Abstract
Background
Norovirus is an important cause of epidemic acute gastroenteritis (AGE), yet the burden of endemic disease in adults has not been well documented. We estimated the prevalence and incidence of outpatient and community-acquired inpatient norovirus AGE at 4 Veterans Affairs Medical Centers (VAMC) (Atlanta, Georgia; Bronx, New York; Houston, Texas; and Los Angeles, California) and examined trends over 4 surveillance years.
Methods
From November 2011 to September 2015, stool specimens collected within 7 days of AGE symptom onset for clinician-requested diagnostic testing were tested for norovirus, and positive samples were genotyped. Incidence was calculated by multiplying norovirus prevalence among tested specimens by AGE-coded outpatient encounters and inpatient discharges, and dividing by the number of unique patients served.
Results
Of 1603 stool specimens, 6% tested were positive for norovirus; GII.4 viruses (GII.4 New Orleans [17%] and GII.4 Sydney [47%]) were the most common genotypes. Overall prevalence and outpatient and inpatient community-acquired incidence followed a seasonal pattern, with higher median rates during November–April (9.2%, 376/100 000, and 45/100 000, respectively) compared to May–October (3.0%, 131/100 000, and 13/100 000, respectively). An alternate-year pattern was also detected, with highest peak prevalence and outpatient and inpatient community-acquired norovirus incidence rates in the first and third years of surveillance (14%–25%, 349–613/100 000, and 43–46/100 000, respectively).
Conclusions
This multiyear analysis of laboratory-confirmed AGE surveillance from 4 VAMCs demonstrates dynamic intra- and interannual variability in prevalence and incidence of outpatient and inpatient community-acquired norovirus in US Veterans, highlighting the burden of norovirus disease in this adult population.
Keywords: norovirus, gastroenteritis, veterans, outpatients, inpatients
We estimate the prevalence and incidence of norovirus acute gastroenteritis at 4 Veterans Affairs Medical Centers over 4 surveillance years. We demonstrate intra- and interannual variability in norovirus prevalence and incidence and highlight the burden of norovirus disease in this population.
Acute gastroenteritis (AGE) is a major cause of morbidity in the United States. An estimated 179 million cases, >1 million hospitalizations, and >11 000 deaths associated with AGE occur annually [1–4]. Norovirus is a leading cause of AGE [5]. Although norovirus AGE is generally self-limiting in healthy adults, the young, elderly, and immunocompromised can be severely affected [3, 6–8]. Adults aged ≥65 years are estimated to account for 320 000 outpatient visits and almost 1000 norovirus-associated deaths annually in the United States [9].
Prior estimates of the US incidence of norovirus AGE in adults have been based on extrapolations of etiologic fractions from studies in other developed countries [2, 10], time-series regression models using electronic healthcare database information [3, 4, 11, 12], and passive surveillance [13–15]. Direct assessments of laboratory-confirmed, age-specific incidence of norovirus AGE in the United States are needed to assess the potential impacts of targeted interventions, such as candidate norovirus vaccines [16]. While norovirus outbreak surveillance and model-based incidence estimates have shown year-to-year variation in prevalence and incidence of norovirus as well as winter seasonality [3, 4, 11, 17–19], previous direct laboratory-based incidence estimates were limited to stool specimens collected over the course of a single year [13–15]. We describe trends in norovirus prevalence and incidence from stool specimens collected from Veterans utilizing 4 Veterans Affairs Medical Centers (VAMCs) between November 2011 and September 2015.
METHODS
Study Population, Specimen Collection, and Testing
The US Department of Veterans Affairs (VA) healthcare system serves 9 million enrollees at >1200 Veterans Health Administration (VHA) sites. This study was conducted at 4 VAMCs (Atlanta VA Medical Center, Atlanta, Georgia; Michael E. DeBakey VA Medical Center, Houston, Texas; Greater Los Angeles VA Healthcare System, Los Angeles, California; and James J. Peters VA Medical Center, Bronx, New York). Each site was selected based on their willingness to participate in the study. This study protocol was reviewed and approved by the institutional review board (IRB) at the Centers for Disease Control and Prevention (CDC) and by the IRB and VA Research and Development Office at each participating VAMC.
Three sites (Atlanta VAMC, Michael E. DeBakey VAMC, and James J. Peters VAMC) participated in all 4 surveillance years (November 2011–October 2012; November 2012– October 2013; November 2013–October 2014; and November 2014–September 2015). Greater Los Angeles VAMC did not participate during the second surveillance year (November 2012–October 2013) due to study personnel turnover.
From 1 November 2011 to 30 September 2015, stool specimens collected for clinician-requested diagnostic testing at each VAMC and associated community-based outpatient clinics were shipped to CDC for supplemental norovirus testing. Specimens were tested for norovirus RNA using the AgPath-ID One-Step reverse-transcription polymerase chain reaction (PCR) kit (Applied Biosystems, Foster City, California) on the 7500 Real-Time PCR platform (Applied Biosystems) [20]. Norovirus-positive specimens were genotyped by comparing region C sequences to reference strains using phylogenetic analysis per CaliciNet protocols [20].
Data Sources, Incidence Calculations, and Statistical Analysis
Clinical and epidemiological data associated with each stool specimen (excluding personal identifiers) were extracted from VAMC electronic medical records. These data included age, race, and sex; presence of vomiting or diarrhea; dates of specimen collection, AGE symptom onset, and admission (for inpatients); if gastroenteritis was a reason for admission (for inpatients); if the specimen was tested for Clostridium difficile or C. difficile toxin, ova, parasites, or enteric bacteria by culture at the VAMC hospital laboratory; and results of those tests. Patients were included in analyses if either vomiting or diarrhea were noted in the medical chart and the specimen was collected within 7 days of symptom onset. For inpatients, only specimens from patients who presented for medical care with AGE symptoms were included in the analysis, and are thus referred to as inpatients with community-acquired norovirus in this analysis.
Population estimates, including unique patients served and AGE-related inpatient discharges and outpatient encounters, were provided by VHA Public Health Surveillance and Research and defined using a previously described set of International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes (Supplementary Table). We considered an outpatient encounter or inpatient discharge to be an episode of AGE if any of these ICD-9-CM codes were present at any outpatient encounter code or discharge code position. Monthly AGE-related inpatient discharges and outpatient encounters were provided for November 2011 through September 2015, as well as the annual numbers of unique patients served for fiscal years 2012 to 2015. Unique patients served includes all Veterans who utilized any VA inpatient or outpatient services in a given year, and is therefore the best indicator of population denominator for incidence calculations.
Site-, age-, and surveillance season–specific norovirus incidence among VAMC patients seeking care was calculated on the basis of norovirus prevalence in outpatient or inpatient specimens (p(noroi)), number of AGE outpatient encounters or inpatient discharges (Ei), and total number of unique patients served (N). Outpatient norovirus incidence per 100 000 population for a given age category or site (j) was calculated as:
Likewise, inpatient community-acquired norovirus incidence per 100 000 patients for a given age category or site (j) was calculated as:
Annualized biannual (November–April, May–October) norovirus incidence was calculated with the formulas above, using the cumulative number of unique patients served in the first half of each fiscal year in the denominator. Combined norovirus season (ie, November 2011–April 2012; November 2012–April 2013; November 2013–April 2014; November 2014–April 2015) and combined low norovirus transmission season (ie, May–October 2012; May–October 2013; May–October 2014; May–September 2015) incidence was calculated by adding together (p(noroi)), (Ei), and the estimated biannual unique patients served for each specified period.
Statistical significance of categorical variables was assessed by calculating univariate odds ratios (ORs) and χ2P values. For categorical variables with >2 categories, logistic regression using indicator variables was used to calculate univariate ORs and 95% confidence interval (CIs). External median quartile calculations were used to calculate quartiles for norovirus prevalence by month. Confidence intervals for incidence calculations were obtained using bootstrapping.
RESULTS
Characteristics of Patients and Specimens
A total of 1603 stool specimens were collected from 1 November 2011 to 30 September 2015. Median patient age was 62 years (range, 25–99 years). Ninety percent (n = 1443) of patients were male (Table 1). Forty-nine percent (n = 788) were seen in outpatient settings; 51% (n = 815) were inpatients admitted to VAMCs for community-acquired AGE. Ninety-six percent (n = 1041) had diarrhea only, 35% (n = 556) had diarrhea with vomiting, and 4% (n = 58) had vomiting only. Median time between onset of AGE symptoms and of stool specimen collection was 3 days. Eighty-two percent (n = 1320) of specimens were tested for C. difficile toxin, 64% were tested using bacterial culturing, and 49% (n = 749) were tested for the presence of ova and parasites.
Table 1.
Characteristics of Patients on Whom Specimens Were Tested for Norovirus at 4 Veterans Affairs Medical Centers, November 2011–September 2015 (N = 1603)
| Characteristic | Total No. (%) (N = 1603) |
No. (%) Norovirus-positive (n = 103) |
Univariate OR (95% CI) | P Value |
|---|---|---|---|---|
| Age category, y | ||||
| 18–44 | 256 (16) | 21 (8) | Ref. | … |
| 45–64 | 704 (44) | 42 (6) | 0.71 (.41–1.22) | .218 |
| 65–84 | 556 (35) | 32 (6) | 0.68 (.39–1.21) | .192 |
| ≥85 | 83 (5) | 8 (10) | 1.19 (.51–2.81) | .685 |
| Not provided | 4 (<1) | 0 (0) | NA | NA |
| Race | ||||
| White | 752 (47) | 62 (8) | Ref. | … |
| African-American | 689 (43) | 32 (5) | 0.54 (.35–.84) | .006 |
| Othera | 162 (10) | 9 (6) | 0.66 (.32–1.36) | .257 |
| Sex | ||||
| Male | 1443 (90) | 93 (6) | 1.01 (.52–1.99) | .971 |
| Female | 157 (10) | 10 (6) | … | … |
| Not provided | 3 (<1) | 0 (0) | Ref. | … |
| Setting | ||||
| Outpatient | 788 (49) | 57 (7) | 1.30 (.87–1.95) | .195 |
| Inpatient | 815 (51) | 46 (6) | Ref. | … |
| Site | ||||
| A (Bronx) | 410 (26) | 25 (6) | 1.39 (.78–2.47) | .261 |
| B (Houston) | 388 (24) | 38 (10) | 2.33 (1.37–3.95) | .002 |
| C (Los Angeles) | 267 (17) | 16 (6) | 1.37 (.71–2.62) | .348 |
| D (Atlanta) | 538 (34) | 24 (5) | Ref. | … |
| Surveillance year (November–October) | ||||
| 2011–2012 | 410 (26) | 36 (8) | 2.48 (1.29–4.76) | .007 |
| 2012–2013 | 363 (23) | 18 (5) | 1.39 (.67–2.87) | .382 |
| 2013–2014 | 472 (29) | 37 (8) | 2.26 (1.18–4.31) | .014 |
| 2014–2015 | 358 (22) | 13 (4) | Ref. | … |
| Clinical symptoms | ||||
| Vomiting | 556 (35) | 65 (12) | NA | … |
| Diarrhea | 1545 (97) | 101 (7) | NA | … |
Abbreviations: CI, confidence interval; NA, not applicable; OR, odds ratio.
aIncludes 21 Asian/Pacific Islanders, 8 American Indian/Alaska Natives, 7 with >1 reported race, and 126 individuals with no reported race.
Norovirus Prevalence
Six percent (103/1603) of stool specimens were norovirus-positive; prevalence by site ranged from 5% to 10% (Table 1). Prevalence varied by surveillance year, with higher norovirus detection rates in the first (8%) and third (8%) years of surveillance and lower rates in the second (5%) and fourth (4%) years. Prevalence was significantly higher during the first (OR, 2.48 [95% CI, 1.29–4.76]) and third (OR, 2.26 [95% CI, 1.18–4.31]) years of surveillance compared to year 4. African-American patients were half as likely to be infected with norovirus compared to white patients (OR, 0.54 [95% CI, .35–.84]). Norovirus prevalence was not significantly different by age, sex, or setting.
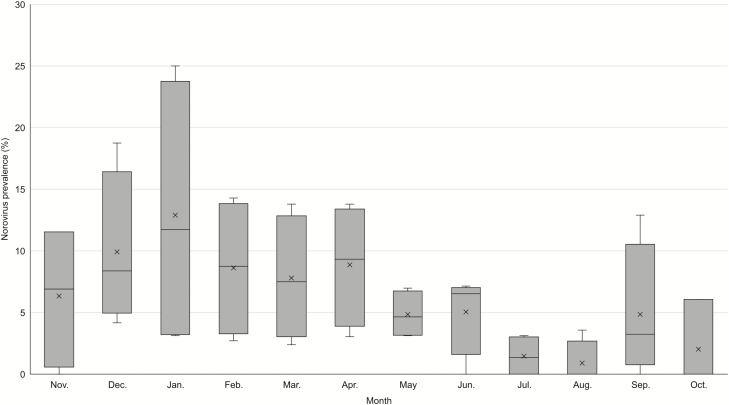
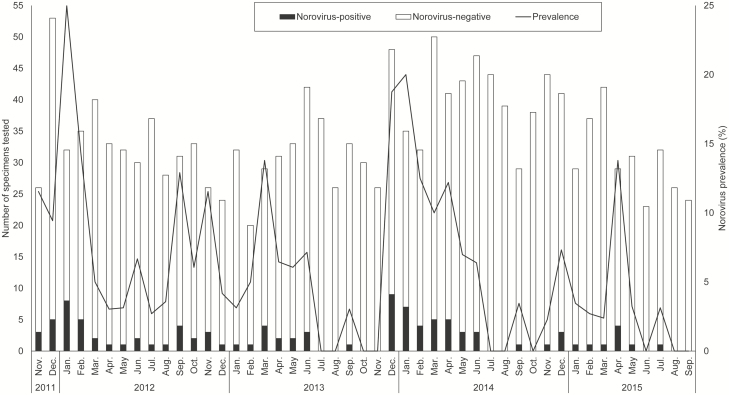
Most (75%) norovirus-positive stool specimens were collected during November–April (Figures 1 and 2). Peak prevalence for norovirus was noted earlier (December–January) in the first and third years of surveillance, and reached a seasonal high of 20%–25%, while the second and fourth years of surveillance had slightly later (March–April) and lower seasonal peaks (14% for both years; Figure 2).
Figure 1.
Box-and-whisker plot of norovirus prevalence by month from stool specimens collected from patients at 4 Veterans Affairs Medical Centers, November 2011–September 2015. Boxes represent the interquartile range by surveillance year; horizontal lines indicate median prevalence; and “X” indicates mean norovirus prevalence for each month.
Figure 2.
Number of stool specimens tested for norovirus and norovirus prevalence by month at 4 Veterans Affairs Medical Centers, November 2011–September 2015.
Coinfections and Genotyping
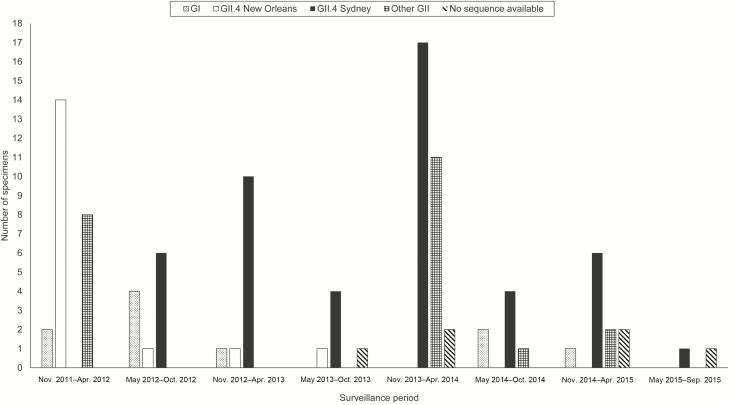
Fourteen percent (n = 14) of norovirus-positive patients were coinfected with ≥1 additional pathogen, most frequently C. difficile (n = 8). GII.4 New Orleans was the most common genotype (17% [n = 17]) in the first year of surveillance. GII.4 Sydney was most common in subsequent surveillance years, in 47% (n = 48) of norovirus-positive specimens (Figure 3). Other GII genotypes represented 21% (n = 22) of norovirus-positive specimens; the genotype of 6% (n = 6) norovirus-positive specimens could not be determined. Ten percent (n = 10) of norovirus-positive specimens were positive for genotype GI.
Figure 3.
Norovirus genotypes among positive stool specimens at 4 Veterans Affairs Medical Centers, by surveillance season, November 2011–September 2015.
Incidence Estimates
Approximately 38 000 AGE-associated outpatient encounters and 5400 AGE-associated inpatient discharges were reported during November 2011–September 2015 (Table 2). Stool specimens corresponded with approximately 2% of all outpatient AGE-related encounters and 15% of all inpatient AGE-related discharges.
Table 2.
Estimates of Incidence Among Outpatients and Inpatients With Community-acquired Norovirus Infection at 4 Veterans Affairs Medical Centers, November 2011–September 2015
| Outpatient | Inpatient Community-acquired | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Total Annual Unique Patients Served, No. | Outpatient AGE Encounters, No. | No. of Stool Specimens Collected (% of AGE Encounters) | No. Norovirus-positive (%) | Norovirus Incidence per 100 000 Patient-years (95% CI) | Inpatient AGE Discharges | No. of Stool Specimens Collected (% of AGE Discharges) | No. Norovirus Positive (%) | Norovirus Incidence per 100 000 Patient-years (95% CI) |
| Site | |||||||||
| Bronx | 97 754 | 4826 | 166 (3.4) | 15 (9.0) | 446 (238–654) | 683 | 244 (35.7) | 10 (4.1) | 29 (11–49) |
| Houston | 384 772 | 12 963 | 224 (1.7) | 22 (9.8) | 331 (196–466) | 2225 | 164 (7.4) | 16 (9.8) | 56 (28–85) |
| Los Angeles | 253 189 | 8356 | 152 (1.8) | 4 (2.6) | 87 (22–174) | 719 | 115 (16.0) | 12 (10.4) | 30 (15–49) |
| Atlanta | 366 961 | 12 178 | 246 (2.0) | 16 (6.5) | 216 (121–324) | 1839 | 292 (15.9) | 8 (2.7) | 14 (5–24) |
| Age category, y | |||||||||
| 18–44 | 209 904 | 8069 | 174 (2.2) | 14 (8.0) | 309 (155–486) | 508 | 82 (16.1) | 7 (8.5) | 21 (9–35) |
| 45–64 | 410 409 | 15 769 | 313 (2.0) | 30 (9.6) | 368 (246–491) | 2388 | 391 (16.4) | 12 (3.1) | 18 (9–30) |
| 65–84 | 416 835 | 12 408 | 268 (2.2) | 11 (4.1) | 122 (56–200) | 2178 | 288 (13.2) | 21 (7.3) | 38 (24–54) |
| ≥85 | 65 528 | 2077 | 32 (1.5) | 2 (6.3) | 198 (0–495) | 392 | 51 (13.0) | 6 (11.8) | 70 (23–129) |
| Surveillance year | |||||||||
| 2011–2012 | 279 152 | 7983 | 161 (2.0) | 14 (8.7) | 239 (124–382) | 1298 | 249 (28.9) | 21 (8.4) | 38 (24–54) |
| 2012–2013a | 208 003 | 7364 | 190 (2.6) | 12 (6.3) | 234 (130–354) | 1141 | 173 (15.2) | 6 (3.5) | 19 (6–35) |
| 2013–2014 | 303 630 | 11 462 | 233 (2.0) | 24 (10.3) | 389 (243–535) | 1516 | 239 (15.7) | 13 (5.4) | 28 (15–42) |
| 2014–2015 | 311 891 | 11 514 | 204 (1.8) | 7 (3.4) | 129 (36–217) | 1511 | 154 (10.1) | 6 (3.9) | 19 (6–35) |
| Total | 1 102 676 | 38 323 | 788 (2.1) | 57 (7.2) | 252 (194–313) | 5466 | 815 (14.9) | 46 (5.6) | 28 (20–36) |
Abbreviations: AGE, acute gastroenteritis; CI, confidence interval.
aData from Los Angeles for 2012–2013 were not available.
Overall norovirus outpatient incidence was estimated at 252 per 100 000 patient-years from November 2011 to September 2015 (range by site, 87–446/100 000 patient-years). Inpatient community-acquired norovirus incidence was estimated at 28 per 100 000 patient-years (range by site: 14–56/100 000 patient-years). Higher rates were observed in the first and third surveillance years (outpatient: 239 and 389/100 000, respectively; community-acquired inpatient: 38 and 28/100 000, respectively), with lower rates in alternate years (outpatient: 234 and 129/100 000, respectively; community-acquired inpatient: 19 and 19/100 000, respectively).
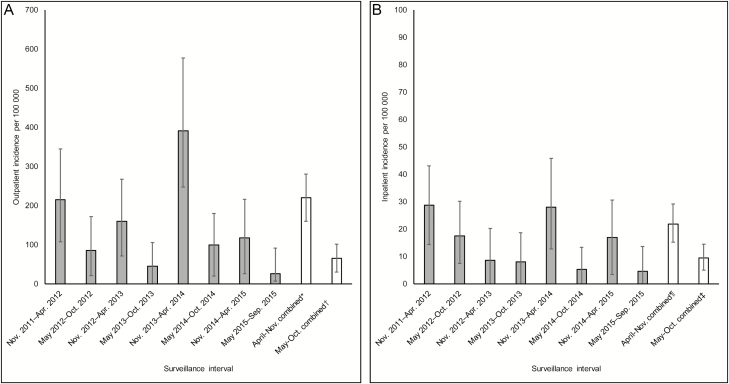
Norovirus incidence peaked during the expected November–April norovirus season. Outpatient norovirus incidence was highest during November–April of each surveillance year, and had higher peaks in the first and third years of surveillance (356/100 000 [95% CI, 178–570/100 000 patient-years] patients in 2011–2012 and 626/100 000 [95% CI, 395–922/100 000 patient-years] patients in 2013–2014). Inpatient community-acquired norovirus incidence demonstrated a similar pattern, with higher peaks in alternate years (48/100 000 [95% CI, 24–71/100 000] and 45/100 000 [95% CI, 20–73/100 000] in 2011–2012 and 2013–2014, respectively). Norovirus incidence during November–April (annualized outpatient incidence: 441/100 000 [95% CI, 321–561/100 000]; annualized inpatient community-acquired incidence: 44/100 000 [95% CI, 31–58/100 000]) was higher than that during May–October (outpatient incidence: 132/100 000 [95% CI, 61–203/100 000]; inpatient community-acquired incidence: 19/100 000 [95% CI, 10–29/100 000]) (Figure 4A and 4B). Median incidence rates were 3 times higher during November–April (376/100 000 and 45/100 000 for outpatients and inpatients with community-acquired norovirus infection, respectively) than May–October (45/100 000 and 13/100 000 for outpatients and inpatients with community-acquired norovirus infection, respectively).
Figure 4.
Biannual annualized norovirus incidence among outpatients (A) and inpatients with community-acquired infection (B) at 4 Veterans Affairs Medical Centers, November 2011–September 2015. *Includes outpatient specimens collected during November 2011–April 2012; November 2012–April 2013; November 2013–April 2014; and November 2014–April 2015. †Includes outpatient specimens collected during May 2012–October 2012; May 2013–October 2013; May 2014–October 2014; and May 2015–September 2015. ¶Includes inpatient specimens collected from patients admitted for acute gastroenteritis during November 2011–April 2012; November 2012–April 2013; November 2013–April 2014; and November 2014–April 2015. ‡Includes inpatient specimens collected from patients admitted for acute gastroenteritis during May 2012–October 2012; May 2013–October 2013; May 2014–October 2014; and May 2015–September 2015.
Among outpatients, Veterans 45–64 years of age had the highest estimated norovirus incidence (368/100 000 patient-years [95% CI, 246–491/100 000 patient-years]); those 65–84 years of age had the lowest (122/100 000 patient-years [95% CI, (56–200/100 000 patient-years]). Among inpatients with community-acquired norovirus infection, Veterans 18–44 years and 45–64 years of age had similar estimated incidence (21/100 000 patient-years [95% CI, 9–35/100 000 patient-years] and 18/100 000 patient-years [95% CI, 9–30/100 000 patient-years], respectively); estimated incidence was higher among older patients (38/100 000 [95% CI, 24–54/100 000] among patients 65–84 years of age) and highest in the oldest age group (70/100 000 [95% CI, 23–129/100 000] among patients ≥85 years of age)
Discussion
Among US Veterans utilizing 4 VAMCs from different geographic regions, norovirus was detected year-round with distinct seasonal peaks. Consistent with previous observations, norovirus incidence was higher among outpatients and ranged from 129 to 389 per 100 000 patients by year, with seasonal peaks up to 349 to 613 per 100 000, whereas inpatient community-acquired norovirus incidence ranged from 19 to 38 per 100 000 patients by year, with seasonal peaks up to 43 to 46 per 100 000.
While overall norovirus prevalence in this study (6%) among AGE specimens tested is similar to other single-year studies conducted using passively collected stool specimens (4%–12%) [13–15], conducting year-round surveillance over multiple norovirus seasons allowed us to examine fluctuation in rates both within and between surveillance years. Prevalence peaked in alternate years; prevalence was higher and peaked earlier in December and January of 2011–2012 and 2013–2014, compared with lower and later spring peaks in March and April of 2012–2013 and 2014–2015. In all 4 surveillance years, winter seasonal highs were observed with a peak prevalence of 14%–25%. While the winter seasonality of norovirus in temperate climates has been well described, few studies have demonstrated distinct peaks by alternate years [21]. This biennial pattern is characteristic of US rotavirus activity in the postvaccine era, with years of low activity and erratic or absent seasonality alternating with years of moderately increased activity and winter–spring seasonality [22–24]. The observed year-to-year variation in norovirus prevalence could partly be an artifact of biennial reductions in rotavirus, leading to a greater relative proportion of AGE attributable to norovirus during those years [5, 25]. Continuing year-round surveillance for norovirus over many years in different populations and settings is therefore necessary to confirm these observations and further characterize trends over time.
Norovirus incidence estimates varied between surveillance years. Inpatient community-acquired norovirus incidence was highest in 2011–2012 and 2013–2014 and lower in the alternate years 2012–2013 and 2014–2015. Outpatient norovirus incidence was highest in the 2011–2012 and 2013–2014 surveillance years, and lowest in 2014–2015; however, the CIs for these point estimates overlapped.
Outpatient incidence rates reported here are generally consistent with results from the first year of surveillance of Veterans at these 4 VAMCs (172–200 cases/100 000) [14], as well as studies of outpatient norovirus incidence rates among adults in the United States (172–790/100 000), United Kingdom (110–380/100 000), and Germany (38–1340/100 000) [11, 13, 14, 26–29]. The inpatient community-acquired norovirus incidence estimates we report here are within the range reported from studies that utilized retrospective regression models, prospective cohorts, or passive surveillance [4, 14, 30, 31]. Other studies that examined laboratory-confirmed or physician-coded norovirus infections among active-duty personnel reported much lower overall norovirus incidence estimates (1.3–5.7/100 000) [32, 33], as did 2 studies that utilized only ICD codes (0.04–6/100 000) [34, 35]; however, these studies were limited by low stool specimen collection and norovirus testing rates and poor utilization of norovirus-specific ICD codes. Another study that utilized time-series regression models among dependent beneficiaries of members of active-duty military reported much higher rates among adults (240–370/100 000) [36]; methods can greatly affect the magnitude of estimates, even when conducted in similar populations.
Some variation in estimates can be attributed to differences in the burden by age group, with young children and older adults (≥65 years of age) disproportionately affected. In our study, the highest rates among inpatients with community-acquired norovirus infection were in older adults (38/100 000 in 65- to 84-year-olds, and 70/100 000 in those ≥85 years old), compared to 18 per 100 000 observed among 18- to 64-year-olds. However, rates among outpatients did not follow this pattern, with the highest outpatient norovirus incidence in 45- to 64-year-olds (368/100 000) and lower rates in those aged 65–84 and ≥85 years (122/100 000 and 198/100 000, respectively). This finding could be in part due to the small sample size of patients in hospitalized oldest age groups, or it could represent increased risk among younger Veterans due to differences in exposures.
Norovirus prevalence was significantly lower among African American patients compared with white patients, possibly due to differences in secretor or Lewis status, which is associated with susceptibility to norovirus infection [37, 38]. Ancestral differences in fut2 gene expression and susceptibility to norovirus infection have also been shown to be significantly associated with Hispanic ethnicity [37]. Although in this study, Hispanic ethnicity was not systematically collected and therefore prevalence in this group of patients was not assessed, future studies may examine the relationship between secretor status, race, and ethnicity to better understand risk factors for norovirus susceptibility.
This study has limitations. Our estimates rely on passive collection of stool specimens from clinician-ordered diagnostic tests for gastrointestinal pathogens. Providers may be less likely to order diagnostic tests for patients with suspected viral AGE due to the self-limiting nature of the illness as well as treatment limitations, which may have biased our sampled populations toward underestimation of norovirus prevalence. In this study, proportions of collected stool specimens among all AGE encounters were low (1.5%–12% by age group and setting). Additionally, norovirus incidence may be underestimated as norovirus-positive patients may have had other symptoms of AGE (eg, vomiting), but not diarrhea or loose stools [39]. While inclusion of multiple years of surveillance allowed us to develop time- and age group–specific norovirus incidence estimates, we still had relatively low numbers of specimens among some categories of time and/or age; consequently, some incidence estimates may be relatively imprecise. Our norovirus incidence estimates are limited to Veterans who utilized the VA for gastroenteritis-associated medical care; some may have sought care through non-VA providers, which would also bias our estimates downward. However, most Veterans utilize VA health services exclusively [40]. We intentionally used a population estimate of Veterans who have utilized VA inpatient or outpatient services in their catchment area within the past year rather than the total number of enrolled Veterans, and excluded patients who only used VA services for prescriptions or laboratory services, to limit this bias. Third, our results may not be generalizable to all US Veterans, as our study included 4 VAMCs classified as “high complexity” facilities, meaning they have high patient volumes, high levels of teaching and research, and the largest number and breadth of physician specialists [41].
This study demonstrates dynamic variability in prevalence and incidence of norovirus AGE by season and between surveillance years for US Veterans seeking care in outpatient and inpatient VAMCs. Our approach demonstrates the feasibility of conducting passive surveillance for norovirus AGE, as well as advantages of conducting systematic, year-round surveillance over multiple years. Ongoing surveillance is needed to further characterize temporal trends identified during passive surveillance, and will allow for refinement of norovirus incidence estimates. Additional surveillance will facilitate detection of emergent strains of norovirus. Future norovirus surveillance among US Veterans will be especially helpful in the event of norovirus vaccine introduction, as older adults are considered an important potential target group for vaccination efforts.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments.The authors acknowledge Daniel Aaronson, E. Clayton Carruth, Brenda Clark, Bailey Fay, Fletcher Fernau, Christopher Graber, Rondeen Mindley, Adrienne Perea, Azra Shahidi, S. Hannah Shirley, Johane Simelane, and Chu-Tang (Sean) Yee for assisting with laboratory specimen and clinical data collection for this study.
Disclaimer.The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the Centers for Disease Control and Prevention (CDC), Department of Veterans Affairs, or the US government.
Financial support.This work was supported in part by the Agriculture and Food Research Initiative (competitive grant number 2011-68003-30395 from the US Department of Agriculture, National Institute of Food and Agriculture). H. B., M. B. G., and M. C. R.-B. received grants from the CDC during the conduct of the study.
Potential conflicts of interest.B. L. has received personal fees from Takeda Pharmaceutical, the CDC Foundation, and Hall Booth Smith, P.C. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Jones TF, McMillian MB, Scallan E, et al. A population-based estimate of the substantial burden of diarrhoeal disease in the United States; FoodNet, 1996–2003. Epidemiol Infect 2007; 135:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Scallan E, Griffin PM, Angulo FJ, Tauxe RV, Hoekstra RM. Foodborne illness acquired in the United States—unspecified agents. Emerg Infect Dis 2011; 17:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hall AJ, Curns AT, McDonald LC, Parashar UD, Lopman BA. The roles of Clostridium difficile and norovirus among gastroenteritis-associated deaths in the United States, 1999–2007. Clin Infect Dis 2012; 55:216–23. [DOI] [PubMed] [Google Scholar]
- 4. Lopman BA, Hall AJ, Curns AT, Parashar UD. Increasing rates of gastroenteritis hospital discharges in US adults and the contribution of norovirus, 1996–2007. Clin Infect Dis 2011; 52:466–74. [DOI] [PubMed] [Google Scholar]
- 5. Hall AJ, Lopman BA, Payne DC, et al. Norovirus disease in the United States. Emerg Infect Dis 2013; 19:1198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harris JP, Edmunds WJ, Pebody R, Brown DW, Lopman BA. Deaths from norovirus among the elderly, England and Wales. Emerg Infect Dis 2008; 14:1546–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Asten L, Siebenga J, van den Wijngaard C, et al. Unspecified gastroenteritis illness and deaths in the elderly associated with norovirus epidemics. Epidemiology 2011; 22:336–43. [DOI] [PubMed] [Google Scholar]
- 8. Lindsay L, Wolter J, De Coster I, Van Damme P, Verstraeten T. A decade of norovirus disease risk among older adults in upper-middle and high income countries: a systematic review. BMC Infect Dis 2015; 15:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cardemil CV, Parashar UD, Hall AJ. Norovirus infection in older adults: epidemiology, risk factors, and opportunities for prevention and control. Infect Dis Clin North Am 2017; 31:839–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mead PS, Slutsker L, Dietz V, et al. Food-related illness and death in the United States. Emerg Infect Dis 1999; 5:607–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gastanaduy PA, Hall AJ, Curns AT, Parashar UD, Lopman BA. Burden of norovirus gastroenteritis in the ambulatory setting—United States, 2001–2009. J Infect Dis 2013; 207:1058–65. [DOI] [PubMed] [Google Scholar]
- 12. Verstraeten T, Cattaert T, Harris J, Lopman B, Tam CC, Ferreira G. Estimating the burden of medically attended norovirus gastroenteritis: modeling linked primary care and hospitalization datasets. J Infect Dis 2017; 216:957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grytdal SP, DeBess E, Lee LE, et al. Incidence of norovirus and other viral pathogens that cause acute gastroenteritis (AGE) among Kaiser Permanente member populations in the United States, 2012–2013. PLoS One 2016; 11:e0148395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grytdal SP, Rimland D, Shirley SH, et al. Incidence of medically-attended norovirus-associated acute gastroenteritis in four Veteran’s Affairs Medical Center populations in the United States, 2011–2012. PLoS One 2015; 10:e0126733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hall AJ, Rosenthal M, Gregoricus N, et al. Incidence of acute gastroenteritis and role of norovirus, Georgia, USA, 2004–2005. Emerg Infect Dis 2011; 17:1381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pringle K, Lopman B, Vega E, Vinje J, Parashar UD, Hall AJ. Noroviruses: epidemiology, immunity and prospects for prevention. Future Microbiol 2015; 10:53–67. [DOI] [PubMed] [Google Scholar]
- 17. Hall AJ, Wikswo ME, Manikonda K, Roberts VA, Yoder JS, Gould LH. Acute gastroenteritis surveillance through the national outbreak reporting system, United States. Emerg Infect Dis 2013; 19:1305–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wikswo ME, Hall AJ; Centers for Disease Control and Prevention Outbreaks of acute gastroenteritis transmitted by person-to-person contact—United States, 2009–2010. MMWR Surveill Summ 2012; 61:1–12. [PubMed] [Google Scholar]
- 19. Shah MP, Wikswo ME, Barclay L, et al. Near real-time surveillance of U.S. norovirus outbreaks by the norovirus sentinel testing and tracking network—United States, August 2009–July 2015. MMWR Morb Mortal Wkly Rep 2017; 66:185–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vega E, Barclay L, Gregoricus N, Williams K, Lee D, Vinjé J. Novel surveillance network for norovirus gastroenteritis outbreaks, United States. Emerg Infect Dis 2011; 17:1389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Siqueira JA, Linhares Ada C, Gonçalves Mdos S, et al. Group A rotavirus and norovirus display sharply distinct seasonal profiles in Belém, northern Brazil. Mem Inst Oswaldo Cruz 2013; 108:661–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aliabadi N, Tate JE, Haynes AK, Parashar UD; Centers for Disease Control and Prevention Sustained decrease in laboratory detection of rotavirus after implementation of routine vaccination—United States, 2000–2014. MMWR Morb Mortal Wkly Rep 2015; 64:337–42. [PMC free article] [PubMed] [Google Scholar]
- 23. Leshem E, Tate JE, Steiner CA, Curns AT, Lopman BA, Parashar UD. Acute gastroenteritis hospitalizations among US children following implementation of the rotavirus vaccine. JAMA 2015; 313:2282–4. [DOI] [PubMed] [Google Scholar]
- 24. Shah MP, Dahl RM, Parashar UD, Lopman BA. Annual changes in rotavirus hospitalization rates before and after rotavirus vaccine implementation in the United States. PLoS One 2018; 13:e0191429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Centers for Disease Control and Prevention. Norovirus activity—United States, 2006–2007. MMWR Morb Mortal Wkly Rep 2007; 56:842–6. [PubMed] [Google Scholar]
- 26. O’Brien SJ, Donaldson AL, Iturriza-Gomara M, Tam CC. Age-specific incidence rates for norovirus in the community and presenting to primary healthcare facilities in the United Kingdom. J Infect Dis 2016; 213(Suppl 1):S15–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Phillips G, Tam CC, Conti S, et al. Community incidence of norovirus-associated infectious intestinal disease in England: improved estimates using viral load for norovirus diagnosis. Am J Epidemiol 2010; 171:1014–22. [DOI] [PubMed] [Google Scholar]
- 28. Bernard H, Hohne M, Niendorf S, Altmann D, Stark K. Epidemiology of norovirus gastroenteritis in Germany 2001–2009: eight seasons of routine surveillance. Epidemiol Infect 2014; 142:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Werber D, Hille K, Frank C, et al. Years of potential life lost for six major enteric pathogens, Germany, 2004–2008. Epidemiol Infect 2013; 141:961–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chan MC, Leung TF, Chung TW, et al. Virus genotype distribution and virus burden in children and adults hospitalized for norovirus gastroenteritis, 2012–2014, Hong Kong. Sci Rep 2015; 5:11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haustein T, Harris JP, Pebody R, Lopman BA. Hospital admissions due to norovirus in adult and elderly patients in England. Clin Infect Dis 2009; 49:1890–2. [DOI] [PubMed] [Google Scholar]
- 32. Hill SE, Poss DE, Harris S. Incidence of gastrointestinal infections among U.S. active component service members stationed in the U.S. compared to U.S. civilians, 2012–2014. MSMR 2017; 24:20–5. [PubMed] [Google Scholar]
- 33. Clark LL, Stahlman S, Oh GT. Using records of diagnoses from healthcare encounters and laboratory test results to estimate the incidence of norovirus infections, active component, U.S. Armed Forces, 2007–2016: limitations to this approach. MSMR 2017; 24:16–9. [PubMed] [Google Scholar]
- 34. Chui KK, Jagai JS, Griffiths JK, Naumova EN. Hospitalization of the elderly in the United States for nonspecific gastrointestinal diseases: a search for etiological clues. Am J Public Health 2011; 101:2082–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ruzante JM, Majowicz SE, Fazil A, Davidson VJ. Hospitalization and deaths for select enteric illnesses and associated sequelae in Canada, 2001–2004. Epidemiol Infect 2011; 139:937–45. [DOI] [PubMed] [Google Scholar]
- 36. Rha B, Lopman BA, Alcala AN, Riddle MS, Porter CK. Incidence of norovirus-associated medical encounters among active duty United States military personnel and their dependents. PLoS One 2016; 11:e0148505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Currier RL, Payne DC, Staat MA, et al. Innate susceptibility to norovirus infections influenced by FUT2 genotype in a United States pediatric population. Clin Infect Dis 2015; 60:1631–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kambhampati A, Payne DC, Costantini V, Lopman BA. Host genetic susceptibility to enteric viruses: a systematic review and metaanalysis. Clin Infect Dis 2016; 62:11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Glass RI, Parashar UD, Estes MK. Norovirus gastroenteritis. N Engl J Med 2009; 361:1776–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Justice AC, Dombrowski E, Conigliaro J, et al. Veterans Aging Cohort Study (VACS): overview and description. Med Care 2006; 44:S13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Veterans Health Administration. Veterans Health Administration: summary of VHA facility complexity model. Available at: https://www.vendorportal.ecms.va.gov/FBODocumentServer/DocumentServer.aspx?DocumentId=2793591&FileName=VA118-16-R-1059-A00002002.docx. Accessed 4 April 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.