Abstract
Feminizing hormone therapy (FHT) may interact with human immunodeficiency virus preexposure prophylaxis (PrEP). We found that transgender women who took FHT exhibited a 7-fold lower rectal tissue ratio of PrEP’s active metabolites vs competing deoxynucleotides compared to cisgender women and men (P = .03) that inversely correlated with estradiol (ρ = –0.79; P < .05). Thus, FHT may negatively impact PrEP efficacy.
Clinical Trials Registration. NCT02983110.
Keywords: transgender, preexposure prophylaxis, lower gastrointestinal tract, HIV, feminizing hormone therapy
Transfeminine populations have an estimated global human immunodeficiency virus (HIV) prevalence of 19% with 49-fold increased odds of infection [1]. Thus, HIV preexposure prophylaxis (PrEP) represents a significant opportunity for improving the health of transfeminine communities. Yet, less than 10% of study participants enrolled in PrEP clinical trials identified as transgender. Subgroup analyses of 1 trial (iPrEx) demonstrated lower PrEP blood concentrations in transgender women (TGW) compared to cisgender men (CGM) who have sex with men (MSM) and no benefit of PrEP compared to placebo among TGW [2]. Investigators attributed this failure to lower adherence and reported no infections in TGW who exhibited concentrations commensurate with 4 doses/week [2]. However, this interpretation was predicated on pharmacokinetics in healthy, cisgender individuals and assumed the populations were matched, which is likely inaccurate. TGW are more likely to have a sexually transmitted infection (STI), use cocaine or methamphetamines, and take high doses of female sex hormones and antiandrogens as feminizing hormone therapy (FHT) [2], all of which may impact pharmacokinetic–pharmacodynamic relationships. In TGW who use FHT, the median estradiol concentrations (258 pg/mL) [3] fall within the clinical reference range of peak estradiol concentrations observed during the periovulatory phase of the menstrual cycle (96–436 pg/mL), typically lasting less than 5 days.
Currently, only a fixed-dose combination tablet of tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC) is approved for PrEP by the US Food and Drug Administration. TDF and FTC are intracellularly phosphorylated to their deoxynucleotide analogs (tenofovir-diphosphate [TFVdp] and emtricitabine-triphosphate [FTCtp]), which block reverse transcription by competing with host cells’ naturally occurring deoxynucleotides (deoxyadenosine-triphosphate [dATP] and deoxycytidine-triphosphate and [dCTP], respectively). Clinical investigation established the importance of nucleotide balance for this class of antiretrovirals by demonstrating that mycophenolate increased abacavir’s potency through decreasing deoxyguanosine triphosphate (dGTP) concentrations [4]. Later in vitro investigation demonstrated that TFVdp’s potency is inversely related to dATP concentrations [5]. High doses of estradiol (13 620 pg/mL) increase the in vitro activity of 5’nucleotidase enzymes, which maintain nucleotide balance [6]. Depending on the cellular location, 5’nucleotidases can facilitate transfer of dATP’s precursor into the cell or impede TFVdp formation [7]. Thus, increased 5’nucleotidase activity can increase dATP and/or decrease TFVdp. In CD4+ T cells isolated from female genital tissues and treated ex vivo with TFV, estradiol, and progesterone, TFVdp formation was decreased by 25% [8].
Since cellular function is highly modulated by sex hormones within mucosal tissues associated with HIV transmission [9] and nucleotide balance is an important effector of TDF/FTC potency, FHT may diminish PrEP efficacy. Therefore, we explored whether FHT alters PrEP pharmacology in blood and rectal tissue of TGW compared to cisgender women (CGW) and CGM.
Post submission changes have increased this above the 50 word limit. HIV PrEP was not spelled out in the submitted version. HIV is common enough terminology that it is unnecessary to spell out here given the tight word constraints. PrEP is defined in the manuscript title.
METHODS
The University of North Carolina (UNC) Biomedical Institutional Review Board approved this study. All participants provided written informed consent prior to enrollment.
We enrolled HIV-infected and HIV-uninfected volunteers aged 18–65 years who were taking TDF/FTC as part of clinical care with intact gastrointestinal tracts into 3 cohorts: TGW on FHT, CGM, and postmenopausal CGW. Pertinent exclusion criteria were past colorectal resection, detectable plasma HIV RNA, signs or symptoms of STIs, <80% self-reported TDF/FTC adherence, estimated glomerular filtration rate (eGFR) <60 mL/min, and taking hormone therapy (cisgender only). Adverse events (AEs), assessed at all visits, were graded according to the National Institute of Allergy and Infectious Diseases Division of AIDS AE grading table. All visits were conducted at the UNC Health Care’s Clinical Trials Research Center. Safety labs (basic metabolic panel, complete blood count, rapid plasma reagin, and prothrombin time and international normalized ratio/activated partial thromboplastin time) were collected at screening. HIV-uninfected individuals were also screened for PrEP adherence by plasma and upper layer packed cells (ULPC) to confirm ≥4 doses/week [10]. eGFR was calculated using the Cockcroft-Gault equation according to natal sex (assigned at birth) and gender identity. Blood and rectal tissue were collected at a sampling visit 16–24 hours after the last self-reported TDF/FTC dose. Participants adhered to a low-fiber diet for 72 hours and clear liquids only for 12 hours prior to sampling. Adherence was assessed by 30-day self-report of missed doses and 5-day dosing record. A follow-up visit was conducted within 14 days.
Sample Analysis
Specimens were collected and processed for sex steroid hormones in serum quantified by specific enzyme immunoassay; pharmacology (parents, metabolites, and deoxynucleotides) in plasma, peripheral blood mononuclear cells (PBMCs), and rectal tissue homogenates was quantified by liquid chromatography–tandem mass spectrometry; and HIV RNA and DNA in rectal tissue homogenates were quantified by droplet digital polymerase chain reaction assay (details in the Supplementary Methods).
Data Analysis
Drug concentrations below limits of quantification were imputed at the lower limit of quantification. Between-cohort comparisons were made using Wilcoxon analysis of variance with the Dwass, Steel, Critchlow-Fligner multiple comparisons procedure. Ratio data were compared between transgender and cisgender participants using the Mann-Whitney rank sum t test. A Spearman rank correlation was performed to quantify the relationships between pharmacologic and hormone measurements, and Hochberg multiple comparison P value adjustments were applied (allowable false-discovery rate = 5%). SAS version 9.4 was used for statistical analyses.
RESULTS
Participant Characteristics
Study participants were enrolled from May 2017 to October 2018. Demographics and other clinical data are summarized in Supplementary Table S1. There were no statistically significant differences in age, weight, body mass index, or creatinine clearance (all P > .07). Ten participants were taking TDF/FTC as part of suppressive HIV treatment and 2 (both CGM) were taking PrEP. Serum estradiol was highest among TGW (P < .001), all of whom were taking spironolactone for androgen suppression.
Blood and Rectal TDF/FTC Pharmacology
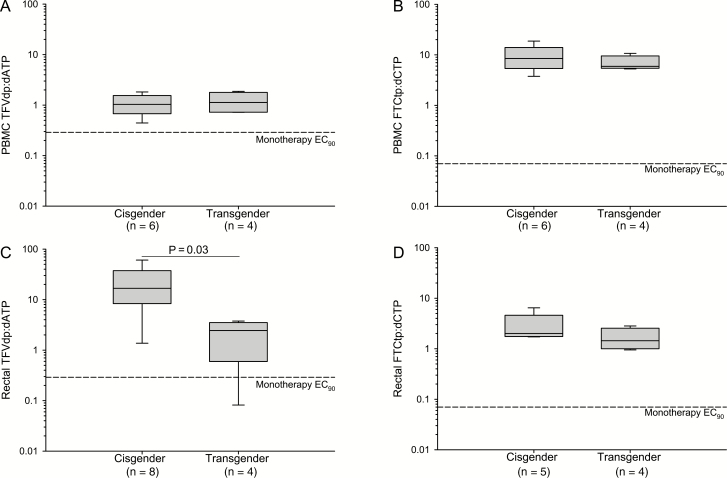
In blood and rectal tissue, parent (TFV/FTC), metabolite (TFVdp/FTCtp), and deoxynucleotide (dATP/dCTP) concentrations were similar across groups (Supplementary Figures S1 and S2), with nonsignificant trends toward higher PBMC FTCtp and lower rectal deoxynucleotides in CGW vs CGM (P = .06). The median TFVdp:dATP was 7-fold lower in rectal tissue (but not PBMCs) of transgender vs cisgender participants (P < .05; Figure 1) and inversely correlated with estradiol and progesterone (ρ = –0.79 and –0.70, respectively; Hochberg adjusted P < .05). FTCtp:dCTP did not differ between transgender and cisgender participants. FTCtp:dCTP in rectal tissue was inversely correlated with serum progesterone (ρ = –0.87; Hochberg P < .05) but not estradiol.
Figure 1.
Tenofovir disoproxil fumarate:emtricitabine nucleotide ratios in blood and rectal tissue of cisgender vs transgender participants. Ratios of active metabolites (TFVdp and FTCtp) to competitive substrates (dATP and dCTP) are plotted in the blood (A, B) and rectal tissue (C, D) for cisgender men and women (n = 8) vs transgender women (n = 4). PBMC dATP and dCTP values were missing for 2 cisgender men omitted from analysis. In rectal tissue, 2/12 dATP and 1/12 dCTP measurements were BLQ and imputed at the sample-specific lower limit of quantification to calculate the nucleotide ratio. The rectal FTCtp:dCTP nucleotide ratio for 3 individuals (2 cisgender women and 1 cisgender man) for whom both concentration values were BLQ were omitted from analysis. Previously published [11] monotherapy 90% effective concentration (EC90) target ratios are denoted by dashed reference lines on each graph. A Mann-Whitney rank sum t test was performed to detect between-cohort differences. The ratio of TFVdp:dATP in rectal tissue was 7-fold lower among transgender women compared to cisgender participants (P = .03). Abbreviations: BLQ, below the limit of quantification; dATP, deoxyadenosine-triphosphate; dCTP, deoxycytidine-triphosphate; FTCtp, emtricitabine-triphosphate; EC90, PBMC, peripheral blood mononuclear cell; TFVdp, tenofovir-diphosphate.
DISCUSSION
TGW could substantially benefit from PrEP but were underrepresented in PrEP clinical trials. Here, we aimed to characterize PrEP pharmacology in TGW within their primary HIV transmission site. We found that rectal TFVdp:dATP, an important effector of PrEP potency [4, 5], was 7-fold lower in transgender vs cisgender participants (P < .05) and inversely correlated with female sex hormones (P < .05). We did not observe differences in TFVdp:dATP in blood or differences in FTCtp:dCTP in blood or rectal tissue. In a recently reported Baltimore cohort, TGW exhibited 68% lower rectal TFVdp concentrations when compared to cisgender MSM, but dATP was not measured [12]. This study and a Thailand cohort of TGW who take PrEP with or without FHT [13] also associated small but statistically significant plasma TFV reductions (13%–27%) with FHT. These 3 cohort studies provide evidence that FHT may alter PrEP pharmacology in a direction associated with reduced efficacy or stricter adherence requirements.
Unlike the aforementioned studies, we did not observe lower plasma TFV. Given our proposed mechanism, this decrease would not be expected. While 5’nucleotidases impede TFVdp formation by degrading TFV [7], only intracellularly located TFV is subject to degradation; however, blood and rectal tissue measurements do not exclusively represent intracellular molecules. Additionally, molar TFVdp concentrations (exclusively intracellularly located) are small relative to TFV concentrations (0.5% and 1% in blood and rectal tissue, respectively, in our study). Thus, degradation of intracellular TFV could result in substantial TFVdp decreases but immeasurable TFV decreases.
Despite significantly lower rectal TFVdp:dATP in TGW, we did not observe increased rectal HIV RNA or DNA (Supplementary Table S1). While sample size prohibits drawing definitive conclusions, we postulated that spironolactone containing FHT may inhibit transcription [14]. Consistent with this postulate, plasma spironolactone (Supplementary Table S1) was lowest among TGW with the highest rectal HIV DNA. Alternatively, estrogen receptor 1 (a well-characterized latency regulator [15]) could be acting to suppress transcription. Characterizing the influence of TGWs’ unique hormonal environment on HIV replication may have important implications for HIV cure strategies.
Our study has some limitations. While we demonstrated significantly lower rectal TFVdp:dATP, we did not observe significant differences in the individual components, although median TFVdp was 1.75-fold lower and dATP was 21-fold higher in TGW. Our small sample size may have precluded detection of such differences. Enrolling postmenopausal CGW (intended as a low-estrogen control group and to avoid introducing variability of the female reproductive cycle’s highly dynamic hormonal profile) may limit generalizability. Physiological changes (ie, altered muscle mass and development of secondary sex characteristics) are present on a spectrum based on FHT selection, duration, and age at initiation. Thus, FHT duration that ranges from 1 to 9 years and heterogeneous FHT and antiretroviral regimens are all potential sources of variability. Last, we relied on self-reported adherence, undetectable viral load, or plasma/ULPC drug concentrations as evidence of adherence rather than directly observed dosing. However, because we did not observe lower blood concentrations, our rectal tissue data are unlikely to be confounded by lower adherence among TGW.
This is the first description of PrEP’s active metabolites and competing deoxynucleotides in rectal tissue of TGW who take FHT. Here, we demonstrate 7-fold lower rectal TFVdp:dATP in TGW vs cisgender participants, which decreased with increasing estradiol. These data confirm in vitro studies that suggest FHT may decrease TDF/FTC’s potency and indicate that the degree of feminization may be an important consideration. Additional studies are needed to determine the clinical implications of our findings and whether PrEP dosing strategies should be reconsidered in TGW.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH). The study team was wholly composed of cisgender individuals, and the authors’ reporting of these data may reflect inherent cisgender bias.
Financial support. This work was supported by a 2018 developmental pilot grant jointly supported by the University of North Carolina–Chapel Hill Center for AIDS Research, an NIH funded program (P30 AI50410), and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1TR002489. A. F. P. receives support from an NIH Research Career Development Award (K23DK113225).
Potential conflicts of interest. F. Z. S. serves as an independent consultant for Agile Therapeutics, TherapeuticsMD, Mithra Pharmaceuticals, and Dr Reddy’s Laboratories. All other authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: AIDS 2018. Amsterdam, the Netherlands, 23–27 July 2018. Abstract TUPDX0106.
References
- 1. Baral SD, Poteat T, Strömdahl S, Wirtz AL, Guadamuz TE, Beyrer C. Worldwide burden of HIV in transgender women: a systematic review and meta-analysis. Lancet Infect Dis 2013; 13:214–22. [DOI] [PubMed] [Google Scholar]
- 2. Deutsch MB, Glidden DV, Sevelius J, et al. ; iPrEx Investigators HIV pre-exposure prophylaxis in transgender women: a subgroup analysis of the iPrEx trial. Lancet HIV 2015; 2:e512–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deutsch MB, Bhakri V, Kubicek K. Effects of cross-sex hormone treatment on transgender women and men. Obstet Gynecol 2015; 125:605–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Margolis DM, Kewn S, Coull JJ, et al. . The addition of mycophenolate mofetil to antiretroviral therapy including abacavir is associated with depletion of intracellular deoxyguanosine triphosphate and a decrease in plasma HIV-1 RNA. J Acquir Immune Defic Syndr 2002; 31:45–9. [DOI] [PubMed] [Google Scholar]
- 5. García-Lerma JG, Aung W, Cong ME, et al. . Natural substrate concentrations can modulate the prophylactic efficacy of nucleotide HIV reverse transcriptase inhibitors. J Virol 2011; 85:6610–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shen Z, Fahey JV, Bodwell JE, et al. . Estradiol regulation of nucleotidases in female reproductive tract epithelial cells and fibroblasts. PLoS One 2013; 8:e69854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hunsucker SA, Mitchell BS, Spychala J. The 5’-nucleotidases as regulators of nucleotide and drug metabolism. Pharmacol Ther 2005; 107:1–30. [DOI] [PubMed] [Google Scholar]
- 8. Shen Z, Fahey JV, Bodwell JE, Rodriguez-Garcia M, Kashuba AD, Wira CR. Sex hormones regulate tenofovir-diphosphate in female reproductive tract cells in culture. PLoS One 2014; 9:e100863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wira CR, Fahey JV, Rodriguez-Garcia M, Shen Z, Patel MV. Regulation of mucosal immunity in the female reproductive tract: the role of sex hormones in immune protection against sexually transmitted pathogens. Am J Reprod Immunol 2014; 72:236–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Corneli AL, Deese J, Wang M, et al. ; FEM-PrEP Study Group FEM-PrEP: adherence patterns and factors associated with adherence to a daily oral study product for pre-exposure prophylaxis. J Acquir Immune Defic Syndr 2014; 66:324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cottrell ML, Yang KH, Prince HM, et al. . A translational pharmacology approach to predicting outcomes of preexposure prophylaxis against HIV in men and women using tenofovir disoproxil fumarate with or without emtricitabine. J Infect Dis 2016; 214:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shieh E, Marzinke M, Fuchs E, et al. . Transgender women on estrogen have significantly lower tenofovir/emtricitabine concentrations during directly observed dosing when compared to cis men. [abstract OA23.03]. In: HIV Research For Prevention (HIVR4P), Madrid, Italy, 21–25 October 2018.
- 13. Hiransuthikul A, Himmad K, Kerr S, et al. . Drug-drug interactions between the use of feminizing hormone therapy and pre-exposure prophylaxis among transgender women: the iFACT study. [abstract TUPDX0107LB]. In: AIDS2018, Amsterdam, Netherlands, 23–27 July 2018.
- 14. Lacombe B, Morel M, Margottin-Goguet F, Ramirez BC. Specific inhibition of HIV infection by the action of spironolactone in T cells. J Virol 2016; 90:10972–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Das B, Dobrowolski C, Luttge B, et al. . Estrogen receptor-1 is a key regulator of HIV-1 latency that imparts gender-specific restrictions on the latent reservoir. Proc Natl Acad Sci U S A 2018; 115:E7795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.