Supplemental digital content is available in the text.
Key Words: lurasidone, atypical antipsychotic, treatment-resistant schizophrenia, cognition
Abstract
Purpose/Background
In addition to clozapine, other atypical antipsychotic drugs pharmacologically similar to clozapine, for example, olanzapine, risperidone, and melperone, are also effective in a similar proportion of treatment-resistant schizophrenia (TRS) patients, ~40%. The major goal of this study was to compare 2 doses of lurasidone, another atypical antipsychotic drug, and time to improvement in psychopathology and cognition during a 6-month trial in TRS patients.
Methods/Procedures
The diagnosis of TRS was based on clinical history and lack of improvement in psychopathology during a 6-week open trial of lurasidone 80 mg/d (phase 1). This was followed by a randomized, double-blind, 24-week trial of lurasidone, comparing 80- and 240-mg/d doses (phase 2).
Findings/Results
Significant non–dose-related improvement in the Positive and Negative Syndrome Scale—Total and subscales and in 2 of 7 cognitive domains, speed of processing and executive function, were noted. Twenty-eight (41.8%) of 67 patients in the combined sample improved ≥20% in the Positive and Negative Syndrome Scale—Total. Of the 28 responders, 19 (67.9%) first reached ≥20% improvement between weeks 6 and 24 during phase 2, including some who had previously failed to respond to clozapine.
Implications/Conclusions
Improvement with lurasidone is comparable with those previously reported for clozapine, melperone, olanzapine, and risperidone in TRS patients. In addition, this study demonstrated that 80 mg/d lurasidone, an effective and tolerable dose for non-TRS patients, was also effective in TRS patients but required longer duration of treatment. Direct comparison of lurasidone with clozapine in TRS patients is indicated.
An accepted definition of treatment-resistant schizophrenia (TRS), which is estimated to include ~30% of schizophrenia patients, is persistent moderate-to-severe delusions and/or hallucinations, despite 2 or more trials of at least 6 weeks in duration, with either typical (T) or atypical (A) antipsychotic drugs (APDs).1–3 Clozapine, the only AAPD approved for TRS achieved ≥20% improvement in the Brief Psychiatric Rating Scale4—Total in 30% of TRS patients within 6 weeks,1 with 60% to 70% response rates in trials lasting 6 to 48 months.5–10 Thirty percent of TRS patients experience little or no benefit from clozapine.5–12 Other AAPDs, for example, melperone,13 olanzapine,7,14 oral risperidone,14,15 long-acting injectable risperidone,16 aripiprazole,17 and quetiapine,15,18 which are safer in some respect than clozapine, have also been reported to be effective in subgroups of TRS patients. Response to these AAPDs, as well as clozapine, required 6 months or even longer treatment in 30% to 50% of these patients.5,7,14–18 Two recent meta-analyses published since the completion of this study examined the comparative efficacy of clozapine and other AAPDs in TRS, with attention to the issue of duration of treatment and dose.19,20 These will be discussed subsequently.
There is some evidence that the average doses of AAPDs needed for improvement in psychopathology in TRS patients are significantly higher than those for non-TRS patients.7,14 For example, in a blinded study of olanzapine in TRS patients, with clozapine as an active comparator, the mean daily dose of olanzapine required for efficacy was 35 mg/d compared with 550 mg/d clozapine.7 On the other hand, 2 studies of TRS patients, one with long-acting injectable risperidone (50 vs 100 mg 4 times a day for 2 weeks)16 and the other with oral quetiapine (600 vs 1200 mg/d),18 did not find efficacy to be dose dependent. Factors other than dose and duration of treatment that do affect response to AAPDs in TRS patients include genetic and epigenetic differences, concomitant medications, duration of illness, number of prior episodes, and age.14–20 It is possible that the variability of dose-response relationships among AAPDs is related to differences in their pharmacokinetics, metabolism, and relative differences in affinities for a variety of receptors, including but not limited to the multiple types of serotonin (5-HT), dopamine (DA), cholinergic, and noradrenergic receptors, which have been shown to have a role in psychopathology and response to APDs. These drugs also differ in their effects on glutamatergic and γ-aminobutyric acid (GABA)ergic neurons and in direct and indirect effects on neurotrophins, including neuregulin and brain-derived neurotrophic factor.21,22 Some actions of these drugs, for example, indirect DA D1 receptor stimulation, may be characterized by an inverted U-shaped function, where higher doses may be less efficacious than lower doses. As will be discussed, the absence of D4 DA receptor antagonism may be important to the efficacy of AAPDs other than clozapine. Also, higher doses of some AAPDs may produce greater DA D2 receptor blockade, which may limit the beneficial effects of 5-HT2A receptor blockade.23
Cognitive impairment, often regarded as the key to better functional outcome in schizophrenia,2,10 has been reported to be more impaired in TRS than non-TRS patients in some24,25 but not all studies.26 Clozapine, melperone, olanzapine, and risperidone are among the AAPDs that have been reported to improve some but not all cognitive domains in TRS, with greater improvement more likely in trials lasting up to 6 months compared with briefer trials.7,16,27–29 Longer exposure to AAPDs would be expected to facilitate neurogenesis, reestablishment of connectivity between neurons, and restoration or establishment of functional circuits.30–32 The differences in the pharmacologic profiles of the AAPDs that affect their dose-related abilities to improve psychopathology, including 5-HT1A partial agonism, 5-HT7 antagonism, and lack of D4 receptor blockade, may also affect their ability to improve cognition.29–33
Lurasidone is a widely used AAPD that shares key pharmacologic features with clozapine, for example, more potent 5-HT2A than D2 receptor antagonism. Like other AAPDs, effects on other 5-HT and DA receptors, for example, 5-HT1A, 5-HT7, and D1 receptors, contribute to its efficacy. All of the AAPDs enhance acetylcholine efflux in the prefrontal cortex and hippocampus, distinguishing them from the TAPDs.31 The most important additional actions of lurasidone are 5-HT1A partial agonism and 5-HT7 antagonism, which have been shown in preclinical studies to be important for its ability to treat psychosis and normalize cognition.31,32 These actions are shared by clozapine. Risperidone also has strong 5-HT7 receptor affinity, but olanzapine does not. Lurasidone differs from clozapine in not blocking DA D4 receptors, which has been shown to be an important advantage over clozapine with regard to improving episodic memory in the phencyclidine model of cognitive impairment associated with schizophrenia.33
Lurasidone produced highly significant improvement in non-TRS patient in 6-week studies at doses of 40 to 160 mg/d.34–38 One clinical trial with non-TRS patients reported that improvement in psychopathology was greater with higher doses of lurasidone (160 mg/d) compared with lower doses.36 As previously mentioned, the duration of treatment and dosages with AAPDs for TRS may differ from that for non-TRS patients.5,7,14–18 The major goal of this study was to test the hypotheses that, in TRS patients, lurasidone at both 80 and 240 mg/d would be more effective to improve psychopathology and cognition at 24 weeks than at 6 weeks with no difference between the 2 doses. As in the pivotal clozapine TRS trial,1 the randomized controlled phase was preceded by a prospective 6-week open trial of the test drug, lurasidone 80 mg/d in this case, to determine if a standard dose trial for 6 weeks, which if not, would provide prospective confirmation of treatment resistance.
MATERIALS AND METHODS
Patient Sample
After obtaining written informed consent, 133 outpatients with clinical diagnoses of TRS recruited from 3 community mental health centers were screened for eligibility using the Structured Clinical Interview for DSM-IV-TR Axis I Disorders39 to identify those meeting the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) schizophrenia or schizoaffective disorder criteria. Most importantly, we reviewed medical records and interviewed previous prescribers, case managers, or relatives, when available, to confirm compliance with prior treatment and severity and refractoriness of positive symptoms, the key criteria for resistance to APDs. Only subjects with verified histories of failure to respond to ≥2 adequate trials with APDs of at least 6 weeks in duration and had scores ≥4 (1–7 scale) on the Positive and Negative Syndrome Scale (PANSS)40 items [P1] delusions, [P3] hallucinations, or [G9] unusual thought content at phase 1 baseline were included. Patients currently receiving clozapine were excluded. Most patients had received one or more AAPDs. Eleven patients had failed clozapine trials; of these, none had received clozapine for at least 3 months before admission to phase 1. Their representation in each group in phase 2 was not significantly different (8/34 in the 80-mg group, 3/33 in the 240-mg group). Phase 2 baseline PANSS-Total scores were not significantly different between those who had prior clozapine trials and those who did not (data not shown).
The total sample size of subjects estimated for the randomized clinical trial in phase 2 (n = 70) was determined by a power analysis based on prior trials of AAPDs in TRS patients.7,16 The average correlation coefficient between ratings during the 24-week period was ~0.70. In order to achieve 80% power with a type I error rate of 0.05 with a 2-sided test, it was estimated that at least 30 subjects per group were needed to detect an effect size (ES) of 0.7 (or less). The study protocol was approved by Centerstone Research Institute and Sterling Institutional Review Board for the 2 sites in Nashville, Tennessee, and the institutional review board of Northwestern University Feinberg School of Medicine for Chicago, Illinois, sites. The protocol was registered on www.ClinicalTrials.gov (NCT01569659). This study was carried out between October 2011 and July 2015.
Study Design
In phase 1, current APDs were cross-tapered over a 1- to 2-week period, whereas treatment with open-label lurasidone 80 mg/d was administered for a total of 6 weeks to verify TRS. Only those patients with ≥4 ratings on the core PANSS positive symptom inclusion items at the end of phase 1 entered phase 2, with subsequent randomization to lurasidone 80 or 240 mg/d. Details of drug administration, dose titration, and blinding in phase 2 are given in the Supplemental Material, http://links.lww.com/JCP/A669. We chose lurasidone rather than another AAPD to confirm TRS in phase 1 because most patients had already failed to respond to olanzapine, risperidone, or quetiapine and none had previously received lurasidone. The choice of lurasidone also enabled us to exclude patients who could respond to an open trial of lurasidone for what we predicted would be an inadequate period. Mood stabilizers, antidepressants, anxiolytics, benzodiazepines, and anticholinergics prescribed before enrollment in phase 1 were continued during phase 1 and phase 2, if considered clinically necessary at the end of phase 1. In the combined groups, 10 patients received concomitant benzodiazepines, another 10 patients with anticholinergic drugs, 20 patients with antidepressants, and 7 patients with mood stabilizers. Importantly, other APDs drugs were prohibited. The distribution of those medications between groups was not significantly different (data not shown). Any adjunctive psychosocial supportive treatments available to patients before phase 1 were continued throughout the study. Weekly visits in phase 1 and biweekly in phase 2 were conducted to monitor adherence, clinical response, and tolerability. Subjects and raters, including the principal investigator, were blinded to treatment group during phase 2.
The primary end point was reduction in PANSS-Total at 24 weeks. In addition, global assessments of psychopathology and functional assessment were evaluated with Clinical Global Impression (CGI)—Severity41 and Personal and Social Performance Scale (PSP).42 All psychopathology ratings were administered at baseline of both phases, and at 6-week intervals during phase 2. All raters had extensive training and experience with the PANSS and other rating instruments, and were tested for reliability during the course of the study.
A full neurocognitive battery was assessed at baseline for both phases, and at weeks 6 and 24 of phase 2. Cognitive function evaluation included the following tests: the Wisconsin Card Sorting Test (WCST),43 Wechsler Intelligence Scale for Children—Revised (WISC-R) Mazes,44 Letter Fluency,45 Category Fluency: Animal Naming,46 the Brown-Peterson Auditory Consonant Trigrams Test,47 Digit Symbol Substitution Test (DSST),48 and Rey Auditory Verbal Learning Test.49 These tests were chosen to evaluate executive function/reasoning, speed of processing, working memory, verbal learning and memory, and attention. The rationale for this battery has been previously described.27
Safety and tolerability were measured at biweekly intervals using the Abnormal Involuntary Movement Scale,41 the Barnes Akathisia Rating Scale,50 the Simpson-Angus Scale,51 and monitoring of adverse events along with collection of vital signs, electrocardiogram, physical examination, and laboratory safety evaluations. Plasma prolactin levels, which were determined by the Northwestern Diagnostic Testing Center, were assessed at baseline of both phases and at 6-week intervals of phase 2.
Data Analysis
The primary outcome measure was the change in the PANSS-Total score from baseline at phase 2 to the end of 24 weeks. Treatment effects over time were analyzed using a mixed-model analysis of covariance with repeated measures after adjusting for baseline, with time as the within-subject factor and treatment group as the between-subject factor.52 Sex, race, age, and duration of illness were also included as covariates. When the group × time effect was significant, post hoc analysis by least square (LS) analysis was adjusted using Bonferroni criteria for multiplicity of testing. Effect sizes were determined using the Cohen d statistic.53 All main effects were tested at a 2-tailed α level of 0.05. All analyses were performed using SAS (SAS Institute, Inc, Cary, North Carolina) statistical software. Secondary outcome measures included assessing changes in the 5 PANSS subscales, the cognitive test scores, PSP, CGI, tolerability assessments, and metabolic measures. Multiple regression analysis was applied to examine relationships between PANSS ratings and cognition (age and sex included as independent variables). χ2 Analysis or Fisher exact test was performed where appropriate.
RESULTS
Patient Demographic and Clinical Characteristics
One hundred one patients were enrolled in phase 1 (consort figure; Fig. 1). At the end of phase 1, 3 (4%) of 70 patients, who completed phase 1, no longer met the TRS criteria and were not eligible for inclusion in phase 2. No patients who entered phase 2 had ≥20% improvement in PANSS-Total (responder criteria) during phase 1. Reasons for discontinuation in phase 1 are given in Supplemental Table 1A, http://links.lww.com/JCP/A668.
FIGURE 1.
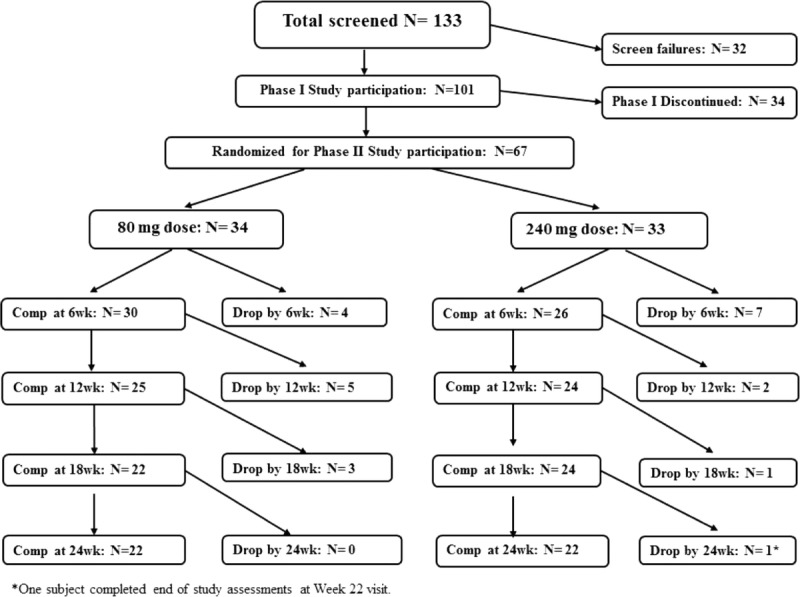
Consort diagram.
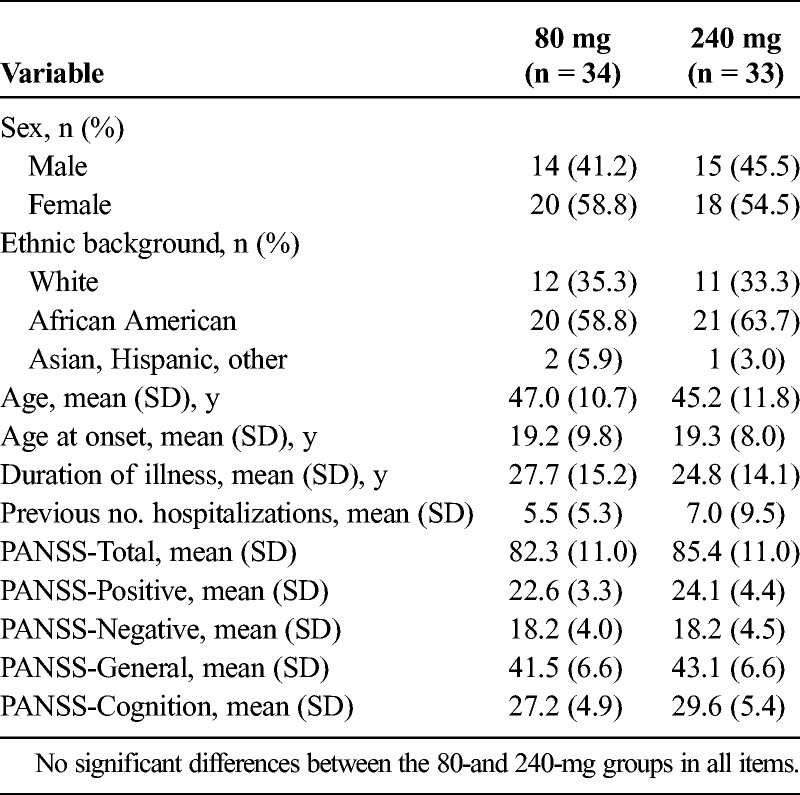
Baseline characteristics for phase 2 showed no significant differences in percent diagnosed as schizophrenia or schizoaffective disorder, race, age, duration of illness, and number of hospitalizations (Table 1). Fifty-six (83.6%) of 67 patients completed phase 2 6-week assessments and were included in the mixed-model analysis of covariance with repeated measure. Twenty-two patients in each group (65.7%) completed phase 2. Reasons for discontinuation in phase 2 are in Supplemental Table 1A, http://links.lww.com/JCP/A668.
TABLE 1.
Demographic Data for 80- and 240-mg Lurasidone Dose Groups
Psychopathology Ratings
Psychopathology data are presented in Figures 2A–F and Supplemental Table 2, http://links.lww.com/JCP/A668. During phase 1, a slight but statistically significant within-group improvement in PANSS-Total (mean change, 2.4; P = 0.03; ES = 0.25) and PANSS-Positive (mean change, 1.4; P = 0.002; ES = 0.94) was noted (Figs. 2A, B). By contrast, analysis of PANSS-Total data between phase 2 baseline and week 24 (Fig. 2A) revealed a highly significant time effect (F = 50.59, P = 0.0001, ES = 1.17), but no significant group × time interaction. In phase 2, the LS mean changes in PANSS-Total between baseline and week 24 in the 80- and 240-mg/d groups were 12.7 and 14.1, respectively. Similarly, there was a significant time effect for the PANSS-Positive subscale between phase 2 baseline and week 24 (F = 27.33, P ≤ 0.0001, ES = 0.86), without significant group × time interaction (Fig. 2B). The LS mean changes in the PANSS-Positive subscale between phase 2 baseline and week 24 in the 80- and 240-mg/d groups were 2.9 and 4.5, respectively (ES = 0.86 for both groups combined). Significant time effects were also found for the PANSS-Negative (combined ES = 0.74), PANSS-Cognitive (combined ES = 1.07), and PANSS-General (combined ES = 1.06) subscales (Figs. 2C–E), without significant group differences for any subscales.
FIGURE 2.
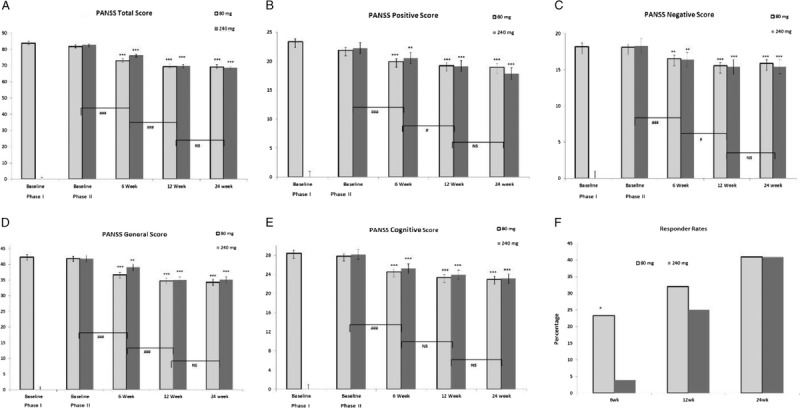
A–F, Changes in psychopathology as measured by the PANSS-Total, Positive, Negative, General and Cognitive subscales, and responder rates during phases 1 and 2. ***P < 0.001, **P < 0.01 denotes significant differences, and +P < 0.1 denotes a trend compared with phase 2 baseline for each dose in panels A-E. ###P < 0.001, #P < 0.05 or NS (not significant) denotes the time effect for adjacent time points for the combined doses. Graph F, *P < 0.05 denotes a significant difference of 6wk responder rates between 2 doses in phase 2.
Improvement Within Phase 2
The within group improvement in PANSS-Total and subscales in phase 2 was significant at week 6 and later time points (Fig. 2); the improvements in phase 2 between weeks 6 and 12 in PANSS-Total and subscales were statistically significant (P < 0.05), but not between weeks 12 and 24. There were no significant interactions in improvement in PANSS-Total or any PANSS subscales with regard to race, age, sex, age at onset of illness, or duration of illness between baseline and the subsequent time points for the entire sample or either group. There was no evidence that concomitant medications affected the response in PANSS scores to either dose of lurasidone (data not presented).
Responder Analysis
The patients with ≥20% improvement in PANSS-Total scores met the response criteria. The proportions of patients with ≥20% improvement in PANSS-Total at 6, 12, and 24 weeks were 28.6%, 28.6%, and 42.9% for the 80-mg/d group (total 14 subjects), and 3.0%, 24.2%, and 42.4% for the 240-mg/d group (total 14 subjects), respectively (Fig. 2F). Twenty-three (82.1%) of 28 subjects first improved by ≥20% in PANSS-Total at week 12 or 24 of phase 2, indicating the importance of the longer trial. Thus, in the total sample, responder rates at 12 (P = 0.02) and 24 weeks (P = 0.002) were significantly greater than at 6 weeks. The proportions of responders by ≥20% in PANSS-Total, but not to an equivalent extent in PANSS-Positive or PANSS-Negative, in the 80-mg/d group at 6 weeks in phase 2 was significantly greater than that of the 240-mg/d group (χ2 = 4.31, P = 0.04), but did not differ at subsequent times (Fig. 2F).
Effect on Cognitive Test Scores
Cognitive test data are presented in Figures 3A–D and Supplemental Table 3, http://links.lww.com/JCP/A668. There were no significant changes in any of the neurocognitive test scores between baseline and end of phase 1 (Figs. 3A–D; Supplemental Table 3, http://links.lww.com/JCP/A668). In the combined group, in phase 2, there were significant time effects at week 24 but no time × group interactions for the WCST-Categories (F = 4.10, P = 0.01, ES = 0.43) and the DSST (F = 5.70, P = 0.002, ES = 0.49). Although both groups showed improvement, significant improvement from baseline was present only in the 80-mg/d group for both tests (WCST-Categories, P = 0.02; DSST, P = 0.001). A group × time effect in the WISC-R Mazes (F = 3.34, P = 0.001, ES = 0.38) was noted; only the 240-mg/d group improved significantly at week 6 (P = 0.01, ES = 0.49), with a trend (P = 0.08) for improvement at week 24. Thus, at 6 weeks, the WISC-R Mazes score was significantly higher in the 240-mg/d group compared with the 80-mg/d group (P = 0.01, ES = 0.70), but not at week 24. WCST-Percent Perseveration also showed a group × time effect (F = 3.48, P = 0.04, ES = 0.39); this interaction was due to significant worsening in the 240-mg group (P = 0.01, ES = 0.55) at week 6 and a trend (P = 0.07) of worsening at week 24, which resulted in the 80-mg/d group performing significantly better at weeks 6 (P = 0.01, ES = 0.57) and 24 (P = 0.01, ES = 0.62) compared with the 240-mg/d group. These cognitive tests were examined after excluding patients receiving benzodiazepines or anticholinergic drugs, which have been reported to impair cognition.54,55 The results were unchanged (data not shown).
FIGURE 3.
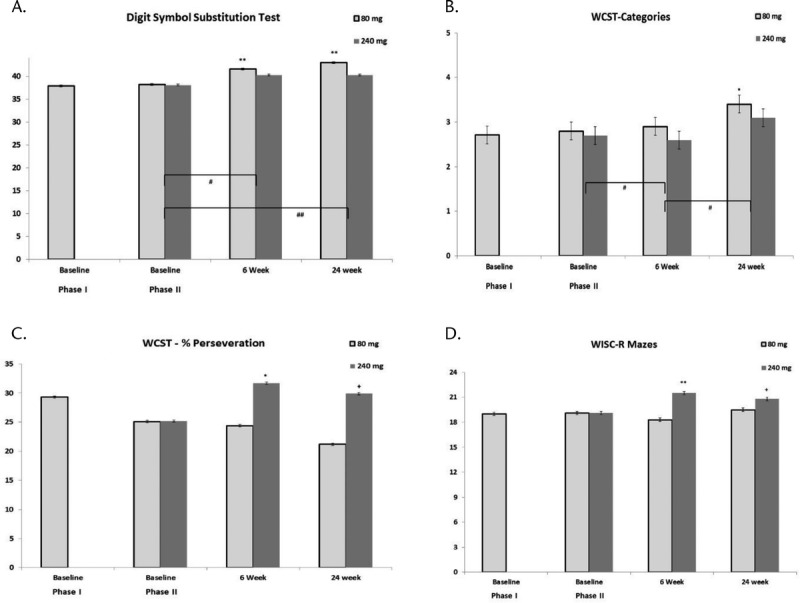
A–D, Improvement in cognitive domains during phases 1 and 2. WCST-Categories, WCST-Percent Perseveration, WISC-R Maze: executive function/reasoning and problem solving; DSST: speed of processing. **P < 0.01, *P < 0.05 denotes significant or +P < 0.1 denotes a trend of differences compared with phase 2 baseline for each dose. ##P < 0.01, #P < 0.05 denotes the time effect for adjacent time points for the combined doses.
Correlations Between Changes in Cognition Measures, and PANSS and PSP
Because the DSST and WCST-Category showed a significant time effect but no time × group interaction, the correlations between these measures, and PANSS and PSP were examined for the combined group. The improvement in the PANSS-Cognition (P = 0.03, β = 0.71), PANSS-Positive (P = 0.02, β = 0.78), and PANSS-Total (P = 0.03, β = 0.28) subscales in the combined group were significantly positively correlated with improvement in the DSST, but not the WCST-Categories, at 24 weeks. Similarly, improvement in PSP at 24 weeks was significantly positively correlated with improvement in DSST (P = 0.03, β = 0.30), but not with the WCST-Categories.
Improvement in Global and Functional Measures
Significant time effects were found in measures of global function in TRS, including improvement in the CGI and PSP (Figs. S1A, B, and Supplemental Table 4, http://links.lww.com/JCP/A668). The CGI improved at 6 weeks (P = 0.004). The LS mean improvements in CGI for the 80- and 240-m/d groups were −0.6 and −0.5, respectively, at 24 weeks (F = 14.70, P = 0.0001, ES = 0.65) without group difference. The PSP showed a trend of improvement at week 6 (P = 0.07) and was significantly improved in both groups, beginning at week 12 of phase 2 (F = 13.22, P = 0.0001, ES = 0.60).
Tolerability and Dropouts
Common adverse events (≥5%) and safety assessments are listed in the Supplemental Table 1B, http://links.lww.com/JCP/A668. A non–study-related cardiac death occurred in an obese patient in the 240-mg group in phase 2 (Supplemental Table 1A, http://links.lww.com/JCP/A668). Three patients from each group (9%) withdrew because of adverse events in phase 2, mainly for mild-moderate nausea, insomnia, and vomiting. There were no significant differences between groups in extrapyramidal side effects, including akathisia, weight gain, or BMI in phase 1 or 2 (Supplemental Table 1C, http://links.lww.com/JCP/A668). Changes in plasma prolactin levels were analyzed in male and female patients separately, as the prolactin increase in response to APD treatment has a strong sex effect.56 Time (P > 0.28) or time × dose group interactions (P > 0.17) were not significant for male or female patients (Supplemental Table 1C, http://links.lww.com/JCP/A668). Galactorrhea occurred in 1 patient receiving lurasidone 80 mg/d. Three patients in the 80-mg/d group and 1 patient in the 240-mg/d group (6.0% in combined group) withdrew because of lack of efficacy, which is not significantly different (Fisher exact test, P = 0.61). The all-cause dropout rate was 34.3%, which is relatively low for a study of this duration.
DISCUSSION
The major findings of this study are as follows: (1) during the 6-week open treatment with lurasidone 80 mg/d (phase 1), only 3 patients met a priori criteria for significant improvement confirming the treatment resistance of the subjects; (2) in phase 2, lurasidone 80 and 240 mg/d produced statistically significant improvement in PANSS-Total, subscales, and functional measures between 6 and 24 weeks of additional treatment with lurasidone, without evidence for a dose-related effect; (3) the rate and time course of response to lurasidone in the combined group at 24 weeks, 41.8% (28/67), are comparable with that previously reported for clozapine, olanzapine and risperidone in TRS patients; (4) lurasidone significantly improved executive function and speed of processing at both doses, with the lower dose achieving greater improvement in executive function; and (5) both doses of lurasidone were well tolerated, with minimal side effects. Thus, lurasidone improved psychopathology in a significant proportion of TRS patients at a rate and time course comparable with that previously reported for clozapine and some other AAPDs with similar but not identical pharmacology, suggesting that they may be differentially beneficial for some TRS patients.
Improvement in Psychopathology in Phases 1 and 2
Time to Response
Only 3 (4.47%) of 67 adjudged on clinical grounds to meet the TRS criteria improved during phase 1, confirming that the patient population was resistant to treatment of schizophrenia. These 3 responders had all received adequate trials of other AAPDs, raising the possibility that they may have met the criteria for TRS but were rapid responders to lurasidone, just as non-TRS patients who respond rapidly to lurasidone have been identified.34–37 We have reported 4 classes of genes, including some schizophrenia risk genes and a number of genes that affect synaptic plasticity, which predict improvement in PANSS-Total scores, including the PANNS cognitive subscale, in clinical trials with lurasidone in non-TRS patients, some in a time-dependent manner.57,58 Significant improvement in psychopathology in the TRS patients occurred by week 6 of phase 2, 12 weeks after beginning treatment with 80 mg/d lurasidone. However, first improvement in psychopathology was more frequent at later times. The responder rate by the 12th week of phase 2 was significantly greater than after 6-week treatment. The differences in time to improvement in both psychopathology and cognition may be based on differences in time for neuroplastic changes in synapses to emerge and stabilize.30 In addition, of the 28 of 67 patients (41.8%) who experienced ≥20% improvement in PANSS-Total at any point, 19 (67.9%) first did so only after 6 weeks of treatment in phase 2. A similar prolonged time to improvement in PANSS scores in TRS patients treated with a variety of AAPDs has been previously noted with clozapine5,7,8 and olanzapine.7
Responder Rate
This trial was similar in design to 2 previous studies of AAPDs in TRS, with the exception that those trials did not include an open treatment phase to confirm TRS.7,16 The response rates at 6 and 24 weeks in those trials were 7% and 60% for clozapine and 18% and 50% for olanzapine,7 comparable with what was found here. Long-acting injectable risperidone response rates at 6 and 24 weeks were 22.2% and 45.8%, and 22.1% and 45.5%, for 50- and 100-mg doses, respectively.16 Although nearly 60% of the TRS patients did not respond to lurasidone with improvement in PANSS-Total scores by ≥20% during the 6-month trial, a similar proportion of TRS patients fail to respond to clozapine.5–7,11 Siskind et al11 performed a meta-analysis of clozapine response in TRS, which reported a 40% response rate. It is unlikely that higher doses of lurasidone would be more effective, but it is possible that the nonresponders might have responded to even longer trials with lurasidone, because the response rate from week 12 through 24 was comparable with that of the earlier periods. We found no evidence that nonresponse to lurasidone in this trial was related to nonadherence or the effect of concomitant meds. It is more likely that heterogeneity in genetics in relation to differences among the AAPDs in pharmacology is responsible. Interestingly, 7 (63.6%) of 11 patients who failed on clozapine responded to lurasidone. This suggests that trials of other AAPDs not previously tried in TRS patients, especially of longer duration, may be of value for those patients who fail clozapine. In addition, trials of augmentation strategies, for example, electroconvulsive shock treatment, magnetic brain stimulation,59,60 or selective pharmacologic ligands effective in preclinical studies, for example, DA D1 or D4 agonists,33,61,62 should be considered in TRS patients who fail monotherapy with clozapine or other AAPDs.
Dosage
We found no advantage to the 240-mg/d lurasidone dose over the 80-mg/d dose, the dose frequently used in non-TRS patients.36,37 On the other hand, the effective olanzapine dosage for patients with TRS in one study was reported to be 30 to 50 mg/d,7 significantly higher than that required for non-TRS patients, 15 to 20 mg/d. However, the design of the olanzapine study did not permit the determination of whether lower doses for longer periods would have produced a comparable response. Long-acting injectable risperidone, 50 versus 100 mg 4 times a day for 2 weeks, was not significantly different in efficacy for TRS patients, although plasma risperidone levels in the 100-mg group was nearly twice that in the 50-m/dg group.16 Taken together, it seems that standard doses of some AAPDs are adequate for TRS patients, if they are going to be effective and should be preferred. Higher doses may cause more severe side effects, including extrapyramidal symptoms, weight gain, sedation, and prolactin elevations. Thus, this study suggests the 80-mg dose of lurasidone, which produces greater improvement in cognition, is preferred over the 240-mg dose.
Comparison With Other AAPDs
Our previous studies with risperidone and olanzapine in TRS patients also found time-dependent improvement in psychopathology,7,16 similar in extent and frequency to the improvement with lurasidone reported here. Considered together, these results indicate that AAPDs with pharmacology similar to that of clozapine benefit many but not all TRS patients, and that time to response is often delayed by several months compared with non-TRS patients. Recently, a network meta-analysis of randomized clinical trials with a mean trial duration of 11 weeks reported that the AAPDs, clozapine, risperidone, olanzapine, and ziprasidone did not differ in efficacy in TRS patients and were, as previously shown for clozapine,1 more effective than TAPDs.19 Another meta-analysis of randomized controlled trials of clozapine versus 25 other comparators, including both AAPDs and TAPDs, concluded that clozapine was superior for total symptoms, but only in the short term, not the long term, which included 6 months in most of the 11 studies surveyed.20 The more complex pharmacology of the AAPDs and the absence of strong D2 receptor blockade are the likely bases for this advantage (see Meltzer10,29 for additional discussion of this issue).
Improvement in Cognition
The selective improvement in DSST and WCST-Categories during phase 2, but not phase 1, indicates that lurasidone produces improvement in some domains of cognition in a time-dependent manner. It is necessary to consider whether this improvement was a practice effect, or secondary to the improvement in psychopathology or secondary to the improvement in psychopathology. There is no evidence to suggest that performance on these 2 tests is more likely to improve because of practice effects than the other tests.63 Other AAPDs that have been shown to improve cognition in schizophrenia also improve some, but not all domains.7,9,16,27–29 The practice effect on cognition in schizophrenia in clinical trials has been found to be small.63 The selective improvement by lurasidone of some cognitive tests could result from differences in the brain circuitry required for specific domains. The pharmacology of lurasidone that may be important for cognition, its potent 5-HT1A partial agonism, 5-HT 7 antagonism, and weak D4 antagonism, complemented by DA, acetylcholine, and a large efflux of glutamate in the cortex and hippocampus, are partially shared by other AAPDs.31,62,64–66 This pharmacology and other mechanisms, such as GABAergic mechanisms, are likely to participate in the ability of the other AAPDs to improve some domains of cognition, with genetically based differences in response to this pharmacology accounting for the patient response differences.29,57,66,67 Cholinergic mechanisms are an example of this. We recently demonstrated that lurasidone's ability to improve episodic memory in rats impaired by subchronic treatment with phencyclidine did not require muscarinic receptor stimulation, whereas that due to clozapine or olanzapine did.68 This suggests that lurasidone might improve episodic memory in patients with minimal muscarinic activity, but clozapine and olanzapine would not. The 80-mg/d dose of lurasidone improved WCST-Categories, a frontal lobe cognitive measure, and did not worsen WCST-Percent Perseveration, as occurred in the patients treated with 240 mg/d, suggesting an advantage for the lower dose for executive function. This is supported by the results of an eye movement study conducted in a subset of the patients who participate in the study reported here.69 Eye movement measures can provide a biomarker to evaluate pharmacologic effects on brain circuits involved in cognition.69 Treatment with low-dose lurasidone was associated with improved executive control of attention reflected by reduced antisaccade errors. High-dose lurasidone resulted in prolonged speed of reflexive and executive shifts of attention and reduced spatial working memory compared with low-dose lurasidone.69 Improvement in WCST-Categories was independent of improvement of PANSS. Improvement in some domains of cognition has been reported with clozapine, olanzapine, and risperidone in prior studies,7,13,16 but only lurasidone produced improvement in executive function, a critically important cognitive domain that is notably resistant to improvement with AAPDs.70,71 The improvement in these 2 domains of cognition is consistent with results in non-TRS schizophrenia patients in a 6-month study of the cognitive effects of lurasidone, which found improvement in processing speed, reasoning, and problem solving.72,73 Improvement in cognition in TRS patients independent of improvement in psychopathology has also been reported for clozapine, olanzapine, and risperidone.7,16,27,74
Tolerability
The benign side effect profiles of lurasidone at both doses are consistent with previous reports in non-TRS patients.34–38 No significant plasma prolactin elevation was observed in both dose groups. An all-cause dropout rate of 34.3% is similar to the prior report from meta-analysis in patients with TRS.19
Limitations of the Study
There are no validated biomarkers with sufficient specificity and sensitivity to make the diagnosis of TRS, although we have reported that L-dopa decarboxylase, a key enzyme in the action of clozapine and formation of trace amines, has some limited values in this regard.75 Some pieces of evidence showing that the patients in this study met the TRS criteria, the baseline PANSS scores, number of prior hospitalizations, and level of function (Table 1) are similar to those of subjects in previous TRS trials7,13,16 as well as the subjects in the recent meta-analysis of TRS clinical trials,19,20 reporting the absence of improvement in the first 6 weeks of lurasidone treatment (phase 1), a period in which PANSS scores improve significantly in non-TRS patients during treatment with lurasidone.34,36–38 Because this study did not include a control group, it is possible that the improvement in psychopathology and cognition that occurred during phase 2 was the result of a beneficial effect of clinical trial participation rather than specific drug effects. The patients in this study received the same level of case management and contact with their referring clinicians during the course of this study, as they have received for years in most cases, with no additional effort from the research staff to supplement the ongoing psychosocial treatment. Study participation would not be expected to provide improvement in specific cognitive domains.
The possibility that the raters were biased to report no change in PANSS ratings during phase 1 and improvement in phase 2 was carefully considered. Raters were instructed to identify improvement in PANNS scores in phase 1 to prevent non-TRS subjects from being enrolled in phase 2, as these individuals would diminish the possibility of finding a time × drug effect in phase 2. The possibility of bias to differentially rate some patients as improved in phase 2 based on dose was minimized by the double-blind design and 2-drug doses. The absence of side effects that were dose related enabled the blinding to be effective throughout the study. The fact that only 42% of patients were rated as responders at the end of phase 2 and that most, but not all, of that improvement occurred after the first 6 weeks of phase 2 is evidence against a bias for expecting improvement in phase 2. Subsequent studies that include less effective treatments for TRS, for example, TAPD, and comparators such as clozapine are needed to provide a definitive confirmation of the results reported here. However, including clozapine as a control group would have required weekly blood drawings and greatly increased the difficulty of conducting this study and its cost.
Mechanism of Action of Lurasidone and Other AAPDs in TRS
The results of this study, together with previous studies of other AAPDs from our laboratory7,13,16 and the meta-analysis of TRS studies with clozapine, risperidone, quetiapine, and olanzapine,19,20 indicate that a significant proportion of TRS patients, approximately 40% in total, respond to AAPDs, but do so more slowly than non-TRS patients. The receptor profile common to these AAPDs, which differentiates them from the TAPDs, is more potent 5-HT2A receptor blockade relative to DA D2 receptor blockade.64 This has added significance because the in vivo ratios of the affinities of D2 to 5-HT2A, as well as ratios of D2 to 5-HT1A and 5-HT2C, in rats were recently reported to predict prefrontal striatal functional connectivity,76 a key measure that has been reported to predict response to AAPDs in first-episode schizophrenia patients,77 supporting the importance of these ratios as relevant to response to AAPDs. Other AAPDs, for example, asenapine, ziprasidone, and iloperidone, also share this core pharmacology of lurasidone and clozapine. Further study is needed to determine if the latter drugs are also effective in some TRS patients.78 Differential effects in TRS patients are to be expected because of the many differences among them with regard to direct and indirect 5-HT1A partial agonism; 5-HT7 antagonism; D4 agonism versus D4 antagonism; GABAergic, cholinergic, noradrenergic, and histaminergic mechanisms; and effects on cortical, hippocampal, and striatal DA, norepinephrine, acetylcholine, and glutamate release, as well as on neurotrophins (eg, brain-derived neurotrophic factor and neuregulin).29,33,64–66 The slow onset of response to lurasidone and other atypicals in most TRS patients suggests that immediate receptor-mediated actions affects gene expression leading to metaplastic changes in synaptic structure and function that are the ultimate basis for improvement in psychopathology, cognition, and ultimately work and social function in TRS patients.30,79–82 We have recently demonstrated that genes associated with prediction of the response of acutely psychotic patients to lurasidone are related to synaptic proteins in both excitatory and inhibitory synapses.57,58 Thus, it is important to determine if TRS patients who do not respond to one AAPD might respond to another. Multiple trials of up to 6 months each may be needed to identify an AAPD, which improves psychopathology and cognition in specific patients. The ultimate goal is to find robust biomarkers that predict individual response (precision medicine) to specific AAPDs, avoiding failed trials, each of which could require months of treatment.
CONCLUSIONS
Lurasidone 80 and 240 mg/d produced comparable, clinically significant improvements in psychopathology, cognition, and functional measures over a 24-week period in 42% of stable TRS patients who showed no significant improvement in any of these measures during a preceding 6-week open trial with lurasidone. The improvement with lurasidone after a total of 30-week treatment during phases 1 and 2 was comparable with that of previous studies, using a similar design, with adequate doses of risperidone, clozapine, or olanzapine.7,16 The value of a 6- to 8-month trial duration to determine improvement with an AAPD in TRS was confirmed. This study also demonstrated that 80 mg/d of lurasidone, which is an effective dose for non-TRS patients, was an effective dose for TRS patients but required longer duration of treatment. In addition, the lower dose was more effective in improving executive function as measured by cognitive testing and an accompanying eye movement study.69 Because of the absence of a comparator in this study, a direct comparison of lurasidone with clozapine and other AAPDs is needed to confirm or reject the efficacy of lurasidone in TRS.
Supplementary Material
AUTHOR DISCLOSURE INFORMATION
This study was supported by a grant from Sunovion, a subsidiary of Dainippon Sumitomo, which developed and markets lurasidone, and was registered on www.ClinicalTrials.gov (NCT01569659).
H.Y.M. receives, or has received, other grant support from ACADIA, Allergan, Dainippon Sumitomo, Sunovion, Eli Lilly, Janssen, Lundbeck, Neurocrine, and Reviva. H.Y.M. also receives support from the Weisman family. None of the other authors have potential conflicts of interest.
Footnotes
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal’s Web site (www.psychopharmacology.com).
REFERENCES
- 1.Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45:789–796. [DOI] [PubMed] [Google Scholar]
- 2.Meltzer HY. Treatment-resistant schizophrenia—the role of clozapine. Curr Med Res Opin. 1997;14:1–20. [DOI] [PubMed] [Google Scholar]
- 3.Elkis H. Treatment-resistant schizophrenia. Psychiatr Clin North Am. 2007;30:511–533. [DOI] [PubMed] [Google Scholar]
- 4.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 5.Meltzer HY. Duration of a clozapine trial in neuroleptic-resistant schizophrenia. Arch Gen Psychiatry. 1989;46:672. [DOI] [PubMed] [Google Scholar]
- 6.Ciapparelli A, Dell'Osso L, Bandettini di Poggio A, et al. Clozapine in treatment-resistant patients with schizophrenia, schizoaffective disorder, or psychotic bipolar disorder: a naturalistic 48-month follow-up study. J Clin Psychiatry. 2003;64:451–458. [DOI] [PubMed] [Google Scholar]
- 7.Meltzer HY, Bobo WV, Roy A, et al. A randomized, double-blind comparison of clozapine and high-dose olanzapine in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2008;69:274–285. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki T, Remington G, Arenovich T, et al. Time course of improvement with antipsychotic medication in treatment-resistant schizophrenia. Br J Psychiatry. 2011;199:275–280. [DOI] [PubMed] [Google Scholar]
- 9.Meltzer HY. Clozapine: balancing safety with superior antipsychotic efficacy. Clin Schizophr Relat Psychoses. 2012;6:134–144. [DOI] [PubMed] [Google Scholar]
- 10.Meltzer HY. Update on typical and atypical antipsychotic drugs. Annu Rev Med. 2013;64:393–406. [DOI] [PubMed] [Google Scholar]
- 11.Siskind D, Siskind V, Kisely S. Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can J Psychiatry. 2017;62:772–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasan A, Falkai P, Wobrock T, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry. 2012;13:318–378. [DOI] [PubMed] [Google Scholar]
- 13.Sumiyoshi T, Jayathilake K, Meltzer HY. A comparison of two doses of melperone, an atypical antipsychotic drug, in the treatment of schizophrenia. Schizophr Res. 2003;62:65–72. [DOI] [PubMed] [Google Scholar]
- 14.Volavka J, Czobor P, Sheitman B, et al. Clozapine, olanzapine, risperidone, and haloperidol in the treatment of patients with chronic schizophrenia and schizoaffective disorder. Am J Psychiatry. 2002;159:255–262. [DOI] [PubMed] [Google Scholar]
- 15.Conley RR, Kelly DL, Nelson MW, et al. Risperidone, quetiapine, and fluphenazine in the treatment of patients with therapy-refractory schizophrenia. Clin Neuropharmacol. 2005;28:163–168. [DOI] [PubMed] [Google Scholar]
- 16.Meltzer HY, Lindenmayer JP, Kwentus J, et al. A six month randomized controlled trial of long acting injectable risperidone 50 and 100mg in treatment resistant schizophrenia. Schizophr Res. 2014;154:14–22. [DOI] [PubMed] [Google Scholar]
- 17.Kane JM, Meltzer HY, Carson WH, et al. Aripiprazole Study Group Aripiprazole for treatment-resistant schizophrenia: results of a multicenter, randomized, double-blind, comparison study versus perphenazine. J Clin Psychiatry. 2007;68:213–223. [PubMed] [Google Scholar]
- 18.Lindenmayer JP, Citrome L, Khan A, et al. A randomized, double-blind, parallel-group, fixed-dose, clinical trial of quetiapine at 600 versus 1200 mg/d for patients with treatment-resistant schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2011;31:160–168. [DOI] [PubMed] [Google Scholar]
- 19.Samara MT, Dold M, Gianatsi M, et al. Efficacy, acceptability, and tolerability of antipsychotics in treatment-resistant schizophrenia: a network meta-analysis. JAMA Psychiat. 2016;73:199–210. [DOI] [PubMed] [Google Scholar]
- 20.Siskind D, McCartney L, Goldschlager R, et al. Clozapine vs first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209:385–392. [DOI] [PubMed] [Google Scholar]
- 21.Pan B, Huang XF, Deng C. Antipsychotic treatment and neuregulin 1-ErbB4 signalling in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:924–930. [DOI] [PubMed] [Google Scholar]
- 22.Mostaid MS, Lee TT, Chana G, et al. Elevated peripheral expression of neuregulin-1 (NRG1) mRNA isoforms in clozapine-treated schizophrenia patients. Transl Psychiatry. 2017;7:1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snigdha S, Horiguchi M, Huang M, et al. Attenuation of phencyclidine-induced object recognition deficits by the combination of atypical antipsychotic drugs and pimavanserin (ACP 103), a 5-hydroxytryptamine (2A) receptor inverse agonist. J Pharmacol Exp Ther. 2010;332:622–631. [DOI] [PubMed] [Google Scholar]
- 24.de Bartolomeis A, Balletta R, Giordano S, et al. Differential cognitive performances between schizoprenic responders and nonresponders to antipsychotics: correlation with course of illness, psychopathology, attitude to the treatment and antipsychotics doses. Psychiatry Res. 2013;210:387–395. [DOI] [PubMed] [Google Scholar]
- 25.Frydecka D, Beszlej JA, Gościmski P, et al. Profiling cognitive impairment in treatment-resistant schizophrenia patients. Psychiatry Res. 2016;235:133–138. [DOI] [PubMed] [Google Scholar]
- 26.Anderson VM, McIlwain ME, Kydd RR, et al. Does cognitive impairment in treatment-resistant and ultra-treatment-resistant schizophrenia differ from that in treatment responders? Psychiatry Res. 2015;230:811–818. [DOI] [PubMed] [Google Scholar]
- 27.Hagger C, Buckley P, Kenny JT, et al. Improvement in cognitive functions and psychiatric symptoms in treatment-refractory schizophrenic patients receiving clozapine. Biol Psychiatry. 1993;34:702–712. [DOI] [PubMed] [Google Scholar]
- 28.Lee MA, Thompson PA, Meltzer HY. Effects of clozapine on cognitive function in schizophrenia. J Clin Psychiatry. 1994;55(suppl B):82–87. [PubMed] [Google Scholar]
- 29.Meltzer HY. Pharmacotherapy of cognition in schizophrenia. Curr Opin Behav Sci. 2015;4:115–121. [Google Scholar]
- 30.Molteni R, Calabrese F, Racagni G, et al. Antipsychotic drug actions on gene modulation and signaling mechanisms. Pharmacol Ther. 2009;124:74–85. [DOI] [PubMed] [Google Scholar]
- 31.Huang M, Panos JJ, Kwon S, et al. Comparative effect of lurasidone and blonanserin on cortical glutamate, dopamine, and acetylcholine efflux: role of relative serotonin (5-HT)2A and DA D2 antagonism and 5-HT1A partial agonism. J Neurochem. 2014;128:938–949. [DOI] [PubMed] [Google Scholar]
- 32.Kusumi I, Boku S, Takahashi Y. Psychopharmacology of atypical antipsychotic drugs: from the receptor binding profile to neuroprotection and neurogenesis. Psychiatry Clin Neurosci. 2015;69:243–258. [DOI] [PubMed] [Google Scholar]
- 33.Miyauchi M, Neugebauer NM, Meltzer HY. Dopamine D4 receptor stimulation contributes to novel object recognition: relevance to cognitive impairment in schizophrenia. J Psychopharmacol. 2017;31:442–452. [DOI] [PubMed] [Google Scholar]
- 34.Meltzer HY, Cucchiaro J, Silva R, et al. Lurasidone in the treatment of schizophrenia: a randomized, double-blind, placebo- and olanzapine-controlled study. Am J Psychiatry. 2011;168:957–967. [DOI] [PubMed] [Google Scholar]
- 35.Citrome L, Cucchiaro J, Sarma K, et al. Long-term safety and tolerability of lurasidone in schizophrenia: a 12-month, double-blind, active-controlled study. Int Clin Psychopharmacol. 2012;27:165–176. [DOI] [PubMed] [Google Scholar]
- 36.Loebel A, Cucchiaro J, Sarma K, et al. Efficacy and safety of lurasidone 80 mg/day and 160 mg/day in the treatment of schizophrenia: a randomized, double-blind, placebo- and active-controlled trial. Schizophr Res. 2013;145:101–109. [DOI] [PubMed] [Google Scholar]
- 37.Nasrallah HA, Silva R, Phillips D, et al. Lurasidone for the treatment of acutely psychotic patients with schizophrenia: a 6-week, randomized, placebo-controlled study. J Psychiatr Res. 2013;47:670–677. [DOI] [PubMed] [Google Scholar]
- 38.Ogasa M, Kimura T, Nakamura M, et al. Lurasidone in the treatment of schizophrenia: a 6-week, placebo-controlled study. Psychopharmacology (Berl). 2013;225:519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.First MB, Williams Janet BW, Spitzer RL, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Clinical Trials Version (SCID-CT). New York: Biometrics Research, Institute New York State Psychiatric Institute; 2007. [Google Scholar]
- 40.Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 41.Guy W. ECDEU Assessment Manual for Psychopharmacology: Revised (DHEW Publication Number ADM 76–338). Rockville, MD: US Department of Health, Education and Welfare, Public Health Service, Alcohol, Drug Abuse and Mental Health Administration, NIMH Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976:534–537. [Google Scholar]
- 42.Morosini PL, Magliano L, Brambilla L, et al. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand. 2000;101:323–329. [PubMed] [Google Scholar]
- 43.Robinson AL, Heaton RK, Lehman RA, et al. The utility of the Wisconsin Card Sorting Test in detecting and localizing frontal lobe lesions. J Consult Clin Psychol. 1980;48:605–614. [DOI] [PubMed] [Google Scholar]
- 44.Wechsler D. Wechsler Intelligence Scale for Children—Revised (WISC-R) Manual. New York, NY: Psychological Corporation; 1974. [Google Scholar]
- 45.Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms and Commentary. 2nd ed New York: Oxford University Press; 1998. [Google Scholar]
- 46.Sager MA, Hermann BP, La Rue A, et al. Screening for dementia in community-based memory clinics. WMJ. 2006;105:25–29. [PubMed] [Google Scholar]
- 47.Peterson LR, Peterson MJ. Short-term retention of individual verbal items. J Exp Psychol. 1959;58:193–198. [DOI] [PubMed] [Google Scholar]
- 48.Wechsler D. Wechsler Adult Intelligence Scale—Revised (WAIS-R). Cleveland, OH: The Psychological Corporation, Harcourt Brace Jovanovich; 1981. [Google Scholar]
- 49.Rey A. L'examen clinique en psychologie [Clinical Tests in Psychology]. Paris, France: Presses Universitaires de France; 1964. [Google Scholar]
- 50.Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–676. [DOI] [PubMed] [Google Scholar]
- 51.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–19. [DOI] [PubMed] [Google Scholar]
- 52.Liu G, Gould AL. Comparison of alternative strategies for analysis of longitudinal trials with dropouts. J Biopharm Stat. 2002;12:207–226. [DOI] [PubMed] [Google Scholar]
- 53.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 1st ed Hillsdale, NJ: Lawrence Erlbaum Associates; 1977. [Google Scholar]
- 54.Stewart SA. The effects of benzodiazepines on cognition. J Clin Psychiatry. 2005;66(suppl 2):9–13. [PubMed] [Google Scholar]
- 55.Ogino S, Miyamoto S, Miyake N, et al. Benefits and limits of anticholinergic use in schizophrenia: focusing on its effect on cognitive function. Psychiatry Clin Neurosci. 2014;68:37–49. [DOI] [PubMed] [Google Scholar]
- 56.López-Rodríguez R, Román M, Novalbos J, et al. DRD2 Taq1A polymorphism modulates prolactin secretion induced by atypical antipsychotics in healthy volunteers. J Clin Psychopharmacol. 2011;31:555–562. [DOI] [PubMed] [Google Scholar]
- 57.Li J, Loebel A, Meltzer HY. Identifying the genetic risk factors for treatment response to lurasidone by genome-wide association study: a meta-analysis of samples from three independent clinical trials. Schizophr Res. 2018;199:203–213. [DOI] [PubMed] [Google Scholar]
- 58.Li J, Yoshikawa A, Brennan MD, et al. Genetic predictors of antipsychotic response to lurasidone identified in a genome wide association study and by schizophrenia risk genes. Schizophr Res. 2018;192:194–204. [DOI] [PubMed] [Google Scholar]
- 59.Arumugham SS, Thirthalli J, Andrade C. Efficacy and safety of combining clozapine with electrical or magnetic brain stimulation in treatment-refractory schizophrenia. Expert Rev Clin Pharmacol. 2016;9:1245–1252. [DOI] [PubMed] [Google Scholar]
- 60.Dold M, Leucht S. Pharmacotherapy of treatment-resistant schizophrenia: a clinical perspective. Evid Based Ment Health. 2014;17:33–37. [DOI] [PubMed] [Google Scholar]
- 61.Meltzer HY, Rajagopal L, Matrisciano F, et al. The allosteric dopamine D1 receptor potentiator, DETQ, ameliorates subchronic phencyclidine-induced object recognition memory deficits and enhances cortical acetylcholine efflux in male humanized D1 receptor knock-in mice. Behav Brain Res. 2019;361:139–150. [DOI] [PubMed] [Google Scholar]
- 62.Huang M, Kwon S, He W, et al. Neurochemical arguments for the use of dopamine D4 receptor stimulation to improve cognitive impairment associated with schizophrenia. Pharmacol Biochem Behav. 2017;157:16–23. [DOI] [PubMed] [Google Scholar]
- 63.Keefe RSE, Davis VG, Harvey PD, et al. Placebo response and practice effects in schizophrenia cognition trials. JAMA Psychiat. 2017;74:807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meltzer HY, Huang M. In vivo actions of atypical antipsychotic drug on serotonergic and dopaminergic systems. Prog Brain Res. 2008;172:177–197. [DOI] [PubMed] [Google Scholar]
- 65.Huang M, Kwon S, Rajagopal L, et al. 5-HT1A parital agonism and 5-HT7 antagonism restore episodic memory in subchronic phencyclidine-treated mice: role of brain glutamate, dopamine, acetylcholine and GABA. Psychopharmacology (Berl). 2018;235:2795–2808. [DOI] [PubMed] [Google Scholar]
- 66.Rajagopal L, Soni D, Meltzer HY. Neurosteroid pregnenolone sulfate, alone and as augmentation of lurasidone or tandospirone, rescues phencyclidine-induced deficits in cognitive function and social interaction. Behav Brain Res. 2018;350:31–43. [DOI] [PubMed] [Google Scholar]
- 67.Harvey PD, Ogasa M, Cucchiaro J, et al. Performance and interview-based assessments of cognitive change in a randomized double-blind comparison of lurasidone vs ziprasidone. Schizophr Res. 2011;127:188–194. [DOI] [PubMed] [Google Scholar]
- 68.Miyauchi M, Neugebauer NM, Sato T, et al. Muscarinic receptor signaling contributes to atypical antipsychotic drug reversal of the phencyclidine-induced deficit in novel object recognition in rats. J Psychopharmacol. 2017;31:1588–1604. [DOI] [PubMed] [Google Scholar]
- 69.Karpouzian-Rogers T, Stocks J, Meltzer HY, et al. The effect of high vs. low dose lurasidone on eye movement biomarkers of prefrontal abilities in treatment-resistant schizophrenia. Schizophr Res. 2020;215:314–321. [DOI] [PubMed] [Google Scholar]
- 70.Harvey PD, Keefe RS. Studies of cognitive change in patients with schizophrenia following novel antipsychotic treatment. Am J Psychiatry. 2001;158:176–184. [DOI] [PubMed] [Google Scholar]
- 71.Woodward ND, Purdon SE, Meltzer HY, et al. A meta-analysis of neuropsychological change to clozapine, olanzapine, quetiapine, and risperidone in schizophrenia. Int J Neuropsychopharmacol. 2005;8:457–472. [DOI] [PubMed] [Google Scholar]
- 72.Harvey PD, Siu CO, Hsu J, et al. Effect of lurasidone on neurocognitive performance in patients with schizophrenia: a short-term placebo- and active-controlled study followed by a 6-month double-blind extension. Eur Neuropsychopharmacol. 2013;23:1373–1382. [DOI] [PubMed] [Google Scholar]
- 73.Harvey PD, Siu CO, Ogasa M, et al. Effect of lurasidone dose on cognition in patients with schizophrenia: post-hoc analysis of a long-term, double-blind continuation study. Schizophr Res. 2015;166:334–338. [DOI] [PubMed] [Google Scholar]
- 74.Bilder RM, Goldman RS, Volavka J, et al. Neurocognitive effects of clozapine, olanzapine, risperidone, and haloperidol in patients with chronic schizophrenia or schizoaffective disorder. Am J Psychiatry. 2002;159:1018–1028. [DOI] [PubMed] [Google Scholar]
- 75.Li J, Meltzer HY. A genetic locus in 7p12.2 associated with treatment resistant schizophrenia. Schizophr Res. 2014;159:333–339. [DOI] [PubMed] [Google Scholar]
- 76.Tollens F, Gass N, Becker R, et al. The affinity of antipsychotic drugs to dopamine and serotonin 5-HT2 receptors determines their effects on prefrontal-striatal functional connectivity. Eur Neuropsychopharmacol. 2018;28:1035–1046. [DOI] [PubMed] [Google Scholar]
- 77.Sarpal DK, Robinson DG, Lencz T, et al. Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA Psychiat. 2015;72:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bark N, Lawson N, Trigoboff E, et al. Among the severely mentally ill, who responds to ziprasidone? Clin Schizophr Relat Psychoses. 2018;12:77–85. [DOI] [PubMed] [Google Scholar]
- 79.Buonaguro EF, Iasevoli F, Marmo F, et al. Re-arrangements of gene transcripts at glutamatergic synapses after prolonged treatments with antipsychotics: a putative link with synaptic remodeling. Prog Neuropsychopharmacol Biol Psychiatry. 2017;76:29–41. [DOI] [PubMed] [Google Scholar]
- 80.Sainz J, Prieto C, Ruso-Julve F, et al. Blood gene expression profile predicts response to antipsychotics. Front Mol Neurosci. 2018;11:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luoni A, Rocha FF, Riva MA. Anatomical specificity in the modulation of activity-regulated genes after acute or chronic lurasidone treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2014;50:94–101. [DOI] [PubMed] [Google Scholar]
- 82.Tarazi FI, Riva MA. The preclinical profile of lurasidone: clinical relevance for the treatment of schizophrenia. Expert Opin Drug Discov. 2013;8:1297–1307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


