Abstract
Background
Despite increased access to highly active antiretroviral therapy (HAART), lung disease remains common in human immunodeficiency virus (HIV)–infected (HIV+) adolescents. There is limited information on changes in lung function over time in perinatally HIV+ adolescents on HAART. The objective was to investigate the progression of spirometry findings over 2 years in HIV+ adolescents on HAART in a prospective cohort, the Cape Town Adolescent Antiretroviral Cohort (CTAAC).
Methods
HIV+ adolescents aged 9–14 years, with at least 6 months of HAART, and a comparator group of healthy HIV-uninfected (HIV–), age-matched controls were enrolled in CTAAC. Spirometry and bronchodilator testing were done at baseline, 12 months, and 24 months. Mixed-effect models were used to compute longitudinal changes in lung function.
Results
Five hundred fifteen HIV+ adolescents, mean age 12 (standard deviation [SD], 1.6) years, 50.4% male, and 110 HIV– adolescents, mean age 11.8 (SD, 1.8) years, 45.6% male, were tested at baseline; 477 (93%) HIV+ and 102 (93%) HIV– adolescents at 12 months; and 473 (92%) HIV+ and 97 (88%) HIV– adolescents at 24 months. Only 5.4% of the HIV+ adolescents had HIV viral load >10 000 copies/mL at baseline. Forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) were lower in the HIV+ compared to the HIV– adolescents and tracked with no deterioration or catch-up over 2 years. Previous pulmonary tuberculosis (PTB) or lower respiratory tract infection (LRTI) was significantly associated with reduced FEV1 and FVC (P < .05 for both).
Conclusions
HIV+ adolescents had lower lung function over 2 years than HIV– adolescents. This study highlights the need for lung function surveillance and prevention of LRTIs and PTB in HIV+ adolescents.
Keywords: HIV, adolescents, chronic lung disease, lung function
Adolescents perinatally infected with human immunodeficiency virus (HIV) had lower lung function over 2 years and more obstructive and mixed-pattern spirometry compared with HIV-uninfected adolescents. Previous pulmonary tuberculosis or lower respiratory tract infection requiring hospitalization were predictors of low lung function.
(See the Editorial Commentary by McCollum on pages 491–92.)
Perinatally acquired human immunodeficiency virus (HIV) has evolved into a chronic disease due to early diagnosis and improved access to effective antiretroviral therapy (ART) [1, 2]. However, of the 2.1 million children <15 years of age living with HIV, only about 43% were on effective ART in 2017 [3]. More than 90% of adolescents with HIV infection (AWH) live in sub-Saharan Africa, with only about 49% accessing effective ART. Chronic lung disease remains one of the most common concerns in AWH in the effective ART era in sub-Saharan Africa [4].
Normal lung growth begins in utero and continues throughout childhood and adolescence till it reaches a plateau in the early 20s and begins to decline [5, 6]. Lung function tracks through life, with those starting at low lung function continuing along the lower centile into adulthood and also being associated with increased respiratory symptoms and illness [7]. However, most of the evidence on lung function in AWH is from cross-sectional studies, with limited information on longitudinal lung function changes, especially in the context of effective ART. Prior studies have shown that AWH on effective ART have lower lung function compared to HIV-uninfected adolescents [8, 9]. Longitudinal studies are needed to understand the lung function trajectory in AWH and the impact of low lung function and the risk of later respiratory disease. The aim of this study was to investigate the progression and determinants of lung function over 2 years in AWH on effective ART in a South African cohort of perinatally infected adolescents.
METHODS
Children were enrolled from October 2013 to March 2015 in a prospective cohort, the Cape Town Adolescent Antiretroviral Cohort (CTAAC), as previously described [8]. AWH, aged 9–14 years on effective ART for >6 months, in whom informed parental consent was obtained and assent provided, were enrolled and followed up every 6 months. Informed consent and assent were renewed annually.
Children were recruited from primary care clinics and hospital-based effective ART clinics in Cape Town, South Africa. An HIV-uninfected age-, sex-, and ethnicity-matched comparator group, with no known preexisting lung disease, was also enrolled. The study was approved by the Faculty of Health Sciences, University of Cape Town Human Research Ethics Committee.
Spirometry and bronchodilator testing were done at baseline, 12 months, and 24 months. Testing was done using NDD EasyOne Pro LAB (Switzerland). Testing adhered to European Respiratory Society/American Thoracic Society guidelines [10, 11]. A minimum of 3 trials to a maximum of 8 attempts were done per patient and at least 3 acceptable, reproducible best quality curves were selected by the respiratory technologist. The highest forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) from any of the acceptable maneuvers was reported. Four hundred micrograms of salbutamol was given via spacer and testing was repeated after 15 minutes.
The Global Lung Initiative [6] African American reference population was used to calculate the lower limit of normal for the spirometry outcome measures. Obstructive spirometry pattern was defined as an FEV1 over FVC ratio less than the lower limit of normal (FEV1/FVC < LLN); a restrictive spirometry pattern was defined as FVC < LLN and normal FEV1/FVC ratio; and a mixed pattern was defined as FEV1/FVC < LLN, FVC < LLN, and FEV1 < LLN. Bronchodilator response was defined as a change in FEV1 ≥12% after 400 µg salbutamol administered via metered dose inhaler spacer.
Clinical data including age, sex, height, weight, CD4 cell count, and viral load were collected annually. Questionnaires were administered semiannually to study participants to collect data on effective ART adherence, smoking, isoniazid prophylaxis, respiratory symptoms, and other intercurrent events. Effective ART adherence was calculated by assessing 30-day recall of any missed doses. Data on previous pulmonary tuberculosis (PTB) and past lower respiratory tract infection (LRTI), date of effective ART initiation, and doctor-diagnosed asthma were extracted from hospital records and validated study questionnaires. Data on medication use were extracted from health records.
Data Analysis
The two-sample t test was used to compute the comparison of lung function outcome between AWH and HIV-uninfected adolescents at baseline and 24 months. Demographic and clinical factors were included to determine associations of lung function. The minimum covariates (age, height, sex, HIV, time, smoke exposure, previous PTB, and previous LRTIs; Supplementary Figure 1) were determined using a directed acyclic graph [12]. Mixed-effects modeling was used to compute longitudinal changes over 2 years and determinants of lung function outcomes.
RESULTS
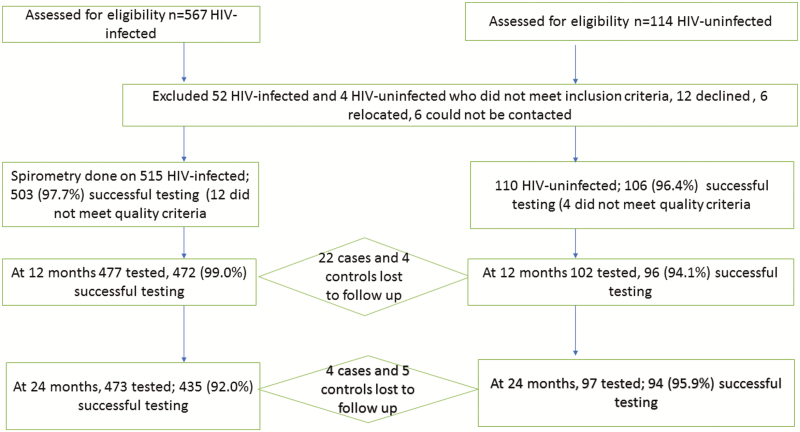
Five hundred fifteen AWH, with a mean age of 12 (standard deviation [SD], 1.6) years, and 110 HIV-uninfected adolescents, with a mean age of 11.8 (SD, 1.8) years, were tested at baseline; 477 (93%) AWH and 102 (93%) HIV-uninfected adolescents were tested at 12 months; and 473 (92%) AWH and 97 (88%) HIV-uninfected adolescents were tested at 24 months, with >90% successfully tested (Figure 1). Four hundred eighty-three (96%) AWH and 88 (83%) HIV-uninfected adolescents had successful bronchodilator testing at baseline; 437 (92.6%) AWH and 91 (94.8%) HIV-uninfected adolescents had bronchodilator testing at 12 months; and 402 (92.4%) AWH and 88 (93.6%) HIV-uninfected adolescents had a successful bronchodilator test at 24 months (Table 1).
Figure 1.
Description of the study cohort. Abbreviation: HIV, human immunodeficiency virus.
Table 1.
Summary of Lung Function at Baseline, 12 Months, and 24 Months
| Baseline | 12 mo | 24 mo | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | HIV+ (n = 503) | HIV– (n = 106) | P Value | HIV+ (n = 472) | HIV– (n = 96) | P Value | HIV+ (n = 435) | HIV– (n = 94) | P Value |
| FEV1z score | –1.0 (1.3) | –0.5 (1.0) | <.001 | –0.9 (1.3) | –0.3 (1.0) | <.001 | –0.8 (1.3) | –0.3 (1.0) | <.001 |
| FVC z score | –1.1 (1.3) | –0.8 (1.0) | .060 | –0.9 (1.2) | –0.3 (1.0) | <.001 | –0.7 (1.2) | –0.3 (0.9) | .003 |
| FEF25-75z score | –0.4 (1.4) | 0.1 (1.0) | <.001 | –0.5 (1.4) | –0.1 (1.1) | .015 | –0.5 (1.4) | –0.0 (1.1) | .004 |
| FEV1/FVC z score | 0.2 (1.4) | 0.9 (1.2) | <.001 | –0.2 (1.3) | 0.0 (1.1) | .231 | –0.3 (1.2) | 0.0 (1.0) | .008 |
| BDR | n = 483 | n = 88 | n = 437 | n = 91 | n = 402 | n = 88 | |||
| BDR (yes), No. (%) | 78 (16.1) | 10 (11.4) | .253 | 45 (10.3) | 5 (5.5) | .155 | 25 (6.2) | 5 (5.7) | .849 |
Data are presented as mean (standard deviation) unless otherwise indicated. P values from 2-samples t test; z scores derived from Global Lung Initiative African American reference values. BDR is positive if change in FEV1 ≥12%.
Abbreviations: BDR, bronchodilator responsiveness; FEF25-75, forced expiratory flow at 25% and 75% of forced vital capacity; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; HIV, human immunodeficiency virus.
At 2 years of study, the median duration of effective ART was 9.8 (interquartile range [IQR], 6.8–11.3) years (Table 2). The median age at effective ART initiation was 4.3 (IQR, 1.9–7.6) years. AWH were shorter and had lower body mass index than HIV-uninfected controls at enrollment and at 2 years (P < .001; Table 3). Only 5%–6% of adolescents had HIV viral load >10 000 copies/mL at both baseline and 24 months (Table 2). History of hospitalization for LRTI or past PTB prior to enrollment visit was more common in AWH compared with uninfected adolescents (P < .001; Table 2). However, intercurrent hospitalization for nontuberculosis LRTI and ambulatory LRTI not requiring hospitalization during the 24-month follow-up period was uncommon in both groups (Table 2). However, AWH had a high incidence rate of intercurrent culture-positive PTB of 3.2% compared to none of the HIV-uninfected adolescents in the 2-year study period. Those with culture-positive Mycobacterium tuberculosis over the 2 years had on average a 0.23 lower FEV1z score (95% confidence interval [CI], –.57 to .10; P = .174, adjusted for time and HIV status, data not shown) compared to those without intercurrent tuberculosis disease.
Table 2.
Characteristics of Participants Tested at Both Baseline and 24 Months, by Human Immunodeficiency Virus Status
| Baseline | 24 mo | |||||
|---|---|---|---|---|---|---|
| Characteristic | HIV+ (n = 503) | HIV– (n = 106) | P Value | HIV+ (n = 435) | HIV– (n = 94) | P Valuea |
| Age, y, mean (SD) | 12.0 (1.6) | 11.9 (1.8) | .533 | 14.1 (1.6) | 13.8 (1.8) | .171 |
| Male sex | 261 (51.9) | 48 (45.2) | .216 | 217 (49.9) | 42 (44.7) | .360 |
| Age at effective ART initiation, y, median (IQR) | 4.3 (1.9–7.6) | … | 4.3 (2.0–7.6) | … | ||
| Effective ART duration, y, median (IQR) | 7.7 (4.6–9.3) | … | 9.8 (6.8–11.3) | … | ||
| CD4 count, cells/µL, median (IQR) | 712 (556–959) | … | 695.5 (529.5–887.5) | … | ||
| Viral load, copies/mL | ||||||
| <50 | 383 (76.1) | … | 260 (59.8) | … | ||
| 50–1000 | 55 (10.9) | … | 108 (24.8) | … | ||
| 1001–10 000 | 37 (7.4) | … | 24 (5.5) | … | ||
| >10 000 | 27 (5.4) | … | 27 (6.2) | … | ||
| Missing | 1 (0.2) | … | 16 (3.7) | … | ||
| Poor ART adherence | 116 (23.0) | … | 41 (9.4) | … | ||
| Effective ART regimen | ||||||
| 2× NRTI + NNRTI | 307 (61.2) | … | 249 (57.2) | … | ||
| 2× NRTI + PI | 166 (33.1) | … | 151 (34.7) | … | ||
| Other | 29 (5.8) | … | 35 (8.1) | … | ||
| Tobacco smoke exposureb | ||||||
| Passive | 127 (25.3) | 21 (19.8) | .236 | 86 (19.8) | 14 (14.9) | .274 |
| Active | 2 (0.4) | … | 5 (1.2) | … | ||
| History of LRTI requiring hospitalization prior to baseline visit | 145 (28.8) | 1 (0.9) | <.001 | … | … | |
| Past history of PTB prior to baseline visit | 303 (60.2) | 2 (1.9) | <.001 | … | … | |
| History of asthma, self-reported (ever) | 62 (12.3) | 6 (5.7) | .048 | 32 (7.4) | 7 (7.5) | .976 |
| Family history of asthma | 78 (15.5) | 13 (12.3) | .395 | … | … | |
| Isoniazid prophylaxis | 115 (22.9) | 4 (3.8) | <.001 | 33 (7.6) | 0 | .006 |
| Inhaled corticosteroids | 13 (2.6) | 2 (1.9) | .674 | 11 (2.5) | 4 (4.3) | .360 |
| Short-course steroids | 13 (2.6) | … | 6 (1.4) | … | ||
| Inhaled salbutamol | 33 (6.6) | 2 (1.9) | .060 | 28 (6.4) | 2 (2.1) | .104 |
| Oral salbutamol | 12 (2.4) | … | 6 (1.4) | … | ||
| Cotrimoxazole prophylaxis | 50 (9.9) | 0 | 33 (7.6) | 0 | ||
| Intercurrent events, during the cumulative 2 y (n = 609) | ||||||
| Hospitalization | ||||||
| Nontuberculosis LRTI | … | … | 5 (0.8) | 1 (0.2) | <.001 | |
| Unconfirmed tuberculosis | … | … | 13 (2.1) | 0 | ||
| Asthma | … | … | 1 (0.2) | 1 (0.2) | 1.000 | |
| Ambulatory LRTI | … | … | 13 (2.1) | 4 (0.7) | <.001 | |
| Culture-positive Mycobacterium tuberculosis | … | … | 19 (3.1) | 0 | ||
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; IQR, interquartile range; LRTI, lower respiratory tract infection; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; PTB, pulmonary tuberculosis; SD, standard deviation.
a P value t test or χ2 test.
bSelf-reported smoking history.
Table 3.
Clinical Characteristics of Participants Tested at Both Baseline and 24 Months
| Baseline | 24 mo | |||||
|---|---|---|---|---|---|---|
| Characteristic | HIV+ (n = 503) | HIV– (n = 106) | P Value | HIV+ (n = 435) | HIV– (n = 94) | P Valuea |
| Height, cm, mean (SD) | 140.0 (10.3) | 144.6 (11.9) | .001 | 150.6 (9.8) | 153.0 (9.7) | .030 |
| BMI, kg/m2, mean (SD) | 17.7 (3.2) | 19.5 (4.2) | <.001 | 19.4 (3.5) | 21.1 (4.6) | <.001 |
| Current history of cough on most daysb | 74 (14.7) | 8 (7.6) | .050 | 51 (11.7) | 11 (11.7) | .995 |
| History of wheezing (ever)b | 55 (10.9) | 6 (5.7) | .100 | 31 (7.1) | 7 (7.5) | .913 |
| Wheezing in the last 12 mob | 31 (6.2) | 3 (2.8) | .174 | 19 (4.4) | 4 (4.3) | .961 |
| SOB in the last 12 mob | 3 (0.6) | 2 (1.9) | .181 | 6 (1.4) | 3 (3.2) | .218 |
| Digital clubbing at baseline | 18 (3.6) | 0 (0) | .048 | … | … | |
| Respiratory rate, breaths/min | 21.5 (3.5) | 20.9 (5.2) | .142 | 21.0 (1.1) | 20.9 (0.9) | .565 |
| Oxygen saturation, % | 98.6 (0.9) | 98.8 (0.4) | .036 | 98.3 (1.7) | 98.5 (0.7) | .199 |
| Tanner stage | ||||||
| I | 242 (48.1) | 34 (32.1) | .003 | 62 (14.3) | 11 (11.7) | .516 |
| II | 123 (24.5) | 30 (28.3) | .406 | 81 (18.6) | 15 (16.0) | .544 |
| III | 69 (13.7) | 18 (17.0) | .383 | 87 (20.0) | 23 (24.5) | .333 |
| IV | 39 (7.8) | 17 (16.0) | .007 | 110 (25.3) | 26 (27.7) | .633 |
| V | 23 (4.6) | 6 (5.7) | .633 | 83 (19.1) | 16 (17.0) | .643 |
| Missing | 7 (1.4) | 1 (0.9) | .713 | 12 (2.8) | 3 (3.2) | .819 |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: BMI, body mass index; HIV, human immunodeficiency virus; SD, standard deviation; SOB, shortness of breath.
aSymptoms are self-reported at each time point.
b t test or χ2 test.
More adolescents reported symptoms of cough or wheeze at enrollment than at the 2-year follow-up period (Table 3). Less than 7% of AWH used asthma medications at baseline and over 2 years (Table 2). There was higher use of isoniazid prophylaxis in the AWH at baseline than at 2 years (115 [22.9%] and 33 [7.6%], respectively; Table 2). Cotrimoxazole prophylaxis use was 10% at baseline and 7.6% at 2 years in the HIV-infected group (Table 2). Less than 20% of AWH reported chronic or recurrent respiratory symptoms at baseline and at 2 years (Table 3).
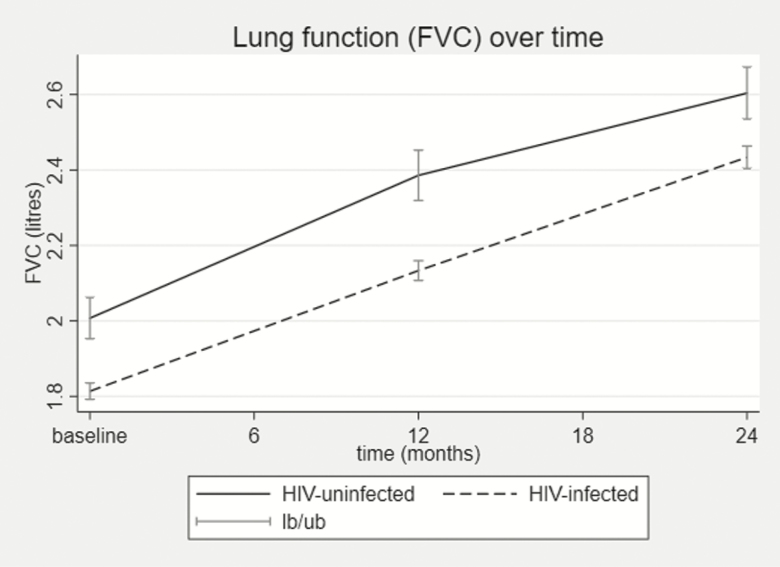
AWH had lower FEV1, FVC, forced expiratory flow at 25% and 75% of FVC, and FEV1/FVC z scores compared with the uninfected adolescents at all time points (Table 1), with AWH having on average a 0.52 FEV1z score lower than the HIV-uninfected adolescents over 2 years (95% CI, –.76 to –.27; P < .001; Table 4). Although HIV infection was associated with lower FEV1 or FVC, the change in either FEV1 or FVC at 2 years was similar between AWH and uninfected adolescents as depicted by a near parallel slope (Figure 2) and a nonsignificant interaction between HIV and time at 2 years for both (coefficient, –0.03 [95% CI, –.17 to .23], P = .764 for FEV1 and –0.14 [95% CI, –.34 to .05], P = .154 for FVC) (Supplementary Table 1).
Table 4.
Effect of Human Immunodeficiency Virus on Lung Function Over 2 Years (n = 609)
| Measure of Lung Function | Coefficient | (95% CI) | P Valuea |
|---|---|---|---|
| FEV1z score | –0.52b | (–.76 to –.27) | <.001 |
| FVC z score | –0.37b | (–.60 to –.14) | .001 |
| FEF25–75z score | –0.42b | (–.68 to –.17) | .001 |
| FEV1/FVC z score | –0.41b | (–.64 to –.19) | <.001 |
Abbreviations: CI, confidence interval; FEF25–75, forced expiratory flow at 25% and 75% of forced vital capacity; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
aLinear mixed-effects model.
bAdjusted for age, sex, height, time.
Figure 2.
Line plots of the trend of lung function over 2 years showing forced expiratory volume in 1 second and FVC absolute values (A and B, respectively) and z scores (C and D, respectively). Baseline: HIV infected (HIV+), n = 503 and HIV uninfected (HIV–), n = 106; 12 months: HIV+, n = 472 and HIV–, n = 96; 24 months: HIV+, n = 435 and HIV–, n = 94. Abbreviations: FVC, forced vital capacity; HIV, human immunodeficiency virus; lb/ub, lower 95% confidence interval /upper 95% confidence interval.
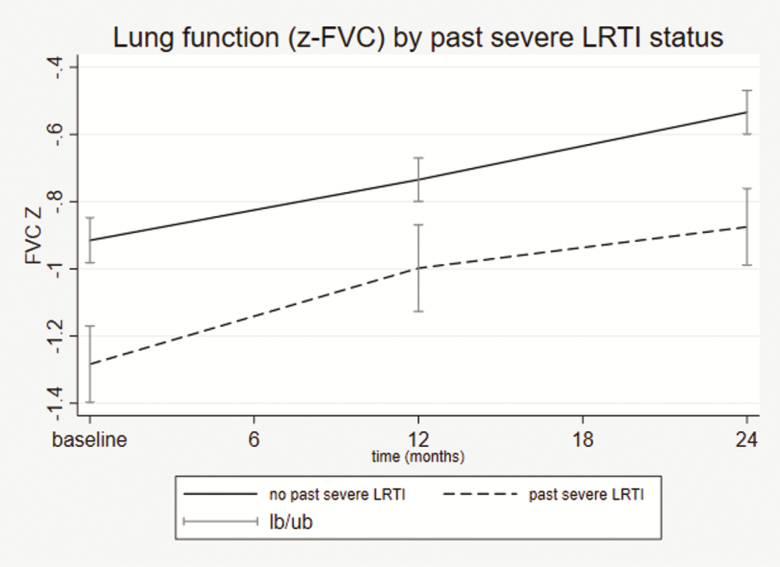
A history of PTB or severe LRTI prior to enrollment was significantly associated with impaired FEV1z score at 2 years (coefficient, –0.27 [95% CI, –.50 to –.03], P = .024 for PTB and –0.37 [95% CI, –.62 to –.13], P = .003 for LRTI) and FVC z score (coefficient, –0.27 [95% CI, –.49 to –.05], P = .015 for PTB and –0.30 [95% CI, –.53 to –.06], P = .013 for LRTI), adjusting for smoke exposure, time, and HIV infection (Table 5 and Supplementary Table 4A and 4B). AWH with past LRTI requiring hospitalization had lower lung function compared to those with no prior severe LRTI (Figure 3).
Table 5.
Association of Forced Expiratory Volume in 1 Second (FEV1), Forced Vital Capacity (FVC), and FEV1/FVC z Scores at 24 Months (n = 515)
| FEV1z Score | FVC z Score | FEV1/FVC z Score | |||||
|---|---|---|---|---|---|---|---|
| Variable | Coefficient | P Valuea | Coefficient | P Valuea | Coefficient | P Valuea | |
| HIV infection | –0.12 | .510 | 0.01 | .946 | –0.34 | .036 | |
| Time at 12 mo | 0.04 | .296 | 0.25 | <.001 | –0.51 | <.001 | |
| Time at 24 mo | 0.17 | <.001 | 0.41 | <.001 | –0.61 | <.001 | |
| Tobacco smoke exposureb | 0.01 | .931 | 0.08 | .192 | –0.18 | .022 | |
| Previous PTB | –0.27 | .024 | –0.27 | .015 | –0.01 | .904 | |
| Previous LRTI | –0.37 | .003 | –0.30 | .013 | –0.27 | .020 | |
Abbreviations: FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; HIV, human immunodeficiency virus; LRTI, lower respiratory tract infection; PTB, pulmonary tuberculosis.
aMultivariate linear mixed model.
bSelf-reported passive smoke exposure (active smoking had too few numbers, thus not reported).
Figure 3.
Lung function at baseline, 12 months, and 24 months by past severe LRTI status in the adolescents with human immunodeficiency virus infection (showing FVC [A] and FEV1 [B] z scores). P values calculated from two-sample independent t test. Missing LRTI data: 29 (5.8%) at baseline, 26 (5.5%) at 12 months, 23 (5.3%) at 24 months. Abbreviations: FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; lb/ub, lower 95% confidence interval /upper 95% confidence interval; LRTI, lower respiratory tract infection.
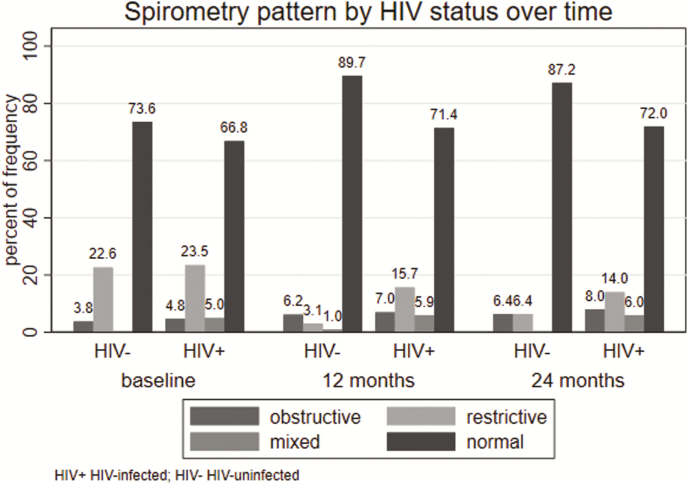
More than 65% of AWH had a normal spirometry pattern at baseline, 12 months, and 24 months (Supplementary Table 2). A mixed spirometry pattern was more common in AWH compared with HIV-uninfected adolescents at each time point (Supplementary Table 2 and Figure 4). Obstructive spirometry pattern increased from a prevalence of 4.8% at baseline to 8.1% at 24 months (Supplementary Table 2). Bronchodilator responsiveness at baseline was 16.1% and 11.4% in AWH and HIV-uninfected adolescents, respectively (Table 1). This decreased to 6.2% and 5.7%, respectively, in the AWH and HIV-uninfected adolescents at 24 months, indicating an increase in fixed airway obstruction over the 24 months. On average, those with abnormal spirometry pattern had a higher odds of having either wheeze, shortness of breath, cough on most days, and history of doctor-diagnosed asthma, adjusted for HIV status (P < .05 for all; Table 6). History of doctor-diagnosed asthma (odds ratio [OR], 2.19 [95% CI, 1.16–4.14]) or past severe LRTI requiring hospitalization (OR, 1.71 [95% CI, 1.02–2.88]) were significantly associated with bronchodilator responsiveness (Supplementary Table 3).
Figure 4.
Spirometry pattern by HIV status at baseline, 12 months, and 24 months. Abbreviation: HIV, human immunodeficiency virus.
Table 6.
Association of Abnormal Spirometry Pattern and Respiratory Symptoms/Signs at Baseline
| Respiratory Symptom/Signa | No. | OR | (95% CI) | P Valueb |
|---|---|---|---|---|
| History of ever wheeze (yes) | 608 | 2.4 | (1.25–4.68) | .009 |
| Cough on most days (yes) | 607 | 2.7 | (1.62–4.62) | < .001 |
| History of asthma ever (yes) | 607 | 3.1 | (1.64–5.83) | < .001 |
| History of shortness of breath ever (yes) | 608 | 4.7 | (1.44–15.25) | .010 |
| Digital clubbing (yes) | 609 | 5.6 | (.89–35.94) | .068 |
Abbreviations: CI, confidence interval; OR, odds ratio.
aAll symptoms are self-reported except digital clubbing.
bAdjusted for human immunodeficiency virus status and time.
DISCUSSION
This study provides novel 2-year follow-up data showing that spirometry in adolescents perinatally infected with HIV established on effective ART tracks lower compared to uninfected adolescents. Prior LRTI requiring hospitalization or PTB was associated with low FEV1 or FVC. Reassuringly, this cohort had minimal chronic respiratory symptoms and few intercurrent ambulatory or hospitalized LRTIs during the 2-year follow-up. The cohort was well established on effective ART with a mean duration of effective ART of 9 years.
The stable trajectory of spirometry outcomes over 2 years suggests that damage to the lung, reflected as lower lung function in the HIV-infected group, may have occurred early in life due to uncontrolled HIV infection as effective ART was only initiated on average at 4 years of age in this cohort. In addition, it suggests that in AWH well established on effective ART, lung growth continues to occur and tracks similar to HIV-uninfected youth. Early damage may have been caused by HIV exposure in utero in the developing lung or in the early childhood years prior to initiation of effective ART. This damage may be attributed to early childhood infection, by HIV-associated inflammation and immune dysregulation, or opportunistic infections in the AWH, who had high rates of prior PTB or LRTI compared with HIV-uninfected participants, as has been reported in other studies [9, 13]. This is supported by the fact that previous LRTI or PTB was associated with lower lung function. The effect of HIV on lung function was reduced after adjusting for LRTI and PTB, suggesting that pulmonary infection may be a major mechanism by which HIV reduces lung function.
The incidence of culture confirmed that tuberculosis in AWH was high over the study period. This is in keeping with published evidence [14] that, while long-term effective ART is associated with a reduction in tuberculosis risk, the incidence remains high in HIV-infected populations. South Africa has a high HIV and tuberculosis burden [15], and previous reports have shown that the adolescent age group has a higher rate of tuberculosis disease [16]. Of concern was the very low use of cotrimoxazole or isoniazid prophylaxis as effective preventive interventions in this cohort. The low use of cotrimoxazole may reflect current program guidelines as prophylaxis is stopped after immune reconstitution, which had occurred in most participants, as reflected by the median CD4 count of around 700 cells/µL.
The higher occurrence of obstructive and mixed-pattern spirometry in AWH may be due to higher rates of bronchiectasis or postinfective bronchiolitis obliterans (BO) in the HIV-infected group. High rates of bronchiectasis in HIV-infected children have been reported [4, 17]. A study on chest computed tomography in AWH reported predominantly mosaic attenuation due to possible BO that was inversely correlated with FEV1 [4]. Bronchiolitis obliterans is a nonreversible condition due to fibrosis. In our study cohort, bronchodilator responsiveness at 2 years was <10%, suggesting irreversible airway pathology, possibly related to BO. Others have reported irreversible airway pathology in HIV-infected youth compared to HIV-exposed uninfected youth [18]. The high rate of restrictive spirometry pattern in both the AWH and HIV-uninfected adolescents at baseline may be for technical reasons as most participants had never done a lung function test before enrollment but became familiar with the technique of performing spirometry and were able to produce better-quality tests in subsequent visits.
The improvement of respiratory symptoms over the 2-year period in our cohort may have been due to close follow-up of study participants, good clinical management, and appropriate treatment or referral when the participant was sick or symptomatic. This cohort also had good adherence to effective ART, reflected by most adolescents having low HIV viral load.
Birth cohort studies from high-income countries in HIV-uninfected populations have shown that lung function tracks from childhood to adulthood [7, 19–21]. Therefore, the lung function of AWH, which was lower than that in HIV-uninfected adolescents, is likely to continue to track along the lower centile over time, provided there are no further insults. Longitudinal studies in HIV-infected children and adolescents are, however, lacking. With the expected physiological growth and development of the lung from in utero to early adulthood [5], it is unclear what effect HIV or opportunistic infections may have on normal physiological growth. In our study, there was no catch-up or deterioration over 2 years between the HIV-infected and the uninfected adolescents, but a longer follow-up period is needed. The lack of deterioration in lung function over the 2 years may reflect that this is a relatively healthy HIV-infected cohort (as evidenced by minimal respiratory morbidity in the 2-year period) who were stable on effective ART for a mean duration of 9 years.
Strengths of the study include a prospective collection of objective measures of lung function, high cohort retention with close follow-up over 2 years, and a well-controlled HIV cohort. Limitations of the study are that data included spirometry only, but this is the most widely used measure of lung function; further bronchodilator responsiveness was also assessed in this study. In the absence of a local South African adolescent spirometry reference equation, we used the Global Lung Initiative 2012 [6] African American reference values, which have been shown to be acceptable for sub-Saharan Africa [22]. Further limitation was the lack of chest computed tomographic imaging to define lung abnormalities and correlate with lung function. These findings may not be generalizable to HIV-infected adolescent populations that are not well controlled on effective ART, where more severe lung function abnormalities have been found [23, 24]. However, as effective ART programs are strengthened, increasing numbers of adolescents perinatally infected with HIV can be expected to be on therapy and to develop this spectrum of lung disease.
CONCLUSIONS
AWH had lower spirometry at all time points compared to HIV-uninfected adolescents over 2 years. Lung growth was, however, preserved in the HIV-infected group and similar in both groups. Previous PTB or previous LRTI was associated with reduced FEV1 and FVC. There was a high incidence rate of culture-confirmed tuberculosis in the AWH. Interventions to prevent childhood LRTI or tuberculosis must be strengthened to optimize lung health. Further longitudinal studies on lung function in HIV-infected children and adolescents on effective ART are needed to show the long-term effect of HIV or opportunistic infections on subsequent respiratory health and to enable timely treatment of lung disease. Surveillance of lung function in AWH from early adulthood and throughout adulthood is necessary to detect disease early.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. L. N. G.: acquisition of data, analysis and interpretation of data, and writing of the manuscript; D. M. G.: conception and design of study and writing of the manuscript; S. H.: acquisition of data; T. M.: statistical analysis; H. J. Z.: conception and design of the study, acquiring of funding for the Cape Town Adolescent Antiretroviral cohort (CTAAC) study, and writing of the manuscript.
Acknowledgments. The authors thank the CTAAC study staff for helping in patient recruitment and assessment and data management, and the CTAAC participants for availing themselves for this project.
Financial support. This work was supported by the National Institute of Child Health and Human Development (grant number R01HD074051); the South Africa Medical Research Council; and the African Partnership for Chronic Diseases Research.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Lowenthal ED, Bakeera-Kitaka S, Marukutira T, Chapman J, Goldrath K, Ferrand RA. Perinatally acquired HIV infection in adolescents from sub-Saharan Africa: a review of emerging challenges. Lancet Infect Dis 2014; 14:627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet 2013; 382:1525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Joint United Nations Programme on HIV/AIDS. Fact sheet 2017. Available at: aidsinfo.unaids.org.Accessed 30 June 2017. [Google Scholar]
- 4. Desai SR, Nair A, Rylance J, et al. Human immunodeficiency virus–associated chronic lung disease in children and adolescents in Zimbabwe: chest radiographic and high-resolution computed tomographic findings. Clin Infect Dis 2018; 66:274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Merkus PJ, ten Have-Opbroek AA, Quanjer PH. Human lung growth: a review. Pediatr Pulmonol 1996; 21:383–97. [DOI] [PubMed] [Google Scholar]
- 6. Quanjer PH, Stanojevic S, Cole TJ, et al. ERS Global Lung Function Initiative. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40:1324–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stern DA, Morgan WJ, Wright AL, Guerra S, Martinez FD. Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet 2007; 370:758–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Githinji LN, Gray DM, Hlengwa S, Myer L, Zar HJ. Lung function in South African adolescents infected perinatally with HIV and treated long-term with antiretroviral therapy. Ann Am Thorac Soc 2017; 14:722–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rylance J, Mchugh G, Metcalfe J, et al. Chronic lung disease in HIV-infected children established on antiretroviral therapy. AIDS 2016; 30:2795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J 2005; 26:319–38. [DOI] [PubMed] [Google Scholar]
- 11. Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005; 26:948–68. [DOI] [PubMed] [Google Scholar]
- 12. Textor J. Drawing and analyzing causal DAGs with DAGitty. 2015. Available at: dagitty.net. Accessed 12 February 2018. [Google Scholar]
- 13. Ferrand RA, Bandason T, Musvaire P, et al. Causes of acute hospitalization in adolescence: burden and spectrum of HIV-related morbidity in a country with an early-onset and severe HIV epidemic: a prospective survey. PLoS Med 2010; 7:e1000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gupta A, Wood R, Kaplan R, Bekker LG, Lawn SD. Tuberculosis incidence rates during 8 years of follow-up of an antiretroviral treatment cohort in South Africa: comparison with rates in the community. PLoS One 2012; 7:e34156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kanabus A. TB statistics for South Africa—National & provincial. UK: Global Health Education, 2016. [Google Scholar]
- 16. Snow KJ, Nelson LJ, Sismanidis C, Sawyer SM, Graham SM. Incidence and prevalence of bacteriologically confirmed pulmonary tuberculosis among adolescents and young adults: a systematic review. Epidemiol Infect 2018; 146:946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Masekela R, Anderson R, Moodley T, et al. HIV-related bronchiectasis in children: an emerging spectre in high tuberculosis burden areas. Int J Tuberc Lung Dis 2012; 16:114–9. [DOI] [PubMed] [Google Scholar]
- 18. Shearer WT, Jacobson DL, Yu W, et al. Pediatric HIV/AIDS Cohort Study. Long-term pulmonary complications in perinatally HIV-infected youth. J Allergy Clin Immunol 2017; 140:1101–11.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tai A, Tran H, Roberts M, et al. Outcomes of childhood asthma to the age of 50 years. J Allergy Clin Immunol 2014; 133:1572–8 e3. [DOI] [PubMed] [Google Scholar]
- 20. Oswald H, Phelan PD, Lanigan A, et al. Childhood asthma and lung function in mid-adult life. Pediatr Pulmonol 1997; 23:14–20. [DOI] [PubMed] [Google Scholar]
- 21. Berry CE, Billheimer D, Jenkins IC, et al. A distinct low lung function trajectory from childhood to the fourth decade of life. Am J Respir Crit Care Med 2016; 194:607–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arigliani M, Canciani MC, Mottini G, et al. Evaluation of the global lung initiative 2012 reference values for spirometry in African children. Am J Respir Crit Care Med 2017; 195:229–36. [DOI] [PubMed] [Google Scholar]
- 23. Mwalukomo T, Rylance SJ, Webb EL, et al. Clinical characteristics and lung function in older children vertically infected with human immunodeficiency virus in Malawi. J Pediatric Infect Dis Soc 2016; 5:161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McHugh G, Rylance J, Mujuru H, et al. Chronic morbidity among older children and adolescents at diagnosis of HIV infection. J Acquir Immune Defic Syndr 2016; 73:275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.