Abstract
Chondrocyte hypertrophy is an essential process required for endochondral bone formation. Proper regulation of chondrocyte hypertrophy is also required in postnatal cartilage homeostasis. Indian hedgehog (Ihh) and PTHrP signaling play crucial roles in regulating the onset of chondrocyte hypertrophy by forming a negative feedback loop, in which Ihh signaling regulates chondrocyte hypertrophy by controlling PTHrP expression. To understand whether there is a PTHrP-independent role of Ihh signaling in regulating chondrocyte hypertrophy, we have both activated and inactivated Ihh signaling in the absence of PTHrP during endochondral skeletal development. We found that upregulating Ihh signaling in the developing cartilage by treating PTHrP−/− limb explants with sonic hedgehog (Shh) protein in vitro, or overexpressing Ihh in the cartilage of PTHrP−/− embryos or inactivating patched 1 (Ptch1), a negative regulator of hedgehog (Hh) signaling, accelerated chondrocyte hypertrophy in the PTHrP−/− embryos. Conversely, when Hh signaling was blocked by cyclopamine or by removing Smoothened (Smo), a positive regulator of Hh signaling, chondrocyte hypertrophy was delayed in the PTHrP−/− embryo. Furthermore, we show that upregulated Hh signaling in the postnatal cartilage led to accelerated chondrocyte hypertrophy during secondary ossification, which in turn caused reduction of joint cartilage. Our results revealed a novel role of Ihh signaling in promoting chondrocyte hypertrophy independently of PTHrP, which is particularly important in postnatal cartilage development and homeostasis. In addition, we found that bone morphogenetic protein (Bmp) and Wnt/β-catenin signaling in the cartilage may both mediate the effect of upregulated Ihh signaling in promoting chondrocyte hypertrophy.
Keywords: Ihh, Patched 1, Cartilage, PTHrP, Chondrocyte hypertrophy
INTRODUCTION
Endochondral ossification is a major bone-forming process in vertebrate skeletal development. The multi-step process of endochondral bone formation begins with the condensation of mesenchymal progenitor cells. These condensed mesenchymal cells then differentiate into chondrocytes to form the cartilage anlagen. In the developing long bones, chondrocytes undergo sequential proliferation and differentiation, which is required for proper longitudinal bone growth. Slowly proliferating periarticular chondrocytes differentiate into highly proliferative columnar chondrocytes, and then proliferative chondrocytes exit cell cycle and undergo hypertrophy. Eventually, the most mature hypertrophic chondrocytes undergo apoptosis. The hypertrophic chondrocytes secrete vascular endothelia growth factor (Vegf) and matrix metalloproteinases (Mmps), which will degrade the extracellular matrix and allow the invasion of blood vessels and osteoblasts differentiated in the perichondrium to form the trabecular bone. Therefore, chondrocyte hypertrophy is an essential step in endochondral bone formation, which is tightly controlled during normal skeletal development by cell-cell signaling and transcription factors (reviewed by de Crombrugghe et al., 2001; Karsenty and Wagner, 2002; Kronenberg, 2003; Zelzer and Olsen, 2003). Furthermore, the proper regulation of chondrocyte hypertrophy is also necessary for maintaining the cartilage lining synovial joint surfaces, as abnormal chondrocyte hypertrophy in articular cartilage is associated with a common cartilage degenerative disease: osteoarthritis (Poole, 1999).
Indian hedgehog (Ihh) is a member of the hedgehog (Hh) family that plays crucial roles in regulating many developmental processes. Ihh is one of the key signaling molecules controlling both chondrocyte hypertrophy and bone formation in the developing skeletal system. Ihh is expressed in prehypertrophic chondrocytes and it regulates the onset of hypertrophic differentiation by forming a negative feedback loop with parathyroid hormone related protein (PTHrP; Pthlh – Mouse Genome Informatics) (St-Jacques et al., 1999; Vortkamp et al., 1996). Ihh activates the expression of PTHrP in the periarticular cells and the articular chondrocytes, and PTHrP signals through its receptor Pthr1 to inhibit chondrocyte hypertrophy and to suppress further Ihh expression by keeping chondrocytes in a proliferating state (Lanske et al., 1996; St-Jacques et al., 1999). Ihh signaling is also required for chondrocyte proliferation and osteoblast differentiation independently of PTHrP signaling (Karp et al., 2000; Long et al., 2004; St-Jacques et al., 1999). However, it remains to be elucidated whether Ihh signaling regulates chondrocyte hypertrophy exclusively through PTHrP, as Ihh signals directly to proliferating and prehypertrophic chondrocytes. In the Ihh−/− embryo, PTHrP is not expressed and yet chondrocyte hypertrophy is delayed at E14.5, although it is accelerated later (St-Jacques et al., 1999). A recent study demonstrates that Ihh promotes the transition from periarticular chondocytes to proliferating chondrocytes and regulates columnar cell mass independently of PTHrP signaling (Kobayashi et al., 2005b). In addition, Gli3, a downstream component of the hedgehog signaling pathway, acts as a repressor to inhibit the transition of periarticular chondrocytes into columnar chondrocytes by a PTHrP-independent mechanism (Hilton et al., 2005; Koziel et al., 2005). As chondrocyte proliferation, which directly affects hypertrophy, was altered when Hh signaling was manipulated in these studies, the PTHrP-independent role of Ihh signaling in regulating chondrocyte hypertrophy was not revealed.
Here, we have tested the role of Ihh in regulating chondrocyte hypertrophy independently of PTHrP, by inactivating or activating Ihh signaling cell autonomously in the developing cartilage in the PTHrP−/− mouse embryo (in which cell proliferation is greatly reduced). All Hh family members signal through two multipass transmembrane proteins, Patched 1 (Ptch1) and Smoothened (Smo). In the absence of Hh ligand, Ptch1 suppresses the activity of Smo. Upon Hh binding to Ptch1, the inhibitory effect on Smo is relieved and Smo transduces the Hh signal to downstream signaling components, which leads to the activation of Hh downstream target gene expression, including Hip1 (Hhip – Mouse Genome Informatics), Gli1 and Ptch1 (reviewed by Huangfu and Anderson, 2006; Lum and Beachy, 2004). Therefore, when Smo is removed, Hh signaling is inactivated, whereas removal of Ptch1 leads to the activation of Hh signaling cell-autonomously. Here, we have investigated the PTHrP-independent function of Ihh signaling in chondrocyte hypertrophy by genetically altering the expression or function of Ihh, Ptch1 and Smo in PTHrP−/− mouse embryos. Our results reveal a novel role of Ihh signaling in promoting chondrocyte hypertrophy independently of PTHrP, which is important in postnatal cartilage development, although it is masked by the dominant effect of PTHrP-dependent Ihh signaling in inhibiting chondrocyte hypertrophy during embryonic development. We also found that canonical Wnt and Bmp signaling may mediate the role of Ihh signaling in promoting chondrocyte hypertrophy.
MATERIALS AND METHODS
Skeletal analysis
Embryos were dissected in PBS, and then skinned, eviscerated and fixed in 95% ethanol. Skeletal preparations were performed as described previously (McLeod, 1980).
Histology, in situ hybridization and immunohistochemistry
Embryos were fixed in 4% paraformaldehyde at 4°C overnight. Fixed samples were embedded in paraffin and sectioned at 5 μm thickness. Histological analysis, immunohistochemistry and radioactive 35S RNA in situ hybridization were performed as described (Yang et al., 2003). Primary antibodies (anti-phospho-Smad1 goat polyclonal IgG (Santa Cruz) at 1:100 and anti-Col2a1 goat polyclonal IgG (Santa Cruz) at 1:200 were used for immunohistochemistry, and signals were detected using the ABC kits (Vector Laboratories) and DAB (Sigma). Probes for in situ hybridization have been described previously: Col10a1, Col2a1 and Ihh (Mak et al., 2006); the Lef1 probe was ordered from ATCC.
Organ cultures of embryonic limb explants
Forelimbs of mouse embryos were dissected free of skin and muscles at E14.5 and cultured for 1.5 days in BGJ-B medium (Invitrogen) with Antibiotic/Antimycotic (Invitrogen) and 0.1% BSA in organ culture dishes under humidified conditions. Cultures were supplemented with 2.5 μg/ml recombinant murine Shh-N (R&D Systems) or 10 μM cyclopamine (BIOMOL). Limb explants were then fixed with 4% paraformaldehyde overnight at 4°C and embedded in paraffin for sectioning.
Preparation of primary chondrocytes
Ventral parts of the rib cages of 0- to 3-day-old wild-type pups were eviscerated of skin and muscles and incubated with 2 mg/ml pronase (Roche) for 30 minutes at 37°C. The samples were then incubated with 3 mg/ml collagenase D (Roche) in DMEM (Gibco) at 37°C for 1.5 hours until all soft tissues had detached from the cartilage. The cartilage was washed with PBS several times and separated from soft tissues by sedimentation. The cartilage was then digested with collagenase for 4 to 5 hours in a Petri dish. Undigested bony parts were discarded and chondrocytes were collected by centrifugation and washed twice with PBS. The primary chondrocytes were cultured in low-serum medium (DMEM, 0.1% FBS) for dual luciferase assays.
Dual luciferase assay
Primary chondrocytes were transfected with the Topflash reporter plasmid using the Human Chondrocyte Nucleofector Kit (Amaxa), following the manufacturer’s protocol. After nucleofection, 0.5×106 cells were seeded in 12-well plate and left to recover for 4-5 hours. Primary chondrocytes were then serum starved overnight before adding 2.5 μg/ml recombinant Shh (R&D Systems) was culturing for 24 hours. Cells were harvested and subjected to luciferase activity measurement using the Dual Luciferase Reporter Assay Kit (Promega), according to the manufacturer’s instructions.
Quantitative RT-PCR
Quantitative PCR was performed to measure the relative expression levels of the Wnt genes and the Wnt target genes Axin2 and Lef1 (see Table 1), using the Platinum SYBR Green Kit (Invitrogen). Samples were normalized to Gapdh expression.
Table 1.
Primer sequences for Wnt ligands
| Gene | Primer sequence |
|---|---|
| Wnt1 | 5′-GACTCGATGGAGCCTTCGGAGCAG-3′ 5′-CCGACAGAACCCGGGGATCCTGCAC-3′ |
| Wnt2 | 5′-GCCAGCATGTCCTCAGAGTACAGG-3′ 5′-GCCAAGGACAGCAAAGGCACCTTC-3′ |
| Wnt2b | 5′-GGGTGACGCGGGTGACCCGAGTTG-3′ 5′-CAGGATGGGGCCAATTTCACAGCAG-3′ |
| Wnt3 | 5′-CATGCAGCTGGCAACAGTCCATGC-3′ 5′-GCCATTGCGTCTTCCACTGGTGC-3′ |
| Wnt3a | 5′-GAGGCACTGTCATACTTGTCCTTG-3′ 5′-GTAGTGAGGACATTGAATTTGGAG-3′ |
| Wnt4 | 5′-CACTCCACCCGCATGTGTGTCAAG-3′ 5′-GTGGCTGTGACCGGACAGTGCACG-3′ |
| Wnt5a | 5′-GGAAGTCCGCCAGCTGCAGCCAGC-3′ 5′-GACTATGGCTACCGCTTCGCCAAG-3′ |
| Wnt5b | 5′-CTTGCGGAACTCGGCCAGCTGGAG-3′ 5′-CGTGGAGTACGGCTACCGCTTTGC-3′ |
| Wnt6 | 5′-CGCGGAACGGAGGCAGCTTCTGCC-3′ 5′-GGCTGCGGAGACGATGTGGACTTC-3′ |
| Wnt7a | 5′-GTCCTTGAGCACGTAGCCTAGCTC-3′ 5′-GCTACGGCATCGGCTTCGCCAAGG-3′ |
| Wnt8 | 5′-GTCAGCCAGCTGCAGCCAACACGTC-3′ 5′-CAGACTCTTCGTGGACAGTTTGGAG-3′ |
| Wnt8b | 5′-GCCTCATTGTTGTGCAGATTCATGGC-3′ 5′-GACAGCATTTGTGCACGCCATCAG-3′ |
| Wnt10a | 5′-CTTCTTCGTCCCCACGTCTGGAGG-3′ 5′-GCCAGCATCAGTTCCGGGACCAGC-3′ |
| Wnt11 | 5′-CACGTCCTGGAGCTCTTGCAGCCC-3′ 5′-GATGTGCGGACAACCTCAGCTACG-3′ |
| Wnt15 | 5′-GCTTCAGCACCTGGCCGGTCTCGC-3′ 5′-CTGAAGTACAGCACCAAGTTCCTC-3′ |
| Wnt16 | 5′-GCCCAATCTTTTCAAAAGAAGACATA-3′ 5′-CCTTGCAGAATGGTGGCTCACCAA-3′ |
Tamoxifen preparation and injection
Tamoxifen (60 mg/ml; Sigma) was dissolved in corn oil (Sigma) and sonicated until the solution became clear. The solution was filtered and 0.075 ml was injected intraperitoneally into the mother on day 3 after the pups were born. Injection continued every other day until the pups were weaned.
RESULTS
Ihh signaling promotes chondrocyte hypertrophy in cultured limb explants in the absence of PTHrP signaling
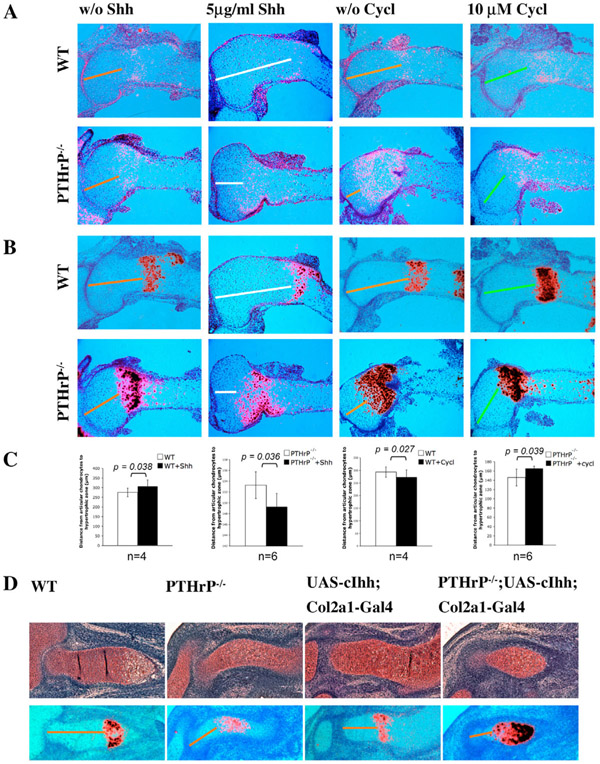
To unravel the direct effect of Ihh signaling on chondrocyte hypertrophy independently of PTHrP signaling, limb explants of E14.5 PTHrP−/− and wild-type control mouse embryos were cultured in the presence of Shh or cyclopamine, a specific inhibitor of Hh signaling (Cooper et al., 1998). Chondrocyte hypertrophy was analyzed by histological staining and in situ hybridization with probes of Ihh and Col10a1, markers for prehypertrophic and hypertrophic chondrocytes, respectively. The distance from the articular end to the prehypertrophic or hypertrophic chondrocytes in the developing cartilage reflects how fast chondrocytes undergo hypertrophy. A shorter distance indicates faster hypertrophy. As expected, treatment of wild-type limbs with Shh resulted in delayed chondrocyte hypertrophy, as shown by an increased distance between the articular end and the Ihh/Col10a1 expression domains (Fig. 1A,B). In the cultured PTHrP−/− mouse limb, chondrocyte hypertrophy was accelerated when compared with the wild-type control (Fig. 1A,B). However, treatment of PTHrP−/− limb explants with Shh further accelerated chondrocyte hypertrophy, when compared with the untreated PTHrP−/− limb cultures (Fig. 1A,B). Furthermore, treatment of the PTHrP−/− limb explants with the Hh inhibitor cyclopamine significantly delayed chondrocyte hypertrophy and increased the domain of proliferating chondrocytes. These results indicate that Ihh signaling promotes chondrocyte hypertrophy in the absence of PTHrP.
Fig. 1. Hedgehog signaling promotes chondrocyte hypertrophy in the absence of PTHrP signaling.
(A,B) Forelimbs of PTHrP−/− or wild-type E14.5 embryos cultured for 36 hours in the presence or absence of recombinant Shh protein or cyclopamine. Treated limbs were compared with untreated contralateral control ones. Serial sections of the proximal humerus of the cultured limbs were hybridized with 35S riboprobes of Ihh (A) or Col10a1 (B). The distances from the articular end to the Ihh/Col10a1 domain in the untreated wild-type and PTHrP−/− embryos are indicated by orange lines. This distance was increased in the Shh-treated wild-type humerus but reduced in the Shh-treated PTHrP−/− humerus (white lines) compared with the untreated contralateral controls. This distance was reduced in the cyclopamine-treated wild type, but increased in the PTHrP−/− humerus (green lines) compared with the untreated controlateral controls. (C) Statistical analysis (paired Student′s t-test) of the distance from articular chondrocytes to the hypertrophic zone between treated and untreated contralateral limbs. Numbers (n) of analyzed limbs are indicated. (D) Serial sections of E14.5 distal humeri of the indicated genotypes stained with Safranin O (upper panel) and hybridized with a 35S Col10a1 riboprobe (lower panel) to detect chondrocyte hypertrophy. The distance from the articular end to the Col10a1 expression domain (orange lines) in the PTHrP−/− embryos was reduced compared with that in the wild-type embryos. This distance in the PTHrP−/−;UAS-cIhh;Col2a1-Gal4 double mutant was further reduced compared with that of the PTHrP−/− embryos.
Overexpression of Ihh in the cartilage in the absence of PTHrP accelerates chondrocyte differentiation in vivo
To rule out the possibility that the PTHrP−/− limb explants may respond to Hh signaling differently in vitro, we analyzed the PTHrP-independent role of Ihh signaling in vivo. We used a UAS-Gal4 bigenic system to overexpress the chicken Ihh gene in the cartilage (Yang et al., 2003) and then crossed it with PTHrP−/− mice to generate a compound mutant UAS-cIhh;Col2a1-Gal4;PTHrP−/−. At E14.5, PTHrP−/− mutant embryos showed accelerated hypertrophy compared with wild-type embryos, as shown by the reduction of the distance from the Col10a1 expression domain to the articular end (Fig. 1C). In the UAS-cIhh;Col2a1-Gal4 mutant embryos, chondrocyte hypertrophy indicated by Col10a1 expression was slightly accelerated compared with that of the wild type (Fig. 1C). Interestingly, in the UAS-cIhh;Col2a1-Gal4;PTHrP−/− compound mutant embryos, the proliferating chondrocyte region before hypertrophy was significantly shorter in length even than that of the PTHrP−/− mutant embryos (Fig. 1C). These results indicate that in the absence of PTHrP signaling, upregulation of Ihh signaling accelerates chondrocyte hypertrophy at an early stage of long bone development in vivo.
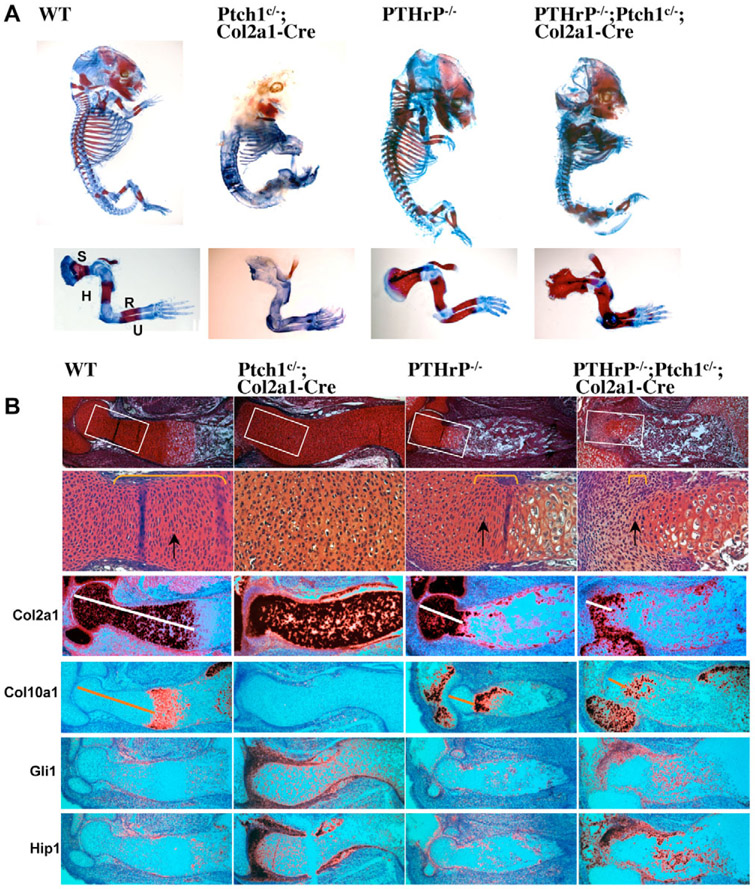
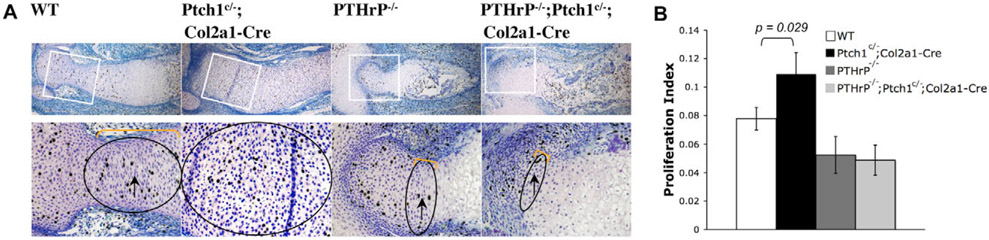
Next, we tested whether Ihh signals directly to the chondrocytes in a PTHrP-independent manner to regulate chondrocyte hypertrophy. We have previously generated a floxed allele of Ptch1 in mice that allows cell-autonomous activation of Hh signaling upon Cre-mediated recombination (Mak et al., 2006). As shown before, we found that in the Ptch1c/−;Col2a1-Cre embryos, Ihh signaling is upregulated, as indicated by the enhanced expression of the Hh signaling target genes Gli1 and Hip1 (Fig. 2B). In addition, chondrocyte hypertrophy was significantly delayed, as there was no Col10a1 expression at E14.5 when it was already strongly expressed in wild-type and PTHrP−/− mutant embryos (Fig. 2B). The delay of chondrocyte hypertrophy is due to the upregulation of PTHrP expression (Mak et al., 2006). Interestingly, when we crossed the Ptch1c/−;Col2a1-Cre mutant to the PTHrP−/− mutant, we found that ossification and mineralization, as indicated by Alizarin Red staining, were strongly enhanced in PTHrP−/−;Ptch1c/−;Col2a1-Cre double mutants when compared with both Ptch1c/−;Col2a1-Cre and the PTHrP−/− single mutant alone (Fig. 2A). When sections of the humerus at E14.5 were examined, we found that chondrocyte hypertrophy was accelerated more than that of the PTHrP−/− embryos. There were a few rows of columnar chondrocytes in the PTHrP−/− embryo, but in the PTHrP−/−;Ptch1c/−;Col2a1-Cre double mutant loss of columnar chondrocytes was so fast that there were few columnar chondrocytes left, and the size of the articular cartilage region was also reduced owing to accelerated articular chondrocyte differentiation (Fig. 2B). In addition, the Col10a1 expression domain in the PTHrP−/−;Ptch1c/−;Col2a1-Cre double mutant was closer to the articular end than that in the PTHrP−/− embryo (Fig. 2B). Furthermore, we performed a BrdU incorporation assay, which shows the percentage of cells in the S phase of mitotic division and allows the comparison of cell proliferation. In the PTHrP−/− embryo, there were still a few BrdU-positive columnar chondrocytes (Fig. 3A). Consistent with the accelerated loss of the fast proliferating columnar chondrocytes in the PTHrP−/−;Ptch1c/−;Col2a1-Cre double mutant, there were hardly any BrdU-positive cells outside of the periarticular region (Fig. 3A). Cell proliferation in the columnar region was similarly reduced in the PTHrP−/− and PTHrP−/−;Ptch1c/−;Col2a1-Cre double mutant (Fig. 3B), supporting the previous notion that PTHrP signaling provides the competence of chondrocytes to respond to Ihh signaling to promote proliferation (Karp et al., 2000). These results demonstrate that upregulation of Ihh signaling in chondrocytes promotes chondrocyte hypertrophy independently of PTHrP.
Fig. 2. Cell autonomous upregulation of Hh signaling in the absence of PTHrP accelerates chondrocyte hypertrophy.
(A) Skeletal preparation of embryos at E14.5. Alizarin Red stains mineralized cartilage and bone tissues; Alcian Blue stains unmineralized cartilage. A higher magnification view of the forelimb is shown in the lower panel. S, scapula; H, humerus; R, radius; U, ulna. (B) Serial sections of distal humerus were stained with Safranin O and hybridized with indicated 35S labeled riboprobes. The boxed articular and columnar chondrocytes regions are shown at higher magnification in the panels below. Columnar chondrocytes are indicated by yellow brackets and arrows. Both Ptch1c/−;Col2a1-Cre and PTHrP−/−;Ptch1c/−;Col2a1-Cre mutants show strong upregulation of Gli1 and Hip1, downstream target genes of Hh signaling, in the perichondrium and synovial joint. The Col2a1-expressing region (white line) is reduced and the Col10a1-expressing domain (yellow line) is closer to the joint in the PTHrP−/−;Ptch1c/−;Col2a1-Cre mutant.
Fig. 3. Cell proliferation is similarly reduced in PTHrP−/− and PTHrP−/−;Ptch1c/−;Col2a1-Cre mutant cartilage.
(A) Comparison of BrdU-labeled chondrocytes in the cartilage of different mutants and in the wild-type control. Boxed regions are shown at higher magnification in the lower panel. The highly proliferating columnar chondrocytes (bracket) were reduced in the PTHrP−/−;Ptch1c/−;Col2a1-Cre mutant. (B) Percentage of BrdU-labeled chondrocytes in the columnar regions (circled in A), calculated from three independent samples to get the mean±s.d. Student′s t-test, P<0.05.
Inhibition of Hh signaling delays chondrocyte hypertrophy independently of PTHrP
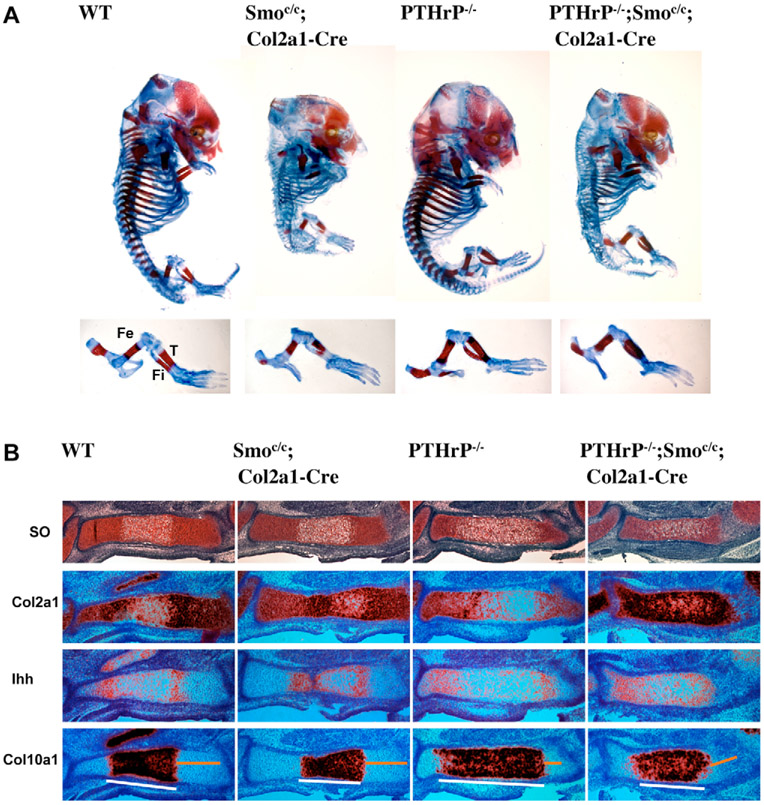
Our finding that upregulated Hh signaling promotes chondrocyte hypertrophy in the absence of PTHrP suggests that inhibition of Ihh signaling would result in delayed chondrocyte hypertrophy in the absence of PTHrP. To test this, we inactivated Hh signaling by removing Smo in chondrocytes of PTHrP−/− mouse embryos and examined the pace of chondrocyte hypertrophy. Skeletal preparations of E15.5 embryos showed that the general morphology of the PTHrP−/−;Smoc/c;Col2a1-Cre double mutant was similar to that of Smoc/c;Col2a1-Cre single mutant, but that mineralization in the double mutant was decreased compared with that in the wild-type control and the PTHrP−/− single mutant (Fig. 4A). More detailed analysis of the E15.5 tibia sections revealed that almost all chondrocytes in the double mutant embryo still expressed Col2a1, a marker for non-hypertrophic chondrocytes, whereas a significant portion of chondrocytes in the PTHrP−/− single mutant embryos had lost Col2a1 expression and undergone hypertrophy (Fig. 4B). In addition, more chondrocytes in the wild-type embryo had lost Col2a1 expression than had those in the Smoc/c;Col2a1-Cre single mutant (Fig. 4B). Consistent with the expression of Col2a1, the expression domains of Col10a1 and the regions between the two Ihh expression domains were smaller in Smoc/c;Col2a1-Cre and PTHrP−/−;Smoc/c;Col2a1-Cre mutant embryos than in wild-type and PTHrP−/− embryos, respectively (Fig. 4B). These data indicate that the progression of chondrocyte hypertrophy is slower when Smo is removed, especially in the absence of PTHrP at early stages of cartilage development. Therefore, in contrast to the role of PTHrP that is activated and maintained by Ihh signaling, Ihh signaling is required to promote chondrocyte hypertrophy in the absence of PTHrP. This agrees with, and explains, the observation that in the Ihh−/− embryo there is an initial delay of chondrocyte hypertrophy at E14.5 (St-Jacques et al., 1999).
Fig. 4. Removal of Hh signaling delays chondrocyte hypertrophy in the absence of PTHrP.
(A) Skeletal preparation of E15.5 embryos. Hindlimbs are shown at higher magnifications in the lower panel. (B) Serial sections of tibia were stained with Safranin O and hybridized with 35S labeled Ihh and Col10a1 riboprobes. Smoc/c;Col2a1-Cre mutant tibia showed a slight delay in chondrocyte hypertrophy compared with that of wild-type embryos. PTHrP−/−;Smoc/c;Col2a1-Cre mutant tibia also showed a delay of chondrocyte hypertrophy, as compared with that of the PTHrP−/− mutant. The proliferating chondrocyte region is indicated by the yellow line; the hypertrophic region is indicated by the white line. Fe, femur; T, tibia; Fi, fibula.
Hh signaling activates the canonical Wnt and Bmp signaling in chondrocytes
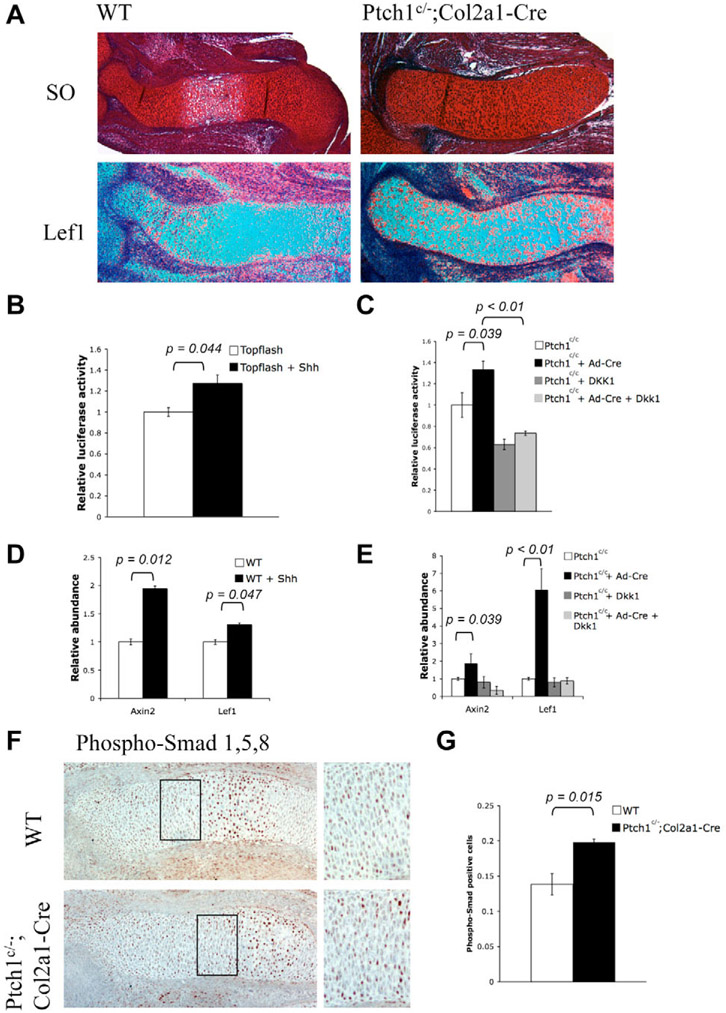
As both Wnt/β-catenin signaling and the PTHrP-independent role of Ihh signaling promote chondrocyte hypertrophy, one likely scenario is that Ihh signaling activates the canonical Wnt signaling in chondrocytes. We have previously shown that delayed chondrocyte hypertrophy caused by upregulated PTHrP expression due to activated Ihh signaling can be further enhanced by the loss of Wnt/β-catenin signaling (Mak et al., 2006), raising the possibility that the loss of Wnt/β-catenin signaling actually diminished the role of Ihh signaling in promoting chondrocyte hypertrophy. To address this, we performed in situ hybridization with Lef1, which is a transcriptional target of Wnt/β-catenin signaling (Hovanes et al., 2001), on E14.5 limb sections of Ptch1c/−;Col2a1-Cre mutants (Fig. 5A). Lef1 is mainly expressed in proliferating chondrocytes (Fig. 5). In the Ptch1c/−;Col2a1-Cre mutants, Lef1 expression was highly upregulated in all chondrocytes, suggesting that Ihh signaling activates Wnt/β-catenin signaling in proliferating chondrocytes. To further confirm the effect of Hh signaling on Wnt/β-catenin signaling, we isolated primary chondrocytes from rib cages of postnatal day one (P1) wild-type mice. The Wnt/β-catenin signaling activity in the cultured primary chondrocytes was measured by the TOPFLASH luciferase activity (Korinek et al., 1997), which is a reporter containing luciferase driven by Lef/Tcf binding sites and a basic promoter. TOPFLASH luciferase expression is activated by β-catenin when it binds Lef/Tcf factors. Consistent with the in vivo observation, activation of the TOPFLASH reporter was detected in the Cre-adenovirus-infected Ptch1c/c primary chondrocytes and in the wild-type primary chondrocytes treated with Shh, when compared with control cells (Fig. 5B,C), indicating that Wnt/β-catenin signaling is activated by Hh signaling. In addition, by quantitative RT-PCR, we found that the expression of two canonical Wnt target genes, Axin2 and Lef1, was significantly increased in the Shh-treated or Ptch1-deficient chondrocytes when compared with the control cells (Fig. 5D,E). By contrast, the upregulation of canonical Wnt signaling activity in response to Hh signaling was abolished when the primarily chondrocytes were treated with Dkk1, a secreted antagonist of the canonical Wnt signaling (Fig. 5C,E). Taken together, our results suggest that upregulated Ihh signaling activates the expression of some Wnt ligands that signal through the canonical Wnt pathway in chondrocytes. Indeed, we found that the expression of several Wnt genes, including Wnt2, Wnt3a, Wnt8 and Wnt8b, that can signal through the canonical pathway was upregulated by activated Hh signaling (data not shown). Furthermore, consistent with the previously observed upregulation of Bmp expression in the developing cartilage of Ptch1c/−;Col2a1-Cre mutant embryos (Mak et al., 2006), we found that Bmp signaling activity was upregulated in Ptch1c/−;Col2a1-Cre mutant embryos, as indicated by the increased phosphorylation of Smad1 in the chondrocytes (Fig. 5F,G).
Fig. 5. Hedgehog signaling activates downstream targets of canonical Wnt signaling both in vivo and in vitro.
(A) Serial sections of E14.5 distal humerus were stained by Safranin O and hybridized with a 35S labeled Lef1 probe. Lef1 expression was strongly upregulated in the cartilage of Ptch1c/−;Col2a1-Cre mutants. (B,C) Dual luciferase assay of primary chondrocytes isolated from wild-type newborn pups. Primary chondrocytes were nucleofected with Topflash reporter vectors as a read out for canonical Wnt signaling. Recombinant Shh or Dkk1 protein was added after serum starvation and luciferase activity was measured 24 hours later. Shh treatment or Cre-adenovirus infection of the Ptch1c/c primary chondrocytes activated TOPFLASH activity. Such activation was diminished by Dkk1. (D,E) Quantitative RT-PCR was performed using RNA isolated from primary chondrocytes. Both Axin2 and Lef1 were significantly increased in Shh-treated primary chondrocytes or Cre-adenovirus-infected Ptch1c/c primary chondrocytes compared with untreated samples. Dkk1 treatment blocked the effect of Hh signaling. (F) Immunohistochemistry of E15.5 limb sections (distal ulna) with antibodies against phospho-Smad1, 5 and 8. More phospho-Smad-positive cells were found in the cartilage of the Ptch1c/−;Col2a1-Cre mutant embryos. Boxed region of columnar/prehypertrophic chondrocytes is shown at a higher magnification on the right-hand side. (G) Statistical analysis of phospho-Smad-positive cells in the boxed region of the cartilage. Three samples in the boxed area were counted, and the mean±s.d. are shown. Student′s t-test, P<0.05.
Hh signaling regulates chondrocyte hypertrophy in postnatal cartilage
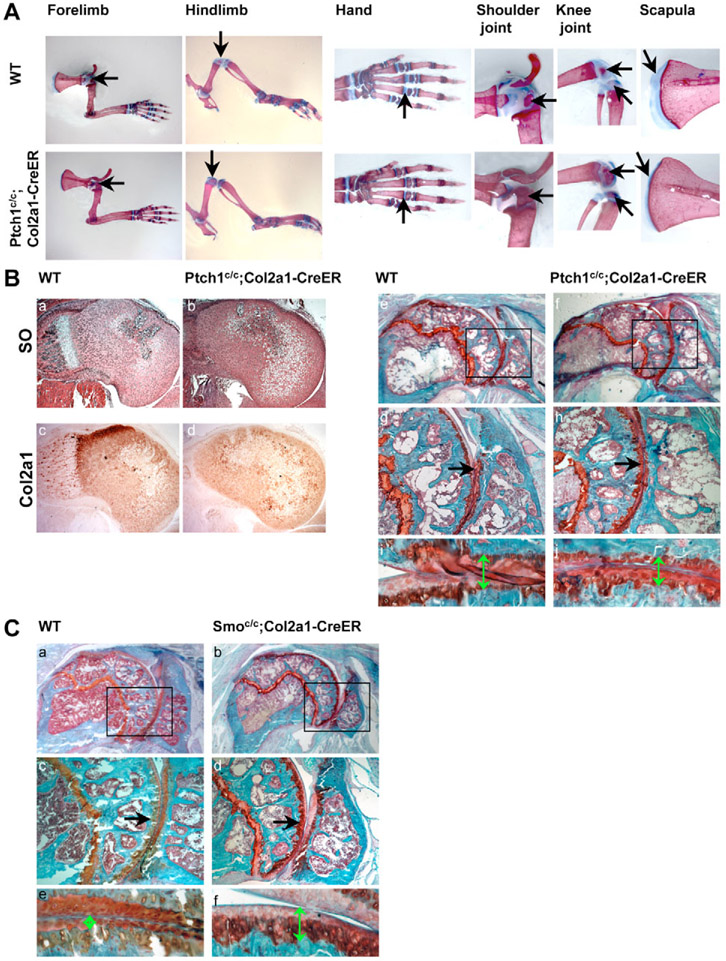
Our results showed that Ihh signaling promotes chondrocyte hypertrophy in the absence of PTHrP in embryonic skeletal development. This function of Ihh signaling may be dominant over the PTHrP-dependent role of Ihh in inhibiting chondrocyte hypertrophy in postnatal cartilage, as PTHrP expression is weaker in adult cartilage (Tsukazaki et al., 1995). To test this, we activated Hh signaling in postnatal cartilage using the floxed Ptch1 allele and an inducible chondrocyte-specific Cre mouse line, Col2a1-CreER (Maeda et al., 2007; Nakamura et al., 2006). Cre recombinase activity was induced in this mouse line by tamoxifen (TM). Skeletal preparations of P15 Ptch1c/c;Col2a1-CreER mice showed a significant acceleration of mineralization, as indicated by increased Alizarin Red staining in both forelimbs and hindlimbs. Conversely, the cartilage region stained by Alcian Blue at the joint was significantly reduced (Fig. 6A). Histological sections of the distal femur showed that chondrocyte hypertrophy around the secondary ossification center in Ptch1c/c;Col2a1-CreER mice was greatly enhanced when compared with the wild-type control (Fig. 6B, parts a,b). In addition, immunohistochemistry with Col2a1 antibodies confirmed that the zone of hypertrophic chondrocytes was larger with lower Col2a1 expression in Ptch1c/c;Col2a1-CreER mice (Fig. 6B, parts c,d). Furthermore, in TM-induced one-year-old Ptch1c/c;Col2a1-CreER mice, the number of chondrocytes lining the joint surface and the proteoglycan content in articular chondrocytes, as indicated by Hematoxylin/Eosin or Safranin O staining, were reduced (Fig. 6B, parts e-j). There was no obvious change in the growth plates in TM-induced Ptch1c/c;Col2al-CreER mice (see Fig. S2 in the supplementary material). Conversely, in 14-month-old Smoc/c;Col2a1-CreER mice, chondrocytes lining the joint surface and the proteoglycan content in articular chondrocytes were increased (Fig. 6C). These data indicate that Hh signaling in postnatal cartilage promotes chondrocyte hypertrophy in the area of secondary ossification. These results further suggest that activated Hh signaling might be a risk factor for osteoarthritis.
Fig. 6. Hedgehog signaling accelerates chondrocyte hypertrophy in postnatal cartilage.
(A) Skeletal preparations of P15 forelimb and hindlimb of the Ptch1c/c;Col2a1-CreER and wild-type mouse. Unmineralized cartilage in the hand, shoulder and knee joints and the scapula was reduced in the mutant, as shown at high magnification. (Ba-d) Serial sections of distal femur of P12 mice were stained with Safranin O and used for immunohistochemistry to detect Col2a1 expression. There were more hypertrophic chondrocytes with reduced Col2a1 expression in the Ptch1c/c;Col2a1-CreER mouse. (Be-j) Sections of proximal humeri from one-year-old mice were stained with Safranin O. Boxed regions in e and f are shown at higher magnifications in g,h. Cartilage lining was thinner in the Ptch1c/c;Col2a1-CreER mouse (arrow). (i,j) Higher magnification of articular cartilage of the proximal humerus. Joint cartilage in the Ptch1c/c;Col2a1-CreER mouse was significantly reduced (double-headed arrows). (Ca-d) Sections of proximal humeri from 14-month-old mice were stained with Safranin O. Boxed regions in a and b are shown at higher magnifications in c,d. Cartilage lining was thicker in the Smoc/c;Col2a1-CreER mouse (arrow). (Ce,f) Higher magnification of articular cartilage of the proximal humerus. Joint cartilage in the Smoc/c;Col2a1-CreER mouse was significantly thicker (double-headed arrows).
DISCUSSION
Here, we report that apart from controlling chondrocyte hypertrophy indirectly through regulating PTHrP expression, Ihh signaling also directly regulates chondrocyte hypertrophy in the absence of PTHrP. The direct and indirect roles of Ihh signaling are opposite to each other. Whereas Ihh-regulated PTHrP signaling inhibits chondrocyte hypertrophy and is dominant in embryonic skeletal development, we found that one PTHrP-independent role of Ihh signaling is to promote chondrocyte hypertrophy, possibly through activating Wnt/β-catenin and Bmp signaling. This function is important in postnatal cartilage development and homeostasis.
The negative-feedback loop of Ihh-PTHrP signaling plays a crucial role in controlling the pace of chondrocyte hypertrophy (Lanske et al., 1996; St-Jacques et al., 1999; Vortkamp et al., 1996). However, Ihh signaling has also been found to promote chondrocyte proliferation and the transition from slowly proliferating periarticular chondrocytes to fast proliferating columnar chondrocytes (periarticular chondrocyte differentiation) independently of PTHrP signaling (Kobayashi et al., 2005b). Our data uncovered another PTHrP-independent activity of Ihh in promoting chondrocyte hypertrophy that was masked by the more potent PTHrP-dependent activity of Ihh, which is to inhibit chondrocyte hypertrophy in embryonic development (Fig. 7). These results highlight the highly cell context-dependent effects of Ihh signaling. Ihh signaling promotes the proliferation of periarticular and columnar chondrocytes, whereas, in more mature prehypertrophic chondrocytes, it may promote hypertrophy by promoting cell cycle exit.
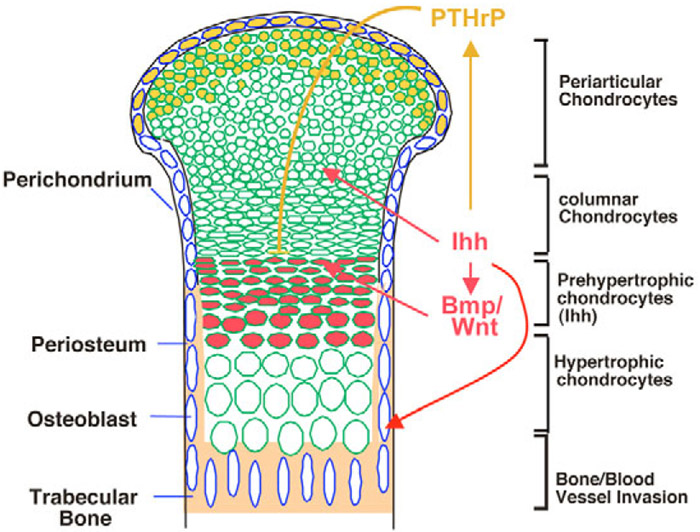
Fig. 7. Model of the PTHrP-dependent and PTHrP-independent function of Ihh signaling in chondrocyte differentiation.
The morphology of a developing long bone is schematically shown. Chondrocytes proliferate and differentiate sequentially along the longitudinal axis. The length of the columnar chondrocyte region is determined by the rate of periarticular chondrocyte differentiation, columnar chondrocyte proliferation and hypertrophy. Ihh expression is shown in red. PTHrP expression is shown in yellow. Ihh acts through PTHrP to inhibit chondrocyte hypertrophy. Ihh acts independently of PTHrP to control chondrocyte proliferation, periarticular chondrocyte differentiation and osteoblast differentiation. Here, we show that Ihh also promotes chondrocyte hypertrophy independently of PTHrP and that this function is likely to be mediated by Bmp and Wnt/β-catenin signaling.
During longitudinal development of the long bone cartilage, periarticular chondrocyte differentiation, which adds cells to the columnar region, is followed by chondrocyte hypertrophy, which reduces cells in the columnar region. Therefore, the length of the columnar chondrocyte region is determined by three parameters: the pace of periarticular chondrocyte differentiation, the pace of chondrocyte hypertrophy and the rate of columnar chondrocyte proliferation (Fig. 7). As upregulated Ihh signaling promotes periarticular chondrocyte differentiation and increases the rate of columnar chondrocyte proliferation (Kobayashi et al., 2005b), the proliferating columnar chondrocyte region would be increased if chondrocyte hypertrophy were not altered. Our observation that the columnar chondrocyte region was shorter in the PTHrP−/−;Ptch1c/−;Col2al-Cre double mutant than in the PTHrP−/− single mutant (Fig. 2B, Fig. 3A) demonstrates that Hh signaling also acts to promote chondrocyte hypertrophy in the absence of PTHrP to reduce columnar chondrocyte number. When Ihh was overexpressed while PTHrP signaling was maintained at a constant level in the caPPR;PTHrP−/−;Ihh-Bg mutant, the periarticular chondrocyte region was smaller but the length of the columnar region was expanded significantly (Kobayashi et al., 2005b). In this case, upregulated Ihh signaling promotes periarticular chondrocyte differentiation and chondrocyte proliferation, and both activities increase the number of proliferating columnar chondrocytes. Although chondrocyte hypertrophy is also accelerated in this case, it is not enough to cancel out the effects of enhanced periarticular chondrocyte differentiation and chondrocyte proliferation. Furthermore, it has been shown that PTHrP signaling is required to provide a competent domain in which Ihh signaling can promote chondrocyte proliferation (Karp et al., 2000). Therefore, the difference between the PTHrP−/−;Ptch1c/−;Col2a1-Cre double mutant and the caPPR;PTHrP−/−;Ihh-Bg mutant is that chondrocyte proliferation is greatly reduced in the PTHrP−/−;Ptch1c/−;Col2a1-Cre double mutant. Hence, the significantly increased columnar chondrocyte region in the caPPR;PTHrP−/−;Ihh-Bg mutant is a result of increased chondrocyte proliferation. These results also suggest that the PTHrP-independent role of Ihh signaling in promoting chondrocyte hypertrophy may be stronger than that in promoting periarticular differentiation in the embryonic cartilage. Overall, the PTHrP-independent function of Ihh signaling is to increase the transition rate through the different chondrocyte zones during endochondral bone formation.
One crucial factor that determines the relative strength of the opposite activities of the PTHrP-dependent and -independent roles of Ihh signaling in chondrocyte hypertrophy is the level of PTHrP expression. PTHrP expression in the cartilage was progressively weaker in older embryos (K.K.M. and Y.Y., unpublished) and is further reduced in adult animals (Tsukazaki et al., 1995). Therefore, when PTHrP signaling is robust in the embryonic cartilage, upregulated Ihh signaling predominantly delays chondrocyte hypertrophy by upregulating PTHrP expression. However, when mammals approach adulthood, cartilage hypertrophy and bone formation accelerate coordinately as longitudinal bone growth declines and the growth plate is either reduced (i.e. in mouse) or closed (i.e. in human). This is consistent with reduced PTHrP levels postnatally. It is also conceivable that when PTHrP signaling is low enough, the PTHrP-independent role of Hh signaling in promoting chondrocyte hypertrophy is significant for postnatal cartilage development and homeostasis. Furthermore, because only periarticular cells and the upper layer of articular chondrocytes are competent to express PTHrP, even when Hh signaling is upregulated throughout the cartilage (Mak et al., 2006), somatic mutations that upregulate Hh signaling only in chondrocytes deep in the joint cartilage and growth plates will not upregulate PTHrP expression. Hence, when the ability to strongly express PTHrP is lost, upregulated Ihh signaling, for instance due to mutations in Ptch1 (as we have shown in the Ptch1c/c;Col2a1-CreER mutant), may be a risk factor for osteoarthritis.
Our finding that Hh signaling activated Wnt/β-catenin and Bmp signaling in chondrocytes has provided some insight into the underlying mechanism of Hh signaling in promoting chondrocyte hypertrophy. Wnt/β-catenin signaling has been shown to promote chondrocyte hypertrophy, at least in part, through downregulating Sox9 protein levels (Akiyama et al., 2004), a transcription factor that is required for chondrocyte formation and also inhibits chondrocyte hypertrophy (Bi et al., 2001). However, Wnt/β-catenin signaling may not be the only downstream target of Hh signaling in regulating chondrocyte hypertrophy. Indeed, upregulated Hh signaling leads to many changes. For example, we have found that the expression and signaling activity of Bmps are upregulated by enhanced Hh signaling (Mak et al., 2006) (this study), and Bmp signaling activity has been shown to promote chondrocyte hypertrophy independently of PTHrP signaling (Kobayashi et al., 2005a). It is noted that, when Hh signaling is reduced by removing Ihh in postnatal cartilage from P1, chondrocyte hypertophy is also accelerated, although PTHrP expression is still detected (Maeda et al., 2007). These results suggest that Hh signaling still inhibits chondrocyte hypertrophy in postnatal cartilage, and this difference can be reconciled by the following considerations. First, Hh signaling was activated cell autonomously in the Ptch1c/c;Col2a1-CreER mutant cartilage by injecting the lactating female mouse at P3. Therefore, Hh signaling activation in our system occurs later and PTHrP expression might be further declined at that time. Second, the effects of Ihh signaling in chondrocyte hypertrophy can be mediated by PTHrP, Bmp and Wnt signaling simultaneously. As PTHrP and Bmp and Wnt signaling exhibit opposite effects in regulating chondrocyte hypertrophy, when Hh signaling is perturbed (up- or downregulated) in the adult cartilage, the outcome of chondrocyte hypertrophy depends on the relative strength of the pro- and anti-hypertrophy signaling. It is likely that in Ptch1c/c;Col2a1-CreER mice when Hh signaling is upregulated after P3, the pro-hypertrophy signaling (i.e. by Bmps and Wnts) is more robust than is the anti-hypertrophy signaling by PTHrP.
Supplementary Material
Acknowledgments
We thank members of the Yang laboratory for stimulating discussion. Work in the Yang and Mackem laboratories is supported by the intramural research programs of NHGRI and NCI of NIH, respectively. Work in the Kronenberg laboratory was suppoted by NIH grant DK56246. Work in the Chuang laboratory was supported by NIH grants HL67822 and HL66600.
Footnotes
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/135/11/1947/DC1
References
- Akiyama H, Lyons JP, Mori-Akiyama Y, Yang X, Zhang R, Zhang Z, Deng JM, Taketo MM, Nakamura T, Behringer RR et al. (2004). Interactions between Sox9 and {beta}-catenin control chondrocyte differentiation. Genes Dev. 18, 1072–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi W, Huang W, Whitworth DJ, Deng JM, Zhang Z, Behringer RR and de Crombrugghe B (2001). Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proc. Natl. Acad. Sci. USA 98, 6698–6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MK, Porter JA, Young KE and Beachy PA (1998). Teratogen-mediated inhibition of target tissue response to Shh signaling. Science 280, 1603–1607. [DOI] [PubMed] [Google Scholar]
- de Crombrugghe B, Lefebvre V and Nakashima K (2001). Regulatory mechanisms in the pathways of cartilage and bone formation. Curr. Opin. Cell Biol 13, 721–727. [DOI] [PubMed] [Google Scholar]
- Hilton MJ, Tu X, Cook J, Hu H and Long F (2005). Ihh controls cartilage development by antagonizing Gli3, but requires additional effectors to regulate osteoblast and vascular development. Development 132, 4339–4351. [DOI] [PubMed] [Google Scholar]
- Hovanes K, Li TW, Munguia JE, Truong T, Milovanovic T, Marsh JL, Holcombe RF and Waterman ML (2001). Beta-catenin-sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nat. Genet 28, 53–57. [DOI] [PubMed] [Google Scholar]
- Huangfu D and Anderson KV (2006). Signaling from Smo to Ci/Gli: conservation and divergence of Hedgehog pathways from Drosophila to vertebrates. Development 133, 3–14. [DOI] [PubMed] [Google Scholar]
- Karp SJ, Schipani E, St-Jacques B, Hunzelman J, Kronenberg H and McMahon AP (2000). Indian hedgehog coordinates endochondral bone growth and morphogenesis via parathyroid hormone related-protein-dependent and -independent pathways. Development 127, 543–548. [DOI] [PubMed] [Google Scholar]
- Karsenty G and Wagner EF (2002). Reaching a genetic and molecular understanding of skeletal development. Dev. Cell 2, 389–406. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Lyons KM, McMahon AP and Kronenberg HM (2005a). BMP signaling stimulates cellular differentiation at multiple steps during cartilage development. Proc. Natl. Acad. Sci. USA 102, 18023–18027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Soegiarto DW, Yang Y, Lanske B, Schipani E, McMahon AP and Kronenberg HM (2005b). Indian hedgehog stimulates periarticular chondrocyte differentiation to regulate growth plate length independently of PTHrP J. Clin. Invest 115, 1734–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B and Clevers H (1997). Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science 275, 1784–1787. [DOI] [PubMed] [Google Scholar]
- Koziel L, Wuelling M, Schneider S and Vortkamp A (2005). Gli3 acts as a repressor downstream of Ihh in regulating two distinct steps of chondrocyte differentiation. Development 132, 5249–5260. [DOI] [PubMed] [Google Scholar]
- Kronenberg HM (2003). Developmental regulation of the growth plate. Nature 423, 332–326. [DOI] [PubMed] [Google Scholar]
- Lanske B, Karaplis AC, Lee K, Luz A, Vortkamp A, Pirro A, Karperien M, Defize LH, Ho C, Mulligan RC et al. (1996). PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science 273, 663–666. [DOI] [PubMed] [Google Scholar]
- Long F, Chung UI, Ohba S, McMahon J, Kronenberg HM and McMahon AP (2004). Ihh signaling is directly required for the osteoblast lineage in the endochondral skeleton. Development 131, 1309–1318. [DOI] [PubMed] [Google Scholar]
- Lum L and Beachy PA (2004). The Hedgehog response network: sensors, switches, and routers. Science 304, 1755–1759. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Nakamura E, Nguyen MT, Suva LJ, Swain FL, Razzaque MS, Mackem S and Lanske B (2007). Indian Hedgehog produced by postnatal chondrocytes is essential for maintaining a growth plate and trabecular bone. Proc. Natl. Acad. Sci. USA 104, 6382–6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak KK, Chen MH, Day TF, Chuang PT and Yang Y (2006). Wnt/beta-catenin signaling interacts differentially with Ihh signaling in controlling endochondral bone and synovial joint formation. Development 133, 3695–3707. [DOI] [PubMed] [Google Scholar]
- McLeod MJ (1980). Differential staining of cartilage and bone in whole mouse fetuses by alcian blue and alizarin red S. Teratology 22, 299–301. [DOI] [PubMed] [Google Scholar]
- Nakamura E, Nguyen MT and Mackem S (2006). Kinetics of tamoxifen-regulated Cre activity in mice using a cartilage-specific CreER(T) to assay temporal activity windows along the proximodistal limb skeleton. Dev. Dyn 235, 2603–2612. [DOI] [PubMed] [Google Scholar]
- Poole AR (1999). An introduction to the pathophysiology of osteoarthritis. Front. Biosci 4, D662–D670. [DOI] [PubMed] [Google Scholar]
- St-Jacques B, Hammerschmidt M and McMahon AP (1999). Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 13, 2072–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukazaki T, Ohtsuru A, Enomoto H, Yano H, Motomura K, Ito M, Namba H, Iwasaki K and Yamashita S (1995). Expression of parathyroid hormone-related protein in rat articular cartilage. Calcif. Tissue Int 57, 196–200. [DOI] [PubMed] [Google Scholar]
- Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM and Tabin CJ (1996). Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science 273, 613–622. [DOI] [PubMed] [Google Scholar]
- Yang Y, Topol L, Lee H and Wu J (2003). Wnt5a and Wnt5b exhibit distinct activities in coordinating chondrocyte proliferation and differentiation. Development 130, 1003–1015. [DOI] [PubMed] [Google Scholar]
- Zelzer E and Olsen BR (2003). The genetic basis for skeletal diseases. Nature 423, 343–348. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.