Abstract
Soil-transmitted helminths (STH) represent a significant public health problem. However, Strongyloides stercoralis is not yet integrated into the control strategy against STH, given limitations to accurately assess its burden. Considering that S. stercoralis shares biological and epidemiological characteristics with hookworms, we describe a new approach for an improved estimation of the burden of infections by S. stercoralis based on the prevalence and burden of hookworms and the relationship between these species. A systematic review of publications reporting prevalence rates for S. stercoralis and hookworms was carried out. The data was classified into two categories: 1) “Community”, with surveys including all age groups, and 2) “SAC”, with surveys limited to school-aged children. The relationship between S. stercoralis and hookworms was characterized in order to estimate the global burden of S. stercoralis infections. The study is registered in PROSPERO (CRD42019131127). Spearman correlation coefficient between S. stercoralis and hookworms was estimated and the global burden of S. stercoralis infections was estimated using a regression model. A total of 119 articles were included, and a significant positive correlation between the burden of S. stercoralis and hookworms was identified. Spearman’s coefficient for Community surveys was 0.94 and for SAC surveys it was 0.63. Based on the linear model, the global burden of S. stercoralis infections was estimated at 386 million (95%CI 324–449 million) people, including 22 million (95%CI 20–24 million) SAC. The significant relationship between S. stercoralis and hookworms allows an estimation of the global burden of S. stercoralis infections in most epidemiologic settings using hookworm burden and justifies the search of integrated control activities.
Trial registration
PROSPERO (registration code CRD42019131127).
Author summary
Evidence on epidemiologic similarities between Strongyloides stercoralis and hookworms was confirmed by our group. A systematic bibliographic search of surveys published between 2001 and 2018 was carried out. Results suggested that the prevalence and burden of infections by hookworms could be used as a proxy to estimate S. stercoralis infections. This approach is based on a linear relationship between the burden of these species. In addition, the models and linearity showed a better fit for surveys using diagnostic methods with high sensitivity for both species. Our estimate of 386 million people infected with S. stercoralis globally, provides an update of the burden of S. stercoralis. This implies an estimate of almost four times higher than what was estimated previously. Our approach allows evidence-based approximation to the estimations of the global burden of S. stercoralis infections, for different age groups and geographic regions. This information highlights the hidden burden of S. stercoralis while also contributing in the estimation of the potential need for ivermectin for the treatment of neglected tropical diseases.
Introduction
Soil-transmitted helminths (STH) are intestinal parasites that need to fulfill part of their life cycle in soil. This group of parasites represents a significant public health problem in terms of prevalence and morbidity, prompting a call from the World Health Assembly in 2001 for their control [1]. STH of relevance to human health currently included in the World Health Organization (WHO) guided strategy for the control of STH as a public health problem are Ascaris lumbricoides, Trichuris trichiura and the hookworms Necator americanus and Ancylostoma duodenale. According to WHO, more than 800 million children around the world concentrated in the most impoverished communities in tropical and sub-tropical areas, should be targeted for preventive chemotherapy through mass drug administration (MDA) of albendazole or mebendazole [2].
Strongyloides stercoralis is an intestinal helminth parasite of importance in human disease that also includes a soil passage in its life cycle, although it has the capacity for internal reproduction and amplification within the human host [3], but is not yet targeted by the control strategy against STH; although the recently issued list of targets for STH control programs by WHO includes the establishment of an efficient strongyloidiasis control program in school age children (SAC) by 2030 [4]. There is a lack of epidemiological information on S. stercoralis with its burden of morbidity and global prevalence largely unknown [5,6]. The main cause for this lack of information is the difficulty in diagnosing S. stercoralis in comparison with the other STH. Reported prevalence estimates range from 30 to 100 million people infected in the world, however this information originates from outdated reports containing estimates based on scarce evidence [7,8]. Moreover, these figures were mostly obtained with laboratory methods inadequate for the diagnosis of S. stercoralis, long before large scale surveys were implemented.
S. stercoralis and hookworms share biological and epidemiological characteristics including an overlapping geographic distribution [9–11]. For these species, the infection occurs when larvae in soils contaminated with fecal matter penetrate the skin. In addition, hookworms and S. stercoralis infections have a similar age distribution range [6]. Global estimates of population infected with hookworm are between 212 and 480 million people [12,13]. Despite these biological similarities, pharmacologic therapies and control strategies for these species of STH differ. Albendazole and alternatively mebendazole are the drugs of choice against hookworms, and ivermectin (IVM) is the preferred therapy against S. stercoralis [14]. With the inclusion of IVM in the latest edition of the Essential Medicines List of WHO for the treatment of STH [15], the growing list of Neglected Tropical Diseases (NTDs) targeted to benefit from the distribution of IVM sets a new stage for the demand of this drug, currently available through the Mectizan Donation Program for the treatment of onchocerciasis and lymphatic filariasis, but excluding other indications [16].
Given the increasing awareness of S. stercoralis as a STH deserving consideration as part of the global strategy against STH, we aimed to investigate whether hookworm burden could serve as proxy to estimate S. stercoralis burden, and contribute to the estimation of the potential global demand of IVM for NTDs control programs.
Methods
Systematic literature search
This systematic review was carried out in accordance with the instructions of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [17], and the Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER) [18]. The protocol for this review was registered with PROSPERO (registration code CRD42019131127). The complete PRISMA and GATHER checklists can be found in the Supporting information (S1 Table and S2 Table). A comprehensive search of the literature was conducted using the following databases: Embase, Medline, CAB, LILACS, ScieLO, Cochrane Library, Cochrane Central Register of Controlled Trials, WHO International Clinical Trials Registry Platform, and PROSPERO. The search was conducted from June 6 to June 25, 2018. The search strategy included: strongyloid* OR “S stercoralis” OR “S. stercoralis” OR estrongyloid* OR estrongiloid* AND “hook worm” OR hook-worm OR hookworm OR ancylostom* OR necator* OR uncinaria*. An example of the search strategy can be found in the Supporting information (S1 File).
The scope of the systematic literature review was defined based on the following criteria:
Population: Human community and school-based population surveys. Inpatient and outpatient populations, whether symptomatic or not, as well as hospital and laboratory records were excluded.
Outcomes: Studies reporting the prevalence of both S. stercoralis and hookworm with appropriate diagnostic methods, particularly for S. stercoralis were included.
Study design: Baseline pre-intervention surveys were included. Reviews, case series, case studies, laboratory database analysis, diagnostic studies and surveys from areas with active MDA programs were excluded.
Articles published in English, Spanish, French, Italian and Portuguese, between 2001 and June 2018 were considered for this review.
Study selection and data extraction
Two study investigators reviewed independently all the titles and abstracts from references identified during the literature search to be potentially relevant to the research question. The references selected after this screening were reviewed in full by two different study investigators to determine final eligibility status. For all eligible studies, data on study characteristics, patient characteristics and outcomes were extracted in duplicate by two investigators. The study characteristics that were extracted included: author, year of publication, country, study design, population, sample size, diagnostic methods used, prevalence and burden of S. stercoralis, hookworms, Ascaris lumbricoides (when available) and Trichuris trichiura (when available). These data were entered manually into an Excel spreadsheet. Any discrepancies identified in each step of study selection and data extraction were resolved through discussion and when discrepancies could not be resolved, a third independent reviewer was consulted.
Risk of bias
To ascertain the validity of eligible studies, two reviewers working independently and with adequate reliability determined the adequacy of subject selection from every survey included in the analysis in order to obtain representative samples. To prevent the risk of underestimating the prevalence of STH, particularly for S. stercoralis, diagnostic methods were classified as high/low sensitivity methods for S. stercoralis and hookworms.
Data analysis
Surveys were classified into two categories according to the target population: 1) “Community”, that includes all age ranges, and 2) “SAC”, representing school-aged children. For each category, two subcategories were analyzed according to the sensitivity of the diagnostic methods used: 1) High Sensitivity (High-S), diagnostic approach including methods that report a high sensitivity for S. stercoralis, and hookworms (serology for S. stercoralis, PCR, qPCR, Harada Mori, Baermann, agar plate, and centrifugation/concentration without conservatives), and 2) Low Sensitivity (Low-S), diagnostic methods that report lower sensitivity techniques for S. stercoralis, and/or hookworm (centrifugation/concentration with preservatives, spontaneous sedimentation, McMaster, Kato Katz, FLOTAC, and Mini FLOTAC).
We estimated weighted Spearman’s correlation between the burdens of S. stercoralis and hookworms and used cubic-spline to identify their relationship. Finally, the data was analyzed using weighted regression, where the weights were defined according to the sample size of each survey. In addition, Cook’s distance was used to find influential outliers. The final model was selected according to the Akaike information criteria (AIC). We performed a cross-validation method in order to avoid over-optimistic expectations of prediction model performance. Statistical analyses were done using R software (version 3.5.3) [19].
Results
Summary of the systematic review
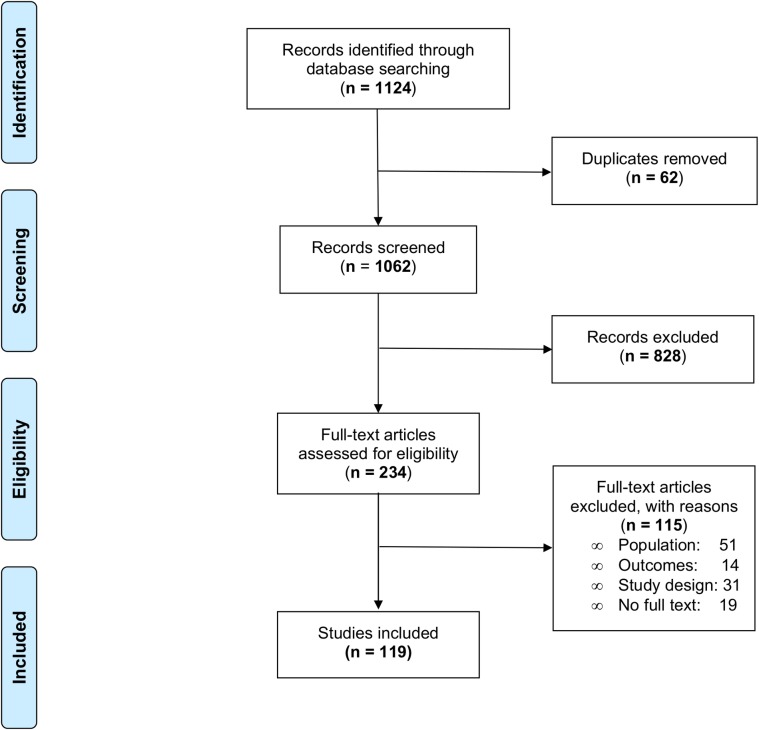
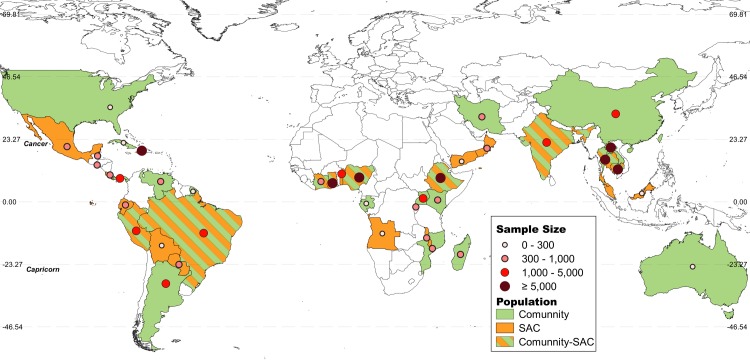
The search strategy yielded 1124 publications across the nine databases. After the initial screening based on titles and abstracts, 890 records were excluded, leaving 234 full-text research articles to be evaluated for eligibility. Of those 234 papers assessed, 19 did not have a full text available, 51 did not meet the population criteria, 14 did not meet the outcomes criteria, and 31 did not meet the study design criteria. Hence, a total of 119 articles, presenting results from 127 different surveys (93958 surveyed individuals) were included (Fig 1). Forty-seven (37%) were from the Americas, 42 (33%) from Africa, 18 (14%) from South East Asia, 17 (13%) from the Western Pacific region and 3 (3%) from the Eastern Mediterranean region (Fig 2). The complete list of included articles with the extracted data can be found in the Supporting information (S3 Table and S2 File).
Fig 1. PRISMA (preferred reporting items for systematic reviews and meta-analyses) flow diagram of systematic literature search.
Fig 2. Map of the geographical distribution of the included surveys (n = 127) [20].
The size of each circle is proportional to the total number of surveyed individuals in each country. Countries are represented in green (community based surveys) or orange (school-aged children). Countries with both type of surveys are represented in green and orange bars.
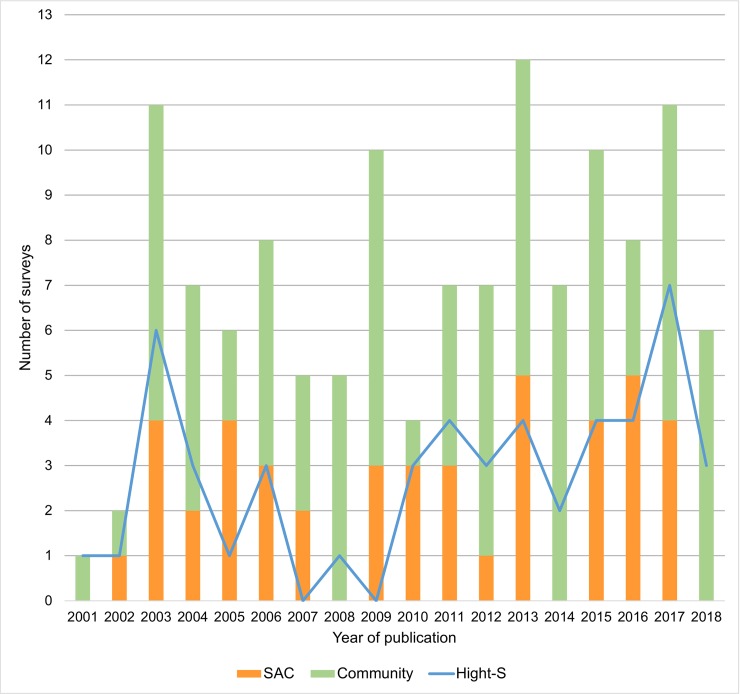
Out of the 127 surveys, 83 (65%) corresponded to Community based surveys including 36 (43%) High-S and 47 (57%) Low-S diagnostic methods; 44 (35%) corresponded to SAC surveys including 14 (32%) High-S methods and 30 (68%) Low-S methods. The number of surveys per year for the community and SAC categories and for the High-S subcategory are presented in Fig 3.
Fig 3. Number of surveys per year.
Orange bars represent the number of surveys in school-aged children (SAC) population. Green bars represent the number of surveys of the Community population. The blue line represents the total number of surveys with highly sensitive diagnostic methods (High-S).
Correlation among STH species
Positive correlation between the S. stercoralis and hookworms burden was identified for both populations, Community and SAC. The estimated Spearman’s correlation was 0.94 for all Community surveys and 0.63 for all SAC surveys.
On the other hand, the correlation between the burden of S. stercoralis and A. lumbricoides and between S. stercoralis and T. trichiura was 0.24 and 0.42, respectively. In addition, a positive correlation was found between A. lumbricoides and T. trichiura, with an estimated Spearman’s coefficient of 0.78.
Regression analysis
Out of the 83 community based surveys included in the systematic review, the marked influence on the regression analysis of a survey, with 20250 surveyed individuals from Ghana (significatively higher than the average of 585 for all other surveys), was identified by Cook’s distance as an outlier (Survey #26 in S3 Table); and was therefore excluded from the regression analysis.
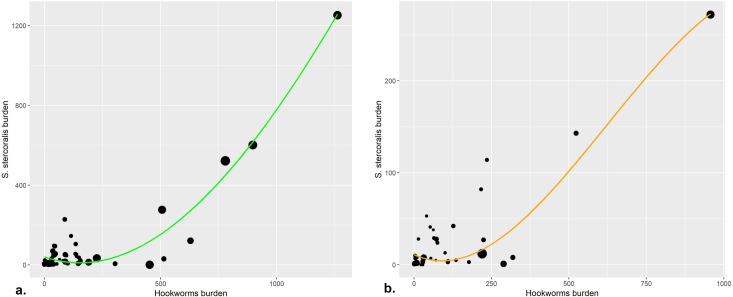
In the exploratory analysis, the shape of the relationship between the burden of S. stercoralis and hookworm was described for both types of surveys (Fig 4).
Fig 4. Spline of different surveys weighted by sample size.
a. In black dots and green line all Community based surveys. b. In black dots and orange line: school-aged children (SAC) based surveys. The size of all dots is proportional to the sample size.
Regression models were performed for both population groups with and without a quadratic term for hookworm prevalence and adjusting for diagnostic sensitivity (Hight-S and Low-S) and WHO region (African Region, Eastern Mediterranean Region, European Region, Region of the Americas, South-East Asian Region and Western Pacific Region). For both populations, the model with a quadratic term for the prevalence of hookworm did not have a better fit than the linear model and it was superior using High-S surveys than all surveys. In addition, cross-validation showed better results for both populations (Community and SAC) using High-S surveys. Thus, we were able to estimate the burden of S. stercoralis for Community and SAC population according to the following equations (Fig 5):
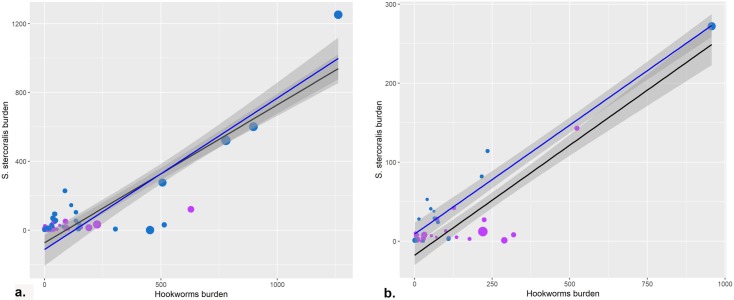
Fig 5. Linear regression.
Blue dots represent surveys with high sensitivity diagnostic methods (Hight-S) and purple dots represent surveys with low sensitivity diagnostic methods (Low-S). a. Community surveys. The black line represents the curve of equation 1 (Table 1), for the linear regression of all surveys (Hight-S and Low-S) of the Community population. The blue line represents the curve of equation 2 (Table 1), for the linear regression of all surveys of the Community population with Hight-S. b. School-aged children (SAC) surveys. The black line represents the curve of equation 3 (Table 1), for the linear regression of all surveys (Hight-S and Low-S) of the SAC population. The blue line represents the curve of equation 4 (Table 1), for the linear regression of all surveys of the SAC population with Hight-S. Gray shading represents the 95% confidence interval.
Stcom_burden = = -112.42585 + 0.88005*HKWcom_burden (2) (Table 1)
Table 1. Linear equations for the different populations.
The total and school-aged children (SAC) population infected with hookworms and the proportion of SAC in each region were obtained from References 12 and 21. St: Strongyloides stercoralis, HKW: hookworm, Com_burden: Community burden, SAC_burden: SAC burden, High-S: high sensitivity methods, 95%CI: 95% confidence interval.
| Population | Equation (Equation number) |
P-value of the slope coefficient | R2 | Infected population with hookworms (millions) | Estimated infected population (millions) with S. stercoralis (95%CI) |
|---|---|---|---|---|---|
| Community | Stcom_burden = -73.26879 + 0.80169*HKWcom_burden (1) | 2*10−16 | 0.82 | ||
| Community + High-S | Stcom_burden = -112.42585 + 0.88005*HKWcom_burden (2) | 3*10−14 | 0.83 | World: 438.9 | 386 (324–449) |
| Asia:281.8 | 248 (206–288) | ||||
| Africa:117.7 | 104 (87–120) | ||||
| America:30.3 | 27 (22–31) | ||||
| SAC | StSAC_burden = -17.7371 + 0.2786*HKWSAC_burden (3) | 2*10−16 | 0.87 | ||
| SAC + High-S | StSAC_burden = 9.0354 + 0.2759*HKWSAC_burden (4) | 4*10−12 | 0.98 | World: 438.9*0.1815 = 79.7 | 22 (20–24) |
| Asia: 281.8*0.1573 = 44.3 | 12 (11–13) | ||||
| Africa: 117.7*0.2623 = 30.9 | 8 (7–9) | ||||
| America:30.3*0.1725 = 5.2 | 14 (13–15) |
And
StSAC_burden = 9.0354 + 0.2759*HKWSAC_burden (4) (Table 1)
Estimated prevalence of S. stercoralis
Based on the estimated regression coefficients and the estimate of population infected by hookworms of 438.9 million [12], we were able to estimate the prevalence of S. stercoralis in 386 million (95%CI 324–449 million) people worldwide. In turn, based on these global numbers and the proportion of SAC worldwide [21], we were able to estimate 22 million (95%CI 20–24 million) SAC infected with S. stercoralis globally.
Discussion
This systematic review of the literature adds further evidence through an innovative approach to the relationship between hookworms and S. stercoralis, which share biologic and epidemiologic features as the host age distribution, infection route, and risk factors [9,22]. Results suggest that the burden of infections by hookworms can be used as a proxy to estimate the burden of individuals infected by S. stercoralis, which is consistent with previous hypothesis [11]. This positive correlation is obtained from a dataset that includes a wide variety of surveys from different geographic regions (Fig 3) performed with different diagnostic methods and is stronger when using a subset of surveys that included in their diagnostic approach laboratory techniques of higher sensitivity for S. stercoralis. While this is encouraging, most of these techniques, including those using molecular biology methods, are still sub-optimal, likely resulting in an underestimated real prevalence of S. stercoralis [23,24].
The diagnosis of hookworm infection, although simpler and with higher sensitivity than for S. stercoralis, is also subject to underestimations due to limitations of the most frequently used techniques, which have an estimated sensitivity of 71 to 78% for high intensity hookworm infections in the 2-slide Kato Katz method and drops to 46 to 60% in low intensity infections [25], a sensitivity that might be even lower when delays occur between sample acquisition and reading [26]. Despite these relevant diagnostic limitations that impact these two species differently, the positive correlation remains significant.
The mechanisms underlying the relationship have not been explored in this analysis and are possibly related to a shared infection route: mainly the lack of adequate sanitation as a common risk factor for both species. This contrasts with A. lumbricoides, and T. trichiura, which have a different and also shared infection route and have been linked to lack of adequate water supply [9]. Interestingly, A. lumbricoides, and T. trichiura have been identified by other authors as having a significant correlation and closely related distribution across surveys in different locations with high and low prevalence, that was not observed for hookworms, which showed a distribution independent of the other species [27]. These observations are consistent and in line with our findings. Specifically, while we found a positive strong correlation between hookworms, and S. stercoralis burdens, a weaker correlation was found between S. stercoralis and A. lumbricoides or T. trichiura. In addition, consistent with previous reports, our study also shows a strong correlation between the two latter species [27].
The relationship between S. stercoralis and hookworms acquires relevance as a consequence of the renewed interest in incorporating the former into the WHO leaded strategy for the control and elimination of STH [4]. While the role of strongyloidiasis in public health has had a growing interest in the recent years [11,28], only its incorporation into an ongoing and successful implementation program, as is WHO`s strategy, puts strongyloidiasis as an achievable goal for control. This recognition as a candidate for incorporation into the STH control strategy, opens two key questions on S. stercoralis; (1) what is the size of the global burden? and (2) what adjustments are needed in the current anthelmintic regimens?
Estimates of the burden of S. stercoralis infections have been the subject of different efforts, all limited by the difficulties in diagnostics and worsened by reports of surveys using inadequate diagnostic methods [28]. Those reports inform a wide range of estimated infected individuals ranging from 3 to 100 million [7,29]. For the purpose of intervention activities, in view of the current target groups for preventive chemotherapy through MDA campaigns, the number of SAC at-risk appears as a necessary piece of information. Based on the approach taken in this analysis, these numbers are conservatively estimated at 386 million individuals worldwide infected with S. stercoralis, including 22 million school-aged children.
While the search of correlations between the different species of STH stimulated the search for combined control with drugs active against all of them as is considered to be the case for albendazole and mebendazole [27,30], these drugs appear as unlikely to achieve elimination goals due to their poor efficacy mainly against T. trichiura [31]. Drug combinations including ivermectin are a prime candidate to improve efficacy against T. trichiura while also including S. stercoralis in the spectrum of activity of the pharmacologic regimens against STH, therefore making elimination goals feasible based on spectrum and efficacy.
A number of limitations should be considered when interpreting results from this study. First, our approach did not explore co-infections between hookworms and S. stercoralis as has been shown by other authors but rather the co-endemicity status between these STH [22]. Also, the estimates of this study are limited to baseline surveys, before chemotherapeutic interventions, which can disrupt the relationship between species as they do with different epidemiologic aspects of these infections [32]. The diagnostic accuracy of the methods used across surveys is probably the biggest limitation, affecting the reported prevalence rates of S. stercoralis more than those of hookworms; an effect that is likely to be responsible for the stronger correlations in the subset of surveys that used diagnostic methods of relatively higher sensitivity. Another limitation is that the equations represent linear functions which might not fully explain the complex relationship between these 2 species. Still, other regressions failed to better explain the variability of S. stercoralis burden. Also, the global and regional burden of hookworm infections was obtained from another estimation which carries its own limitation [12], although acknowledged by the most recent guidelines by WHO [33]. Finally, the potentially different correlation between S. stercoralis with A. duodenale and N. americanus has not been explored since the large majority of surveys reported hookworms as a group, preventing the analysis by separate species. Taking these limitations into account, we were able to find two equations, one for whole communities and one for SAC, for the estimation of the burden of S. stercoralis using the burden of hookworms as an independent variable. According to this results, confirmed by a cross validation, we can state that although it is not possible to fully explain the S. stercoralis burden just with hookworm burden, the strong correlations obtained for both types of surveys offers an evidence based approximation to understand the size of the population infected by S. stercoralis; and that the use of diagnostic approaches of higher sensitivity, allows a reasonable estimate of the prevalence of S. stercoralis in the absence of a better model. It should even be noted that the estimated values of S. stercoralis could have been underestimated if it is taken into account that the burden of hookworm may also be underestimated due to the use of low sensitivity diagnostic techniques.
In conclusion, the significant and consistent relationship between S. stercoralis and hookworms found in our analysis suggests that a simple equation based on the hookworm prevalence could be used as an approximation to estimate the global burden of S. stercoralis infections in most although not all epidemiologic settings. The inclusion of S. stercoralis in STH control programs can be strenghthened by an approach targeting both parasites since they share the route of infection, risk factors and age distribution.
Supporting information
(PDF)
(PDF)
(XLSX)
(PDF)
(PDF)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was funded by an unrestricted grant from Fundación Mundo Sano, Argentina (www.mundosano.org) to AK. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Assembly, 54. (2001). Schistosomiasis and soil-transmitted helminth infections. World Health Organization; Available from: https://apps.who.int/iris/handle/10665/78794. [Google Scholar]
- 2.WHO. Schistosomiasis and soil-transmittes helminthiases: numbers of people treated in 2017. Wkly Epidemiol Rec. 2018;50:681–92. Available from: https://www.who.int/neglected_diseases/resources/who_wer9350/en/ [PubMed] [Google Scholar]
- 3.Streit A. Reproduction in Strongyloides (Nematoda): a life between sex and parthenogenesis. Parasitology. 2008;135:285–94. 10.1017/S003118200700399X [DOI] [PubMed] [Google Scholar]
- 4.WHO. 2030 targets for soil-transmitted helminthiases control programmes. World Health Organization. WHO; 2020. Available from: https://apps.who.int/iris/handle/10665/330611. [Google Scholar]
- 5.Olsen A, van Lieshout L, Marti H, Polderman T, Polman K, Steinmann P, et al. Strongyloidiasis—the most neglected of the neglected tropical diseases? Trans R Soc Trop Med Hyg. 2009;103:967–72. 10.1016/j.trstmh.2009.02.013 [DOI] [PubMed] [Google Scholar]
- 6.Krolewiecki AJ, Lammie P, Jacobson J, Gabrielli A-F, Levecke B, Socias E, et al. A public health response against Strongyloides stercoralis: time to look at soil-transmitted helminthiasis in full. PLoS Negl Trop Dis. 2013. January;7:e2165 10.1371/journal.pntd.0002165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genta R. Global Prevalence of Strongyloidiasis: Critical Review with Epidemiologic Insights into the Prevention of Disseminated Disease Author (s): Robert M. Genta Source: Reviews of Infectious Diseases, Vol. 11, No. 5 (Sep.—Oct., 1989), pp. 755–76. Rev Infect Dis. 1989;11:755–67. 10.1093/clinids/11.5.755 [DOI] [PubMed] [Google Scholar]
- 8.Jorgensen T, Montresor A, Savioli L. Effectively controlling strongyloidiasis. Parasitol Today. 1996;12:164 10.1016/0169-4758(96)80806-4 [DOI] [PubMed] [Google Scholar]
- 9.Echazú A, Bonanno D, Juarez M, Cajal SP, Paredes G, Krolewiecki AJ. Effect of Poor Access to Water and Sanitation As Risk Factors for Soil-Transmitted Helminth Infection: Selectiveness by the Infective Route. PLoS Negl Trop Dis. 2015. September; 30;9(9): e0004111 10.1371/journal.pntd.0004111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forrer A, Khieu V, Schär F, Vounatsou P, Chammartin F, Marti H, et al. Strongyloides stercoralis and hookworm co-infection: spatial distribution and determinants in Preah Vihear Province, Cambodia. Parasites & Vectors; 2018. January; 12;11(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bisoffi Z, Buonfrate D, Montresor A, Requena-Méndez A, Muñoz J, Krolewiecki AJ, et al. Strongyloides stercoralis: A Plea for Action. PLoS Negl Trop Dis. 2013. May; 9;7(5):e2214 10.1371/journal.pntd.0002214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pullan RL, Smith JL, Jasrasaria R, Brooker SJ. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit Vectors. Parasites & Vectors; 2014;7:1–19. 10.1186/1756-3305-7-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 Diseases and Injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018. November 10;392(10159):1789–1858. 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keiser J, Utzinger J. The Drugs We Have and the Drugs We Need Against Major Helminth Infections. Adv Parasitol. 2010;73:197–230. 10.1016/S0065-308X(10)73008-6 [DOI] [PubMed] [Google Scholar]
- 15.WHO. The selection and use of essential medicines: report of the WHO Expert Committee (including the 20th WHO Model List of Essential Medicines and the 6th WHO Model List of Essential Medicines for Children). Geneva; 2017. Available from: http://www.who.int/medicines/publications/essentialmedicines/20th_EML2017_FINAL_amendedAug2017.pdf?ua=1 [Google Scholar]
- 16.Richards FO. Upon entering an age of global ivermectin-based integrated mass drug administration for neglected tropical diseases and malaria. BioMed Central. 2017;16:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. J Clin Epidemiol. 2009. October; 62(10):e1–34. 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 18.Stevens GA, Alkema L, Black RE, Boerma JT, Collins GS, Ezzati M, et al. Guidelines for Accurate and Transparent Health Estimates Reporting: the GATHER statement. Lancet. 2016. December 10;388(10062):e19–e23. 10.1016/S0140-6736(16)30388-9 [DOI] [PubMed] [Google Scholar]
- 19.R Core Team (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: URL https://www.R-project.org/} [Google Scholar]
- 20.QGIS Development Team (2019). QGIS Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.org QGIS. "Paises del Mundo". Downloaded from http://tapiquen-sig.jimdo.com. Carlos Efraín Porto Tapiquén. Orogénesis Soluciones Geográficas. Porlamar, Venezuela 2015. Based on layers of Enviromental Systems Research Institute (ESRI). Free Distribution. [Google Scholar]
- 21.Bartsch SM, Hotez PJ, Asti L, Zapf KM, Bottazzi ME, Diemert DJ, et al. The Global Economic and Health Burden of Human Hookworm Infection. PLoS Negl Trop Dis. 2016. September; 8;10(9):e0004922 10.1371/journal.pntd.0004922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Becker L, Sieto B, Silue KD, Adjossan L, Kone S. Diagnosis, Clinical Features, and Self-Reported Morbidity of Strongyloides stercoralis and Hookworm Infection in a Co-Endemic Setting. PLoS Negl Trop Dis. 2011. August;5(8): e1292 10.1371/journal.pntd.0001292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buonfrate D, Requena-mendez A, Angheben A, Cinquini M, Cruciani M, Fittipaldo A, et al. Accuracy of molecular biology techniques for the diagnosis of Strongyloides stercoralis infection—A systematic review and meta- analysis. PLoS Negl Trop Dis. 2018. February 9;12(2):e0006229 10.1371/journal.pntd.0006229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krolewiecki A, Nutman TB. Strongyloidiasis: A Neglected Tropical Disease. Infect Dis Clin North Am. 2019. Mar;33:135–51. 10.1016/j.idc.2018.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nikolay B, Brooker SJ, Pullan RL. Sensitivity of diagnostic tests for human soil-transmitted helminth infections: a meta-analysis in the absence of a true gold standard. Int J Parasitol. 2014. October 1;44(11):765–74. 10.1016/j.ijpara.2014.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dacombe RJ, Crampin a C, Floyd S, Randall A, Ndhlovu R, Bickle Q, et al. Time delays between patient and laboratory selectively affect accuracy of helminth diagnosis. Trans R Soc Trop Med Hyg. 2007. February;101:140–5. 10.1016/j.trstmh.2006.04.008 [DOI] [PubMed] [Google Scholar]
- 27.Booth M, Bundy DA. Comparative prevalences of Ascaris lumbricoides, Trichuris trichiura and hookworm infections and the prospects for combined control. Parasitology. 1992. August;105 (Pt 1:151–7). [DOI] [PubMed] [Google Scholar]
- 28.Krolewiecki AJ, Lammie P, Jacobson J, Gabrielli A-FF, Levecke B, Socias E, et al. A public health response against Strongyloides stercoralis: time to look at soil-transmitted helminthiasis in full. PLoS Negl Trop Dis. 2013. May 9;7, e2165 10.1371/journal.pntd.0002165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schär F, Trostdorf U, Giardina F, Khieu V, Muth S, Marti H, et al. Strongyloides stercoralis: Global Distribution and Risk Factors. Brooker S, editor. PLoS Negl Trop Dis. 2013. July 11;7(7):7:e2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albonico M, Montresor A, Crompton DWT, Savioli L. Intervention for the control of soil-transmitted helminthiasis in the community. Adv Parasitol. 2006;61:311–48. 10.1016/S0065-308X(05)61008-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Truscott JE, Turner HC, Anderson RM. What impact will the achievement of the current World Health Organisation targets for anthelmintic treatment coverage in children have on the intensity of soil transmitted helminth infections? Parasit Vectors. 2015. October 22;8:551 10.1186/s13071-015-1135-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basáñez MG, French MD, Walker M, Churcher TS. Paradigm lost: How parasite control may alter pattern and process in human helminthiases. Trends Parasitol. 2012. April;28:161–71. 10.1016/j.pt.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 33.WHO.Guideline: preventive chemotherapy to control soil-transmitted helminth infections in at-risk population groups. Geneva: WHO; 2017. Available from: https://www.who.int/nutrition/publications/guidelines/deworming/en/ [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(XLSX)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.