Abstract
Perennial habit and floral scent are major traits that distinguish domesticated cowpeas from their wild relatives. However, the genetic basis of these two important traits remains largely unknown in cowpea. Plant longevity, a perenniality-related trait, and floral scent, an outcrossing trait, were investigated using a RIL population derived from a cross between a domesticated and a wild cowpea. QTL analysis revealed three significant loci, one on chromosome 8 associated with plant longevity and two, on chromosomes 1 and 11, for floral scent. Genes within the QTL regions were identified. Genes encoding an F-box protein (Vigun08g215300) and two kinases (Vigun08g217000, Vigun08g217800), and involved in physiological processes including regulation of flowering time and plant longevity, were identified within the perenniality QTL region. A cluster of O-methyltransferase genes (Vigun11g096800, Vigun11g096900, Vigun11g097000, Vigun11g097600, and Vigun11g097800) was identified within the floral scent QTL region. These O-methyltransferase cowpea genes are orthologs of the Arabidopsis N-acetylserotonin O-methyltransferase (ASMT) gene, which is involved in the biosynthesis of melatonin. Melatonin is an indole derivative, which is an essential molecule for plant interactions with pollinators. These findings lay the foundation for further exploration of the genetic mechanisms of perenniality and floral scent in cowpea. Knowledge from this study can help in the development of new extended-growth cycle lines with increased yield or lines with increased outcrossing for population breeding.
Introduction
Cowpea (Vigna unguiculata [L.] Walp.) is a warm season legume of major importance for worldwide food and nutritional security. It is an annual, diploid (2n = 22) species with a morphologically and genetically diverse gene-pool composed of cultivated forms and several wild taxa [1]. Cowpea was domesticated in Africa [2, 3], from where it spread to other continents. Although there is a lack of consensus on where in Africa cowpea domestication occurred, it is believed that V. unguiculata subsp. dekindtiana is the probable wild progenitor of cultivated cowpea [4, 5]. The main characteristics of V. unguiculata subsp. dekindtiana include perenniality, hairinesss, and small size of pods and seeds [6]. Although this wild gene-pool could be a potential source of favorable alleles for traits related to resistance to biotic and abiotic stresses (e.g., aphid resistance, Striga resistance, and drought and heat tolerance), it has remained largely unexplored by breeders for the development of improved cultivars. Increased knowledge of the genomic regions controlling domestication-related traits (DRTs) is needed to exploit cowpea wild germplasm efficiently.
Cowpea domestication involved considerable phenotypic changes from subsp. dekindtiana, including reduction of pod shattering, increased size of edible organs, loss of perenniality, and changes in flower color and scent. Compared to many other crop species, only a few studies have identified loci associated with DRTs in cowpea. Those studies made use of bi-parental populations of recombinant inbred lines (RILs) derived from a cross between a domesticated and a wild cowpea [7–9]. In particular, Andargie et al. [7] and Lo et al. [9] identified QTLs for several DRTs including ovule number, seed germination, seed coat thickness, pod shattering and days to flowering, while the study of Fatokun et al. [8] focused on seed size. However, the genetics of other important DRTs such as perenniality and flower scent remains unknown.
Developing perennial crops has been a longstanding goal in many breeding programs, especially those of cereal crops [10]. Perennial grain crops have been proposed as a strategy to address agriculture’s global challenges such as land degradation, food insecurity and climate change [10]. Perennial species have longer growing periods leading to increased photosynthate assimilation, and extensive root systems, which help decrease nitrate runoff, increase soil sequestration of carbon and decrease soil erosion [11]. Furthermore, perennial crops are reported to be more resilient to abiotic stress resulting from weather fluctuations and may require less herbicide treatment [12]. In cowpea, developing perennial varieties could be beneficial for grain yields and the improvement of soil fertility. In addition, as cowpea fodder is highly favored by livestock farmers in the dry savanna regions of sub-Saharan Africa, perennial cowpea varieties could provide a reliable source of fodder for livestock by surviving the dry season. However, to our knowledge there have been no reports on efforts to introgress perenniality from wild cowpea into elite lines.
Genes associated with perenniality-related traits have been explored mostly in Arabidopsis. Several of these studies have identified genes known to regulate flowering time and meristem determinacy such as SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 (SOC1), APETALA1(AP1), FRUITFULL (FUL) [13, 14]. Another gene, TERMINAL FLOWER 1 (TFL1), has been suggested to play an important role in perennialism by controlling the juvenile to adult transition phase and contributing to the polycarpic growth habit [15, 16]. Also, orthologs of TFL1 have been shown to be associated in polycarpic growth habit in other perennial species including ryegrass [17], apple [18], Populus sp. [19] and Arabis alpina [20]. In legumes, TFL1 orthologs including Dt1 in soybean [21], PvTFL1y in common bean [22], CcTFL1 in pigeonpea [23] have been reported to control determinate growth habit.
Another domestication syndrome trait in cowpea is floral scent, which facilitates plant-pollinator interaction. Pollination has been reported to contribute to more than one third of crop yield [24] and could assist in population breeding and the development of F1 hybrid varieties. In addition, as cowpea varieties carrying a transgenic resistance gene (Bt) derived from Bacillus thuringiensis are being evaluated in some African countries, understanding the genetics of floral scent would help inform insect pollination behavior and flight distances [25] and environmental biosafety policy and regulation related to transgene scape.
Floral scent-related genes have been characterized from several model plants. Those mostly encode enzymes including Iso-eugenol O-methyltransferase, benzylalcohol acyltransferase, orcinol O-methyltransferase and phloroglucinol O-methyltransferase [26–29]. In cowpea, a previous study identified loci associated with floral scent compounds [30]. The authors identified QTLs influencing 23 volatile compounds including nitrogen compounds. Here we studied the genetic differences in scent production between wild and domesticated cowpea.
In the present study, we identified loci associated with perenniality and floral scent using a wild by cultivated RIL population [9], a high-density SNP genotyping array [31], a reference genome sequence [32] and new phenotypic data. Knowledge from this study can guide the development of cowpea perennial lines leading to an increase of cowpea grain and fodder productivities.
Materials and methods
Plant material and phenotypic data collection
A biparental mapping population of 170 recombinant inbred lines (RILs) derived from a cross between a wild and a domesticated cowpea accession and developed at the International Institute of Tropical Agriculture (IITA) was used to evaluate perenniality and floral scent. The development of this population is described in Lo et al. [9]. Briefly, the wild parent (TVNu-1158) is a small-seeded and aphid-resistant accession with a perennial growth habit and scented flowers. The cultivated parent (IT99K-573-1-1) is an early-maturing, white-seeded, high-yielding and Striga resistant variety with an annual life cycle and non-scented flowers. The parents and the RILs were sown on February 20th, 2017 in pots filled with 5.0 kg topsoil and placed in a screen house at IITA, Ibadan, Nigeria (latitude 7°30′N and longitude 3°54′E, elevation 240 masl). Five to eight seeds of each RIL were sown per pot. When seedlings were well established (as rate of growth initially varied between seedlings of the RILs) the number of plants was reduced to three per pot. The plants were thereafter allowed to grow until death was recorded on the last of the three plants. Perenniality was scored as the number of days from planting to when the plant died. Floral scent was scored as a qualitative trait by human olfaction on five newly opened flowers as “scented” (score = 1) or “non-scented” (score = 0).
QTL analysis and identification of candidate genes
The RIL population was genotyped with the Cowpea iSelect Consortium Array, which includes 51,128 SNPs [31] (Muñoz‐Amatriaín et al., 2017). SNPs were called using the GenomeStudio software V.2011.1 (Illumina, Inc., San Diego, CA, USA). Data curation was performed by removing SNPs with more than 20% missing and/or heterozygous calls and minor allele frequencies <0.25. MSTmap [33] (http://www.mstmap.org/) was used for constructing the genetic map, which consisted of 17,739 SNPs mapped to 1,825 unique positions [9]. The chromosomes were numbered and oriented according to the cowpea pseudomolecules [32].
QTL mapping was performed using a linear mixed model (y = Xβ+Zkak+Wkdk+ξ+ϵ) [34] implemented in R as described in Lo et al. [9]. A genome-wide critical value was calculated with a modified Bonferroni correction and used as the threshold for the detection of significant QTL. The modified Bonferroni used the effective degrees of freedom of the trait as the denominator. The effective degrees of freedom was defined as , where Wk is the Wald test statistic for SNP k. The trait specific Bonferroni corrected critical value was -log10 (0.05/m0). A SNP was declared as significant if its -log10 (P-value) was larger than -log10 (0.05/m0) [9]. The proportion of phenotypic variance contributed by each significant SNP was calculated using the following formula: , where X is a variable holding the genotype code (+1,-1) for SNP k, var(X) is the variance of variable X, a is the estimated effect for SNP k and var(y) is the total phenotypic variance of the trait under study. The effect of each marker was estimated as a fixed effect and tested using the Wald test statistic (squared effect divided by the variance of the estimated effect).
The physical region of the QTL was determined by positioning flanking SNPs in the reference genome sequence [32] and annotated gene models underlying the QTL region were identified.
Results and discussion
Phenotypic variation in the population
DRTs “perenniality” and “flower scent” were evaluated in this study. Phenotypic values obtained from the parents and the 170 lines in the population are reported in S1 Table. Perenniality (plant longevity) was scored as the number of days from planting to when the plant died. IT99K-573-1-1 has an annual life cycle and died 123 days after planting, while TVNu-1158 died 700 days after planting (Table 1). In the RIL population, the number of survived days ranged from 94 to >774 days (Table 1), and mean and standard deviation were calculated. Transgressive segregation was observed suggesting that more than one locus could contribute to plant longevity. The frequency distribution of plant longevity did not fit a normal distribution as it was skewed positively towards a longer life span (S1 Fig).
Table 1. Phenotypic values of the parental lines and the RIL population.
NA: not applicable.
| Trait | IT99K-573-1-1 | TVNu-1158 | RIL population | |
|---|---|---|---|---|
| Mean +/- SE | Range | |||
| Plant longevity (#days) | 123 | 700 | 325 +/- 142 | 94 - >774 |
| Floral scent | non-scented | scented | NA | NA |
Floral scent was scored qualitatively by human olfaction as scented and non-scented flowers. The domesticated parent IT99K-573-1-1 had non-scented flowers while TVNu-1158 had scented flowers. In the population, 51 RILs had scented flowers and 119 lines had non-scented flowers (Table 1). A chi square goodness-of-fit test suggests that the segregation pattern of floral scent in the RIL population did not fit a Mendelian 1:1 ratio (P-value = 1.83E-07).
QTLs for perenniality on chromosome 8 and floral scent on chromosomes 1 and 11
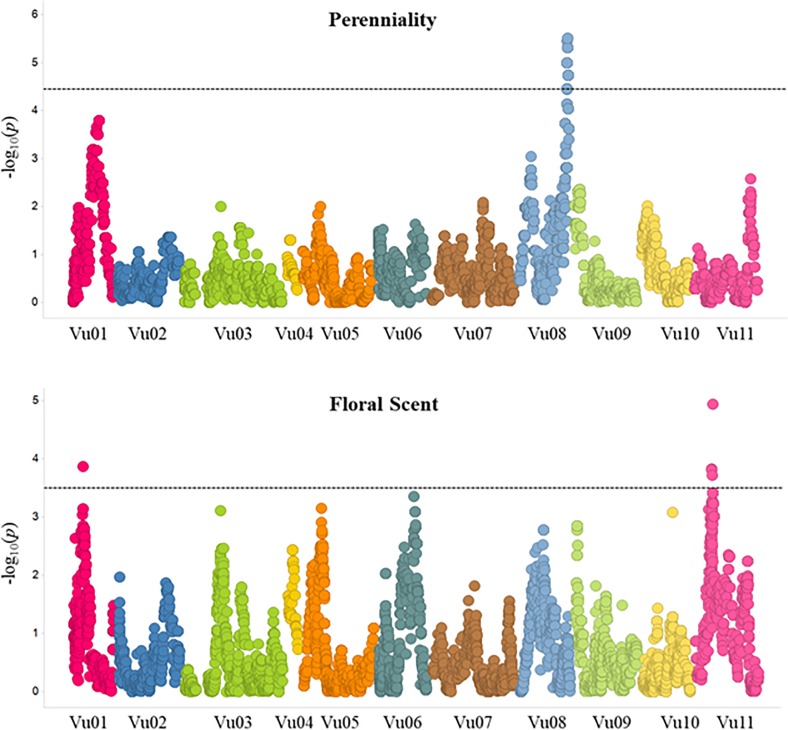
Using a mixed linear model for QTL analysis developed by [34], one QTL for perenniality was identified on chromosome 8 (Vu08) and 2 QTL were identified for floral scent on chromosomes 1 (Vu01) and 11 (Vu11) (Fig 1; Table 2). The effect of each QTL was determined and reported in Table 2. The effect of these QTLs might be artificially increase due to small population size.
Fig 1. QTL plots for perenniality and floral scent.
The horizontal axis indicates the chromosomes, the vertical axis indicates the -log10 of the probability (P-values). The dashed line indicates the significance threshold.
Table 2. QTL for perenniality and flower scent identified by the linear mixed model analysis.
| Trait | Peak SNP | Chr. | Position (cM) | Position (bp) | -Log10(P) | QTL region (cM) | % Phenotypic variation | Effect |
|---|---|---|---|---|---|---|---|---|
| Perenniality (Plant longevity) | 2_14129 | 8 | 79.58 | 37725907 | 5.51 | 78.87–80.67 | 26.45 | 25.16 |
| Flower scent | 2_51494 | 1 | 14.74 | 27704236 | 3.87 | 14.74–15.03 | 9.5 | -0.15 |
| Flower scent | 2_00068 | 11 | 27.4 | 27125507 | 4.94 | 26.81–28 | 13.75 | -0.18 |
The QTL for perenniality explained 26% of the phenotypic variance (Table 2), spanned 527 kb (37,388,250 to 37,914,805 bp) on Vu08 and contained 79 gene models [32] (S2 Table). QTLs associated with perenniality-related traits have been identified mostly in cereal crops including maize [35], wheat [36] and rice [37]. In mungbean (Vigna radiata) the inheritance of perenniality through the presence or absence of tuberous roots was studied by Nguyen et al. [38]. Authors proposed that the formation of tuberous root (a perenniality-related trait) might be conditioned by two complementary dominant loci. However, these cited studies were interested in perennialism based on traits such as regrowth and formation of a rhizome, while the present study focuses on plant longevity. Our results reinforce the view that plant longevity is a component of perennialism [39]. The plant longevity locus could assist breeding programs in the development of perennial cowpea. In cereal crops, loci associated with perenniality-related traits have been used to confer perennial habit on annual elite lines. For example, Cox et al. [40] used loci associated with rhizome production to develop perennial lines derived from crosses between Sorghum bicolor and either S. halepense or S. propinquum with the goal of improving grain yields and seed weights and preventing and reversing soil degradation in the sorghum growing region.
Floral scent is an important trait to attract pollinators and could also be involved in repelling pests [41]. In the present study two QTLs associated with the emission of scented flowers were detected, one each on Vu01 and Vu11 (Fig 1). The QTL on Vu01 explained 9.5% of the variation of the trait while the QTL on Vu11 accounted for ~14% (Table 2). The significant region on Vu01 spanned 196 kb (27,704,236–27,900,691 bp) and contained 14 annotated genes, while the QTL on Vu11 spanned 6 Mb (23,241,654–29,117,703 bp) and contained 201 gene models [32] (S2 Table). A previous study in cowpea reported a total of 63 QTLs influencing 23 floral scent compounds [30]. Because of the unavailability of marker sequences used in that study, the possible overlap between the loci identified here and those from the Andargie et al. [30] could not be determined. In addition to mapping two main QTLs, the present study identified noteworthy candidate genes associated with floral scent. For the QTL on Vu11, the peak SNPs are 800 kb away from a cluster of O-methyltransferase family protein genes, which are involved in the biosynthetic process of aromatic compounds in plants [42].
Identification of candidate genes
A total of 79 genes were identified within the QTL region for perenniality. Among these are a gene encoding histidine kinase (Vigun08g217000), a protein kinase superfamily protein (Vigun08g217800), and an F-box family protein gene (Vigun08g215300). Genes encoding two kinases and an F-box protein have been reported as candidates that potentially impact the life history switch from perennial to annual of different Arabidopsis species [43]. Furthermore, those authors suggested that the F-box gene is the best candidate for future functional studies based on the dN (nonsynonymous substitutions per nonsynonymous site) to dS (synonymous substitutions per synonymous site) ratio. In addition, F-box genes have been known to influence a variety of biological processes essential for plant growth and development [44]. The Arabidopsis ortholog of Vigun08g215300 is AT5G48170 (SNEEZY), which is a regulator of gibberellin (GA) signaling [45, 46]. SNEEZY mutations cause phenotypes resulting from reduced GA response including delayed flowering. Moreover, Ariizumi and Steber [46] reported that SNEEZY overexpression has an impact in apical dominance and growth habit. The other perenniality candidate is Vigun08g217000 encoding a histidine kinase. Vigun08g21700000 is located in the QTL peak region and its Arabidopsis ortholog AHK2 has been shown to regulate plant longevity [47]. Gain-of-function mutation of AHK2 increased plant longevity and prolonged the reproductive growth phase in Arabidopsis [47]. Interestingly, this gene has been identified as a candidate for other domestication-related traits in cowpea including seed size [32, 48]. The last candidate gene, Vigun08g217800, encodes a kinase superfamily protein and is an ortholog of the Arabidopsis gene AT5G55560, which is a serine/threonine-protein kinase member of the WNK gene family. Genes belonging to the WNK family are involved in various physiological processes including regulation of flowering time by modulating the photoperiod pathway [49, 50]. Further studies such as fine mapping, mutant analysis, and gene expression will be required to explore the role these candidate genes in the perennial to annual switch in cowpea.
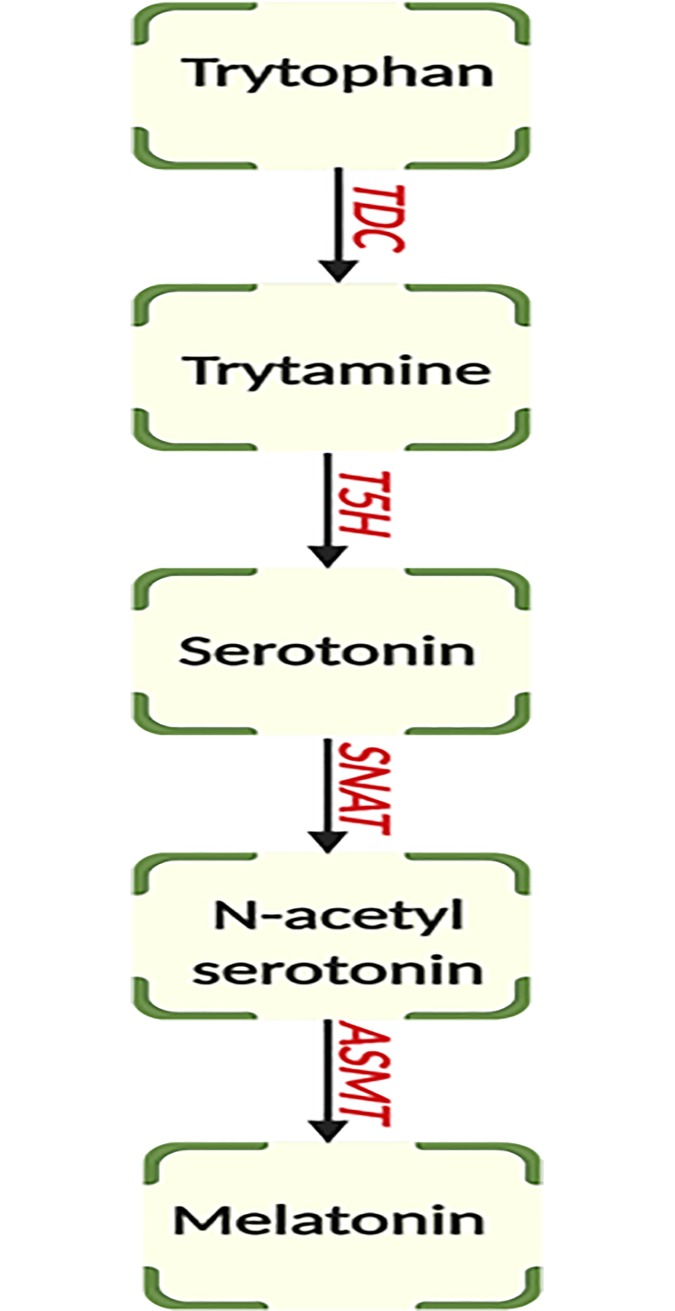
No obvious candidate genes were identified for the minor floral scent QTL on Vu01. However, O-methyltransferase family protein genes were found within the Vu11 QTL. Expression data from Yao et al. [51] available at legumeinfo.org showed that five of these genes (Vigun11g096800, Vigun11g096900, Vigun11g097000, Vigun11g097600 and Vigun11g097800) were expressed in flower tissues (S2 Fig). These genes are orthologs of the Arabidopsis gene AT4G35160 (ASMT), which encodes a cytosolic N-acetylserotonin O-methyltransferase. ASMT, together with other genes such as tryptophan decarboxylase (TDC), tryptamine 5-hydroxylase (T5H) and serotonin N-acyltransferase (SNAT), have been implicated in the process of melatonin synthesis from tryptophan [52–54]. Fig 2 shows a diagram of one of the four pathways proposed in the biosynthesis of melatonin with ASMT responsible for the transformation of N-acetylserotonin to melatonin. Melatonin (N-acetyl-5 methoxytryptamine) is an indole derivative (with an indole nucleus). Indole is one of the major metabolites of scent [55]. In addition, indole is one of the several volatiles that attract pollinators and contribute to defense [56, 57]. Indole is one of the floral scent compounds identified in cowpea [30], which was also associated with two QTLs in that study. Thus, these O-methyltransferase genes are promising candidates to study the genetic mechanism of floral scent in cowpea.
Fig 2. Schematic view of melatonin biosynthesis pathway.
TDC: tryptophan decarboxylase, T5H: tryptamine 5-hydroxylase, SNAT: serotonin N-acetyltransferase, ASMT: N-acetylserotinin methyltransferase.
This study provides a step towards understanding the genetic architecture of perenniality and floral scent in cowpea. Deciphering the genetics of these two DRTs would help efforts to perennialize domesticated cowpea, domesticate new crops from wild cowpea and develop new varieties with the aim of increasing yield and other quality traits.
Supporting information
(TIF)
TPM: Transcripts Per Million; dap: days after pollination. Data from Yao et al (2016) and available at legumeinfo.org.
(TIF)
(XLSX)
(XLSX)
Acknowledgments
The authors thank Stefano Lonardi (University of California Riverside, USA) for assistance with the cowpea genome sequence and annotations Ira Herniter and Yi-Ning Guo (University of California Riverside, USA) for technical assistance.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from the Feed the Future Innovation Lab for Climate Resilient Cowpea (Cooperative Agreement AID-OAA-A-13-00070) T.C., the National Science Foundation BREAD project “Advancing the Cowpea Genome for Food Security” (NSF IOS-1543963) T.C, M.MA and Hatch Project CA-R-BPS-5306-H, T.C. The Bill and Melinda Gates Foundation is acknowledged for funds used to implement the activities carried out at IITA under Tropical Legumes Project, C.F.; O.B.
References
- 1.Pasquet R. Wild cowpea (Vigna unguiculata) evolution. Advances in legume systematics. 1996;8:95–100. [Google Scholar]
- 2.D'Andrea AC, Kahlheber S, Logan AL, Watson DJ. Early domesticated cowpea (Vigna unguiculata) from Central Ghana. Antiquity. 2007;81(313):686–98. [Google Scholar]
- 3.Faris D. The origin and evolution of the cultivated forms of Vigna sinensis. Canadian journal of genetics and cytology. 1965;7(3):433–52. [Google Scholar]
- 4.Lush W, Evans L. The domestication and improvement of cowpeas (Vigna unguiculata (L.) Walp.). Euphytica. 1981;30(3):579–87. [Google Scholar]
- 5.Ng N, Marechal R. Cowpea taxonomy, origin and germplasm Cowpea research, production, and utilization Wiley, Chichester, UK: 1985:11–21. [Google Scholar]
- 6.Padulosi S, Ng N. Origin, taxonomy, and morphology of Vigna unguiculata (L.) Walp. Advances in cowpea research. 1997:1–12. [Google Scholar]
- 7.Andargie M, Pasquet RS, Gowda BS, Muluvi GM, Timko MP. Molecular mapping of QTLs for domestication-related traits in cowpea (V. unguiculata (L.) Walp.). Euphytica. 2014;200(3):401–12. [Google Scholar]
- 8.Fatokun CA, Menancio-Hautea DI, Danesh D, Young ND. Evidence for orthologous seed weight genes in cowpea and mung bean based on RFLP mapping. Genetics. 1992;132(3):841–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lo S, Muñoz-Amatriaín M, Boukar O, Herniter I, Cisse N, Guo Y-N, et al. Identification of QTL controlling domestication-related traits in cowpea (Vigna unguiculata L. Walp). Scientific reports. 2018;8(1):6261 10.1038/s41598-018-24349-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glover JD, Reganold JP, Bell LW, Borevitz J, Brummer EC, Buckler ES, et al. Increased Food and Ecosystem Security via Perennial Grains. Science. 2010;328(5986):1638–9. 10.1126/science.1188761 [DOI] [PubMed] [Google Scholar]
- 11.Kell DB. Breeding crop plants with deep roots: their role in sustainable carbon, nutrient and water sequestration. Annals of Botany. 2011;108(3):407–18. 10.1093/aob/mcr175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox TS, Glover JD, Van Tassel DL, Cox CM, DeHaan LR. Prospects for developing perennial grain crops. American Institute of Biological Sciences; 2006. [Google Scholar]
- 13.Melzer S, Lens F, Gennen J, Vanneste S, Rohde A, Beeckman T. Flowering-time genes modulate meristem determinacy and growth form in Arabidopsis thaliana. Nature Genetics. 2008;40(12):1489–92. 10.1038/ng.253 [DOI] [PubMed] [Google Scholar]
- 14.Sablowski R. Flowering and determinacy in Arabidopsis. Journal of Experimental Botany. 2007;58(5):899–907. 10.1093/jxb/erm002 [DOI] [PubMed] [Google Scholar]
- 15.Liljegren SJ, Gustafson-Brown C, Pinyopich A, Ditta GS, Yanofsky MF. Interactions among APETALA1, LEAFY, and TERMINAL FLOWER1 specify meristem fate. The Plant Cell. 1999;11(6):1007–18. 10.1105/tpc.11.6.1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ratcliffe OJ, Bradley DJ, Coen ES. Separation of shoot and floral identity in Arabidopsis. Development. 1999;126(6):1109–20. [DOI] [PubMed] [Google Scholar]
- 17.Jensen CS, Salchert K, Nielsen KK. A TERMINAL FLOWER1-like gene from perennial ryegrass involved in floral transition and axillary meristem identity. Plant Physiology. 2001;125(3):1517–28. 10.1104/pp.125.3.1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotoda N, Iwanami H, Takahashi S, Abe K. Antisense expression of MdTFL1, a TFL1-like gene, reduces the juvenile phase in apple. Journal of the American Society for Horticultural Science. 2006;131(1):74–81. [Google Scholar]
- 19.Mohamed R, Wang CT, Ma C, Shevchenko O, Dye SJ, Puzey JR, et al. Populus CEN/TFL1 regulates first onset of flowering, axillary meristem identity and dormancy release in Populus. The Plant Journal. 2010;62(4):674–88. 10.1111/j.1365-313X.2010.04185.x [DOI] [PubMed] [Google Scholar]
- 20.Wang R, Albani MC, Vincent C, Bergonzi S, Luan M, Bai Y, et al. Aa TFL1 confers an age-dependent response to vernalization in perennial Arabis alpina. The Plant cell. 2011;23(4):1307–21. 10.1105/tpc.111.083451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu B, Watanabe S, Uchiyama T, Kong F, Kanazawa A, Xia Z, et al. The soybean stem growth habit gene Dt1 is an ortholog of Arabidopsis TERMINAL FLOWER1. Plant Physiology. 2010;153(1):198–210. 10.1104/pp.109.150607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Repinski SL, Kwak M, Gepts P. The common bean growth habit gene PvTFL1y is a functional homolog of Arabidopsis TFL1. Theoretical and Applied Genetics. 2012;124(8):1539–47. 10.1007/s00122-012-1808-8 [DOI] [PubMed] [Google Scholar]
- 23.Mir RR, Kudapa H, Srikanth S, Saxena RK, Sharma A, Azam S, et al. Candidate gene analysis for determinacy in pigeonpea (Cajanus spp.). TAG Theoretical and applied genetics Theoretische und angewandte Genetik. 2014;127(12):2663–78. 10.1007/s00122-014-2406-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein A-M, Vaissiere BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, et al. Importance of pollinators in changing landscapes for world crops. Proceedings of the royal society B: biological sciences. 2006;274(1608):303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasquet RS, Peltier A, Hufford MB, Oudin E, Saulnier J, Paul L, et al. Long-distance pollen flow assessment through evaluation of pollinator foraging range suggests transgene escape distances. Proceedings of the National Academy of Sciences. 2008;105(36):13456–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barkman TJ. Evidence for Positive Selection on the Floral Scent Gene Isoeugenol-O-methyltransferase. Molecular Biology and Evolution. 2003;20(2):168–72. 10.1093/molbev/msg030 [DOI] [PubMed] [Google Scholar]
- 27.Beekwilder J, Alvarez-Huerta M, Neef E, Verstappen FW, Bouwmeester HJ, Aharoni A. Functional characterization of enzymes forming volatile esters from strawberry and banana. Am Soc Plant Biol; 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu S, Watanabe N, Mita S, Dohra H, Ueda Y, Shibuya M, et al. The key role of phloroglucinol O-methyltransferase in the biosynthesis of Rosa chinensis volatile 1,3,5-trimethoxybenzene. Plant physiology. 2004;135(1):95–102. 10.1104/pp.103.037051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu S, Watanabe N, Mita S, Ueda Y, Shibuya M, Ebizuka Y. Two O-methyltransferases isolated from flower petals of Rosa chinensis var. spontanea involved in scent biosynthesis. Journal of bioscience and bioengineering. 2003;96(2):119–28. [PubMed] [Google Scholar]
- 30.Andargie M, Knudsen JT, Pasquet RS, Gowda BS, Muluvi GM, Timko MP. Mapping of quantitative trait loci for floral scent compounds in cowpea (Vigna unguiculata L.). Plant Breeding. 2014;133(1):92–100. [Google Scholar]
- 31.Muñoz‐Amatriaín M, Mirebrahim H, Xu P, Wanamaker SI, Luo M, Alhakami H, et al. Genome resources for climate‐resilient cowpea, an essential crop for food security. The Plant Journal. 2017;89(5):1042–54. 10.1111/tpj.13404 [DOI] [PubMed] [Google Scholar]
- 32.Lonardi S, Muñoz-Amatriaín M, Liang Q, Shu S, Wanamaker SI, Lo S, et al. The genome of cowpea (Vigna unguiculata [L.] Walp.). The Plant Journal. 2019;98(5):767–82. 10.1111/tpj.14349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Y, Bhat PR, Close TJ, Lonardi S. Efficient and accurate construction of genetic linkage maps from the minimum spanning tree of a graph. PLoS Genet. 2008;4(10):e1000212 10.1371/journal.pgen.1000212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu S. Mapping quantitative trait loci by controlling polygenic background effects. Genetics. 2013;195(4):1209–22. 10.1534/genetics.113.157032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Westerbergh A, Doebley J. Quantitative trait loci controlling phenotypes related to the perennial versus annual habit in wild relatives of maize. Theoretical and Applied Genetics. 2004;109(7):1544–53. 10.1007/s00122-004-1778-6 [DOI] [PubMed] [Google Scholar]
- 36.Lammer D, Cai X, Arterburn M, Chatelain J, Murray T, Jones S. A single chromosome addition from Thinopyrum elongatum confers a polycarpic, perennial habit to annual wheat. Journal of Experimental Botany. 2004;55(403):1715–20. 10.1093/jxb/erh209 [DOI] [PubMed] [Google Scholar]
- 37.Hu F, Tao D, Sacks E, Fu B, Xu P, Li J, et al. Convergent evolution of perenniality in rice and sorghum. Proceedings of the National Academy of Sciences. 2003;100(7):4050–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen TD, Lawn R, Bielig L. Expression and inheritance of perenniality and other qualitative traits in hybrids between mungbean cultivars and Australian wild accessions. Crop and Pasture Science. 2012;63(7):619–34. [Google Scholar]
- 39.Thomas H. Ageing in plants. Mechanisms of ageing and development. 2002;123(7):747–53. 10.1016/s0047-6374(01)00420-1 [DOI] [PubMed] [Google Scholar]
- 40.Cox S, Nabukalu P, Paterson A, Kong W, Nakasagga S. Development of perennial grain sorghum. Sustainability. 2018;10(1):172. [Google Scholar]
- 41.Schiestl FP. The evolution of floral scent and insect chemical communication. Ecology Letters. 2010;13(5):643–56. 10.1111/j.1461-0248.2010.01451.x [DOI] [PubMed] [Google Scholar]
- 42.Lavid N, Wang J, Shalit M, Guterman I, Bar E, Beuerle T, et al. O-methyltransferases involved in the biosynthesis of volatile phenolic derivatives in rose petals. Plant physiology. 2002;129(4):1899–907. 10.1104/pp.005330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heidel AJ, Kiefer C, Coupland G, Rose LE. Pinpointing genes underlying annual/perennial transitions with comparative genomics. BMC genomics. 2016;17(1):921 10.1186/s12864-016-3274-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang X, Kalluri UC, Jawdy S, Gunter LE, Yin T, Tschaplinski TJ, et al. The F-Box Gene Family Is Expanded in Herbaceous Annual Plants Relative to Woody Perennial Plants. Plant Physiology. 2008;148(3):1189–200. 10.1104/pp.108.121921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ariizumi T, Lawrence PK, Steber CM. The role of two f-box proteins, SLEEPY1 and SNEEZY, in Arabidopsis gibberellin signaling. Plant physiology. 2011;155(2):765–75. 10.1104/pp.110.166272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ariizumi T, Steber CM. Mutations in the F-box gene SNEEZY result in decreased Arabidopsis GA signaling. Plant Signaling & Behavior. 2011;6(6):831–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bartrina I, Jensen H, Novak O, Strnad M, Werner T, Schmülling T. Gain-of-function mutants of the cytokinin receptors AHK2 and AHK3 regulate plant organ size, flowering time and plant longevity. Plant physiology. 2017:pp. 01903.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lo S, Muñoz-Amatriaín M, Hokin SA, Cisse N, Roberts PA, Farmer AD, et al. A genome-wide association and meta-analysis reveal regions associated with seed size in cowpea [Vigna unguiculata (L.) Walp]. Theoretical and Applied Genetics. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kahle KT, Rinehart J, Ring A, Gimenez I, Gamba G, Hebert SC, et al. WNK protein kinases modulate cellular Cl− flux by altering the phosphorylation state of the Na-K-Cl and K-Cl cotransporters. Physiology. 2006;21(5):326–35. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Liu K, Liao H, Zhuang C, Ma H, Yan X. The plant WNK gene family and regulation of flowering time in Arabidopsis. Plant Biology. 2008;10(5):548–62. 10.1111/j.1438-8677.2008.00072.x [DOI] [PubMed] [Google Scholar]
- 51.Yao S, Jiang C, Huang Z, Torres‐Jerez I, Chang J, Zhang H, et al. The Vigna unguiculata gene expression atlas (VuGEA) from de novo assembly and quantification of RNA‐seq data provides insights into seed maturation mechanisms. The Plant Journal. 2016;88(2):318–27. 10.1111/tpj.13279 [DOI] [PubMed] [Google Scholar]
- 52.Back K, Tan D-X, Reiter RJ. Melatonin biosynthesis in plants: multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. Journal of Pineal Research. 2016;61(4):426–37. 10.1111/jpi.12364 [DOI] [PubMed] [Google Scholar]
- 53.Byeon Y, Lee H-J, Lee HY, Back K. Cloning and functional characterization of the Arabidopsis N-acetylserotonin O-methyltransferase responsible for melatonin synthesis. Journal of Pineal Research. 2016;60(1):65–73. 10.1111/jpi.12289 [DOI] [PubMed] [Google Scholar]
- 54.Lee HY, Byeon Y, Lee K, Lee H-J, Back K. Cloning of Arabidopsis serotonin N-acetyltransferase and its role with caffeic acid O-methyltransferase in the biosynthesis of melatonin in vitro despite their different subcellular localizations. Journal of Pineal Research. 2014;57(4):418–26. 10.1111/jpi.12181 [DOI] [PubMed] [Google Scholar]
- 55.Berstad A, Raa J, Valeur J. Indole—the scent of a healthy 'inner soil'. Microb Ecol Health Dis. 2015;26:27997–. 10.3402/mehd.v26.27997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dötterl S, Füssel U, Jürgens A, Aas G. 1,4-Dimethoxybenzene, a Floral Scent Compound in Willows that Attracts an Oligolectic Bee. Journal of Chemical Ecology. 2005;31(12):2993–8. 10.1007/s10886-005-9152-y [DOI] [PubMed] [Google Scholar]
- 57.Pichersky E, Gershenzon J. The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Current opinion in plant biology. 2002;5(3):237–43. 10.1016/s1369-5266(02)00251-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
TPM: Transcripts Per Million; dap: days after pollination. Data from Yao et al (2016) and available at legumeinfo.org.
(TIF)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.