Abstract
Background
Household contacts (HHCs) of individuals with multidrug-resistant tuberculosis (MDR-TB) are at high risk of infection and subsequent disease. There is limited evidence on the willingness of MDR-TB HHCs to take MDR-TB preventive therapy (MDR TPT) to decrease their risk of TB disease.
Methods
In this cross-sectional study of HHCs of MDR-TB and rifampicin-resistant tuberculosis (RR-TB) index cases from 16 clinical research sites in 8 countries, enrollees were interviewed to assess willingness to take a hypothetical, newly developed MDR TPT if offered. To identify factors associated with willingness to take MDR TPT, a marginal logistic model was fitted using generalized estimating equations to account for household-level clustering.
Results
From 278 MDR-TB/RR-TB index case households, 743 HHCs were enrolled; the median age of HHCs was 33 (interquartile range, 22–49) years, and 62% were women. HHC willingness to take hypothetical MDR TPT was high (79%) and remained high even with the potential for mild side effects (70%). Increased willingness was significantly associated with current employment or schooling (adjusted odds ratio [aOR], 1.83 [95% confidence interval {CI}, 1.07–3.13]), appropriate TB-related knowledge (aOR, 2.22 [95% CI, 1.23–3.99]), confidence in taking MDR TPT (aOR, 7.16 [95% CI, 3.33–15.42]), and being comfortable telling others about taking MDR TPT (aOR, 2.29 [95% CI, 1.29–4.06]).
Conclusions
The high percentage of HHCs of MDR-TB/RR-TB index cases willing to take hypothetical MDR TPT provides important evidence for the potential uptake of effective MDR TPT when implemented. Identified HHC-level variables associated with willingness may inform education and counseling efforts to increase HHC confidence in and uptake of MDR TPT.
Keywords: tuberculosis, contacts, drug resistance, prophylaxis, preventive therapy
In this multi-site, cross-sectional study of household contacts (HHCs) of multidrug-resistant tuberculosis (MDR-TB) index cases, a high percentage of HHCs reported willingness to take hypothetical newly developed MDR TB preventive therapy. Identified factors associated with willingness may inform counseling efforts.
(See the Major Article by Gupta et al on pages 425–35.)
Tuberculosis (TB) is the leading infectious cause of mortality worldwide with an estimated 10.0 million new cases and 1.3 million deaths in 2017 alone [1]. Multidrug-resistant TB (MDR-TB; ie, resistant to at least isoniazid and rifampicin) and rifampicin-resistant TB (RR-TB) are estimated to have caused 558 000 of these new cases and a disproportionately high number of deaths [1]. Household contacts (HHCs) of individuals with active TB are at high risk of infection due to prolonged exposure in shared environments [2, 3]. The development of MDR-TB disease among HHCs [2–4] has severe implications for already TB-affected households due to poor treatment outcomes despite lengthy, costly, and toxic regimens [5, 6]. Prevention of MDR-TB disease, therefore, remains a critical public health priority.
Preventing new TB cases through the treatment of TB infection among persons exposed to an infectious TB case is a pillar of the End TB Strategy. Isoniazid- and rifamycin-containing regimens have been demonstrated to reduce the risk of TB disease among HHCs exposed to drug-susceptible TB [7–9]. However, observational studies on MDR-TB preventive therapy (MDR TPT), primarily describing the use of fluoroquinolone-based regimens, have been inconclusive, resulting in a conditional recommendation for treatment of only high-risk HHCs [4, 9, 10]. Ongoing clinical trials are evaluating new potential regimens to treat MDR-TB infection [11], but little evidence exists on the willingness of HHCs to take MDR TPT were it available.
Studies of knowledge, attitudes, and practices have the potential to provide insights into the willingness of populations to utilize a proposed prevention or treatment strategy, as well as elucidate barriers and enablers of uptake. These findings can provide context to an intervention’s acceptability [12], inform health education efforts [13, 14], and characterize provider opinions and preparedness [15]. We conducted a multicountry, cross-sectional study of HHCs of MDR-TB/RR-TB index cases in diverse high-TB-burden settings to understand how HHCs’ TB-related knowledge, attitudes, and practices (KAP) are associated with their willingness to take a hypothetical MDR TPT.
METHODS
Study Setting
The study was conducted from October 2015 to April 2016 at 16 clinical research sites in 8 countries—Botswana (1 site), Brazil (1), Haiti (1), India (2), Kenya (1), Peru (2), South Africa (7), and Thailand (1), in preparation for the Protecting Households on Exposure to Newly Diagnosed Index Multidrug-resistant Tuberculosis Patients (PHOENIx) trial being conducted by the AIDS Clinical Trials Group (ACTG; https://actgnetwork.org/) and International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT; http://impaactnetwork.org/). Information on local TB program activities, including contact tracing and TB preventive therapy (TPT), was collected through key informant interviews (Table 1).
Table 1.
Routine Practices of Tuberculosis Control Programs Affiliated With Participating Clinical Research Sites
| Routine Practices of TB Control Program | Botswana | Brazil | Haiti | India | Kenya | Peru | South Africa | Thailand | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site 1 | Site 2 | Site 1 | Site 2 | Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | Site 6 | Site 7 | ||||||
| Evaluation of HHCs of MDR-TB patients | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Household visits conducted to evaluate HHCs | Yes | Yes | No | No | Yes | Yes | Yes | Yes | … | Yes | Yes | … | Yes | Yes | Yes | Yes |
| Evaluation of adult contacts of MDR-TB patients | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | … | Yes | Yes | … | Yes | Yes | Yes | Yes |
| Symptom screen | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | … | Yes | Yes | … | Yes | No | Yes | Yes |
| Tuberculin skin test | No | Yes | No | Yes | No | Yes | Yes | Yes | … | No | No | … | No | No | No | Yes |
| Interferon-γ release assay | No | No | No | No | No | No | No | No | … | No | No | … | No | No | No | No |
| Chest radiograph | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | … | Yes | No | … | No | No | No | Yes |
| Follow-up with contacts of MDR-TB patients, mo | Yesa | No | 3 | No | 9 | 24 | 24 | 24 | No | No | 24 | No | No | 6 | 24 | 4 |
| Preventive therapy given to HHCs of MDR-TB patients | No | Yes | No | No | No | No | No | No | No | Yes | Yes | No | Yes | Yes | Yes | No |
| Fluoroquinolone alone | … | No | … | … | … | … | … | … | … | No | No | … | No | No | No | … |
| Fluoroquinolone + ethambutolb | … | No | … | … | … | … | … | … | … | No | No | … | Yes + INH | Yes | Yes + PZA | … |
| Fluoroquinolone + ethionamide | … | No | … | … | … | … | … | … | … | No | No | … | No | No | No | … |
| Isoniazid + rifampicin | … | No | … | … | … | … | … | … | … | No | No | … | No | No | No | … |
| Isoniazid | … | Yes | … | … | … | … | … | … | … | Yes | Yes | … | Yes | Yes | No | … |
Abbreviations: HHCs, household contacts; INH, isoniazid; MDR-TB, multidrug-resistant tuberculosis; PZA, pyrazinamide.
aFollow-up with contacts of MDR-TB patients: unknown duration.
bTwo sites in South Africa routinely provide 3-drug therapy: fluoroquinolone + ethambutol + an additional medication.
Study Participant Eligibility Criteria and Recruitment
Pulmonary MDR-TB/RR-TB index cases were eligible for enrollment if they met the following inclusion criteria: (1) documented rifampicin resistance by Xpert MTB/RIF assay, line probe assay, or phenotypic drug sensitivity testing; (2) MDR-TB treatment initiation within 6 months of study enrollment; (3) ≥1 HHC; (4) permission for the study team to enumerate and screen HHCs; and (5) residing at a distance deemed by the site-level study team close enough for study conduct. HHCs were defined as (1) any person currently living or having lived in the same dwelling unit or plot of land; (2) currently sharing or having shared the same housekeeping arrangements as the index case; and (3) reporting exposure within 6 months prior to the index case starting MDR-TB treatment. Index cases were recruited and all eligible adult and adolescent HHCs (≥13 years of age) without active TB were asked to complete a KAP questionnaire.
Data Collection and Variables
A semi-structured KAP questionnaire was adapted for MDR-TB from a recent World Health Organization guide for tuberculosis KAP survey development [16]. The survey was pilot tested among TB community health workers at one study site (India site 2). Additional HHC information obtained included demographic, social, medical, and household characteristics. All questionnaires were completed in-person by trained field staff or clinicians prior to participant education or counseling by anyone affiliated with the study.
The primary outcome in this analysis was willingness to take hypothetical, newly developed MDR TPT. Additionally, HHC willingness to take this therapy even if it were to cause mild temporary side effects was analyzed as a secondary outcome. MDR TPT was not offered to any HHC participating in the study, and no additional questions were asked about how side effects would change interest in taking MDR TPT. Both outcome variables, as well as HHC willingness to have a blood test (ie, interferon-γ release assay), to provide a sputum sample, and to obtain a chest radiograph to determine if the HHC was a good candidate for TPT were collected as categorical (yes, not sure, no) and dichotomized as yes vs not sure or no for analysis.
TB knowledge was analyzed as a binary variable, where appropriate knowledge was defined as correctly identifying all of the following: cough ≥3 weeks is a TB symptom; TB is a curable disease; TB is transmitted via air when an infected person coughs or sneezes, and MDR-TB cure is possible through directly observed therapy [17]. “Incomplete” knowledge was defined as not correctly identifying all 4 items above. Confidence in taking MDR TPT was defined as HHCs feeling confident or very confident in being able to perform all 5 of the following (5-point Likert scale): meeting with study staff monthly to collect medications, coping with difficulties TPT may cause, taking all doses, continuing to take medications even if feeling healthy, and completing medications. A review of systems was completed differently for HHCs <15 and ≥15 years of age to identify the presence of TB-related symptoms at the time of interview. For adolescents or children (<15 years), TB-related symptoms included any of the following at the time of interview: neck swelling, fever, night sweats, cough ≥10 days, poor weight gain, less playful, convulsion, or decreased consciousness. For adults (≥15 years), symptoms included any of the following in the past month: cough ≥10 days, fever, night sweats, unintentional weight loss, or enlarged lymph nodes.
Statistical Analysis
The primary objective of this analysis was to evaluate the association between HHC-level KAP factors and willingness to take hypothetical, newly developed MDR TPT. Among enrolled HHCs with complete KAP data, aggregate and site-level exploratory data analysis was conducted using summary statistics and scatterplots. Simple (adjusting only for research site) and multivariable marginal logistic models for willingness to take MDR TPT were fitted using generalized estimating equations with robust variance estimates to account for household-level clustering assuming an exchangeable within-household correlation structure [18]. Household contact age was rescaled by dividing the variable by a constant (10 years) to improve interpretability. Fixed-effect dummy variables for research sites were included to adjust for variation between sites. Informed by a literature review and the Health Belief Model (Supplementary Figure 1) [19], covariates of interest were selected prior to analysis from a larger set of variables available through the PHOENIx Feasibility Study.
If any site reported 100% willingness to take MDR TPT, the outcome status of one randomly selected HHC at the respective site was set to “not willing” in order to allow for model fit. The sensitivity of model parameter estimates to this random outcome reassignment was examined. The potential confounding of HHC-level associations by corresponding household-level aggregate variables was also evaluated. Model diagnostics included examining the influence of individual HHCs and research sites on parameter estimates as well as residual plots [20]. All analyses were conducted in Stata version 13.1 software (StataCorp, College Station, Texas) except for the creation of stacked bar charts [21].
Human Research Ethics Approvals
Ethical approval was obtained from each research site’s local institutional review board. Written informed consent was obtained for all participating MDR-TB/RR-TB index cases and household contacts prior to study interviews and procedures.
RESULTS
Study Recruitment
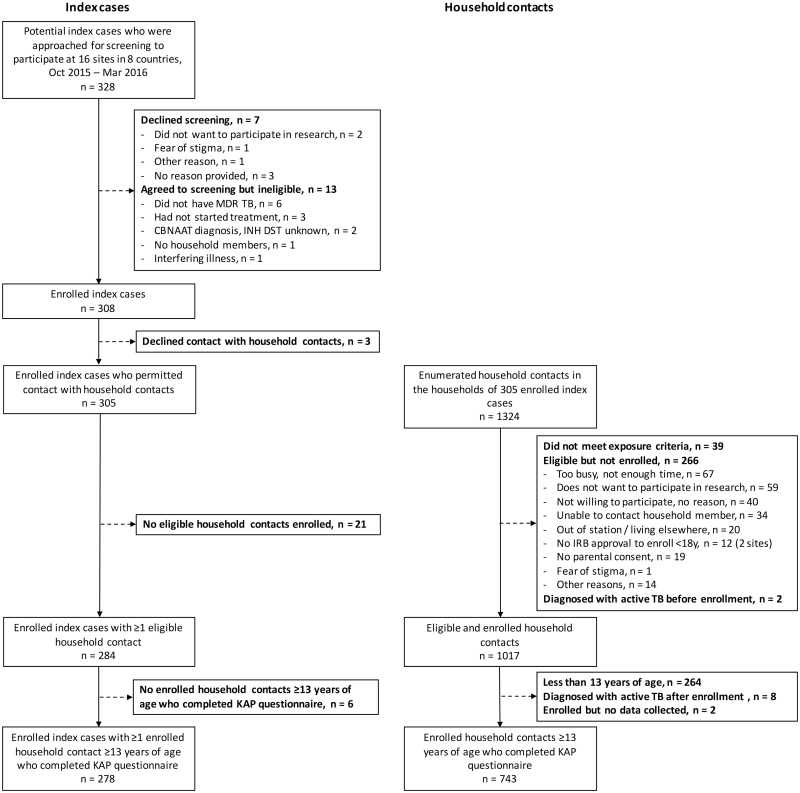
Across all sites, 328 adult pulmonary MDR-TB/RR-TB index cases were screened during the recruitment period; 20 declined screening or were ineligible. Three declined contact with HHCs, and 27 had no eligible, enrolled HHCs who also completed the KAP questionnaire. The final study sample included 278 index cases and their HHCs.
Characteristics of MDR-TB/RR-TB Index Cases and Their Households
Among included index cases (n = 278), the median time between MDR-TB treatment initiation and study enrollment was 68 days (interquartile range [IQR], 29–125 days) with a majority also reporting a history of prior TB (53%). The median number of eligible, enumerated HHCs of all ages in these index case households was 4 (IQR, 2–5) with 32% of households having ≥1 adolescent HHC (13 to <18 years of age), 31% with ≥1 HHC who was <5 years of age, and 6% with ≥1 pregnant HHC.
Characteristics of Enrolled HHCs of MDR-TB/RR-TB Index Cases
For the analysis of factors associated with willingness to take MDR TPT, complete KAP data were available for 743 adult and adolescent HHCs (99.7% of 745 enrolled, and 79% of 946 without active TB and eligible for the KAP study) from 278 MDR-TB/RR-TB index case households (median, 2 enrolled HHCs with complete KAP data per HH [IQR, 1–3]) (Figure 1). The median number of MDR-TB/RR-TB index cases and HHCs enrolled per site and included in this analysis was 14 (IQR, 10–25) and 39 (IQR, 22–70), respectively.
Figure 1.
Eligibility, enrollment, and participation of 743 adult and adolescent household contacts from 278 multidrug-resistant tuberculosis (TB)/rifampicin-resistant TB index case households participating in the Protecting Households on Exposure to Newly Diagnosed Index Multidrug-resistant Tuberculosis Patients (PHOENIx) feasibility TB knowledge, attitudes, and practices study. Abbreviations: CBNAAT, cartridge-based nucleic acid amplification test; DST, drug susceptibility testing; INH, isoniazid; IRB, institutional review board; KAP, knowledge, attitudes, and practices; MDR-TB, multidrug-resistant tuberculosis; TB, tuberculosis.
Among HHCs participating in the KAP study, the median age was 33 years (IQR, 22–49 years), 62% were women, 58% had secondary school education or higher, and 46% were currently employed or in school. Overall, 10% of HHCs reported prior TB treatment and 21% current tobacco use. For alcohol use, 58 HHCs (9%) reported daily or almost daily use in the last year, and 8 (1%) refused to answer the question. Similarly, 57 (8%) reported illegal drug use in the last year and 3 (0.4%) refused to answer (Table 2). Participation in the KAP study was not associated with age, but females were more likely to participate than males (83% vs 72%, P < .001).
Table 2.
Characteristics of Enrolled Multidrug-resistant Tuberculosis (MDR-TB) Household Contacts (n = 743) From 278 Index Case Households and Factors Associated With Their Willingness to Take Newly Developed MDR-TB Preventive Therapy
| Variables | Summary Statistics | Marginal Logistic Models | ||||||
|---|---|---|---|---|---|---|---|---|
| Total No. (Column %)a | Willing to Take MDR TPT, No. (Row %)a | Simple (Adjusted for Site Only) | Multivariable | |||||
| aOR | (95% CI) | P Value | aOR | (95% CI) | P Value | |||
| Sociodemographic characteristics | ||||||||
| Age (rescaled to 10 y for logistic models) | ||||||||
| Median (IQR), y | 33 (22–49) | 33 (22–48) | 1.06 | (.90–1.23) | .495 | 1.06 | (.88–1.28) | .532 |
| Sex | ||||||||
| Female | 461 (62.1) | 366 (79.4) | 1.32 | (.86–2.01) | .201 | 1.26 | (.76–2.08) | .364 |
| Male | 282 (38.0) | 219 (77.7) | 1.00 | ref | 1.00 | ref | ||
| Education (highest completed) | ||||||||
| Secondary, university or higher | 430 (57.9) | 343 (79.8) | 1.11 | (.64–1.93) | .717 | 0.88 | (.44–1.79) | .729 |
| None or primary | 313 (42.1) | 242 (77.3) | 1.00 | ref | 1.00 | ref | ||
| Currently employed or in school | ||||||||
| Yes | 343 (46.2) | 277 (80.8) | 1.34 | (.86–2.09) | .192 | 1.83 | (1.07–3.13) | .028 |
| No/refused to answer | 400 (53.8) | 308 (77.0) | 1.00 | ref | 1.00 | ref | ||
| Medical and social history | ||||||||
| Tobacco use: current smoking | ||||||||
| Yes | 152 (20.5) | 139 (91.5) | 1.40 | (.63–3.08) | .408 | 2.28 | (.89–5.85) | .088 |
| No | 591 (79.5) | 446 (75.5) | 1.00 | ref | 1.00 | ref | ||
| Alcohol use: ever drank alcohol daily in past 12 mo | ||||||||
| Yes | 58 (7.8) | 53 (91.4) | 1.49 | (.52–4.24) | .459 | 1.89 | (.46–7.82) | .382 |
| No/refused to answer | 685 (92.2) | 532 (77.6) | 1.00 | ref | 1.00 | ref | ||
| Drug use: ever used in past 12 mo | ||||||||
| Yes | 57 (7.7) | 47 (82.5) | 0.43 | (.18–1.03) | .057 | 0.37 | (.13–1.08) | .070 |
| No/refused to answer | 686 (92.3) | 538 (78.4) | 1.00 | ref | 1.00 | ref | ||
| Previously treated for TB | ||||||||
| Yes | 77 (10.4) | 67 (87.0) | 0.75 | (.36–1.58) | .447 | 0.42 | (.16–1.13) | .087 |
| No/unknown | 666 (89.6) | 518 (77.8) | 1.00 | ref | 1.00 | ref | ||
| Perceived susceptibility to and severity of MDR-TB | ||||||||
| Knowledge-related to TB | ||||||||
| Appropriate | 493 (66.4) | 445 (90.3) | 2.92 | (1.74–4.90) | < .001 | 2.22 | (1.23–3.99) | .008 |
| Incomplete | 250 (33.7) | 140 (56.0) | 1.00 | ref | 1.00 | ref | ||
| Can die of MDR-TB without treatment | ||||||||
| Yes | 627 (84.4) | 544 (86.8) | 3.89 | (1.77–8.55) | .001 | 2.48 | (1.00–6.13) | .050 |
| No/don’t know | 116 (15.6) | 41 (35.3) | 1.00 | ref | 1.00 | ref | ||
| Typical treatment of person with TB in community | ||||||||
| Most people reject them | 206 (27.7) | 138 (67.0) | 1.19 | (.62–2.29) | .591 | 1.13 | (.62–2.06) | .695 |
| Other responses | 537 (72.3) | 447 (83.2) | 1.00 | ref | 1.00 | ref | ||
| Concerned about getting MDR-TB from recently diagnosed HH member | ||||||||
| Yes | 478 (64.3) | 406 (84.9) | 2.33 | (1.52–3.57) | < .001 | 1.62 | (.94–2.78) | .083 |
| No/neutral | 265 (35.7) | 179 (67.6) | 1.00 | ref | 1.00 | ref | ||
| Sleeping arrangement in HH with index case | ||||||||
| Same room, same bed | 87 (11.7) | 72 (82.8) | 0.77 | (.40–1.48) | .430 | 0.67 | (.31–1.46) | .311 |
| Same room, different bed | 164 (22.1) | 126 (76.8) | 1.06 | (.61–1.82) | .840 | 1.33 | (.71–2.50) | .377 |
| Different room or building | 492 (66.2) | 387 (78.7) | 1.00 | ref | 1.00 | ref | ||
| Cues to action | ||||||||
| Presence of any TB-related symptom | ||||||||
| Yes | 128 (17.2) | 112 (87.5) | 0.92 | (.51–1.66) | .789 | 1.02 | (.53–1.97) | .950 |
| No/unknown | 615 (82.8) | 473 (76.9) | 1.00 | ref | 1.00 | ref | ||
| Perception that TB is serious problem in community | ||||||||
| Yes | 454 (61.1) | 390 (85.9) | 1.29 | (.75–2.20) | .358 | 0.83 | (.44–1.54) | .550 |
| No/neutral | 289 (38.9) | 195 (67.5) | 1.00 | ref | 1.00 | ref | ||
| Barriers and enablers to preventive therapy | ||||||||
| Comfortable telling family about taking MDR TPT | ||||||||
| Yes | 494 (66.5) | 440 (89.1) | 3.07 | (1.88–4.99) | < .001 | 2.29 | (1.29–4.06) | .004 |
| No/neutral | 249 (33.5) | 145 (58.2) | 1.00 | ref | 1.00 | ref | ||
| Confident in properly taking preventive therapy | ||||||||
| Yes | 405 (54.5) | 388 (95.8) | 7.66 | (3.72–15.79) | < .001 | 7.16 | (3.33–15.42) | < .001 |
| No/neutral | 338 (45.5) | 197 (58.3) | 1.00 | ref | 1.00 | ref |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; HH, household; IQR, interquartile range; MDR-TB, multidrug-resistant tuberculosis; MDR TPT, multidrug-resistant tuberculosis preventive therapy; ref, reference group; TB, tuberculosis.
aColumn and row percentages unless otherwise specified; confidence intervals not overlapping the odds ratio null value are bolded.
MDR-TB/RR-TB HHCs and Their TB-related Knowledge and Attitudes
Appropriate MDR-TB knowledge was demonstrated by 66% of enrolled HHCs, with substantial site-level variation (Supplementary Figure 2), notably low knowledge at India site 1 (5%). A majority of HHCs (64%) were concerned about being infected with MDR-TB from their diagnosed household member, and 84% believed that someone could die from MDR-TB without proper treatment. Regarding perceived stigma, 28% of HHCs reported that a person with TB is usually rejected by their community, and 34% stated that they would be uncomfortable telling family members or friends they were taking MDR TPT (Table 2).
Willingness to Take Hypothetical, Newly Developed MDR TPT Among HHCs of MDR-TB/RR-TB Index Cases
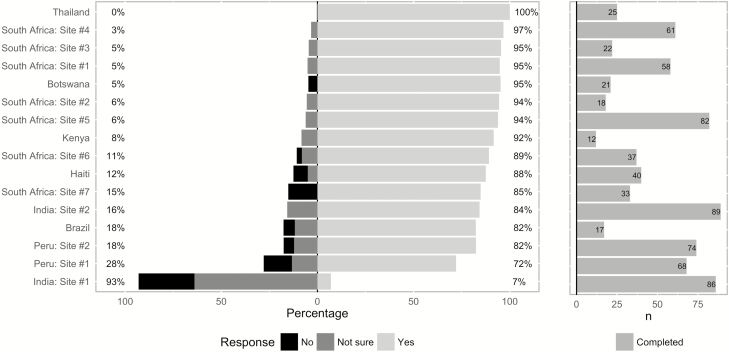
HHC willingness to take hypothetical, newly developed MDR TPT was high overall (79%) with observed site-level variation (site-level median, 90% [IQR, 84%–95%]; Figure 2). At India site 1, only 7% of HHCs reported willingness (29% not willing, 64% not sure). Willingness to take MDR TPT with potential mild temporary side effects was somewhat lower across sites (70% overall; site-level median, 80% [IQR, 66%–91%]). Reported HHC willingness to complete prerequisite steps to determine MDR TPT eligibility was high at all sites except one: blood test (96% [IQR, 88%–98%]), provide sputum sample (97% [IQR, 95%–100%]), and obtain chest radiograph (100% [IQR, 97%–100%]).
In the multivariable model for the primary outcome (Table 2), increased willingness to take MDR TPT was significantly associated with the following HHC characteristics: currently employed or in school (adjusted odds ratio [aOR], 1.83 [95% confidence interval {CI}, 1.07–3.13]), appropriate TB-related knowledge (aOR, 2.22 [95% CI, 1.23–3.99]), confidence in taking MDR TPT (aOR, 7.16 [95% CI, 3.33–15.42]), being comfortable telling family or friends about taking MDR TPT (aOR, 2.29 [95% CI, 1.29–4.06]). Increased willingness was also marginally associated with knowledge that one can die of MDR-TB without treatment (aOR, 2.48 [95% CI, 1.00–6.13]). In a multivariable model including the same set of covariates, the secondary outcome of willingness to take MDR TPT with side effects was significantly associated with increased HHC-level concern about being infected from the index case (aOR, 2.01 [95% CI, 1.30–3.09]) and confidence in taking MDR TPT (aOR, 7.86 [95% CI, 4.41–14.01]).
Site-level variation was observed in the unadjusted associations between willingness to take MDR TPT and covariates of interest (Supplementary Table 2); however, the direction of these associations was highly consistent for the most significant covariates identified through the multivariable model: appropriate TB-related knowledge, comfort telling family or friends about taking MDR TPT, and confidence in taking MDR TPT. After adjustment for all covariates included in the multivariable model, predicted willingness of HHCs at India site 1 remained significantly lower than all other sites on pairwise comparisons with Bonferroni correction. Potential confounding of observed HHC-level associations by between-household effects was examined by creating household-level summary means for all HHC-level covariates [22]. Including all aggregate household-level variables in the final multivariable model did not qualitatively change any HHC-level association except HHC education, which was not significant in either model. In sensitivity analyses, the direction and magnitude of HHC-level variable associations with willingness to take MDR TPT was robust to exclusion of India site 1 and primary outcome reassignment of each of the 25 individual HHCs at the Thailand research site, at which all HHCs reported willingness (Supplementary Figures 3 and 4).
DISCUSSION
In this large multicountry study of HHCs of MDR-TB/RR-TB index cases residing in diverse high-TB-burden regions, willingness to take a hypothetical, newly developed MDR TPT was high (79%), along with willingness to complete prerequisite steps to determine eligibility for treatment. In the context of multiple ongoing clinical trials to identify effective MDR TPT regimens [11], our study provides evidence from diverse geographic settings for the potential high uptake of these therapies when implemented in the high-risk population of household contacts. The identification of factors associated with increased willingness to take TPT can inform counseling efforts, generate hypotheses for more contextualized local studies of KAP factors, and identify populations where implementation of MDR TPT may be particularly effective or challenging.
These findings are similar to prior observational studies and case series from limited settings that documented high levels of MDR TPT initiation among contacts. In studies identified through recent systematic reviews on MDR TPT [4, 9, 23], treatment initiation data have been reported for 8 generally small cohorts (median, 30 contacts [IQR, 22–36]) (Supplementary Table 1). For these studies, treatment initiation, defined as taking any MDR TPT for ≥2 weeks, was reported to be high overall (median, 85.6% [IQR, 71.2%–93.4%]); however, data were not available on factors associated with uptake [24–30]. Considerably more evidence exists for drug-susceptible TPT. In a recent meta-analysis of 25 cohorts, 88% of individuals were estimated to have started TPT if it was recommended; factors associated with initiation included younger age, high perceived risk of TB, and no prior treatment for TB infection [31].
We found that appropriate TB-related knowledge, being comfortable speaking with family and friends about taking MDR TPT, and, most notably, confidence in properly taking TPT were all associated with increased willingness to start treatment. The marginal association between current HHC tobacco smoking, and increased willingness to take MDR TPT suggests a possible opportunity for increased TPT uptake and tobacco cessation services among a population at higher risk of both TB infection and active disease [32]. Many of these factors have been previously identified to be associated with drug-susceptible TPT initiation or completion [31, 33, 34]. Factors included in the present study’s KAP questionnaire were primarily patient-level, social, and lifestyle [34]; however, health system factors (eg, clinic wait times, provider opinions on TPT) and therapy characteristics (eg, duration, side effects) have also been demonstrated to be important predictors of treatment initiation and completion [31, 33–36]. Observed associations may furthermore be confounded by unmeasured HHC- or household-level variables.
The decreased willingness of HHCs to take MDR TPT with mild side effects is also consistent with prior research, which has identified side effects as a primary reason for treatment discontinuation [26, 30]. Although some studies have documented high TPT completion rates among initiators [25, 27, 28], completion has been demonstrated to be one of the main gaps in the TPT cascade of care [31]. Furthermore, self-reported willingness to initiate treatment may not be a strong proxy for HHC treatment completion or even future initiation due to social desirability bias and prohibitive treatment costs as well as changes in beliefs, attitudes, or circumstances over time [37, 38]. Selection bias may also have been introduced if HHCs of eligible index cases who had initiated TB treatment differed from HHCs of ineligible index cases who had not initiated treatment. An additional limitation of this research was variable site-level sample sizes due to a constrained period of enrollment after the overall target of 300 index cases was met. As a result, the study sample was weighted toward sites starting enrollment earlier and with faster rates of recruitment (Figure 2) as well as households with greater numbers of HHCs. Across research sites, unadjusted associations for key risk factors were highly consistent; however, there was insufficient power to detect within-site differences in associations between measured HHC factors and willingness to take MDR TPT. Last, the generalizability of these findings may be limited by a lack of data from some high-burden countries as well as potential future implementation challenges, including patient-level costs.
Figure 2.
Willingness of multidrug-resistant tuberculosis (MDR-TB)/rifampicin-resistant tuberculosis (RR-TB) household contacts to take newly developed MDR-TB preventive therapy, stratified by clinical research site (left panel). Number of enrolled MDR-TB/RR-TB household contacts at each clinical research site (right panel).
In conclusion, the high percentage of HHCs of MDR-TB/RR-TB index cases willing to take hypothetical, newly developed MDR TPT provides important evidence for the potential uptake of effective TPT when implemented. Identified HHC-level variables associated with decreased willingness to take MDR TPT may inform education and counseling efforts to increase HHC confidence in and uptake of MDR TPT. Possible interventions to improve uptake include collaborations with patient advocacy groups, community sensitization workshops, and the provision of educational tools and training to TB healthcare workers. Data on caregiver willingness to provide preventive therapy to children were also collected through this study and are currently being analyzed. While this study focused on willingness to take MDR TPT at the HHC level, further research examining the site-specific context of TB-related KAP through qualitative or mixed-methods studies offers promise in guiding the rollout of MDR TPT to reduce the burden of TB in these high-risk populations.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the study participants and their families, as well as the site investigators, study teams, and protocol team of A5300/I2003.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funding agencies.
Financial support. This work was supported by the National Institute of Allergy Infectious Diseases of the National Institutes of Health with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Institute of Mental Health (NIMH), all of the US National Institutes of Health (grant numbers UM1AI068634, UM1AI068636, UM1AI106701, UM1AI068632, UM1AI068616 and UM1AI106716) and by NICHD contract number HHSN275201800001I. M. T. M. received training support from the Johns Hopkins University Medical Scientist Training Program funded by the National Institute of General Medical Sciences (5T32GM007309-43) as well as the UJMT (US collaborating universities - University of North Carolina at Chapel Hill, Johns Hopkins University, Morehouse School of Medicine and Tulane University) Fogarty Global Health Fellowship (award number D43TW009340) funded by the Fogarty International Center; the National Institute of Neurological Disorders and Stroke; the National Heart, Lung and Blood Institute; and the National Institute Environmental Health Sciences of the NIH.
Potential conflicts of interest. A. G. has received grants from the Centers for Disease Control and Prevention, Wyncote Foundation, Ujala Foundation, and Gilead. S. S. has received grants from Merck and ViiV. L. M. reports grants from Janssen Pharmaceuticals, Merck Sharp & Dohme, ViiV Healthcare, Johnson & Johnson, Pfizer Pharmaceuticals, and Bristol-Myers Squibb; and nonfinancial support from Sanofi Aventis and Kowa Pharmaceuticals America. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Participating sites
Rodney Dawson and Kim Narunsky, University of Cape Town Lung Institute (site 31792), grant 110205; Alberto Mendoza and Fanny Rosas, Barranco CRS (site 11301), grant 5UM1AI069438-10; Pedro Gonzalez and Jessica Rios, San Miguel CRS (site 11302), grant 110199; Nishi Suryavanshi, PhD, Amita Gupta, MD, MHS, Nikhil Gupte, PhD, Vidya Mave, MD, and Sushant Meshram, MD, Byramjee Jeejeebhoy Government Medical College CRS (site 31441), grant UM1AI069465; N. Kumarasamy and Easter Thamburaj, Chennai Antiviral Research and Treatment (CART) CRS (site 11701), grant UM01 A1069432; Kyla Comins and Victoire Ticha, TASK CRS (site 31718), grant UM1AI069521; Francesca Conradie and Ralinah Maepa, Wits Helen Joseph Hospital CRS (site 11101), grant AI069463; Mark Hatherill and Justin Shenje, South African Tuberculosis Vaccine Initiative (SATVI) (site 31793), grant 5UM1AI068636-10; Sandy Nerette Fontain and Stalz Charles Vilbrun, GHESKIO Centers IMIS (site 31730), grant 5UM1AI069421; Anneke Hesseling MD, Anthony Garcia-Prats, and Petra De Koker, Desmond Tutu TB Centre, Stellenbosch University (DTTC-SU) (site 31790); Aida Asmelash and Ayotunde Omozoarhe, Gaborone CRS (site 12701), grant UM1AI069456; Supalert Nedsuwan and Tim R Cressey, PHPT-Chiangrai Prachanukroh Hospital (site 5116), grant HHSN275201300003C; Lerato Mohapi and Debra Peters, Soweto ACTG CRS (site 12301), grant 5UM1AI069453; Umesh G. Lalloo and Rosie Mngqibisa, Durban International CRS, Enhancing Care Foundation (site 11201), grant 2UM1AI069432-08; Ana Cristina Garcia Ferreira and Maria Regina Rocha, Instituto Nacional de Infectologia–INI/Fiocruz (site 12101), grant 5UM1AI069476-09; Christopher Mugah and Elisha Okeyo, Kisumu CRS (site 31460), Emory-CDC HIV/AIDS Clinical Trials Unit grant UM1AI069418.
References
- 1. World Health Organization (WHO) Global tuberculosis report, 2018. WHO/HTM/TB/2017.23. Geneva, Switzerland: WHO, 2018. [Google Scholar]
- 2. Becerra MC, Appleton SC, Franke MF, et al. Tuberculosis burden in households of patients with multidrug-resistant and extensively drug-resistant tuberculosis: a retrospective cohort study. Lancet 2011; 377:147–52. [DOI] [PubMed] [Google Scholar]
- 3. Shah NS, Yuen CM, Heo M, Tolman AW, Becerra MC. Yield of contact investigations in households of patients with drug-resistant tuberculosis: systematic review and meta-analysis. Clin Infect Dis 2014; 58:381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fox GJ, Schaaf HS, Mandalakas A, Chiappini E, Zumla A, Marais BJ. Preventing the spread of multidrug-resistant tuberculosis and protecting contacts of infectious cases. Clin Microbiol Infect 2017; 23:147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ahuja SD, Ashkin D, Avendano M, et al. Collaborative Group for Meta-Analysis of Individual Patient Data in MDR-TB Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLoS Med 2012; 9:e1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nathanson E, Gupta R, Huamani P, et al. Adverse events in the treatment of multidrug-resistant tuberculosis: results from the DOTS-Plus initiative. Int J Tuberc Lung Dis 2004; 8:1382–4. [PubMed] [Google Scholar]
- 7. Akolo C, Adetifa I, Shepperd S, Volmink J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev 2010; CD000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sharma SK, Sharma A, Kadhiravan T, Tharyan P. Rifamycins (rifampicin, rifabutin and rifapentine) compared to isoniazid for preventing tuberculosis in HIV-negative people at risk of active TB. Cochrane Database Syst Rev 2013; CD007545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization (WHO) Latent tuberculosis infection: updated and consolidated guidelines for programmatic management. WHO/CDS/TB/2018.4. Geneva, Switzerland: WHO, 2018. [PubMed] [Google Scholar]
- 10. van der Werf MJ, Langendam MW, Sandgren A, Manissero D. Lack of evidence to support policy development for management of contacts of multidrug-resistant tuberculosis patients: two systematic reviews. Int J Tuberc Lung Dis 2012; 16:288–96. [DOI] [PubMed] [Google Scholar]
- 11. Moore DAJ. What can we offer to 3 million MDRTB household contacts in 2016? BMC Med 2016; 14:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Madhivanan P, Li T, Srinivas V, Marlow L, Mukherjee S, Krupp K. Human papillomavirus vaccine acceptability among parents of adolescent girls: obstacles and challenges in Mysore, India. Prev Med 2014; 64:69–74. [DOI] [PubMed] [Google Scholar]
- 13. Shu E, Sobieszczyk ME, Sal Y Rosas VG, et al. Knowledge of tuberculosis and vaccine trial preparedness in Lima, Peru. Int J Tuberc Lung Dis 2017; 21:1288–93. [DOI] [PubMed] [Google Scholar]
- 14. Chang SH, Cataldo JK. A systematic review of global cultural variations in knowledge, attitudes and health responses to tuberculosis stigma. Int J Tuberc Lung Dis 2014; 18:168–73, i–iv. [DOI] [PubMed] [Google Scholar]
- 15. Karris MY, Beekmann SE, Mehta SR, Anderson CM, Polgreen PM. Are we prepped for preexposure prophylaxis (PrEP)? Provider opinions on the real-world use of PrEP in the United States and Canada. Clin Infect Dis 2014; 58:704–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organization (WHO) Advocacy, communication and social mobilization for TB control a guide to developing knowledge, attitude and practice surveys. WHO/HTM/STB/2008.46. Geneva, Switzerland: WHO/Stop TB Partnership, 2008. [Google Scholar]
- 17. Sagili KD, Satyanarayana S, Chadha SS. Is knowledge regarding tuberculosis associated with stigmatising and discriminating attitudes of general population towards tuberculosis patients? Findings from a community based survey in 30 districts of India. PLoS One 2016; 11:e0147274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liang KY,, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986; 73:13–22. [Google Scholar]
- 19. Becker MH. The health belief model and personal health behavior. Health Educ Monogr 1974; 2:324–473. [Google Scholar]
- 20. Hosmer DW Jr, Lemeshow S, Sturdivant RX.. Applied logistic regression. Hoboken, New Jersey: John Wiley & Sons, 2013: 354–75. [Google Scholar]
- 21. Bryer J, Speerschneider K. Likert: analysis and visualization Likert items. R package version 1.3.5.2016. Available at: https://CRAN.R-project.org/package=likert. Accessed 21 January 2017.
- 22. Rabe-Hesketh S, Skrondal A.. Multilevel and longitudinal modeling using Stata. 2nd ed.College Station, TX: STATA Press, 2012:109–22. [Google Scholar]
- 23. Fraser A, Paul M, Attamna A, Leibovici L. Treatment of latent tuberculosis in persons at risk for multidrug-resistant tuberculosis: systematic review. Int J Tuberc Lung Dis 2006; 10:19–23. [PubMed] [Google Scholar]
- 24. Adler-Shohet FC, Low J, Carson M, Girma H, Singh J. Management of latent tuberculosis infection in child contacts of multidrug-resistant tuberculosis. Pediatr Infect Dis J 2014; 33:664–6. [DOI] [PubMed] [Google Scholar]
- 25. Bamrah S, Brostrom R, Dorina F, et al. Treatment for LTBI in contacts of MDR-TB patients, Federated States of Micronesia, 2009–2012. Int J Tuberc Lung Dis 2014; 18:912–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bedini A, Garlassi E, Stentarelli C, et al. Multidrug-resistant tuberculosis outbreak in an Italian prison: tolerance of pyrazinamide plus levofloxacin prophylaxis and serial interferon gamma release assays. New Microbes New Infect 2016; 12:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Denholm JT, Leslie DE, Jenkin GA, et al. Long-term follow-up of contacts exposed to multidrug-resistant tuberculosis in Victoria, Australia, 1995–2010. Int J Tuberc Lung Dis 2012; 16:1320–5. [DOI] [PubMed] [Google Scholar]
- 28. Garcia-Prats AJ, Zimri K, Mramba Z, Schaaf HS, Hesseling AC. Children exposed to multidrug-resistant tuberculosis at a home-based day care centre: a contact investigation. Int J Tuberc Lung Dis 2014; 18:1292–8. [DOI] [PubMed] [Google Scholar]
- 29. Trieu L, Proops DC, Ahuja SD. Moxifloxacin prophylaxis against MDR TB, New York, New York, USA. Emerg Infect Dis 2015; 21:500–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Younossian AB, Rochat T, Ketterer JP, Wacker J, Janssens JP. High hepatotoxicity of pyrazinamide and ethambutol for treatment of latent tuberculosis. Eur Respir J 2005; 26:462–4. [DOI] [PubMed] [Google Scholar]
- 31. Alsdurf H, Hill PC, Matteelli A, Getahun H, Menzies D. The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. Lancet Infect Dis 2016; 16:1269–78. [DOI] [PubMed] [Google Scholar]
- 32. Lin HH, Ezzati M, Murray M. Tobacco smoke, indoor air pollution and tuberculosis: a systematic review and meta-analysis. PLoS Med 2007; 4:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Talbot EA, Kenyon TA, Halabi S, Moeti TL, More K, Binkin NJ. Knowledge, attitudes and beliefs regarding tuberculosis preventive therapy for HIV-infected persons, Botswana, 1999. Int J Tuberc Lung Dis 2000; 4:1156–63. [PubMed] [Google Scholar]
- 34. Sebastian MS, Bothamley GH. Tuberculosis preventive therapy: perspective from a multi-ethnic community. Respir Med 2000; 94:648–53. [DOI] [PubMed] [Google Scholar]
- 35. Fox GJ, Dobler CC, Marais BJ, Denholm JT. Preventive therapy for latent tuberculosis infection—the promise and the challenges. Int J Infect Dis 2017; 56:68–76. [DOI] [PubMed] [Google Scholar]
- 36. Pease C, Hutton B, Yazdi F, et al. Efficacy and completion rates of rifapentine and isoniazid (3HP) compared to other treatment regimens for latent tuberculosis infection: a systematic review with network meta-analyses. BMC Infect Dis 2017; 17:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stanton BF, Clemens JD, Aziz KM, Rahman M. Twenty-four-hour recall, knowledge-attitude-practice questionnaires, and direct observations of sanitary practices: a comparative study. Bull World Health Organ 1987; 65:217–22. [PMC free article] [PubMed] [Google Scholar]
- 38. Launiala A. How much can a KAP survey tell us about people’s knowledge, attitudes and practices? Some observations from medical anthropology research on malaria in pregnancy in Malawi. Anthropol Matters 2009; 11:1–13. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.