Abstract
Introduction:
External cervical resorption (ECR) has been challenging for its diagnosis, prevention, and treatment. Its etiology and pathogenesis are largely unknown. This study characterized microRNA (miRNA) expression patterns of human tissues from ECR lesions and identified potential messenger RNA targets and pathways.
Methods:
Granulomatous tissues from ECR (n = 5) and their adjacent nonaffected asymptomatic gingival connective tissues (n = 5) were collected. Similarly, chronic periodontitis (CP) and control samples were collected (n = 3). Quantitative reverse transcription polymerase chain reaction array analysis compared the expression profiles of 88 miRNAs between diseases. Differentially expressed miRNAs were identified using the Student t test. Bioinformatics for messenger RNA (miRWalk) and KEGG pathway analyses were performed to identify predicted target genes and biological/cellular functions and signaling pathways.
Results:
Three miRNAs (miR-20a-5p, miR-210–3p, and miR-99a-4p) were significantly down-regulated and 1 miRNA (miR-122–5p) was significantly up-regulated in ECR (P < .05). One up-regulated and 1 down-regulated miRNA reached the significance threshold in CP. A comparison of miRNA expression in ECR and CP identified 3 differentially expressed miRNAs, indicating differences in disease pathobiology. Inflammation-associated Wnt, PI3K-Akt, mitogen-activated protein kinases signaling, and bone formation–associated transforming growth factor beta pathways were identified and predicted to be modulated by differentially expressed miRNAs in both ECR and CP. Biological processes unique to each disease entity were identified, such as T- and B-cell receptor signaling pathways, osteoclast differentiation, and extracellular matrix–receptor interaction for CP. Glycosaminoglycan biosynthesis, mineral absorption, and insulin signaling pathways for ECR were identified.
Conclusions:
This proof-of-principle in vivo study indicated that ECR has both common and unique miRNA expression profiles in comparison with CP, which are predicted to target genes regulating inflammation, immunity, and metabolism of mineralized tissues.
Keywords: Chronic periodontitis, external cervical resorption, inflammation, microRNA
External cervical resorption (ECR) is defined as the loss of cementum and dentin because of odontoclastic activity. Two types of resorption are commonly known: a physiologic process that occurs during exfoliation of the deciduous dentition and pathologic processes, which negatively affect the permanent dentition. Cementum is considered protective to the underlining root dentin, and it is broadly accepted that damage to, or deficiency of, this protective cementum layer apical to the epithelial attachment exposes the root surface to osteoclasts, which then resorb the dentin1,2. Pathologic root resorption is usually of unfavorable prognosis and may result in irreversible damage and eventual tooth loss.
The etiology of pathologic ECR is uncertain but has been associated with orthodontic treatment, trauma, intracoronal bleaching, surgical procedures, viral infections, and periodontal therapy. The nature of the resorptive process can be regarded as either purely inflammatory or possibly combined with a microbial and viral presence3–8. However, mechanistic studies of root resorption have primarily focused on permanent tooth eruption and orthodontic treatment9–11 so that the exact etiology and pathological mechanisms underlying ECR remain unclear and poorly understood.
MicroRNAs (miRNAs) are evolutionarily conserved, small (~22 nucleotide), noncoding RNA molecules that play pivotal roles in posttranscriptional modulation of gene expression by binding to complementary sequences within the 3′ untranslated region of target genes, evoking messenger RNA (mRNA) degradation or translational inhibition. MiRNAs have emerged as important regulators of gene expression and diverse biological processes, including inflammation, immune response, and osteoclastic bone resorption12,13. The impact of miRNA on gene regulation can be noted in the most fundamental and diverse biological processes, ranging from cell development to apoptosis14,15, and it is now evident that aberrant miRNA expression in the immune system is sufficient to cause disease16–19.
miRNA is recognized for its significant therapeutic potential. A recent query (1/112019) of the National Institutes of Health ClinicalTrials.gov search engine identified (recruiting, not yet recruiting, and available) 291 studies using miRNA for therapeutic purposes (http://www.clinicaltrials.gov/ct2/results?term5mirna&recr5Open). This highlights their significant therapeutic potential to address numerous pathological conditions affecting humankind. Of interest, inflammation, a key component of endodontic/periodontal pathology, is among the areas of therapeutic focus. Unfortunately, the understanding of the role of miRNAs in dental diseases is in its infancy, and no reports are available regarding their role in ECR.
Our incomplete understanding of the regulatory networks governing periodontal inflammation (granulomatous tissue formation) at the cervical root level represents a critical barrier in the prevention and treatment of ECR. Considering the periodontal literature, numerous investigators have documented both in vitro and in vivo transcriptional responses associated with periodontal pathosis and the inflammatory response to periodontal pathogens20–22. Although numerous gene regulatory networks have been studied in detail, it is not always possible to reconcile transcriptional events with actual proteomic outcomes. Because miRNA regulates gene expression at the posttranscriptional level that affects the protein level, the knowledge gained in miRNA expression network changes associated with periodontal disease would shed light on our understanding of ECR at the molecular level. Therefore, the purpose of this study was to characterize the miRNAs expressed in the granulomatous tissue found adjacent to lesions with ECR in humans to non–ECR-involved clinically healthy gingival tissue and to identify their potential gene targets/pathways. In addition, in order to compare the unique and common molecular miRNA signatures in different oral disease processes, this study characterized the granulomatous tissue associated with chronic periodontitis (CP) lesions in the same manner as the ECR lesions with clinically healthy gingival tissues serving as the control. Comparing these 2 distinct disease processes occurring in similar anatomic sites may help elucidate the role of miRNA in inflammation surrounding dental roots and their detrimental outcomes (ie, root resorption vs alveolar bone loss). The elucidation of these inflammatory mechanisms can lead to potential diagnostic, preventive, and intervention measures for these destructive conditions. Our specific aims were to
characterize the miRNA expression profiles in pathological ECR and CP and
perform a bioinformatics assessment of miRNA-mRNA target interactions, with a specific focus on signaling pathways linked to inflammation and immunity.
MATERIALS AND METHODS
Study Participants and Sample Collection
This was a single-center pilot study that evaluated the miRNA levels in individuals with ECR or CP. Granulomatous tissue (test) samples and clinically asymptomatic or clinically healthy gingival biopsy (control) samples were collected from participants with ECR or CP at the time of the surgical treatment. In total, 8 participants (5 with ECR and 3 with CP) were recruited from a pool of individuals seeking dental treatment at the University of North Carolina at Chapel Hill School of Dentistry’s clinics.
This study protocol was approved by the institutional review board of the University of North Carolina at Chapel Hill (#13–1279). The inclusion criteria were as follows:
individuals between 18 and 80 years of age;
clinical and/or radiographic evidence of ECR; and
general good health, including individuals classified as American Society of Anesthesiologists type 1 or 2. Individuals were excluded from the study if they had a chronic disease with oral manifestation (eg, autoimmune conditions, such as lichen planus, scleroderma, pemphigoid, and so on). Other exclusions included patients with gross oral pathology, patients who were currently under chronic treatment with nonsteroidal anti-inflammatory drugs, and patients who had taken aspirin within 1 month of the screening examination or had taken ongoing medications initiated less than 3 months before recruitment. Patients using tobacco or who were pregnant were also excluded. A history of previous orthodontic treatment was not among the exclusion criteria. The following clinical data were collected from each participant: demographic information, health history, dental history, radiographic images, endodontic diagnosis, and presence or absence of infection. For CP patients, the test CP samples were collected from the site with a probing depth >5 mm with the clinical attachment level >4 mm. Supplementary Table S1 (available online at www.jendodon.com) combines the demographic and clinical data of the participants.
Participants had a soft tissue sample collected (~1–2 mm2) from the area adjacent to the external root resorption site or the tooth with the deepest probing depth upon surgical treatment of CP(test). Inaddition, a secondsmall (~1–2 mm2) connective tissue biopsy (gingiva) was collected from a noninvolved site to serve as the control. After local anesthesia, a gingival flap surrounding the involved tooth was elevated to expose the external root resorption lesion, and the granulomatous tissue was removed using a periodontal curette. A similar procedure was performed for the CP lesions. The harvested tissue samples were placed in sterile round-bottom polypropylene tubes and immediately immersed in 1.0 mL RNAlater (Applied Biosystems/Ambion, Austin, TX) solution and incubated at 4°C for at least 24 hours. After this step, theprocessed samples weretransferred to a −80°C freezer for long-term storage.
Tissue Lysis and RNA Preparation
Samples were thawed on ice and centrifuged at 4°C for 2 minutes at 12,000 rpm to remove the stabilizer (RNAlater) reagent. Tissues were washed twice with 1× phosphate-buffered saline, and 700 mL Qiazol (Qiagen, Germantown, MD) was added to each sample before homogenization using a TissueLyzer (Qiagen). Total RNA (including miRNAs) was isolated using the miRNeasy kit (Qiagen) according to the manufacturer’s instructions. The RNA was quantitated using a NanoDrop (Thermo Scientific, Wilmington, DE), and RNA integrity was assessed using the 2100 Bioanalyzer (Agilent, Foster City, CA).
Reverse Transcription and Quantitative Reverse Transcription Polymerase Chain Reaction Array
Five hundred nanograms of total RNA from each sample was reverse transcribed to complementary DNA (20 μL reaction) using miScript II RT Kit (Qiagen) per the manufacturer’s instructions. After completion, 90 μL RNAse-free water was added to each tube. For mature miRNA quantitation, an miFinder PCR array plate (96 well) containing 84 different miRNAs, 6 endogenous controls, and 6 experimental controls was purchased from Qiagen (catalog no.: MIHS-001ZA). One hundred microliters complementary DNA from each sample was added to a cocktail containing 2× master mix (Taq polymerase+deoxyribonucleotide triphosphates + buffer), universal reverse primer, and water to make 1.5 mL reaction mix. From each tube, 15 μL reaction mix was added to each well of the 96-well plate already containing forward miRNA specific primers. Reverse transcription polymerase chain reaction was performed using a StepOnePlus thermocycler (Applied Biosystems, Carlsbad, CA). miRNA polymerase chain reaction array data were analyzed using the Qiagen GeneGlobe Data Analysis Center (https://www.qiagen.com/us/shop/genes-and-pathways/data-analysis-center-overview-page/). Expression levels of miRNAs across the different groups were normalized with respect to at least 3 most consistently expressed endogenous controls in all the samples.
Bioinformatics miRNA Analysis and Target Selection
Potential mRNA target genes for differentially expressed miRNAs were identified using miRWalk (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2/miRretsys-self.html). miRWalk is a comprehensive, multispecies miRNA database that provides information on predicted as well as validated binding sites on their target genes. It is based on a comparison of computed mRNA 3′ untranslated regions and their miRNA binding sites with 12 miRNA-target prediction programs, of which we selected 8 algorithms including Diana-microT (http://diana.cslab.ece.ntua.gr/microT), miRanda (http://www.microrna.org/microrna/home.do), miRDB (http://mirdb.org/miRDB), Pictar (http://pictar.mdc-berlin.de), Pita (http://genie.weizmann.ac.il/pubs/mir07/mir07_prediction.html), RNA22 (http://cbcsrv.watson.ibm.com/rna22.html), RNAhybrid (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/submission.html), and Targetscan (http://www.targetscan.org). Candidate mRNAs were selected if they were identified as miRNA targets in at least 5 of 8 databases, as mentioned previously, and linked to immunity, inflammation, and pain by the Gene Ontology biological process (www.geneontology.org). Results from both miRWalk and a PubMed search were integrated for interpretation of the final results.
Statistical Analysis
The fold change of miRNA changes was calculated using the 2(−ΔCT) method with respect to the healthy control. miRNAs exhibiting a ≥1.5- or ≤0.8-fold change were included in the data. These cutoffs are chosen because given that multiple microRNAs work in concert to exert biological function, summation of even small differences can yield biological changes when miRNAs acting on similar pathways change. These fold change cutoffs have been used in previous publications23,24. P values are calculated based on a Student t test of the replicate 2(−ΔCT) values for each miRNA in the control asymptomatic and diseased (ECR or periodontitis) samples and ECR and CP lesions. miRNAs with the P values ≤.05 (highlighted by asterisks) and 0.1 are listed in Tables 1–3. P values <.05 were considered significant.
TABLE 1 -.
Differentially Expressed MicroRNAs in External Cervical Resorption Samples Relative to Controls
| MicroRNA Identification | Fold change | P value |
|---|---|---|
| hsa-miR-122–5p* | 3.59† | .04 |
| hsa-let-7i-5p | 1.92† | .08 |
| hsa-miR-181b-5p | 1.89† | .05 |
| hsa-miR-140–3p | 1.60† | .08 |
| hsa-miR-103a-3p | 0.80‡ | .07 |
| hsa-miR-30b-5p | 0.67‡ | .06 |
| hsa-miR-30c-5p | 0.58‡ | .05 |
| hsa-miR-27a-3p | 0.58‡ | .07 |
| hsa-miR-20a-5p* | 0.55‡ | .04 |
| hsa-miR-18a-5p | 0.51‡ | .08 |
| hsa-miR-19a-3p | 0.41‡ | .09 |
| hsa-miR-210–3p* | 0.34‡ | .03 |
| hsa-miR-200c-3p | 0.34‡ | .08 |
| hsa-miR-99a-5p* | 0.25‡ | .01 |
P < .05.
Up-regulation in comparison with asymptomatic controls.
Down-regulation in comparison with asymptomatic controls.
TABLE 3 -.
Differentially Expressed MicroRNAs in External Cervical Resorption Samples Relative to Chronic Periodontitis Lesion Samples
| MicroRNA Identification | Fold change | P value |
|---|---|---|
| hsa-miR-302b-3p | 2.58† | .08 |
| hsa-miR-223p-3p* | 0.48‡ | .01 |
| hsa-miR-143b-3p | 0.54‡ | .08 |
P < .05.
Up-regulation in comparison with chronic periodontitis.
Down-regulation in comparison with chronic periodontitis.
RESULTS
Demographic, clinical, and pulp diagnosis data of the study participants are shown in Supplemental Table S1 (available online at www.jendodon.com). All ECR-involved teeth were either maxillary or mandibular anterior teeth. The teeth with ECR, having had a history of trauma, all had the diagnosis of necrotic pulp. Interestingly, the cases of unknown etiology for ECR had vital pulp status. Figure 1 depicts radiographic evidence of 2 ECR-involved teeth (#6 and #24) from different patients. ECR samples were collected from the lesion site during repair surgeries. For CP gingival biopsies, 1 piece from the distal site of tooth #31 with a probing depth of 7 mm, 1 piece from the midbuccal site of tooth #18 with a probing depth of 11 mm, and 1 from the distal site of tooth #11 with a probing depth of 10 mm were collected during periodontal surgeries. All sites exhibited bleeding upon probing before surgeries.
FIGURE 1 -.
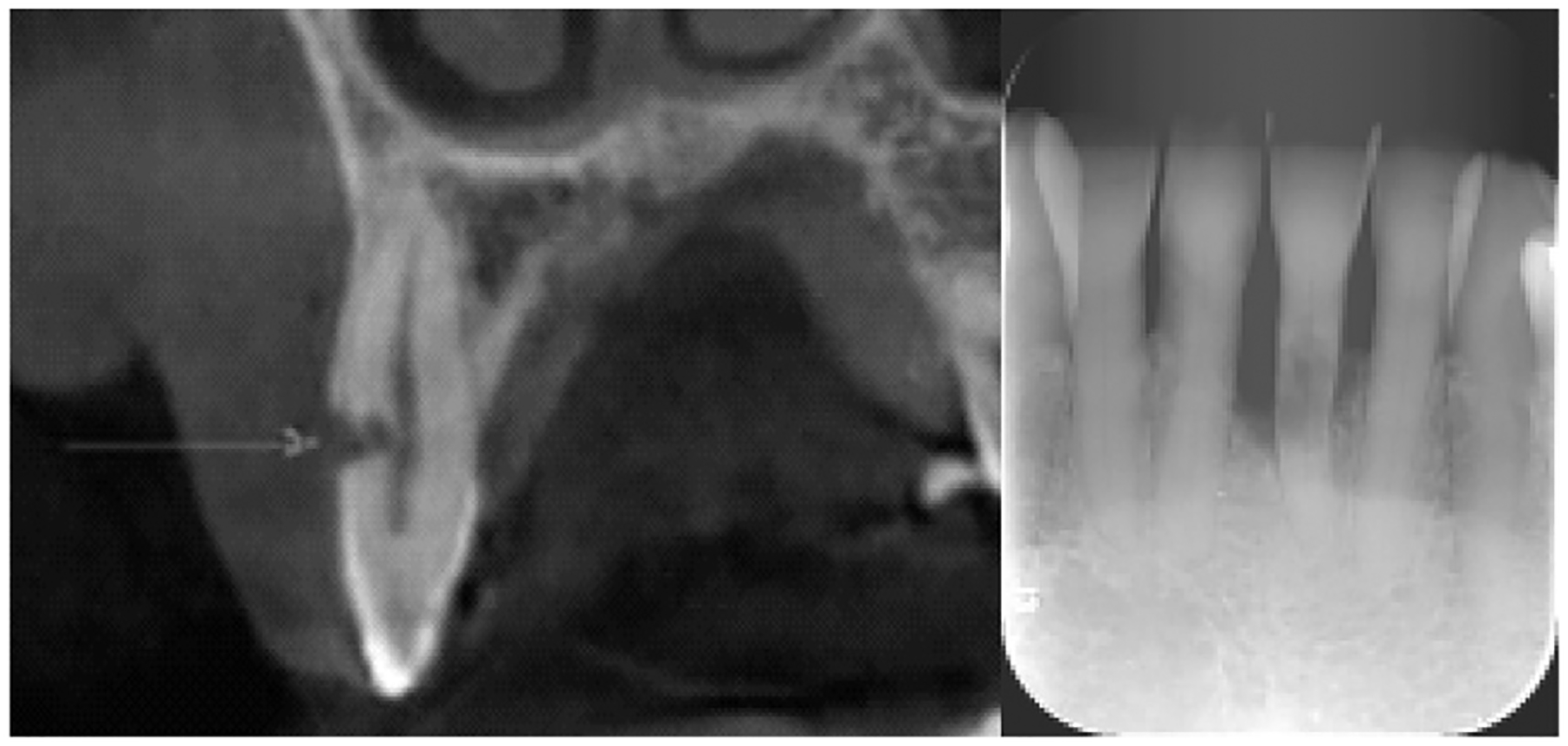
Representative ECR lesions shown on radiography. ECR is present on (left) tooth #6 and (right) tooth #24. The arrow indicates an ECR lesion at the buccal side of #6 on radiography.
Differentially Expressed miRNAs in ECR and CP
This study first compared the differential expression levels of individual miRNA species in ECR and CP with their adjacent asymptomatic control gingival connective tissues biopsied from the same participants. In ECR analysis, there were 5 ECR tissues and 5 control connective tissues. Two control samples were discarded during data analysis because of very low miRNA values for all the RNA species detected compared with the remaining samples of the same group. We did not include those 2 outliers in the control group for any data analysis. Four miRNAs with significantly increased expression were identified in ECR granulomatous tissues (n = 5) when compared with the adjacent control connective tissues (n = 3) (Table 1, P < .1). Among these, miR-122–5p exhibited a 3.58-fold increase and was the most elevated miRNA in ECR in comparison with the controls (P < .05). In addition, significant down-regulation of 10 miRNAs was observed in ECR lesions (Table 1, P < .1), with miR-99a-5p being the most down-regulated at approximately 25% of the expression level compared with control tissues. Figure 2A shows the levels of differentially expressed miRNA species with a threshold of P < .05 in ECR compared with the corresponding controls.
FIGURE 2 -.
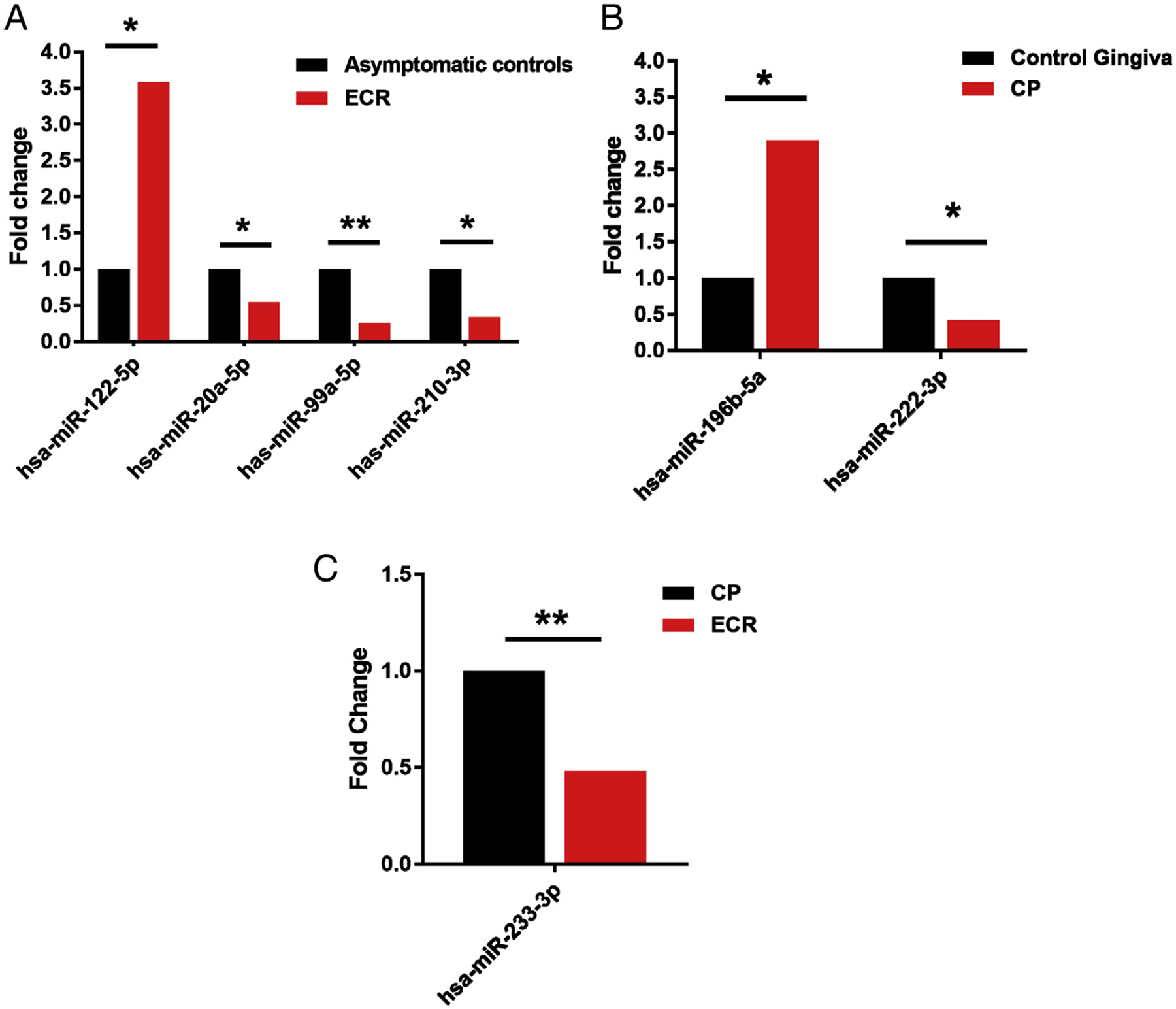
Histograms showing fold changes of significantly differentially expressed miRNAs in (A) ECR tissue compared with asymptomatic controls, (B) CP tissues compared with healthy gingiva, and (C) ECR and CP tissues. *P < .05. **P < .01.
miRNA profile analysis in healthy (n = 3) and CP (n = 3) tissues showed differential expression of 6 miRNAs in CP tissues (P < .1). Among these, 3 miRNAs were significantly increased, and 3 miRNA species were significantly down-regulated in CP tissues compared with the controls (Table 2, P < .1). In CP granulomatous tissues, miR-143–3p increased 3.5-fold and was the most up-regulated miRNA, whereas the expression of miR-222–3p in CP tissues was down-regulated more than 2-fold when compared with control connective tissues. Figure 2B shows the levels of differentially expressed miRNA species with a threshold of P < .05 in CP compared with controls.
TABLE 2 -.
Differentially Expressed MicroRNAs in Chronic Periodontitis Samples Relative to the Controls
| MicroRNA identification | Fold change | P value |
|---|---|---|
| hsa-miR-143–3p | 3.50† | .07 |
| hsa-miR-29b-3p | 3.23† | .09 |
| hsa-miR-196b-5p* | 2.90† | .049 |
| hsa-miR-27p-3p | 0.47‡ | .078 |
| hsa-miR-30b-5p | 0.46‡ | .07 |
| hsa-miR-222–3p* | 0.42‡ | .02 |
P < .05.
Up-regulation in comparison with asymptomatic controls.
Down-regulation in comparison with asymptomatic controls.
One miRNA target, miR-302b-3p, was up-regulated in ECR samples, whereas miR-233–3p and miR-143–3p targets were down-regulated, with miR-233–3p reaching the significance threshold (P = .0054) in ECR samples compared with periodontitis lesions (Table 3, P < .1; Fig. 2C).
Predicted Targets and Biological Processes of Differentially Expressed miRNAs
We performed bioinformatics analysis (using miRWalkversion 2.0) to identify the human mRNA targets of the differentially expressed miRNAs in both inflammatory disease entities. The purpose of this analysis was to explore the common host target mRNAs or biological activities involved in ECR and CP lesions that clinically result in hard tissue resorption. To narrow the scope of target mRNAs, the study specifically focused on the miRNAs with a P value <.05. As seen in Table 4, the targeted transcripts of mammalian genes were involved in a wide spectrum of biological activities, including chemokine activity, extracellular matrix activity, protein turnover, control of mRNA stability, regulation of cell proliferation, and immune and inflammatory responses. For example, the dampened expression of miR-222–3p in CP lesions may presumably increase the mRNA level of matrix metalloproteinases 19, which is a key molecule for extracellular matrix degradation and responsible for resorption of mineralized tissues25. Both targets of miR-122–5p, which were shown to be up-regulated in ECR lesions, VAMP3 and XIAP, are all involved in inflammatory or immune responses26,27.
TABLE 4 -.
Potential miRNA Target Genes Identified with miRWalk and Gene Product Functions Determined by GO Biologic Process
| MicroRNA | Target mammalian gene | Gene product function | GO term (accession, ontology) |
|---|---|---|---|
| miR-196–5p | CCL23, chemokine (C-C motif) ligand 23 | Chemotactic activity for monocytes, resting T lymphocytes, and neutrophils | GO:0008009, chemokine activity (molecular function) |
| RAB29, member RAS oncogene familylike 1 | Late endosome-/lysosome-associated small GTPase that regulate endosomal sorting, biogenesis of lysosome and phagocytosis | GO:0017137, Rab GTPase binding (molecular function) | |
| CTSO, cathepsin O | Proteolytic enzyme involved in cellular protein degradation and turnover | GO:0004197, cysteine-type endopeptidase activity (molecular function) | |
| miR-222–3p | MMP19, matrix metallopeptidase 19 | Endopeptidase that degrades various components of the extracellular matrix during development, hemostasis, and pathological conditions | GO:0004222, metalloendopeptidase activity (molecular function) |
| PLCE1; phospholipase C, epsilon 1 | By generating secondary messengers, this gene plays a role in cell survival, cell growth, actin organization, and T-cell activation | GO:0004435, phosphatidylinositol phospholipase C activity (molecular function) | |
| AGO3, argonaute RISC catalytic component 3 | Binds to short RNAs such as microRNAs (miRNAs) and represses the translation of mRNAs, which are complementary to them | GO:0035278, miRNA-mediated inhibition of translation (biological process) | |
| miR-99a-5p | ETV3, ETS translocation variant 3 | Transcriptional repressor that contributes to growth arrest during terminal macrophage differentiation | GO:0008285, negative regulation of cell proliferation (biological process) |
| TRIM71, E3 ubiquitin-protein ligase TRIM71 | Binds to miRNAs and associates with AGO2, participates in posttranscriptional repression of transcripts in miRNA-dependent and -independent manner | GO:0061158, 3’-UTR-mediated mRNA destabilization (biological process) | |
| miR-210–3p | E2F3, E2F transcription factor 3 | Involved in cell cycle regulation or DNA replication | GO:0008284; positive regulation of cell proliferation (Biological process) |
| FGFRL1, fibroblast growth factor receptorlike 1 | Negatively regulates cell proliferation | GO:0017134, fibroblast growth factor binding (molecular function) | |
| SCARA3; scavenger receptor class A, member 3 | Protect cells by scavenging oxidative molecules or harmful products of oxidation | GO:0006979, response to oxidative (biological process) | |
| miR-20a-5p | EIF4E, eukaryotic translation initiation factor 4E | Involved in translation by binding to the 7-methylguanosine-containing mRNA cap during the initiation of protein synthesis and facilitates ribosome binding | GO:0003743, translation initiation factor activity (molecular function) |
| ITGB6; integrin, beta 6 | Integrin alpha-V/beta-6 are involved in cell invasion, cell adhesion; receptor for herpesvirus entry into host cells | GO:0005178; integrin binding (molecular function) | |
| miR-122–5p | VAMP3, vesicle-associated membrane protein 3 | SNARE involved in vesicular transport from the late endosomes to the trans-Golgi network | GO:0002479; antigen processing and presentation of exogenous peptide antigen via MHC class I, TAP-dependent (molecular function) |
| XIAP, X-linked inhibitor of apoptosis | Direct caspase inhibitor; modulates inflammatory signaling and immunity, copper homeostasis, mitogenic kinase signaling, cell proliferation | GO:0043027, cysteine-type endopeptidase inhibitor activity involved in apoptotic process (molecular function) |
GO, Gene Ontology; GTPase, guanosine triphosphate; MHC, major histocompatibility complex; mNRA, messenger RNA; TAP, transporter associated with antigen processing; UTR, untranslated region.
KEGG Pathway Analyses of Targeted Genes
This study further investigated the biological pathways potentially regulated by differentially expressed miRNAs in ECR and CP through their predicted gene targets. KEGG pathway analysis based on the miRNAs with P < .05 showed that several pathways were targeted in both ECR and CP including the ErbB signaling pathway, which is involved in cell proliferation, differentiation, apoptosis, and cell motility, and the PI3K-Akt signaling pathway, which participates in diverse cellular processes such as metabolism, growth, transcription and protein synthesis, and focal adhesion (Supplemental Table S2 is available online at www.jendodon.com). We also identified disease-specific biological pathways. For example, the mitogen-activated protein kinases signaling pathway, which plays important roles in inflammation, and the transforming growth factor beta signaling pathway, which is critically involved in bone formation and the anti-inflammatory response, neurotrophin pathway, and mineral absorption pathway, were significantly activated only in ECR lesions, whereas extracellular matrix–receptor interaction was uniquely activated in CP. The convergence of pathways from ECR and CP may indicate that common biological processes, such as inflammation, bone remodeling, development, cell contact, and cell survival and proliferation, are involved in the pathogenesis of both diseases.
DISCUSSION
In this report, we sought to uncover the potential role of miRNAs in ECR and CP, both of which are inflammatory diseases that possess unique pathogenicity. Clinically, ECR and CP have a common feature regarding the resorption of the adjacent hard tissues in which ECR affects the cementum and dentin and CP affects the alveolar bone. To our knowledge, this pilot study is the first to identify differential expression of miRNAs in ECR. By comparing the differentially regulated miRNA species in ECR and CP lesions with the clinically healthy counterparts, this study investigated the convergence and divergence of miRNA-targeted mammalian gene activities and the biological processes in the pathogenesis of these 2 distinct disease entities. Differential miRNA profiles noticed in our comparative analysis of ECR and CP lesions further support their distinct pathobiology.
Differential expression (both up- and down-regulation) of miRNA species was identified in the diseased granulomatous tissues for both ECR and CP. This study revealed an altered expression of some miRNAs in both diseases. For example, miR-30b-5p expression was down-regulated in both diseased states. This microRNA species can potentially regulate inflammatory genes, such as interleukin (IL)-1β. Studies from our laboratory have also shown that miR-30b-5p is down-regulated upon challenge by lipopolysaccharide or immunoglobulin G–coated beads28. The overexpression of miR-30b alters tumor necrosis factor alpha, IL-6, and IL-12p70 secretion by myeloid cells challenged with whole Escherichia coli or LPS29,30. In an experimental contusive spinal cord injury rat model, miR-30b-5p was down-regulated in the injury epicenter31. The reduced expression of miR-30b-5p was predicted to up-regulate the tissue level of IL-1β as well as other inflammatory mediators. Another study showed miR-30b-5p was significantly up-regulated in rat islets exposed to inflammatory cytokines in vitro32. A wide range of genes with diverse activities and functions targeted by those differentially expressed miRNAs were identified in both ECR and CP diseases. These genes play critical roles in a plethora of physiological and pathological processes, including the immune response (eg, CCL23, MAP2K6, and XIAP), remodeling extracellular matrix (eg, matrix metalloproteinases 19 and ITGB6), cell proliferation, differentiation or apoptosis (eg, PLCE1, ETV3, E2F3, and FGFRL1), and regulation of basic cellular functions (eg, CTSO and EIF4E) (Table 4).
Based on the KEGG pathway analysis, this study identified a wide range of diverse biological pathways significantly regulated by miRNAs. The shared common pathways activated in both ECR and CP may indicate that common biological processes, such as inflammation, bone remodeling, development, cell contact, and cell survival and proliferation, are involved in the pathogenesis of these conditions. The convergence of these pathways may also point to the shared molecular mechanisms in the pathogenesis of both ECR and CP.
In contrast, we also observed certain pathways that are uniquely identified in either ECR or CP. For example, the mineral absorption pathway was only significantly associated with ECR. However, extracellular matrix–receptor interaction pathway activation was only observed in CP lesions. In addition, the neurotrophin signaling pathway was also significantly associated with ECR. The neuropathway activation has been reported in other oral inflammatory conditions. For example, Offenbacher et al33 reported that in an experimental gingivitis clinical model, genes related to the neuronal process were the second largest pathway next to the immune response pathway. The close association between the neural pathway and dental abnormalities remains to be further explored. The divergence of these pathways suggests that disease-specific molecular and biological activities may account for the unique clinical characteristics of ECR and CP in which resorption occurs in the cementum and dentin, whereas in CP the disease process affects mostly the alveolar bone.
The identified miRNA species in both inflammatory diseases entities and the convergence and divergence profile of the targeted pathways may serve as the foundation for developing novel therapeutic interventions. miRNA targeting has been recently appreciated as a potential efficient approach to treat several diseases, including those in the craniofacial region34. Synthetic miRNAs designed to rescue the disease-associated abnormally expressed genes have been already used in clinical trials34. The ultimate goals for the current research are to initiate the elucidation of potential destructive pathways causing ECR and eventually develop therapeutic vehicles to prevent and/or arrest ECR.
One limitation of this study is the selection of tissue controls. The clinically disease-free–appearing gingival connective tissue may not match the cell components present in the ECR granulomatous lesion. In addition, those control tissues that were collected approximate to the periodontitis lesion may have undergone histologic or molecular changes mimicking the initial periodontal disease or inflammation. A clear demarcation between inflamed granulomatous lesion tissue in periodontitis and its adjacent healthy connective tissue is usually lacking. Another limitation is the relatively small sample size for both disease entities. The data from this proof-of-principle study need to be replicated in follow-up investigations with larger sample sizes and global miRnome profiles. Our in silico findings are definitely based on bioinformatics analysis; several of these targets are very likely bona fide targets based on the ΔG values, strong sequence complementary of miRNA, and the target. To provide more convincing evidence on the role of dysregulated miRNAs in ECR pathogenesis, further studies are required to confirm miRNA targets identified here and to characterize biological function of altered miRNAs.
We conclude that this proof-of-principle in vivo study indicated that ECR has both common and unique miRNA expression profiles in comparison with CP, which are predicted to target genes regulating inflammation, immunity, and metabolism of mineralized tissues.
Supplementary Material
SIGNIFICANCE.
We profile miRNAs expressed in granulomatous tissues adjacent to lesions with external cervical resorption in humans and identified potential gene targets/pathways. Elucidation of these inflammatory mechanisms can lead to potential diagnostic, preventive, and intervention measures for this destructive condition.
ACKNOWLEDGMENTS
Supported in part by the American Association of Endodontists Foundation and the National Institutes of Health/National Institute of Dental and Craniofacial Research (grant nos. R21 DE026259-01A1, R01 DE027980, and R03 DE027147 to A.R.N.; R01 DE02105201A1 to S.N.; and 1K99DE027086 to S.Z.).
Footnotes
The authors deny any conflicts of interest related to this study.
SUPPLEMENTARY MATERIAL
Supplementary material associated with this article can be found in the online version at www.jendodon.com (https://doi.org/10.1016/j.joen.2019.06.001).
REFERENCES
- 1.Hammarstrom L, Lindskog S. Factors regulating and modifying dental root resorption. Proc Finn Dent Soc 1992;88(Suppl 1):115–23. [PubMed] [Google Scholar]
- 2.Gold SI, Hasselgren G. Peripheral inflammatory root resorption. A review of the literature with case reports. J Clin Periodontol 1992;19:523–34. [DOI] [PubMed] [Google Scholar]
- 3.Harrington GW, Natkin E. External resorption associated with bleaching of pulpless teeth. J Endod 1979;5:344–8. [DOI] [PubMed] [Google Scholar]
- 4.Fuss Z, Tsesis I, Lin S. Root resorption–diagnosis, classification and treatment choices based on stimulation factors. Dent Traumatol 2003;19:175–82. [DOI] [PubMed] [Google Scholar]
- 5.Patel S, Kanagasingam S, Pitt Ford T. External cervical resorption: a review. J Endod 2009;35:616–25. [DOI] [PubMed] [Google Scholar]
- 6.Hofmann-Lehmann R, Berger M, Sigrist B, et al. Feline immunodeficiency virus (FIV) infection leads to increased incidence of feline odontoclastic resorptive lesions (FORL). Vet Immunol Immunopathol 1998;65:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar V, Chawla A, Kaur A. Multiple idiopathic cervical root resorptions in patients with hepatitis B virus infection. J Endod 2018;44:1575–7. [DOI] [PubMed] [Google Scholar]
- 8.Mavridou AM, Bergmans L, Barendregt D, Lambrechts P. Descriptive analysis of factors associated with external cervical resorption. J Endod 2017;43:1602–10. [DOI] [PubMed] [Google Scholar]
- 9.Hartsfield JK Jr. Pathways in external apical root resorption associated with orthodontia. Orthod Craniofac Res 2009;12:236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tyrovola JB, Spyropoulos MN, Makou M, Perrea D. Root resorption and the OPG/RANKL/RANK system: a mini review. J Oral Sci 2008;50:367–76. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z, McCauley LK. Osteoclasts and odontoclasts: signaling pathways to development and disease. Oral Dis 2011;17:129–42. [DOI] [PubMed] [Google Scholar]
- 12.Sonkoly E, Stahle M, Pivarcsi A. MicroRNAs and immunity: novel players in the regulation of normal immune function and inflammation. Semin Cancer Biol 2008;18:131–40. [DOI] [PubMed] [Google Scholar]
- 13.Sugatani T, Hruska KA. Impaired micro-RNA pathways diminish osteoclast differentiation and function. J Biol Chem 2009;284:4667–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambros V. The functions of animal microRNAs. Nature 2004;431:350–5. [DOI] [PubMed] [Google Scholar]
- 15.Flynt AS, Lai EC. Biological principles of microRNA-mediated regulation: shared themes amid diversity. Nat Rev Genet 2008;9:831–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol 2010;10:111–22. [DOI] [PubMed] [Google Scholar]
- 17.Baltimore D, Boldin MP, O’Connell RM, et al. MicroRNAs: new regulators of immune cell development and function. Nat Immunol 2008;9:839–45. [DOI] [PubMed] [Google Scholar]
- 18.Pedersen I, David M. MicroRNAs in the immune response. Cytokine 2008;43:391–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao C, Rajewsky K. MicroRNA control in the immune system: basic principles. Cell 2009;136:26–36. [DOI] [PubMed] [Google Scholar]
- 20.Nares S, Moutsopoulos NM, Angelov N, et al. Rapid myeloid cell transcriptional and proteomic responses to periodontopathogenic Porphyromonas gingivalis. Am J Pathol 2009;174:1400–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorensen LK, Havemose-Poulsen A, Sonder SU, et al. Blood cell gene expression profiling in subjects with aggressive periodontitis and chronic arthritis. J Periodontol 2008;79:477–85. [DOI] [PubMed] [Google Scholar]
- 22.Ryder MI, Hyun W, Loomer P, Haqq C. Alteration of gene expression profiles of peripheral mononuclear blood cells by tobacco smoke: implications for periodontal diseases. Oral Microbiol Immunol 2004;19:39–49. [DOI] [PubMed] [Google Scholar]
- 23.Campbell JD, Liu G, Luo L, et al. Assessment of microRNA differential expression and detection in multiplexed small RNA sequencing data. RNA 2015;21:164–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moradifard S, Hoseinbeyki M, Ganji SM, Minuchehr Z. Analysis of microRNA and gene expression profiles in Alzheimer’s disease: a meta-analysis approach. Sci Rep 2018;8:4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prideaux M, Staines KA, Jones ER, et al. MMP and TIMP temporal gene expression during osteocytogenesis. Gene Expr Patterns 2015;18:29–36. [DOI] [PubMed] [Google Scholar]
- 26.Veale KJ, Offenhauser C, Lei N, et al. VAMP3 regulates podosome organisation in macrophages and together with Stx4/SNAP23 mediates adhesion, cell spreading and persistent migration. Exp Cell Res 2011;317:1817–29. [DOI] [PubMed] [Google Scholar]
- 27.Chirieleison SM, Rathkey JK, Abbott DW. Unique BIR domain sets determine inhibitor of apoptosis protein-driven cell death and NOD2 complex signal specificity. Sci Signal 2018;11:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naqvi AR, Fordham JB, Nares S. MicroRNA target Fc receptors to regulate Ab-dependent Ag uptake in primary macrophages and dendritic cells. Innate Immun 2016;22:510–21. [DOI] [PubMed] [Google Scholar]
- 29.Fordham JB, Naqvi AR, Nares S. Regulation of miR-24, miR-30b, and miR-142–3p during macrophage and dendritic cell differentiation potentiates innate immunity. J Leukoc Biol 2015;98:195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naqvi AR, Fordham JB, Nares S. miR-24, miR-30b, and miR-142–3p regulate phagocytosis in myeloid inflammatory cells. J Immunol 2015;194:1916–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu NK, Wang XF, Lu QB, Xu XM. Altered microRNA expression following traumatic spinal cord injury. Exp Neurol 2009;219:424–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bravo-Egana V, Rosero S, Klein D, et al. Inflammation-mediated regulation of MicroRNA expression in transplanted pancreatic islets. J Transplant 2012;2012:723614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Offenbacher S, Barros SP, Paquette DW, et al. Gingival transcriptome patterns during induction and resolution of experimental gingivitis in humans. J Periodontol 2009;80:1963–82. [DOI] [PubMed] [Google Scholar]
- 34.Chakraborty C, Sharma AR, Sharma G, et al. Therapeutic miRNA and siRNA: moving from bench to clinic as next generation medicine. Mol Ther Nucleic Acids 2017;8:132–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


