Abstract
Background
Elevated parathyroid hormone (PTH) levels in secondary hyperparathyroidism (SHPT) lead to vascular calcification, which is associated with cardiovascular events and mortality. Increased PTH production is caused by the excessive proliferation of parathyroid gland cells, which is accelerated by abnormal mineral homeostasis. Evocalcet, an oral calcimimetic agent, inhibits the secretion of PTH from parathyroid gland cells and has been used for the management of SHPT in dialysis patients. We observed the effects of evocalcet on ectopic calcification and parathyroid hyperplasia using chronic kidney disease (CKD) rats with SHPT.
Methods
CKD rats with SHPT induced by adenine received evocalcet orally for 5 weeks. The calcium and inorganic phosphorus content in the aorta, heart and kidney was measured. Ectopic calcified tissues were also assessed histologically. To observe the effects on the proliferation of parathyroid gland cells, parathyroid glands were histologically assessed in CKD rats with SHPT induced by 5/6 nephrectomy (Nx) after receiving evocalcet orally for 4 weeks.
Results
Evocalcet prevented the increase in calcium and inorganic phosphorus content in the ectopic tissues and suppressed calcification of the aorta, heart and kidney in CKD rats with SHPT by reducing the serum PTH and calcium levels. Evocalcet suppressed the parathyroid gland cell proliferation and reduced the sizes of parathyroid cells in CKD rats with SHPT.
Conclusions
These findings suggest that evocalcet would prevent ectopic calcification and suppress parathyroid hyperplasia in patients with SHPT.
Introduction
Secondary hyperparathyroidism (SHPT), which is characterized by serum parathyroid hormone (PTH) elevation, is a common mineral metabolism abnormality in patients with chronic kidney disease (CKD). Excessive PTH secretion disturbs the calcium (Ca) and phosphate metabolism, which is considered to be the main cause of ectopic calcification [1]. Accelerated coronary artery calcification is often found in dialysis patients with SHPT and its presence is associated with increased cardiovascular events and mortality [2]. Since there is no treatment that can efficiently reverse vascular calcification, its prevention is important in improving prognosis of patients with SHPT.
Parathyroid hyperplasia is also a characteristic feature of SHPT and its progression leads to hypersecretion of PTH from the parathyroid glands and this in turn leads to altered mineral metabolism [3]. Hence, preventing the progression of parathyroid cell growth and gland enlargement is important to suppress the elevation of serum PTH and Ca.
Cinacalcet is the first approved calcimimetic agent in 2004, allosterically modulates the calcium receptor (CaR) on parathyroid gland cells and suppresses PTH secretion [4, 5]. Cinacalcet has been widely used clinically for more than 10 years in the world for management of SHPT in dialysis patients [6–11] and succeeded to reduce the number of parathyroidectomies [12]. It has been reported that the progression of cardiovascular calcification was attenuated by combination therapy with cinacalcet and low-dose vitamin D receptor activator (VDRA) in comparison to monotherapy with higher doses of VDRA in SHPT patients [13]. It has also been suggested that cinacalcet contributes to reducing the size of the parathyroid glands in SHPT patients [14–16].
Although cinacalcet has excellent PTH lowering effect, it has problems of causing upper gastro-intestinal (GI) side effects such as nausea and vomiting at a certain rate [17]. These problems sometimes become an obstacle to the long-term use of cinacalcet or treatment with increased doses of cinacalcet [18, 19]. Evocalcet has been developed to address these issues. Although evocalcet suppresses PTH secretion with a similar pharmacological profile to cinacalcet, it has lesser effect on the GI tract than cinacalcet [20]. Nevertheless, the effects of evocalcet on ectopic calcification and parathyroid hyperplasia need to be evaluated further.
In this study, we assessed the effects of evocalcet on vascular and tissue calcification using CKD rats with SHPT induced by adenine feeding. We also measured the effects of evocalcet on parathyroid gland cell proliferation and cell size in CKD rats with SHPT induced by 5/6 nephrectomy (Nx).
Materials and methods
Evocalcet
Evocalcet was synthesized at Mitsubishi Tanabe Pharma Corporation (Lot No. 134016 for the single administration study, Lot No. 45AP80110001 for the long term administration studies, Osaka, Japan).
Animals
Male Sprague Dawley rats (13 weeks of age) were purchased from Japan SLC Inc. (Shizuoka, Japan) for the study using CKD rats with SHPT induced by adenine. Male Sprague Dawley rats (6 weeks of age) were purchased from Charles River Laboratories Japan, Inc. (Kanagawa, Japan), for the study using CKD rats with SHPT induced by 5/6 Nx.
The rats were kept at 20–26°C and 30–70% humidity under a 12-hour light-dark cycle with ad libitum access to tap water and commercial chow (for the study using CKD rats with SHPT induced by adenine: CE-2, CLEA Japan, Inc., Shizuoka, Japan. For the study using CKD rats with SHPT induced by 5/6 Nx: FR-2, Funabashi Farm Co., Ltd., Chiba, Japan. Both diets are normal diets) prior to the experiments. All experimental animals were monitored at least once per working day throughout the course of the study. Humane endpoints were defined as reduced physical activity level, weight loss, hunched posture, and other signs of distress. All rats reaching humane endpoints or in the single administration study were euthanized by carbon dioxide inhalation after the completion of studies. Euthanasia by carbon dioxide inhalation was conducted in the home cage. An optimal flow rate is 20% replacement of the home cage volume/min. We observed the respiratory and cardiac arrest in rats, and maintained CO2 flow for at least 3 minutes after respiratory and cardiac arrest. After both signs were observed, rats were removed from the cage. The rats in the long term studies were euthanized by exsanguination via the abdominal aorta/vena cava under isoflurane anaesthesia. All animal studies were carried out in strict accordance with the Standards for Proper Conduct of Animal Experiments at Kyowa Kirin Co., Ltd. The protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Kyowa Kirin Co., Ltd. (protocol number APS 18J0188 for the single administration study, 17J0078 for the five-week administration study using CKD rats with SHPT induced by adenine, 14J0052 for the four-week administration study using CKD rats with SHPT induced by 5/6 Nx), and all efforts were made to minimize patient distress and suffering.
CKD rats with SHPT induced by adenine
Single administration study
To establish CKD rats with SHPT induced by adenine, eighteen rats were fed with a CE-2 diet containing 0.75% adenine and 2.5% protein (adenine diet; CLEA, Japan, Inc., Shizuoka, Japan). Six rats in the control group were fed with a CE-2 diet containing 25% protein (control diet). After three weeks of the adenine-diet feeding, rats were randomly divided into three groups matched for body weight as well as blood urea nitrogen (BUN) and serum creatinine. The adenine diet was then changed to a normal diet and vehicle (0.5% methyl cellulose solution) or evocalcet (0.03 or 0.3 mg/kg) was orally administered. Blood samples were obtained from the tail vein before and 2, 4, 8, and 24 hours after the administration.
Five-week administration study
CKD rats with SHPT induced by adenine by the methods described above, were used. After adenine-diet feeding, sixteen rats were randomly divided into two groups. The adenine diet was then changed to a normal diet, and vehicle (0.5% methyl cellulose solution) or evocalcet (0.3 mg/kg) were orally administered once daily for five weeks. Blood samples were obtained from the jugular vein 24 hours after the last administration. At the end of the study, the thoracic aorta, abdominal aorta, heart and kidney were removed and their Ca and inorganic phosphorus (IP) content and calcification levels were measured.
Biochemical analyses
The serum PTH levels were measured using a Rat Intact PTH ELISA kit (Immutopics, Inc., San Clemente, CA). The serum Ca, IP, BUN and creatinine levels were measured using an auto analyzer (Hitachi High-Technologies Corporation., Tokyo, Japan). For the single administration study, the serum Ca level was measured using a Calcium E-test Wako (FUJIFILM Wako Pure Chemical Co., Ltd., Osaka, Japan).
Evaluation of the Ca and IP content in the thoracic aorta, heart and kidney
The thoracic aorta, heart and kidney were defatted with chloroform and methanol (2:1) for two days and dehydrated by acetone for three hours. The samples were incinerated to ashes at 550°C for 12 hours using an electric muffle furnace, then extracted with hydrochloric acid and diluted with distilled water. The levels of Ca and IP in the tissue were measured using a Calcium E-test Wako and Phospha C-test Wako (FUJIFILM Wako Pure Chemical Co., Ltd., Osaka, Japan) respectively and were represented as the weight of Ca or IP per dry tissue weight.
Evaluation of calcification with von Kossa staining
The thoracic aorta, abdominal aorta, heart and kidney were fixed in a 10% neutral-buffered formalin and embedded in paraffin and sectioned by standard methods. Paraffin blocks were sectioned into slices of approximately 3 μm in thickness. The sections were stained using the von Kossa method and scored by visually estimating the percentage of the stained area within the samples as: 0% (none), ±: <25% (slight), +: 25–50% (mild), 2+: 50–75% (moderate), 3+: >75% (marked).
CKD rats with SHPT induced by 5/6 Nx
Four-week administration study
Rats were 5/6 nephrectomized in two steps. Under anesthesia (pentobarbital, 50 mg/kg; intraperitoneally) and analgesia (lidocaine; topically), two-thirds of the left kidney was removed, and then the right kidney was removed after a seven-day interval. Seven days after the completion of 5/6 Nx, the FR-2 diet was changed to a high-phosphate diet, containing 0.6% calcium and 0.9% phosphate (Oriental Yeast Co., Ltd., Tokyo, Japan). Approximately two weeks after the initiation of the high-phosphate diet, sixty four 5/6 Nx rats were divided into three groups matched for body weight as well as BUN and serum PTH and Ca. Vehicle or evocalcet (0.1 or 0.3 mg/kg) was orally administered to each group once daily for four weeks. To evaluate the cellular proliferation, the rats were subcutaneously infused with 5-bromo-2’-deoxyuridine (BrdU) by osmotic pump from seven days before necropsy. At necropsy, the weight of the parathyroid gland was measured.
Evaluation of parathyroid gland cell proliferation and cell size
The parathyroid glands were fixed with 10% (vol) neutral-buffered formalin and routinely processed with paraffin. The paraffin blocks were sectioned into slices of approximately 5 μm in thickness. After dewaxing and rehydration, the sections were incubated with 3% (vol) hydrogen peroxide/PBS, to quench the endogenous peroxidase, and treated with 0.05% (w/v) pronase E for deproteinization. The sections were incubated with an anti-BrdU antibody (Clone: Bu20a; DAKO Inc., Carpinteria, CA) and with horseradish peroxidase-labelled polymer conjugated to anti-mouse immunoglobulins (Envision+; DAKO Inc.). The signal was visualized with 3,3-diaminobenzidine. Hematoxylin was applied for nuclear counterstaining.
The ratio of BrdU-positive cells was calculated from the number of BrdU-positive nuclei, the number of total nuclei, and the area of a section. The area of parathyroid gland cells was expressed as the cell size and determined using a hematoxylin-stained section. The cell size was calculated using the following formula: Cell size (μm2) = the total area of section/the total number of nucleus in a section. These parameters were analyzed using the Aperio ImageScope and Nuclear (ver. 9) software programs (Aperio Technologies Inc., Vista, CA).
Statistical analysis
All values are expressed as the mean + S.E. The statistical analyses were all performed using a statistical analysis software program (SAS, release 9.4; SAS Institute, Inc., Cary, USA). Differences between the control or sham groups and the vehicle-treated CKD rat group were determined by Fisher’s t test followed by Student’s t-test or the Aspin-Welch test. When significant differences between the vehicle-treated CKD rat group and the evocalcet-treated groups were identified by Bartlett test, differences were determined by a one-way ANOVA followed by Dunnett’s test. When significant differences were not identified by Bartlett test, differences were assessed by the Kruskal-Wallis test followed by the Steel test. P values of <0.05 were considered to indicate statistical significance in all of the analyses.
Results
Effects of evocalcet on serum PTH and Ca in CKD rats with SHPT induced by adenine (single administration study)
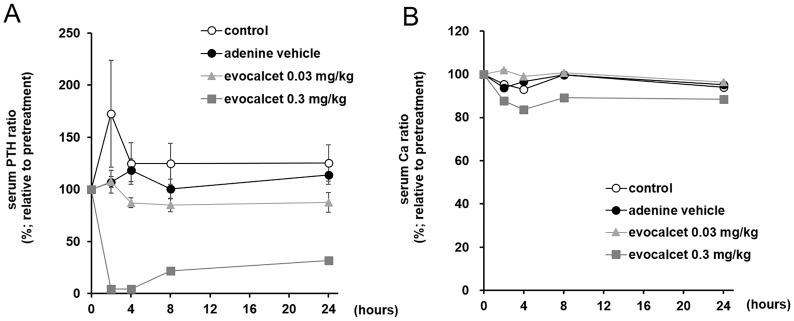
We observed the effects of evocalcet on serum PTH and Ca in CKD rats with SHPT induced by adenine. The rats, which were fed an adenine diet for three weeks, showed a significant increase in serum PTH levels in comparison to control rats (7750±901 pg/mL vs. 2737±592 pg/mL), suggesting the development of SHPT. Oral treatment with evocalcet (0.3 mg/kg) obviously decreased the serum PTH levels in comparison to vehicle-treated CKD rats at 2 hours after administration; the effect lasted for 24 hours (Fig 1A). The serum Ca levels were also decreased by treatment with evocalcet (0.3 mg/kg) (Fig 1B). On the other hand, evocalcet (0.03 mg/kg) did not clearly affect either serum PTH or Ca at any point (Fig 1A and 1B).
Fig 1. Effects of evocalcet on serum PTH and Ca in CKD rats (single administration study).
Vehicle or evocalcet (0.03 or 0.3 mg/kg) was orally administered to control rats and CKD rats with SHPT induced by adenine. (A) Serum PTH and (B) Ca levels. The data are presented as the mean + S.E. (n = 6/group).
Effects of evocalcet on biochemical parameters in CKD rats with SHPT induced by adenine (five-week administration study)
CKD rats with SHPT induced by adenine were treated with evocalcet for five weeks. There were no differences in body weight or food consumption between vehicle-treated CKD rats and evocalcet-treated CKD rats (Table 1).
Table 1. Biochemical parameters of evocalcet after 5-week-administration to CKD rats.
| Parameters | Sham | Adenine | |
|---|---|---|---|
| Vehicle | Evocalcet | ||
| body weight (BW; g) | 500 ± 17 | 370 ± 15## | 394 ± 8 |
| cumulative food intake (g) | 1265 ± 33 | 791 ± 28## | 801 ± 11 |
| serum PTH (pg/mL) | 991 ± 235 | 7630 ± 2020# | 1359 ± 416** |
| serum Ca (mg/dL) | 10.7 ± 0.1 | 10.5 ± 0.2 | 10.0 ± 0.1 |
| blood urea nitrogen (mg/dL) | 20.9 ± 0.8 | 109.5 ± 11.7## | 121.1 ± 13.0 |
| serum creatinine (mg/dL) | 0.41 ± 0.01 | 1.84 ± 0.23## | 2.05 ± 0.24 |
| serum IP (mg/dL) | 7.1 ± 0.2 | 9.1 ± 0.8# | 9.7 ± 0.7 |
| Ca x IP (mg2/dL2) | 75.9 ± 2.7 | 94.4 ± 6.7# | 97.5 ± 7.5 |
| heart weight (mg/g BW) | 2.45 ± 0.03 | 3.76 ± 0.23## | 3.58 ± 0.11 |
| kidney weight (mg/g BW) | 5.5 ± 0.2 | 22.5 ± 3.3## | 22.6 ± 2.2 |
#P < 0.05,
##P < 0.01 vs. control group (Student’s t-test or Aspin-Welch test);
**P < 0.01 vs. vehicle-treated CKD rat group (Steel test).
At the end of the study period, in comparison to the control rats, CKD rats exhibited significant increases in their serum PTH and IP levels as well as BUN and serum creatinine levels (Table 1). Evocalcet treatment reduced the serum PTH and Ca levels, while the serum IP level tended to increase, resulting in no significant differences in the serum calcium phosphate product (Ca x IP), which is presumed to be a risk factor for aortic calcification. The relative heart and kidney weight were increased in vehicle-treated CKD rats; no significant improvement was observed in the evocalcet-treated group.
Effects of evocalcet on ectopic calcification in CKD rats with SHPT induced by adenine
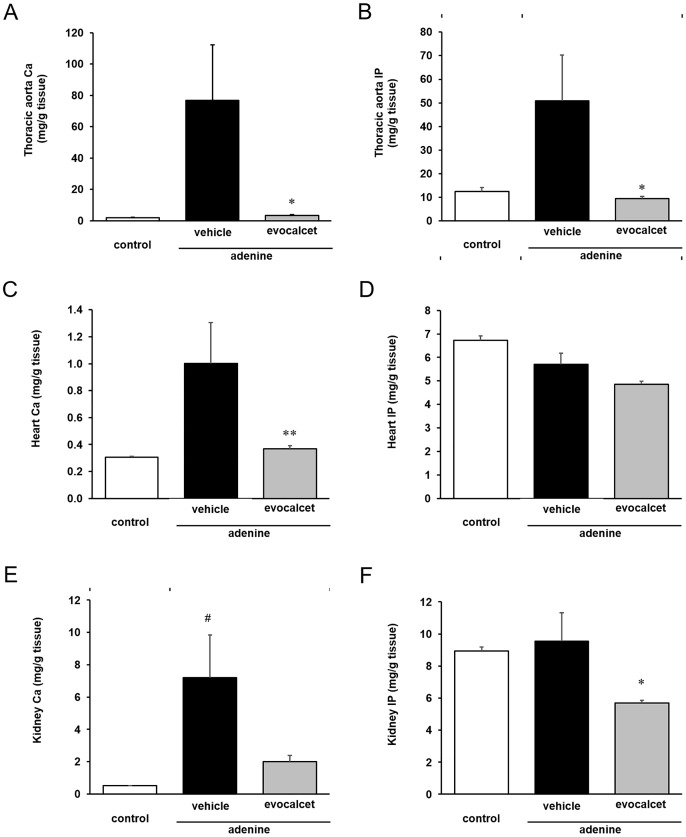
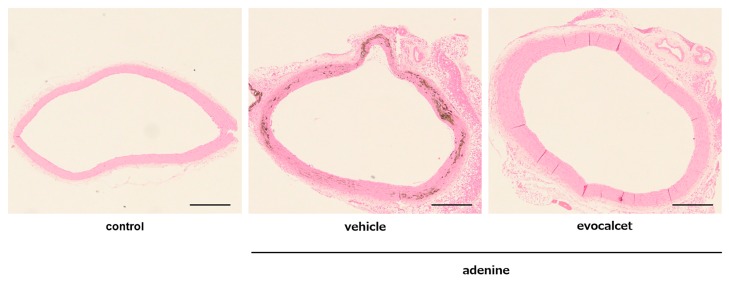
In order to examine the effectiveness of evocalcet in preventing ectopic calcification of soft tissues, we analyzed the Ca and IP levels of the thoracic aorta, heart and kidney, and calcification of the thoracic aorta, abdominal aorta, heart and kidney histologically by von Kossa staining. In the vehicle-treated CKD rats group, the tissue Ca and IP content in the thoracic aorta was significantly increased in comparison to the control group (Fig 2A and 2B), which displayed Mönckeberg arterial calcification, characterized by Ca deposition in the medial smooth muscle cells (Fig 3). Evocalcet reduced the content of both Ca and IP, as well as calcification of the aorta in CKD rats (Figs 2A, 2B and 3), which was also shown by von Kossa staining score (Table 2). Similarly, the tissue Ca content and calcification scores in the heart and kidney were increased in CKD rats in comparison to the control group, and were reduced by the administration of evocalcet (Fig 2C–2F and Table 2).
Fig 2. Effects of evocalcet on Ca and IP content in tissues in CKD rats.
Vehicle or evocalcet (0.3 mg/kg) was orally administered to control and CKD rats with SHPT induced by adenine once daily for five weeks. The data are presented as the mean + S.E. (n = 5-10/group). (A) The Ca and (B) IP content in the thoracic aorta. (C) The Ca and (D) IP content in the heart. (E) The Ca and (F) IP content in kidney. #P < 0.05 vs. control group (Aspin-Welch test); *P < 0.05, and **P < 0.01 vs. vehicle-treated CKD rat group (Steel test).
Fig 3. Representative von Kossa staining of the CKD rat aorta.
Thoracic aortas from control rats and CKD rats with SHPT induced by adenine were subjected to von Kossa staining. Scale bar, 500 μm. (A) Control; (B) vehicle-treated CKD; (C) evocalcet-treated CKD.
Table 2. Von Kossa stain scoring.
| Grade | |||||
|---|---|---|---|---|---|
| - | ± | + | 2+ | 3+ | |
| Thoracic aorta | |||||
| control | 5 | 0 | 0 | 0 | 0 |
| adenine vehicle | 3 | 1 | 1 | 2 | 2 |
| adenine evocalcet | 10 | 0 | 0 | 0 | 0 |
| Abdominal aorta | |||||
| control | 5 | 0 | 0 | 0 | 0 |
| adenine vehicle | 4 | 1 | 0 | 2 | 2 |
| adenine evocalcet | 10 | 0 | 0 | 0 | 0 |
| Heart | |||||
| control | 5 | 0 | 0 | 0 | 0 |
| adenine vehicle | 6 | 1 | 2 | 0 | 0 |
| adenine evocalcet | 9 | 1 | 0 | 0 | 0 |
| Kidney | |||||
| control | 5 | 0 | 0 | 0 | 0 |
| adenine vehicle | 0 | 0 | 4 | 5 | 0 |
| adenine evocalcet | 0 | 2 | 8 | 0 | 0 |
The data are presented as the number of samples with each von Kossa staining score for each group. Scoring: -: 0% (none), ±: <25% (slight), +: 25–50% (mild), 2+: 50–75% (moderate), 3+: >75% (marked).
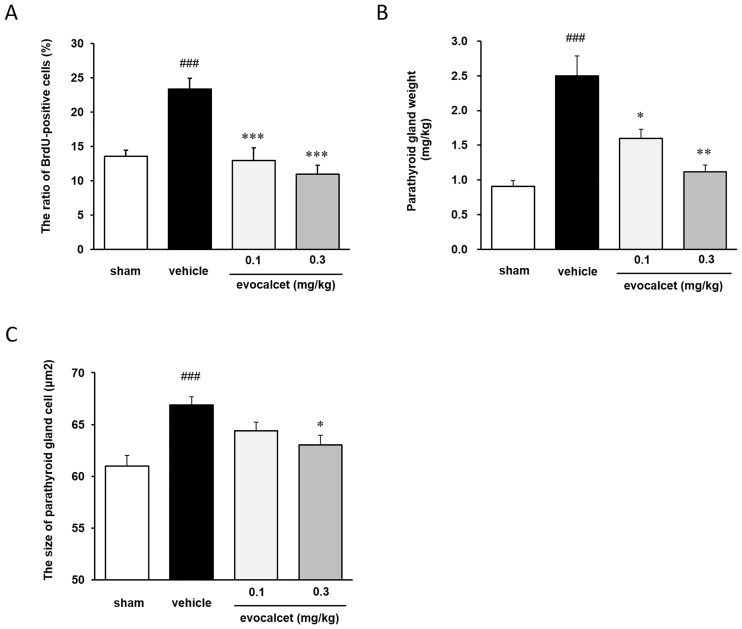
Effects of evocalcet on parathyroid gland cell proliferation and size in CKD with SHPT rats induced by 5/6 Nx
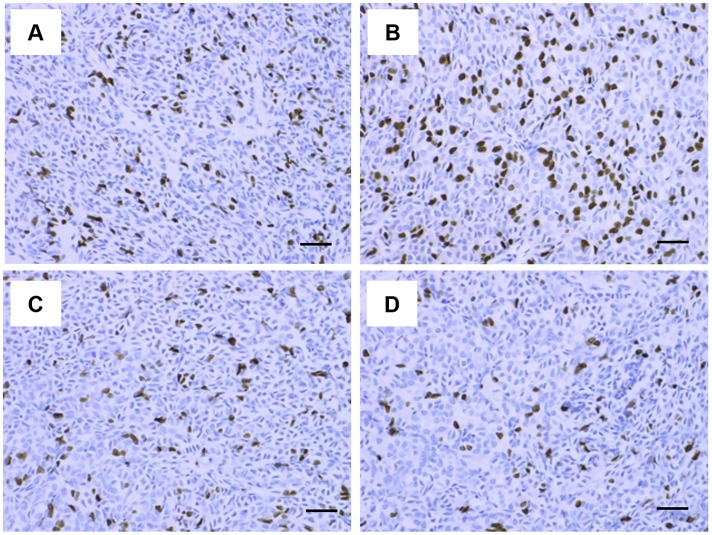
Next, to evaluate the effects of evocalcet on parathyroid gland cell proliferation, CKD rats with SHPT induced by 5/6 Nx were treated with evocalcet (0.1 and 0.3 mg/kg) once daily for four weeks. The dose of 0.1 mg/kg was associated with a significant reduction in serum PTH and Ca levels and an even higher reduction was observed at a dose of 0.3 mg/kg in this model, as we showed previously [20]. The ratio of BrdU-positive cells (%) in the vehicle-treated CKD rat group was significantly increased in comparison to the sham group (Figs 4 and 5). Parathyroid gland cell proliferation was suggested to be induced by 5/6 Nx. Evocalcet treatment significantly decreased the ratio of BrdU-positive cells in comparison to vehicle-treated CKD rats. The comparison of the vehicle-treated CKD rat group and the sham group revealed that the weight of the parathyroid gland and the size of parathyroid gland cells were significantly increased, whereas these increases were significantly ameliorated by evocalcet treatment (Fig 5).
Fig 4. Representative BrdU staining of a parathyroid gland specimen from a CKD rats.
The parathyroid glands from sham-operated and CKD rats with SHPT induced by 5/6 Nx were stained with BrdU. (A) Sham; (B) vehicle-treated CKD; (C) evocalcet-treated CKD (0.1 mg/kg); (D) evocalcet-treated CKD (0.3 mg/kg). Scale bar, 50 μm. Nx, nephrectomy.
Fig 5. Effects of evocalcet on parathyroid gland cell proliferation and sizes in CKD rats.
Vehicle or evocalcet (0.1 or 0.3 mg/kg) was orally administered to sham-operated and CKD rats with SHPT induced by 5/6 Nx once daily for four weeks. The data are presented as the mean + S.E. (n = 10-11/group). (A) Parathyroid gland cell proliferation. (B) Parathyroid gland weight. (C) The size of the parathyroid gland cells. ###P < 0.001 vs. Sham group (Student’s t-test or Aspin-Welch test); *P < 0.05, **P < 0.01, and ***P < 0.001 vs. vehicle-treated CKD group (Dunnett’s test or Steel test). Nx, nephrectomy.
Discussion
Evocalcet is a newly synthesized calcimimetic compound that improves several issues with cinacalcet, including the high rate of GI tract side-effects, the inhibition of the CYP2D6 enzyme and low bioavailability due to rapid metabolization by the CYP3A4 enzyme. Meanwhile, its pharmacological profile as an allosteric modulator of CaR and the effect of suppressing PTH secretion from parathyroid gland cells are similar to cinacalcet [17, 21–24].
In this study, we investigated whether evocalcet would prevent ectopic calcification and suppress parathyroid hyperplasia. We used CKD rats with SHPT induced by adenine to evaluate the effect on calcification because progressive ectopic calcification is induced in this model by high levels of serum PTH followed by the accumulation of Ca and phosphate in soft tissues [25]. The dosage of evocalcet (0.3 mg/kg) that showed clear suppressive effect on serum PTH and Ca levels by single administration was used in this study [20]. As a result of five weeks continuous treatment, evocalcet reduced the Ca and IP content and prevented calcification of the aorta, heart and kidney. This preventive effect of evocalcet on calcification is likely caused by the inhibition of PTH secretion from parathyroid gland cells followed by the reduction of serum Ca, since we previously showed that PTH depletion by parathyroidectomy suppressed calcification in CKD rats with SHPT induced by 5/6 Nx [26].
Although evocalcet tends to decrease the serum phosphate levels in patients with SHPT [27], we could not observe the reduction in serum IP levels in CKD rats with SHPT induced by adenine. The discrepancy is possibly due to the remnant kidney function of this animal model of CKD. In fact, due to the negation of phosphaturic effect of PTH by these calcimimetic agents, phosphorus levels actually increase in CKD patients [28]. Since PTH reduces phosphate reabsorption at the proximal renal tubule, the inhibition of PTH by evocalcet might reduce the excretion of phosphate from the renal tubules [29, 30], which negates the decrease in serum phosphate level resulting from the prevention of bone resorption.
Ectopic calcification is formed at an early stage of SHPT, and is accelerated by the increase of serum Ca and phosphate levels and associated with high-turnover bone disease with high serum PTH levels [1, 31, 32]. The processes involved in vascular calcification have been identified [33]. Briefly, Ca is actively deposited in the vessel wall and forms amorphous calcium phosphate under hypercalemia. This compound gradually transforms into the insoluble apatite form within tissue leading to vascular calcification. In circulation, calcium phosphate crystals coexist with chaperone-binding proteins that inhibit the crystallization of Ca and IP particles at physiological concentrations. These mineral-protein complexes are called calciprotein particles, CPP, and contain fetuin-A and Matrix Gla protein, well-known circulating inhibitors of calcification [34, 35]. Since it was suggested that suppressive effect of evocalcet on serum Ca contributed to prevent the ectopic calcification, there is room for further study to evaluate whether evocalcet suppresses CPP production and there is the effects of evocalcet on these factors for calcification.
We also observed the effect of evocalcet on parathyroid gland growth using CKD rats with SHPT induced by 5/6 Nx. We found that evocalcet significantly suppressed the parathyroid gland enlargement and cell proliferation. By reducing parathyroid hyperplasia, evocalcet might suppress the development of SHPT, thereby inhibiting the increase of serum PTH and thus prevent ectopic calcification at an initial stage.
Reduced expression of the CaR is one of the main cause of proliferation of parathyroid grand cells and cinacalcet shows the preventive effect on the growth of parathyroid gland cells [36–39]. NPS R-568, a calcimimetic compound, was shown to reduce parathyroid gland cell volume in CKD rats with SHPT induced by 5/6 Nx [40]. These reports suggest that activation of CaR is important in the suppression of parathyroid cell proliferation.
Parathyroid hyperplasia is caused by a complex cascade of events which has not been completely clarified. Nevertheless, there are some hypotheses that can explain the regression of the parathyroid gland by calcimimetic agents. First, calcimimetic agents may affect the cell cycle. Cinacalcet has been shown to increase the expression of p21, a cell cycle inhibitor, which controls cell entry into the actively dividing S phase, and thus prevents parathyroid gland cells from entering a proliferative hyperplastic state [37, 39]. Second, calcimimetic agents may promote cell apoptosis. High concentrations of cinacalcet have been shown to induce apoptosis in parathyroid cells from uremic rats in vitro, although this result is controversial [14]. Third, calcimimetic agents may increase the oxyphil/chief cell ratio in the parathyroid gland. Parathyroid glands are composed of many chief cells and fewer oxyphil cells, and the correlation between cinacalcet therapy and a high oxyphil/chief cell ratio was shown in hemodialysis patients. Since the oxyphil cells have a lower level of proliferation than the chief cells, the increase in the oxyphil/chief cell ratio may reduce parathyroid gland cell proliferation [41]. As a calcimimetic agent that allosterically activates the CaR, these hypotheses might be applied to evocalcet. However, the size of the oxyphil cell is larger than that of the chief cell, so none of these hypotheses explains why the parathyroid gland cell sizes were reduced in this study. Further studies are needed to verify the effect of evocalcet on these factors.
In summary, the present study suggests that evocalcet can improve ectopic calcification and parathyroid hyperplasia by inhibiting PTH secretion in SHPT patients similarly to cinacalcet. These effects seemed important to improve prognosis in patients with SHPT. Evocalcet is therefore expected to significantly contribute to the management of SHPT through early intervention.
Supporting information
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
We thank Mitsubishi Tanabe Pharma Corporation for providing evocalcet. We thank Yasuhiro Ina, Yuki Tanbo, Miho Araki and Youji Shoukei for their assistance in the in vivo studies. We also thank Toyoko Kashiwagi and Naoya Kimoto for their support in pathological analysis. Ecpotic calcification study was supported by Takahiro Sugiura (Nihon Bioresearch Inc.).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Mitsubishi Tanabe Pharma Corporation provided evocalcet. All studies were performed and the cost of them were supported by Kyowa Kirin Co., Ltd. Mariko Sakai, Shin Tokunaga, Mika Kawai, Miki Murai, Misaki Kobayashi, Tetsuya Kitayama, Satoshi Saeki, and Takehisa Kawata are employees of Kyowa Kirin Co., Ltd. Kyowa Kirin Co., Ltd provided support in the form of salaries for authors, MS, ST, MK, MM, MK, TK, SS and TK, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.Hujairi NM, Afzali B, Goldsmith DJ. Cardiac calcification in renal patients: what we do and don’t know. Am J Kidney Dis. 2004; 43: 234–43. 10.1053/j.ajkd.2003.10.014 [DOI] [PubMed] [Google Scholar]
- 2.Wang XR, Zhang JJ, Xu XX, Wu YG. Prevalence of coronary artery calcification and its association with mortality, cardiovascular events in patients with chronic kidney disease: a systematic review and meta-analysis. Ren Fail. 2019; 41: 244–56. 10.1080/0886022X.2019.1595646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tokumoto M, Taniguchi M, Matsuo D, Tsuruya K, Hirakata H, Iida M. Parathyroid cell growth in patients with advanced secondary hyperparathyroidism: vitamin D receptor, calcium sensing receptor, and cell cycle regulating factors. Ther Apher Dial. 2005;9 Suppl 1: S27–34. 10.1111/j.1744-9987.2005.00302.x [DOI] [PubMed] [Google Scholar]
- 4.Nemeth EF, Heaton WH, Miller M, Fox J, Balandrin MF, Van Wagenen BC, et al. Pharmacodynamics of the type II calcimimetic compound cinacalcet HCl. J Pharmacol Exp Ther. 2004; 308: 627–35. 10.1124/jpet.103.057273 [DOI] [PubMed] [Google Scholar]
- 5.Kawata T, Imanishi Y, Kobayashi K, Onoda N, Okuno S, Takemoto Y, et al. Direct in vitro evidence of the suppressive effect of cinacalcet HCl on parathyroid hormone secretion in human parathyroid cells with pathologically reduced calcium-sensing receptor levels. J Bone Miner Metab. 2006; 24: 300–6. 10.1007/s00774-006-0687-y [DOI] [PubMed] [Google Scholar]
- 6.Nagano N. Pharmacological and clinical properties of calcimimetics: calcium receptor activators that afford an innovative approach to controlling hyperparathyroidism. Pharmacol Ther. 2006; 109: 339–65. 10.1016/j.pharmthera.2005.06.019 [DOI] [PubMed] [Google Scholar]
- 7.Fukagawa M, Yumita S, Akizawa T, Uchida E, Tsukamoto Y, Iwasaki M, et al. Cinacalcet (KRN1493) effectively decreases the serum intact PTH level with favorable control of the serum phosphorus and calcium levels in Japanese dialysis patients. Nephrol Dial Transplant. 2008; 23: 328–35. 10.1093/ndt/gfm534 [DOI] [PubMed] [Google Scholar]
- 8.Wetmore JB, Gurevich K, Sprague S, Da Roza G, Buerkert J, Reiner M, et al. A Randomized Trial of Cinacalcet versus Vitamin D Analogs as Monotherapy in Secondary Hyperparathyroidism (PARADIGM). Clin J Am Soc Nephrol. 2015; 10: 1031–40. 10.2215/CJN.07050714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akizawa T, Kido R, Fukagawa M, Onishi Y, Yamaguchi T, Hasegawa T, et al. Decreases in PTH in Japanese hemodialysis patients with secondary hyperparathyroidism: associations with changing practice patterns. Clin J Am Soc Nephrol. 2011; 6: 2280–8. 10.2215/CJN.11501210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukagawa M, Fukuma S, Onishi Y, Yamaguchi T, Hasegawa T, Akizawa T, et al. Prescription patterns and mineral metabolism abnormalities in the cinacalcet era: results from the MBD-5D study. Clin J Am Soc Nephrol. 2012; 7: 1473–80. 10.2215/CJN.13081211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukuma S, Kurita N, Fukagawa M, Akizawa T, Fukuhara S. Impact of cinacalcet introduction on MBD management: the MBD-5D study in Japan. Kidney Int Suppl (2011). 2013; 3: 436–41. 10.1038/kisup.2013.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tominaga Y, Kakuta T, Yasunaga C, Nakamura M, Kadokura Y, Tahara H. Evaluation of Parathyroidectomy for Secondary and Tertiary Hyperparathyroidism by the Parathyroid Surgeons’ Society of Japan. Ther Apher Dial. 2016; 20: 6–11. 10.1111/1744-9987.12352 [DOI] [PubMed] [Google Scholar]
- 13.Raggi P, Chertow GM, Torres PU, Csiky B, Naso A, Nossuli K, et al. The ADVANCE study: a randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol Dial Transplant. 2011; 26: 1327–39. 10.1093/ndt/gfq725 [DOI] [PubMed] [Google Scholar]
- 14.Komaba H, Nakanishi S, Fujimori A, Tanaka M, Shin J, Shibuya K, et al. Cinacalcet effectively reduces parathyroid hormone secretion and gland volume regardless of pretreatment gland size in patients with secondary hyperparathyroidism. Clin J Am Soc Nephrol. 2010; 5: 2305–14. 10.2215/CJN.02110310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ichii M, Ishimura E, Okuno S, Chou H, Kato Y, Tsuboniwa N, et al. Decreases in parathyroid gland volume after cinacalcet treatment in hemodialysis patients with secondary hyperparathyroidism. Nephron Clin Pract. 2010; 115: c195–202. 10.1159/000313035 [DOI] [PubMed] [Google Scholar]
- 16.Yamada S, Tokumoto M, Taniguchi M, Toyonaga J, Suehiro T, Eriguchi R, et al. Two Years of Cinacalcet Hydrochloride Treatment Decreased Parathyroid Gland Volume and Serum Parathyroid Hormone Level in Hemodialysis Patients With Advanced Secondary Hyperparathyroidism. Ther Apher Dial. 2015; 19: 367–77. 10.1111/1744-9987.12292 [DOI] [PubMed] [Google Scholar]
- 17.Palmer SC, Nistor I, Craig JC, Pellegrini F, Messa P, Tonelli M, et al. Cinacalcet in patients with chronic kidney disease: a cumulative meta-analysis of randomized controlled trials. PLoS Med. 2013; 10: e1001436 10.1371/journal.pmed.1001436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Block GA, Martin KJ, de Francisco AL, Turner SA, Avram MM, Suranyi MG, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004; 350: 1516–25. 10.1056/NEJMoa031633 [DOI] [PubMed] [Google Scholar]
- 19.Gincherman Y, Moloney K, McKee C, Coyne DW. Assessment of adherence to cinacalcet by prescription refill rates in hemodialysis patients. Hemodial Int. 2010; 14: 68–72. 10.1111/j.1542-4758.2009.00397.x [DOI] [PubMed] [Google Scholar]
- 20.Kawata T, Tokunaga S, Murai M, Masuda N, Haruyama W, Shoukei Y, et al. A novel calcimimetic agent, evocalcet (MT-4580/KHK7580), suppresses the parathyroid cell function with little effect on the gastrointestinal tract or CYP isozymes in vivo and in vitro. PLoS One. 2018; 13: e0195316 10.1371/journal.pone.0195316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris RZ, Salfi M, Sullivan JT, Padhi D. Pharmacokinetics of cinacalcet hydrochloride when administered with ketoconazole. Clin Pharmacokinet. 2007; 46: 495–501. 10.2165/00003088-200746060-00003 [DOI] [PubMed] [Google Scholar]
- 22.Nakashima D, Takama H, Ogasawara Y, Kawakami T, Nishitoba T, Hoshi S, et al. Effect of cinacalcet hydrochloride, a new calcimimetic agent, on the pharmacokinetics of dextromethorphan: in vitro and clinical studies. J Clin Pharmacol. 2007; 47: 1311–9. 10.1177/0091270007304103 [DOI] [PubMed] [Google Scholar]
- 23.Block GA, Bushinsky DA, Cheng S, Cunningham J, Dehmel B, Drueke TB, et al. Effect of Etelcalcetide vs Cinacalcet on Serum Parathyroid Hormone in Patients Receiving Hemodialysis With Secondary Hyperparathyroidism: A Randomized Clinical Trial. JAMA. 2017; 317: 156–64. 10.1001/jama.2016.19468 [DOI] [PubMed] [Google Scholar]
- 24.Hamano N, Komaba H, Fukagawa M. Etelcalcetide for the treatment of secondary hyperparathyroidism. Expert Opin Pharmacother. 2017; 18: 529–34. 10.1080/14656566.2017.1303482 [DOI] [PubMed] [Google Scholar]
- 25.Price PA, Roublick AM, Williamson MK. Artery calcification in uremic rats is increased by a low protein diet and prevented by treatment with ibandronate. Kidney Int. 2006; 70: 1577–83. 10.1038/sj.ki.5001841 [DOI] [PubMed] [Google Scholar]
- 26.Kawata T, Nagano N, Obi M, Miyata S, Koyama C, Kobayashi N, et al. Cinacalcet suppresses calcification of the aorta and heart in uremic rats. Kidney Int. 2008; 74: 1270–7. 10.1038/ki.2008.407 [DOI] [PubMed] [Google Scholar]
- 27.Yokoyama K, Shimazaki R, Fukagawa M, Akizawa T, Evocalcet Study G. Long-Term Efficacy and Safety of Evocalcet in Japanese Patients with Secondary Hyperparathyroidism Receiving Hemodialysis. Sci Rep. 2019; 9: 6410 10.1038/s41598-019-42017-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charytan C, Coburn JW, Chonchol M, Herman J, Lien YH, Liu W, et al. Cinacalcet hydrochloride is an effective treatment for secondary hyperparathyroidism in patients with CKD not receiving dialysis. J Kidney Dis. 2005; 46: 58–67. 10.1053/j.ajkd.2005.04.013 . [DOI] [PubMed] [Google Scholar]
- 29.Lederer E. Regulation of serum phosphate. J Physiol. 2014; 592: 3985–95. 10.1113/jphysiol.2014.273979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bergwitz C, Juppner H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu Rev Med. 2010; 61: 91–104. 10.1146/annurev.med.051308.111339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cozzolino M, Ciceri P, Galassi A, Mangano M, Carugo S, Capelli I, et al. The Key Role of Phosphate on Vascular Calcification. Toxins (Basel). 2019; 11 10.3390/toxins11040213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coen G. Calcimimetics, parathyroid hormone, and vascular calcification in chronic kidney disease. Kidney Int. 2008; 74: 1229–31. 10.1038/ki.2008.417 [DOI] [PubMed] [Google Scholar]
- 33.Kakani E, Elyamny M, Ayach T, El-Husseini A. Pathogenesis and management of vascular calcification in CKD and dialysis patients. Semin Dial. 2019. 10.1111/sdi.12840 [DOI] [PubMed] [Google Scholar]
- 34.Schafer C, Heiss A, Schwarz A, Westenfeld R, Ketteler M, Floege J, et al. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003; 112: 357–66. 10.1172/JCI17202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997; 386: 78–81. 10.1038/386078a0 [DOI] [PubMed] [Google Scholar]
- 36.Colloton M, Shatzen E, Miller G, Stehman-Breen C, Wada M, Lacey D, et al. Cinacalcet HCl attenuates parathyroid hyperplasia in a rat model of secondary hyperparathyroidism. Kidney Int. 2005; 67: 467–76. 10.1111/j.1523-1755.2005.67103.x [DOI] [PubMed] [Google Scholar]
- 37.Miller G, Davis J, Shatzen E, Colloton M, Martin D, Henley CM. Cinacalcet HCl prevents development of parathyroid gland hyperplasia and reverses established parathyroid gland hyperplasia in a rodent model of CKD. Nephrol Dial Transplant. 2012; 27: 2198–205. 10.1093/ndt/gfr589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Imanishi Y, Kawata T, Kenko T, Wada M, Nagano N, Miki T, et al. Cinacalcet HCl suppresses Cyclin D1 oncogene-derived parathyroid cell proliferation in a murine model for primary hyperparathyroidism. Calcif Tissue Int. 2011; 89: 29–35. 10.1007/s00223-011-9490-4 [DOI] [PubMed] [Google Scholar]
- 39.Taniguchi M, Tokumoto M, Matsuo D, Tsuruya K, Hirakata H, Iida M. Parathyroid growth and regression in experimental uremia. Kidney Int. 2006; 69: 464–70. 10.1038/sj.ki.5000090 [DOI] [PubMed] [Google Scholar]
- 40.Wada M, Furuya Y, Sakiyama J, Kobayashi N, Miyata S, Ishii H, et al. The calcimimetic compound NPS R-568 suppresses parathyroid cell proliferation in rats with renal insufficiency. Control of parathyroid cell growth via a calcium receptor. J Clin Invest. 1997; 100: 2977–83. 10.1172/JCI119851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lomonte C, Vernaglione L, Chimienti D, Bruno A, Cocola S, Teutonico A, et al. Does vitamin D receptor and calcium receptor activation therapy play a role in the histopathologic alterations of parathyroid glands in refractory uremic hyperparathyroidism? Clin J Am Soc Nephrol. 2008; 3: 794–9. 10.2215/CJN.04150907 [DOI] [PMC free article] [PubMed] [Google Scholar]