, Iturbe D, Katsumoto TR. et al. Outcomes in systemic sclerosis-related lung disease after lung transplantation. Transplantation 2013;95:975–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saggar R, Khanna D, Furst DE. et al. Systemic sclerosis and bilateral lung transplantation: a single centre experience. Eur Respir J 2010;36:893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Launay D, Savale L, Berezne A. et al. Lung and heart-lung transplantation for systemic sclerosis patients. A monocentric experience of 13 patients, review of the literature and position paper of a multidisciplinary Working Group. Presse Med 2014;43(10 Pt 2):e345–63. [DOI] [PubMed] [Google Scholar]
- 10. Pradère P, Tudorache I, Magnusson J. et al. Lung transplantation for scleroderma lung disease: an international, multicenter, observational cohort study. J Heart Lung Transplant 2018;37:903–11. [DOI] [PubMed] [Google Scholar]
- 11. Puri V, Patterson GA, Meyers BF.. Single versus bilateral lung transplantation: do guidelines exist? Thorac Surg Clin 2015;25:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoffman EA, Clough AV, Christensen GE. et al. The comprehensive imaging-based analysis of the lung: a forum for team science. Acad Radiol 2004;11:1370–80. [DOI] [PubMed] [Google Scholar]
- 13. Hartley PG, Galvin JR, Hunninghake GW. et al. High-resolution CT-derived measures of lung density are valid indexes of interstitial lung disease. J Appl Physiol (1985) 1994;76:271–7. [DOI] [PubMed] [Google Scholar]
- 14. Tashkin DP, Volkmann ER, Tseng CH. et al. Relationship between quantitative radiographic assessments of interstitial lung disease and physiological and clinical features of systemic sclerosis. Ann Rheum Dis 2016;75:374–81. [DOI] [PubMed] [Google Scholar]
- 15. van den Hoogen F, Khanna D, Fransen J. et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2013;72:1747–55. [DOI] [PubMed] [Google Scholar]
- 16. Stewart S, Fishbein MC, Snell GI. et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant 2007;26:1229–42. [DOI] [PubMed] [Google Scholar]
- 17. Meyer KC, Raghu G, Verleden GM. et al. An international ISHLT/ATS/ERS clinical practice guideline: diagnosis and management of bronchiolitis obliterans syndrome. Eur Respir J 2014;44:1479–503. [DOI] [PubMed] [Google Scholar]
- 18. Sato M, Waddell TK, Wagnetz U. et al. Restrictive allograft syndrome (RAS): a novel form of chronic lung allograft dysfunction. J Heart Lung Transplant 2011;30:735–42. [DOI] [PubMed] [Google Scholar]
- 19. Jin GY, Lynch D, Chawla A. et al. Interstitial lung abnormalities in a CT lung cancer screening population: prevalence and progression rate. Radiology 2013;268:563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Travis WD, Costabel U, Hansell DM. et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Travis WD, Hunninghake G, King TE Jr. et al. Idiopathic nonspecific interstitial pneumonia: report of an American Thoracic Society project. Am J Respir Crit Care Med 2008;177:1338–47. [DOI] [PubMed] [Google Scholar]
- 22. Wheeler GL, Shi R, Beck SR. et al. Pericardial and visceral adipose tissues measured volumetrically with computed tomography are highly associated in type 2 diabetic families. Invest Radiol 2005;40:97–101. [DOI] [PubMed] [Google Scholar]
- 23. Busacker A, Newell JD Jr, Keefe T. et al. A multivariate analysis of risk factors for the air-trapping asthmatic phenotype as measured by quantitative CT analysis. Chest 2009;135:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heremans A, Verschakelen JA, Van fraeyenhoven L, Demedts M.. Measurement of lung density by means of quantitative CT scanning. A study of correlations with pulmonary function tests. Chest 1992;102:805–11. [DOI] [PubMed] [Google Scholar]
- 25. Verleden SE, Ruttens D, Vandermeulen E. et al. Predictors of survival in restrictive chronic lung allograft dysfunction after lung transplantation. J Heart Lung Transplant 2016;35:1078–84. [DOI] [PubMed] [Google Scholar]
- 26. Schultz HH, Andersen CB, Steinbruuchel D. et al. Recurrence of sarcoid granulomas in lung transplant recipients is common and does not affect overall survival. Sarcoidosis Vasc Diffuse Lung Dis 2014;31:149–53. [PubMed] [Google Scholar]
- 27. Banga A, Sahoo D, Lane CR, Farver CF, Budev MM.. Disease recurrence and acute cellular rejection episodes during the first year after lung transplantation among patients with sarcoidosis. Transplantation 2015;99:1940–5. [DOI] [PubMed] [Google Scholar]
- 28. Ionescu DN, Hunt JL, Lomago D, Yousem SA.. Recurrent sarcoidosis in lung transplant allografts: granulomas are of recipient origin. Diagn Mol Pathol 2005;14:140–5. [DOI] [PubMed] [Google Scholar]
- 29. Kazerooni EA, Jackson C, Cascade PN.. Sarcoidosis: recurrence of primary disease in transplanted lungs. Radiology 1994;192:461–4. [DOI] [PubMed] [Google Scholar]
- 30. Etienne B, Bertocchi M, Gamondes JP. et al. Relapsing pulmonary Langerhans cell histiocytosis after lung transplantation. Am J Respir Crit Care Med 1998;157:288–91. [DOI] [PubMed] [Google Scholar]
- 31. King MB, Jessurun J, Hertz MI.. Recurrence of desquamative interstitial pneumonia after lung transplantation. Am J Respir Crit Care Med 1997;156:2003–5. [DOI] [PubMed] [Google Scholar]
- 32. Mortani Barbosa EJ Jr, Shou H, Simpsom S. et al. Quantitative computed tomography metrics from the transplanted lung can predict forced expiratory volume in the first second after lung transplantation. J Thorac Imaging 2018;33:112–23. [DOI] [PubMed] [Google Scholar]
- 33. Leung AN, Fisher K, Valentine V. et al. Bronchiolitis obliterans after lung transplantation: detection using expiratory HRCT. Chest 1998;113:365–70. [DOI] [PubMed] [Google Scholar]
- 34. Horie M, Salazar P, Saito T. et al. Quantitative chest CT for subtyping chronic lung allograft dysfunction and its association with survival. Clin Transplant 2018;32:e13233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.