Abstract
Aedes aegypti is the primary vector of dengue, chikungunya, Zika, and urban yellow fever. Insecticides are often the most effective tools to rapidly decrease the density of vector populations, especially during arbovirus disease outbreaks. However, the intense use of insecticides, particularly pyrethroids, has selected for resistant mosquito populations worldwide. Mutations in the voltage gated sodium channel (NaV) are among the principal mechanisms of resistance to pyrethroids and DDT, also known as “knockdown resistance,” kdr. Here we report studies on the origin and dispersion of kdr haplotypes in samples of Ae. aegypti from its worldwide distribution. We amplified the IIS6 and IIIS6 NaV segments from pools of Ae. aegypti populations from 15 countries, in South and North America, Africa, Asia, Pacific, and Australia. The amplicons were barcoded and sequenced using NGS Ion Torrent. Output data were filtered and analyzed using the bioinformatic pipeline Seekdeep to determine frequencies of the IIS6 and IIIS6 haplotypes per population. Phylogenetic relationships among the haplotypes were used to infer whether the kdr mutations have a single or multiple origin. We found 26 and 18 haplotypes, respectively for the IIS6 and IIIS6 segments, among which were the known kdr mutations 989P, 1011M, 1016I and 1016G (IIS6), 1520I, and 1534C (IIIS6). The highest diversity of haplotypes was found in African samples. Kdr mutations 1011M and 1016I were found only in American and African populations, 989P + 1016G and 1520I + 1534C in Asia, while 1534C was present in samples from all continents, except Australia. Based primarily on the intron sequence, IIS6 haplotypes were subdivided into two well-defined clades (A and B). Subsequent phasing of the IIS6 + IIIS6 haplotypes indicates two distinct origins for the 1534C kdr mutation. These results provide evidence of kdr mutations arising de novo at specific locations within the Ae. aegypti geographic distribution. In addition, our results suggest that the 1534C kdr mutation had at least two independent origins. We can thus conclude that insecticide selection pressure with DDT and more recently with pyrethroids is selecting for independent convergent mutations in NaV.
Author summary
Insecticide resistance is a global threat for the control of Aedes aegypti, the mosquito vector of aboviruses such as dengue, chikungunya and Zika. Mutations in the voltage gated sodium channel (NaV), known as kdr, are one of the principal mechanisms related to resistance to pyrethroids, the class of insecticide most employed worldwide inside and around residences. We investigate whether the same kdr mutations found in Ae. aegypti populations from distinct regions of the world have a common origin and subsequently dispersed or if they emerged in unrelated populations at distinct moments. By evaluating the sequences of two fragments of the NaV gene, obtained from DNA collections of Ae. aegypti from several countries, we found at least two independent origins for the F1534C kdr mutation in American, African and Asian populations. There was no evidence for multiple origins of the common kdr mutations V1016I and P989S + V1016G, which were exclusive to American and Asian populations. Our results increase our knowledge of insecticide resistance evolution in one of the main arboviral mosquito vectors of major global diseases.
Introduction
Aedes aegypti is considered one of the most successful invasive species worldwide. Originally from Africa and now found throughout the tropics and subtropics, i t has been divided into two subspecies Aedes aegypti aegypti (Aaa) and Aedes aegypti formosus (Aaf). The subspecies Aaa, hereafter referred simply as Ae. aegypti, spread to the five continents, starting from Africa to the Americas during the human slave trade that began in the 1500’s, and later (1800’s) from the Americas to Asia and Australia [1]. Ae. aegypti is generally referred to as the yellow fever mosquito, as it was the vector of yellow fever virus in the urban cycle of the disease in the Americas until the first half of the last century and more recently in West Africa [2]. Vaccination against yellow fever and intense vector control eliminated the urban cycle of the disease in most countries by mid-20th Century [3]. However, decreasing entomological surveillance along with rapid increases in urbanization and demographic changes has led to the re-establishment of this species. Currently Ae. aegypti occurs on six continents [4], reaching high densities in urban centers of tropical and subtropical cities and acts as the primary vector of viruses causing dengue, chikungunya and, Zika, as well as re-emerging yellow fever [5]. Today, the risk of epidemic arboviral diseases is the highest in history [6] and it is predicted that in response to increased urbanization and climate change, 2.25 billion more people (or 60% of global population) will be at risk of dengue in 2080 compared to 2015 [7].
The first line of effective control of Ae. aegypti should be improvement of sanitary infrastructure, with insecticides employed as a complementary tool during high density infestation and arboviruses epidemic seasons [8]. However, insecticides have been extensively used as the primary front of action, even during low-density seasons, increasing selection for resistance. Insecticide resistance is now recognized as a major threat for the control of arboviral diseases and has likely contributed to their re-emergence and spread. Important knowledge gaps remain on mosquito insecticide resistance, including its global distribution, dynamics, mechanisms, fitness costs, and impact on vector control [9].
In contrast to the numerous insecticidal compounds available for agriculture, only a few classes of insecticides are permitted for public health purposes, such as larvicides in drinkable water tanks. Fewer are approved for spatial spraying against adult mosquitoes, for example, only pyrethroids are recommended for indoor spraying [10] and treatment of mosquito nets [11]. Pyrethroids are the most frequently used insecticides globally, due to their low toxicity to mammals, limited impact on the environment, and rapid effects on insects. This rapid action is known as the knockdown effect, which includes intense nerve firings, followed by paralysis and death. DDT has similar effects, as both compounds interact with the voltage gated sodium channel (NaV), a transmembrane protein of neuron axons [12]. Common mechanisms of resistance against pyrethroids include an increased expression of detoxifying enzyme genes, especially P450 cyp genes, or single nucleotide polymorphisms (SNPs) in the pyrethroid target NaV gene, known as kdr (knockdown resistance).
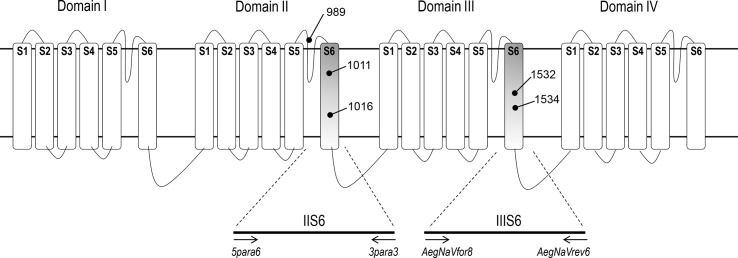
The Ae. aegypti NaV protein contains 2061 aa (amino acids) [13], with four similar domains (I-IV), each composed of six transmembrane segments (S1-S6) Fig 1. The first kdr mutation was identified in Musca domestica, at the 1014 aa site in the IIS6 segment [14]. In several other insects, including mosquitoes of the genus Anopheles and Culex, kdr mutations were identified at the same site. Mutations at several other NaV sites have also been classified as kdr mutations due to a correlated resistance to pyrethroids and/or DDT. The principal kdr mutations described in Ae. aegypti are V410L (IS6), P989S (in the link IIS5-S6), I1011M, V1016I and V1016G (IIS6), T1520I and F1534C (IIIS6) [9].
Fig 1. Scheme of the voltage gated sodium channel.
The IIS6 and IIIS6 segments are highlighted, indicating the primers (arrows showing orientation) used to amplify their corresponding DNA regions in this study. The kdr mutation sites previously found in Aedes aegypti in these segments (989, 1011, 1016, 1532 and 1534) are also indicated.
Some of these mutations, such as F1534C, are present in multiple populations around the world. This could be the due to a single origin event that spread widely or a consequence of multiple independent events. The same mutation could arise independently if there were functional restrictions on the number of resistance-inducing substitutions that would retain proper physiological function of the sodium channel, as is the case of the L1014F in An. gambiae [15]. Here, we use a next generation sequencing approach to explore the variation in the nucleotide regions encoding part of the IIS6 and IIIS6 segments of the NaV of Ae. aegypti, where the most important kdr mutations are found. We then reconstruct the evolutionary relationships among haplotypes to determine the origin of worldwide kdr mutations in Ae. aegypti.
Methods
The Ion Torrent PGM platform (Thermo Fisher Scientific Inc.) was used to sequence ~400 bases of the IIS6 and IIIS6 segments of the voltage gated sodium channel gene of Aedes aegypti (AaNaV), which contain most of the described kdr mutations.
DNA amplification
Ae. aegypti DNA was obtained from the nucleic acid collection maintained at the Powell lab at the Ecology and Evolutionary Biology Department of Yale University; generated from previous studies [16–18]. An aliquot of 1 μL (~30 ng) from single mosquito DNA preparations from the same population samples were pooled. Each pool contained an average of 30 samples (see S1 Table). Pooled DNA was then used to amplify segments IIS6 and IIIS6 of the NaV gene in separate PCR reactions, with the Phusion High-Fidelity DNA Polymerase PCR kit (New England BioLabs). Each reaction consisted of 40 μL, containing 1X Phusion Hf buffer, 200 μM dNTP, 3% DMSO, 0.4 U Polymerase, 1.0 μM of each primer, 2 μL of pooled DNA and H2O q.s. 40 μL. The primers employed were 5para6: CGGGTATTATGCGGCGAGTG x 3para3: TGGACAAAAGCAAGGCTAAG for the IIS6 segment, and AegNaVfor8: GTGGGAAAGCAGCCGATTCGC x AegNaVrev6: TGTTGAACCCGATGAACAAC for IIIS6 segment (all primers described in 5’to 3’orientation) (Fig 1). Thermocycling conditions were 93°C/30”followed by 35 cycles of 98°C/15”, 60°C/30” and 72°C/60”, with a final extension of 72°C/5’, for both reactions. The PCR products were run on a 3% agarose LE electrophoresis gel, and 400 bp bands were excised and purified with the QIAquick PCR Purification Kit (QIAGEN), following manufacturer instructions. Purified DNA was quantified in a 2100 Bioanalyzer instrument (Agilent Technologies Inc.). Purified PCR amplicons for the two NaV segments from the same population were pooled in equimolar concentrations for library preparation.
NGS Library preparation
We barcoded each population using the Ion Plus Fragment Library Kit (Thermo Fischer Scientific Inc.). Briefly, the pooled amplicons of each population were end repaired and purified with magnetic AMPure XP beads (Agencourt, Beckman Coulter), before ligation to adapters (Ion P1 Adapter and Ion Xpress Barcode X) and nick repair. The list of barcode sequences and their respective populations are presented in S1 Table. Libraries were then purified with magnetic beads and quantified with a High Sensitivity DNA chip in a 2100 Bioanalyzer (Agilent Technologies Inc.). Next, libraries were PCR amplified with Platinum PCR SuperMix High fidelity (100 μL), Library Amplification Primer Mix (5 μL), unamplified library (10 μL) and low TE buffer (15 μL), in five cycles, according to the conditions indicated in the manufacturer protocol. Amplified barcoded libraries were purified with magnetic beads and molar concentrations measured with the High Sensitivity DNA Kit (Agilent Technologies Inc.) in the Bioanalyzer. We then pooled 100 pM of each library to prepare the sequencing template. Two pooled templates were prepared, the first with library containing barcodes 1 to 48 and the second with 49 to 96.
Enriched template preparation by emulsion PCR (emPCR)
First, the template-positive Ion Sphere Particle (ISP) was prepared with the kit Ion PGM Template OT2 400 kit (ThermoFischer), using the Ion OneTouche 2 Instrument. Then, the resulting template-positive ISP suspension was enriched in the Ion OneTouch ES system, continuing the procedures indicated in the same kit. Both procedures followed the manufacturer protocol.
Sequencing
The enriched template-positive ISP was sequenced using the PGM Sequencing 400 kit (Thermo Fischer Scientific Inc.), as per manufacturer protocol. Two runs were performed, one for each pooled library template, both with an Ion 318 Chip v2.
Data analysis
Raw sequencing data was de-multiplexed, clustered and analyzed using the software package SeekDeep [19] (http://baileylab.umassmed.edu/SeekDeep). See details in S1 Text. All data generated or analyzed during this study are included in this published article and the Sequence Read Archive available at www.ncbi.nlm.nih.gov/sra (SRA Accession number: PRJNA579141, Biosample number: SAMN13092781). Haplotypes were aligned by Muscle in the Geneious software V 11.1.5 [20]. The alignments were submitted to a TCS analysis in PopArt [21] to reconstruct the haplotype networks. Phylogenetic trees were built employing the maximum likelihood model in MEGA 7 [22].
We first evaluated genetic diversity, built the haplotypic network, and performed phylogenetic analyses for each segment independently. The haplotypes were named as 2s6 or 3s6 (in reference to IIS6 and IIIS6 segments, respectively), followed by an identifying number. As evident in the Results section, the IIS6 haplotypes were grouped into A or B clades. Accordingly, we added A or B, following 2s6, to the names of IIS6 haplotypes. Although IIS6 and IIIS6 segments are ~ 44.5 Kb apart, since linkage disequilibrium in Ae. aegypti is about 50–80 Kb [distance at which correlation is halved from maximum [23, 24]], we expect variation in the two segments to be correlated. As we could not phase the haplotypes when both segments were polymorphic, haplotypes phase could be determined only in populations monomorphic for one of the two segments. In these cases, phased haplotypes were noted with the prefix “Phased” followed by their respective IIS6 and IIIS6 haplotype designations.
Temporal variation
As sequences of several Ae. aegypti populations from Brazil spanning 2001 to 2015 were available, we evaluated whether the frequencies of the observed NaV haplotypes increased or decreased over time in this country.
Results
Quality filtering
After quality filtering we obtained IIS6 and IIIS6 segment sequences for 79 and 71 populations, respectively. Specifics on the number of sequences used for each locality are listed in S2 Table.
Haplotype analyses
Spatial analyses
Analyses of the IIS6 segment resulted in 26 haplotypes, ranging from 324 to 352 bp, among which 16 were exclusive to a single population (private alleles), all in Africa (S3 Table). The sequences of these haplotypes were submitted to Genebank (accession numbers: MN602753-MN602778) and their frequencies per locality are reported in S3 Table and S1 Fig. An alignment of the haplotypes (S2 Fig) revealed that the intron had insertions and deletions (indels). The maximum likelihood tree groups these haplotypes into two well supported clades (S3 Fig), Clade A (N = 6) and Clade B (N = 20), consistent with previous reports [25]. Eighteen of the 20 haplotypes in Clade B are exclusive to Africa. Exon regions were conserved, with four non-synonymous substitutions in codons 989 (TCC/CCC: S/P), 1011 (ATG/ATA: I/M) and 1016 (GTA/ATA/GGA: V/I/G). All non-synonymous substitutions have been previously described as kdr mutations [9]. There were four codons with synonymous substitutions: amino acid 993 (TTG/TGC, haplotype 2S6_B_21) and 1002 (ATT/ATA, haplotype 2S6_B_24) in Clade B; and 1000 + 1016 (respectively GTA/GTG and TCC/TCT in the same haplotype 2S6_A_19) in clade A (Fig 2).
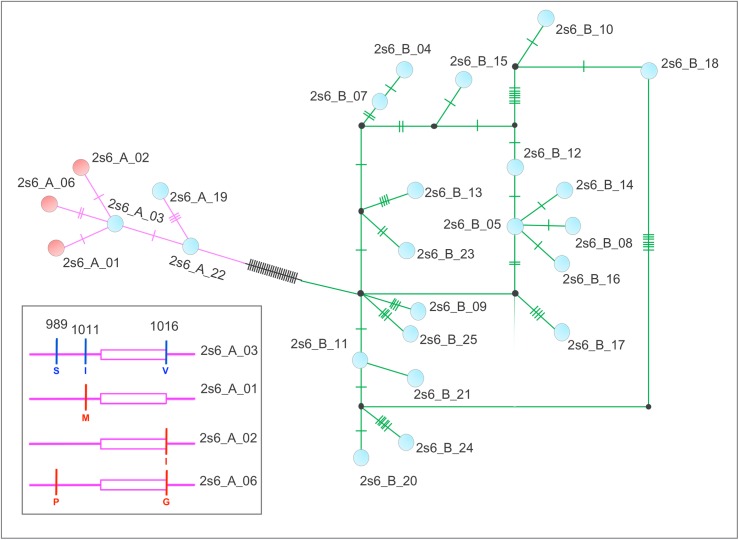
Fig 2. Haplotype Network of nucleotides corresponding to the IIS6 segment of the voltage gated sodium channel gene of Aedes aegypti populations from diverse continents.
The network was designed based on the genealogies calculated by the TCS method [21]. Each dot represents a haplotype, connected by lines in which each tick mark indicates a mutational step between connected haplotypes. Lines are color-coded to indicate Clade A (purple) or Clade B (green). Dot colors indicate haplotypes with (red) or without (blue) kdr mutations. A schematic representation of the clade with kdr haplotypes is in the box, with kdr SNPs in red.
The most common haplotype was the 2S6_B_00 (Clade B), found in 69 out of 79 populations. However, among the seven African populations sampled, only Sedhiou (Senegal) had this haplotype. Also in Clade B, the 2s6_B_04 haplotype was found in Africa (Kenya), Australia and Brazil. Interestingly, the oldest samples (2001–2009) from Brazil contained this haplotype.
In clade A, only six haplotypes were detected. The 2S6_A_03 allele was present in all continents included in the analysis. All haplotypes containing a kdr allele were placed in clade A: 2S6_A_01 (1011M), 2S6_A_02 (1016I) and 2S6_A_06 (989P + 1016G), all identical to the 2S6_A_03 haplotype and differing only at the site that defines kdr. Haplotype 2S6_A_01 was found in populations from Brazil, Colombia, and Dominica; the 2S6_A_02 in Brazil, Colombia, Haiti, Dominica, Mexico, USA and Senegal; and 2S6_A_06 only in Asian populations: Saudi Arabia, Thailand and Philippines. We did not find any haplotype carrying only the 989P or 1016G alone. The other two haplotypes in clade A, 2S6_A_19 and 2S6_A_22, were exclusive to African localities (Cameroon and Senegal, respectively).
A IIS6 haplotype network analysis shows the three kdr haplotypes (2s6_A_01, 2s6_A_02 and 2s6_A_06) directly linked to the 2s6_A_03 haplotype (Fig 2). The 2s6_A_03 haplotype was widely distributed, present in Americas (Tucson, Haiti, Venezuela and Brazil), Africa (Sedhiou and Nairobi), Asia (Cebu) and Pacific (Hawaii), and Australia (S6 Fig). The results of the network analyses together with the wide distribution of the 2s6_A_03 haplotype suggests a single origin, with an haplotype similar to 2s6_A_03 as the likely ancestor of the kdr haplotypes with derived SNPs in sites 989, 1011 and 1016.
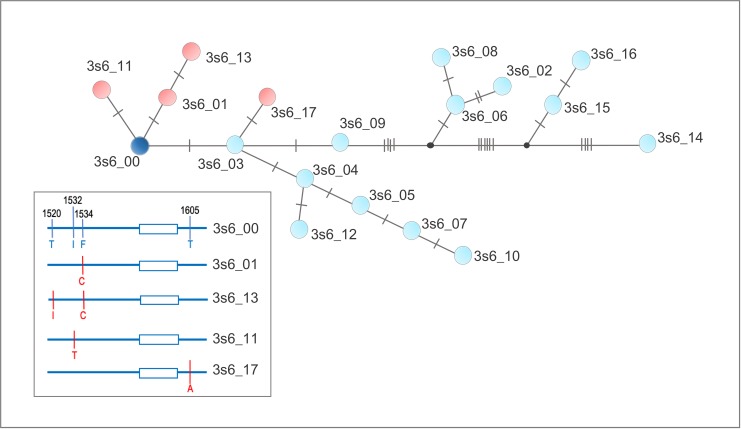
Similar analyses with sequences of the IIIS6 segment reveled 18 haplotypes between 353 and 354 bp. Nine of the haplotypes only occurred in a single population. Out of these nine, six belonged to African populations (S3 Table). Sequences are available on GenBank (accession numbers: MN602779-MN602796). The greatest variation was observed inside the intron, with 18 variable sites, and 11 SNPs found in the exons (seven synonymous and four non-synonymous (S4 and S5 Figs). The most frequent haplotype (3s6_00) was found in all continents and eleven haplotypes were specific to Africa. Haplotype 3s6_02 and 3s6_03 were found in three (Africa, South America, and Asia) and two (Africa and South America) continents, respectively. Four haplotypes presented non-synonymous substitution: 3s6_01 (1534C, found in Africa, Americas and Asia), 3s6_13 (1520I + 1534C, in Asia), 3s6_11 (1523T, in Haiti) and 3s6_17(1605A, a novel SNP found in Brazil). Contrary to the phylogenetic analyses for the IIS6 segment haplotypes, a ML tree using the IIIS6 segment haplotypes did not provide support for distinct clades (S6 Fig).
The TSC haplotype network analysis for the IIIS6 haplotypes is consistent with a single origin of the 1534C kdr mutation, which despite occurring worldwide, it was only found in two IIIS6 haplotypes (3s6_01 and 3s6_13). This network also implies that the Asian 1520I + 1534C haplotype (3s6_13) originated from 3s6_01 (Fig 3). The frequencies of both IIS6 and IIIS6 haplotypes with and without a known kdr SNP in each population are represented in Fig 4.
Fig 3. Haplotype Network of the IIIS6 segment of the voltage gated sodium channel gene of Aedes aegypti populations from diverse continents.
The network is the genealogy calculated by the TCS method [21]. Each dot represents a haplotype, connected by lines in which each slash indicates a mutational step between connected haplotypes. Red and blue dots indicate synonymous and non-synonymous substitutions, respectively. The most common haplotype 3s6_00 is in dark blue. A schematic representation with haplotypes containing non-synonymous SNPs is in the box, with SNPs in red.
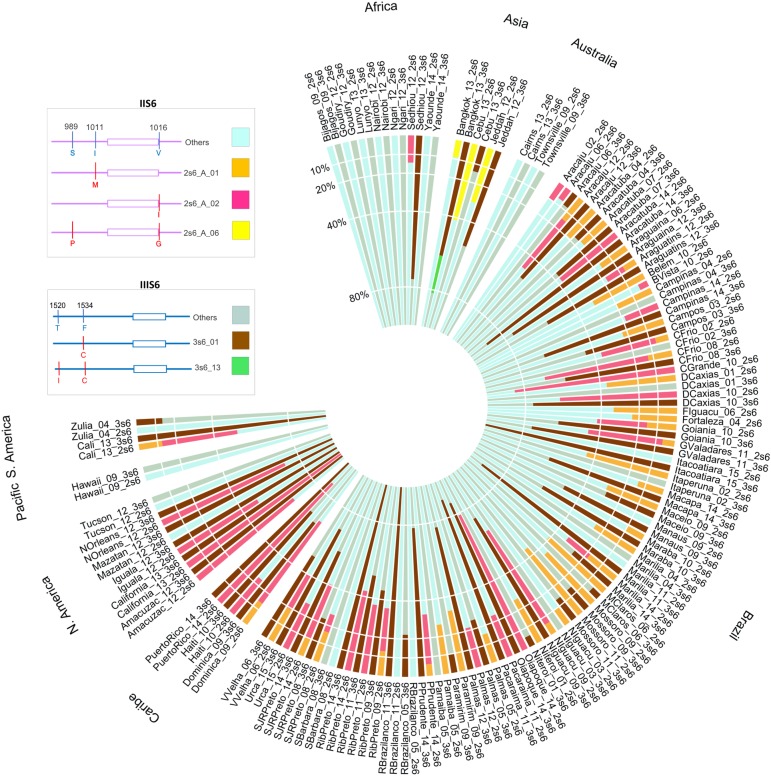
Fig 4. Frequencies of haplotypes with or without non-synonymous substitutions in IIS6 and IIIS6 NaV segments of Aedes aegypti worldwide populations.
The populations contain one bar for each of the two NaV segments. In each bar it is presented the frequency of the haplotypes with non-synonymous SNPs and the sum of the remaining haplotypes (wild-type). Populations are grouped according to their continent.
Reconstructing Nav evolution by merging information of IIS6 and IIIS6 haplotypes
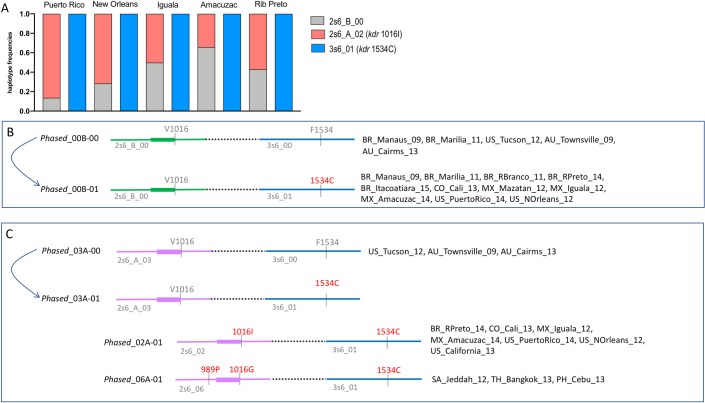
The variable intron in the haplotypes of the IIS6 segment, which grouped the sequences in clades A and B was usedto determine if the kdr mutations in this segment had single or multiple origins. As 1011M, 1016I and 989P + 1016G were present in single haplotypes, there was no evidence of multiple origins of these kdr mutations. In the IIIS6 segment, the intron was not highly variable and although the kdr 1534C was present in two haplotypes, they were identical except for a difference in one nucleotide (3s6_01 and 3s6_13). This is consistent with a single origin for the kdr 1534C mutation. We phased IIS6 and IIIS6 to combine the variability from both segments, and thus increase our analysis power. The phasing could only be determined in populations which haplotypes were monomorphic in at least one of the segments, because it is not possible to indicate which haplotypes are on the same chromosome when populations were polymorphic in both segments. Six populations (Puerto Rico, New Orleans, Iguala, Amacuzac and Ribeirão Preto) satisfied this condition of being monomorphic for IIIS6, all presenting the kdr 1534C haplotype 3s6_01, and two IIS6 haplotypes: 2s6_B_00 and the kdr 1016I 2s6_A_02 (Fig 5A). Therefore, in these cases the only possible phased haplotypes were 2S6_B_00 + 3s6_01 (Phased_00B-01) and 2S6_A_02 + 3s6_01 (Phased_02A-01). As 2s6_B_00 and 2s6_A_02 are in clades A and B, respectively (see Fig 2), and both phased haplotypes carry the kdr 1534C mutation in the IIIS6 segment, evidence suggests that this mutation had at least two independent origins. This information was not available when looking only at the IIIS6 haplotype network alone (Fig 3).
Fig 5. Evidence for at least two independent origins for the 1534C kdr mutation in Aedes aegypti.
Bars show the haplotype frequencies of IIS6 and IIIS6 NaV segments found in five localities that are fixed for 3s6-01 haplotype (A). The IIS6 haplotypes from Clades A or B (see Fig 2) are represented as purple and green lines, respectively. Two different origins are proposed for the 1534C kdr mutation in B and C panels. The single origin for the other kdr mutations is also suggested in panel C. Phased haplotypes are followed by the name of the populations where they were found. Kdr SNPs are shown in red.
We suggest that the kdr 1534C Phased 00B-01 present in Brazil, Colombia, Mexico, Puerto Rico and USA could have derived from Phased_00B-00 found in Brazil, USA and Australia (Fig 5B). In addition, we hypothesize that the Phased_03A-00, found in USA and Australia, likely originated a Phased 03A-01. This should have been the ancestor of the other 1534C haplotypes, which also contained the kdr mutations in the IIS6 segment: 1016I (Phased_02A-01) in Brazil, Colombia, Mexico, Puerto Rico and USA, and 989P + 1016G (Phased_06A-01) in Saudi Arabia, Thailand and Philippines. There was no evidence of multiple origins for the kdr mutations in the IIS6 segment (Fig 5C).
In summary, we have inferred that there are at least two wildtype (insecticide susceptible) NaV phased haplotype sources of the kdr mutation (insecticide resistant): Phased_00B-00 and Phased_03A-00 (Fig 5), probably originally in Africa.
Temporal variation
Samples from Brazil from years spanning 2001 to 2015 were used in order to evaluate changes in frequency of the NaV haplotypes. Five haplotypes in the IIS6 segment were found in these populations: 2s6_B_00, 2s6_B_04, 2s6_A_01, and the kdr 2s6_A_02 and 2s6_A_03. We observed that the kdr 1016 Ile (2s6_A_02) first appeared in samples from 2002, only achieving higher frequencies from 2007 onwards. On the other hand, the 1011 Met (2s6_A_01) was present during the whole period, however seems to decrease in frequency. Among the non kdr haplotypes, 2s6_A_03 and 2s6_A_04 also tended to decrease, whilst 2s6_B_00 was generally the most frequent haplotype (S7 Fig). Five haplotypes were observed in the IIIS6 segment of Ae. aegypti populations from Brazil: 3s6_00, 3s6_02, 3s6_03, 3s6_17 and the kdr 3s6_01 (1534 Cys). The kdr haplotype was present in 2001, at low frequency, with a very clear progressive increase in frequency until 2015, in parallel to the decrease of 3s6_00. The 3s6_02 haplotype generally occured at low frequencies, except in a population where was fixed, and the other two haplotypes were rare (S8 Fig). The remarked increase of 3s6_01 makes sense, since it is part of both Phased_00B-01 (1534 Cys) and Phased_02A-01 (1016 Ile + 1534 Cys), as afore mentioned. This is consistent with the increase of kdr resistance in Ae. aegypti from Brazil in the last decade and indicates that the kdr 1534C from both distinct origins are present in this country.
Discussion
Molecules that are targeted by neurotoxic insecticides are remarkably conserved among distant taxa, implying the encoded proteins are under selective constraints even in the absence of insecticides [26]. This explains the repeated changes at the classical kdr 1014 site (Leu to Phe or Ser) in several insect species, including other mosquitoes in the genera Culex and Anopheles. In Aedes, however, due to a codon constraint, two nucleotide substitutions in the relevant codon are needed to produce the amino acid changes found in other insects, reducing its likelihood [27]. Thus, in Aedes, alternative mutations that change the channel conformation making it resistant to pyrethroids without losing its physiological role have arisen. Mutations at NaV sites 1016 and 1534 produce resistance and are found not only in many populations of Ae. aegypti, but also in Aedes albopictus [28, 29], a likely result of convergent adaptations to pyrethroids pressure. This indicates that the relevant mutations may arise multiple times in a species and thus the geographic distributions of resistant alleles is not always the result of migration from a single geographic origin. Our analyses found evidence of multiple origins of the 1534C kdr mutation in Ae. aegypti, as previously hypothesized [30, 31]. Similar analyses of intronic sequences near the 1014 site also indicated convergent evolution of the kdr mutation in several insects, such as An. gambiae [15], in the house fly Musca domestica [32], the codling moth Cydia pomonella [33] and the aphid Myzus persicae [34].
Spatial analyses
Here we found the largest diversity of NaV haplotypes in Africa with 14 out of 17 and 23 out of 25 of IIS6 and IIIS6 haplotypes, respectively. This higher diversity in African populations was expected since they are older than populations outside Africa [1] and is consistent with the high diversity observed with other genetic markers in Africa [(e.g. microsatellites, [35])]. In addition to being younger, populations outside Africa may be subject to selective sweeps around the NaV gene due to exposure to insecticides. There is remarkably little data on insecticide resistance in Ae. aegypti from Africa [9], in contrast to the information for Anopheles. The lack of attention to insecticide resistance in African Ae. aegypti may be due to insecticides rarely being employed specifically against this mosquito in Africa. We suggest that exposure could be a byproduct of extensive use against malaria vectors. Among the five African countries from which we had samples, we found a kdr haplotype only in Senegal. It is known that Senegal has populations with mixed genetic signature of Aaf and Aaa [35, 36], so it is possible this kdr haplotype came back from the Americas.
In Africa, the first detection of kdr was in resistant populations from Ghana (West African coast) from samples collected in 2012 and 2014 [37]. The kdr 1534C mutation was observed in individuals with scaling patterns usually found in both subspecies (Aaa and AAf). As in our analyses and previous work [38], the IIS6 haplotypes belonged to two distinct clades for IIS6 in those African populations. The kdr 1534C African sequences were associated to non-kdr IIS6 haplotypes in clade A [37], a result confirmed by our analyses, as the kdr 1534C haplotype (in this study haplotype 3s6_01, Fig 3) is also associated with IIS6 haplotypes in Clade A (2s6_A_02: 1016I, Fig 2). However, we also show that this association goes beyond Africa, as it is found in populations from Brazil, Mexico, Puerto Rico, and USA. In addition, the kdr 1534C haplotype (3s6_01) was also found with a non-kdr IIS6 haplotype in clade B (2s6_B_00) in populations from Brazil, Mexico, Colombia, Puerto Rico and USA. Therefore, the linkage of kdr 1534C haplotypes with IIS6 sequences in both distinct clades (Fig 5) indicates that the 1534C kdr mutation has emerged independently at least twice. This also explains the high frequency of the haplotype 2s6_B_00 (i.e. without a kdr mutation in this fragment), since it is non-randomly associated (“hitchhiked”) with the 1534C kdr mutation in the IIIS6 segment.
Our samples from Asia were polymorphic for both IIS6 and IIIS6 segments but in these populations we could not infer associations between IIS6 and IIIS6 haplotypes given the impossibility to phase the haplotypes for the two segments. Unlike in the Americas where the 1016I kdr mutation generally occurs together with 1534C, 1016G occurs alone or in conjugation with 1534C in Asian populations [39]. Here, we observed only one haplotype with 1016G which also harbored the 989P kdr mutation (haplotype 2s6_A_06) found in Saudi Arabia, Thailand, and Philippine populations. Based on the sequence of the intron, this haplotype is in IIS6 clade A, as observed in Taiwanese populations [40]. Additionally, 1534C was linked to IIS6 clade B, i.e. without mutations in the sites 989 and 1016 [40]. This suggests that in Asian populations 1016G and 989P + 1016G haplotypes emerged from a F1534 haplotype. This is different from what was found with American haplotypes, where 1016I emerged from a 1534C haplotype as also suggested by other studies [30, 31]. In Ae. aegypti from Saudi Arabia, the 989P + 1016G + F1534 is in high frequency, with a small portion of the triple mutant 989P + 1016G + 1534C [41]. This is consistent with 1534C having more than one origin. Likewise, in Sri Lanka although the triple mutant genotype was found, no individual was homozygote for the three mutations [42].
The substitution T1520I with 1534C (haplotype 3s6_13) was found in Thailand samples, and also in Delhi, India [43]. However, it remains to be elucidated whether this haplotype is resistant to pyrethroids or DDT. Electrophysiological assays of distinct kdr mutations introduced in an AaNaV1 cDNA expressed in a Xenopus oocytes heterologous system, evidenced that the 1520I alone did not alter the channel sensitivity to pyrethroids and DDT. However, resistance to permethrin was higher with 1520I + 1534C than with 1534C alone [44]. To our knowledge, the non-synonymous substitution I1532T found in the haplotype 3s6_11 in a population from Haiti has not been previously described in Ae. aegypti, but interestingly is found in Italian and Chinese populations of Ae. albopictus [45].
Temporal variation
The substitution I1011M (designated here as 2s6_A_01) was found in the oldest collections we studied (1995 and 1998 from Brazil, Guyana, and Martinique) and only in samples from the New World. Neurophysiological assays showed that mosquitoes with that mutation required higher concentrations of pyrethroids to induce repetitive firing compared to susceptible mosquitoes [46]. Further studies confirmed that I1011M was in higher frequency in pyrethroid resistant individuals in a Brazilian population [25]. However, these previous studies examined only the sequence in the IIS6 segment, meaning that it is possible that the resistant allele 1534C in the IIIS6 segment might also have been present. Indeed, the Brazilian samples we examined here from 2001–2005 had the 1534C mutation (haplotype 3s6_01). In addition, 1011M appeared only as an apparent heterozygote in several natural populations from Brazil. This led to the suggestion that it may be a duplication event [47], a possibility that remains to be confirmed. An Ae. aegypti line selected to carry this mutation was not resistant to deltamethrin [48]. However, what is certain is that the frequency of 1011M has been decreasing in parallel to an increase of 1016I (1016I + 1534C) over time, as observed in this study (S7 and S8 Figs).
Focusing on the polymorphism at sites 1016 (Val or Ile) and 1534 (Phe or Cys) in Ae. aegypti from Mexico collected between 2000 and 2012 [31], in some localities kdr mutations increased from almost zero to near fixation in 12 years. The haplotype V1016 + 1534C increased first, but began to decline when the double kdr 1016I + 1534C started increasing. In addition, the haplotype 1016I + F1534 was observed at low frequencies in some Mexican populations but not elsewhere. Vera-Maloof et al. (2015) [31] speculated that low fitness of 1016I + F1534 accounts for their rarity. They also suggested that haplotype V1016 + 1534C arose first, followed by 1016I + 1534C with even greater pyrethroid resistance.
A similar scenario was found in Brazil [49]. The relative level of resistance among these haplotypes was confirmed in laboratory studies: a homozygote line for V1016 + 1534C was less resistant to deltamethrin than the double kdr 1016I + 1534C in Ae. aegypti with homogeneous genetic background [48]. Results observed in our study corroborate the increase of kdr frequency in Ae. aegypti from Brazil, which in part justify the spread of pyrethroid resistance and support the genotyping tools currently used for surveillance of 1016I and 1534C kdr mutations in natural populations [8].
Conclusions and future directions
Based on our sequencing of two distinct segments of the NaV gene in a spatially and temporally diverse group of populations, we show that the 1534C kdr mutation circulating in Ae. aegypti populations around the world has emerged at least two distinct times, likely as convergent selection for resistance to DDT and/or pyrethroids. We found no evidence however of multiple origins of other kdr mutations: 1016I (Americas and Africa), 989P + 1016G and 1520I + 1534C (Asia). A temporal analysis with Ae. aegypti populations from Brazil showed that the kdr 1534C from both origins are present, where the V1016 + 1534C haplotype arose first and more recently the double mutant 1016I + 1534C has been expanding in this country. The combination of these results with global genetic population data will help us to better understand how insecticide resistance arises, is selected, and expands in the major worldwide arbovirus vector, Ae. aegypti. Future work will aim at further elucidating specific questions such as how insecticide resistance is evolving in complex landscape scenarios, considering genetic structure, migration as well as vector competence in local populations of Ae. aegypti.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
Haplotypic frequencies are displayed for each population (panel above), indicating continent, country and year of collection. The panel bellow shows the populations from Brazil.
(TIFF)
Nucleotides in the exon and intron regions are in upper- and lower-case, respectively. Dots indicate the same nucleotides as in the 2S6_B_00 haplotype, whereas nonsynonymous changes are in red. The amino acid translation is indicated above the alignment, with changes numbered according to the NaV protein of Musca domestica, as usual.
(PDF)
Evolutionary history inferred using Maximum Likelihood with the Tamura-Nei model in MEGA7 [22]. Bootstrap consensus tree (1000 replicates), only. values over 90% are shown. Branch lengths are in scale with the number of substitutions per site. Colored symbols indicate the continent where the haplotypes were found, according to the legend. Haplotypes found in more than one country are in circles and those found exclusively in one country are indicated with a triangular symbol. Haplotypes with non-synonymous substitutions are outlined in red.
(TIF)
Nucleotides in the exon and intron regions are in upper- and lowercase letters, respectively. Dots indicate the same nucleotides as in the 3S6_00 haplotype. Non-synonymous SNPs changes are in red. The amino acid translation is indicated above the alignment, numbered according to the NaV protein of Musca domestica, as reference.
(PDF)
Haplotypic frequencies are displayed for each population (panel above), indicating continent, country and year of collection. The panel bellow shows the populations from Brazil.
(TIFF)
This tree is the bootstrap consensus tree (1000 replicates) inferred by Maximum Likelihood and Tamura-Nei model using MEGA7 software [22], rooted on the 3s6_00 haplotype. Bootstraps values over 90% are shown and branch lengths are in scale with the number of substitutions per site. Colored symbols indicate the continent where the haplotypes were found, according to the legend. Haplotypes found in more than one country are in circles and those found exclusively in one country are indicated with a triangular symbol. Red lined squares denote haplotypes with non-synonymous substitutions.
(TIF)
Each dot represents the haplotype frequency for a given population (see S3 Table). The frequencies of the haplotypes from IIS6 segment.
(TIF)
Each dot represents the haplotype frequency for a given population (see S3 Table). The frequencies of the haplotypes from IIIS6 segment.
(TIF)
Acknowledgments
We would like to thank Carol Mariani (Yale University, USA), for her outstanding technical assistance, José Bento Pereira Lima (IOC/Fiocruz, Brazil) and Maria de Lourdes Macoris (Sucen-Marilia, Brazil) for collaboration in obtaining samples from Brazil, and to Heloiza Diniz (IOC/Fiocruz, Brazil) for the support with the figures in this paper. We are grateful for support from the Coordination for the Improvement of Higher Education Personnel (CAPES).
Data Availability
All data generated or analyzed during this study are included within the manuscript and its Supporting Information files, as well as in the Sequence Read Archive (SRA Accession number: PRJNA579141, Biosample number: SAMN13092781). The sequences of the haplotypes were submitted to Genebank (accession numbers: MN602753-MN602796).
Funding Statement
This study was funded by the National Institute of Health (NIH, Grant no. UO1 AI115595) to JP, the Instituto Nacional de Ciência e Tecnologia em Entomologia Molecular (INCT-EM, Grant n0 465678/2014-9) to AJM, and the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ, Grants no E-26/203.177/2016, E-26/201.836/2017) also to AJM. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Powell JR, Gloria-Soria A, Kotsakiozi P. Recent History of Aedes aegypti: Vector Genomics and Epidemiology Records. Bioscience. 2018;68(11):854–60. Epub 2018/11/23. 10.1093/biosci/biy119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ingelbeen B, Weregemere NA, Noel H, Tshapenda GP, Mossoko M, Nsio J, et al. Urban yellow fever outbreak-Democratic Republic of the Congo, 2016: Towards more rapid case detection. PLoS Negl Trop Dis. 2018;12(12):e0007029 Epub 2018/12/12. 10.1371/journal.pntd.0007029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soper FL. The elimination of urban yellow fever in the Americas through the eradication of Aedes aegypti. Am J Public Health Nations Health. 1963;53:7–16. Epub 1963/01/01. 10.2105/ajph.53.1.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kraemer MU, Sinka ME, Duda KA, Mylne AQ, Shearer FM, Barker CM, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife. 2015;4:e08347 10.7554/eLife.08347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler D. Fears rise over yellow fever's next move. Nature. 2016;532(7598):155–6. Epub 2016/04/15. 10.1038/532155a . [DOI] [PubMed] [Google Scholar]
- 6.Corbel V, Durot C, Achee NL, Chandre F, Coulibaly MB, David JP, et al. Second WIN International Conference on "Integrated approaches and innovative tools for combating insecticide resistance in vectors of arboviruses", October 2018, Singapore. Parasit Vectors. 2019;12(1):331 Epub 2019/07/05. 10.1186/s13071-019-3591-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Messina JP, Brady OJ, Golding N, Kraemer MUG, Wint GRW, Ray SE, et al. The current and future global distribution and population at risk of dengue. Nat Microbiol. 2019;4(9):1508–15. Epub 2019/06/12. 10.1038/s41564-019-0476-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valle D, Bellinato DF, Viana-Medeiros PF, Lima JBP, Martins Junior AJ. Resistance to temephos and deltamethrin in Aedes aegypti from Brazil between 1985 and 2017. Mem Inst Oswaldo Cruz. 2019;114:e180544 Epub 2019/05/01. 10.1590/0074-02760180544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moyes CL, Vontas J, Martins AJ, Ng LC, Koou SY, Dusfour I, et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl Trop Dis. 2017;11(7):e0005625 10.1371/journal.pntd.0005625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO. WHO recommended insecticides for space spraying against mosquitoes: World Health Organization; 2016 [cited 2018 06/10/2018]. Available from: who.int/neglected_diseases/vector_ecology/vector‐control/Space_Spray_products_February_2016.pdf
- 11.WHO. WHO recommended long-lasting insecticidal nets: World Health Organization; 2017 [cited 2018 06/10/2018]. Available from: https://www.who.int/neglected_diseases/vector_ecology/vector-control/en/.
- 12.Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000;26(1):13–25. Epub 2000/05/08. 10.1016/s0896-6273(00)81133-2 . [DOI] [PubMed] [Google Scholar]
- 13.Du Y, Nomura Y, Satar G, Hu Z, Nauen R, He SY, et al. Molecular evidence for dual pyrethroid-receptor sites on a mosquito sodium channel. Proc Natl Acad Sci U S A. 2013. Epub 2013/07/04. 10.1073/pnas.1305118110 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williamson MS, Martinez-Torres D, Hick CA, Devonshire AL. Identification of mutations in the housefly para-type sodium channel gene associated with knockdown resistance (kdr) to pyrethroid insecticides. Mol Gen Genet. 1996;252(1–2):51–60. Epub 1996/08/27. 10.1007/bf02173204 . [DOI] [PubMed] [Google Scholar]
- 15.Pinto J, Lynd A, Vicente JL, Santolamazza F, Randle NP, Gentile G, et al. Multiple origins of knockdown resistance mutations in the Afrotropical mosquito vector Anopheles gambiae. PloS one. 2007;2(11):e1243 Epub 2007/11/29. 10.1371/journal.pone.0001243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gloria-Soria A, Ayala D, Bheecarry A, Calderon-Arguedas O, Chadee DD, Chiappero M, et al. Global genetic diversity of Aedes aegypti. Mol Ecol. 2016;25(21):5377–95. 10.1111/mec.13866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kotsakiozi P, Gloria-Soria A, Caccone A, Evans B, Schama R, Martins AJ, et al. Tracking the return of Aedes aegypti to Brazil, the major vector of the dengue, chikungunya and Zika viruses. PLoS Negl Trop Dis. 2017;11(7):e0005653 10.1371/journal.pntd.0005653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown JE, McBride CS, Johnson P, Ritchie S, Paupy C, Bossin H, et al. Worldwide patterns of genetic differentiation imply multiple 'domestications' of Aedes aegypti, a major vector of human diseases. Proc Biol Sci. 2011;278(1717):2446–54. 10.1098/rspb.2010.2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hathaway NJ, Parobek CM, Juliano JJ, Bailey JA. SeekDeep: single-base resolution de novo clustering for amplicon deep sequencing. Nucleic Acids Res. 2018;46(4):e21 Epub 2017/12/05. 10.1093/nar/gkx1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28(12):1647–9. 10.1093/bioinformatics/bts199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clement M, Snell Q, Walker P, Posada D, Crandall K, editors. TCS: Estimating gene genealogies. Parallel and Distributed Processing Symposium, International Proceedings; 2002.
- 22.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol Biol Evol. 2016;33(7):1870–4. Epub 2016/03/24. 10.1093/molbev/msw054 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matthews BJ, Dudchenko O, Kingan SB, Koren S, Antoshechkin I, Crawford JE, et al. Improved reference genome of Aedes aegypti informs arbovirus vector control. Nature. 2018;563(7732):501–7. Epub 2018/11/16. 10.1038/s41586-018-0692-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans O, Caragata EP, McMeniman CJ, Woolfit M, Green DC, Williams CR, et al. Increased locomotor activity and metabolism of Aedes aegypti infected with a life-shortening strain of Wolbachia pipientis. J Exp Biol. 2009;212(Pt 10):1436–41. 10.1242/jeb.028951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martins AJ, Lins RM, Linss JG, Peixoto AA, Valle D. Voltage-gated sodium channel polymorphism and metabolic resistance in pyrethroid-resistant Aedes aegypti from Brazil. Am J Trop Med Hyg. 2009;81(1):108–15. Epub 2009/06/27. . [PubMed] [Google Scholar]
- 26.ffrench-Constant RH, Pittendrigh B, Vaughan A, Anthony N. Why are there so few resistance-associated mutations in insecticide target genes? Philos Trans R Soc Lond B Biol Sci. 1998;353(1376):1685–93. Epub 1999/02/18. 10.1098/rstb.1998.0319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saavedra-Rodriguez K, Urdaneta-Marquez L, Rajatileka S, Moulton M, Flores AE, Fernandez-Salas I. A mutation in the voltage-gated sodium channel gene associated with pyrethroid resistance in Latin American Aedes aegypti. Insect Mol Biol. 2007;166 10.1111/j.1365-2583.2007.00774.x [DOI] [PubMed] [Google Scholar]
- 28.Kasai S, Caputo B, Tsunoda T, Cuong TC, Maekawa Y, Lam-Phua SG, et al. First detection of a Vssc allele V1016G conferring a high level of insecticide resistance in Aedes albopictus collected from Europe (Italy) and Asia (Vietnam), 2016: a new emerging threat to controlling arboviral diseases. Euro surveillance: bulletin europeen sur les maladies transmissibles = European communicable disease bulletin. 2019;24(5). Epub 2019/02/07. 10.2807/1560-7917.ES.2019.24.5.1700847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kasai S, Ng LC, Lam-Phua SG, Tang CS, Itokawa K, Komagata O, et al. First detection of a putative knockdown resistance gene in major mosquito vector, Aedes albopictus. Japanese journal of infectious diseases. 2011;64(3):217–21. Epub 2011/05/28. . [PubMed] [Google Scholar]
- 30.Saavedra-Rodriguez K, Maloof FV, Campbell CL, Garcia-Rejon J, Lenhart A, Penilla P, et al. Parallel evolution of vgsc mutations at domains IS6, IIS6 and IIIS6 in pyrethroid resistant Aedes aegypti from Mexico. Scientific reports. 2018;8(1):6747 Epub 2018/05/02. 10.1038/s41598-018-25222-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vera-Maloof FZ, Saavedra-Rodriguez K, Elizondo-Quiroga AE, Lozano-Fuentes S, Black Iv WC. Coevolution of the Ile1,016 and Cys1,534 Mutations in the Voltage Gated Sodium Channel Gene of Aedes aegypti in Mexico. PLoS Negl Trop Dis. 2015;9(12):e0004263 10.1371/journal.pntd.0004263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rinkevich FD, Hedtke SM, Leichter CA, Harris SA, Su C, Brady SG, et al. Multiple origins of kdr-type resistance in the house fly, Musca domestica. PloS one. 2012;7(12):e52761 Epub 2013/01/04. 10.1371/journal.pone.0052761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franck P, Siegwart M, Olivares J, Toubon JF, Lavigne C. Multiple origins of the sodium channel kdr mutations in codling moth populations. PloS one. 2012;7(8):e43543 Epub 2012/08/23. 10.1371/journal.pone.0043543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anstead JA, Williamson MS, Denholm I. Evidence for multiple origins of identical insecticide resistance mutations in the aphid Myzus persicae. Insect Biochem Mol Biol. 2005;35(3):249–56. Epub 2005/02/12. 10.1016/j.ibmb.2004.12.004 . [DOI] [PubMed] [Google Scholar]
- 35.Gloria-Soria A, Kellner DA, Brown JE, Gonzalez-Acosta C, Kamgang B, Lutwama J, et al. Temporal genetic stability of Stegomyia aegypti (= Aedes aegypti) populations. Med Vet Entomol. 2016;30(2):235–40. Epub 2016/01/09. 10.1111/mve.12153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown JE, Evans BR, Zheng W, Obas V, Barrera-Martinez L, Egizi A, et al. Human impacts have shaped historical and recent evolution in Aedes aegypti, the dengue and yellow fever mosquito. Evolution. 2014;68(2):514–25. 10.1111/evo.12281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawada H, Higa Y, Futami K, Muranami Y, Kawashima E, Osei JH, et al. Discovery of Point Mutations in the Voltage-Gated Sodium Channel from African Aedes aegypti Populations: Potential Phylogenetic Reasons for Gene Introgression. PLoS Negl Trop Dis. 2016;10(6):e0004780 10.1371/journal.pntd.0004780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martins AJ, Lima JB, Peixoto AA, Valle D. Frequency of Val1016Ile mutation in the voltage-gated sodium channel gene of Aedes aegypti Brazilian populations. Trop Med Int Health. 2009;14(11):1351–5. Epub 2009/09/09. 10.1111/j.1365-3156.2009.02378.x . [DOI] [PubMed] [Google Scholar]
- 39.Du Y, Nomura Y, Zhorov BS, Dong K. Sodium Channel Mutations and Pyrethroid Resistance in Aedes aegypti. Insects. 2016;7(4). 10.3390/insects7040060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung HH, Cheng IC, Chen YC, Lin C, Tomita T, Teng HJ. Voltage-gated sodium channel intron polymorphism and four mutations comprise six haplotypes in an Aedes aegypti population in Taiwan. PLoS Negl Trop Dis. 2019;13(3):e0007291 Epub 2019/03/30. 10.1371/journal.pntd.0007291 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al Nazawi AM, Aqili J, Alzahrani M, McCall PJ, Weetman D. Combined target site (kdr) mutations play a primary role in highly pyrethroid resistant phenotypes of Aedes aegypti from Saudi Arabia. Parasit Vectors. 2017;10(1):161 10.1186/s13071-017-2096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernando SD, Hapugoda M, Perera R, Saavedra-Rodriguez K, Black WCt, De Silva NK. First report of V1016G and S989P knockdown resistant (kdr) mutations in pyrethroid-resistant Sri Lankan Aedes aegypti mosquitoes. Parasit Vectors. 2018;11(1):526 Epub 2018/09/28. 10.1186/s13071-018-3113-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kushwah RB, Dykes CL, Kapoor N, Adak T, Singh OP. Pyrethroid-resistance and presence of two knockdown resistance (kdr) mutations, F1534C and a novel mutation T1520I, in Indian Aedes aegypti. PLoS Negl Trop Dis. 2015;9(1):e3332 10.1371/journal.pntd.0003332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen M, Du Y, Wu S, Nomura Y, Zhu G, Zhorov BS, et al. Molecular evidence of sequential evolution of DDT- and pyrethroid-resistant sodium channel in Aedes aegypti. PLoS Negl Trop Dis. 2019;13(6):e0007432 Epub 2019/06/04. 10.1371/journal.pntd.0007432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Auteri M, La Russa F, Blanda V, Torina A. Insecticide Resistance Associated with kdr Mutations in Aedes albopictus: An Update on Worldwide Evidences. BioMed research international. 2018;2018:3098575 Epub 2018/09/04. 10.1155/2018/3098575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brengues C, Hawkes NJ, Chandre F, McCarroll L, Duchon S, Guillet P, et al. Pyrethroid and DDT cross-resistance in Aedes aegypti is correlated with novel mutations in the voltage-gated sodium channel gene. Med Vet Entomol. 2003;17(1):87–94. Epub 2003/04/12. 412 [pii]. 10.1046/j.1365-2915.2003.00412.x . [DOI] [PubMed] [Google Scholar]
- 47.Martins AJ, Brito LP, Linss JG, Rivas GB, Machado R, Bruno RV, et al. Evidence for gene duplication in the voltage-gated sodium channel gene of Aedes aegypti. Evolution, medicine, and public health. 2013;2013(1):148–60. 10.1093/emph/eot012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brito LP, Carrara L, Freitas RMd, Lima JBP, Martins AJ. Levels of Resistance to Pyrethroid among Distinct kdr Alleles in Aedes aegypti Laboratory Lines and Frequency of kdr Alleles in 27 Natural Populations from Rio de Janeiro, Brazil. BioMed research international. 2018;2018:10 10.1155/2018/2410819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Linss JG, Brito LP, Garcia GA, Araki AS, Bruno RV, Lima JB, et al. Distribution and dissemination of the Val1016Ile and Phe1534Cys Kdr mutations in Aedes aegypti Brazilian natural populations. Parasit Vectors. 2014;7:25 10.1186/1756-3305-7-25 [DOI] [PMC free article] [PubMed] [Google Scholar]